Abstract
The emergence of a novel coronavirus and global pandemic raised the need for the rapid development of new vaccines to reduce the morbidity and mortality associated with Covid-19. Common side effects of these vaccines such as myalgia, arthralgia, nausea, fatigue, and injection site reaction are usually self-resolving. Recognition of other potential adverse effects of these novel vaccines is important due to their rapid and widespread distribution. We report a case of a 51-year-old man admitted to Parkland Memorial Hospital with headache, nausea, vomiting, malaise, and diffuse arthralgias 3 days after he received his second mRNA-1273 SARS-CoV-2 vaccination. He was found to have hyponatremia and a low serum cortisol level. Further workup revealed hypopituitarism with central hypothyroidism, hypogonadism, and a subnormal response to cosyntropin. Magnetic resonance imaging revealed a diffusely enlarged pituitary gland consistent with acute hypophysitis. The patient responded well to glucocorticoid and thyroid hormone supplementation and was discharged after 2 days in the hospital. This is the first reported case of hypopituitarism potentially associated with Covid-19 immunization.
Keywords: Covid-19, mRNA-1273 SARS-CoV-2, hypopituitarism, hypophysitis, pituitary, vaccine, adrenal insufficiency
Introduction
On December 13, 2020, the Food and Drug Administration granted Pfizer-BioNTech the first approval of a vaccine effective against the Sars-CoV-2 virus responsible for COVID-19 infections. A week later, the Moderna vaccine was also approved. Most patients have tolerated the vaccines with mild to moderate self-limited symptoms including injection site tenderness, fevers, chills, and myalgias. As of June 2021, approximately 177 million people in the United States have received at least one dose of vaccine.1 It is important that rare but serious adverse effects potentially related to these vaccines are reported to allow for earlier identification and intervention.
Case Presentation
A 51-year-old man with no significant medical history was in his usual state of health when he received his first dose of the Moderna Covid-19 vaccine. Three days later, he noted the onset of nausea, malaise, and arthralgias. He used nonprescription non-steroidal anti-inflammatory drugs with minimal relief. Despite this, he received his second dose of Moderna vaccine 28 days later. Two days following this, he began having worsening nausea, vomiting, and mid-epigastric abdominal pain prompting him to present to the emergency department.
On presentation, the pulse was 68 beats per minute, the blood pressure 114/78 mm Hg, and the weight 77.1 kg. He was tired-appearing though the physical examination was otherwise unremarkable.
Laboratory evaluation revealed a serum sodium level of 116 mmol/L (reference range, 135-145 mmol/L). Complete blood count and kidney and liver function tests were normal.
Thyroid function tests and random cortisol were checked as part of the workup of hyponatremia. The thyroid-stimulating hormone (TSH) was 0.13 µIU/mL (reference range, 0.40-4.50 µIU/mL) and free thyroxine (free T4) level was 0.4 ng/dL (reference range, 0.8-1.8 ng/dL), consistent with central hypothyroidism. Both random and morning cortisol levels were below detectable limits (<1.0 µg/dL). He was treated with intravenous dexamethasone 4 mg and a high-dose cosyntropin (250 µg) stimulation test was performed. The adrenal response was markedly abnormal. The baseline cortisol level of <1.0 µg/dL increased to 2.4 µg/dL at 30 minutes and to 3.0 µg/dL at 60 minutes (normal response is > 18 µg/dL at either time point). Baseline adrenocorticotropic hormone (ACTH) was also low at 1.9 pg/mL (reference range, 7.2-63.3 pg/mL) which confirmed secondary adrenal insufficiency.
The patient also reported symptoms of low libido and erectile dysfunction for the past month and additional hormonal workup revealed a prolactin level of 12.9 ng/mL (reference range, 4.0-15.2 ng/mL), follicle-stimulating hormone (FSH) of 3.3 mIU/mL (reference range, 1.5-12.4 mIU/mL), luteinizing hormone (LH) of 1.9 mIU/mL (reference range, 1.7-8.6 mIU/mL), and 8 a.m. testosterone level of 108 ng/dL (reference range, 193-740 ng/dL).
The patient did not have polyuria, nocturia, polydipsia, or laboratory findings, such as elevated serum sodium and osmolality, to suggest diabetes insipidus.
As hormonal workup was consistent with secondary adrenal insufficiency, central hypothyroidism, and central hypogonadism, computed tomography (CT) of the brain was obtained. This showed eccentric 10 × 15 × 9 mm enlargement of the pituitary gland which was new compared with previous images from 2011.
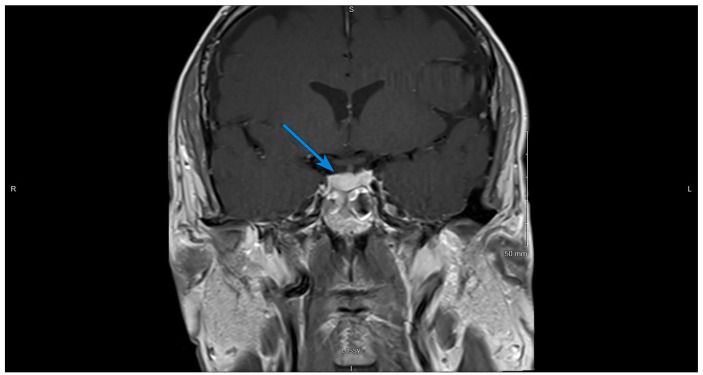
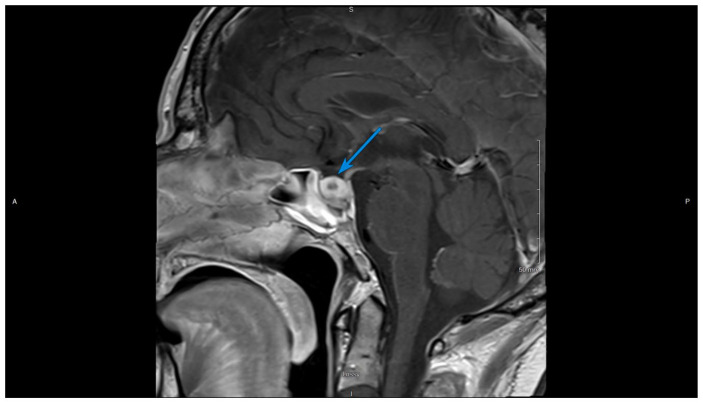
Dedicated pituitary protocol magnetic resonance imaging (MRI) showed asymmetric enlargement of the pituitary gland with a larger more globular component on the right. The total gland measured 1.2 cm AP by 1.8 cm transverse by 0.8 cm craniocaudal, and there was thickening of the pituitary stalk, findings seen with hypophysitis (Figures 1 and 2).
Figure 1.
Contrast-enhanced T1-weighted coronal image of the brain, on initial presentation. The arrow shows an asymmetric enlargement of the pituitary gland with a larger more globular component on the right.
Figure 2.
Contrast-enhanced T1-weighted sagittal image of the brain, on initial presentation. The arrow shows a homogeneous enlargement of the pituitary with thickening of the stalk.
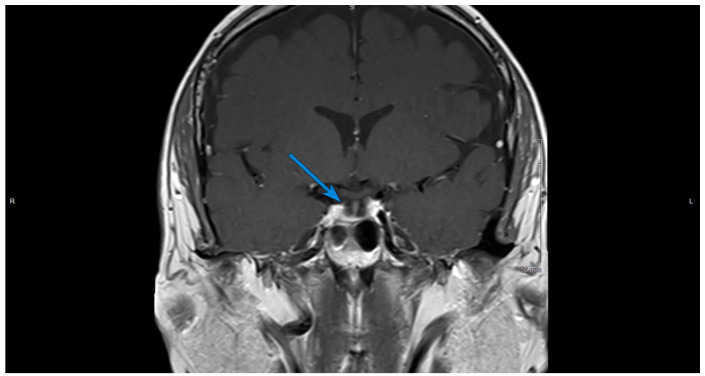
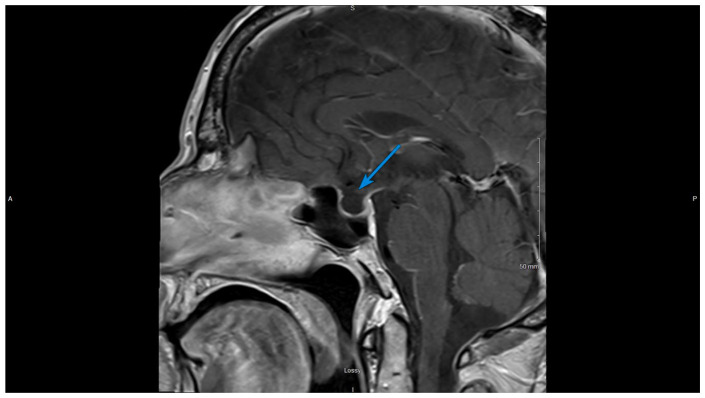
Treatment was initiated with high-dose steroids and thyroid hormone replacement. Over the next 2 days, his blood sodium level normalized and symptoms resolved. He was discharged on prednisone and levothyroxine. After 1 month, follow-up magnetic resonance imaging (MRI) of the pituitary revealed markedly diminished enlargement of the gland with a mostly empty sella (Figures 3 and 4). Plasma testosterone level normalized without testosterone replacement therapy. The hypothalamic-pituitary-adrenal axis and the hypothalamic-pituitary-thyroid axis will be evaluated on planned outpatient follow-up.
Figure 3.
Contrast-enhanced T1-weighted coronal image of the brain, 1 month after initial presentation. The arrow shows a diminished enlargement of the gland with a mostly empty sella.
Figure 4.
Contrast-enhanced T1-weighted sagittal image of the brain, 1 month after initial presentation. The arrow shows a mostly empty sella.
Discussion
Hypopituitarism is defined as deficiency of one or more pituitary hormones resulting from diseases of the pituitary gland or of the hypothalamus.2,3 The incidence of anterior pituitary deficiency has been estimated to be approximately 4 cases per 100,000 population with approximately half caused by neoplasms. Non-tumor etiologies of hypopituitarism include stroke, infection, trauma, surgery, radiation, and inflammatory disorders.
Hypophysitis is an extremely rare disorder characterized by lymphocytic, granulomatous, plasmacytic, or xanthomatous infiltration of the gland and the pituitary stalk. Patients with hypophysitis typically present with severe headaches and biochemical work up consistent with pituitary/hypothalamic dysfunction, such as adrenal insufficiency, hypothyroidism, and hypogonadism.4 Pituitary biopsy and histopathological analysis remains the gold standard, but due to the invasiveness, it is rarely performed. The diagnosis is based mainly on clinical presentation, laboratory tests, and imaging.5,6 MRI often shows a pituitary enlargement often mimicking an adenoma, pituitary stalk thickening, with diffuse homogeneous anterior pituitary contrast enhancement, and loss of posterior pituitary bright spot on T1-weighted imaging.5,7
Lymphocytic hypophysitis, also referred to as autoimmune hypophysitis, is the most common chronic inflammatory condition affecting the pituitary gland although the cause is often unknown.8
Our patient presented in a similar fashion to those experiencing autoimmune hypophysitis as a complication of immune checkpoint inhibitor therapy. Hypophysitis induced by anti-cytotoxic T-lymphocyte-associated antigen 4 antibody therapy (anti-CTLA-4) has been reported in 0.4% to 17% of treated patients and more commonly in men.9 ACTH and TSH deficiencies are the most commonly reported deficits in these patients, though hypogonadotropic hypogonadism has also been reported. The time of onset of hypophysitis averages 6 to 12 weeks following initiation of therapy.9 The mechanism of anti-CTLA4 induced hypophysitis remains unclear, though pituitary autoantibody induction and pituitary expression of CTLA4 have been suggested to play roles.10
Currently, three vaccines are authorized and recommended in the United States to prevent COVID-19: Pfizer-BioNTech vaccine, Moderna vaccine, and Johnson & Johnson/Janssen vaccine. The first two are mRNA vaccines while the latter is a vector vaccine. Reported side effects of these vaccines include fever, myalgia, arthralgia, nausea, fatigue, and injection site reactions.
Delayed large local reactions to the mRNA-1273 vaccine have been reported in which skin-biopsy specimens showed lymphohistiocytic infiltrates with CD3+ (including CD8+ and CD4+) T cells and some eosinophils.11,12
Our patient received the mRNA-1273 SARS-CoV-2 vaccine, a lipid-nanoparticle (LNP)–encapsulated mRNA vaccine expressing the prefusion-stabilized spike glycoprotein developed by Moderna.13
Recently Frara et al published the main pituitary manifestations of Covid-19, reviewed several reported cases of pituitary apoplexy in the context of pre-existing macroadenomas, but no data are yet available on possible occurrence of hypophysitis associated with Covid-19 infection.14
To our knowledge, hypophysitis or pituitary apoplexy has never been reported with any of the COVID-19 vaccines.
Conclusion
We present a 51-year-old man with no significant medical history who developed acute hypophysitis and panhypopituitarism within days of receiving the first dose of the Moderna Covid-19 vaccine.
We suspect this case of hypophysitis and acute panhypopituitarism is a result of vaccination for SARS CoV-19 based on the time correlation between his symptoms and receipt of the vaccine. We can find no other potential etiologies based on his history, physical examination, or medication list.
We have not seen any other reports of acute hypophysitis following immunization for Covid-19, and we have reported this case to the Department of Health Human Services Vaccine Adverse Event Reporting System (VAERS) as a potential complication of the vaccination. Given the life-threatening nature of untreated hypopituitarism, it is crucial that any additional cases are recognized and are urgently managed.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Verbal informed consent was obtained from the patient for their anonymized information to be published in this article.
References
- 1.Centers for Disease Control and Prevention. CDC COVID data tracker. https://covid.cdc.gov/covid-data-tracker/#vaccinations.
- 2.Ascoli P, Cavagnini F. Hypopituitarism. Pituitary. 2006;9(4):335-342. doi: 10.1007/s11102-006-0416-5. [DOI] [PubMed] [Google Scholar]
- 3.Higham CE, Johannsson G, Shalet SM. Hypopituitarism. Lancet. 2016;388(10058):2403-2415. doi: 10.1016/S0140-6736(16)30053-8. [DOI] [PubMed] [Google Scholar]
- 4.Powrie JK, Powell M, Ayers AB, Lowy C, Sönksen PH. Lymphocytic adenohypophysitis: magnetic resonance imaging features of two new cases and a review of the literature. Clin Endocrinol (Oxf). 1995;42(3):315-322. doi: 10.1111/j.1365-2265.1995.tb01881.x. [DOI] [PubMed] [Google Scholar]
- 5.Angelousi A, Cohen C, Sosa S, et al. Clinical, endocrine and imaging characteristics of patients with primary hypophysitis. Horm Metab Res. 2018;50(4):296-302. doi: 10.1055/s-0044-101036. [DOI] [PubMed] [Google Scholar]
- 6.Gubbi S, Hannah-Shmouni F, Verbalis JG, Koch CA. Hypophysitis: an update on the novel forms, diagnosis and management of disorders of pituitary inflammation. Best Pract Res Clin Endocrinol Metab. 2019;33(6):101371. doi: 10.1016/j.beem.2019.101371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunn BH, Martin WG, Simpson S, Jr, Mclean CA. Idiopathic granulomatous hypophysitis: a systematic review of 82 cases in the literature. Pituitary. 2014;17(4):357-365. doi: 10.1007/s11102-013-0510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caturegli P, Newschaffer C, Olivi A, Pomper MG, Burger PC, Rose NR. Autoimmune hypophysitis. Endocr Rev. 2005;26(5):599-614. doi: 10.1210/er.2004-0011. [DOI] [PubMed] [Google Scholar]
- 9.Corsello SM, Barnabei A, Marchetti P, De Vecchis L, Salvatori R, Torino F. Endocrine side effects induced by immune checkpoint inhibitors. J Clin Endocrinol Metab. 2013;98(4):1361-1375. doi: 10.1210/jc.2012-4075. [DOI] [PubMed] [Google Scholar]
- 10.Byun DJ, Wolchok JD, Rosenberg LM, Girotra M. Cancer immunotherapy—immune checkpoint blockade and associated endocrinopathies. Nat Rev Endocrinol. 2017;13(4):195-207. doi: 10.1038/nrendo.2016.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baeck M, Marot L, Belkhir L. Delayed large local reactions to mRNA vaccines. N Engl J Med. 2021;384(24):e98. doi: 10.1056/NEJMc2104751. [DOI] [PubMed] [Google Scholar]
- 12.Blumenthal KG, Saff RR, Freeman EE. Delayed large local reactions to mRNA vaccines. Reply. N Engl J Med. 2021;384(24):e98. doi: 10.1056/NEJMc2104751. [DOI] [PubMed] [Google Scholar]
- 13.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frara S, Allora A, Castellino L, Filippo L, Loli P, Giustina A. COVID-19 and the pituitary. Pituitary. 2021;24(3):465-481. doi: 10.1007/s11102-021-01148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]