Abstract
A significant increase in the incidence of caseous lesions in the lymph nodes of slaughter pigs prompted a large-scale investigation in five slaughterhouses in The Netherlands. In total, 158,763 pigs from 2,899 groups underwent gross examination. At least one pig with caseous lesions in the submaxillary and/or mesenteric lymph nodes was observed in each of 154 of the 2,899 groups examined (5%). In total, 856 pigs (0.5%) were affected. As many as five pigs in each of 141 of the 154 positive groups (91.5%) had lymph node lesions. Greater numbers of pigs with affected lymph nodes were found in 13 groups (8.5%). Four pigs had lesions in the kidneys, liver, or spleen. Acid-fast bacteria were detected by microscopic examination of 121 of 292 Ziehl-Neelsen-stained smears of caseous lesions (41%). In a follow-up study, Mycobacterium avium complex (MAC) bacteria were isolated from 219 of 402 affected lymph nodes (54.2%). Ninety-one of the isolated strains were analyzed by restriction fragment length polymorphism (RFLP) typing with insertion sequence IS1245 as a probe. All but 1 of these 91 strains contained IS1245 DNA, indicating that pigs in The Netherlands carried almost exclusively M. avium bacteria and no other bacteria of MAC. Only one pig isolate exhibited the bird-type RFLP pattern. MAC isolates from 191 human patients in The Netherlands in 1996 were also typed by RFLP analysis. Computer-assisted analysis showed that the RFLP patterns of 61% of the human isolates and 59% of the porcine isolates were at least 75% similar to the RFLP patterns of the other group of strains. This indicates that pigs may be an important vehicle for M. avium infections in humans or that pigs and humans share common sources of infection.
Severe Mycobacterium avium complex (MAC) infections in humans, especially in human immunodeficiency virus-positive and other immunodeficient individuals, have been reported (8, 13). The origin of MAC infections in humans is still a matter of speculation. Previous studies have shown that the MAC bacteria are present in birds, soil, compost, water, animals, pigs, and even cigarettes (2, 3, 5, 6, 8, 11, 19). As suggested by the designation M. avium, infections were once thought to be derived from birds. Later, serotyping showed that only some of the MAC bacteria isolated from humans represent serotypes 1, 2, and 3, which are the most common serotypes among bird isolates (1, 7).
Recently, new molecular tools like restriction fragment length polymorphism (RFLP) typing with the insertion sequence IS1245 (IS1245 RFLP analysis) have become available (2, 6, 12). Genotyping of M. avium strains from various sources in Switzerland indicated that both pigs and humans were infected with strains carrying a large number of IS1245 elements (2). IS901 and IS1245 RFLP typing showed that 47 M. avium isolates from birds in The Netherlands invariably belonged to a well-conserved separate taxon within MAC. Bird-type RFLP patterns were observed only as an exception among isolates from other hosts (2, 12). These facts rule birds out as significant sources of M. avium infections in humans in The Netherlands (12).
The current study was undertaken to determine the prevalence of MAC in the lymph nodes of pigs. Furthermore, in order to examine the significance of M. avium infections in slaughter pigs with regard to public health aspects, the IS1245 RFLP patterns of porcine isolates were compared with those of the M. avium strains isolated from humans in The Netherlands in 1996.
MATERIALS AND METHODS
Gross examination of pigs.
In an initial study, special attention was given to the gross examination of the submaxillary and mesenteric lymph nodes of pigs in five slaughterhouses during a 2-week period at the end of 1996. The submaxillary lymph nodes were incised, and the mesenteric lymph nodes were palpated. The following information was collected: the farm identification numbers of the pigs slaughtered, the number of pigs slaughtered per farm, the number of pigs with caseous lesions in the submaxillary or mesenteric lymph nodes, and the number of pigs whose spleen, liver, or kidneys were also affected. Whenever possible, up to three specimens per group were studied by microscopic examination of Ziehl-Neelsen-stained smears.
Sampling, culture, and identification of mycobacteria from pigs.
In a follow-up study performed in early 1997, the presence of mycobacteria in caseous lesions was determined by culture. For this purpose, macroscopically positive submaxillary and mesenteric lymph nodes were collected at six slaughterhouses and were frozen at −20°C. In the first part of the follow-up study, samples were taken from each of three to four pigs in 44 groups in which several animals were affected. In the second part of the follow-up study, 144 groups with only one or two affected animals each were sampled. After arrival at the laboratory, the samples were thawed, and direct smears were produced. Ziehl-Neelsen-stained material was then examined microscopically. In addition, cultures were grown from all lesions by the following procedure: all lesions were ground, decontaminated by oxalic acid-sodium hydroxide treatment, and inoculated onto Löwenstein-Jensen medium, Stonenbrink egg medium, and Middlebrook 7H10 agar, followed by incubation for 4 weeks at 37°C.
Subcultures were made from colonies suspected of representing MAC bacteria. The MAC bacteria of the subcultures were identified by the following characteristics: growth after 2 to 4 weeks of incubation, negative or doubtful acid phosphatase reaction, negative nitrate reductase reaction, weakly positive catalase reaction (<45 mm) at room temperature, variable catalase reaction at 68°C, negative β-d-galactosidase reaction, positive nicotinamidase and pyrazinamidase activities, and negative urease activity by the amidase test of Bönicke. All but 1 of the 91 isolated MAC strains contained IS1245, which is characteristic of M. avium (2, 6, 12). To ensure this identification, 30 IS1245-containing MAC isolates were subjected to the Accuprobe test specific for M. avium, and they were found to be positive.
MAC bacteria from humans.
In 1996, 191 MAC isolates originating from 35 peripheral laboratories were received at the National Institute of Public Health and the Environment (RIVM) in The Netherlands. This number covers at least 80% of all human MAC strains isolated in The Netherlands in 1996.
Serotyping.
MAC isolates were tested by slide agglutination, as described by Engel et al. (4), to determine their serotypes. The panel of test sera represented serotypes 1 to 4 and 8.
DNA fingerprinting.
M. avium isolates were DNA fingerprinted by RFLP typing; IS1245 was used as a probe, as described previously (12, 16). Internal and external molecular size markers and high-resolution gels (24 cm) were applied to facilitate computer-assisted analysis.
Computer-assisted RFLP analysis.
Analysis of the IS1245 fingerprints was done with computer assistance, using GelCompar software, version 4.1 (Applied Maths, Kortrijk, Belgium), as described in a proposal for standardization of IS1245 RFLP typing (16). The band positions of the IS1245-containing restriction fragments were compared with those of a set of internal molecular weight markers by superimposing the autoradiograms of the IS1245 DNA fingerprints and the autoradiograms of the internal markers. The patterns were compared by the unweighted pair group method with the arithmetic averages clustering method and with the Dice coefficient according to the instructions of the manufacturer of GelCompar.
RESULTS
Examination of affected lymph nodes by microscopy and culture.
A total of 158,763 pigs in 2,899 groups were inspected during the initial study at the end of 1996. Each of 154 groups (5%) included at least one pig with caseous lesions in the submaxillary and/or the mesenteric lymph nodes. Altogether, 856 pigs (0.5%) were affected. For practical reasons, only 292 lesion smears were microscopically examined. Acid-fast bacteria were seen in 121 of them. Five or fewer pigs in each of 141 of the affected groups had caseous lesions. Greater numbers of affected pigs were detected in the remaining 13 groups. The average percentage of affected pigs in these 13 groups amounted to 31, and the range was between 8 and 78%. Only four pigs also had macroscopic deviations in the kidney, liver, or spleen.
In order to examine whether M. avium was the etiologic agent of these caseous lesions, a follow-up study was planned for early 1997. In the first part of the follow-up study, 239 lymph nodes with caseous lesions from pigs from 44 farms were examined (Table 1). These farms were not the same ones as those in the initial study. MAC bacteria were isolated from 166 of the lymph nodes (69%) from 39 of the groups examined (89%). Seventy-eight percent of the affected mesenteric lymph nodes and 52% of the submaxillary lymph nodes yielded growth of MAC bacteria. In the second part of the follow-up study, lymph nodes from 163 pigs from 144 farms were examined (Table 1). MAC bacteria were isolated from 53 of the pigs (33%), which originated from 46 of all groups examined (32%). Forty-nine percent of the affected mesenteric lymph nodes and 23% of the submaxillary lymph nodes were found to be positive for MAC by culture. From the mesenteric and submaxillary lymph nodes with a positive culture for MAC bacteria, a total of 82 and 80% of the samples, respectively, were also found to be positive by microscopic examination (Table 1). However, also a total of 79 and 83% of the mesenteric and submaxillary lymph nodes with negative cultures for MAC bacteria, respectively, yielded acid-fast bacilli in the microscopic examination (Table 1). Furthermore, rapidly growing mycobacteria from 53 lymph nodes were cultured and were found to have an orange pigment. These non-M. avium mycobacteria were mainly (40 of the 53) isolated from the submaxillary lymph nodes.
TABLE 1.
Correlation of culture results and microscopic examination of affected lymph nodes in the first and second parts of the follow-up studya
| Study part and lymph node | No. of samples | No. (%) of samples with positive cultures
|
No. (%) of samples with negative cultures
|
||||
|---|---|---|---|---|---|---|---|
| Total | ZN pos | ZN neg | Total | ZN pos | ZN neg | ||
| First part | |||||||
| Mesenteric | 159 | 124 | 100 (81) | 24 | 35 | 27 (77) | 8 |
| Submaxillary | 80 | 42 | 32 (76) | 10 | 38 | 30 (79) | 8 |
| Total | 239 | 166 | 132 | 73 | 57 | ||
| Second part | |||||||
| Mesenteric | 61 | 30 | 26 (87) | 4 | 31 | 24 (77) | 7 |
| Submaxillary | 102 | 23 | 20 (87) | 3 | 79 | 67 (85) | 12 |
| Total | 163 | 53 | 46 | 110 | 91 | ||
ZN, microscopic examination of Ziehl-Neelsen-stained material; pos, positive; neg, negative.
Serotyping.
The serotypes of the MAC isolates were determined by the slide agglutination method, and the results are given in Fig. 1. Most strains were of serotype 3 (18 strains) or 4 (20 strains), and 39 isolates did not react with the panel of sera that we used. A minority of the isolates were of serotype 2, 8, or 4/8. No correlation was found between the serotypes and the IS1245 RFLP patterns.
FIG. 1.
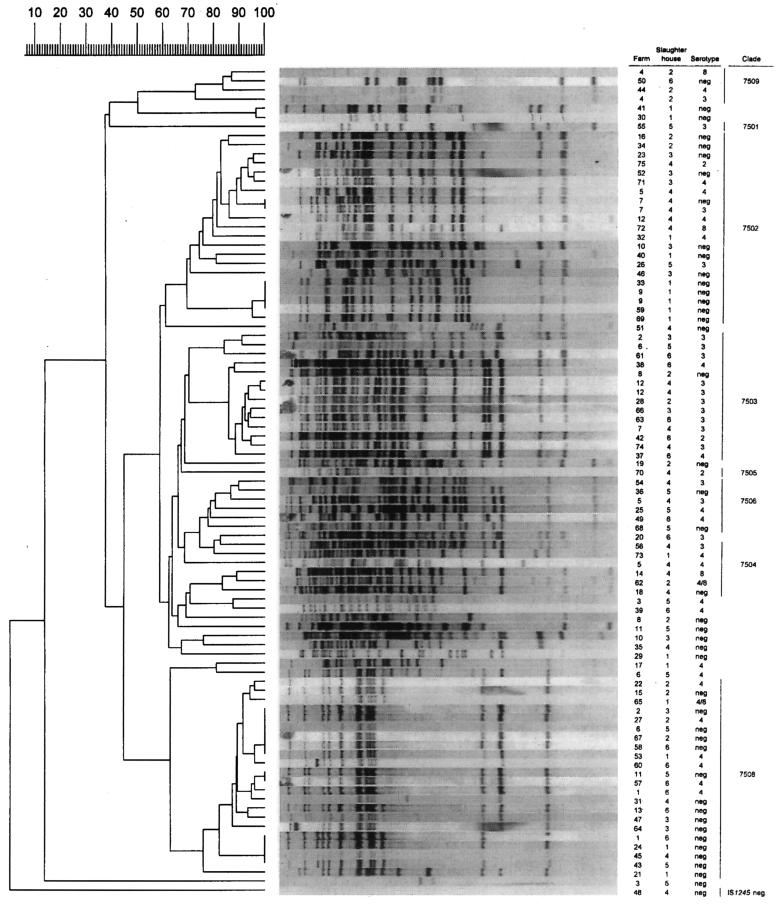
Dendrogram of the 91 IS1245 RFLP patterns of M. avium complex isolates from pigs. The columns indicate (i) the farms where the pigs originated, (ii) the slaughterhouses where the pigs were processed, (iii) the serotypes of the isolates, and (iv) the IS1245 RFLP genotype family (clade) to which the isolate belongs. The numbers at the top represent percent relatedness.
IS1245 RFLP typing of porcine isolates.
To get an impression of the occurrence of IS1245 RFLP types in various geographic regions, 10 to 20 isolates from pigs from each of the six slaughterhouses enrolled in this study were genotyped. Figure 1 shows a dendrogram of all 91 DNA fingerprint patterns. Only one of the IS1245 RFLP patterns, consisting of three bands, represented the bird-type DNA fingerprint (2, 6, 12). One other MAC isolate was devoid of IS1245 DNA, indicating that this strain represents a grouping other than M. avium in the MAC. The number of copies for the other 89 strains ranged from 9 to 34, with an average of 21 per strain. In general, the degree of polymorphism among the DNA fingerprints of pig isolates was large. However, most of the isolates could be grouped into genotype families that shared a similarity of at least 75% among the IS1245 RFLP patterns (Fig. 1).
The M. avium isolates subjected to RFLP typing originated from 91 pigs from 75 farms. A single pig from each of 63 farms was examined, and two or three pigs from each of 12 farms were inspected. In the case of 11 of the 12 multiple isolates from the same farm, two or more different DNA fingerprints were found (Fig. 1). This indicates the presence of multiple M. avium strains in pigs from 11 of 12 farms from which more than one porcine M. avium isolate was obtained. In contrast, identical DNA fingerprints were found among isolates from different farms. In total, nine clusters, with a cluster size of two to six isolates, comprised 30 strains originating from 28 farms in a widespread geographic area.
Comparison of RFLP patterns of human and porcine M. avium isolates.
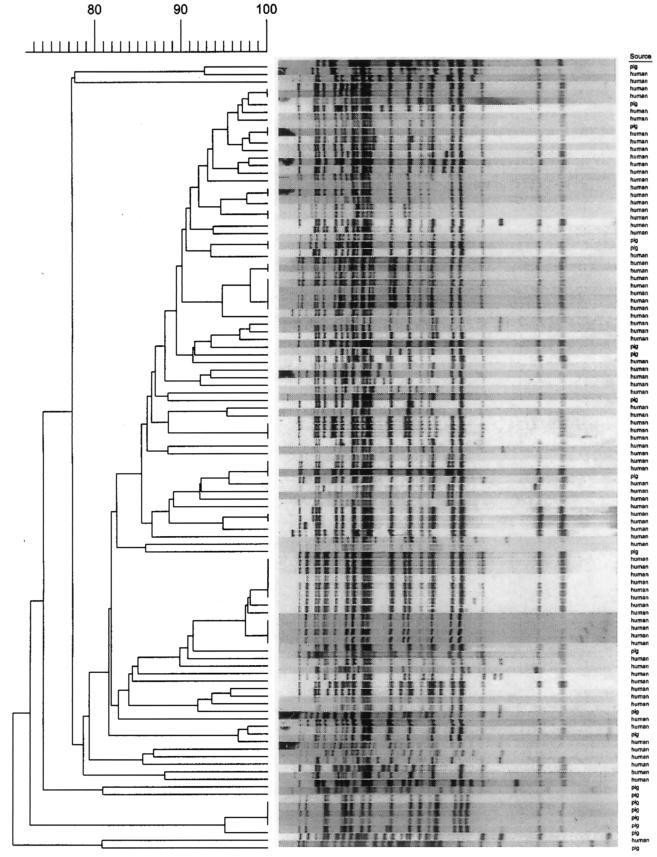
In 1996, 191 MAC isolates from the same number of human patients were subjected to IS1245-based RFLP typing in the framework of an epidemiological population-based study on MAC infections in The Netherlands (13). Forty-eight of the 191 isolates (25%) lacked IS1245 DNA, indicating that these strains do not represent M. avium but represent other groupings within MAC. Computer-assisted analysis helped compare the 90 porcine M. avium isolates with the 143 IS1245-containing human M. avium isolates from 1996. Nine genotype families were defined on the basis of at least 75% similarity between the IS1245 RFLP patterns of human and porcine isolates. The occurrence of isolates from both sources in these nine clades is given in Table 2. In total, 59% of the pig isolates and 61% of the human strains were in common genotype families. The largest family (clade 7502) comprised 21 isolates from pigs and 83 isolates from humans (Fig. 2). Two genotype families comprised only four human isolates (clade 7507; data not shown) and only 22 pig isolates (clade 7508; Fig. 1).
TABLE 2.
Occurrence of MAC isolates from humans and pigs in IS1245 RFLP genotype families with at least 75% similarity
| Genotype family | No. of isolates from the following:
|
||
|---|---|---|---|
| Total | Pigs | Human | |
| 7501 | 4 | 1 | 3 |
| 7502 | 16 | 14 | 2 |
| 7503 | 16 | 8 | 8 |
| 7504 | 91 | 20 | 71 |
| 7505 | 4 | 4 | |
| 7506 | 7 | 6 | 1 |
| 7507 | 22 | 22 | |
| 7508 | 16 | 4 | 12 |
| IS1245 negative | 41 | 1 | 40 |
| Not in a clade | 41 | 15 | 26 |
| Total | 258 | 91 | 167 |
FIG. 2.
Dendrogram of IS1245 DNA fingerprints of pig and human isolates in clade 7502. The numbers at the top represent percent relatedness.
DISCUSSION
The average prevalence of caseous lesions in slaughtered pigs was 0.5%, which is unexpectedly high, taking into account the fact that positive pigs were selected only by eye on the basis of deviations in lymph nodes. In an earlier study in Switzerland, Offermann (10) isolated M. avium from the mesenteric lymph nodes from 48 of 345 (13.9%) healthy slaughter pigs without any lesions in these lymph nodes. Therefore, the true prevalence of M. avium in slaughter pigs in The Netherlands might be much higher.
Molecular typing and computer-assisted analysis facilitate the comparison of human and porcine isolates on a large scale. Although no identical DNA fingerprints of porcine and human origin were found, 60% of the isolates from both sources had a similarity of at least 75% among the IS1245 RFLP patterns. This means that, for IS1245 RFLP patterns consisting of 20 bands, at least 15 band positions are shared. Taking into account the high degree of IS1245-based polymorphism among M. avium strains in general, this justifies the conclusion that humans and pigs are infected with the same types of M. avium strains. It is currently not clear whether humans and pigs share common sources of infection or that pork products prepared without appropriate heating may infect susceptible humans. Long-term epidemiological studies are needed to examine this hypothesis. Such studies might find direct links between the consumption of contaminated pork products and infections in humans. However, such studies are complicated by the fact that pigs from various parts of The Netherlands are slaughtered at about 26 large and 30 small slaughterhouses scattered over the whole country. In addition, approximately 70% of the pork and pork products are exported.
Isolation of M. avium by culture is considered the “gold standard” test for the diagnosis of porcine mycobacterial infections. A sensitivity for microscopic examination of Ziehl-Neelsen-stained smears of 15% for MAC culture-positive lymph nodes has been reported by Margolis et al. (9). In the follow-up part of the current study, a much greater sensitivity was found by microscopic examination: in total, 80% for the submaxillary lymph nodes and 82% for the mesenteric lymph nodes. However, 81% of all samples with a negative MAC culture result also yielded acid-fast bacilli by microscopic examination. Furthermore, large differences between the predictive value of positive microscopic examinations of submaxillary lymph nodes (26%) and that of positive microscopic examinations of mesenteric lymph nodes (71%) were observed. This low predictive value regarding positive microscopic examinations of the submaxillary lymph nodes is presumably due to a high prevalence of other, non-MAC bacteriological infections caused by injuries as a result of fighting and/or cutting of dents. In our study we found more than 50 positive cultures that yielded orange-pigmented acid-fast mycobacterial rapid growers.
The occurrence of IS1245 is restricted to M. avium (6, 12). Only 1 of 91 porcine isolates lacked IS1245 DNA in this study, revealing that the porcine MAC isolates almost invariably represented true M. avium. Among the human isolates, 25% of the strains did not hybridize to the IS1245 probe. This indicates that a proportion of the human MAC isolates much larger than that of the porcine isolates represented other groupings within MAC. This presumably reflects the fact that humans have sources of infection not shared with pigs. The identification of the IS1245-negative MAC strains is described elsewhere (13).
In the current study, MAC isolates from pigs at one farm were usually infected with various genotypes of M. avium, and identical fingerprints were found among isolates from pigs from different farms. This suggests that there is no ongoing transmission among pigs but, rather, that pigs are infected from environmental sources, and these may be shared by farms at different geographic locations. In a study by Engel et al. (5) of three farms in The Netherlands in 1977, M. avium serotype 2 was isolated from 12 of 13 pigs on one farm and occasionally from pigs on two other farms. Since serotypes 1, 2, and 3 were commonly found among bird isolates, this finding at that time strongly suggested a role of birds in the transmission of “avian” tuberculosis. However, in our previous study (12), we found multicopy IS1245 RFLP patterns among M. avium serotype 2 and 3 strains apart from the frequently found bird-type RFLP pattern. The multicopy patterns clearly do not represent the bird type RFLP pattern. This means that serotyping is not a reliable method of recognizing M. avium strains that originate in birds. The serotyping results in this study also reflect this. Although 21 of the 91 porcine isolates represented serotype 2 or 3, only one of these 21 strains had the bird-type IS1245 RFLP pattern. This finding, combined with the fact that 47 M. avium strains from birds in The Netherlands invariably exhibited the bird-type IS1245 RFLP pattern (12), excludes birds as significant sources of MAC infections in pigs.
Engel et al. (5) used a pig infection model to demonstrate that tuberculous lymphadenitis can be induced by feeding pigs compost. However, it is assumed that compost can no longer be suspected as a main factor in the etiology of M. avium infections in pigs, because compost is disinfected nowadays by heating and is thought not to contain viable M. avium bacteria.
In this study, slaughter pigs were examined by selecting lymph nodes with caseous lesions. Macroscopically negative lymph nodes and the dissemination of MAC infections to other organs must be examined to estimate the true prevalence of MAC bacteria in pigs. Furthermore, detailed studies are needed to further investigate possible sources of infection at farms with a high incidence of MAC-positive pigs.
ACKNOWLEDGMENTS
We acknowledge the contributions of the inspection teams of the slaughterhouses in Boxtel, Doetichem, Druten, Roosendaal, Rotterdam, and Zevenaar, The Netherlands, for gross examination and collection of the lymph nodes of slaughter pigs in the bacteriological survey.
REFERENCES
- 1.Askgaard D S, Giese S B, Thybo S, Lerche A, Bennedsen J. Serovars of Mycobacterium avium complex isolated from patients in Denmark. J Clin Microbiol. 1994;32:2880–2882. doi: 10.1128/jcm.32.11.2880-2882.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bono M, Jemmi T, Bernasconi C, Burki D, Telenti A, Bodmer T. Genotypic characterisation of Mycobacterium avium strains recovered from animals and their comparison to human strains. Appl Environ Microbiol. 1995;61:371–373. doi: 10.1128/aem.61.1.371-373.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eaton T, Falkingham J O, von Reyn C F. Recovery of Mycobacterium avium from cigarettes. J Clin Micobiol. 1995;33:2757–2758. doi: 10.1128/jcm.33.10.2757-2758.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engel H W B, Berwald L G. A simplified agglutination test for serologic typing of mycobacteria. Am Rev Respir Dis. 1970;101:112–115. doi: 10.1164/arrd.1970.101.1.112. [DOI] [PubMed] [Google Scholar]
- 5.Engel H W B, Groothuis D G, Wouda W, König C D W, Lenders L H M. ‘Pig-compost’ as a source of Mycobacterium avium infections in swine. Zentbl Vet Med B. 1977;25:373–382. doi: 10.1111/j.1439-0450.1978.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 6.Guerrero C, Bernasconi C, Burki D, Bodmer T, Telenti A. A novel insertion element from Mycobacterium avium, IS1245, is a specific target for analysis of strain relatedness. J Clin Microbiol. 1995;33:304–307. doi: 10.1128/jcm.33.2.304-307.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffner S E, Kallenius G, Petrini B, Brennan P J, Tsang A Y. Serovars of Mycobacterium avium complex isolated from patients in Sweden. J Clin Microbiol. 1990;28:1105–1107. doi: 10.1128/jcm.28.6.1105-1107.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inderlied C B, Kemper C A, Bermudez L E M. The Mycobacterium avium complex. Clin Microbiol Rev. 1993;6:266–310. doi: 10.1128/cmr.6.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Margolis M J, Hutchinson L J, Kephart K B, Hattel A L, Whitlock R H, Payeur J B. Results of using histological examination and acid-fast staining to confirm a diagnosis of swine mycobacteriosis made on the basis of gross examination. J Am Vet Med Assoc. 1994;204:1571–1572. [PubMed] [Google Scholar]
- 10.Offermann, U. 1997. Personal communication.
- 11.Portaels F. Epidemiology of mycobacterial diseases. Clin Dermatol. 1995;13:207–222. doi: 10.1016/0738-081x(95)00004-y. [DOI] [PubMed] [Google Scholar]
- 12.Ritacco V, Kremer K, van der Laan T, Pijnenburg J E M, de Haas P E W, van Soolingen D. Use of IS901 and IS1245 in RFLP typing of Mycobacterium avium complex: relatedness among serovar reference strains, human and animal isolates. Int J Tuberc Lung Dis. 1998;2:242–251. [PubMed] [Google Scholar]
- 13.Schneider M M E, de Haas P E W, Komijn R E, Hoepelmans I M, van Soolingen D. A one-year population-based study on the occurrence of Mycobacterium avium complex in humans in the Netherlands. Ph.D. thesis. Utrecht, The Netherlands: University of Utrecht; 1998. [Google Scholar]
- 14.Schneider M M E, Reiss P, Borleffs J C C, Rozenberg-Arska M, Hoepelman I M. Mycobacterium avium-infectie bij HIV-geïnfecteerde patiënten: epidemiologie, diagnose, profylaxe en behandeling. Nederlands Tijdschr Geneeskd. 1997;141:80–83. [PubMed] [Google Scholar]
- 15.Thorel M F, Kriechevsky M, Levi-Frebault V V. Numerical taxonomy of mycobactin-dependent mycobacteria, emended description of Mycobacterium avium, and description of Mycobacterium avium subsp. avium subsp. nov., Mycobacterium avium subsp. paratuberculosis subsp. nov., and Mycobacterium avium subsp. silvaticum subsp. nov. Int J Syst Bacteriol. 1990;40:254–260. doi: 10.1099/00207713-40-3-254. [DOI] [PubMed] [Google Scholar]
- 16.Van Soolingen D, Bauer J, Ritacco V, Leao S, Pavlik I, Vincent V, Rastogi N, Gori A, Bodmer T, Garcia M. IS1245 restriction fragment length polymorphism typing of Mycobacterium avium isolates: a proposal for standardization. J Clin Microbiol. 1998;36:3051–3054. doi: 10.1128/jcm.36.10.3051-3054.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Soolingen D, de Haas P E W, Hermans P W M, van Embden J D A. DNA fingerprinting of Mycobacterium tuberculosis. Methods Enzymol. 1993;235:196–205. doi: 10.1016/0076-6879(94)35141-4. [DOI] [PubMed] [Google Scholar]
- 18.Van Soolingen D, Qian L, de Haas P E W, Douglas J T, Traore H, Portaels F, Qing H Z, Enkhsaikan D, Nymadawa P, van Embden J D A. Predominance of a single genotype of Mycobacterium tuberculosis in countries of East Asia. J Clin Microbiol. 1995;33:3234–3238. doi: 10.1128/jcm.33.12.3234-3238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Von Reyn C F, Maslow J N, Barber T W, Falkiham III J O, Arbeit R D. Persistent colonisation of potable water as a source of Mycobacterium avium infection in AIDS. Lancet. 1994;343:1137–1141. doi: 10.1016/s0140-6736(94)90239-9. [DOI] [PubMed] [Google Scholar]
- 20.Wayne L G, Good R C, Tsang A, Butler R, Dawson D, Groothuis D, Gross W, Hawkins J, Kilburn J, Kubin M, Schroder K H, Silcox V A, Smith C, Thorel M F, Woodley C, Yakrus M A. Serovar determination and molecular taxonomic correlation in Mycobacterium avium, Mycobacterium intracellulare, and Mycobacterium scrofulaceum: a cooperative study of the International Working Group on Mycobacterial Taxonomy. Int J Syst Bacteriol. 1993;43:482–489. doi: 10.1099/00207713-43-3-482. [DOI] [PubMed] [Google Scholar]