Abstract
Objectives
The aim of this case-control study was to identify early-life risk factors associated with the occurrence of owner-reported mobility changes in 6-year-old cats by examining prospective data from a longitudinal cohort study of pet cats, the Bristol Cats study.
Methods
Data on potential risk factors were obtained from seven sequential questionnaires completed between the ages of 2–4 months and 5 years. Mobility-related questions from the study questionnaire distributed at the age of 6 years were used to calculate each cat’s mobility score. Cats with mobility scores of ⩾2 and 0 were allocated to the case and control groups, respectively, and the cat’s status was the outcome variable.
Results
Of the 799 cats included for analysis, 238 (29.8%) had owner-reported mobility changes. Binomial logistic regression using backwards elimination identified four risk factors for owner-reported mobility changes at 6 years of age: entire neuter status at 6 months of age (odds ratio [OR] 1.97; 95% confidence interval [CI] 1.26–3.07), sustained trauma before 6 years of age (OR 1.85; 95% CI 1.30–2.60), outdoor access at 6 years of age (OR 1.67; 95% CI 0.96–2.90) and overweight/obese status at 6 years of age (OR 1.62; 95% CI 1.13–2.33).
Conclusions and relevance
Risk factor analysis demonstrated that obesity, outdoor access and a history of trauma may predispose cats to developing owner-reported mobility changes associated with degenerative joint disease, whereas neutering before 6 months of age appears to decrease that risk.
Keywords: Degenerative joint disease, osteoarthritis, mobility, Bristol Cats study, risk factors, obesity, trauma, outdoor access, neutering
Introduction
Feline degenerative joint disease (DJD) is one of the leading causes of long-term pain in cats, 1 and – based on radiographical studies – is estimated to occur in 61–99% of cats of all ages.2,3 Although the terms DJD and osteoarthritis have been used interchangeably, osteoarthritis refers to the degenerative process affecting synovial joints only, whereas DJD also includes cartilaginous joints.
Depending on the aetiology, feline DJD is classified as primary or secondary. Scottish Fold osteochondrodysplasia4,5 and mucopolysaccharidosis6,7 are primary causes of DJD. Secondary DJD occurs as a result of a specific disease process, and a plethora of recognised and postulated factors have been linked with its development (Table 1). However, most cats with DJD have no obvious initiating cause; this has been termed age-related cartilage degeneration 8 and it is possible that unrecognised risk factors are responsible for the occurrence of DJD in these cats.
Table 1.
List of recognised and postulated primary and secondary causes of feline degenerative joint disease
| Primary | • Scottish Fold osteochondrodysplasia4,5
• Mucopolysaccharidosis VI6,7 • Age-related cartilage degeneration 8 |
| Secondary | • Congenital/developmental ○ Hip dysplasia9–11 ○ Patellar luxation10,12,13 ○ Elbow dysplasia14–16 ○ Elbow luxation17,18 |
| • Traumatic ○ Cranial cruciate ligament injury19–23 ○ Other trauma24–28 |
|
| • Nutritional – hypervitaminosis A 29 | |
| • Endocrine – hypersomatotropism30,31 | |
| • Neoplastic ○ Synovial osteochondromatosis32,33 ○ Osteosarcoma 26 |
|
| • Immune-mediated34,35
○ Erosive polyarthropathies • Feline periosteal proliferative polyarthritis • Feline rheumatoid-like arthritis ○ Non-erosive polyarthropathies • Primary or idiopathic • Secondary (reactive polyarthritis, systemic lupus erythematosus) |
|
| • Infectious
35
○ Mycoplasma species36–38 ○ Bartonella species 39 ○ Histoplasma capsulatum 40 ○ Cryptococcus neoformans 41 ○ Feline leukaemia virus42,43 ○ Feline syncytia-forming virus43,44 |
Despite the high prevalence, little is known about risk factors predisposing cats to DJD. The only identified risk factor for feline DJD to date is age,2,3 whereas in prospective and retrospective studies other risk factors such as sex, neuter status, breed, time spent outdoors and vaccination status were not shown to be associated with the development of DJD.2,3,25,27,45–47 Contrary to humans 48 and dogs 49 where the association between obesity and the development of DJD has been established, several studies have failed to demonstrate a causal relationship between weight or body condition score (BCS) and DJD in cats.2,3,25,46,47 No specific disease process was associated with DJD severity in one previous study; 27 however, in a more recent study, cats with concurrent chronic kidney disease (CKD) and DJD had higher levels of feline DJD-related pain. 50 An association between the severity of dental disease – another chronic inflammatory process associated with age – and the development of CKD has also been suggested. 51 These associations may support the concept of a common pathway linking chronic inflammatory processes such as dental disease, CKD and DJD.
The aim of this study was to identify novel risk factors associated with the occurrence of feline DJD, as well as to evaluate previously investigated risk factors identified in the literature. This was accomplished by examining prospectively collected data related to husbandry and health from a large-scale longitudinal cohort study of pet cats (Bristol Cats [BC] study). Previous studies have been cross-sectional in their design; however, this longitudinal cohort study data set provided an opportunity to examine the time sequencing of outcome and exposures. Elucidating the risk factors that are found in cats with DJD would allow the development of successful preventative strategies, thereby decreasing the prevalence of the disease and improving feline health and welfare.
Materials and methods
This study was approved by the University of Bristol’s Health Sciences Faculty Research Ethics Committee (69041; 04/07/2018).
The BC study (http://www.bristol.ac.uk/vet-school/research/projects/cats/) is an ongoing longitudinal study of health, behaviour and environment of client-owned cats that has been approved by the Ethics of Human Research Committee and by the Animal Welfare and Ethical Review Body (UIN/17/049). 52 Data are being collected prospectively from owners and veterinary surgeons via the use of questionnaires and the sharing of clinical records. Questionnaires include information about cat and owner demographics, cat behaviour, husbandry, clinical signs of disease and veterinary treatment. These are constructed based on the evidence available at the time of questionnaire development by the BC team which consists of specialist veterinary surgeons, behaviourists and epidemiologists. Questionnaires were completed by owners at specific intervals during their cat’s life; questionnaires one (Q1) at 2–4 months, two (Q2) at 6 months, three (Q3) at 12 months, four (Q4) at 18 months, five (Q5) at 2.5 years, six (Q6) at 4 years, then annually thereafter.
Participants included the entire BC study cohort of 2444 cats. The owners of included cats needed to have completed the BC study questionnaire (File 1 in the supplementary material) when their cat turned 6 years of age (Q8).
Twelve mobility-related questions were selected by the authors out of a total of 15 questions from Q8 (section E2) based on owner-reported changes that were most likely to occur as a result of DJD rather than other disease processes according to the literature to date.3,24,27,53–55 With this in mind, ‘cries out loudly for no apparent reason’ and ‘appears forgetful or disorientated’ were excluded. ‘Has difficulty getting in or out of the cat flap’ was also excluded as it would only apply to a subpopulation of cats with outdoor access. In these 12 questions, owners were asked to rate their agreement to statements relating to their cat’s ability to perform different activities, with the options ‘yes’, ‘maybe’, ‘no’ and ‘not applicable’. Answers were scored as follows: 0 = ‘no’, 1 = ‘maybe’ and 2 = ‘yes’. Total mobility scores (MS) were used to classify cats as cases or controls. Cats were excluded where two or more questions were not answered, or where the owner replied ‘not applicable’ to six or more questions. This was done to ensure that enough data were available per cat to classify them confidently. Control cats were required to have no owner-assessed mobility impairment (MS = 0), whereas cats with owner-assessed mobility impairment (MS >1) were assigned to the case group. Cats with a MS of 1, corresponding to a single ‘maybe’ answer, were excluded to eliminate uncertain responses.
Data and statistical analysis
Participating cats were identified using their unique BC study identification number. Data on present husbandry and health were obtained from the questionnaire completed at 6 years of age (Q8), and data on potential early-life risk factors were obtained from questionnaires completed between the ages of 2–4 months and 5 years (Q1–Q7). A total of 17 variables were considered as predictors in a logistic regression model (File 2 in the supplementary material). Thirteen variables were included directly from the questionnaires (File 1 in the supplementary material) and, where original explanatory variables had low numbers for individual categories, these were combined to form new derived variables. Cats were organised in two additional categories based on their breed: according to the cephalic index, the ratio between the width and length of a cat’s skull (brachycephalic, mesocephalic, dolichocephalic) 56 and according to the average breed body size (small/toy, medium, large/giant). 57 Two composite variables were also created by combining information concerning each cat’s vaccination and trauma history, respectively. Answers to the vaccination questions were grouped according to previous research 51 as (a) never vaccinated; (b) primary vaccinations only; (c) occasional vaccination (>2-year interval); (d) frequent or annual vaccination (every 1–2 years); and (e) unknown vaccination status. Owing to the small number of cats in each, the first two categories were combined. Trauma-related information was acquired by analysing questions regarding injuries that received veterinary treatment and injuries that the owners deemed were not serious enough to seek veterinary attention for (Q1–Q7). Trauma was classified as road traffic accident (RTA), fall from height (eg, high-rise syndrome), fracture/dislocation, dog or cat bite and abscess, and soft tissue trauma (STT). When the exact nature of lameness was unclear, it was classified as STT. These categories were grouped from least to most severe as (a) STT; (b) dog or cat bites and abscesses; and (c) RTAs/falls /fractures/dislocations. Finally, only the most severe injury was included for cats that had sustained multiple injuries, and only the oldest injury was included for cats that had sustained the same type of injury multiple times. In the end, all trauma-related data were collapsed for analysis to reflect if trauma had occurred or not as data were sparse for some trauma categories. The age when the oldest and most severe trauma occurred was also noted.
Analyses were performed using SPSS (version 24.0.0.2; IBM). The outcome variable used was the cat’s status, which reflected the absence or presence of owner-reported early DJD-related signs at 6 years of age.
All explanatory variables were of a categorical nature. In order to test for multicollinearity between all explanatory variables, a correlation matrix was initially constructed. If two or more explanatory variables were highly correlated (Spearman’s ρ >|0.8|), only one was taken forward to the univariable models. Univariable analysis was conducted using binomial logistic regression models to examine the association of potential explanatory variables with the outcome of owner-reported presence of early DJD-related signs at 6 years of age; only variables with a Wald test P <0.2 were considered for inclusion in the multivariable model. A multivariable model was then constructed to explore and quantify the presence of independent associations between different predictor variables and the odds of a cat having owner-reported early DJD-related signs at 6 years of age. This was built using a backwards elimination method, and removal of variables was undertaken based on minimising the log-likelihood-ratio statistic (–2LL).
Results
Of the 986 questionnaires distributed when the cats turned 6 years of age that owners completed, 799 were retained for further analysis. Questionnaires were excluded where the responses to mobility questions were either incomplete (n = 35) or ambiguous (n = 152).
Demographic data
Missing data were present in 2/799 (0.3%), 35/799 (4.4%), 5/799 (0.6%) and 2/799 (0.3%) cats for sex, neuter status at 6 months of age, neuter status at 6 years of age and outdoor access, respectively. There was also missing information on the breed of 6/799 (0.8%) cats which precluded their classification according to cephalic index and average breed body size. Demographic information for all cats is listed in Table 2.
Table 2.
Demographic data for 799 cats
| n (%) | ||
|---|---|---|
| Sex | Male | 423 (53.1) |
| Female | 374 (46.9) | |
| Total | 797 (99.7) | |
| Neuter status at 6 months of age | Entire | 111 (14.5) |
| Neutered | 653 (85.5) | |
| Total | 764 (95.6) | |
| Neuter status at 6 years of age | Entire | 7 (0.9) |
| Neutered | 787 (99.1) | |
| Total | 794 (99.4) | |
| Breed category | DSH, DLH and their crossbreeds | 637 (80.3) |
| Purebred | 156 (19.7) | |
| Total | 793 (99.2) | |
| Breed category (cephalic index) | Mesocephalic | 713 (89.9) |
| Brachycephalic | 52 (6.6) | |
| Dolichocephalic | 28 (3.5) | |
| Total | 793 (99.2) | |
| Breed category (body size) | Medium | 661 (83.4) |
| Small/toy | 13 (1.6) | |
| Large/giant | 119 (15.0) | |
| Total | 793 (99.2) | |
| Outdoor access | No – inside only | 92 (11.5) |
| Yes – only in an enclosed run or on a lead | 86 (10.8) | |
| Yes – inside and outside | 618 (77.5) | |
| Yes – outside only | 1 (0.1) | |
| Total | 797 (99.7) |
As there was a small number of cats in each breed, they formed the purebred group; domestic shorthairs (DSH), domestic longhairs (DLH) and their crossbreeds formed the other. Two breed-derived variables were used to further investigate the possible effect of breed: the cephalic index (the ratio between the width and length of a cat’s skull) and the average breed body size
Most cats (n = 553/799; 69.2%) lived in a single-cat household, whereas 214/799 (26.8%), 24/799 (3.0%) and 8/799 (1.0%) cats lived in a household with a total of two, three and four cats, respectively. Joint supplements or medications were being administered to 2/799 (0.3%) and 9/799 (1.1%) cats, respectively; six cats were receiving steroids and three cats were receiving non-steroidal anti-inflammatory drugs.
BCS
Using the 5-point system, 58 the owner-reported BCS range was 1–5 with a median of 3 at all time points (Figure 1). The interquartile range (IQR) was 3 in cats aged 12 months, 18 months, 2.5 years, 4 years and 5 years, and the IQR was 3–4 in cats aged 6 years. Missing data were present in 161/799 (20.2%), 172/799 (21.5%), 97/799 (12.1%), 150/799 (18.8%), 163/799 (20.4%) and 23/799 (2.9%) of cats aged 12 months, 18 months, 2.5 years, 4 years, 5 years and 6 years of age, respectively.
Figure 1.
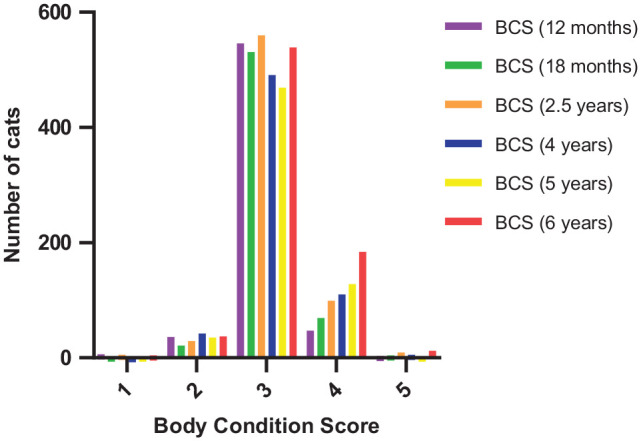
Owner-reported body condition score (BCS) at all time points
Health-related information
It was not possible to establish the vaccination status in 16/799 (2.0%) cats. Most cats had been vaccinated occasionally in intervals longer than 2 years (n = 377/783; 48.1%) or frequently/annually (308/783; 39.3%). With regard to CKD, no answer was given in 285/799 (35.7%) cats, and a diagnosis of CKD was made only in 4/514 (0.8%) cats. Finally, there was missing information on the dental health of 296/799 (37.0%) cats and most cats (n = 376/503; 74.8%) were reported to have good dental health. Health-related information for all cats is listed in Table 3.
Table 3.
Health-related information for 799 cats
| n (%) | ||
|---|---|---|
| Vaccination history | Frequent / annual vaccination (every 1–2 years) | 308 (39.3) |
| Occasional vaccination (>2-year interval) | 377 (48.1) | |
| Primary vaccinations only | 85 (10.9) | |
| Never vaccinated | 13 (1.7) | |
| Total | 783 (98) | |
| CKD diagnosis | No | 510 (99.2) |
| Yes | 4 (0.8) | |
| Total | 514 (64.3) | |
| Dental health | Good health | 376 (74.8) |
| Some dental disease | 104 (20.7) | |
| S&P advised | 12 (2.4) | |
| Additional treatment advised | 11 (2.2) | |
| Total | 503 (63.0) |
Answers to the vaccination questions were grouped according to previous research 51 to create the composite vaccination history variable. Dental health was assessed by veterinary surgeons or nurses in the last 12 months prior the completion of the questionnaire
CKD = chronic kidney disease; S&P = scale and polish
Trauma incidence
The incidence of different types of trauma until the age of 6 years is shown in Table 4.
Table 4.
Owner-reported incidence of different types of trauma until the age of 6 years (n = 799)
| n (%) | ||
|---|---|---|
| Road traffic accident | No | 767 (96.0) |
| Once | 32 (4.0) | |
| Fracture/dislocation | No | 788 (98.6) |
| Once | 11 (1.4) | |
| Fall from height | No | 792 (99.1) |
| Once | 7 (0.9) | |
| Cat bite and/or abscess | No | 692 (86.6) |
| Once | 89 (11.1) | |
| Twice | 16 (2.0) | |
| Three times | 2 (0.3) | |
| Dog bite | No | 791 (99.0) |
| Once | 8 (1.0) | |
| Soft tissue trauma | No | 737 (92.2) |
| Once | 54 (6.8) | |
| Twice | 8 (1.0) |
This information was acquired by analysing questions regarding injuries that received veterinary treatment and injuries that the owners deemed were not serious enough to seek veterinary attention for when the cats were between the age of 2 months and 6 years
Following retention of only the oldest and most severe injury for each cat, trauma was reported in 206/799 (25.8%) cats and, when grouped according to their severity, 110/206 (53.4%) were dog/cat bites and/or abscesses, followed by 50/206 (24.3%) RTAs, falls, fractures/dislocations and 46/206 (22.3%) STTs.
In most cats, trauma was reported either at the age of 2.5 (n = 64/206; 31.1%) or 4 (n = 49/206; 23.8%) years. Trauma was reported in 27/206 (13.1%) cats at the age of 12 months, and in 22/206 (10.7%) cats at the age of 6 months, 18 months or 5 years.
Univariable logistic regression analysis
The outcome variable used was the cat’s status as defined by the MS (MS ⩾2 for cases and MS = 0 for controls). MS range was 0–15 with a median of 0 (IQR 0–2). Case cats (238/799; 29.8%) had a median MS of 2 (IQR 2–4).
Only seven (0.9%) cats were entire at the age of 6 years and thus this variable was not analysed statistically. Similarly, owing to a small number of cats in all remaining categories, neither breed-derived additional variables (cephalic index and body size) were analysed statistically. Only four cats were diagnosed with CKD and therefore this variable was also not analysed statistically. Thirteen variables were considered for univariable analysis (Table 5).
Table 5.
List of explanatory variables considered for univariable analysis of risk factors for feline degenerative joint disease in 6-year-old cats (n = 799)
| Variable | All cats | Cases (MS ⩾2) | Controls (MS = 0) | |
|---|---|---|---|---|
| Sex | Male | 423 (53.1) | 121 (50.8) | 302 (54.0) |
| Female | 374 (46.9) | 117 (49.2) | 257 (46.0) | |
| Total | 797 (99.7) | 238 (100.0) | 559 (99.6) | |
| Neuter status at 6 months of age | Entire | 111 (14.5) | 43 (19.0) | 68 (12.6) |
| Neutered | 653 (85.5) | 183 (81.0) | 470 (87.4) | |
| Total | 764 (95.6) | 226 (95.0) | 538 (95.9) | |
| Breed category | DSH, DLH and their crossbreeds | 637 (80.3) | 186 (78.5) | 451 (81.1) |
| Purebred | 156 (19.7) | 51 (21.5) | 105 (18.9) | |
| Total | 793 (99.2) | 237 (99.6) | 556 (99.1) | |
| Outdoor access | No outdoor access | 92 (11.5) | 21 (8.9) | 71 (12.7) |
| Outdoor access | 705 (88.5) | 216 (91.1) | 489 (87.3) | |
| Total | 797 (99.7) | 237 (99.6) | 560 (99.8) | |
| BCS (6 years old) | Not overweight | 580 (74.7) | 157 (68.0) | 423 (77.6) |
| Overweight/obese | 196 (25.3) | 74 (32.0) | 122 (22.4) | |
| Total | 776 (97.1) | 231 (97.1) | 545 (97.1) | |
| BCS (5 years old) | Not overweight | 506 (79.6) | 145 (76.7) | 361 (80.8) |
| Overweight/obese | 130 (20.4) | 44 (23.3) | 86 (19.2) | |
| Total | 636 (79.6) | 189 (79.4) | 447 (79.7) | |
| BCS (4 years old) | Not overweight | 534 (82.3) | 148 (77.9) | 386 (84.1) |
| Overweight/obese | 115 (17.7) | 42 (22.1) | 73 (15.9) | |
| Total | 649 (81.2) | 190 (79.8) | 459 (81.8) | |
| BCS (2.5 years old) | Not overweight | 594 (84.6) | 174 (84.1) | 420 (84.8) |
| Overweight/obese | 108 (15.4) | 33 (15.9) | 75 (15.2) | |
| Total | 702 (87.9) | 207 (87.0) | 495 (88.2) | |
| BCS (18 months old) | Not overweight | 554 (88.4) | 172 (86.4) | 382 (89.3) |
| Overweight/obese | 73 (11.6) | 27 (13.6) | 46 (10.7) | |
| Total | 627 (78.5) | 199 (83.6) | 428 (76.3) | |
| BCS (12 months old) | Not overweight | 588 (92.2) | 186 (91.2) | 402 (92.6) |
| Overweight/obese | 50 (7.8) | 18 (8.8) | 32 (7.4) | |
| Total | 638 (79.8) | 204 (85.7) | 434 (77.4) | |
| Dental health | Good health | 376 (74.8) | 112 (74.7) | 264 (74.8) |
| Dental disease | 127 (25.2) | 38 (25.3) | 89 (25.2) | |
| Total | 503 (63.0) | 150 (63.0) | 353 (62.9) | |
| Vaccination history | Never/primary vaccinations only | 98 (12.5) | 34 (14.7) | 64 (11.6) |
| Occasional vaccination | 377 (48.1) | 115 (49.6) | 262 (47.5) | |
| Frequent or annual vaccination | 308 (39.3) | 83 (35.8) | 225 (40.8) | |
| Total | 783 (98.0) | 232 (97.5) | 551 (98.2) | |
| Trauma incidence | None reported | 593 (74.2) | 157 (66.0) | 436 (77.7) |
| Reported | 206 (25.8) | 81 (34.0) | 125 (22.3) | |
| Total | 799 (100.0) | 238 (100.0) | 561 (100.0) |
Data are presented as n (%). As data were sparse for the lower body condition score (BCS) using the 5-point system, cats were grouped for further analysis as ‘overweight/obese’ for BCS 4–5, which included both overweight (BCS 4) and obese (BCS 5) cats, or ‘not overweight’ for BCS 1–3, which included underweight (BCS 1–2) cats and cats of ideal weight (BCS 3). Data were also sparse for some dental health categories, and thus these were collapsed for analysis as 0 = ‘good health’ or 1 = ‘dental disease’ (everything else)
MS = mobility score; DLH = domestic longhair; DSH = domestic shorthair
Prior to univariable analysis, a Spearman rank correlation matrix was constructed; no significant collinearity was detected between variables and therefore all were taken forward to univariable analysis. Five variables were significant at P <0.2 in univariable analysis and were therefore retained for multivariable analysis (File 2 in the supplementary material). These were neuter status at 6 months of age (P = 0.023), outdoor access (P = 0.125), BCS at 4 (P = 0.061) and 6 (P = 0.005) years of age, and trauma incidence (P = 0.001).
Multivariable logistic regression analysis
The initial multivariable model included only the intercept, had a −2LL of 897.197 and classified 70.5% of cats correctly. The final multivariable model included 740 cats and four variables (Table 6); it had a −2LL of 866.849, classified 70.9% of cats correctly and had an effect size of 0.057, explaining 5.7% of the variability in the model. Outdoor access was retained in the model as it resulted in a 3.524 change in −2LL and explained an additional 0.8% variability.
Table 6.
Multivariable logistic regression model of risk factors for feline degenerative joint disease in 6-year-old cats
| B | SE | Wald χ2 | df | Sig | OR (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Included in the model | |||||||
| Neuter status at 6 months of age | Neutered | Reference | |||||
| Entire | 0.678 | 0.226 | 8.974 | 1 | 0.003 | 1.97 (1.264–3.071) | |
| Outdoor access | No outdoor access | Reference | |||||
| Outdoor access | 0.513 | 0.283 | 3.298 | 1 | 0.069 | 1.671 (0.96–2.907) | |
| BCS (6 years old) | Not overweight | Reference | |||||
| Overweight/obese | 0.485 | 0.184 | 6.964 | 1 | 0.008 | 1.624 (1.133–2.328) | |
| Trauma incidence | None | Reference | |||||
| Yes | 0.613 | 0.179 | 11.759 | 1 | 0.001 | 1.846 (1.3–2.62) | |
| Intercept | −1.745 | 0.284 | 37.61 | 1 | 0 | 0.175 | |
| Not included in the model | |||||||
| BCS (4 years old) | Not overweight | Reference | |||||
| Overweight/obese | −0.023 | 0.285 | 0.007 | 1 | 0.935 | 0.977 (0.559–1.707) | |
B is the unstandardised regression weight which reflects the change in the logit of the outcome variable (status = case or control) associated with a one-unit change in the predictor variable, and SE is the standard error of B. Wald χ2 is the test statistic for each predictor variable and is associated with different degrees of freedom (df). Sig refers to the P value associated with each Wald χ2 statistic. The odds ratio (OR) is a measurement of likelihood for each predictor variable and is presented with its 95% confidence interval (CI). The multivariable logistic regression was built using a backwards elimination method, and removal of variables was undertaken based on minimising the log-likelihood ratio statistic (–2LL), rather than the P value associated with the Wald χ2 statistic. The model χ2 is the difference between the model –2LL and the baseline –2LL; this was χ2 (4) = 30.348 (P <0.001). Cox and Snell’s measure was 0.040 and Nagelkerke’s measure was 0.057; these provided an approximate effect size measure for the model
BCS = body condition score
Discussion
Previous studies have failed to identify risk factors, other than age, for feline DJD.2,3,25,27,45–47 This is the first study to examine prospective data from a longitudinal cohort study of pet cats in order to identify early-life risk factors associated with the occurrence of owner-reported mobility changes at 6 years of age.
Cats that were entire at 6 months of age were twice as likely to have owner-reported early DJD-related signs than cats that were neutered before that age. In dogs, neutering before the age of 6 months has been associated with an increased incidence of primary DJD and musculoskeletal problems that can result in secondary DJD.59,60 Retrospective feline cohort studies to date have not established similar associations;61,62 therefore the postulated explanations for the effect of neutering in dogs do not appear to apply in cats. However, the immunosuppressive effect of testosterone during the early stages of development has been recognised in both sexes in humans and other species.63,64 Although neutering has not been established as a risk factor for the development of chronic inflammatory processes, cats neutered before the age of 5.5 months are less likely to suffer from feline asthma and gingivitis than cats neutered later in life. 62 A possible explanation for the protective effect of neutering before the age of 6 months that was demonstrated in this study could therefore be that neutering resulted in reduced circulating levels of androgens during that developmental period, thereby decreasing the incidence of owner-reported early DJD-related signs at 6 years of age.
Cats that had sustained trauma were twice as likely to have owner-reported early DJD-related signs than cats that had not sustained trauma. Although trauma is a confirmed risk factor for the development of secondary DJD in humans, there is little evidence to support this in dogs or cats. 8 This is the first study where the occurrence of feline DJD following joint trauma has been evaluated. The mechanisms by which secondary DJD may have developed in the cats of this study can be explained by relevant literature to date. Skeletal fractures/dislocations involve direct trauma to the bones or joints and were reported in 60% and 68.9% of cats that were alive on arrival following RTAs 65 or falls from height, 66 respectively. Cat and dog bites can cause not only penetrating STT and fractures, 67 but also secondary DJD by instigating bacterial arthritis as resulting wounds contain a plethora of aerobic and anaerobic bacteria. Although it is unlikely that a single STT could have contributed to the development of DJD, this category may have included more severe trauma that was not witnessed, resulting in owners only reporting the lameness rather than the cause of the lameness.
Cats with outdoor access were twice as likely to have owner-reported early DJD-related signs than indoor-only cats. Outdoor access has been hypothesised to increase the risk of accidental injuries in cats, 68 thus increasing the risk of developing secondary DJD. Nevertheless, there was no multicollinearity between outdoor access and owner-reported trauma incidence in this study. The increase in DJD-related signs may be explained by the cats with outdoor access being more likely to undergo repetitive microtrauma which was not noted by the owners. Indeed, repetitive microtrauma has been shown to result in altered biomechanics that leads to DJD in dogs, 69 horses 70 and humans.71,72
Overweight/obese cats were twice as likely to have owner-reported early DJD-related signs than cats that were not overweight. Obesity has been established as a risk factor for the development of DJD in humans and dogs.73,74 This was initially attributed to abnormal joint loading; however, DJD has also been shown to develop in the non-weightbearing joints of obese patients, 75 indicating that obesity-related biochemical factors may also be involved in the development of DJD. Circulating levels of leptin, which reflect body fat mass, are increased in overweight cats, dogs and humans, 76 and the proinflammatory role of leptin in the development of DJD has been established in humans 77 and dogs.73,74,78,79 Although there is a paucity of studies investigating the role of mechanical and biochemical factors in the relationship between obesity and feline DJD, it is possible that these postulations could also apply to cats.
Cohort studies can assess causality and thus provide strong scientific evidence; 80 however, this study also has potential limitations. The study’s population was generally similar to what was reported in a large cross-sectional UK study. 81 The proportion of purebred cats was higher in the present study, most likely because a pedigree breeder was a more common source of cats for the BC study cohort 82 than the UK population. 83 Nevertheless, no breed-specific risk factor was implicated in the prevalence of owner-reported early DJD-related signs.
One of the limitations of this study’s design was that a sample size calculation was not performed as, based on the literature to date, it was not possible to estimate the anticipated incidence of early DJD-related signs.2,3,24,26 In the absence of this information, a pragmatic approach was taken and all 2444 cats initially recruited in the BC study were included where they met the inclusion criteria.
Another study design limitation relates to the fact that neither the mobility questions contained in the BC questionnaire nor the scoring system were validated against clinical assessments. Although it would have been preferable to ask owners to complete a questionnaire that has been validated for the clinical assessment of DJD-associated chronic pain in cats, such as the Feline Musculoskeletal Pain Index, 84 this would have been outside of the scope of this study.
A further limitation is that the study depended on owner-reported data, possibly introducing reporting bias. This could have been mitigated if clinical information pertaining to health-related risk factors was compared against veterinary records; however, this was not possible within the time frame of the study. Owners of both BC 85 and non-BC cats86,87 tend to underestimate the BCS of their cats. The prevalence of obesity in this study may therefore be an underestimation, indicating that the relationship between obesity and the development of early DJD-related signs is stronger than that detected by the model.
Conclusions
This study not only evaluated early life risk factors for the first time, but also used prospective data from a longitudinal cohort study, further expanding research on feline DJD by identifying novel risk factors for its development. Cats that were entire at 6 months of age, cats that were obese at 6 years of age, cats with outdoor access and cats with a history of trauma were more likely to have early DJD-related changes in owner-reported mobility at 6 years of age. Further research is needed to determine if other aspects of a cat’s husbandry, diet, lifestyle and clinical history are implicated in the development of DJD. Additional research on the possible link between chronic inflammatory processes such as DJD, CKD and dental disease is also warranted. Future risk factor analysis on older BC study cats could corroborate the findings of the present study and identify additional risk factors for the development of DJD, as well as compare owner-reported signs of early DJD with well-established DJD.
Supplemental Material
Explanatory variables considered for univariable analysis of risk factors for feline DJD in 6-year-old cats
BC questionnaire 8 for 6-year-old cats
Acknowledgments
The authors would like to thank all owners and their cats for their ongoing participation in the Bristol Cats study, as well as the Bristol Cats study team for assisting with data acquisition. Many thanks to Dr Jennifer McDonald for her helpful comments on the manuscript.
Footnotes
Accepted: 1 January 2021
Supplementary material: The following files are available online:
File 1: BC questionnaire 8 for 6-year-old cats.
File 2: Explanatory variables considered for univariable analysis of risk factors for feline DJD in 6-year-old cats.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This study was funded by Zoetis as part of Evangelia Maniaki’s Feline Scholar role. The Bristol Cats study is funded by Cats Protection and Waltham Petcare Science Institute.
Ethical approval: This work involved the use of non-experimental animals only (including owned or unowned animals and data from prospective or retrospective studies). Established internationally recognised high standards (‘best practice’) of individual veterinary clinical patient care were followed. Ethical approval from a committee, while not specifically required for publication in JFMS, was nonetheless obtained, as stated in the manuscript.
Informed consent: Informed consent (either verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (either experimental or non-experimental animals) for the procedure(s) undertaken (either prospective or retrospective studies). No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Evangelia Maniaki  https://orcid.org/0000-0001-8508-1361
https://orcid.org/0000-0001-8508-1361
Jo Murrell  https://orcid.org/0000-0003-0456-5159
https://orcid.org/0000-0003-0456-5159
Sorrel J Langley-Hobbs  https://orcid.org/0000-0003-4397-5150
https://orcid.org/0000-0003-4397-5150
References
- 1. Robertson S, Lascelles D. Long-term pain in cats: how much do we know about this important welfare issue? J Feline Med Surg 2010; 12: 188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lascelles BD, Henry JB, 3rd, Brown J, et al. Cross-sectional study of the prevalence of radiographic degenerative joint disease in domesticated cats. Vet Surg 2010; 39: 535–544. [DOI] [PubMed] [Google Scholar]
- 3. Slingerland LI, Hazewinkel HA, Meij BP, et al. Cross-sectional study of the prevalence and clinical features of osteoarthritis in 100 cats. Vet J 2011; 187: 304–309. [DOI] [PubMed] [Google Scholar]
- 4. Gandolfi B, Alamri S, Darby WG, et al. A dominant TRPV4 variant underlies osteochondrodysplasia in Scottish fold cats. Osteoarthritis Cartilage 2016; 24: 1441–1450. [DOI] [PubMed] [Google Scholar]
- 5. Malik R, Allan GS, Howlett CR, et al. Osteochondrodysplasia in Scottish Fold cats. Aust Vet J 1999; 77: 85–92. [DOI] [PubMed] [Google Scholar]
- 6. Crawley AC, Muntz FH, Haskins ME, et al. Prevalence of mucopolysaccharidosis type VI mutations in Siamese cats. J Vet Intern Med 2003; 17: 495–498. [DOI] [PubMed] [Google Scholar]
- 7. Macri B, Marino F, Mazzullo G, et al. Mucopolysaccharidosis VI in a Siamese/Short-Haired European cat. J Vet Med A Physiol Pathol Clin Med 2002; 49: 438–442. [DOI] [PubMed] [Google Scholar]
- 8. Lascelles BD. Feline degenerative joint disease. Vet Surg 2010; 39: 2–13. [DOI] [PubMed] [Google Scholar]
- 9. Keller GG, Reed AL, Lattimer JC, et al. Hip dysplasia: a feline population study. Vet Radiol Ultrasound 1999; 40: 460–464. [DOI] [PubMed] [Google Scholar]
- 10. Langenbach A, Green P, Giger U, et al. Relationship between degenerative joint disease and hip joint laxity by use of distraction index and Norberg angle measurement in a group of cats. J Am Vet Med Assoc 1998; 213: 1439–1443. [PubMed] [Google Scholar]
- 11. Loder RT, Todhunter RJ. Demographics of hip dysplasia in the Maine Coon cat. J Feline Med Surg 2018; 20: 302–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Loughin CA, Kerwin SC, Hosgood G, et al. Clinical signs and results of treatment in cats with patellar luxation: 42 cases (1992–2002). J Am Vet Med Assoc 2006; 228: 1370–1375. [DOI] [PubMed] [Google Scholar]
- 13. Smith GK, Langenbach A, Green PA, et al. Evaluation of the association between medial patellar luxation and hip dysplasia in cats. J Am Vet Med Assoc 1999; 215: 40–45. [PubMed] [Google Scholar]
- 14. Freire M, Meuten D, Lascelles D. Pathology of articular cartilage and synovial membrane from elbow joints with and without degenerative joint disease in domestic cats. Vet Pathol 2014; 51: 968–978. [DOI] [PubMed] [Google Scholar]
- 15. Freire M, Robertson I, Bondell HD, et al. Radiographic evaluation of feline appendicular degenerative joint disease vs. macroscopic appearance of articular cartilage. Vet Radiol Ultrasound 2011; 52: 239–247. [DOI] [PubMed] [Google Scholar]
- 16. Staiger BA, Beale BS. Use of arthroscopy for debridement of the elbow joint in cats. J Am Vet Med Assoc 2005; 226: 401–403. [DOI] [PubMed] [Google Scholar]
- 17. Rossi F, Vignoli M, Terragni R, et al. Bilateral elbow malformation in a cat caused by radio-ulnar synostosis. Vet Radiol Ultrasound 2003; 44: 283–286. [DOI] [PubMed] [Google Scholar]
- 18. Valastro C, Di Bello A, Crovace A. Congenital elbow subluxation in a cat. Vet Radiol Ultrasound 2005; 46: 63–64. [DOI] [PubMed] [Google Scholar]
- 19. Harasen GL. Feline cranial cruciate rupture: 17 cases and a review of the literature. Vet Comp Orthop Traumatol 2005; 18: 254–257. [PubMed] [Google Scholar]
- 20. Herzog W, Adams ME, Matyas JR, et al. Hindlimb loading, morphology and biochemistry of articular cartilage in the ACL-deficient cat knee. Osteoarthritis Cartilage 1993; 1: 243–251. [DOI] [PubMed] [Google Scholar]
- 21. Leumann A, Leonard T, Nuesch C, et al. The natural initiation and progression of osteoarthritis in the anterior cruciate ligament deficient feline knee. Osteoarthritis Cartilage 2019; 27: 687–693. [DOI] [PubMed] [Google Scholar]
- 22. Wessely M, Reese S, Schnabl-Feichter E. Aetiology and pathogenesis of cranial cruciate ligament rupture in cats by histological examination. J Feline Med Surg 2017; 19: 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu JZ, Herzog W, Epstein M. Joint contact mechanics in the early stages of osteoarthritis. Med Eng Phys 2000; 22: 1–12. [DOI] [PubMed] [Google Scholar]
- 24. Clarke SP, Bennett D. Feline osteoarthritis: a prospective study of 28 cases. J Small Anim Pract 2006; 47: 439–445. [DOI] [PubMed] [Google Scholar]
- 25. Clarke SP, Mellor D, Clements DN, et al. Prevalence of radiographic signs of degenerative joint disease in a hospital population of cats. Vet Rec 2005; 157: 793–799. [DOI] [PubMed] [Google Scholar]
- 26. Godfrey DR. Osteoarthritis in cats: a retrospective radiological study. J Small Anim Pract 2005; 46: 425–429. [DOI] [PubMed] [Google Scholar]
- 27. Hardie EM, Roe SC, Martin FR. Radiographic evidence of degenerative joint disease in geriatric cats: 100 cases (1994–1997). J Am Vet Med Assoc 2002; 220: 628–632. [DOI] [PubMed] [Google Scholar]
- 28. Johnston SA. Osteoarthritis. Joint anatomy, physiology, and pathobiology. Vet Clin North Am Small Anim Pract 1997; 27: 699–723. [DOI] [PubMed] [Google Scholar]
- 29. Polizopoulou ZS, Kazakos G, Patsikas MN, et al. Hypervitaminosis A in the cat: a case report and review of the literature. J Feline Med Surg 2005; 7: 363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peterson ME, Taylor RS, Greco DS, et al. Acromegaly in 14 cats. J Vet Intern Med 1990; 4: 192–201. [DOI] [PubMed] [Google Scholar]
- 31. Wassenaar MJ, Biermasz NR, van Duinen N, et al. High prevalence of arthropathy, according to the definitions of radiological and clinical osteoarthritis, in patients with long-term cure of acromegaly: a case-control study. Eur J Endocrinol 2009; 160: 357–365. [DOI] [PubMed] [Google Scholar]
- 32. Tan C, Allan GS, Barfield D, et al. Synovial osteochondroma involving the elbow of a cat. J Feline Med Surg 2010; 12: 412–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tas O, De Cock H, Lemmens P, et al. Synovial osteochondromatosis and sclerosing osteosarcoma in a cat. Vet Comp Orthop Traumatol 2013; 26: 160–164. [DOI] [PubMed] [Google Scholar]
- 34. Gao X, Lee J, Malladi S, et al. Feline degenerative joint disease: a genomic and proteomic approach. J Feline Med Surg 2013; 15: 466–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lemetayer J, Taylor S. Inflammatory joint disease in cats: diagnostic approach and treatment. J Feline Med Surg 2014; 16: 547–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liehmann L, Degasperi B, Spergser J, et al. Mycoplasma felis arthritis in two cats. J Small Anim Pract 2006; 47: 476–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moise NS, Crissman JW, Fairbrother JF, et al. Mycoplasma gateae arthritis and tenosynovitis in cats: case report and experimental reproduction of the disease. Am J Vet Res 1983; 44: 16–21. [PubMed] [Google Scholar]
- 38. Zeugswetter F, Hittmair KM, de Arespacochaga AG, et al. Erosive polyarthritis associated with Mycoplasma gateae in a cat. J Feline Med Surg 2007; 9: 226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tomas A, Pultorak EL, Gruen ME, et al. Relationship between degenerative joint disease, pain, and Bartonella spp. seroreactivity in domesticated cats. J Vet Intern Med 2015; 29: 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wolf AM. Histoplasma capsulatum osteomyelitis in the cat. J Vet Intern Med 1987; 1: 158–162. [DOI] [PubMed] [Google Scholar]
- 41. Tisdall PL, Martin P, Malik R. Cryptic disease in a cat with painful and swollen hocks: an exercise in diagnostic reasoning and clinical decision-making. J Feline Med Surg 2007; 9: 418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Oohashi E, Yamada K, Oohashi M, et al. Chronic progressive polyarthritis in a female cat. J Vet Med Sci 2010; 72: 511–514. [DOI] [PubMed] [Google Scholar]
- 43. Pedersen NC, Pool RR, O′Brien T. Feline chronic progressive polyarthritis. Am J Vet Res 1980; 41: 522–535. [PubMed] [Google Scholar]
- 44. Inkpen H. Chronic progressive polyarthritis in a domestic shorthair cat. Can Vet J 2015; 56: 621–623. [PMC free article] [PubMed] [Google Scholar]
- 45. Godfrey D, Vaughan L. Historical prevalence of radiological appendicular osteoarthritis in cats (1972–1973). J Am Anim Hosp Assoc 2018; 54: 209–212. [DOI] [PubMed] [Google Scholar]
- 46. Ohlund M, Palmgren M, Holst BS. Overweight in adult cats: a cross-sectional study. Acta Vet Scand 2018; 60: 5. DOI: 10.1186/s13028-018-0359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Scarlett JM, Donoghue S. Associations between body condition and disease in cats. J Am Vet Med Assoc 1998; 212: 1725–1731. [PubMed] [Google Scholar]
- 48. Vina ER, Kwoh CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol 2018; 30: 160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zoran DL. Obesity in dogs and cats: a metabolic and endocrine disorder. Vet Clin North Am Small Anim Pract 2010; 40: 221–239. [DOI] [PubMed] [Google Scholar]
- 50. Chiu K, Gruen M, Marino C, et al. IRIS stage influences pain level in cats with degenerative joint disease (DJD) [abstract]. Vet Surg 2019; 48: 1101. [Google Scholar]
- 51. Finch NC, Syme HM, Elliott J. Risk factors for development of chronic kidney disease in cats. J Vet Intern Med 2016; 30: 602–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Murray JK, Casey RA, Gale E, et al. Cohort profile: the ‘Bristol Cats Study’ (BCS) – a birth cohort of kittens owned by UK households. Int J Epidemiol 2017; 46: 1749–1750e. [DOI] [PubMed] [Google Scholar]
- 53. Bennett D, Morton C. A study of owner observed behavioural and lifestyle changes in cats with musculoskeletal disease before and after analgesic therapy. J Feline Med Surg 2009; 11: 997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Klinck MP, Frank D, Guillot M, et al. Owner-perceived signs and veterinary diagnosis in 50 cases of feline osteoarthritis. Can Vet J 2012; 53: 1181–1186. [PMC free article] [PubMed] [Google Scholar]
- 55. Lascelles BD, Hansen BD, Roe S, et al. Evaluation of client-specific outcome measures and activity monitoring to measure pain relief in cats with osteoarthritis. J Vet Intern Med 2007; 21: 410–416. [DOI] [PubMed] [Google Scholar]
- 56. Farnworth MJ, Packer RMA, Sordo L, et al. In the eye of the beholder: owner preferences for variations in cats’ appearances with specific focus on skull morphology. Animals (Basel) 2018; 8. DOI: 10.3390/ani8020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Purina. Cat breeds. https://www.purina.com/cats/cat-breeds (2020, accessed January 19, 2021).
- 58. Laflamme D. Development and validation of a body condition score system for cats: a clinical tool. Feline Practice 1997; 25: 13–18. [Google Scholar]
- 59. Howe LM. Current perspectives on the optimal age to spay/castrate dogs and cats. Vet Med (Auckl) 2015; 6: 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sanderson S. The epidemic of canine obesity and its role in osteoarthritis. Isr J Vet Med 2012; 67: 195–202. [Google Scholar]
- 61. Howe LM, Slater MR, Boothe HW, et al. Long-term outcome of gonadectomy performed at an early age or traditional age in cats. J Am Vet Med Assoc 2000; 217: 1661–1665. [DOI] [PubMed] [Google Scholar]
- 62. Spain CV, Scarlett JM, Houpt KA. Long-term risks and benefits of early-age gonadectomy in cats. J Am Vet Med Assoc 2004; 224: 372–379. [DOI] [PubMed] [Google Scholar]
- 63. Nunn CL, Lindenfors P, Pursall ER, et al. On sexual dimorphism in immune function. Philos Trans R Soc Lond B Biol Sci 2009; 364: 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Martin JT. Sexual dimorphism in immune function: the role of prenatal exposure to androgens and estrogens. Eur J Pharmacol 2000; 405: 251–261. [DOI] [PubMed] [Google Scholar]
- 65. Rochlitz I. Clinical study of cats injured and killed in road traffic accidents in Cambridgeshire. J Small Anim Pract 2004; 45: 390–394. [DOI] [PubMed] [Google Scholar]
- 66. Vnuk D, Pirkic B, Maticic D, et al. Feline high-rise syndrome: 119 cases (1998–2001). J Feline Med Surg 2004; 6: 305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dendle C, Looke D. Management of mammalian bites. Aust Fam Physician 2009; 38: 868–874. [PubMed] [Google Scholar]
- 68. Buffington CA. External and internal influences on disease risk in cats. J Am Vet Med Assoc 2002; 220: 994–1002. [DOI] [PubMed] [Google Scholar]
- 69. Marcellin-Little DJ, Levine D, Canapp SO., Jr. The canine shoulder: selected disorders and their management with physical therapy. Clin Tech Small Anim Pract 2007; 22: 171–182. [DOI] [PubMed] [Google Scholar]
- 70. Magnusson LE, Ekman S. Osteoarthrosis of the antebrachiocarpal joint of 7 riding horses. Acta Vet Scand 2001; 42: 429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rehmani R, Endo Y, Bauman P, et al. Lower extremity injury patterns in elite ballet dancers: ultrasound/MRI imaging features and an institutional overview of therapeutic ultrasound guided percutaneous interventions. HSS Journal 2015; 11: 258–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yang J, Tibbetts AS, Covassin T, et al. Epidemiology of overuse and acute injuries among competitive collegiate athletes. J Athl Train 2012; 47: 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Guilak F. Biomechanical factors in osteoarthritis. Best Pract Res Clin Rheumatol 2011; 25: 815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Marshall W, Bockstahler B, Hulse D, et al. A review of osteoarthritis and obesity: current understanding of the relationship and benefit of obesity treatment and prevention in the dog. Vet Comp Orthop Traumatol 2009; 22: 339–345. [DOI] [PubMed] [Google Scholar]
- 75. Cicuttini FM, Baker JR, Spector TD. The association of obesity with osteoarthritis of the hand and knee in women: a twin study. J Rheumatol 1996; 23: 1221–1226. [PubMed] [Google Scholar]
- 76. Radin MJ, Sharkey LC, Holycross BJ. Adipokines: a review of biological and analytical principles and an update in dogs, cats, and horses. Vet Clin Pathol 2009; 38: 136–156. [DOI] [PubMed] [Google Scholar]
- 77. Yan M, Zhang J, Yang H, et al. The role of leptin in osteoarthritis. Medicine (Baltimore) 2018; 97: e0257. DOI: 10.1097/MD.0000000000010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kleine SA, Sanderson SL, George C, et al. Correlation of serum and synovial leptin concentrations with body condition scores in healthy and osteoarthritic dogs. Vet Surg 2019; 48: 780–785. [DOI] [PubMed] [Google Scholar]
- 79. Schmidli MR, Fuhrer B, Kurt N, et al. Inflammatory pattern of the infrapatellar fat pad in dogs with canine cruciate ligament disease. BMC Vet Res 2018; 14: 161. DOI: 10.1186/s12917-018-1488-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Song JW, Chung KC. Observational studies: cohort and case-control studies. Plast Reconstr Surg 2010; 126: 2234–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. O′Neill DG, Church DB, McGreevy PD, et al. Longevity and mortality of cats attending primary care veterinary practices in England. J Feline Med Surg 2015; 17: 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wilson JL, Gruffydd-Jones TJ, Murray JK. Risk factors for road traffic accidents in cats up to age 12 months that were registered between 2010 and 2013 with the UK pet cat cohort (‘Bristol Cats’). Vet Rec 2017; 180: 195. DOI: 10.1136/vr.103859. [DOI] [PubMed] [Google Scholar]
- 83. Murray JK, Gruffydd-Jones TJ. Proportion of pet cats registered with a veterinary practice and factors influencing registration in the UK. Vet J 2012; 192: 461–466. [DOI] [PubMed] [Google Scholar]
- 84. Gruen ME, Griffith EH, Thomson AE, et al. Criterion validation testing of clinical metrology instruments for measuring degenerative joint disease associated mobility impairment in cats. PLoS One 2015; 10: e0131839. DOI: 10.1371/journal.pone.0131839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rowe EC, Browne WJ, Casey RA, et al. Early-life risk factors identified for owner-reported feline overweight and obesity at around two years of age. Prev Vet Med 2017; 143: 39–48. [DOI] [PubMed] [Google Scholar]
- 86. Cave NJ, Allan FJ, Schokkenbroek SL, et al. A cross-sectional study to compare changes in the prevalence and risk factors for feline obesity between 1993 and 2007 in New Zealand. Prev Vet Med 2012; 107: 121–133. [DOI] [PubMed] [Google Scholar]
- 87. Colliard L, Paragon BM, Lemuet B, et al. Prevalence and risk factors of obesity in an urban population of healthy cats. J Feline Med Surg 2009; 11: 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Explanatory variables considered for univariable analysis of risk factors for feline DJD in 6-year-old cats
BC questionnaire 8 for 6-year-old cats


