Abstract
Background:
Fatigue is a common, debilitating symptom of multiple sclerosis (MS) without a current standardised treatment.
Objective:
The aim of this systematic review with network meta-analyses was to estimate the relative effectiveness of both fatigue-targeted and non-targeted exercise, behavioural and combined (behavioural and exercise) interventions.
Methods:
Nine electronic databases up to August 2018 were searched, and 113 trials (n = 6909) were included: 34 were fatigue-targeted and 79 non-fatigue-targeted trials. Intervention characteristics were extracted using the Template for Intervention Description and Replication guidelines. Certainty of evidence was assessed using GRADE.
Results:
Pairwise meta-analyses showed that exercise interventions demonstrated moderate to large effects across subtypes regardless of treatment target, with the largest effect for balance exercise (SMD = 0.84). Cognitive behavioural therapies (CBTs) showed moderate to large effects (SMD = 0.60), with fatigue-targeted treatments showing larger effects than those targeting distress. Network meta-analysis showed that balance exercise performed significantly better compared to other exercise and behavioural intervention subtypes, except CBT. CBT was estimated to be superior to energy conservation and other behavioural interventions. Combined exercise also had a moderate to large effect.
Conclusion:
Treatment recommendations for balance and combined exercise are tentative as the certainty of the evidence was moderate. The certainty of the evidence for CBT was high.
Keywords: Fatigue, multiple sclerosis, network meta-analysis, randomised controlled trials, quasi-randomised controlled trials, behavioural interventions, exercise interventions, TIDieR
Introduction
Fatigue affects around two-thirds of people with multiple sclerosis (pwMS).1,2 MS-related fatigue is a complex and subjective symptom characterised by a lack of energy or overwhelming sense of physical and/or mental tiredness.3–5 Fatigue is associated with poorer quality of life (QoL) even when controlling for disease severity and is a major reason for stopping work in pwMS.6
Treating fatigue has been identified as priority by pwMS.7 In routine clinical care, pharmacological treatments tend to be the treatment of choice, with behavioural and exercise interventions considered as alternative or adjunctive treatment options.8 In many cases, patients are never offered these adjunctive treatments. This is concerning as the current evidence base suggests pharmacological interventions to date are largely ineffective, while exercise and behavioural interventions have larger effects.9
So, why are behavioural treatments for fatigue not part of standard treatment? One reason may be there are many randomised controlled trials (RCTs) of very different behavioural interventions for fatigue in MS. There is little standardisation of these interventions across the studies, making it unclear which of these should be part of the routine offer. Standard pairwise meta-analytic systematic reviews of exercise and/or behavioural interventions have attempted to summarise the findings across studies.9–17 However, control conditions of included trials within these reviews differ considerably, making it challenging to directly compare effect sizes across intervention types. In contrast to pairwise meta-analysis which has been used in all previous reviews, network meta-analysis (NMA) allows for trial arm data to be compared across trials (e.g. aerobic exercise to energy conservation (EC) methods) even when these direct head-to-head comparisons do not exist.18 This allows a ranking of the most effective treatments against each other19 and provides greater clarity of which may be the best to take forward.
In most previous systematic reviews, interventions designed specifically for fatigue have not been distinguished from those where fatigue is one of the secondary outcomes.10,11,12,16 Combining these in one analysis may dilute the effect. The overarching aim of the current meta-analysis was to elucidate which non-pharmacological interventions are likely to be the most effective for the management of fatigue in MS by:
Thoroughly reviewing the interventions included in the trials so that subgroups of exercise, behavioural and combined interventions could be clearly defined including whether they are targeting fatigue as a primary or secondary outcome;
Directly comparing these defined intervention groupings using NMA at the end of treatment and at follow-up;
Exploring if fatigue-targeted interventions (intervention exclusively designed with an aim to reduce fatigue) have larger effects than non-fatigue-targeted interventions, including some predefined within category comparisons (1) cognitive behavioural therapy (CBT) for depression, where fatigue is measured as a secondary outcome, versus CBT for fatigue which is a different protocol where fatigue is the primary target, and (2) whether systematic differences in treatment effects exist depending on setting of general exercise delivery, that is, aquatic versus land-based;
Defining the quality of the evidence using GRADE criteria;
Exploring how treatment effects vary according to type of MS, duration of health care professional (HCP) contact and study quality (i.e. risk-of-bias) in sensitivity/subgroup analyses.
Methods
This review was conducted according to PRISMA guidelines20,21 and a full description of methods used can be found in the protocol. Our registered protocol22 had two additional aims which are not detailed in this paper: (1) to conduct pairwise meta-analyses for pooled treatment effects across intervention categories and estimate statistical heterogeneity and (2) to explore fatigability as a secondary outcome. Fatigability has been defined as ‘the magnitude or rate of change in a performance criterion relative to a reference value or given time of task performance or measure of mechanical output’.23 We have conducted the pairwise comparisons but as they overlap with previous publications, we have focused this paper on the novel NMA. We did not find any papers with fatiguability as an outcome.22,24 The inclusion/exclusion criteria are detailed in Table 1.
Table 1.
PICOS inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria | |
|---|---|---|
| Study design | Randomised controlled trials (RCTs) or quasi-RCTs (feasibility, efficacy and naturalistic/pragmatic trials) | Uncontrolled intervention studies, or case series studies, or observational studies |
| Population | Adults with any type of MS | Children, adolescents (<18 years old) |
| Intervention | Behavioural and/or exercise interventions either fatigue-targeted or non-targeted versus any comparator (no intervention, usual care, medication, placebo treatment or another active intervention) | Trials were excluded if they were solely pharmacological and/or dietary interventions |
| Outcome | Fatigue measured using validated patient-reported outcome measures (PROMs) of fatigue severity and/or impact as either a primary or secondary outcome | |
| Language | There were no language restrictions | |
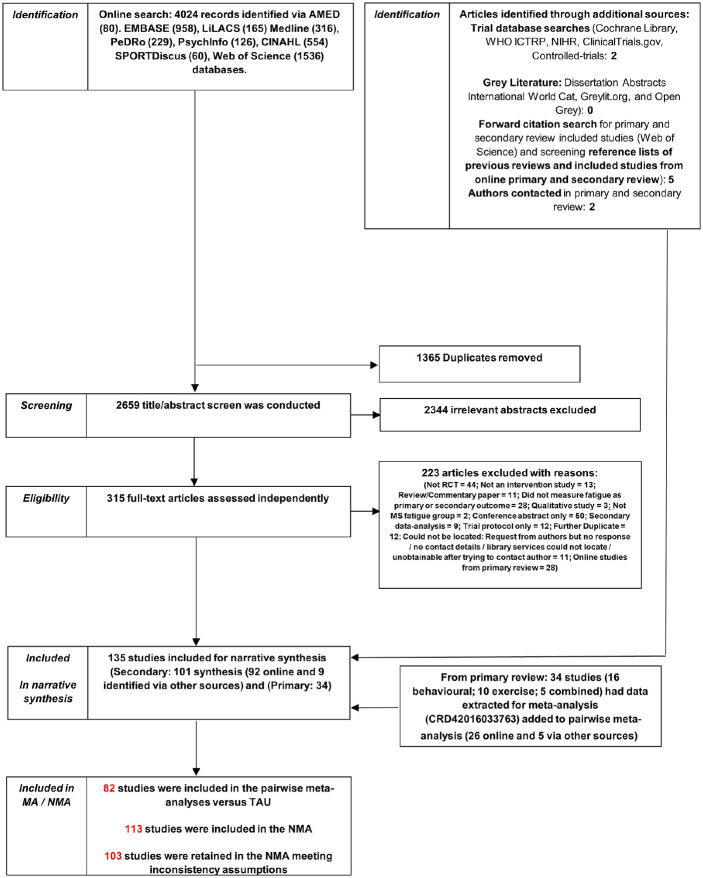
Studies were identified though a systematic online search of nine databases shown in Figure 1 undertaken in December 2015, and repeated in July 2017 and August 2018, using search terms presented in Supplementary Material A. As part of the updated searches, the same search strategy was used in the nine databases as the original search, but restricted to the date of the last search to capture any new hits since. Studies were also identified through a search of trial and grey literature databases, forward citation, reference lists of included studies and previous reviews, and contacting authors of published studies. Initial screening of titles/abstracts and full-text articles was performed independently by co-authors A.M.H. and R.S. and disagreements resolved by team consensus. The 2017 and 2018 updated searches and screening were conducted by M.L.v.d.L. and Georgia Andreopoulou (see Acknowledgements).
Figure 1.
PRISMA flowchart diagram.
Data extraction and risk of bias assessment
A data extraction tool, including Cochrane Risk of Bias (RoB) tool, was developed a priori based on the Cochrane Handbook for Systematic Reviews25 and elements of the Template for Intervention Description and Replication (TIDieR).26 Data extracted were sample sizes, pre-post change scores between groups, means and standard deviations per arm at each post-randomisation assessment and related information (e.g. standard errors, confidence intervals and test statistics). Details of study participant characteristics and key intervention components or techniques were also extracted.
Data extraction and RoB assessments were performed by R.S. and S.G. for exercise and by A.M.H. and L.S. for behavioural or combined trials. Agreement for RoB, based on 15 exercise and 15 behavioural or mixed studies, ranged from Kappa = 0.54–0.86 across each rater pairing indicating ‘good’ consistency.27 All remaining studies, including the update searches, were single-extracted by S.G., L.S., M.L.v.d.L., G.A., R.S. and A.M.H., and meta-analytic data and RoB were cross-checked by M.L.v.d.L., R.S. and A.M.H.
Data analysis
Descriptive data and categorisation of studies
All studies were tabulated, summarising demographics of the samples, fatigue outcome measures, end-of-treatment timepoint, attrition at follow-up, long-term follow-up if available and detailed intervention characteristics (see tables in Supplementary Material B). For fatigue-targeted studies, this information is available elsewhere.28
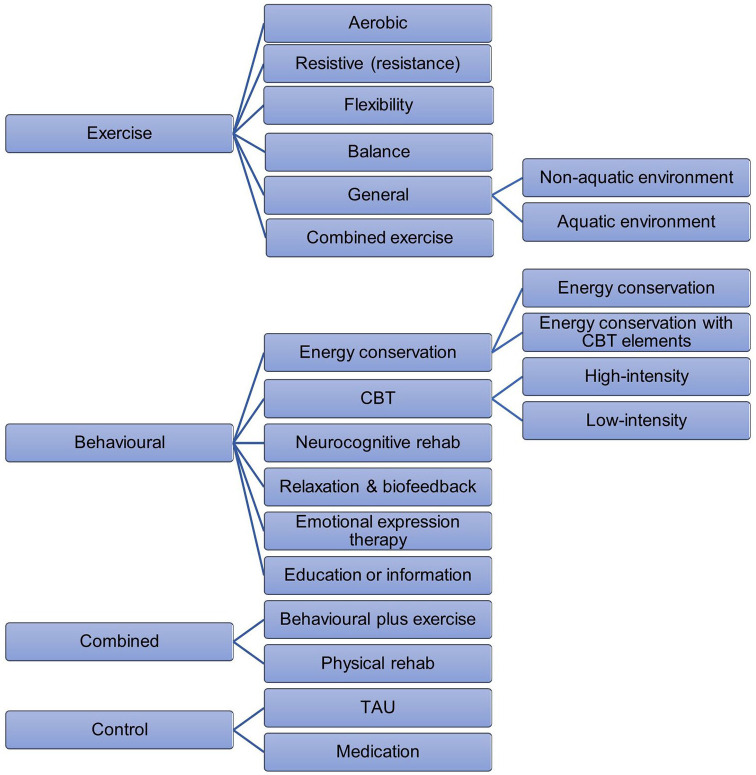
The primary outcome was fatigue severity and/or impact at ⩽ 3 months post-treatment (end-of-treatment), while the secondary outcome was fatigue at longer follow-up, >3 to 6 and >6 months post-treatment. A three-level categorisation of interventions was used for the analysis (Figure 2; Table 2) based on a component breakdown conducted as part of a previous review.28 At the top-level interventions are coded as exercise, behavioural, combined and control treatments such as treatment as usual (TAU) and medication. The second level includes subgroups, defined in Table 2, to account for heterogeneity in intervention types and is the main unit for analysis. There were six subgroups of exercise, five for behavioural and two for combined. A further level of categorisation reflects heterogeneity in the nature of the intervention (e.g. intensity or environment). FACETS, consisting of CBT with some EC techniques for improved patient acceptability,36,37 was classified as EC, similar to two other interventions.38,39 These interventions differ from what is considered a typical exemplar of this type of category and sensitivity analyses have been conducted to assess whether this affects the outcome. At post-treatment relative to TAU, EC with elements of CBT, SMD = −0.06, 95% CI = (−0.38, 0.27), appeared marginally less effective than EC alone, SMD = −0.23, 95% CI = (−0.41, −0.05). Whether methodological heterogeneity appears to result in statistical heterogeneity was explored in sensitivity analysis. Discrepancies between category assignment were resolved in discussion with M.L.d.v.L. and R.M.M. until consensus was reached.
Figure 2.
Hierarchical categorisation of interventions included in the pairwise meta-analysis and network meta-analysis.
Table 2.
Description of intervention subtypes based on total number of studies identified (N=135).
| Intervention subtype | Descriptor | N studies included |
|---|---|---|
| Exercise | ||
| Aerobic Both targeted and non-targeted interventions included |
Main fitness component in exercise in which the body’s large muscles move in a rhythmic manner for a sustained period of time. Aerobic activity, also called endurance activity, improves cardiorespiratory fitness. Examples include walking, running, cycling and swimming. | 25 |
| Resistive (also referred to as
resistance) Both targeted and non-targeted interventions included |
Main physical fitness component in exercise aiming at increasing skeletal muscle strength, power, endurance and mass (e.g. progressive resistance training (PRT) – fixed weights, PRT-free weights, resistance training and body-weight-resistance exercise). | 8 |
| Flexibility Non-targeted intervention |
Main fitness component in exercises aimed at increasing the range of motion possible at a joint. Flexibility is specific to each joint and depends on specific variables, including but not limited to the tightness of specific ligaments and tendons. | 1 |
| Balance Both targeted and non-targeted interventions included |
Main fitness component in static and dynamic exercises that are designed to improve individuals’ ability to withstand challenges from postural sway or destabilising stimuli caused by self-motion, the environment or other objects. | 9 |
| General exercise (aquatic and
land-based) Both targeted and non-targeted interventions included |
Exercise involving combination of two or more of the above components, no dominant fitness component focus provided. | 28 of which 6 aquatic |
| Combined exercise Both targeted and non-targeted interventions included |
Exercise explicitly aimed at more than one of the exercise components listed above, often using a progressive overload principle. | 24 |
| Behavioural | ||
| Energy conservation Only fatigue-targeted interventions included |
Energy effectiveness strategies or energy conservation (EC) education is defined as the ‘the identification and development of activity modifications to reduce fatigue through a systematic analysis of daily work, home and leisure activities in all relevant environments’ (p. 592).69 EC strategies include analysing and modifying activities to reduce energy expenditures; taking frequent rests; prioritising activities; planning; delegating some activities, using the body efficiently, organising tools, materials and work area, using assistive technologies to conserve energy; adopting good posture; leading a healthy lifestyle (regular exercise, healthy diet and stress management, examining and modifying standards and priorities). | 12 of which 3 incorporated elements of CBT |
| Cognitive behavioural therapy Both targeted and non-targeted interventions included |
CBT is founded on the premise that physiological, cognitive (thinking), emotional and behavioural responses influence one another in a reciprocal way within the context of the social environment, where change in any one of these responses may produce changes in others.70 CBT was originally developed to treat anxiety and depression. CBT has also been developed to treat fatigue specifically and MS-specific versions have been developed. CBT for fatigue is based on guided discovery where individuals identify which perpetuating factors may be relevant to them and are provided techniques to alter or manage these behavioural, cognitive, emotional and external factors.71 Protocols vary but tend to include creating consistent activity routines (including sleep wake cycles), graded increase of daily activity, identifying and managing unhelpful thoughts in relation to fatigue, and managing stress. | 14, of which 4 low intensity |
| Neurocognitive rehabilitation Only non-targeted interventions included |
Neurocognitive rehabilitation aims to reduce cognitive deficits, through learning and memory-based training to improve cognitive skills, like attention, or identification of alternative skills to compensate for cognitive deficits in daily living.72 | 7 |
| Relaxation and biofeedback Both targeted and non-targeted interventions included |
Relaxation techniques include ‘a number of practices such as progressive relaxation, guided imagery, biofeedback, self-hypnosis, and deep breathing exercises. The goal is similar in all: to produce the body’s natural relaxation response, characterized by slower breathing, lower blood pressure, and a feeling of increased well-being’.73 | 5 |
| Emotional expression therapy Only non-targeted interventions included |
The aim of emotional expression therapy is to facilitate and promote acceptance, expression, regulation and understanding of emotions.74 | 3 |
| Education or information Both targeted and non-targeted interventions included |
8, predominantly as active control plus 3 active controls re-categorised as education for inclusion in the analysis | |
| Combined (behavioural and exercise) | ||
| Behavioural plus exercise Both targeted and non-targeted interventions included |
Behavioural techniques to promote physical activity/exercise or behavioural interventions with integrated exercise (e.g. EC plus aerobic exercise). | 11 |
| Physical rehabilitation Both targeted and non-targeted interventions included |
Occupational therapy and physiotherapy. | 6 |
| Control | ||
| Treatment as usual | 82 | |
| Medication | 2, of which Nedeljkovic et al.,75 Methylprednisolone + TAU | |
Note. The number of studies for each intervention subtype captures studies where each intervention subtype was evaluated at least in one arm based on the total number of studies identified (N=135). In text, the number of studies and arms are reported for the top-level intervention groupings after exclusion of studies that did not provide sufficient data to be included in the analyses and combining/removing arms that were in the same intervention subcategory.
Statistical analysis
All analyses were conducted using Stata 15.1. First, separate pairwise random-effects meta-analyses were conducted (using admetan package) including only studies comparing interventions against a TAU control arm (Supplementary Material C). Second, using restricted maximum likelihood by the mvmeta command and network packages, an NMA including all studies in a single analysis combining direct and indirect effect estimates was conducted. Effect sizes were expressed as standardised mean differences (SMD) between groups, calculated as Hedge’s g with a small sample correction applied.29 SMDs were calculated using the between-group post-treatment mean change from baseline scores where available and post-treatment means otherwise. The consistency assumption, which is an extension of the pairwise meta-analytic assumption of statistical homogeneity,30 was tested using loop-specific and node-splitting approaches. The final model was estimated excluding studies that contributed to violations of the consistency assumption. Treatments were ranked according to estimates of the surface under the cumulative ranking curve (SUCRA), where this ranges between 0 and 1 and higher values indicate a greater likelihood of treatment being more effective relative to other treatments.
Interventions in two-arm studies categorised within the same subgroup (e.g. two aerobic exercise arms) could not be included in the same analysis. If a study included an additional arm/s not in the intervention subgroup (e.g. a common TAU control arm), the treatment arms within the same subgroup were combined by calculating the weighted mean and pooled standard deviation.31 Combining arms in this way does not impact on the treatment effect for that subgroup but ensures that the standard error is calculated correctly.32 If all arms were in the same subgroup, the control arm was re-categorised to allow study inclusion where possible or excluded from the analyses (the control arms for Mackay et al.40 and van Kessel et al.41 were both categorised as education arms, whereas originally the control arms mimicked the intervention arms but without biofeedback or without email support, respectively. In Straudi et al.,42 both arms were categorised as general exercise, and consequently, the control arm consisting of walking therapy was categorised as aerobic exercise for the purpose of analysis).
Results
Description of the included studies
Overall, 135 studies (292 arms) were identified from the literature search (Figure 1). These studies included 119 two-arm, 12 three-arm and 4 four-arm trials. Twenty-two studies did not provide sufficient data for inclusion in the pairwise or NMAs. After combining/removing arms that were in the same intervention subcategory, data from 6909 participants across 235 arms from 113 studies were included in the analyses (studies with multiple arms in the same category combined were Briken et al.,43 Garrett et al.,44 Hogan et al.,45 Seebacher et al.,46 Seebacher et al.47 and Shanazari et al.48 Both arms from Samaei et al.49 were aerobic exercise and could not be included in the analysis). There were 78 arms (51 studies) involving entirely or mainly exercise interventions, 56 arms (43 studies) involving entirely or mainly behaviour interventions, and 19 arms (19 studies) involving combined exercise and behavioural interventions. Table 2 shows the number of studies in each type of the intervention subtypes based on the total number of studies identified (N=135). Few studies directly compared an intervention involving an entirely or mainly exercise intervention with an entirely or mainly behavioural intervention. No trials compared either CBT or EC to an exercise intervention. Figure 3 summarises the subcategory comparisons included in the network. The results focus on the NMA, but the results of the pairwise meta-analysis are available in Supplementary Material C.
Figure 3.
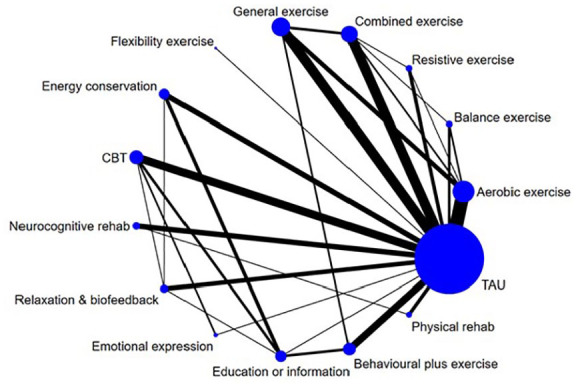
Network of intervention comparisons based on model meeting inconsistency assumptions. Node (circle) sizes indicate the number of studies and edge (line) widths the number of direct comparisons.
NMA of post-treatment effects
The final NMA based on the model meeting the consistency assumption (inconsistency χ2(26) = 34.93; p = 0.113) involved data from 6430 participants across 213 arms from 103 studies (Figure 4). The median sample size was 20 per arm with an interquartile range from 14 to 37 (range = 4–204).
Figure 4.
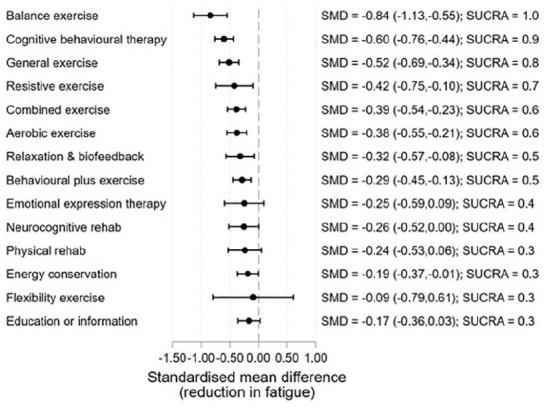
Treatment effects relative to TAU at the end of treatment.
Ten of the 113 studies identified were excluded from the final model due to issues with assumption of consistency between direct and indirect treatment effect estimates in preliminary analysis (χ2(29) = 43.5; p = 0.041). The exclusion of six studies with treatment effect estimates that were clear outliers (outside 99% limits on funnel plot) reduced the overall test for inconsistency to non-significant.33–35,50–52 However, issues with loop-specific inconsistency remained for physical rehabilitation, relaxation/biofeedback and education/information until a further four studies were removed.53–56 The excluded studies involved general exercise, combined exercise, physical rehabilitation and relaxation/biofeedback as active control arms, where most other studies involved these treatments as the experimental arm. Estimates from the preliminary NMA model involving all 113 studies are presented in Supplementary Material D.
Treatment effect estimates relative to TAU from the final NMA model are presented in Figure 4. The ordering of the interventions in the graph is based on the SUCRA. The largest effect is observed for balance exercise (SMD = −0.84, 5 studies, 124 participants). Along with CBT (SMD = −0.60, 15 studies, 594 participants) (separating CBT into high vs low intensity indicated no difference in the treatment effect estimates: high intensity SMD = −0.62, 95% CI = (−0.82, −0.41), low intensity SMD = −0.51, 95% CI = (−0.89, −0.13)), general exercise (SMD = −0.52, 16 studies, 373 participants) (separating general exercise into exercise undertaken in a non-aquatic vs aquatic environment indicated no difference in the treatment effect estimates: non-aquatic SMD = −0.50, 95% CI = (−0.80, −0.21), aquatic SMD = −0.39, 95% CI = (−0.80, 0.03). The setting of delivery for general exercise may therefore not be an important factor); resistive exercise (SMD = −0.42, 5 studies, 90 participants); combined exercise (SMD = −0.39, 15 studies, 531 participants); aerobic exercise (SMD = −0.38, 22 studies, 352 participants); relaxation/biofeedback (SMD = −0.32, 8 studies, 182 participants); behavioural plus exercise (SMD = −0.29, 12 studies, 633 participants); and EC (SMD = −0.19, 10 studies, 436 participants) were all estimated to have statistically significant moderate effects on fatigue.
Table 3 provides an integrated overview of treatment effects based on the pairwise and NMAs and GRADE rating of the certainty of the evidence. In most categories, certainty of the evidence was moderate and the effects for the pairwise and network analyses were very similar. The certainty of the evidence presented for CBT, behavioural plus exercise and EC was high and for combined exercise and rehabilitation, it was low.
Table 3.
Treatment effects based on pairwise and network meta-analyses by intervention subcategories taking into consideration certainty of the evidence based on GRADE assessment.
| Classification of interventions | Intervention | Pairwise meta-analysis |
NMAb |
Certainty of the evidenced
(GRADE)e |
|||
|---|---|---|---|---|---|---|---|
| Ncomparisons; Nparticipants (median) | SMDa (95% CI) | I2 (%) | SMD (95% CI) | SUCRAc | |||
| Moderate to large effect | Balance | 3; 168 (67) | −0.87 (−1.18, −0.55) | 0.00 | −0.84 (−1.13, 0.55) | 1.0 | ⊕⊕⊕⊝Moderatef |
| Cognitive behavioural therapy | 9; 775 (50) | −0.54 (−0.69, −0.40) | 3.40 | −0.60 (−0.76, −0.44) | 0.9 | ⊕⊕⊕⊕High | |
| General | 11; 544 (34) | −0.47 (−0.65, −0.30) | 32.60 | −0.52 (−0.69, −0.34) | 0.8 | ⊕⊕⊕⊝Moderateg | |
| Small to moderate effect | Resistive | 4; 161 (32.5) | −0.46 (−0.77, −0.15) | 0.00 | −0.42 (−0.75, −0.10) | 0.7 | ⊕⊕⊕⊝Moderatef |
| Combined exercise | 12; 767 (41.5) | −0.35 (−0.50, −0.20) | 68.20 | −0.39 (−0.54, −0.23) | 0.6 | ⊕⊕⊝⊝Lowg,h | |
| Aerobic | 15; 475 (30) | −0.41 (−0.62, −0.22) | 18.50 | −0.38 (−0.55, −0.21) | 0.6 | ⊕⊕⊕⊝Moderateg | |
| Relaxation and biofeedback | 5; 217 (48) | −0.53 (−0.80, −0.26) | 0.00 | −0.32 (−0.57, −0.08) | 0.5 | ⊕⊕⊕⊝Moderatef | |
| Behavioural plus exercise | 8; 728 (94.5) | −0.28 (−0.43, −0.13) | 0.00 | −0.29 (−0.45, −0.13) | 0.5 | ⊕⊕⊕⊕High | |
| Emotional expression therapy | 1; 60 (NA) | NAi | NA | −0.25 (−0.59, −0.09) | 0.4 | NA | |
| Neurocognitive rehabilitation | 6; 269 (41.5) | −0.24 (−0.51, 0.02) | 12.30 | −0.26 (−0.52, −0.00) | 0.4 | ⊕⊕⊕⊝Moderatef | |
| Physical rehabilitation | 4; 211 (43) | −0.16 (−0.44, 0.11) | 0.00 | −0.24 (−0.53, 0.06) | 0.3 | ⊕⊕⊝⊝Lowg,f | |
| Negligible to small effect | Energy conservation | 6; 632 (108.5) | −0.19 (−0.35, −0.03) | 0.00 | −0.19 (−0.37, −0.01) | 0.3 | ⊕⊕⊕⊕High |
| Education or information | 1; 49 (NA) | NA | NA | −0.17 (−0.36, 0.03) | 0.3 | NA | |
| Uncertain | Flexibility | 1; 38 (NA) | NA | NA | −0.09 (−0.79, 0.61) | 0.3 | NA |
| Overall groupings | Overall exercise intervention | 46; 2153 (32) | −0.44 (−0.53, −0.35) | 42.5 | NA | NA | ⊕⊝⊝⊝Very lowg,h,j |
| Overall behavioural intervention | 28; 2002 (50) | −0.37 (−0.47, −0.28) | 12.40 | NA | NA | ⊕⊕⊝⊝Lowh,j | |
| Overall combined intervention | 12; 939 (85) | −0.25 (−0.38, −0.12) | 0.00 | NA | NA | ⊕⊕⊕⊝Moderatej | |
Standardised mean difference.
Network meta-analysis.
Surface under the cumulative ranking curve.
GRADE Working Group grades of evidence: High quality: further research is very unlikely to change our confidence in the estimate of effect; Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; Very low quality: we are very uncertain about the estimate.
Grading of Recommendations, Assessment, Development and Evaluations.
Total sample size is small.
Most information is from studies with inadequate allocation concealment or incomplete accounting for outcome data.
Wide variation in effect size exists across studies or large I2 indicates large proportion of the variation in effect size due to among-study differences.
Not applicable.
Small sample effect on the estimated effect due to asymmetrical funnel plot.
Table 4 details the significant comparative efficacy between interventions from the NMA. Balance exercise performed significantly better compared to aerobic exercise, combined exercise, EC, neurocognitive rehabilitation, relaxation/biofeedback, emotional expression therapy, education/information, behavioural interventions including exercise or physical activity, and physical rehabilitation. CBT was estimated to perform significantly better than EC, education/information, behavioural interventions including exercise or physical activity, and physical rehabilitation.
Table 4.
Indirect standardised mean differences (95% confidence intervals) below diagonal and p-values above diagonal based on network meta-analysis.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exercise interventions | Aerobic exercise | – | 0.033 | 0.826 | 0.513 | 0.939 | 0.307 | 0.224 | 0.143 | 0.495 | 0.740 | 0.573 | 0.196 | 0.523 | 0.327 | 0.002 |
| Balance exercise | 0.46 (0.14, 0.78) |
– | 0.138 | 0.128 | 0.028 | 0.129 | 0.006 | 0.252 | 0.025 | 0.041 | 0.048 | 0.005 | 0.015 | 0.017 | 0.000 | |
| Resistive exercise | 0.05 (−0.31, 0.40) |
−0.42 (−0.85, 0.01) |
– | 0.481 | 0.856 | 0.670 | 0.308 | 0.429 | 0.490 | 0.667 | 0.539 | 0.278 | 0.535 | 0.353 | 0.050 | |
| Flexibility exercise | −0.28 (−1.00, 0.43) |
−0.75 (−1.50, 0.004) |
−0.33 (−1.09, 0.43) |
– | 0.499 | 0.345 | 0.821 | 0.262 | 0.722 | 0.608 | 0.732 | 0.861 | 0.646 | 0.855 | 0.819 | |
| Combined exercise | 0.01 (−0.20, 0.21) |
−0.45 (−0.76, −0.15) |
−0.04 (−0.38, 0.31) |
0.29 (−0.42, 1.00) |
– | 0.304 | 0.185 | 0.140 | 0.455 | 0.695 | 0.541 | 0.163 | 0.463 | 0.298 | 0.000 | |
| General exercise | 0.14 (−0.08, 0.35) |
−0.32 (−0.65, 0.003) |
0.09 (−0.27, 0.45) |
0.42 (−0.29, 1.14) |
0.13 (−0.07, 0.33) |
– | 0.046 | 0.548 | 0.181 | 0.295 | 0.267 | 0.040 | 0.123 | 0.117 | 0.000 | |
| Behavioural interventions | Energy conservation | −0.19 (−0.43, 0.05) |
−0.65 (−0.99, −0.31) |
−0.24 (−0.60, 0.13) |
0.09 (−0.62, 0.81) |
−0.20 (−0.43, 0.03) |
−0.33 (−0.57, −0.08) |
– | 0.009 | 0.738 | 0.451 | 0.779 | 0.861 | 0.459 | 0.939 | 0.106 |
| Cognitive behavioural therapy | 0.22 (−0.01, 0.45) |
−0.24 (−0.57, 0.09) |
0.18 (−0.18, 0.54) |
0.51 (−0.20, 1.22) |
0.21 (−0.01, 0.43) |
0.08 (−0.15, 0.32) |
0.41 (0.19, 0.64) |
– | 0.080 | 0.113 | 0.099 | 0.004 | 0.031 | 0.052 | 0.000 | |
| Neurocognitive rehabilitation | −0.13 (−0.44, 0.18) |
−0.59 (−0.98, −0.20) |
−0.17 (−0.59, 0.24) |
0.16 (−0.58, 0.89) |
−0.14 (−0.44, 0.16) |
−0.27 (−0.57, 0.04) |
0.06 (−0.25, 0.37) |
−0.35 (−0.65, −0.05) |
– | 0.735 | 0.999 | 0.664 | 0.824 | 0.719 | 0.138 | |
| Relaxation & biofeedback | −0.06 (−0.35, 0.24) |
−0.52 (−0.90, −0.14) |
−0.10 (−0.51, 0.30) |
0.23 (−0.51, 0.96) |
−0.07 (−0.35, 0.22) |
−0.20 (−0.49, 0.10) |
0.13 (−0.15, 0.42) |
−0.28 (−0.55, −0.01) |
0.07 (−0.28, 0.42) |
– | 0.768 | 0.388 | 0.847 | 0.521 | 0.049 | |
| Emotional expression therapy | −0.13 (−0.51, 0.25) |
−0.59 (−1.03, −0.15) |
−0.17 (−0.64, 0.29) |
0.16 (−0.61, 0.93) |
−0.14 (−0.51, 0.23) |
−0.27 (−0.64, 0.11) |
0.06 (−0.31, 0.44) |
−0.35 (−0.67, −0.03) |
0.0003 (−0.42, 0.42) |
−0.07 (−0.48, 0.33) |
– | 0.707 | 0.855 | 0.769 | 0.246 | |
| Education or information | −0.21 (−0.47, 0.04) |
−0.67 (−1.02, −0.33) |
−0.26 (−0.63, 0.12) |
0.07 (−0.65, 0.79) |
−0.22 (−0.46, 0.02) |
−0.35 (−0.60, −0.10) |
−0.02 (−0.23, 0.19) |
−0.43 (−0.65, −0.22) |
−0.08 (−0.40, 0.24) |
−0.15 (−0.44, 0.13) |
−0.08 (−0.46, 0.29) |
– | 0.326 | 0.973 | 0.178 | |
| Combined interventions | Behavioural plus exercise | −0.09 (−0.32, 0.14) |
−0.55 (−0.88, −0.22) |
−0.14 (−0.49, 0.22) |
0.20 (−0.52, 0.91) |
−0.10 (−0.32, 0.12) |
−0.23 (−0.45, −0.002) |
0.10 (−0.12, 0.32) |
−0.31 (−0.52, −0.10) |
0.04 (−0.26, 0.34) |
−0.03 (−0.31, 0.25) |
0.04 (−0.33, 0.41) |
0.12 (−0.08, 0.32) |
– | 0.560 | 0.008 |
| Physical rehabilitation | −0.21 (−0.54, 0.13) |
−0.67 (−1.07, −0.26) |
−0.25 (−0.68, 0.18) |
0.08 (−0.67, 0.83) |
−0.21 (−0.54, 0.11) |
−0.34 (−0.68, −0.01) |
−0.01 (−0.35, 0.32) |
−0.43 (−0.75, −0.10) |
−0.08 (−0.43, 0.28) |
−0.15 (−0.52, 0.23) |
−0.08 (−0.52, 0.37) |
0.01 (−0.34, 0.35) |
−0.12 (−0.44, 0.21) |
− | 0.338 | |
| Treatment as usual | −0.38 (−0.55, −0.21) |
−0.84 (−1.13, −0.55) |
−0.42 (−0.75, −0.10) |
−0.09 (−0.79, 0.60) |
−0.39 (−0.54, −0.23) |
−0.51 (−0.68, −0.35) |
−0.19 (−0.36, 0.01) |
−0.60 (−0.76, −0.44) |
−0.25 (−0.51, 0.01) |
−0.32 (−0.56, −0.08) |
−0.25 (−0.59, 0.09) |
−0.17 (−0.36, 0.02) |
−0.29 (−0.45, −0.13) |
−0.17 (−0.46, 0.11) |
– |
Comparisons are for column versus row (i.e. negative values favour column and positive values favour row).
Significant (p < 0.05) differences denoted in bold. Please note that order of interventions is not ranked in this table.
NMA of follow-up data
Data from 33 studies (35 arms) were available at mid-term follow-up (3–6 months) allowing for assessment of treatment effects for 9 treatment types. The effects were generally comparable to end-of-treatment, with some attenuation evident for most interventions (Figure 5). However, these estimates need to be interpreted with caution as the number of studies per intervention type is typically low and the effect estimates generally provide considerable uncertainty as to the true effect sizes. Data at longer-term follow-up (7–12 months) were available in 9 studies, which was insufficient for analysis.
Figure 5.
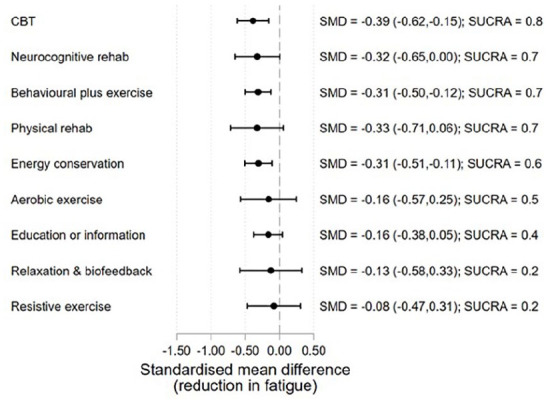
Treatment effects relative to TAU at mid-term follow-up (3–6 months).
Estimates of the effect at longer-term follow-up versus TAU where only one study was available are not presented (balance exercise, combined exercise and emotional expression therapy).
Moderator and sensitivity analyses
Planned analyses were conducted to assess whether effect sizes vary according to the following pre-specified characteristics.
Type of MS and total contact hours with HCP
Most studies included mixed MS samples or did not specify MS type. Samples of 19 studies consisted only of participants with RRMS. Of these, data were too sparse for NMA, with a maximum of five studies evaluating combined exercise interventions and only one CBT intervention. Similarly, there were only six studies with samples consisting exclusively of participants with PPMS, and therefore insufficient for NMA. The number of studies evaluating subcategories of interventions by MS subtype is detailed in Supplementary Material E.
Predominantly, interventions included over 80 minutes of HCP contact (except for 11 arms) and consequently, this precluded us from examining treatment effects by intensity of HCP contact.
Fatigue-targeted versus non-fatigue-targeted interventions
A common effect of fatigue-targeted versus non-fatigue-targeted interventions was estimated across all interventions, rather than on an intervention-by-intervention basis, due to some intervention categories having small numbers of either targeted or non-targeted interventions. The magnitude of the difference in the SMD for fatigue-targeted versus non-target interventions was negligible and non-significant (g = −0.01, p = 0.940, 95% CI = (−0.23, 0.21)).
Since some CBT interventions specifically targeted fatigue, the NMA was re-estimated splitting CBT into a targeted and non-targeted groups. For the targeted CBT interventions, the effect was larger (targeted −0.78, p < 0.001, 95% CI = (−1.04, −0.53); non-targeted −0.47, p < 0.001, 95% CI = (−0.68, −0.26)); however, the difference was not statistically reliable (difference g = 0.31, p = 0.062, 95% CI = (−0.02, 0.64)).
RoB
A summary of RoB assessment as percentage across all included studies and for each included study is presented in Supplementary Material F. Behavioural studies had the lowest RoB relative to exercise studies, followed by combined intervention studies. Exercise studies had the highest RoB relative to behavioural and combined intervention studies. In assessing RoB, performance bias was not considered for the overall quality judgement as lack of blinding of participants and healthcare professional is an inherent limitation in studies of behavioural and exercise interventions. For the other five RoB domains, behavioural studies and combined behavioural and exercise studies had low RoB compared to exercise studies. In exercise studies (both fatigue-targeted and non-targeted interventions), the method for random allocation to arms was either unclear or inappropriate in 9.2% of studies (n = 7). Furthermore, 53.9% of studies (n = 41) did not provide sufficient information on allocation concealment, and 40.8% of studies (n = 31) had incomplete outcome data, whereas in behavioural studies and combined behavioural and exercise intervention studies, these ratings were 6.8% (n = 3), 29.5% (n = 13), 25.0% (n = 11) and 5.9% (n = 1), 29.4% (n = 5), 17.65% (n = 3), respectively. Reporting bias was identified in 10.5% of exercise (n = 8) studies compared to 13.6% of behavioural studies (n = 6) and 23.5% of combined intervention studies (n = 4). Planned sensitivity analysis was not possible to examine RoB on an intervention-by-intervention basis, so a common effect was estimated pooling across all studies. Studies with low RoB were estimated to have SMDs relative to TAU that were less favourable of the intervention compared to those that were not considered low RoB, though the difference was non-significant (0.15, p = 0.257, 95% CI = (−0.11, 0.41)).
Discussion
Summary of evidence
The overarching aim of the current meta-analysis was to elucidate which subtypes of exercise, behavioural and combined interventions are likely to be the most effective for the management of fatigue in MS using NMA. NMA reflects a novel and powerful quantitative synthesis tool that allows for both direct and indirect comparisons of multiple interventions that have not been directly compared within the same trial. By using the NMA approach, we were able to (1) generate rankings that account for uncertainty in the treatment effect estimates and (2) provide comparative evidence between interventions based on direct and indirect evidence.57 This approach to evidence synthesis has also provided a clear overview of the existing evidence for different interventions and direct comparisons that have been drawn between interventions to date. The large number of trials (many of them small) evaluating a myriad of interventions with few head-to-head (direct) comparisons between them represents a key barrier to improving standard fatigue care in MS. The NMA approach provides a more robust method of comparing and ranking the efficacy of the wide range of treatments when compared to pairwise meta-analysis. The geometry of the graphic display of the network also provides a clear visual summary of how much evidence (based on number and size of trials) exists for each treatment.57
A total of 135 studies were identified from the literature search; 113 studies (including 235 arms) had sufficient data to be included in the NMA, but only 103 studies were retained based on the model meeting inconsistency assumptions. The largest intervention category was TAU (82 studies), followed by entirely or mainly exercise interventions (51 studies), entirely or mainly behavioural interventions (43 studies) and finally, combined exercise and behavioural interventions (19 studies). Studies evaluating behavioural and combined intervention were of better quality according to Cochrane’s RoB tool, compared to exercise studies. A sensitivity analysis across all interventions showed that studies which included fatigue as a primary outcome had similar effects to those where fatigue was a secondary outcome. Overall, sensitivity analysis indicated that high RoB studies resulted in larger estimates of the common effect across all studies; however, this was not statistically significant and the magnitude of the difference was small (difference g = 0.15, p = 0.257, 95% CI = (−0.11, 0.41)). Notably, many exercise studies had inadequate allocation concealment (53.9%) or incomplete outcome data (40.8%), which, with respect to quality of evidence, lowered our confidence in the overall estimate of treatment effect of exercise interventions, and in particular, of aerobic, general and combined exercise interventions.
The findings of the current review that synthesised evidence on both fatigue-targeted and non-fatigue-targeted interventions using the NMA approach are similar to the findings of our previous review that focused exclusively on interventions aimed at fatigue using pairwise meta-analysis.28 However, the NMA approach allowed us to gain precision and additional insights into less studied interventions, such as balance exercise (n = 2,28 current review n = 5) and aerobic exercise (n = 3,28 current review n = 22).
In terms of end-of-treatment effects, balance exercise had the largest effect on fatigue when compared to TAU in the NMA (SMD = −0.84). This effect was significantly larger than all other types of exercise and behavioural interventions except CBT. CBT had the next largest effect (SMD = −0.60) and was significantly superior to all other behavioural treatments. The GRADE rating for certainty of the CBT effect was high. The rating for balance exercise was moderate so needs to be treated with some caution as it is based on small studies (N range = 12–88) and differences in the nature of the interventions. Balance exercise interventions included hippotherapy,58 vestibular rehabilitation,59 and balance and eye movement exercises.94 As there are only end-of-treatment effects in this category, we do not know if these effects will sustain. Follow-up data on CBT showed that this ranked the highest of intervention types where follow-up (3–6 months) was available and the effect was still significant although somewhat attenuated (SMD = 0.39). It also needs to be noted that heterogeneity was also identified in the CBT category, in terms of type and target of CBT (fatigue versus depression or stress), the mode of delivery (web, telephone and face-to-face delivery), amount of therapist contact and type of therapists. Pre-planned subgroup analysis suggests that CBT which specifically targets fatigue may have larger effects that CBT where fatigue is a secondary target.
General exercise (defined as including two or more of the key exercise types – balance, aerobic, strength and/or flexibility) also had a moderate to large effect. Other interventions which had significant moderate effects on fatigue at the end of treatment were resistive exercise, combined exercise, aerobic exercise, relaxation/biofeedback interventions and behavioural interventions which included an exercise or physical activity component. The certainty of the evidence in these categories was low or moderate except for behavioural plus exercise where the GRADE rating was high.
EC which was specifically developed to treat fatigue in MS had a marginally significant effect on fatigue at end-of-treatment and was ranked one of the least effective treatments, followed by flexibility exercise and education. The certainty of the small to negligible effect for EC is high. It is worth noting that EC had a significant small effect at longer-term follow-up. The study that contributed most to the follow-up effect combined EC with elements of CBT36 which may have enhanced the effect. Alternately, small positive effects for EC maybe be experienced later on. Behavioural interventions which included an exercise or physical activity component appeared to retain significant effects at longer-term follow-up, but aerobic and resistive exercise did not. No data were available on longer-term follow-up of combined exercise.
Evidence in favour of end-of-treatment effects of different subtypes of exercise interventions (except flexibility on its own) suggests there is no single optimal exercise modality for MS fatigue, but rather a choice of exercise subtype may depend on MS symptoms, level of disability, and the needs and preferences of pwMS. The mechanisms through which exercise improves fatigue will therefore differ by subtypes of exercise interventions.60–62 For example, impaired balance is common in MS, affecting 85% of pwMS, and has been linked to fatigue.63 Impaired postural control and increased likelihood of falls can lead to reduced ability to perform physical activity and further deconditioning. In addition, extreme and continuous efforts to maintain postural control may not only lead to further muscle strain and subsequently pain, spasticity and inefficient movement patterns, but also demand constant attention–all resulting in increased fatigue.
Further work should focus on tailoring exercise interventions to the needs and preferences of pwMS. Needs-based assessment should include an understanding of possible mechanisms of fatigue which may include the balance ones discussed above or physiological deconditioning due to fatigue related to activity. For this reason, it is of paramount importance for studies to specify MS subtypes within their samples and for future research to explore the treatment needs and systematic patterns in treatment effects by MS subtype. A focus is needed on maintaining effects at long-term follow-up. As interventions which combined exercise and behavioural treatments show sustained significant effects, combining approaches may be beneficial. Combining the two types of interventions may improve adherence to exercise and maintenance of benefits over time. Behavioural treatments may also tackle fatigue mechanisms not addressed by exercise such as poor sleep or disrupted circadian rhythms. To date, no studies have combined CBT with exercise. As CBT ranked highest in the behavioural category, has an empirically validated theoretical underpinning64 – a combined CBT and exercise intervention may be one way to enhance existing treatment effects.
Based on the common effect across all intervention types, there was no significant difference between fatigue-targeted versus non-fatigue-targeted interventions; however, the limited number of fatigue-targeted interventions in some intervention categories precluded us from exploring the effect of treatment target on an intervention-by-intervention basis so this finding should be treated with caution. In terms of CBT, pre-planned moderator analysis suggested that CBT designed to treat fatigue had greater effects than CBT for depression or stress. This may be in part because CBT for fatigue includes a focus on grading or increasing activity levels, whereas CBT for depression focuses on activities which improve mood, suggesting that treatment recommendations should specify type of CBT. This has also been previously highlighted in a meta-analytic systematic review of non-pharmacological interventions for cancer fatigue.65
Limitations
To our knowledge, this is the first systematic review of non-pharmacological interventions for MS fatigue that adopted a detailed approach to intervention coding and utilised NMA. Nonetheless, limitations of this review need to be noted. First, for the analyses, categorisation of interventions was necessary given the limited number of some subtypes of exercise, behavioural and combined interventions, for example, mindfulness interventions were grouped together with CBT and FACETS which combined CBT and EC36,37 was grouped under EC. Important nuances may have therefore been obscured because of data reduction. This may explain some of the heterogeneity in these subcategories. Any inferences about the effect size relate to the broad category and not necessarily to any specific type of intervention within that category. It is important to also note the heterogeneity related to the measurement of fatigue across studies with 13 different fatigue measures used, an inherent weakness of the fatigue literature.66,67 However, evidence in support of the convergent validity between the different self-report measures of fatigue, particularly the Modified Fatigue Impact Scale (MFIS) and Fatigue Severity Scale (FSS) (the most commonly used measures in the studies here, n = 49 and n = 43, respectively),66,68 and the use of standardised mean differences circumvents the uncertainty related to measurement heterogeneity.
Second, this review included feasibility, efficacy and naturalistic/pragmatic trials. The aims and scope are likely to differ between these types of trials, which may have important implications on treatment effects. Finally, statistical heterogeneity was high for most of the pairwise meta-analyses (Supplementary Material C), which relates to the NMA assumption of consistency. The final NMA model met consistency assumptions after the exclusion of 10 studies and, therefore, most treatment effect estimates can be interpreted without too much concern regarding the level of statistical heterogeneity initially observed. However, there was some indication of inconsistency in the estimates for the relaxation/biofeedback and education/information intervention treatment effect estimates. These estimates must be interpreted more cautiously.
Conclusion
The findings of this review provide insights and specific recommendations for treatment guidelines for fatigue in MS. These need to be more prescriptive in terms of type of behavioural intervention. Balance exercise and CBT interventions appear to be the most promising interventions for fatigue based on direct and indirect comparisons. CBT that is fatigue-focused should maximise effects. Other exercise modalities also appear effective, including aerobic and strength/resistive exercise and combined exercise (balance, aerobic, strength and flexibility) in the short-term, but more work is needed to sustain these effects. This may include allowing patient preference in terms of choice of exercise and behavioural methods to enhance regular exercise habits. More support to maintain exercise is needed over the trajectory of illness, particularly around how to adapt exercise for relapse and progression. EC, which is still offered as a treatment to pwMS, has minimal effects as does flexibility exercise on their own. Just educating people on fatigue is also not an effective intervention. To date, a combined exercise and CBT approach for MS fatigue has not been evaluated, but this warrants further exploration. Although there is a clear need for adequately powered trials with evaluation of treatment mechanisms, and a focus on maintaining treatment effects, there is sufficient pooled evidence of more than 100 trials to suggest that the methods mentioned above should become part of standard treatment for fatigue in MS. Future work should focus on how to effectively implement these treatments into routine care including the most cost-effective ways of delivering treatment.
Note: Studies that were included in the review but not mentioned in the main text are the following.76–184
Supplemental Material
Supplemental material, sj-pdf-1-msj-10.1177_1352458521996002 for Which exercise and behavioural interventions show most promise for treating fatigue in multiple sclerosis? A network meta-analysis by Anthony M Harrison, Reza Safari, Tom Mercer, Federica Picariello, Marietta L van der Linden, Claire White, Rona Moss-Morris and Sam Norton in Multiple Sclerosis Journal
Supplemental material, sj-pdf-2-msj-10.1177_1352458521996002 for Which exercise and behavioural interventions show most promise for treating fatigue in multiple sclerosis? A network meta-analysis by Anthony M Harrison, Reza Safari, Tom Mercer, Federica Picariello, Marietta L van der Linden, Claire White, Rona Moss-Morris and Sam Norton in Multiple Sclerosis Journal
Supplemental material, sj-pdf-3-msj-10.1177_1352458521996002 for Which exercise and behavioural interventions show most promise for treating fatigue in multiple sclerosis? A network meta-analysis by Anthony M Harrison, Reza Safari, Tom Mercer, Federica Picariello, Marietta L van der Linden, Claire White, Rona Moss-Morris and Sam Norton in Multiple Sclerosis Journal
Supplemental material, sj-pdf-4-msj-10.1177_1352458521996002 for Which exercise and behavioural interventions show most promise for treating fatigue in multiple sclerosis? A network meta-analysis by Anthony M Harrison, Reza Safari, Tom Mercer, Federica Picariello, Marietta L van der Linden, Claire White, Rona Moss-Morris and Sam Norton in Multiple Sclerosis Journal
Supplemental material, sj-pdf-5-msj-10.1177_1352458521996002 for Which exercise and behavioural interventions show most promise for treating fatigue in multiple sclerosis? A network meta-analysis by Anthony M Harrison, Reza Safari, Tom Mercer, Federica Picariello, Marietta L van der Linden, Claire White, Rona Moss-Morris and Sam Norton in Multiple Sclerosis Journal
Supplemental material, sj-pdf-6-msj-10.1177_1352458521996002 for Which exercise and behavioural interventions show most promise for treating fatigue in multiple sclerosis? A network meta-analysis by Anthony M Harrison, Reza Safari, Tom Mercer, Federica Picariello, Marietta L van der Linden, Claire White, Rona Moss-Morris and Sam Norton in Multiple Sclerosis Journal
Supplemental material, sj-pdf-7-msj-10.1177_1352458521996002 for Which exercise and behavioural interventions show most promise for treating fatigue in multiple sclerosis? A network meta-analysis by Anthony M Harrison, Reza Safari, Tom Mercer, Federica Picariello, Marietta L van der Linden, Claire White, Rona Moss-Morris and Sam Norton in Multiple Sclerosis Journal
Acknowledgments
The authors express thanks to the MS Society for funding this research. They would also like to thank Dr Jane Petty, King’s College London, Stephanie Hanna, Carole Bennett and Kay-Anne Sheen, MS Society, patient and public involvement members, for their help and support with the broader project. With thanks also to Sam Goodliffe and Louise Sweeney for their support with data extraction, and Georgia Andreopoulou for her involvement in the updated search and screening process.
Footnotes
Author Contribution: All authors contributed to the design of the review, where previously R.M.M., T.M., C.W. and M.L.v.d.L. developed the funding proposal, and R.M.M., T.M. and M.L.v.d.L. were grant holders for the awarded MS Society UK grant; A.M.H. and R.S. conducted all searches, formal screening, data extraction and quality assessment of studies with support from research assistants (see Acknowledgements); S.N. undertook all statistical analyses and interpretation, with assistance from F.P.; A.M.H., R.S., F.P., S.N. and R.M.M. drafted and revised the manuscript with support from the other authors.
Declaration of Conflicting Interest: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: R.M.M. has published RCTs of behavioural interventions for fatigue in MS, which were included in the current review. However, preliminary searches, formal screening of search results against eligibility criteria, data extraction, RoB assessment and data analysis were conducted independently of R.M.M. Remaining authors declare no financial or other conflicts of interest. R.M.M. acknowledges the financial support of the Department of Health via the National Institute for Health Research (NIHR) Specialist Biomedical Research Centre for Mental Health award to the South London and Maudsley NHS Foundation Trust (SLaM) and the Institute of Psychiatry at King’s College London. The views expressed in this article are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Multiple Sclerosis Society UK (Grant No. 26). The funder of the review had no role in study design, data collection, data analysis, data interpretation or writing of the report. The authors had access to all study data and final responsibility for the decision to submit for publication.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Anthony M Harrison, Department of Clinical and Health Psychology, Leeds Teaching Hospitals National Health Service Trust, Leeds, UK.
Reza Safari, Health and Social Care Research Centre, College of Health, Psychology and Social Care, University of Derby, Derby, UK.
Tom Mercer, Centre for Health, Activity and Rehabilitation Research, Queen Margaret University, Edinburgh, UK.
Federica Picariello, Health Psychology Section, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK.
Marietta L van der Linden, Centre for Health, Activity and Rehabilitation Research, Queen Margaret University, Edinburgh, UK.
Claire White, School of Population Health & Environmental Sciences, Faculty of Life Sciences & Medicine, King’s College London, London, UK.
Rona Moss-Morris, Health Psychology Section, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK.
Sam Norton, Health Psychology Section, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK.
References
- 1.Weiland TJ, Jelinek GA, Marck CH, et al. Clinically significant fatigue: Prevalence and associated factors in an international sample of adults with multiple sclerosis recruited via the internet. PLoS ONE 2015; 10(2): e0115541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood B, van der Mei IA, Ponsonby AL, et al. Prevalence and concurrence of anxiety, depression and fatigue over time in multiple sclerosis. Mult Scler 2013; 19(2): 217–224. [DOI] [PubMed] [Google Scholar]
- 3.Krupp LB. Fatigue in multiple sclerosis. CNS Drugs 2003; 17: 225–234. [DOI] [PubMed] [Google Scholar]
- 4.Bleijenberg G. Chronic fatigue and chronic fatigue syndrome in the general population. Health Qual Life Outcomes 2003; 1: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NICE. Tiredness/fatigue in adults, https://cks.nice.org.uk/tirednessfatigue-in-adults#!topicSummary (2015, accessed 19 August 2019).
- 6.Kobelt G, Langdon D, Jönsson L. The effect of self-assessed fatigue and subjective cognitive impairment on work capacity: The case of multiple sclerosis. Mult Scler 2019; 25(5): 740–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James Lind Alliance. Multiple Sclerosis Top 10, https://www.jla.nihr.ac.uk/priority-setting-partnerships/multiple-sclerosis/top-10-priorities/
- 8.NICE. Multiple sclerosis in adults: Management (NICE Guidelines CG186), 2014, https://www.nice.org.uk/guidance/cg186/resources/multiple-sclerosis-in-adults-management-35109816059077
- 9.Asano M, Finlayson ML. Meta-analysis of three different types of fatigue management interventions for people with Multiple Sclerosis: Exercise, education, and medication. Mult Scler Int 2014; 2014: 798285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pilutti LA, Greenlee TA, Motl RW, et al. Effects of exercise training on fatigue in multiple sclerosis: a meta-analysis. Psychosomat Med 2013; 75: 575–580. [DOI] [PubMed] [Google Scholar]
- 11.Cramer H, Lauche R, Azizi H, et al. Yoga for multiple sclerosis: a systematic review and meta-analysis. PLoS ONE 2014; 9: e112414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blikman LJ, Huisstede BM, Kooijmans H, et al. Effectiveness of energy conservation treatment in reducing fatigue in multiple sclerosis: A systematic review and meta-analysis. Arch Phys Med Rehabil 2013; 94(7): 1360–1376. [DOI] [PubMed] [Google Scholar]
- 13.van den Akker LE, Beckerman H, Collette EH, et al. Effectiveness of cognitive behavioral therapy for the treatment of fatigue in patients with multiple sclerosis: A systematic review and meta-analysis. J Psychosom Res 2016; 90: 33–42. [DOI] [PubMed] [Google Scholar]
- 14.Wendebourg MJ, Heesen C, Finlayson M, et al. Patient education for people with multiple sclerosis-associated fatigue: A systematic review. PLoS ONE 2017; 12(3): e0173025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motl RW, Gosney JL. Effect of exercise training on quality of life in multiple sclerosis: A meta-analysis. Mult Scler 2008; 14(1): 129–135. [DOI] [PubMed] [Google Scholar]
- 16.Heine M, van de Port I, Rietberg MB, et al. Exercise therapy for fatigue in multiple sclerosis. Cochrane Database Syst Rev 2015; 9: CD009956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Safari R, Van der Linden ML, Mercer TH. Effect of exercise interventions on perceived fatigue in people with multiple sclerosis: Synthesis of meta-analytic reviews. Neurodegener Dis Manag 2017; 7(3): 219–230. [DOI] [PubMed] [Google Scholar]
- 18.Faltinsen EG, Storebø OJ, Jakobsen JC, et al. Network meta-analysis: The highest level of medical evidence. BMJ Evid Based Med 2018; 23(2): 56–59. [DOI] [PubMed] [Google Scholar]
- 19.Moss-Morris R, Norton S. Aerobic exercise, cognitive behavioural therapy and energy conservation management for multiple sclerosis (MS) fatigue: Are three trials better than one. Mult Scler 2017; 23(11): 1436–1440. [DOI] [PubMed] [Google Scholar]
- 20.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Annals of Internal Medicine 2015; 162: 777–784. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 2010; 340: c869–c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moss-Morris R, Mercer T, White C, et al. Efficacy of targeted versus non-targeted exercise and behavioural interventions on fatigue in multiple sclerosis: Systematic review and meta-analysis. PROSPERO 2016, http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016036671
- 23.Kluger BM, Krupp LB, Enoka RM. Fatigue and fatigability in neurologic illnesses: Proposal for a unified taxonomy. Neurology 2013; 80: 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrison AM, das Nair R, Moss-Morris R. Operationalising cognitive fatigability in multiple sclerosis: A Gordian knot that can be cut. Mult Scler 2017; 23(13): 1682–1696. [DOI] [PubMed] [Google Scholar]
- 25.Higgins J, Altman D, Gøtzsche P, et al. Cochrane bias methods group, Cochrane statistical methods group (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffmann TC, Walker MF. ‘TIDieR-ing up’ the reporting of interventions in stroke research: The importance of knowing what is in the ‘black box’. Int J Stroke 2015; 10(5): 657–658. [DOI] [PubMed] [Google Scholar]
- 27.Altman D. Practical statistics for medical research: London: Chapman and Hall, 1991. [Google Scholar]
- 28.Moss-Morris R, Harrison AM, Safari R, et al. Which behavioural and exercise interventions targeting fatigue show the most promise in multiple sclerosis? A systematic review with narrative synthesis and meta-analysis. Behav Res Therapy 2019; 137: 103464. [DOI] [PubMed] [Google Scholar]
- 29.White IR, Thomas J. Standardized mean differences in individually-randomized and cluster-randomized trials, with applications to meta-analysis. Clin Trials 2005; 2(2): 141–151. [DOI] [PubMed] [Google Scholar]
- 30.Higgins J, Jackson D, Barrett J, et al. Consistency and inconsistency in network meta-analysis: Concepts and models for multi-arm studies. Res Synth Methods 2012; 3(2): 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borenstein M, Hedges L, Higgins J, et al. Introduction to Meta-Analysis. Chichester: John Wiley & Sons, 2009. [Google Scholar]
- 32.Borenstein M, Hedges LV, Higgins JP, et al. Introduction to meta-analysis. Chichester: John Wiley & Sons, 2011. [Google Scholar]
- 33.Razazian N, Yavari Z, Farnia V, et al. Exercising impacts on fatigue, depression, and paresthesia in female patients with multiple sclerosis. Med Sci Sports Exerc 2016; 48(5): 796–803. [DOI] [PubMed] [Google Scholar]
- 34.Cakt BD, Nacir B, Genç H, et al. Cycling progressive resistance training for people with multiple sclerosis: A randomized controlled study. Am J Phys Med Rehabil 2010; 89(6): 446–457. [DOI] [PubMed] [Google Scholar]
- 35.Sangelaji B, Nabavi SM, Estebsari F, et al. Effect of combination exercise therapy on walking distance, postural balance, fatigue and quality of life in multiple sclerosis patients: A clinical trial study. Iran Red Crescent Med J 2014; 16(6): e17173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas PW, Thomas S, Kersten P, et al. One year follow-up of a pragmatic multi-centre randomised controlled trial of a group-based fatigue management programme (FACETS) for people with multiple sclerosis. BMC Neurology 2014; 14: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas S, Thomas PW, Kersten P, et al. A pragmatic parallel arm multi-centre randomised controlled trial to assess the effectiveness and cost-effectiveness of a group-based fatigue management programme (FACETS) for people with multiple sclerosis. J Neurol Neurosurg Psychiatry 2013; 84(10): 1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ehde DM, Elzea JL, Verrall AM, et al. Efficacy of a telephone-delivered self-management intervention for persons with multiple sclerosis: A randomized controlled trial with a one-year follow-up. Arch Phys Med Rehabil 2015; 96(11): 1945–1958. [DOI] [PubMed] [Google Scholar]
- 39.Mohr DC, Hart S, Vella L. Reduction in disability in a randomized controlled trial of telephone-administered cognitive-behavioral therapy. Health Psychol 2007; 26(5): 554–563. [DOI] [PubMed] [Google Scholar]
- 40.Mackay AM, Buckingham R, Schwartz RS, et al. The effect of biofeedback as a psychological intervention in multiple sclerosis: A randomized controlled study. Int J MS Care 2015; 17(3): 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Kessel K, Wouldes T, Moss-Morris R. A New Zealand pilot randomized controlled trial of a web-based interactive self-management programme (MS Invigor8) with and without email support for the treatment of multiple sclerosis fatigue. Clin Rehabil 2016; 30(5): 454–462. [DOI] [PubMed] [Google Scholar]
- 42.Straudi S, Fanciullacci C, Martinuzzi C, et al. The effects of robot-assisted gait training in progressive multiple sclerosis: A randomized controlled trial. Mult Scler 2016; 22(3): 373–384. [DOI] [PubMed] [Google Scholar]
- 43.Briken S, Gold SM, Patra S, et al. Effects of exercise on fitness and cognition in progressive MS: A randomized, controlled pilot trial. Mult Scler 2014; 20(3): 382–390. [DOI] [PubMed] [Google Scholar]
- 44.Garrett M, Hogan N, Larkin A, et al. Exercise in the community for people with minimal gait impairment due to MS: An assessor-blind randomized controlled trial. Mult Scler Journal 2012; 19: 782–789. [DOI] [PubMed] [Google Scholar]
- 45.Hogan N, Kehoe M, Larkin A, et al. The effect of community exercise interventions for people with MS who use bilateral support for gait. Mult Scler Int 2014; 2014: 109142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seebacher B, Kuisma R, Glynn A, et al. Rhythmic cued motor imagery and walking in people with multiple sclerosis: A randomised controlled feasibility study. Pilot Feasibility Stud 2015; 1: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seebacher B, Kuisma R, Glynn A, et al. The effect of rhythmic-cued motor imagery on walking, fatigue and quality of life in people with multiple sclerosis: A randomised controlled trial. Mult Scler J 2017; 23: 286–296. [DOI] [PubMed] [Google Scholar]
- 48.Shanazari Z, Marandi SM, Minasian V. Effect of 12-week Pilates and aquatic training on fatigue in women with multiple sclerosis. J Mazandaran Univ Med Sci 2013; 23: 257–264 (in Persian). [Google Scholar]
- 49.Samaei A, Bakhtiary AH, Hajihasani A, et al. Uphill and downhill walking in multiple sclerosis: A randomized controlled trial. Int J MS Care 2016; 18(1): 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kargarfard M, Etemadifar M, Baker P, et al. Effect of aquatic exercise training on fatigue and health-related quality of life in patients with multiple sclerosis. Arch Phys Med Rehabil 2012; 93(10): 1701–1708. [DOI] [PubMed] [Google Scholar]
- 51.Patti F, Ciancio MR, Reggio E, et al. The impact of outpatient rehabilitation on quality of life in multiple sclerosis. J Neurol 2002; 249(8): 1027–1033. [DOI] [PubMed] [Google Scholar]
- 52.Kargarfard M, Shariat A, Ingle L, et al. Randomized controlled trial to examine the impact of aquatic exercise training on functional capacity, balance, and perceptions of fatigue in female patients with multiple sclerosis. Arch Phys Med Rehabil 2018; 99(2): 234–241. [DOI] [PubMed] [Google Scholar]
- 53.Pompa A, Morone G, Iosa M, et al. Does robot-assisted gait training improve ambulation in highly disabled multiple sclerosis people? A pilot randomized control trial. Mult Scler 2017; 23(5): 696–703. [DOI] [PubMed] [Google Scholar]
- 54.Castro-Sanchez AM, Mataran-Penarrocha GA, Lara-Palomo I, et al. Hydrotherapy for the treatment of pain in people with multiple sclerosis: A randomized controlled trial. Evid Based Complement Alternat Med 2012; 2012: 473963–473963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ozkul C, Guclu-Gunduz A, Irkec C, et al. Effect of combined exercise training on serum brain-derived neurotrophic factor, suppressors of cytokine signaling 1 and 3 in patients with multiple sclerosis. J Neuroimmunol 2018; 316: 121–129. [DOI] [PubMed] [Google Scholar]
- 56.Smith DC, Lanesskog D, Cleeland L, et al. Motivational interviewing may improve exercise experience for people with multiple sclerosis: A small randomized trial. Health Soc Work 2012; 37(2): 99–109. [DOI] [PubMed] [Google Scholar]
- 57.Mills EJ, Thorlund K, Ioannidis JP. Demystifying trial networks and network meta-analysis. BMJ 2013; 346: f2914. [DOI] [PubMed] [Google Scholar]
- 58.Vermöhlen V, Schiller P, Schickendantz S, et al. Hippotherapy for patients with multiple sclerosis: A multicenter randomized controlled trial (MS-HIPPO). Mult Scler 2018; 24(10): 1375–1382. [DOI] [PubMed] [Google Scholar]
- 59.Hebert JR, Corboy JR, Manago MM, et al. Effects of vestibular rehabilitation on multiple sclerosis–related fatigue and upright postural control: A randomized controlled trial. Phys Ther 2011; 91(8): 1166–1183. [DOI] [PubMed] [Google Scholar]
- 60.Langeskov-Christensen M, Bisson EJ, Finlayson ML, et al. Potential pathophysiological pathways that can explain the positive effects of exercise on fatigue in multiple sclerosis: A scoping review. J Neurol Sci 2017; 373: 307–320. [DOI] [PubMed] [Google Scholar]
- 61.O’Connor PJ, Puetz TW. Chronic physical activity and feelings of energy and fatigue. Med Sci Sports Exerc 2005; 37(2): 299–305. [DOI] [PubMed] [Google Scholar]
- 62.Puetz TW, O’Connor PJ, Dishman RK. Effects of chronic exercise on feelings of energy and fatigue: A quantitative synthesis. Psychol Bull 2006; 132(6): 866–876. [DOI] [PubMed] [Google Scholar]
- 63.Hebert J, Corboy JR. The association between multiple sclerosis-related fatigue and balance as a function of central sensory integration. Gait Posture 2013; 38(1): 37–42. [DOI] [PubMed] [Google Scholar]
- 64.van Kessel K, Moss-Morris R. Understanding multiple sclerosis fatigue: A synthesis of biological and psychological factors. J Psychosom Res 2006; 61(5): 583–585. [DOI] [PubMed] [Google Scholar]
- 65.Kangas M, Bovbjerg DH, Montgomery GH. Cancer-related fatigue: A systematic and meta-analytic review of non-pharmacological therapies for cancer patients. Psychol Bull 2008; 134(5): 700–741. [DOI] [PubMed] [Google Scholar]
- 66.Whitehead L. The measurement of fatigue in chronic illness: A systematic review of unidimensional and multidimensional fatigue measures. J Pain Symptom Manage 2009; 37(1): 107–128. [DOI] [PubMed] [Google Scholar]
- 67.Elbers RG, Rietberg MB, van Wegen EE, et al. Self-report fatigue questionnaires in multiple sclerosis, Parkinson’s disease and stroke: A systematic review of measurement properties. Qual Life Res 2012; 21(6): 925–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chipchase S, Lincoln N, Radford K. Measuring fatigue in people with multiple sclerosis. Disabil Rehabil 2003; 25: 778–784. [DOI] [PubMed] [Google Scholar]
- 69.Mathiowetz VG, Finlayson ML, Matuska KM, et al. Randomized controlled trial of an energy conservation course for persons with multiple sclerosis. Mult Scler 2005; 11: 592–601. [DOI] [PubMed] [Google Scholar]
- 70.Beck AT. Cognitive therapy: A 30-year retrospective. Am Psychol 1991; 46: 368–375. [DOI] [PubMed] [Google Scholar]
- 71.van Kessel K, Moss-Morris R, Willoughby E, et al. A randomized controlled trial of cognitive behavior therapy for multiple sclerosis fatigue. Psychosom Med 2008; 70: 205–213. [DOI] [PubMed] [Google Scholar]
- 72.Mitolo M, Venneri A, Wilkinson ID, et al. Cognitive rehabilitation in multiple sclerosis: A systematic review. J Neurol Sci 2015; 354: 1–9. [DOI] [PubMed] [Google Scholar]
- 73.NCCIH. Relaxation Techniques for Health. https://www.nccih.nih.gov/health/relaxation-techniques-for-health
- 74.Greenberg LS. Emotion-focused therapy. American Psychological Association, 2011. [Google Scholar]
- 75.Nedeljkovic U, Raspopovic ED, Ilic N, et al. Effectiveness of rehabilitation in multiple sclerosis relapse on fatigue, self-efficacy and physical activity. Acta Neurol Belg 2016; 116(3): 309–315. [DOI] [PubMed] [Google Scholar]
- 76.Ahmadi A, Arastoo AA, Nikbakht M, et al. Comparison of the effect of 8 weeks aerobic and yoga training on ambulatory function, fatigue and mood status in MS patients. Iran Red Crescent Med J 2013; 15(6): 449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alguacil Diego IM, Pedrero Hernandez C, Molina Rueda F, et al. Effects of vibrotherapy on postural control, functionality and fatigue in multiple sclerosis patients. Neurologia 2012; 27(3): 143–153. [DOI] [PubMed] [Google Scholar]
- 78.Atri AE, Saeedi M, Sorouri F, et al. The effect of aquatic exercise program on fatigue in women with multiple sclerosis [Persian]. J Mazandaran Univ Med Sci 2012; 22: 53–61. [Google Scholar]
- 79.Aydin T, Akif Sariyildiz M, Guler M, et al. Evaluation of the effectiveness of home based or hospital based calisthenic exercises in patients with multiple sclerosis. Europ Rev Med Pharmacol Sci 2014; 18: 1189–1198. [PubMed] [Google Scholar]
- 80.Azimzadeh E, Hosseini M, Nourozi Tabrizi K. Effect of Tai Chi Chuan on quality of life in women with multiple sclerosis. HAYAT 2013; 19: 1–13. [DOI] [PubMed] [Google Scholar]
- 81.Bansi J, Bloch W, Gamper U, et al. Training in MS: Influence of two different endurance training protocols (aquatic versus overland) on cytokine and neurotrophin concentrations during three week randomized controlled trial. Mult Scler 2013; 19(5): 613–621. [DOI] [PubMed] [Google Scholar]
- 82.Brichetto G, Spallarossa P, de Carvalho MLL, et al. The effect of Nintendo (R) Wii (R) on balance in people with multiple sclerosis: A pilot randomized control study. Mult Scler Journal 2013; 19: 1219–1221. [DOI] [PubMed] [Google Scholar]
- 83.Brichetto G, Piccardo E, Pedulla L, et al. Tailored balance exercises on people with multiple sclerosis: A pilot randomized, controlled study. Mult Scler 2015; 21(8): 1055–1063. [DOI] [PubMed] [Google Scholar]
- 84.Bulguroglu I, Guclu-Gunduz A, Yazici G, et al. The effects of Mat Pilates and Reformer Pilates in patients with multiple sclerosis: A randomized controlled study. Neurorehabilitation 2017; 41(2): 413–422. [DOI] [PubMed] [Google Scholar]
- 85.Collett J, Dawes H, Meaney A, et al. Exercise for multiple sclerosis: A single-blind randomized trial comparing three exercise intensities. Mult Scler 2011; 17(5): 594–603. [DOI] [PubMed] [Google Scholar]
- 86.Coote S, Uszynski M, Herring MP, et al. Effect of exercising at minimum recommendations of the multiple sclerosis exercise guideline combined with structured education or attention control education–secondary results of the step it up randomised controlled trial. BMC Neurology 2017; 17: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dalgas U, Stenager E, Jakobsen J, et al. Fatigue, mood and quality of life improve in MS patients after progressive resistance training. Mult Scler 2010; 16(4): 480–490. [DOI] [PubMed] [Google Scholar]
- 88.Dettmers C, Sulzmann M, Ruchay-Plossl A, et al. Endurance exercise improves walking distance in MS patients with fatigue. Acta Neurol Scand 2009; 120(4): 251–257. [DOI] [PubMed] [Google Scholar]
- 89.Dodd KJ, Taylor NF, Shields N, et al. Progressive resistance training did not improve walking but can improve muscle performance, quality of life and fatigue in adults with multiple sclerosis: A randomized controlled trial. Mult Scler J 2011; 17: 1362–1374. [DOI] [PubMed] [Google Scholar]
- 90.Ebrahimi A, Eftekhari E, Etemadifar M. Effects of whole body vibration on hormonal & functional indices in patients with multiple sclerosis. Indian J Med Res 2015; 142: 450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Escudero-Uribe S, Hochsprung A, Heredia-Camacho B, et al. Effect of training exercises incorporating mechanical devices on fatigue and gait pattern in persons with relapsing-remitting multiple sclerosis. Physiother Can 2017; 69(4): 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Feys P, Moumdjian L, Van Halewyck F, et al. Effects of an individual 12-week community-located ‘start-to-run’ program on physical capacity, walking, fatigue, cognitive function, brain volumes, and structures in persons with multiple sclerosis. Multiple Sclerosis Journal 2019; 25: 92–103. [DOI] [PubMed] [Google Scholar]
- 93.Frevel D, Mäurer M. Internet-based home training is capable to improve balance in multiple sclerosis: A randomized controlled trial. Eur J Phys Rehabil Med 2015; 51(1): 23–30. [PubMed] [Google Scholar]
- 94.Gandolfi M, Geroin C, Picelli A, et al. Robot-assisted versus sensory integration training in treating gait and balance dysfunctions in patients with multiple sclerosis: A randomized controlled trial. Frontiers in Human Neuroscience 2014; 8: 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gandolfi M, Munari D, Geroin C, et al. Sensory integration balance training in patients with multiple sclerosis: A randomized, controlled trial. Mult Scler J 2015; 21: 1453–1462. [DOI] [PubMed] [Google Scholar]
- 96.Geddes EL, Costello E, Raivel K, et al. The effects of a twelve-week home walking program on cardiovascular parameters and fatigue perception of individuals with multiple sclerosis: A pilot study. Cardiopulm Phys Ther J 2009; 20(1): 5–12. [PMC free article] [PubMed] [Google Scholar]
- 97.Gervasoni E, Cattaneo D, Jonsdottir J. Effect of treadmill training on fatigue in multiple sclerosis: A pilot study. Int J Rehab Research 2014; 37: 54-60. [DOI] [PubMed] [Google Scholar]
- 98.Hayes HA, Gappmaier E, LaStayo PC. Effects of high-intensity resistance training on strength, mobility, balance, and fatigue in individuals with multiple sclerosis: A randomized controlled trial. J Neurol Phys Ther 2011; 35(1): 2–10. [DOI] [PubMed] [Google Scholar]
- 99.Hebert JR, Corboy JR, Vollmer T, et al. Efficacy of balance and eye-movement exercises for persons with multiple sclerosis (BEEMS). Neurology 2018; 90: e797–e807. [DOI] [PubMed] [Google Scholar]
- 100.Heine M, Verschuren O, Hoogervorst EL, et al. Does aerobic training alleviate fatigue and improve societal participation in patients with multiple sclerosis? A randomized controlled trial. Mult Scler 2017; 23(11): 1517–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hugos CL, Bourdette D, Chen Y, et al. A group-delivered self-management program reduces spasticity in people with multiple sclerosis: A randomized, controlled pilot trial. Mult Scler J Exp Transl Clin 2017; 3(1): 2055217317699993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kalron A, Rosenblum U, Frid L, et al. Pilates exercise training vs. physical therapy for improving walking and balance in people with multiple sclerosis: A randomized controlled trial. Clin Rehabil 2017; 31(3): 319–328. [DOI] [PubMed] [Google Scholar]
- 103.Karbandi S, Gorji MAH, Mazloum SR, et al. Effectiveness of group versus individual yoga exercises on fatigue of patients with multiple sclerosis. N Am J Med Sci 2015; 7(6): 266–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kerling A, Keweloh K, Tegtbur U, et al. Effects of a short physical exercise intervention on patients with multiple sclerosis (MS). Int J Mol Sci 2015; 16: 15761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kern C, Elmenhorst J, Oberhoffer R. Effect of sport climbing on patients with multiple sclerosis – Hints or evidence? [Wirkung Von Therapeutischem Klettern Bei Personen Mit Multipler Sklerose – Hinweise Oder Nachweise?] Neurologie Und Rehabil 2013; 19: 247–256 (in German). [Google Scholar]
- 106.Kooshiar H, Moshtagh M, Sardar MA, et al. Fatigue and quality of life of women with multiple sclerosis: A randomized controlled clinical trial. J Sports Med Phys Fitness 2015; 55(6): 668–674. [PubMed] [Google Scholar]
- 107.Küçük F, Kara B, Poyraz EÇ, et al. Improvements in cognition, quality of life, and physical performance with clinical Pilates in multiple sclerosis: A randomized controlled trial. J Phys Ther Sci 2016; 28(3): 761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Learmonth YC, Paul L, Miller L, et al. The effects of a 12-week leisure centre-based, group exercise intervention for people moderately affected with multiple sclerosis: A randomized controlled pilot study. Clin Rehabil 2011; 26: 579–593. [DOI] [PubMed] [Google Scholar]
- 109.Learmonth YC, Adamson BC, Kinnett-Hopkins D, et al. Results of a feasibility randomised controlled study of the guidelines for exercise in multiple sclerosis project. Contemp Clin Trials 2017; 54: 84–97. [DOI] [PubMed] [Google Scholar]
- 110.Lutz C, Kersten S, Haas CT. Short-term and long-term effects of an exercise-based patient education programme in people with multiple sclerosis: A pilot study. Mult Scler Int 2017; 2017: 2826532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McCullagh R, Fitzgerald AP, Murphy RP, et al. Long-term benefits of exercising on quality of life and fatigue in multiple sclerosis patients with mild disability: A pilot study. Clin Rehabil 2008; 22: 206–214. [DOI] [PubMed] [Google Scholar]
- 112.Mokhtarzade M, Ranjbar R, Majdinasab N, et al. Effect of aerobic interval training on serum IL-10, TNFα, and adipokines levels in women with multiple sclerosis: Possible relations with fatigue and quality of life. Endocrine 2017; 57(2): 262–271. [DOI] [PubMed] [Google Scholar]
- 113.Mostert S, Kesselring J. Effects of a short-term exercise training program on aerobic fitness, fatigue, health perception and activity level of subjects with multiple sclerosis. Mult Scler 2002; 8(2): 161–168. [DOI] [PubMed] [Google Scholar]
- 114.Negahban H, Rezaie S, Goharpey S. Massage therapy and exercise therapy in patients with multiple sclerosis: A randomized controlled pilot study. Clin Rehabil 2013; 27: 1126–1136. [DOI] [PubMed] [Google Scholar]
- 115.Oken BS, Kishiyama S, Zajdel D, et al. Randomized controlled trial of yoga and exercise in multiple sclerosis. Neurology 2004; 62: 2058–2064. [DOI] [PubMed] [Google Scholar]
- 116.Petajan JH, Gappmaier E, White AT, et al. Impact of aerobic training on fitness and quality of life in multiple sclerosis. Ann Neurol 1996; 39(4): 432–441. [DOI] [PubMed] [Google Scholar]
- 117.Pilutti LA, Paulseth JE, Dove C, et al. Exercise training in progressive multiple sclerosis: A comparison of recumbent stepping and body weight-supported treadmill training. Int J MS Care 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Plow MA, Mathiowetz V, Lowe DA. Comparing individualized rehabilitation to a group wellness intervention for persons with multiple sclerosis. Am J Health Promot 2009; 24(1): 23–26; 18: 221–229. [DOI] [PubMed] [Google Scholar]
- 119.Romberg A, Virtanen A, Ruutiainen R. Long-term exercise improves functional impairment but not quality of life in multiple sclerosis. J Neurol 2005; 252: 839–845. [DOI] [PubMed] [Google Scholar]
- 120.Sabapathy NM, Minahan CL, Turner GT, et al. Comparing endurance- and resistance-exercise training in people with multiple sclerosis: A randomized pilot study. Clin Rehabil 2011; 25: 14–24. [DOI] [PubMed] [Google Scholar]
- 121.Sangelaji B, Hatamizadhe N, Rashvafnd F, et al. Study about the effects of rehabilitation on quality of life in multiple sclerosis patients. J Nurs Midwifery 2011; 20: 56–56. [Google Scholar]
- 122.Schulz K-H, Gold SM, Witte J, et al. Impact of aerobic training on immune-endocrine parameters, neurotrophic factors, quality of life and coordinative function in multiple sclerosis. J Neurol Sci 2004; 225: 11–18. [DOI] [PubMed] [Google Scholar]
- 123.Siengsukon CF, Aldughmi M, Kahya M, et al. Randomized controlled trial of exercise interventions to improve sleep quality and daytime sleepiness in individuals with multiple sclerosis: A pilot study. Mult Scler J Exp Transl Clin 2016; 2: 2055217316680639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Skjerbæk AG, Næsby M, Lützen K, et al. Endurance training is feasible in severely disabled patients with progressive multiple sclerosis. Mult Scler 2014; 20(5): 627–630. [DOI] [PubMed] [Google Scholar]
- 125.Straudi S, Martinuzzi C, Pavarelli C, et al. A task-oriented circuit training in multiple sclerosis: A feasibility study. BMC Neurol 2014; 14: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tallner A, Tzschoppe R, Peters S, et al. Web-based physical activity enhancement in persons with multiple sclerosis [Internetgestutzte Bewegungsforderung Bei Personen Mit Multipler Sklerose]. Neurologie Und Rehabil 2013; 19: 35–46 (in German). [Google Scholar]
- 127.Tallner A, Streber R, Hentschke C, et al. Internet-supported physical exercise training for persons with multiple sclerosis – A randomised, controlled study. Int J Mol Sci 2016; 17: 1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tarakci E, Yeldan I, Huseyinsinoglu BE, et al. Group exercise training for balance, functional status, spasticity, fatigue and quality of life in multiple sclerosis: A randomized controlled trial. Clin Rehabil 2013; 27: 813–822. [DOI] [PubMed] [Google Scholar]
- 129.Uszynski M, Purtill H, Donnelly A, et al. The feasibility of comparing whole body vibration intervention to the same duration and dose of exercise for people with Multiple Sclerosis. Physiother Pract Res 2014; 35: 75–86. [Google Scholar]
- 130.Uszynski MK, Purtill H, Donnelly A, et al. Comparing the effects of whole-body vibration to standard exercise in ambulatory people with Multiple Sclerosis: A randomised controlled feasibility study. Clin Rehabil 2015; 30: 657–668. [DOI] [PubMed] [Google Scholar]
- 131.van den Berg M, Dawes H, Wade DT, et al. Treadmill training for individuals with multiple sclerosis: A pilot randomised trial. J Neurol Neurosurg Psychiatry 2006; 77(4): 531–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vaney C, Gattlen B, Lugon-Moulin V, et al. Robotic-assisted step training (Lokomat) not superior to equal intensity of over-ground rehabilitation in patients with multiple sclerosis. Neurorehabil Neural Repair 2012; 26: 212–221. [DOI] [PubMed] [Google Scholar]
- 133.Velikonja O, Čurić K, Ozura A, et al. Influence of sports climbing and yoga on spasticity, cognitive function, mood and fatigue in patients with multiple sclerosis. Clin Neurol Neurosurg 2010; 112(7): 597–601. [DOI] [PubMed] [Google Scholar]
- 134.Alisaleh E, Shahrbanoo G. Effectiveness of mindfulness-based stress reduction (MBSR) in stress and fatigue in patients with multiple sclerosis (MS). Int J Med Res Health Sci 2016; 5: 486–491. [Google Scholar]
- 135.Amato MP, Goretti B, Viterbo RG, et al. Computer-assisted rehabilitation of attention in patients with multiple sclerosis: Results of a randomized, double-blind trial. Mult Scler 2014; 20(1): 91–98. [DOI] [PubMed] [Google Scholar]
- 136.Blikman LJ, van Meeteren J, Twisk JW, et al. Effectiveness of energy conservation management on fatigue and participation in multiple sclerosis: A randomized controlled trial. Mult Scler 2017; 23(11): 1527–1541. [DOI] [PubMed] [Google Scholar]
- 137.Bogosian A, Chadwick P, Windgassen S, et al. Distress improves after mindfulness training for progressive MS: A pilot randomised trial. Mult Scler 2015; 21(9): 1184–1194. [DOI] [PubMed] [Google Scholar]
- 138.Carletto S, Tesio V, Borghi M, et al. The effectiveness of a body-affective mindfulness intervention for multiple sclerosis patients with depressive symptoms: A randomized controlled clinical trial. Front Psychol 2017; 8: 2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cavalera C, Rovaris M, Mendozzi L, et al. Online meditation training for people with multiple sclerosis: A randomized controlled trial. Mult Scler J 2019; 25: 610–617. [DOI] [PubMed] [Google Scholar]
- 140.Choobforoushzadeh A, Neshat-Doost HT, Molavi H, et al. Effect of neurofeedback training on depression and fatigue in patients with multiple sclerosis. Appl Psychophysiol Biofeedback 2015; 40(1): 1–8. [DOI] [PubMed] [Google Scholar]
- 141.Eyssen ICJM, Steultjens MPM, de Groot V, et al. A cluster randomised controlled trial on the efficacy of client-centred occupational therapy in multiple sclerosis: Good process, poor outcome. Disabil Rehabil 2013; 35: 1636–1646. [DOI] [PubMed] [Google Scholar]
- 142.Finlayson M, Preissner K, Cho C, et al. Randomized trial of a teleconference-delivered fatigue management program for people with multiple sclerosis. Mult Scler 2011; 17(9): 1130–1140. [DOI] [PubMed] [Google Scholar]
- 143.Fischer A, Schroder J, Pottgen J, et al. Effectiveness of an internet-based treatment programme for depression in multiple sclerosis: A randomized controlled trial. Mult Scler 2013; 1: 350–351. [Google Scholar]
- 144.Flachenecker P, Meissner H, Frey R, et al. Neuropsychological training of attention improves MS-related fatigue: Results of a randomized, placebo-controlled, double-blind pilot study. Eur Neurol 2017; 78(5-6): 312–317. [DOI] [PubMed] [Google Scholar]
- 145.Garcia-Burguillo MP, Aguila-Maturana AM. Energy-saving strategies in the treatment of fatigue in patients with multiple sclerosis: A Pilot Study. Revista De Neurologia 2009; 49: 181–185. [PubMed] [Google Scholar]
- 146.Garcia Jalon EG, Lennon S, Peoples L, et al. Energy conservation for fatigue management in multiple sclerosis: A pilot randomized controlled trial. Clin Rehabil 2012; 27: 63–74. [DOI] [PubMed] [Google Scholar]
- 147.Ghahari S, Leigh Packer T, Passmore AE. Effectiveness of an online fatigue self-management programme for people with chronic neurological conditions: A randomized controlled trial. Clin Rehabil 2010; 24(8): 727–744. [DOI] [PubMed] [Google Scholar]
- 148.Graziano F, Calandri E, Borghi M, et al. The effects of a group-based cognitive behavioral therapy on people with multiple sclerosis: A randomized controlled trial. Clin Rehabil 2014; 28(3): 264–274. [DOI] [PubMed] [Google Scholar]
- 149.Grossman P, Kappos L, Gensicke H, et al. MS quality of life, depression, and fatigue improve after mindfulness training: A randomized trial. Neurology 2010; 75: 1141–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hildebrandt H, Lanz M, Hahn HK, et al. Cognitive training in MS: Effects and relation to brain atrophy. Restor Neurol Neurosci 2007; 25(1): 33–43. [PubMed] [Google Scholar]
- 151.Kiropoulos LA, Kilpatrick T, Holmes A, et al. A pilot randomized controlled trial of a tailored cognitive behavioural therapy based intervention for depressive symptoms in those newly diagnosed with multiple sclerosis. BMC Psychiatry 2016; 16: 435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kos D, Duportail M, D’hooghe M, et al. Multidisciplinary fatigue management programme in multiple sclerosis: A randomized clinical trial. Mult Scler 2007; 13(8): 996–1003. [DOI] [PubMed] [Google Scholar]
- 153.Kos D, Duportail M, Meirte J, et al. The effectiveness of a self-management occupational therapy intervention on activity performance in individuals with multiple sclerosis-related fatigue: A randomized-controlled trial. Int J Rehabil Res 2016; 39(3): 255–262. [DOI] [PubMed] [Google Scholar]
- 154.Mathiowetz VG, Matuska KM, Finlayson ML, et al. One-year follow-up to a randomized controlled trial of an energy conservation course for persons with multiple sclerosis. Int J Rehabil Res 2007; 30(4): 305–313. [DOI] [PubMed] [Google Scholar]
- 155.Mattioli F, Stampatori C, Bellomi F, et al. A RCT comparing specific intensive cognitive training to a specific psychological intervention in RRMS: The SMICT Study. Front Neurol 2014; 5: 278–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mohr DC, Hart SL, Goldberg A. Effects of treatment for depression on fatigue in multiple sclerosis. Psychosom Med 2003; 65(4): 542–547. [DOI] [PubMed] [Google Scholar]
- 157.Moss-Morris R, McCrone P, Yardley L, et al. A pilot randomised controlled trial of an Internet-based cognitive behavioural therapy self-management programme (MS Invigor8) for multiple sclerosis fatigue. Behav Res Ther 2012; 50(6): 415–421. [DOI] [PubMed] [Google Scholar]
- 158.Nazari F, Shahreza MS, Shaygannejad V, et al. Comparing the effects of reflexology and relaxation on fatigue in women with multiple sclerosis. Iran J Nurs Midwifery Res 2015; 20(2): 200–204. [PMC free article] [PubMed] [Google Scholar]
- 159.Pérez-Martín MY, González-Platas M, Eguía-del Río P, et al. Efficacy of a short cognitive training program in patients with multiple sclerosis. Neuropsychiatr Dis Treat 2017; 13: 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pöttgen J, Moss-Morris R, Wendebourg JM, et al. Randomised controlled trial of a self-guided online fatigue intervention in multiple sclerosis. J Neurol Neurosurg Psychiatry 2018; 89(9): 970–976. [DOI] [PubMed] [Google Scholar]
- 161.Rosti-Otajärvi E, Mäntynen A, Koivisto K, et al. Neuropsychological rehabilitation has beneficial effects on perceived cognitive deficits in multiple sclerosis during nine-month follow-up. J Neurol Sci 2013; 334: 154–160. [DOI] [PubMed] [Google Scholar]
- 162.Sgoifo A, Bignamini A, La Mantia L, et al. Integrated imaginative distention therapy to cope with fatigue. Neurol Ther 2017; 6(2): 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Simpson R, Mair FS, Mercer SW. Mindfulness-based stress reduction for people with multiple sclerosis–a feasibility randomised controlled trial. BMC Neurology 2017; 17: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sterz C, Heimes S, Blessing T, et al. Creative arts therapy improves quality of life in MS – Results of a randomized controlled trial during inpatient rehabilitation [Kunsttherapie steigert die Lebensqualitat bei Multipler Sklerose: Ergebnisse einer randomisierten, kontrollierten Studie wahrend einer stationaren Rehabilitationsbehandlung]. Neurologie Und Rehabil 2013; 19: 176–182 (in German). [Google Scholar]
- 165.Sutherland G, Andersen MB, Morris T. Relaxation and health-related quality of life in multiple sclerosis: The example of autogenic training. J Behav Med 2005; 28(3): 249–256. [DOI] [PubMed] [Google Scholar]
- 166.Tesar N, Bandion K, Baumhackl U. Efficacy of a neuropsychological training programme for patients with multiple sclerosis — A randomised controlled trial. Wien Klin Wochenschr 2005; 117(21–22): 747–754. [DOI] [PubMed] [Google Scholar]
- 167.van den Akker LE, Beckerman H, Collette EH, et al. Cognitive behavioral therapy positively affects fatigue in patients with multiple sclerosis: Results of a randomized controlled trial. Mult Scler 2017; 23(11): 1542–1553. [DOI] [PubMed] [Google Scholar]
- 168.Bombardier CH, Cunniffe M, Wadhwani R, et al. The efficacy of telephone counseling for health promotion in people with multiple sclerosis: A randomized controlled trial. Arch Phys Med Rehabil 2008; 89(10): 1849–1856. [DOI] [PubMed] [Google Scholar]
- 169.Carter A, Daley A, Humphreys L, et al. Pragmatic intervention for increasing self-directed exercise behaviour and improving important health outcomes in people with multiple sclerosis: A randomised controlled trial. Mult Scler 2014; 20(8): 1112–1122. [DOI] [PubMed] [Google Scholar]
- 170.Craig J, Young CA, Ennis M, et al. A randomised controlled trial comparing rehabilitation against standard therapy in multiple sclerosis patients receiving intravenous steroid treatment. J Neurol Neurosurg Psychiatry 2003; 74(9): 1225–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Di Fabio RP, Soderberg J, Choi T, et al. Extended outpatient rehabilitation: Its influence on symptom frequency, fatigue, and functional status for persons with progressive multiple sclerosis. Arch Phys Med Rehabil 1998; 79(2): 141–146. [DOI] [PubMed] [Google Scholar]
- 172.Ennis M, Thain J, Boggild M, et al. A randomized controlled trial of a health promotion education programme for people with multiple sclerosis. Clin Rehabil 2006; 20(9): 783–792. [DOI] [PubMed] [Google Scholar]
- 173.Grasso MG, Broccoli M, Casillo P, et al. Evaluation of the impact of cognitive training on quality of life in patients with multiple sclerosis. Eur Neurol 2017; 78(1–2): 111–117. [DOI] [PubMed] [Google Scholar]
- 174.Hugos CL, Copperman LF, Fuller BE, et al. Clinical trial of a formal group fatigue program in multiple sclerosis. Mult Scler 2010; 16(6): 724–732. [DOI] [PubMed] [Google Scholar]
- 175.Hugos CL, Cameron MH, Chen Z, et al. A multicenter randomized controlled trial of two group education programs for fatigue in multiple sclerosis: Long-term (12-month) follow-up at one site. Mult Scler J 2019; 25: 817–875. [DOI] [PubMed] [Google Scholar]
- 176.Hugos CL, Chen Z, Chen Y, et al. A multicenter randomized controlled trial of two group education programs for fatigue in multiple sclerosis: Short-and medium-term benefits. Mult Scler 2019; 25(2): 275–285. [DOI] [PubMed] [Google Scholar]
- 177.O’Hara L, Cadbury H, De Ide L. Evaluation of the effectiveness of professionally guided self-care for people with multiple sclerosis living in the community: A randomized controlled trial. Clin Rehabil 2002; 16(2): 119–128. [DOI] [PubMed] [Google Scholar]
- 178.Rietberg MB, van Wegen EE, Eyssen IC, et al. Effects of multidisciplinary rehabilitation on chronic fatigue in multiple sclerosis: A randomized controlled trial. PLoS ONE 2014; 9(9): e107710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Pilutti L, Dlugonski D, Sandroff B, et al. Randomized controlled trial of a behavioral intervention targeting symptoms and physical activity in multiple sclerosis. Mult Scler 2014; 20(5): 594–601. [DOI] [PubMed] [Google Scholar]
- 180.Storr LK, Sorensen PS, Ravnborg M. The efficacy of multidisciplinary rehabilitation in stable multiple sclerosis patients. Mult Scler 2006; 12(2): 235–242. [DOI] [PubMed] [Google Scholar]
- 181.Stuifbergen AK, Becker H, Blozis S, et al. A randomized clinical trial of a wellness intervention for women with multiple sclerosis. Arch Phys Med Rehabil 2003; 84(4): 467–476. [DOI] [PubMed] [Google Scholar]
- 182.Thomas S, Fazakarley L, Thomas PW, et al. Mii-vitaliSe: A pilot randomised controlled trial of a home gaming system (Nintendo Wii) to increase activity levels, vitality and well-being in people with multiple sclerosis. BMJ Open 2017; 7: e016966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Turner AP, Hartoonian N, Sloan AP, et al. Improving fatigue and depression in individuals with multiple sclerosis using telephone-administered physical activity counseling. J Consult Clin Psychol 2016; 84(4): 297–309. [DOI] [PubMed] [Google Scholar]
- 184.Zalisova K, Havrdová E. Effect of a comprehensive physiotherapeutic programme (incl. an aerobic load) on physical fitness, fatigue and neurological deficiency in patients with multiple sclerosis-Pilot study. Ceska Slovenska Neurol Neuro 2001; 64: 173–177. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-msj-10.1177_1352458521996002 for Which exercise and behavioural interventions show most promise for treating fatigue in multiple sclerosis? A network meta-analysis by Anthony M Harrison, Reza Safari, Tom Mercer, Federica Picariello, Marietta L van der Linden, Claire White, Rona Moss-Morris and Sam Norton in Multiple Sclerosis Journal
Supplemental material, sj-pdf-2-msj-10.1177_1352458521996002 for Which exercise and behavioural interventions show most promise for treating fatigue in multiple sclerosis? A network meta-analysis by Anthony M Harrison, Reza Safari, Tom Mercer, Federica Picariello, Marietta L van der Linden, Claire White, Rona Moss-Morris and Sam Norton in Multiple Sclerosis Journal
Supplemental material, sj-pdf-3-msj-10.1177_1352458521996002 for Which exercise and behavioural interventions show most promise for treating fatigue in multiple sclerosis? A network meta-analysis by Anthony M Harrison, Reza Safari, Tom Mercer, Federica Picariello, Marietta L van der Linden, Claire White, Rona Moss-Morris and Sam Norton in Multiple Sclerosis Journal
Supplemental material, sj-pdf-4-msj-10.1177_1352458521996002 for Which exercise and behavioural interventions show most promise for treating fatigue in multiple sclerosis? A network meta-analysis by Anthony M Harrison, Reza Safari, Tom Mercer, Federica Picariello, Marietta L van der Linden, Claire White, Rona Moss-Morris and Sam Norton in Multiple Sclerosis Journal
Supplemental material, sj-pdf-5-msj-10.1177_1352458521996002 for Which exercise and behavioural interventions show most promise for treating fatigue in multiple sclerosis? A network meta-analysis by Anthony M Harrison, Reza Safari, Tom Mercer, Federica Picariello, Marietta L van der Linden, Claire White, Rona Moss-Morris and Sam Norton in Multiple Sclerosis Journal
Supplemental material, sj-pdf-6-msj-10.1177_1352458521996002 for Which exercise and behavioural interventions show most promise for treating fatigue in multiple sclerosis? A network meta-analysis by Anthony M Harrison, Reza Safari, Tom Mercer, Federica Picariello, Marietta L van der Linden, Claire White, Rona Moss-Morris and Sam Norton in Multiple Sclerosis Journal
Supplemental material, sj-pdf-7-msj-10.1177_1352458521996002 for Which exercise and behavioural interventions show most promise for treating fatigue in multiple sclerosis? A network meta-analysis by Anthony M Harrison, Reza Safari, Tom Mercer, Federica Picariello, Marietta L van der Linden, Claire White, Rona Moss-Morris and Sam Norton in Multiple Sclerosis Journal