Abstract
Complete visualization of lesions is critical for the accurate diagnosis and management of dermatological diseases. Currently, the most readily available technologies used by dermatologists include dermoscopy and photography. Nevertheless, ultrasound has emerged as a useful non-invasive modality in dermatology, which can be added to the clinical examination supporting an early and more accurate diagnosis. Moreover, there are significant technological advances in recent years, such as the development of handheld devices and ultra-high frequency probes that have expanded the integration of ultrasound into daily dermatology practice. In this article, we reviewed the most common applications of ultrasound in the field of dermatology.
Keywords: ultrasound, high‐frequency ultrasound, skin sonography, imaging
Introduction
In recent years, the use of ultrasound (US) in dermatology has expanded and gained popularity. High‐frequency ultrasound (HFUS), ≥15 megahertz (MHz) wavelength, allows a high-resolution visualization of the skin layers with sufficient depth to capture the full thickness of the skin.1
US is a safe imaging modality that uses acoustic waves to travel through the skin; the echoes reflected by the different cutaneous tissues return to the transducer forming a visual image.2 The echogenicity of each skin structure is determined by its density affecting the acoustic wave’s speed passing through it.3The primary sources of echogenicity in the skin layers are keratinocytes in the epidermis, collagen in the dermis, and fat in the subcutis.3
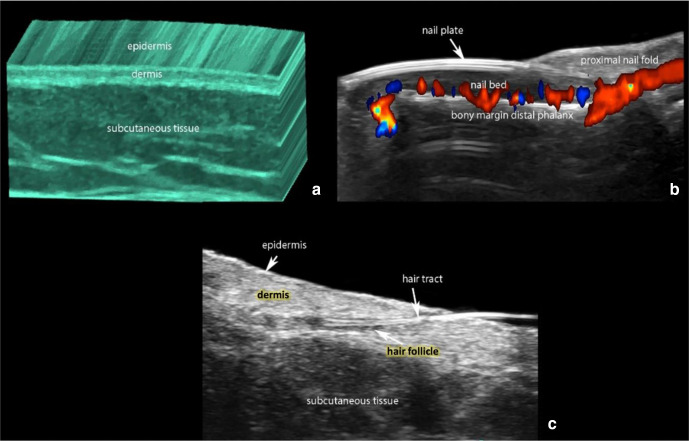
At 15-20 MHz, the normal skin layers of the non-glabrous skin appear as a hyperechoic epidermal line, then a hyperechoic dermal band, and a much larger hypoechoic subcutis layer.4 In the glabrous skin of the palms and soles, the epidermis presents as a hyperechoic bilaminar structure due to a thicker stratum corneum (Figure 1).4
Figure 1.
(a) Normal ultrasound anatomy of the skin (3D reconstruction of greyscale with color filter). (b) Nail (color Doppler longitudinal view of the nail of the index finger with the blood flow in colors). (c) Hair.
Color Doppler US is an application of US that enables the real-time visualization of the blood flow, which is increased in neo-angiogenesis, tumors, and inflammation.5
Wortsman et al. proposed guidelines to standardize the performance of US examinations in dermatology.1 They recommend a minimum frequency of 15 MHz, the performance of a minimum of 300 skin examinations per physician annually to ensure competence, and the routine use of color Doppler US to assess vascularity.1
US has the advantage of being non-invasive and inexpensive compared to other imaging techniques such as magnetic resonance imaging (MRI). It has a higher axial resolution than MRI and computerized tomography.3 The axial resolution of US can vary from 100 µm at 15 MHz to 30 µm, which is closer to the lower magnification of histology.2
The main applications of US in dermatology are the study of benign and malignant tumors, nail pathology, inflammatory dermatoses , and to reduce complications of esthetic procedures. Moreover, it is radiation-free and can help avoid skin biopsy, which is especially useful in the pediatric population.6,7 Sonographic findings of common dermatological diseases are summarized in Table 1.
Table 1.
Sonographic Findings of Common Dermatological Diseases.
| Epidermal Cyst | Intact cysts show well defined round anechoic or hypoechoic structures in the dermis and subcutaneous layer, without blood flow. |
| Pilomatricoma | Target sign, with a hypoechoic rim and a calcium-rich hyperechoic center. |
| Hemangioma | In proliferative phase, they present as a hypoechoic solid and hypervascular mass with arterial, venous, and sometimes arteriovenous shunts. |
| Basal Cell Carcinoma | Hyperechoic spots within the lesion (pathognomonic sign). |
| Dermatofibrosarcoma Protuberans | Jellyfish-like sign, an oval-shaped hypoechoic body within the dermis with tentacle-like or pseudopods projections spreading through the subcutaneous tissue. |
| Hidradenitis Suppurativa | Widening of the hair follicles. |
| Morphea | Inflammatory phase lesions show increased thickness and decreased echogenicity of the dermis, increased echogenicity of the subcutaneous tissue, and increased dermal and subcutaneous blood flow. |
The US limitations include the inability to detect lesions that measure <0.1 mm at 15 MHz and <0.03 mm at 70 MHz or intraepidermal macular lesions such as solar lentigines and cafe-au-lait macules.6
With the increased interest in technology, especially in non-invasive bedside tools, we present an up-to-date review of the use of US in dermatology. We searched MEDLINE, EMBASE, and Cochrane Central Register from their respective conception dates to January 4, 2020. The search included keywords related to US and cutaneous diseases that usually need further investigation, such as skin biopsy or imaging.
In this review, dermatological conditions will be divided into benign tumors, followed by vascular lesions, malignant tumors, inflammatory conditions, hair diseases, nail diseases, and exogenous material.
Benign Tumors
Epidermal cyst
Distinguishing features on US depend on the phase of the cyst. Intact cysts show well defined round anechoic or hypoechoic structures in the dermis and subcutis (Supplemental Figure S1).8 Ruptured cysts present an ill-defined irregular or lobulated shape and increased blood flow at the periphery.9,10 Usually, within the cysts, there are anechoic bands that correspond to the cholesterol crystals. Underneath the cyst, it is frequent to see a posterior acoustic enhancement artifact.11
Sometimes on the cyst’s surface, an anechoic or hypoechoic band is detected, which correlates with the punctum seen on clinical examination.12
Pilomatricoma
For this common benign hair matrix tumor, the diagnostic accuracy of physical exam alone is only 16%.13 On US exam, it commonly presents as a target-like lesion, with a hypoechoic rim and a calcium-rich hyperechoic center.14 Solivetti et al. classified US findings of pilomatrixoma into five types; type 1: fully calcified, type 2: partially calcified or target, type 3: complex lesion, type 4: pseudocysts, and type 5: pseudo-tumoral.15
Bandera et al. defined a characteristic sign, both hyperechoic dots, which represent calcification and echogenic dots that represent proteinaceous material, mostly keratin (Supplemental Figure S2).16
Vascular Lesions
Infantile hemangiomas
Infantile hemangiomas are the most common soft tissue tumors in the pediatric age. Although they are usually diagnosed clinically, the depth of involvement would be difficult to assess by clinical examination alone.
The US appearance of infantile hemangiomas varies according to the phase. In the proliferative phase, they present as hypoechoic solid and hypervascular mass with arterial, venous, and sometimes arteriovenous shunts (Supplemental Figure S3).17 During the partial involuting phase, they present a mixed echogenicity with hyperechoic and hypoechoic areas and an intermediate degree of vascularity.18 In the involuted phase, the lesion appears hyperechoic, hypovascular, and fibrofatty tissue can be seen.19
Vascular Malformations
Vascular malformations are classified as either high-flow; including arterial and arteriovenous, or low flow; such as venous, capillary, and lymphatic.
In contrast with hemangiomas, vascular malformations are errors in morphogenesis and don’t present a mass-effect.9 Arterial, venous, and lymphatic vascular malformations show anechoic tubules or lacunar areas. Capillary malformations usually demonstrate changes in the echogenicity of the cutaneous layers that can vary according to the location. Importantly, color Doppler allows for assessing the type and velocity of flow using the spectral curve analysis. Arterial flow typically demonstrates a systolic and diastolic morphology while the venous flow shows a monophasic curve. Conversely, the lymphatic and capillary malformations have no flow (Supplemental Figure S4).18
In arteriovenous malformations, a turbulent arterialized venous type of flow is usually found.18 Low flow venous malformation is prone to develop painful intralesional thrombosis; US can help detect these blood clots.20An important clue to differentiate deep infantile hemangiomas from venous malformation is the presence of acoustic shadow on US, indicative of phleboliths in venous lesions.21
Malignant Tumors
Melanoma
There is some evidence that US can assist in the clinical diagnosis and surgical management of melanoma. However, it is not incorporated into melanoma clinical practice guidelines and is not part of the standard of care.
Since the 1990s, investigators have shown that using US allows a precise preoperative assessment of tumor thickness that correlates with histology.22-24 Machet et al. used US to plan a one step surgical excision in 31 patients with lesions suspicious for melanoma. The authors demonstrated that Breslow thickness correlates well with the ultrasonographic thickness in 26 out of the 31 studied cases [74%, 95% CI 55-88], sparing these patients a second operation.21 The authors also retrieved data from 7 studies assessing a total of 1611 melanomas, where the ultrasonographic thickness was used to predict histologic thickness.21 Among these studies, the predictive value to determine the tumor thickness ranged from 72%(95% CI 65-79) to 89% (95% CI 80-94).25,26 In another prospective study evaluating 131melanoma patients, HFUS correlated well with histology and showed excellent inter-rater reproducibility, making HFUS a reliable in vivo assessment tool for melanoma thickness.27 These findings have led to its implementation in the routine dermatology assessment at some institutions.28 Supplemental Figure S5 shows color Doppler US findings in melanoma.
Satellite lesions are cutaneous or subcutaneous melanoma lesions occurring within 2 cm of the primary tumor, whereas in-transit metastases occur beyond 2 cm.27 These metastatic lesions appear as hypoechoic and hypervascular subcutaneous nodules on HFUS.29,30 In a cohort study of 600 melanoma patients, US was able to detect in-transit or satellite metastases in 63 clinically negative patients.31The same group showed that HFUS was superior to positron emission tomography-computed tomography and thermography for the diagnosis of in-transit metastases.32
While HFUS can aid preoperative evaluation and early detection of clinically occult satellite or in-transit melanoma metastases, it is at present unreliable in differentiating benign from malignant melanocytic lesions. This is due to the limitation of US in detecting melanin and superficial, particularly epidermal lesions. However, in theses cases, US can be used as a complementary tool along with standard dermoscopy to assess the thickness of the lesions.33
Nonmelanoma Skin Cancers
Basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) are the most common forms of skin cancer.34 Non-invasive detection of tumor thickness guided by HFUS was studied in 100 patients with either SCC or BCC before Mohs micrographic surgery to evaluate the ultrasonographic tumor thickness compared to histology.34 In this study, the sensitivity of HFUS was low (32%); the specificity reached 88%.34 However, it is not clear in this study if the US technicians who performed the US examinations were trained on skin examination.In contrast, Bobadilla et al. studied 25 patients with 29 suspicious lesions of BCC that were surgically removed, and histologic tumoral thickness was correlated with their US measurements, showing a very good correlation between US and histology.35 The authors concluded, based on their results, that the application of HFUS was a useful technique for the preoperative planning of BCC.
Interestingly, US can help distinguish histological subtypes associated with high-risk or low-risk for recurrence in BCC.This discrimination is done by identifying hyperechoic spots within the lesions, which are almost pathognomonic signs of BCC (Supplemental Figure S6). A cut-off of 7 or more hyperechoic spots suggests a high-risk histological BCC subtype such as the micronodular, infiltrative, morpheiform, sclerosing, and metatypical variants.36,37 Supplemental Figure S7 shows an US image of SCC.
Dermatofibrosarcoma Protuberans
Dermatofibrosarcoma protuberans is a rare and locally aggressive cutaneous malignancy that initially presents as an asymptomatic ill-defined flesh-colored plaque.
Early diagnosis and surgical intervention are crucial yet challenging due to its initial subtle presentation.38
Dermatofibrosarcoma protuberans presents a “jellyfish-like” image on HFUS that was described as an oval-shaped hypoechoic body within the dermis with tentacle-like or pseudopods projections spreading through the subcutaneous tissue.38 Other findings included hyperechoic areas within the tumor related to stromal infiltrate on histology and increased vascularity seen with color Doppler (Supplemental Figure S8).38 These ultrasound findings support the implementation of HFUS in the diagnostic process and preoperative planning of this malignancy.
Inflammatory Conditions
Hidradenitis suppurativa
In the evaluation of hidradenitis suppurativa (HS), US has demonstrated utility in diagnosing, staging, and assessing disease activity. This helps select medical treatment, determining the extent of surgical excision, and monitoring response to treatment.39,40
It is difficult to determine the extent of HS by relying only on physical examination. US has a value in recognizing these subclinical deeper findings such as fistulas or fluid collections.41
Currently, there are ultrasonographic signs for diagnosing HS that include the presence of dilated hair follicles, pseudocysts, fluid collections, and fistulas, which are called the main key lesions.40,42 80% of HS patients present ectopic hair tract fragments within the fluid collections or fistulae.43
Wortsman et al. developed sonographic diagnostic criteria shown in Table 2.44 They found that US use led to modification of management in 82% of the patients, including a switch in 24% of the cases from medical to surgical management. Similarly, a recent study showed that 44.7% of HS patients diagnosed clinically as Hurley stage 1were restaged to Hurley stage 2 or 3 after using US, which resulted in management modification.45
Table 2.
Sonographic Criteria of Hidradenitis Suppurativa.44
| Criteria |
|---|
| Widening of the hair follicles |
| Thickening or abnormal echogenicity of the dermis |
| Dermal pseudocystic nodules (round or oval-shaped hypoechoic or anechoic nodular structures) |
| Fluid collections (anechoic or hypoechoic fluid deposits, in the dermis or hypodermis connected to the base of widened hair follicles) |
| Fistulous tracts (anechoic or hypoechoic band-like structures across skin layers in the dermis or hypodermis connected to the base of widened hair follicles) |
aTo diagnose HS, you need three or more of the above signs.
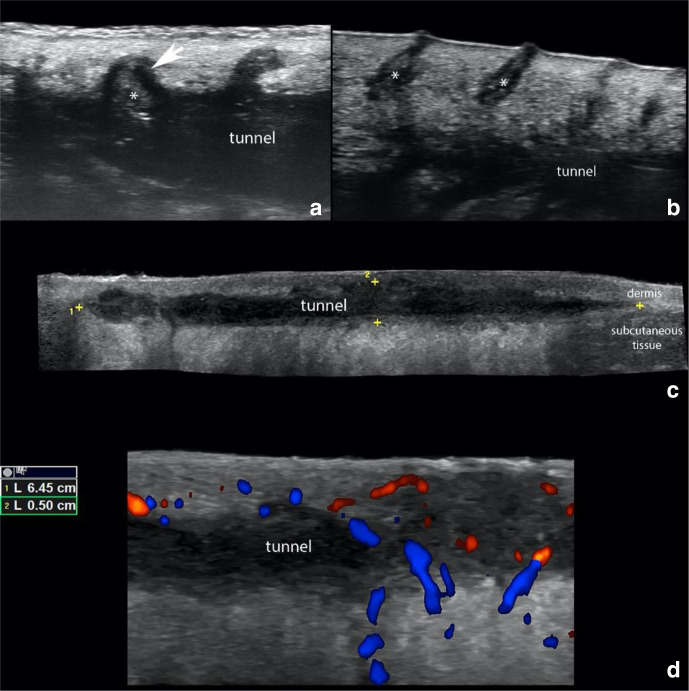
US examination is also used in assessing disease activity; color and power Doppler can evaluate the degree of vascularization, which correlates with the degree of active inflammation and pain in the HS lesions (Figure 2).46
Figure 2.
Hidradenitis Suppurativa. (a) shows ballooning of a hair follicle (*, arrow) that is protruding into the periphery of a tunnel (donor of keratin sign). (b) presents dilation of the regional hair follicles (*). (c) demonstrate a 6.45 cm (length) x 0.5 cm (thickness) hypoechoic band-like structure that corresponds to a tunnel that runs in the dermis and upper subcutaneous tissue. (d) Doppler ultrasounds demonstrate hypervascularity in the periphery of the tunnel.
On color Doppler US, the fistulous tracts have been classified according to their degree of edema and fibrosis into three types of severity.47 Type 1: low fibrotic scar with high or low edema, type 2: high fibrotic scar with low edema, and Type 3:high fibrotic scar with high edema.47
It has been reported that the presence of communicating sinuses is more frequent in type three (71%) and type two (29%), and these two types represent 63% of patients with multiple tracts, which correlate with the severity of the disease.47
Martorell et al. described 4 different fistulae patterns: Type A; dermal fistula, Type B; dermo-epidermal fistula, Type C; complex fistula, and Type D; subcutaneous fistula. After 6 months of medical therapies, a complete resolution has been described in type A (95%) and type B (65%). Conversely, types C and D showed no significant response after medical management.48
A standardized technique for reporting US findings in HS has been proposed and includes documenting the type and extent of the pseudocysts, fluid collections, and fistulas, besides the locoregional vascularity.49 Online supplemental video 1 showing US-guided needle insertion into a sinus tract (available online supplemental video 1).
Systemic Sclerosis and Morphea
Color Doppler US is usually used to assess the activity in systemic sclerosis and morphea.50,51 Furthermore, US examination can distinguish between the inflammatory and atrophic stages of the disease, which is essential for prognosis and management.52
Wortsman et al. found that the sensitivity of the US in determining the active phase of morphea was 100% (95% CI 84% to 100%) and specificity was 98.8% (95% CI 93% to 99%) when compared to the gold standard histologic examination.53
They found the most accurate signs of morphea activity are increased echogenicity of subcutaneous tissue and increased blood flow.53 The inflammatory phase lesions show increased thickness and decreased echogenicity of the dermis, increased echogenicity of the subcutaneous tissue, and increased dermal and subcutaneous blood flow (Supplemental Figure S9).53 Conversely, the lesions in the atrophic phase present decreased dermal and subcutaneous thickness with a lack of blood flow.54,55
Calcinosis in systemic sclerosis manifests on US examination as a hyperechoic subcutaneous focal deposit that commonly generates a posterior acoustic shadowing artifact.56
US can also be used in defining the pathology and extent of skin ulcers in Systemic sclerosis.57 A study by Sulli et al. showed that US examination was superior to clinical assessment using the modified Rodnan skin score in assessing the extent and degree of skin sclerosis.58
Panniculitis
US can help in differentiating septal from lobular panniculitis. In a study of 64 patients, the sensitivity and specificity of US examination in distinguishing mostly lobular from mostly septal panniculitis was 85% and 88%, respectively.59 Lobular panniculitis shows diffuse hyperechogenicity of the fatty lobules, while septal panniculitis presents a hypoechoic thickening of the septa between the hyperechoic fatty lobules (Supplemental Figure S10).60
Hair Diseases
The evaluation of hair diseases is performed mainly by clinical assessment supported by trichoscopic examination while scalp biopsy is reserved for challenging cases.Despite the development in trichoscopy techniques, the findings obtained are limited to the skin surface, and direct assessment in the dermis and subcutaneous tissue is not feasible. Therefore, other non-invasive modalities facilitating the evaluation of the deeper skin tissues are still in demand and US might potentially replace or support the currently-available diagnostic tools. In addition, the detection of fibrosis and inflammation around hair follicles, which is not directly observable by trichoscopy, might be possible by US serving as an effective tool to evaluate severity and activity of hair diseases. On US examination, the normal hair presents 2 distinct morphologies. The first is terminal hair which is common in the scalp displays a trilaminar hyperechoic appearance with an inner hyperechoic medulla and an outer cortex-cuticle complex. The corporal hair, also called villus hair, presents as a bilaminar hyperechoic structure without a medulla.61,62
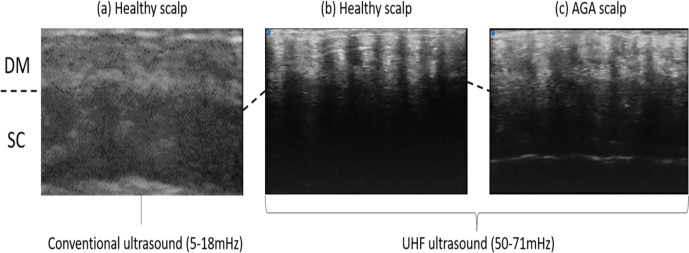
Given the tiny size of the hair shaft/follicle ranging from 1.2 to 3.1 mm2 on average, conventional US is of limited value in assessing hair disorders at frequencies of 15-18 MHz.63-65 However, newly developed ultra-high-frequency (UHF) transducers of up to 70 MHz allowing excellent visualization of the hair and surrounding tissues.66 Figure 3 a and b demonstrate a comparison between conventional US with 5-18 mHz transducer and UHF-US with 50-71 mHz transducer (VevoMD; VisualSonics, Toronto, Ontario, Canada). As evident in the images, conventional US fails to capture individual hair shaft/follicle and skin appendages, whereas UHF-US successfully detects each hair follicle.
Figure 3.
The ultrasonographic images of conventional and newly developing ultrasound. (a) Healthy scalp depicted by conventional ultrasound. (b) Healthy scalp depicted by ultra-high-frequency (UHF) ultrasound. (c) Scalp of androgenetic alopecia depicted by UHF ultrasound. AGA, androgenetic alopecia; DM, dermis; SC, subcutis.
Figure 3 (c) shows the scalp of a patient with androgenetic alopecia (AGA), while (b) is taken from a healthy control.AGA is characterized by a diversity of hair shaft diameters whereas the normal scalp demonstrates homogeneously arranged hair shafts with similar diameters. Another difference exists in telogen/anagen ratios. All the hairs depicted in the normal scalp (3b) are anagen hairs as they end in subcutaneous tissue illustrating the “bottom-heavy” pattern. Conversely, AGA scalp (3 c) contains small ovoid and relatively hyperechoic structures in keeping with telogen hair follicles. Further assessment, such as the calculation of hair density, average hair shaft/follicle diameter, telogen/anagen hair ratio, is possible by combining these sequential vertical images with specialized image processing software.
Nail Diseases
The use of US can help avoid nail biopsy which is peculiarly painful and associated with the risk of permenant nail destruction. The main applications include tumoral conditions, inflammatory entities, and growth abnormalities.
Glomus Tumor
Glomus tumor is a lesion derived from the neuromyoarterial plexus and on US shows as an oval-shaped hypoechoic nodule in the nail bed that generates scalloping of the bony margin of the distal phalanx (Supplemental Figure S11).67 On color Doppler, they frequently present hypervascularity.67
Subungual Exostosis
This bony outgrowth protrudes into the nail bed and may simulate other nail conditions. The US examination demonstrates a band-like hyperechoic structure with a posterior acoustic shadowing artifact and connected to the underlying bony margin (Supplemental Figure S11).68
Onychocriptosis
This is the embedding of the nail plate into the periungual nail fold. The US examination shows the hyperechoic bilaminar fragment in the periungual region and the hypoechoic inflammatory in the surrounding tissue. On color Doppler, there is a variable degree of hypervascularity.69
Exogenous Material
US can be useful in diagnosing foreign bodies, (Supplemental Figure S12) and cosmetic fillers. A study by Bray et al. showed that US had a Sensitivity of 9% (CI 89% to 97%) and specificity of 99% (CI 96% to 100%) in detecting foreign bodies.70 They appear as hyperechoic linear structures surrounded by a hypoechoic halo due to inflammation or granuloma formation. This is useful in determining the location of foreign bodies to guide surgical removal.70
US also can detect the most common types of cosmetic fillers. Thus, US is sometimes used in the management of the complications of cosmetic fillers such as granuloma or inflammation.71
Conclusion
US is a useful non-invasive modality in the field of dermatology that can provide critical information to complement the clinical examination. It can accurately assess disease severity and activity in several inflammatory dermatoses. It is also useful in the diagnosis and preoperative planning of skin tumors. Moreover,it could mitigate the need for skin biopsy in some situations which is especially helpful in the pediatric population, nail/hair disorders.
Supplemental Material
Supplemental material, Supplementary Material 1, for Overview of Ultrasound Imaging Applications in Dermatology by Nouf Almuhanna, Ximena Wortsman, Iris Wohlmuth-Wieser, Misaki Kinoshita-Ise and Raed Alhusayen in Journal of Cutaneous Medicine and Surgery
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and publication of the article: Dr. Iris Wohlmuth-Wieser´s work was supported by the Austrian Science Fund (FWF) (J 4382-B).
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Nouf Almuhanna https://orcid.org/0000-0002-4828-4562
Ximena Wortsman https://orcid.org/0000-0003-3359-5023
Misaki Kinoshita-Ise https://orcid.org/0000-0002-9440-6663
References
- 1.Wortsman X., Alfageme F., Roustan Get al. Guidelines for performing dermatologic ultrasound examinations by the dermus group. J Ultrasound Med. 2016;35(3):577-580. 10.7863/ultra.15.06046 [DOI] [PubMed] [Google Scholar]
- 2.Rallan D., Harland CC. Ultrasound in dermatology–basic principles and applications. Clin Exp Dermatol. 2003;28(6):632-638. 10.1046/j.1365-2230.2003.01405.x [DOI] [PubMed] [Google Scholar]
- 3.Kleinerman R., Whang TB., Bard RL., Marmur ES. Ultrasound in dermatology: principles and applications. J Am Acad Dermatol. 2012;67(3):478-487. 10.1016/j.jaad.2011.12.016 [DOI] [PubMed] [Google Scholar]
- 4.Marmur ES., Berkowitz EZ., Fuchs BS., Singer GK., Yoo JY. Use of high-frequency, high-resolution ultrasound before Mohs surgery. Dermatol Surg. 2010;36(6):841-847. 10.1111/j.1524-4725.2010.01558.x [DOI] [PubMed] [Google Scholar]
- 5.Alfageme Roldán F. Ultrasound skin imaging. Actas Dermo-Sifiliográficas. 2014;105(10):891-899. 10.1016/j.adengl.2014.10.002 [DOI] [PubMed] [Google Scholar]
- 6.Schneider SL., Kohli I., Hamzavi IH., Council ML., Rossi AM., Ozog DM. Emerging imaging technologies in dermatology: Part II: applications and limitations. J Am Acad Dermatol. 2019;80(4):1121-1131. 10.1016/j.jaad.2018.11.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wortsman X., Wortsman J. Clinical usefulness of variable-frequency ultrasound in localized lesions of the skin. J Am Acad Dermatol. 2010;62(2):247-256. 10.1016/j.jaad.2009.06.016 [DOI] [PubMed] [Google Scholar]
- 8.Yuan W-H., Hsu H-C., Lai Y-C., Chou Y-H., Li AF-Y. Differences in sonographic features of ruptured and unruptured epidermal cysts. J Ultrasound Med. 2012;31(2):265-272. 10.7863/jum.2012.31.2.265 [DOI] [PubMed] [Google Scholar]
- 9.Wortsman X. Common applications of dermatologic sonography. J Ultrasound Med. 2012;31(1):97-111. 10.7863/jum.2012.31.1.97 [DOI] [PubMed] [Google Scholar]
- 10.Lee HS., Joo KB., Song HTet al. Relationship between sonographic and pathologic findings in epidermal inclusion cysts. J Clin Ultrasound. 2001;29(7):374-383. 10.1002/jcu.1052 [DOI] [PubMed] [Google Scholar]
- 11.Kim HK., Kim SM., Lee SH., Racadio JM., Shin MJ. Subcutaneous epidermal inclusion cysts: ultrasound (US) and MR imaging findings. Skeletal Radiol. 2011;40(11):1415-1419. 10.1007/s00256-010-1072-4 [DOI] [PubMed] [Google Scholar]
- 12.Lee DH., Yoon CS., Lim BJet al. Ultrasound feature-based diagnostic model focusing on the “submarine sign” for epidermal cysts among superficial soft tissue lesions. Korean J Radiol. 2019;20(10):1409-1421. 10.3348/kjr.2019.0241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones CD., Ho W., Robertson BF., Gunn E., Morley S. Pilomatrixoma: a comprehensive review of the literature. Am J Dermatopathol. 2018;40(9):631-641. 10.1097/DAD.0000000000001118 [DOI] [PubMed] [Google Scholar]
- 14.Choo HJ., Lee SJ., Lee YHet al. Pilomatricomas: the diagnostic value of ultrasound. Skeletal Radiol. 2010;39(3):243-250. 10.1007/s00256-009-0678-x [DOI] [PubMed] [Google Scholar]
- 15.Solivetti FM., Elia F., Drusco A., Panetta C., Amantea A., Di Carlo A. Epithelioma of malherbe: new ultrasound patterns. J Exp Clin Cancer Res. 2010;29(1):42. 10.1186/1756-9966-29-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodríguez Bandera AI., Sebaratnam DF., Feito Rodríguez M., Lucas Laguna R. Cutaneous ultrasound and its utility in pediatric dermatology. Part I: lumps, bumps, and inflammatory conditions. Pediatr Dermatol. 2020;37(1):29-39. 10.1111/pde.14033 [DOI] [PubMed] [Google Scholar]
- 17.Dubois J., Patriquin HB., Garel Let al. Soft-tissue hemangiomas in infants and children: diagnosis using Doppler sonography. AJR Am J Roentgenol. 1998;171(1):247-252. 10.2214/ajr.171.1.9648798 [DOI] [PubMed] [Google Scholar]
- 18.Paltiel HJ., Burrows PE., Kozakewich HP., Zurakowski D., Mulliken JB. Soft-tissue vascular anomalies: utility of US for diagnosis. Radiology. 2000;214(3):747-754. 10.1148/radiology.214.3.r00mr21747 [DOI] [PubMed] [Google Scholar]
- 19.Shi H., Song H., Wang Jet al. Ultrasound in assessing the efficacy of propranolol therapy for infantile hemangiomas. Ultrasound Med Biol. 2014;40(11):2622-2629. 10.1016/j.ultrasmedbio.2014.06.021 [DOI] [PubMed] [Google Scholar]
- 20.van Es J., Kappelhof NA., Douma RA., Meijers JCM., Gerdes VEA., van der Horst CMAM. Venous thrombosis and coagulation parameters in patients with pure venous malformations. Neth J Med. 2017;75(8):328-334. [PubMed] [Google Scholar]
- 21.Ding A., Gong X., Li J., Xiong P. Role of ultrasound in diagnosis and differential diagnosis of deep infantile hemangioma and venous malformation. J Vasc Surg Venous Lymphat Disord. 2019;7(5):715-723. 10.1016/j.jvsv.2019.01.065 [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann K., Jung J., el Gammal S., Altmeyer P. Malignant melanoma in 20-MHz B scan sonography. Dermatology. 1992;185(1):49-55. 10.1159/000247403 [DOI] [PubMed] [Google Scholar]
- 23.Dummer W., Blaheta HJ., Bastian BC., Schenk T., Bröcker EV., Remy W. Preoperative characterization of pigmented skin lesions by epiluminescence microscopy and high-frequency ultrasound. Arch Dermatol. 1995;131(3):279-285. 10.1001/archderm.1995.01690150043010 [DOI] [PubMed] [Google Scholar]
- 24.Lassau N., Mercier S., Koscielny Set al. Prognostic value of high-frequency sonography and color Doppler sonography for the preoperative assessment of melanomas. AJR Am J Roentgenol. 1999;172(2):457-461. 10.2214/ajr.172.2.9930803 [DOI] [PubMed] [Google Scholar]
- 25.Pellacani G., Seidenari S. Preoperative melanoma thickness determination by 20-MHz sonography and digital videomicroscopy in combination. Arch Dermatol. 2003;139(3):293-298. 10.1001/archderm.139.3.293 [DOI] [PubMed] [Google Scholar]
- 26.Serrone L., Solivetti FM., Thorel MF., Eibenschutz L., Donati P., Catricalà C. High frequency ultrasound in the preoperative staging of primary melanoma: a statistical analysis. Melanoma Res. 2002;12(3):287-290. 10.1097/00008390-200206000-00013 [DOI] [PubMed] [Google Scholar]
- 27.Meyer N., Lauwers-Cances V., Lourari Set al. High-frequency ultrasonography but not 930-nm optical coherence tomography reliably evaluates melanoma thickness in vivo: a prospective validation study. Br J Dermatol. 2014;171(4):799-805. 10.1111/bjd.13129 [DOI] [PubMed] [Google Scholar]
- 28.Chaput L., Laurent E., Pare Aet al. One-step surgical removal of cutaneous melanoma with surgical margins based on preoperative ultrasound measurement of the thickness of the melanoma. Eur J Dermatol. 2018;28(2):202-208. 10.1684/ejd.2018.3298 [DOI] [PubMed] [Google Scholar]
- 29.Balch CM., Soong SJ., Gershenwald JEet al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American joint committee on cancer melanoma staging system. J Clin Oncol. 2001;19(16):3622-3634. 10.1200/JCO.2001.19.16.3622 [DOI] [PubMed] [Google Scholar]
- 30.Mandava A., Ravuri PR., Konathan R. High-resolution ultrasound imaging of cutaneous lesions. Indian J Radiol Imaging. 2013;23(3):269-277. 10.4103/0971-3026.120272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solivetti FM., Di Luca Sidozzi A., Pirozzi G., Coscarella G., Brigida R., Eibenshutz L. Sonographic evaluation of clinically occult in-transit and satellite metastases from cutaneous malignant melanoma. Radiol Med. 2006;111(5):702-708. 10.1007/s11547-006-0067-7 [DOI] [PubMed] [Google Scholar]
- 32.Solivetti FM., Desiderio F., Guerrisi Aet al. HF ultrasound vs PET-CT and telethermography in the diagnosis of in-transit metastases from melanoma: a prospective study and review of the literature. J Exp Clin Cancer Res. 2014;33(1):96. 10.1186/s13046-014-0096-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maj M., Warszawik-Hendzel O., Szymanska Eet al. High frequency ultrasonography: a complementary diagnostic method in evaluation of primary cutaneous melanoma. G Ital Dermatol Venereol. 2015;150(5):595-601. [PubMed] [Google Scholar]
- 34.Jambusaria-Pahlajani A., Schmults CD., Miller CJet al. Test characteristics of high-resolution ultrasound in the preoperative assessment of margins of basal cell and squamous cell carcinoma in patients undergoing Mohs micrographic surgery. Dermatol Surg. 2009;35(1):9-15. 10.1111/j.1524-4725.2008.34376.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bobadilla F., Wortsman X., Muñoz C., Segovia L., Espinoza M., Jemec GBE. Pre-Surgical high resolution ultrasound of facial basal cell carcinoma: correlation with histology. Cancer Imaging. 2008;8(1):163-172. 10.1102/1470-7330.2008.0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vega N., Wortsman X., Navarrete N., Sazunic I. Color Doppler ultrasound supports early diagnosis of mixed high and low risk of recurrence subtypes in the same basal cell carcinoma lesion. Dermatol Surg. 2018;44(5):741-743. 10.1097/DSS.0000000000001328 [DOI] [PubMed] [Google Scholar]
- 37.Wortsman X., Vergara P., Castro Aet al. Ultrasound as predictor of histologic subtypes linked to recurrence in basal cell carcinoma of the skin. J Eur Acad Dermatol Venereol. 2015;29(4):702-707. 10.1111/jdv.12660 [DOI] [PubMed] [Google Scholar]
- 38.Rodríguez Bandera AI., Moreno Bonilla G., Feito Rodríguez M., Beato Merino MJ., de Lucas Laguna R. Jellyfish-like sonographic pattern can help recognition of dermatofibrosarcoma protuberans. Report of 3 new cases and review of the literature. Australas J Dermatol. 2019;60(2):148-150. 10.1111/ajd.12922 [DOI] [PubMed] [Google Scholar]
- 39.Ingram JR. Refining the hidradenitis suppurativa Hurley staging system for mild, moderate and severe disease. Br J Dermatol. 2019;180(5):991-992. 10.1111/bjd.17769 [DOI] [PubMed] [Google Scholar]
- 40.Elkin K., Daveluy S., Avanaki KM. Hidradenitis suppurativa: current understanding, diagnostic and surgical challenges, and developments in ultrasound application. Skin Res Technol. 2020;26(1):11-19. 10.1111/srt.12759 [DOI] [PubMed] [Google Scholar]
- 41.Nazzaro G., Passoni E., Muratori Set al. Comparison of clinical and sonographic scores in hidradenitis suppurativa and proposal of a novel ultrasound scoring system. G Ital Dermatol Venereol. 2018;10(4) 10.23736/S0392-0488.18.06196-504 Oct 2018. [DOI] [PubMed] [Google Scholar]
- 42.Wortsman X., Jemec GBE. Real-time compound imaging ultrasound of hidradenitis suppurativa. Dermatol Surg. 2007;33(11):1340-1342. 10.1111/j.1524-4725.2007.33286.x [DOI] [PubMed] [Google Scholar]
- 43.Wortsman X., Wortsman J. Ultrasound detection of retained hair tracts in hidradenitis suppurativa. Dermatol Surg. 2015;41(7):867-869. 10.1097/DSS.0000000000000388 [DOI] [PubMed] [Google Scholar]
- 44.Wortsman X., Moreno C., Soto R., Arellano J., Pezo C., Wortsman J. Ultrasound in-depth characterization and staging of hidradenitis suppurativa. Dermatol Surg. 2013;39(12):1835-1842. 10.1111/dsu.12329 [DOI] [PubMed] [Google Scholar]
- 45.Martorell A., Alfageme Roldán F., Vilarrasa Rull Eet al. Ultrasound as a diagnostic and management tool in hidradenitis suppurativa patients: a multicentre study. J Eur Acad Dermatol Venereol. 2019;33(11):2137-2142. 10.1111/jdv.15710 [DOI] [PubMed] [Google Scholar]
- 46.Nazzaro G., Passoni E., Calzari Pet al. Color Doppler as a tool for correlating vascularization and pain in hidradenitis suppurativa lesions. Skin Res Technol. 2019;25(6):830-834. 10.1111/srt.12729 [DOI] [PubMed] [Google Scholar]
- 47.Wortsman X., Castro A., Figueroa A. Color Doppler ultrasound assessment of morphology and types of fistulous tracts in hidradenitis suppurativa (HS). J Am Acad Dermatol. 2016;75(4):760-767. 10.1016/j.jaad.2016.05.009 [DOI] [PubMed] [Google Scholar]
- 48.Martorell A., Giovanardi G., Gomez-Palencia P., Sanz-Motilva V. Defining Fistular patterns in hidradenitis suppurativa. Dermatologic Surg. 2019;45(10):1237-1244. [DOI] [PubMed] [Google Scholar]
- 49.Martorell A., Wortsman X., Alfageme Fet al. Ultrasound evaluation as a complementary test in hidradenitis suppurativa: proposal of a Standarized report. Dermatol Surg. 2017;43(8):1065-1073. 10.1097/DSS.0000000000001147 [DOI] [PubMed] [Google Scholar]
- 50.Hesselstrand R., Scheja A., Wildt M., Åkesson A. High-frequency ultrasound of skin involvement in systemic sclerosis reflects oedema, extension and severity in early disease. Rheumatology. 2008;47(1):84-87. 10.1093/rheumatology/kem307 [DOI] [PubMed] [Google Scholar]
- 51.Ihn H., Shimozuma M., Fujimoto Met al. Ultrasound measurement of skin thickness in systemic sclerosis. Br J Rheumatol. 1995;34(6):535-538. 10.1093/rheumatology/34.6.535 [DOI] [PubMed] [Google Scholar]
- 52.Porta F., Gargani L., Kaloudi Oet al. The new frontiers of ultrasound in the complex world of vasculitides and scleroderma. Rheumatology. 2012;51 Suppl 7(7):vii26-vii30. 10.1093/rheumatology/kes336 [DOI] [PubMed] [Google Scholar]
- 53.Wortsman X., Wortsman J., Sazunic I., Carreño L. Activity assessment in morphea using color Doppler ultrasound. J Am Acad Dermatol. 2011;65(5):942-948. 10.1016/j.jaad.2010.08.027 [DOI] [PubMed] [Google Scholar]
- 54.Ranosz-Janicka I., Lis-Święty A., Skrzypek-Salamon A., Brzezińska-Wcisło L. An extended high-frequency ultrasound protocol for assessing and quantifying of inflammation and fibrosis in localized scleroderma. Skin Res Technol. 2019;25(3):359-366. 10.1111/srt.12660 [DOI] [PubMed] [Google Scholar]
- 55.Nezafati KA., Cayce RL., Susa JSet al. 14-MHz ultrasonography as an outcome measure in morphea (localized scleroderma. Arch Dermatol. 2011;147(9):1112-1115. 10.1001/archdermatol.2011.243 [DOI] [PubMed] [Google Scholar]
- 56.Saavedra T., Mardones F., Sazunic I., Wortsman X. Facial dystrophic calcinosis cutis secondary to acne. Actas Dermo-Sifiliográficas. 2009;100(7):622-624. 10.1016/S1578-2190(09)70136-2 [DOI] [PubMed] [Google Scholar]
- 57.Suliman YA., Kafaja S., Fitzgerald Jet al. Ultrasound characterization of cutaneous ulcers in systemic sclerosis. Clin Rheumatol. 2018;37(6):1555-1561. 10.1007/s10067-018-3986-5 [DOI] [PubMed] [Google Scholar]
- 58.Sulli A., Ruaro B., Smith Vet al. Subclinical dermal involvement is detectable by high frequency ultrasound even in patients with limited cutaneous systemic sclerosis. Arthritis Res Ther. 2017;19(1):61. 10.1186/s13075-017-1270-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Romaní J., Giavedoni P., Roé E., Vidal D., Luelmo J., Wortsman X. Inter- and Intra-rater agreement of dermatologic ultrasound for the diagnosis of lobular and septal panniculitis. J Ultrasound Med. 2020;39(1):107-112. 10.1002/jum.15080 [DOI] [PubMed] [Google Scholar]
- 60.Wortsman X. Sonography of dermatologic emergencies. J Ultrasound Med. 2017;36(9):1905-1914. 10.1002/jum.14211 [DOI] [PubMed] [Google Scholar]
- 61.Wortsman X., Wortsman J., Matsuoka Let al. Sonography in pathologies of scalp and hair. Br J Radiol. 2012;85(1013):647-655. 10.1259/bjr/22636640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wortsman X., Carreño L., Ferreira-Wortsman Cet al. Ultrasound characteristics of the hair follicles and tracts, sebaceous glands, Montgomery glands, apocrine glands, and Arrector pili muscles. J Ultrasound Med. 2019;38(8):1995-2004. 10.1002/jum.14888 [DOI] [PubMed] [Google Scholar]
- 63.Sperling LC. Hair density in African Americans. Arch Dermatol. 1999;135(6):656-658. 10.1001/archderm.135.6.656 [DOI] [PubMed] [Google Scholar]
- 64.Whiting DA. Diagnostic and predictive value of horizontal sections of scalp biopsy specimens in male pattern androgenetic alopecia. J Am Acad Dermatol. 1993;28(5 Pt 1):755-763. 10.1016/0190-9622(93)70106-4 [DOI] [PubMed] [Google Scholar]
- 65.Lee H-J., Ha S-J., Lee J-H., Kim J-W., Kim H-O., Whiting DA. Hair counts from scalp biopsy specimens in Asians. J Am Acad Dermatol. 2002;46(2):218-221. 10.1067/mjd.2002.119558 [DOI] [PubMed] [Google Scholar]
- 66.Kinoshita-Ise M., Foster FS., Shear NH. Immune checkpoint inhibitor-related alopecia: insight into the pathophysiology utilizing non-invasive diagnostic techniques. J Dermatol. 2019;46(5):e152-e153. 10.1111/1346-8138.14736 [DOI] [PubMed] [Google Scholar]
- 67.Baek HJ., Lee SJ., Cho KHet al. Subungual tumors: clinicopathologic correlation with us and MR imaging findings. Radiographics. 2010;30(6):1621-1636. 10.1148/rg.306105514 [DOI] [PubMed] [Google Scholar]
- 68.Chiou HJ., Chou YH., Chiou SY., Wang HK. High-resolution ultrasonography in superficial soft tissue tumors. J Med Ultrasound. 2007;32(9):1287-1297. [DOI] [PubMed] [Google Scholar]
- 69.Aluja Jaramillo F., Quiasúa Mejía DC., Martínez Ordúz HM., González Ardila C. Nail unit ultrasound: a complete guide of the nail diseases. J Ultrasound. 2017;20(3):181-192. 10.1007/s40477-017-0253-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bray PW., Mahoney JL., Campbell JP. Sensitivity and specificity of ultrasound in the diagnosis of foreign bodies in the hand. J Hand Surg Am. 1995;20(4):661-666. 10.1016/S0363-5023(05)80287-4 [DOI] [PubMed] [Google Scholar]
- 71.Wortsman X., Wortsman J., Orlandi C., Cardenas G., Sazunic I., Jemec GBE. Ultrasound detection and identification of cosmetic fillers in the skin. J Eur Acad Dermatol Venereol. 2012;26(3):292-301. 10.1111/j.1468-3083.2011.04047.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary Material 1, for Overview of Ultrasound Imaging Applications in Dermatology by Nouf Almuhanna, Ximena Wortsman, Iris Wohlmuth-Wieser, Misaki Kinoshita-Ise and Raed Alhusayen in Journal of Cutaneous Medicine and Surgery