Abstract
The prevalence of pancreatic cysts has increased significantly over the last decade, partly secondary to increased quality and frequency of cross-sectional imaging. While the majority never progress to cancer, a small number will and need to be followed. The management of pancreatic cysts can be both confusing and intimidating due to the multiple guidelines with varying recommendations. Despite the differences in the specifics of the guidelines, they all agree on several high-risk features that should get the attention of any clinician when assessing a pancreatic cyst: presence of a mural nodule or solid component, dilation of the main pancreatic duct (or presence of main duct intraductal papillary mucinous neoplasm), pancreatic cyst size ⩾3–4 cm, or positive cytology on pancreatic cyst fluid aspiration. Other important criteria to consider include rapid cyst growth (⩾5 mm/year), elevated serum carbohydrate antigen 19-9 levels, new-onset diabetes mellitus, or acute pancreatitis thought to be related to the cystic lesion.
Keywords: mucinous cystic neoplasms, pancreatic cyst, pancreatic cyst guidelines, pancreatic cystic neoplasms, pancreatic cystic tumors, pancreatic neoplasia
Pancreatic cystic neoplasms (PCNs) are a variable group of cystic lesions which are typically diagnosed incidentally.1 More than 70% of incidentally discovered PCNs are asymptomatic; however, a subset of these lesions are premalignant, thus raising significant clinical concern.1–5 Despite the bourgeoning literature on the topic, the workup and management of PCNs remain both confusing and problematic for many clinicians. There are multiple guidelines on the management of PCNs, though each varies, and the most ideal balance of surveillance and intervention for specific lesions remains highly debated. The proper management of PCNs requires a detailed understanding of the various types of PCNs, their associated malignancy risk, the guidelines for surveillance and intervention, and the cost analysis of such guidelines.
Improvement in the quality of cross-sectional imaging studies coupled with an aging population has led to an increase in the incidental detection of asymptomatic pancreatic cysts. Studies have shown higher rates of PCNs in older patients, although the true prevalence of these lesions remains unclear and varies significantly between studies based on the timing of the study and the age of the population included. For example, an older study with a younger patient population showed the rate of incidentally detected PCNs to be as low as 2.4%, whereas a more recent study with an older patient population showed the rate as high as 50%.2,3,6–11 Kromrey and colleagues2 analyzed magnetic resonance imaging (MRI)/magnetic resonance cholangiopancreatography (MRCP) studies obtained in 1077 patients participating in the Study of Health in Pomerania, a prospective-based cohort study in Northern Germany designed to better determine the incidence and prevalence of various diseases. They showed the weighted prevalence of PCNs to be 49.1%, with older patients having a higher prevalence, number, and size of PCNs. Moris and colleagues12 studied a sample of 500 MRIs obtained for other indications at the Mayo Clinic over a 10-year period and found an incidental PCN in 41.6% of patients as well as a significant increase in PCN detection with newer MRI hardware and software compared with older models. Despite the high overall prevalence of PCNs, few of these lesions are large. In fact, a review of five studies including 25,195 patients found the prevalence of PCN >2 cm to be only 0.8%.13,14
The malignant potential of various types of PCNs differ greatly, which highlights the importance of distinguishing benign lesions from those that harbor significant risk for the development of pancreatic cancer. Serous cystic neoplasms (SCNs), for example, have no malignant potential, whereas intraductal papillary mucinous neoplasms (IPMNs) do. A review of 99 studies including 9249 patients with IPMNs, many of whom contained high-risk/worrisome features resulting in surgical resection, found the incidence of pancreatic cancer or high-grade dysplasia to be 42% at the time of surgical resection.13 However, the risks of surgery are not negligible. In fact, the mortality rate from surgical intervention for pancreatic cysts is estimated to be between 1% and 7%, and the morbidity rate is as high as 64% (average of 30%), so surgical intervention must be carefully considered, particularly given the increased incidence of PCNs in older patients.13,15
Understanding of the various types of PCNs can help guide surveillance and intervention recommendations (see Table 1). PCNs are typically divided into two categories: mucinous and non-mucinous. Those that are mucinous (IPMNs, MCNs) are lined by a columnar epithelium which produces mucus and have malignant potential. When aspirated by endoscopic ultrasound (EUS), these lesions will typically have viscous contents with a positive ‘string sign’. The string sign is tested by placing a drop of aspirated fluid from the cyst between two gloved fingers or between two glass slides and gently pulling the two fingers or slides apart (see Figure 1). If this creates a string longer than 3.5 mm, this is indicative of a mucinous PCN. While this test is somewhat subjective and has a sensitivity of 58%, it has been shown to have a specificity of 95% and a positive predictive value of 94%.7,16,17 In addition, fluid carcinoembryonic antigen (CEA) levels can be helpful in distinguishing mucinous and non-mucinous PCNs. CEA is secreted from columnar epithelial cells that are derived from the endoderm and line mucinous PCNs, whereas non-mucinous PCNs are lined by non-endodermally derived simple cuboidal epithelium and do not produce CEA.7 The majority of studies and guidelines use a cyst fluid CEA level of 192 ng/ml as a cut-off value, above which the CEA is considered elevated, indicating the PCN in a mucinous lesion. Several studies assessing this cut-off level show an accuracy of 72–79% for distinguishing mucinous from non-mucinous PCN, although a lower CEA level does not completely exclude a mucinous lesion.18,19 The utility of PCN fluid CEA to predict an underlying malignancy within the PCN has been shown to be low. For example, in a study of 198 patients with tissue obtained from a PCN who also underwent EUS-guided PCN sampling, there was no difference in the fluid CEA level between benign and malignant mucinous PCNs.20 Finally, elevated amylase in PCN fluid indicates a connection with the pancreatic duct and is typically elevated in IPMNs and pseudocysts and low in other types of PCNs. In a study21 assessing PCN fluid collected by EUS sampling from 442 different PCNs, a fluid amylase of <350 U/l was found in 85% SCNs, and in a similar study22 of 450 patients, a fluid amylase of <250 U/l had a 98% specificity of excluding pseudocysts. Another study showed a cut-off value of amylase >479 U/l had a sensitivity of 73% and specificity of 90% for distinguishing a pseudocyst from other forms of PCNs.23,24 In addition, cross-sectional imaging studies are helpful in distinguishing different types of PCNs (refer to Table 1).
Table 1.
Characteristics of the major types of cystic lesions of the pancreas.
| Type of PCN | Age at diagnosis (years) | Gender distribution | Connection to main duct | Characteristics on imaging | Fluid CEA | Fluid amylase | Solitary or multifocal | Malignancy rate |
|---|---|---|---|---|---|---|---|---|
| Pseudocyst | Any | Equal | Some | Well-circumscribed, oval, or round, anechoic on EUS, low attenuation on CT | Low | High | Either | None |
| Serous cystic neoplasm | 40s–60s | 75% female | No | Microcystic/honeycomb, 30% have central scar | Low | Low | Solitary | None |
| Solid-pseudopapillary neoplasms | 20s | 90% female | No | Well-demarcated mixed solid-cystic tumors. Occur anywhere in pancreas | Low | Low | Solitary | High |
| Cystic pancreatic neuroendocrine tumor | 30s–50s | Equal | No | Well-circumscribed cystic lesion with peripheral rim enhancement | Low | Low | Typically solitary | <2 cm in size: Low >2 cm in size: Moderate |
| Mucinous cystic neoplasm | 40s–60s | Almost exclusively females | No | May be unilocular or septated, some have peripheral calcifications, most located in tail of pancreas | High | Low | Solitary | <3 cm in size: <5% >3 cm in size: high |
| Main duct IPMN | 40s–60s | Equal | Yes | Dilation of PD | High | High | Either | Very high |
| Branch duct IPMN | 40s–60s | Equal | Yes | Dilation of side branches of PD; grape-like cystic lesion | High | High | Either | Variable (depending on high-risk features)a |
Figure 1.

Demonstration of a positive ‘string sign’ where a drop of aspirated pancreatic cystic fluid is placed between two glass slides, and as the two glass slides are slowly pulled apart, there is a string of mucous >3.5 mm in length. This is consistent with a mucinous pancreatic cyst.
Types of pancreatic cystic neoplasms
Pseudocyst
Pseudocysts are a collection of debris, inflammatory cells, and blood surrounded by a thick fibrous wall and are considered ‘pseudo’ cystic lesions because they are not lined by a true epithelium. Pseudocysts can develop either within or outside of the pancreatic parenchyma and exclusively occur as a complication of acute pancreatitis.25 These harbor no malignancy risk and therefore require no surveillance or intervention from the standpoint of malignancy risk reduction. If the diagnosis of pseudocyst is unclear, EUS can be pursued to assess the lesion, and fluid sampling typically reveals a high fluid amylase and a low fluid CEA level (<192 ng/ml).13,14,25
Serous cystic neoplasm
An SCN, also known as a serous cystadenoma, is a collection of multiple microcysts, each of which is lined by a single layer of cuboidal epithelium.25 These lesions typically appear in microcystic or honeycomb pattern on imaging, and up to 30% will have a central scar (see Figure 2(a) and (b)). They occur most commonly in women (approximately 75% predominant in women) in the fifth to seventh decades of life.14,25 There is no connection with the main pancreatic duct (PD), so the fluid amylase is low, and, given that they are lined by simple cuboidal epithelium, the fluid CEA is low.7,25 SCNs are thought to have virtually no malignancy risk and therefore require no surveillance, although these lesions can grow and become symptomatic (i.e. cause pancreatitis, abdominal pain, biliary obstruction, or gastric outlet/duodenal obstruction), requiring surgical resection.7,14
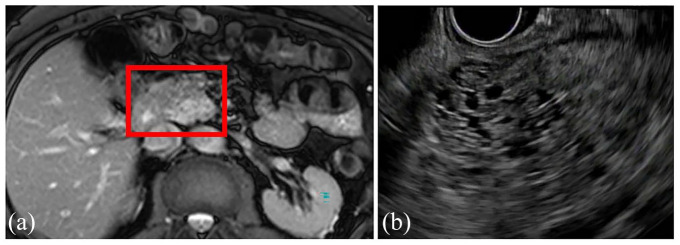
Figure 2.
Coronal view of MRI (a) and EUS (b) showing a 4.2-cm serous cystic neoplasm with classic honeycombing and microcystic pattern in the uncinated process of the pancreas of a 57-year-old woman, which was discovered incidentally during the workup for a ventral hernia. She was asymptomatic, and no additional surveillance was recommended.
Solid pseudopapillary neoplasm (SPN)
SPNs are mixed solid-and-cystic lesions that are lined by monomorphic cuboidal cells and have a fibrous pseudocapsule.25 Smaller lesions tend to be mostly solid, whereas larger lesions contain more cystic components (see Figure 3(a) and (b)). These are typically seen in women in their 20s but can be seen at any age and can occur anywhere within the pancreas.14,25 There is no connection with the pancreatic duct, so fluid amylase is low. SPNs do have at least a moderate malignancy risk, and surgical resection is typically recommended, although long-term prognosis is excellent.26–28
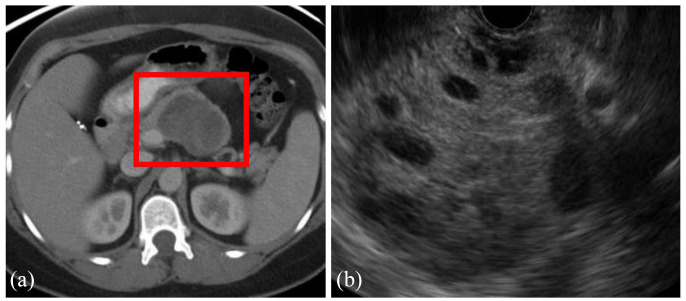
Figure 3.
Axial view of CT (a) and EUS (b) of a 5.2-cm solid pseudopapillary neoplasm in a 43-year-old woman who presented with abdominal pain. The lesion is well demarcated with mixed solid and cystic features.
Cystic pancreatic neuroendocrine tumors (cNETs)
cNETs represent almost approximately 15% of all pancreatic neuroendocrine tumors and typically arise in the fourth to sixth decades of life. These have a robust blood supply, and imaging studies typically show a well-circumscribed cystic lesion with peripheral rim enhancement, most commonly located in the head of the pancreas (see Figure 4(a) and (b)).25,29 It is not easy to distinguish cNETs from SPNs on cross-sectional imaging studies, and EUS with fine needle aspiration (FNA) is often required. cNETs have a low CEA level and do not communicate with the pancreatic duct, so they have a low amylase content, and a cytology smear reveals round cells that are loosely cohesive. The sensitivity of EUS-guided FNA cytology in cNETs has been shown to range from 70% to 88.5%.30,31 Asymptomatic cNETs <2 cm in size are typically observed because these lesions tend to be less aggressive than solid NETs.32,33 Up to one-quarter of cNETs are associated with an underlying multiple endocrine neoplasia type 1, but the majority are nonfunctional.34–36
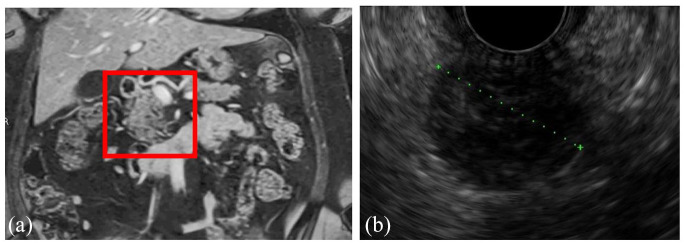
Figure 4.
Coronal view of MRI (a) and EUS (b) of a cystic pancreatic neuroendocrine tumor found in a 55-year-old woman during the workup for abdominal pain. The lesion is well circumscribed and solitary.
Mucinous cystic neoplasm (MCN)
MCNs have a tall columnar epithelium surrounded by an ovarian-type stroma which differentiates them from other mucin-producing neoplasms, so they are found almost exclusively in women.37,38 These typically present in middle age (mean age of 48 years)25,39 and are located in the body or tail of the pancreas in 90–95% of cases.14 Imaging classically shows a macrocystic lesion, some of which contain peripheral calcifications (see Figure 5(a) and (b)). There is no communication with the PD, so fluid amylase is low. The columnar epithelial cells can produce CEA, so these lesions typically have a high CEA level. MCNs are at risk of malignant transformation, although the degree of this risk is somewhat debated, and more recent studies indicate this risk may be lower than previously thought, particularly in lesions less than 3 cm in diameter without other high-risk features.37,39–42
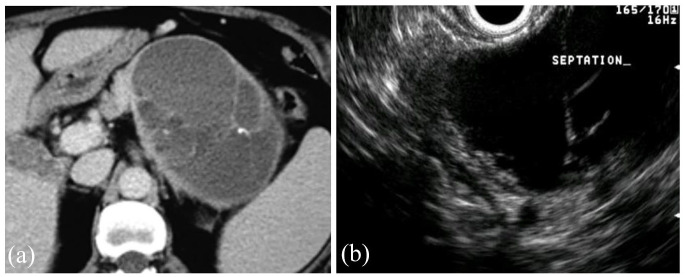
Figure 5.
Axial view of CT (a) and EUS (b) of a mucinous cystic neoplasm found incidentally in the tail of the pancreas of a 53-year-old woman. Several thin septations can be seen on both CT and EUS. The patient underwent lateral pancreatectomy which confirmed the diagnosis, and no malignancy was present.
Intraductal papillary mucinous neoplasms (IPMNs)
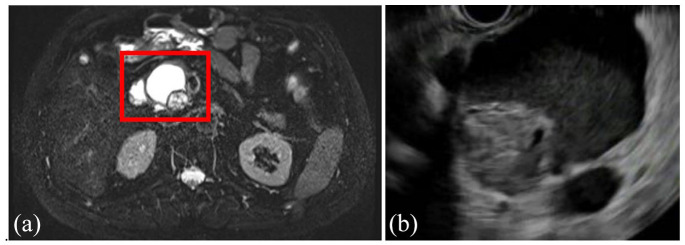
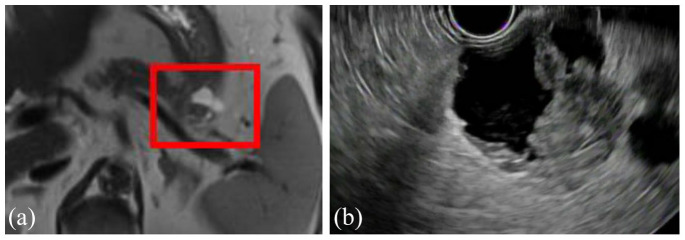
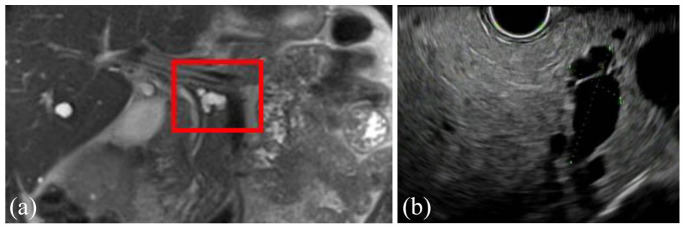
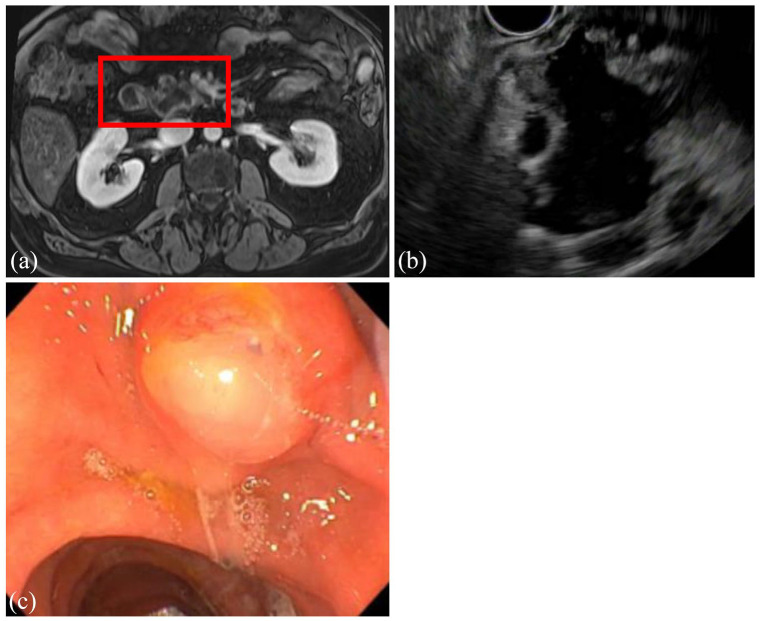
IPMNs are the most common type of PCN and are lined by a columnar epithelium and therefore produce mucin.14,43,44 IPMNs most commonly occur in the head of the pancreas but can occur anywhere within the pancreas, can be singular or multifocal, and while they can arise at any age they are most commonly found in the fifth to seventh decades of life.7 These are divided into three subtypes based on the type of pancreatic duct they involve: branch duct (BD-IPMN), main duct (MD-IPMN), and mixed (mixed IPMN) (see Figures 6(a)–9(c)). All IPMNs have a malignancy risk which varies significantly based on which ducts are involved, the IPMN size, growth rate, the presence of mural nodules or solid component, and several other features detailed below. BD-IPMNs are the most common subtype of IPMN and harbor a malignancy risk, although this is less than that of MD-IPMNs which have the highest malignancy risk.7,14,45,46 In the 2012 International Consensus Guidelines, Tanaka and colleagues47 summarized the results of 20 studies from 2003 to 2010, including a total of 3568 high-risk IPMNs which underwent surgical resection and found a mean rate of malignancy of 61.6% (range, 36–100%) in MD-IPMNs and 25.5% (range, 6.3–46.5%) in BD-IPMNs.48–67 A meta-analysis13 of 22 case series estimated 0.24% of the 6240 patients with PCNs (over a median of 18,079 patient years) developed malignancy. However, this is generally thought to be an underestimation of the risk of malignant transformation, and other studies have shown the rate of invasive cancer to be 31.1% in resected BD-IPMNs (range, 14.4–47.9%).68,69 MD-IPMNs secrete thick mucin into the PD, which typically results in focal or segmental dilation of the main duct and can often be seen endoscopically extruding from the pancreatic os (known as a ‘fish eye’ appearance, which is pathognomonic for this diagnosis) (see Figure 9(a)–(c)). The thick mucin may result in pancreatic ductal obstruction leading to pancreatitis, although this is rare. Most MD-IPMNs should be considered for surgical resection, and the guidelines differ slightly on when resection should be considered. In general, there is agreement that an MD-IPMN with an associated dilation of the PD 5–9 mm should be closely assessed with EUS and referral to surgery should be considered (particularly if there are other high-risk features present such as a mural nodule or solid component, upstream atrophy of the pancreas, or increase in size), whereas there is agreement that an MD-IPMN with an associated dilation of the PD ⩾10 mm should always be referred for surgery.14,44,68,70 The management of BD-IPMNs is the major focus of the multiple guidelines and is discussed in detail below.
Figure 6.
Axial view of MRI (a) and EUS (b) showing a 4.7-cm BD-IPMN in the head of the pancreas with an associated mural nodule in a 62-year-old man who was incidentally found to have a pancreatic cystic lesion on imaging obtained as part of the workup for COPD. EUS-guided fine needle aspiration was consistent with poorly differentiated carcinoma. He subsequently underwent pancreatoduodenectomy, with pathology showing the same.
Figure 7.
Axial view of MRI (a) and EUS (b) of a 3-cm branch duct IPMN in the tail of the pancreas with a solid component in a 79-year-old patient. The patient underwent EUS-guided fine needle aspiration of the solid component with cytology revealing adenocarcinoma. The patient then underwent lateral pancreatectomy with splenectomy and regional lymphadenectomy. Pathology revealed an invasive mucinous adenocarcinoma, and all lymph nodes were negative.
Figure 8.
Coronal view of MRI (a) and EUS (b) showing a 2.3-cm branch duct IPMN in the body of the pancreas of a 69-year-old man with no worrisome or high-risk features.
Figure 9.
Axial view of MRI (a) and EUS (b) of a main duct intraductal papillary mucinous neoplasm (MD-IPMN) identified in a 66-year-old man. The main pancreatic duct is dilated to 16.4 mm extending from the head to the genu of the pancreas. Endoscopic view (c) of the major papilla showing spontaneous extrusion of mucous (‘Mucorrhea’), which is pathognomonic for an MD-IPMN. This patient underwent a pancreatoduodenectomy with pathology revealing focally invasive carcinoma arising from an IPMN with high-grade dysplasia.
Guidelines
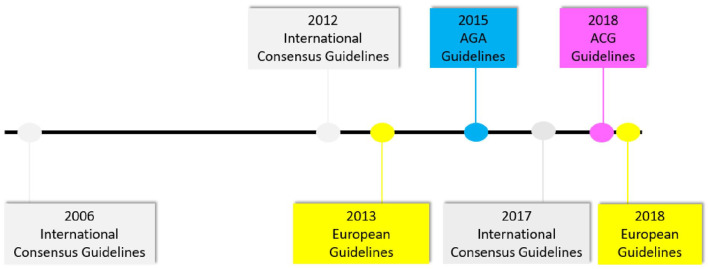
There have been numerous guidelines and publications throughout the years related to the management of PCNs (see Figure 10). The first major guideline was the 2006 International Consensus Guidelines (also known as the Sendai Guidelines as they were written in Sendai, Japan)71 which have since been updated in 201247 and again in 2017.68 Similarly, the European Guidelines were first published in 20139 and were revised in 2018.70 The American Gastroenterological Association (AGA) Guidelines were published in 2015,72 and the American College of Gastroenterology (ACG) published their guidelines in 2018.14 The guidelines vary somewhat on the timing and type of interval follow-up imaging studies based on PCN size. The AGA Guidelines72 are the first (and only) guidelines to recommend cessation of additional surveillance in the absence of changes in the PCN after 5 years of surveillance or if the patient underwent surgical resection and a non-malignant IPMN was diagnosed.
Figure 10.
Timeline of major guideline publications related to the management of pancreatic cystic neoplasms.
AGA, American Gastroenterological Association; ACG, American College of Gastroenterology.
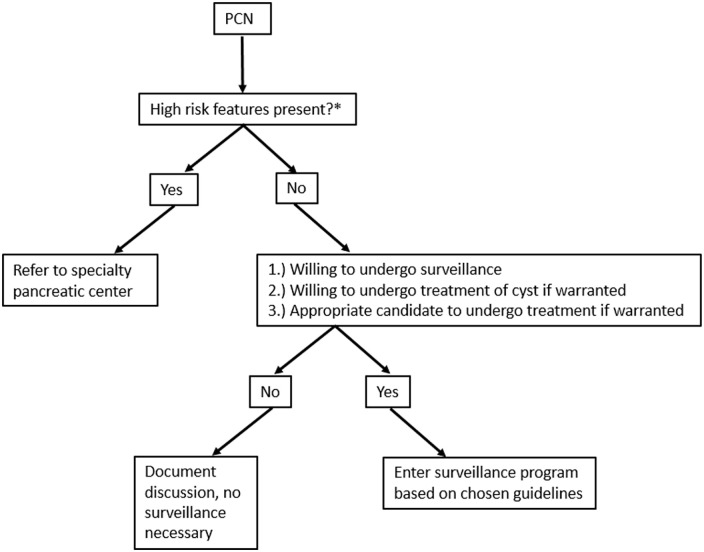
Figure 11.
Algorithm to approach of pancreatic cysts.
*High-risk features: presence of mural nodule or solid component, dilation of main pancreatic duct (⩾5 mm), cyst size ⩾3–4 cm, and positive cytology on fluid aspiration.
Prior to initiating a surveillance program for a PCN, there are many patient-specific aspects that must be considered, and it is important to discuss the potential risks and benefits of undergoing a surveillance program with the patient, as the risk tolerance varies greatly between patients. As the 2015 AGA Guidelines72 emphasize, the majority of PCNs will never become malignant, so some patients will elect to defer surveillance. In addition, one must consider the morbidity and mortality associated with pancreatic surgery. In fact, larger and more recent studies estimate a morbidity of 30–46% and a mortality of 4% following pancreatic surgery.13,73,74 In recent years, there have been emerging data for EUS-guided pancreatic cyst ablation (in the form of injection of ethanol or antitumor agents or through radiofrequency ablation) in patients unable or unwilling to undergo surgery. While the data for these interventions are promising for selected patients, it is not yet considered routine practice and is not discussed in the guidelines, but the future is likely bright for this technology.75–77 Finally, one must consider the psychological burden of patients undergoing routine surveillance of PCN. One study assessed the psychological impact on 109 patients with a PCN (31 prior to starting surveillance versus 78 already undergoing surveillance) and found patients already undergoing surveillance had more difficulty sleeping (30% versus 13%, p = 0.035) and found follow-up more burdensome (33% versus 13%, p = 0.044) compared with those who had not yet entered surveillance; however, 82% overall felt as if surveillance reduced their concerns of developing pancreatic cancer and 94% overall felt as if the potential advantages of surveillance outweighed the potential disadvantages.78 No matter which guideline is followed, the patient’s willingness and candidacy for surgical intervention or chemotherapy must be strongly considered. If the patient is not a candidate for or unwilling to undergo surgery or chemotherapy or if they have a limited life expectancy, surveillance is not indicated as it would not alter management.
Prior to delving into the specifics of the guidelines, it is important to understand that the majority of these guidelines are exclusively discussing IPMNs and MCNs (premalignant mucinous cysts). SCNs do not need surveillance as their malignancy risk is considered to be essentially zero, and surgery is only recommended if they are symptomatic.79,80 Surgical resection, however, is classically recommended for all solid pseudopapillary tumors and cystic neuroendocrine tumors >2 cm.
The guideline management of MCNs is summarized in Table 2. Surgical resection is recommended for any MCN in a surgically fit patient in the 2017 International Consensus Guidelines.68 The 2018 European Guidelines70 treat MCNs similarly to IPMNs in that surgical resection is recommended for MCNs ⩾4 cm, if symptomatic, or with the presence of high-risk features (i.e. mural nodules, PD dilation, cytology that is suspicious for or consistent with malignancy), and surveillance is recommended for all MCNs <3 cm. The 2015 AGA Guidelines72 and the 2018 ACG Guidelines14 also treat MCNs similar to IPMNs.
Table 2.
Management of mucinous cystic neoplasms of the pancreas.
| 2015 AGA |
2017 International Consensus | 2018 ACG |
2018 European |
|
|---|---|---|---|---|
| Management of MCN | Same as management for IPMN | Surgical resection for all surgically fit patients | Same as management for IPMN | Surgical resection if any of the following: size ⩾4 cm,
symptomatic, have high-risk features (same as those for
IPMNs) If size <3 cm, surveillance (same as IPMNs) |
ACG, American College of Gastroenterology; AGA, American Gastroenterological Association; IPMN, intraductal papillary mucinous neoplasm; MCN, mucinous cystic neoplasm.
There are multiple high-risk features on radiographic and EUS studies that can help distinguish IPMNs that harbor increased malignancy risk from those that can be considered low risk and, with proper surveillance and intervention, potentially prevent the development of pancreatic cancer. The guidelines differ somewhat in their criteria for recommending EUS or surgery, but they do agree on several features that are considered high-risk or worrisome: large cyst size (⩾3 cm in most guidelines), dilation of the PD (⩾5 mm), and the presence of a solid component within the PCN. PCNs without high-risk features enter a surveillance program which is determined by the size of the PCN. Tables 3 summarizes the various guidelines’ recommendations for interval surveillance based on PCN size. If high-risk features (which are detailed below) are present, the patient may be referred for EUS (the various guidelines’ recommendations for indications for EUS are summarized in Table 4) or surgery (the various guidelines’ recommendations for surgical indication are listed in Table 5).
Table 3.
Summary of major guideline publications related to the management of pancreatic cystic neoplasms based on cyst size.
| Cyst size | 2015 AGA |
2017 International Consensus | 2018 ACG |
2018 European |
|---|---|---|---|---|
| <1 cm | If no solid component and no dilated PD and cyst <3 cm: MRI in 1 year then every 2 years for 5 years if no change (then can stop if no change) | MRI or CT in 6 months, then every 2–3 years if no change | MRI every 2 years × 4 years (then consider lengthening) | Year 1: MRI or EUS every 6 months (in addition to serum CA-19-9
level and clinical evaluation) After Year 1: MRI and/or EUS every 1 year (in addition to serum CA-19-9 level and clinical evaluation) ⩾4 cm: resection |
| 1–2 cm | MRI or CT: Year 1: every 6 months Years 2–3: yearly After 3 years: every 2 years if no change |
MRI every 1 year × 3 years, then every 2 years × 4 years (then considering lengthening) | ||
| 2–3 cm | EUS in 3–6 months, then every year (can alternate with MRI) | MRI or EUS every 6–12 months × 3 years, then MRI every 1 year × 4 years (then lengthen) | ||
| >3 cm | Alternate EUS and MRI every 3–6 months | Refer to multidisciplinary group and alternate EUS and MRI every 6 months × 3 years, then every 1 year × 4 years (then consider lengthening) |
ACG, American College of Gastroenterology; AGA, American Gastroenterological Association; CA-19-9, carbohydrate antigen 19-9; EUS, endoscopic ultrasound; PD, pancreatic duct.
Table 4.
Indications for endoscopic ultrasound.
| Indications for endoscopic ultrasound | |
|---|---|
| 2015 AGA | ⩾2 high-risk features: - Cyst size ⩾3 cm - Dilated PD - Presence of a solid component |
| 2017 International Consensus | If any of the following present: - Pancreatitis due to cyst - Cyst size ⩾3 cm - Enhancing mural nodule <5 mm - Thickened/enhancing cyst walls - PD 5–9 mm - Abrupt change in diameter of PD with distal pancreatic atrophy - Lymphadenopathy - Elevated CA-19-9 - Rapid growth of cyst (>5 mm/2 years) |
| 2018 ACG | If any of the following present: - PD ⩾5 mm - IPMN or MCN ⩾3 cm - Change in PD caliber with upstream atrophy - Size increase of ⩾3 mm/year during surveillance - Jaundice due to cyst - Pancreatitis due to cyst - Presence of a mural nodule or solid component |
| 2018 European | Clinical or radiological features of concern for
malignancy Can be alternated or done in conjunction with MRI during surveillance |
ACG, American College of Gastroenterology; AGA, American Gastroenterological Association; CA-19-9, carbohydrate antigen 19-9; MRI, magnetic resonance imaging; PCN, pancreatic cystic neoplasm; PD, main pancreatic duct.
Table 5.
Indications for surgical resection.
| Indications for surgical resection | |
|---|---|
| 2015 AGA | Positive cytology on EUS-guided FNA; both a solid component and dilated PD |
| 2017 International Consensus | Obstructive jaundice with PCN in head of pancreas; enhanced mural nodule ⩾5 mm; PD ⩾10 mm; MD-IPMN; cytology suspicious or positive for malignancy |
| 2018 ACG | All MD-IPMNs; cytology with high-grade dysplasia or malignancy; mural nodule; concerning features on EUS |
| 2018 European |
Absolute indications: Cytology suspicious or
positive for malignancy or high-grade dysplasia; solid
component; obstructive jaundice with PCN in head of pancreas;
enhancing mural nodule >5 mm; PD ⩾10 mm; symptoms due to
PCN Relative indications: PCN growth rate ⩾5 mm/year; elevated CA-19-9 level; PD 5–9.9 mm; PCN size ⩾40 mm; new-onset diabetes mellitus; acute pancreatitis (due to PCN); enhancing mural nodule <5 mm |
ACG, American College of Gastroenterology; AGA, American Gastroenterological Association; BD-IPMN, branch duct intraductal papillary mucinous neoplasm; CA-19-9 carbohydrate antigen 19-9; EUS, endoscopic ultrasound; FNA, fine needle aspiration; IPMN, intraductal papillary mucinous neoplasm; MCN, mucinous cystic neoplasm; PCN, pancreatic cystic neoplasm; PD, main pancreatic duct.
Associated symptoms
If the patient is symptomatic from their PCN, surgical resection should be strongly considered to alleviate their symptoms and to obviate the potentially increased malignancy risk from PCNs, driving symptoms.81 If the patient presents with nonspecific symptoms like abdominal pain, back pain, or weight loss, it can be quite challenging to determine whether the PCN is actually the source of the patient’s symptoms. However, symptoms that are directly attributed to the PCN such as jaundice from biliary obstruction or nausea and vomiting from gastric outlet obstruction are more clear-cut.14 The association with recently diagnosed acute pancreatitis and the malignancy risk of an underlying IPMN is somewhat controversial, but most studies do show this risk to be increased.14,82–84 The 2017 International Consensus Guidelines68 and the 2018 ACG Guidelines14 recommend EUS assessment of the PCN if it is felt to be the etiology for acute pancreatitis, and the 2018 European Guidelines70 consider this a relative indication for surgical resection. The association of newly diagnosed diabetes mellitus with an underlying IPMN’s risk of malignancy is a bit more controversial. Several studies have correlated an increased risk of high-grade dysplasia or pancreatic cancer in patients with PCNs who have diabetes mellitus, and as almost two-thirds of patients with pancreatic cancer have underlying diabetes mellitus, many guidelines recommend careful consideration in this setting, and the 2018 European Guidelines70 consider this a relative indication for surgical resection.14,52,83,85,86
IPMN size
The frequency and format of imaging for surveillance of BD-IPMNs differ between guidelines (see Table 3). IPMN size ⩾3 cm has been associated with an increased risk of underlying malignancy compared with smaller cysts. In a meta-analysis by Anand and colleagues87 which included 1058 patients, the presence of an IPMN size ⩾3 cm had an odds ratio (OR) of 62.4 (30.8–126.3) for underlying malignancy, which was the strongest predictive factor. Other studies, however, have not found such a large OR. For example, a systematic review of six studies including 644 patients (381 of whom had cyst >3 cm) found an OR of 2.97 (1.82–4.85) for a cyst size >3 cm to have high-grade dysplasia or cancer; of the 381 patients with cyst >3 cm, 163 (43%) had a malignancy; in the 263 patients with cyst <3 cm, however, only 57 (22%) had a malignancy.13 All guidelines except the 2018 European Guidelines70 recommend consideration of EUS or referral to a multidisciplinary group for IPMN or MCN ⩾3 cm; the 2018 European Guidelines70 recommend surgery for IPMN or MCN size ⩾4 cm even in the absence of other worrisome features. Table 6 provides examples of the differences in the various guidelines’ recommendations based on PCN size alone.
Table 6.
Comparison of different guidelines’ recommendations based on examples of cyst size after initial discovery (assuming no high-risk or worrisome features).
| PCN size | 2015 AGA |
2017 International Consensus | 2018 ACG |
2018 European |
|---|---|---|---|---|
| 1–2 cm | MRI in 1 year | MRI or CT in 1 year | MRI in 1 year | MRI or EUS in 6 months (along with serum CA-19-9 level and clinical evaluation) |
| 2–3 cm | MRI in 1 year | EUS in 3–6 months | MRI or EUS in 6–12 months | MRI and/or EUS in 6 months (along with serum CA-19-9 level and clinical evaluation) |
| 3–4 cm | MRI in 1 year | MRI or EUS in 3–6 months | MRI or EUS every 6–12 months (and refer to multidisciplinary group) | MRI or EUS in 6 months (along with serum CA-19-9 level and clinical evaluation) |
ACG, American College of Gastroenterology; AGA, American Gastroenterological Association; CA-19-9, carbohydrate antigen 19-9; EUS, endoscopic ultrasound.
Solid component and mural nodule
Solid components and mural nodules (see Figures 6(a) and 7(b)) are technically separate features but are occasionally grouped together in guidelines and studies. A solid component is typically considered a solid region within the pancreatic parenchyma that involves or neighbors a PCN, whereas a mural nodule is typically considered a solid component in the wall of the PCN. Both solid components and mural nodules associated with IPMNs have independently been associated with an increased risk of underlying malignancy. For example, a meta-analysis of seven studies including 816 patients with PCN who underwent surgery found a solid component in 186 (23%) of these cysts, of which 136 (73%) were found to harbor malignancy; in the 630 patients without a solid component to their cyst, only 147 (23%) were found to have malignancy (OR, 7.73; 3.38–17.67).13,72 A large meta-analysis by Anand and colleagues87 studied 1452 patients and found the presence of a mural nodule had an OR of 9.3 (5.3–16.1) for developing malignancy. Similarly, another systemic review13 showed an OR of 7.73 (3.38–17.67) for developing malignancy with an underlying mural nodule, whereas another meta-analysis88 of 23 studies including 1373 patients found the presence of mural nodule had an OR of 6.0 (4.1–8.8) for malignancy. There are little data to support the size of a mural nodule at which malignancy should be suspected, although there are studies supporting a cut-off of 5 mm.89 The presence of a solid component is an indication for surgery in the 2018 European Guidelines70 and the 2018 ACG Guidelines.14 The 2015 AGA Guidelines72 also consider a solid component a high-risk feature and recommend surgery if the combination of a solid component and a dilated PD is present. The 2017 International Consensus Guidelines68 and the 2018 European Guidelines70 recommend surgery for mural nodules ⩾5 mm.
PD dilation
The association of a dilated PD (see Figure 9(a) and (b)) with underlying malignancy has been debated. The large meta-analysis by Anand and colleagues87 studied 328 patients and found a PD ⩾6 mm had an OR of 7.27 [95% confidence interval (CI), 3.0–17.4] for developing malignancy. However, another meta-analysis of four studies including 609 patients (148 with a dilated PD and 461 without a dilated PD) who underwent surgery for their PCN found an underlying malignancy in 69 (47%) patients with a dilated PD and in 150 (33%) patients without a dilated PD (OR, 2.38; 95% CI, 0.71–8.00).13 The size of the PD at which surgical resection should be strongly considered varies somewhat between the guidelines. Both the 2017 International Guidelines47,68 and the 2018 European Guidelines recommend surgery for PD ⩾10 mm. The 2015 AGA Guidelines72 also consider a dilated PD a high-risk feature and recommend surgery if the combination of a solid component and a dilated PD is present. The 2018 ACG Guidelines14 recommend EUS for a PD ⩾5 mm.
Serum CA-19-9 levels
Elevated serum carbohydrate antigen 19-9 (CA-19-9) levels (which is considered >37 units/ml) have also been associated with an increased risk of malignancy. A meta-analysis of 15 studies including 1629 patients with a PCN and elevated serum CA-19-9 levels found a pooled sensitivity of malignancy to be 40% and a pooled specificity to be 89%.90 In addition, a study of biopsy-proven malignant IPMNs in 117 patients showed elevated serum CA-19-9 levels had an accuracy of 73.8%, sensitivity of 34.2%, and specificity of 92.4% for predicting malignancy.91 Elevated serum CA-19-9 levels are a relative indication for surgery in the 2018 European Guidelines9,70 and are an indication for EUS in the 2017 International Consensus Guidelines68 but are not specified in the criteria for EUS or surgery in either the 2015 AGA Guidelines72 or the 2018 ACG Guidelines14
Cytology
Cytology consistent with or highly suspicious for high-grade dysplasia or cancer is an indication for surgical resection in all guidelines. In a meta-analysis of four studies with 96 patients, cytology was shown to have a relatively low sensitivity (64.8% with 95% CI of 0.44–0.82) and high specificity (90.6% with 95% CI of 0.81–0.96) for pancreatic cancer. Therefore, negative results do not rule out the presence of a high-risk lesion, but positive results are an indication for surgical resection in all of the guidelines.
Rate of PCN growth
Rapid growth of a PCN is classically heralded as an increased risk of malignancy. However, there are little data to support this. For example, a meta-analysis of five studies included 572 patients with PCNs, of which 125 (22%) had interval growth in PCN size. Of these five studies, no single study showed a statistically significant association between cancer and PCN growth rate, and no difference was seen when these results were pooled (OR, 1.65; 95% CI, 0.52–5.23).13 In addition, significant interobserver variability has been seen between radiologists when estimating the size of a PCN. For example, a study of 144 PCNs seen on MRI ranging in size from 5 to 35 mm showed a mean interobserver variability of 4.0 mm in size estimation per cyst. Despite these limited data, many guidelines recommend proceeding to EUS if the cyst increases in size, and the 2018 European Guidelines70 include a relative indication for surgery if the PCN size increases by ⩾5 mm/year. The 2017 International Consensus Guidelines68 recommend EUS if the PCN increases by >5 mm in 2 years, and the 2018 ACG Guidelines14 recommend EUS if the PCN increases by ⩾3 mm/year. The 2015 AGA Guidelines72 do not contain criteria for recommending EUS or surgery based on the rate of PCN size increase.
Other diagnostic modalities
Despite decades of research in pancreatic cystic lesions, there remain much controversy and debate regarding the best methods for accurately diagnosing and risk-stratifying PCNs, and many of the PCNs that undergo surgical resection only contain low-grade dysplasia or no dysplasia at all, indicating a potential toward overutilization of surgical resection.92 In recent years, new markers from EUS-guided fluid samples from PCNs and new technical modalities of tissue acquisition and assessment have emerged, which could improve our ability to accurately diagnose PCNs and understand their risk for malignant transformation. However, these are not currently recommended for usage by any of the guidelines, so we will only briefly discuss them.
Next-generation sequencing of PCN fluid: The presence of KRAS or GNAS mutations in the cyst fluid has a high specificity for the diagnosis of mucinous PCNs.93,94 Additional mutations (TP53, SMAD4, PIK3CA, PTEN, CDKN2A) have been shown to be associated with preoperative risks for advanced neoplasia in mucinous cysts, but need validation in multicenter studies.93,95–98
Cyst fluid glucose level: Multiple studies have shown glucose levels to be lower in mucinous compared with non-mucinous PCNs.24,99,100 For example, a meta-analysis of eight studies including 609 PCNs found that when PCN fluid glucose was compared with PCN fluid CEA, glucose had a higher sensitivity (91% versus 56%) and diagnostic accuracy for detecting mucinous lesions (94% versus 85%), with no difference in specificity between the tests.101
Microbiopsy: EUS-guided through-the-needle biopsies of the PCN wall using microbiopsy forceps may increase the diagnosis yield and further help differentiate non-mucinous from mucinous PCNs and improve presurgical assessment of malignancy risk.102–104
Confocal laser endomicroscopy: In this modality, a confocal laser endomicroscope probe is introduced through an EUS-directed 19-guage needle, and the PCN epithelium can be microscopically imaged in real time to further assist in identifying the type of PCN and risk-stratify IPMNs.105–109
Another controversial aspect of PCN management includes longevity of surveillance. The 2015 AGA Guidelines72 uniquely give a conditional recommendation for discontinuation of surveillance if there has been no change in the PCN after 5 years. The AGA Technical Review on PCNs13 does leave the caveat that these decisions need to be individualized and that surveillance could be extended in surgically fit patients under 70 years of age. This is in distinction to other guidelines that either do not alter surveillance timelines after 5 years of no change (2017 International Consensus Guidelines68 and the 2018 European Guidelines)70 or call to lengthen the interval (2018 ACG Guidelines)14.
The utility of continued surveillance beyond 5 years has been assessed in several studies, and the data are mixed. A study110 of 804 patients with BD-IPMNs who were followed prospectively found the overall incidence of pancreatic cancer to be 3.5% at 10 years and 12.0% at 15 years from the time of initial diagnosis; similarly, another study111 of 131 presumed low-risk BD-IPMNs which were surveyed for ⩾5 years showed 73 (55.7%) progressed in some form, both of which support continued surveillance beyond 5 years. On the contrary, a multicenter study112 of 310 patients with PCNs followed for at least 5 years found only 1% of patients developed pancreatic cancer and that mortality unrelated to pancreatic cancer was 8 times higher than that from pancreatic cancer. Crippa and colleagues113 followed 144 patients with BD-IPMNs and showed 26 (18%) developed high-risk stigmata after a median of 77.5 months from the time of diagnosis, which indicates that these would have been missed if following the 2015 AGA Guidelines,72 although only 2% developed pancreatic malignancy, and the overall 12-year disease-specific survival was 98.6%. In addition, when they applied the 2015 AGA Guidelines72 criteria for high-risk features (PCN >3 cm, dilated PD, mural nodule), they showed the risk of developing pancreatic cancer was 0% with no high-risk features, 1% with one high-risk feature, and 15% with two high-risk features. They, therefore, concluded that continued surveillance beyond 5 years is not warranted in patients with ⩽1 high-risk feature; however, patients with two high-risk features should continue surveillance beyond 5 years.
While there are some similarities between the various PCN surveillance guidelines, each varies significantly in its frequency of radiographic testing and its threshold for EUS or surgery. There have been several studies comparing the efficacy of the various aspects of these studies.
Lekkerkerker and colleagues114 studied 115 patients who underwent pancreatic resection for PCN and assessed final histopathological diagnosis and the initial indication for surgery and compared the 2012 International Consensus Guidelines,47 the 2013 European Guidelines,9 and the 2015 AGA Guidelines.72 They found the preoperative diagnosis (based on imaging or EUS) was correct in 83 (72%) patients, and in the 75 patients with IPMNs, the indication for surgery was correctly justified in 36 (54%) of 67 patients using the 2012 International Consensus Guidelines,47 in 36 (53%) of 68 using the 2013 European Guidelines,9 and in 32 (59%) of 54 using the 2015 AGA Guidelines.72 Surgery could have been avoided in 8 (11%) of 75 patients when the 2012 International Consensus Guidelines47 were applied, in 7 (9%) of 75 when the 2013 European Guidelines9 were applied, and in 21 (28%) of 75 when the 2015 AGA Guidelines72 were applied. No patients with high-grade dysplasia or malignancy would have been missed by applying the 2012 International Consensus Guidelines47 or the 2013 European Guidelines,9 whereas 4 (12%) of 33 patients would have been missed by applying the 2015 AGA Guidelines.72 The authors concluded that while the 2015 AGA Guidelines72 may avoid more unnecessary surgeries, more malignancies will be missed by following this guideline compared with the others.
Lobo and colleagues115 came to a similar conclusion by creating a Monte Carlo simulation model for the evaluation of a cohort of 10,000 patients with pancreatic cysts to compare the 2015 AGA Guidelines72 (which are considered to be less intensive) with the 2017 International Consensus Guidelines68 (which are considered to be more intensive). The model included patients aged 55–70 years and assumed a rate of malignant transformation to be 0.12% per year, which was based on the lower end of the 95% CI found in the AGA Technical Review13 and was chosen due to the younger age of the population in the cohort (the rate of malignant transformation is assumed to be higher in older age patients). This showed the two guidelines were similar in terms of deaths related to PCN management and quality-adjusted life years. The 2017 International Consensus Guidelines68 had more surgeries (711 versus 163), more surgery-related deaths (18.5 versus 3.5), more imaging studies (an average of 11.70 versus 6.89 imaging studies per patient), and higher total cost per cancer identified ($1,384,896 versus $898,760 or a total cost per patient of $16,825 versus $8,939), which corresponds to a $3.6 million higher cost per additional cancer detected. The 2015 AGA Guidelines,72 however, had more missed cancers (71 versus 49) and more cancer-related deaths (47.3 versus 32.1). Finally, the model predicted more overall deaths that were unrelated to PCN management than those who died of pancreatic cancer (1422 versus125). Therefore, the 2015 AGA Guidelines72 resulted in a similar number of deaths at a much lower cost.
The study by Lobo and colleagues115 is paramount in understanding the population-based approach to PCN management. As the authors discuss, clinicians’ primary goal is to detect a potentially preventable or curable malignancy; however, clinicians may fail to fully appreciate the harm, including cost, morbidity, and mortality, that accompanies more aggressive surveillance or early intervention. When taken as a whole, this study highlights the low-risk nature of many PCNs and the overscreening and overintervention of many of the guidelines in these low-risk lesions. However, one must remember that other studies have shown a much higher rate of malignant transformation than 0.12% when certain high-risk features are present, so caution is prudent when generalizing the results of this study to the patient sitting before you.
While the differences between the guidelines may seem trivial at initial glance, the data comparing the multiple guidelines in specific patient populations highlight the significant variances in long-term costs, number of imaging procedures and surgeries performed, as well as the number of malignancies missed. A less-intensive strategy is advisable when there are no high-risk or worrisome features, but in the small percentage of patients who develop such features, a more intensive strategy performs better but at a higher cost. While there are variations in the specifics of the guidelines, they all agree on several high-risk features that should get the attention of any clinician when assessing a PCN:
Presence of a mural nodule or solid component;
Dilation of the PD (or presence of MD-IPMN);
PCN size ⩾3–4 cm;
Positive cytology on PCN fluid aspiration.
For gastroenterologists who come across PCNs, if patients are good surgical candidates and willing, they should enter a surveillance program. It is advisable to follow one of the guidelines and document the guideline used as well as the rationale for the interval and method of imaging. As there are many centers that have pancreatic cyst clinics or screening programs, referral is always a reasonable option as well. Fortunately for most of our patients, the majority of cystic lesions will not impact their ultimate survival.
Footnotes
Author contributions: Ross CD Buerlein and Vanessa M Shami were involved in writing and editing of the manuscript.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Vanessa M Shami is consultant for both Interpace Diagnostics and Olympus America. Ross CD Buerlein has no conflicts of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Ross C.D. Buerlein  https://orcid.org/0000-0002-1033-9783
https://orcid.org/0000-0002-1033-9783
Vanessa M. Shami  https://orcid.org/0000-0001-7528-5141
https://orcid.org/0000-0001-7528-5141
Contributor Information
Ross C.D. Buerlein, University of Virginia Digestive Health, Charlottesville, VA, USA
Vanessa M. Shami, University of Virginia Digestive Health, 1215 Lee Street, Charlottesville, VA 22903, USA.
References
- 1.Ferrone CR, Correa-Gallego C, Warshaw AL, et al. Current trends in pancreatic cystic neoplasms. Arch Surg 2009; 144: 448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kromrey ML, Bülow R, Hübner J, et al. Prospective study on the incidence, prevalence and 5-year pancreatic-related mortality of pancreatic cysts in a population-based study. Gut 2018; 67: 138–145. [DOI] [PubMed] [Google Scholar]
- 3.Stark A, Donahue TR, Reber HA, et al. Pancreatic cyst disease: a review. JAMA 2016; 315: 1882–1893. [DOI] [PubMed] [Google Scholar]
- 4.Tada M, Kawabe T, Arizumi M, et al. Pancreatic cancer in patients with pancreatic cystic lesions: a prospective study in 197 patients. Clin Gastroenterol Hepatol 2006; 4: 1265–1270. [DOI] [PubMed] [Google Scholar]
- 5.Matsubara S, Tada M, Akahane M, et al. Incidental pancreatic cysts found by magnetic resonance imaging and their relationship with pancreatic cancer. Pancreas 2012; 41: 1241–1246. [DOI] [PubMed] [Google Scholar]
- 6.de Jong K, Nio CY, Hermans JJ, et al. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol 2010; 8: 806–811. [DOI] [PubMed] [Google Scholar]
- 7.van Huijgevoort NCM, Del Chiaro M, Wolfgang CL, et al. Diagnosis and management of pancreatic cystic neoplasms: current evidence and guidelines. Nat Rev Gastroenterol Hepatol 2019; 16: 676–689. [DOI] [PubMed] [Google Scholar]
- 8.Lee KS, Sekhar A, Rofsky NM, et al. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol 2010; 105: 2079–2084. [DOI] [PubMed] [Google Scholar]
- 9.Del Chiaro M, Verbeke C, Salvia R, et al. European experts consensus statement on cystic tumours of the pancreas. Dig Liver Dis 2013; 45: 703–711. [DOI] [PubMed] [Google Scholar]
- 10.Girometti R, Intini S, Brondani G, et al. Incidental pancreatic cysts on 3D turbo spin echo magnetic resonance cholangiopancreatography: prevalence and relation with clinical and imaging features. Abdom Imaging 2011; 36: 196–205. [DOI] [PubMed] [Google Scholar]
- 11.Lennon AM, Canto MI. Pancreatic cysts—part 2: should we be less cyst centric. Pancreas 2017; 46: 745–750. [DOI] [PubMed] [Google Scholar]
- 12.Moris M, Bridges MD, Pooley RA, et al. Association between advances in high-resolution cross-section imaging technologies and increase in prevalence of pancreatic cysts from 2005 to 2014. Clin Gastroenterol Hepatol 2016; 14: 585–593. [DOI] [PubMed] [Google Scholar]
- 13.Scheiman JM, Hwang JH, Moayyedi P. American gastroenterological association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015; 148: 824–848. [DOI] [PubMed] [Google Scholar]
- 14.Elta GH, Enestvedt BK, Sauer BG, et al. ACG clinical guideline: diagnosis and management of pancreatic cysts. Am J Gastroenterol 2018; 113: 464–479. [DOI] [PubMed] [Google Scholar]
- 15.Scheiman JM. Pancreatic cysts—part 1: using the American Gastroenterological Association guidelines for the management of pancreatic cysts-a practical approach. Pancreas 2017; 46: 742–744. [DOI] [PubMed] [Google Scholar]
- 16.Bick BL, Enders FT, Levy MJ, et al. The string sign for diagnosis of mucinous pancreatic cysts. Endoscopy 2015; 47: 626–631. [DOI] [PubMed] [Google Scholar]
- 17.Oh SH, Lee JK, Lee KT, et al. The combination of cyst fluid carcinoembryonic antigen, cytology and viscosity increases the diagnostic accuracy of mucinous pancreatic cysts. Gut Liver 2017; 11: 283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park WG, Mascarenhas R, Palaez-Luna M, et al. Diagnostic performance of cyst fluid carcinoembryonic antigen and amylase in histologically confirmed pancreatic cysts. Pancreas 2011; 40: 42–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology 2004; 126: 1330–1336. [DOI] [PubMed] [Google Scholar]
- 20.Cizginer S, Turner BG, Bilge AR, et al. Cyst fluid carcinoembryonic antigen is an accurate diagnostic marker of pancreatic mucinous cysts. Pancreas 2011; 40: 1024–1028. [DOI] [PubMed] [Google Scholar]
- 21.Snozek CL, Mascarenhas RC, O’Kane DJ. Use of cyst fluid CEA, CA19-9, and amylase for evaluation of pancreatic lesions. Clin Biochem 2009; 42: 1585–1588. [DOI] [PubMed] [Google Scholar]
- 22.van der Waaij LA, van Dullemen HM, Porte RJ. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: a pooled analysis. Gastrointest Endosc 2005; 62: 383–389. [DOI] [PubMed] [Google Scholar]
- 23.Rockacy M, Khalid A. Update on pancreatic cyst fluid analysis. Ann Gastroenterol 2013; 26: 122–127. [PMC free article] [PubMed] [Google Scholar]
- 24.Lopes CV. Cyst fluid glucose: an alternative to carcinoembryonic antigen for pancreatic mucinous cysts. World J Gastroenterol 2019; 25: 2271–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdelkader A, Hunt B, Hartley CP, et al. Cystic lesions of the pancreas: differential diagnosis and cytologic-histologic correlation. Arch Pathol Lab Med 2020; 144: 47–61. [DOI] [PubMed] [Google Scholar]
- 26.Law JK, Ahmed A, Singh VK, et al. A systematic review of solid-pseudopapillary neoplasms: are these rare lesions. Pancreas 2014; 43: 331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tipton SG, Smyrk TC, Sarr MG, et al. Malignant potential of solid pseudopapillary neoplasm of the pancreas. Br J Surg 2006; 93: 733–737. [DOI] [PubMed] [Google Scholar]
- 28.Lennon AM, Wolfgang C. Cystic neoplasms of the pancreas. J Gastrointest Surg 2013; 17: 645–653. [DOI] [PubMed] [Google Scholar]
- 29.Caglià P, Cannizzaro MT, Tracia A, et al. Cystic pancreatic neuroendocrine tumors: to date a diagnostic challenge. Int J Surg 2015; 21(Suppl. 1): S44–S49. [DOI] [PubMed] [Google Scholar]
- 30.Morales-Oyarvide V, Yoon WJ, Ingkakul T, et al. Cystic pancreatic neuroendocrine tumors: the value of cytology in preoperative diagnosis. Cancer Cytopathol 2014; 122: 435–444. [DOI] [PubMed] [Google Scholar]
- 31.Mitra V, Nayar MK, Leeds JS, et al. Diagnostic performance of endoscopic ultrasound (EUS)/endoscopic ultrasound–fine needle aspiration (EUS-FNA) cytology in solid and cystic pancreatic neuroendocrine tumours. J Gastrointestin Liver Dis 2015; 24: 69–75. [DOI] [PubMed] [Google Scholar]
- 32.Koh YX, Chok AY, Zheng HL, et al. A systematic review and meta-analysis of the clinicopathologic characteristics of cystic versus solid pancreatic neuroendocrine neoplasms. Surgery 2014; 156: 83–96. [DOI] [PubMed] [Google Scholar]
- 33.Zhu JK, Wu D, Xu JW, et al. Cystic pancreatic neuroendocrine tumors: a distinctive subgroup with indolent biological behavior? A systematic review and meta-analysis. Pancreatology 2019; 19: 738–750. [DOI] [PubMed] [Google Scholar]
- 34.Bordeianou L, Vagefi PA, Sahani D, et al. Cystic pancreatic endocrine neoplasms: a distinct tumor type. J Am Coll Surg 2008; 206: 1154–1158. [DOI] [PubMed] [Google Scholar]
- 35.Goh BK, Ooi LL, Tan YM, et al. Clinico-pathological features of cystic pancreatic endocrine neoplasms and a comparison with their solid counterparts. Eur J Surg Oncol 2006; 32: 553–556. [DOI] [PubMed] [Google Scholar]
- 36.Hurtado-Pardo LJAC, A Cienfuegos J, Ruiz-Canela M, et al. Cystic pancreatic neuroendocrine tumors (cPNETs): a systematic review and meta-analysis of case series. Rev Esp Enferm Dig 2017; 109: 778–787. [DOI] [PubMed] [Google Scholar]
- 37.Nilsson LN, Keane MG, Shamali A, et al. Nature and management of pancreatic mucinous cystic neoplasm (MCN): a systematic review of the literature. Pancreatology 2016; 16: 1028–1036. [DOI] [PubMed] [Google Scholar]
- 38.Compagno J, Oertel JE. Mucinous cystic neoplasms of the pancreas with overt and latent malignancy (cystadenocarcinoma and cystadenoma). Am J Clin Pathol 1978; 69: 573–580. [DOI] [PubMed] [Google Scholar]
- 39.Yamao K, Yanagisawa A, Takahashi K, et al. Clinicopathological features and prognosis of mucinous cystic neoplasm with ovarian-type stroma: a multi-institutional study of the Japan pancreas society. Pancreas 2011; 40: 67–71. [DOI] [PubMed] [Google Scholar]
- 40.Goh BK, Tan YM, Chung YF, et al. A review of mucinous cystic neoplasms of the pancreas defined by ovarian-type stroma: clinicopathological features of 344 patients. World J Surg 2006; 30: 2236–2245. [DOI] [PubMed] [Google Scholar]
- 41.Park JW, Jang JY, Kang MJ, et al. Mucinous cystic neoplasm of the pancreas: is surgical resection recommended for all surgically fit patients. Pancreatology 2014; 14: 131–136. [DOI] [PubMed] [Google Scholar]
- 42.Postlewait LM, Ethun CG, McInnis MR, et al. Association of preoperative risk factors with malignancy in pancreatic mucinous cystic neoplasms: a multicenter study. JAMA Surg 2017; 152: 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Werner J, Fritz S, Büchler MW. Intraductal papillary mucinous neoplasms of the pancreas—a surgical disease. Nat Rev Gastroenterol Hepatol 2012; 9: 253–259. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka M. Clinical management and surgical decision-making of IPMN of the pancreas. Methods Mol Biol 2019; 1882: 9–22. [DOI] [PubMed] [Google Scholar]
- 45.Hackert T, Fritz S, Klauss M, et al. Main-duct intraductal papillary mucinous neoplasm: high cancer risk in duct diameter of 5 to 9 mm. Ann Surg 2015; 262: 875–880; discussion 880–881. [DOI] [PubMed] [Google Scholar]
- 46.Shimizu Y, Yamaue H, Maguchi H, et al. Predictors of malignancy in intraductal papillary mucinous neoplasm of the pancreas: analysis of 310 pancreatic resection patients at multiple high-volume centers. Pancreas 2013; 42: 883–888. [DOI] [PubMed] [Google Scholar]
- 47.Tanaka M, Fernández-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012; 12: 183–197. [DOI] [PubMed] [Google Scholar]
- 48.Serikawa M, Sasaki T, Fujimoto Y, et al. Management of intraductal papillary-mucinous neoplasm of the pancreas: treatment strategy based on morphologic classification. J Clin Gastroenterol 2006; 40: 856–862. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt CM, White PB, Waters JA, et al. Intraductal papillary mucinous neoplasms: predictors of malignant and invasive pathology. Ann Surg 2007; 246: 644–651; discussion 651. [DOI] [PubMed] [Google Scholar]
- 50.Nagai K, Doi R, Kida A, et al. Intraductal papillary mucinous neoplasms of the pancreas: clinicopathologic characteristics and long-term follow-up after resection. World J Surg 2008; 32: 271–278; discussion 279. [DOI] [PubMed] [Google Scholar]
- 51.Hwang DW, Jang JY, Lee SE, et al. Clinicopathologic analysis of surgically proven intraductal papillary mucinous neoplasms of the pancreas in SNUH: a 15-year experience at a single academic institution. Langenbecks Arch Surg 2012; 397: 93–102. [DOI] [PubMed] [Google Scholar]
- 52.Mimura T, Masuda A, Matsumoto I, et al. Predictors of malignant intraductal papillary mucinous neoplasm of the pancreas. J Clin Gastroenterol 2010; 44: e224–e229. [DOI] [PubMed] [Google Scholar]
- 53.Bournet B, Kirzin S, Carrère N, et al. Clinical fate of branch duct and mixed forms of intraductal papillary mucinous neoplasia of the pancreas. J Gastroenterol Hepatol 2009; 24: 1211–1217. [DOI] [PubMed] [Google Scholar]
- 54.Crippa S, Fernández-Del Castillo C, Salvia R, et al. Mucin-producing neoplasms of the pancreas: an analysis of distinguishing clinical and epidemiologic characteristics. Clin Gastroenterol Hepatol 2010; 8: 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sugiyama M, Izumisato Y, Abe N, et al. Predictive factors for malignancy in intraductal papillary-mucinous tumours of the pancreas. Br J Surg 2003; 90: 1244–1249. [DOI] [PubMed] [Google Scholar]
- 56.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg 2004; 239: 788–797; discussion 797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salvia R, Crippa S, Partelli S, et al. Differences between main-duct and branch-duct intraductal papillary mucinous neoplasms of the pancreas. World J Gastrointest Surg 2010; 2: 342–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suzuki Y, Atomi Y, Sugiyama M, et al. Cystic neoplasm of the pancreas: a Japanese multiinstitutional study of intraductal papillary mucinous tumor and mucinous cystic tumor. Pancreas 2004; 28: 241–246. [DOI] [PubMed] [Google Scholar]
- 59.Lee SY, Lee KT, Lee JK, et al. Long-term follow up results of intraductal papillary mucinous tumors of pancreas. J Gastroenterol Hepatol 2005; 20: 1379–1384. [DOI] [PubMed] [Google Scholar]
- 60.Schnelldorfer T, Sarr MG, Nagorney DM, et al. Experience with 208 resections for intraductal papillary mucinous neoplasm of the pancreas. Arch Surg 2008; 143: 639–646; discussion 646. [DOI] [PubMed] [Google Scholar]
- 61.Kim SC, Park KT, Lee YJ, et al. Intraductal papillary mucinous neoplasm of the pancreas: clinical characteristics and treatment outcomes of 118 consecutive patients from a single center. J Hepatobiliary Pancreat Surg 2008; 15: 183–188. [DOI] [PubMed] [Google Scholar]
- 62.Ohno E, Hirooka Y, Itoh A, et al. Intraductal papillary mucinous neoplasms of the pancreas: differentiation of malignant and benign tumors by endoscopic ultrasound findings of mural nodules. Ann Surg 2009; 249: 628–634. [DOI] [PubMed] [Google Scholar]
- 63.Nara S, Onaya H, Hiraoka N, et al. Preoperative evaluation of invasive and noninvasive intraductal papillary-mucinous neoplasms of the pancreas: clinical, radiological, and pathological analysis of 123 cases. Pancreas 2009; 38: 8–16. [DOI] [PubMed] [Google Scholar]
- 64.Rodriguez JR, Salvia R, Crippa S, et al. Branch-duct intraductal papillary mucinous neoplasms: observations in 145 patients who underwent resection. Gastroenterology 2007; 133: 72–79; quiz 309–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jang JY, Kim SW, Lee SE, et al. Treatment guidelines for branch duct type intraductal papillary mucinous neoplasms of the pancreas: when can we operate or observe? Ann Surg Oncol 2008; 15: 199–205. [DOI] [PubMed] [Google Scholar]
- 66.Sadakari Y, Ienaga J, Kobayashi K, et al. Cyst size indicates malignant transformation in branch duct intraductal papillary mucinous neoplasm of the pancreas without mural nodules. Pancreas 2010; 39: 232–236. [DOI] [PubMed] [Google Scholar]
- 67.Kanno A, Satoh K, Hirota M, et al. Prediction of invasive carcinoma in branch type intraductal papillary mucinous neoplasms of the pancreas. J Gastroenterol 2010; 45: 952–959. [DOI] [PubMed] [Google Scholar]
- 68.Tanaka M, Fernández-Del Castillo C, Kamisawa T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017; 17: 738–753. [DOI] [PubMed] [Google Scholar]
- 69.Goh BK, Thng CH, Tan DM, et al. Evaluation of the Sendai and 2012 International Consensus Guidelines based on cross-sectional imaging findings performed for the initial triage of mucinous cystic lesions of the pancreas: a single institution experience with 114 surgically treated patients. Am J Surg 2014; 208: 202–209. [DOI] [PubMed] [Google Scholar]
- 70.European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018; 67: 789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 2006; 6: 17–32. [DOI] [PubMed] [Google Scholar]
- 72.Vege SS, Ziring B, Jain R, et al. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015; 148: 819–822; quiz e12–e13. [DOI] [PubMed] [Google Scholar]
- 73.Pergolini I, Sahora K, Ferrone CR, et al. Long-term risk of pancreatic malignancy in patients with branch duct intraductal papillary mucinous neoplasm in a referral center. Gastroenterology 2017; 153: 1284–1294. [DOI] [PubMed] [Google Scholar]
- 74.Kneuertz PJ, Pitt HA, Bilimoria KY, et al. Risk of morbidity and mortality following hepato-pancreato-biliary surgery. J Gastrointest Surg 2012; 16: 1727–1735. [DOI] [PubMed] [Google Scholar]
- 75.Barthet M, Giovannini M, Lesavre N, et al. Endoscopic ultrasound-guided radiofrequency ablation for pancreatic neuroendocrine tumors and pancreatic cystic neoplasms: a prospective multicenter study. Endoscopy 2019; 51: 836–842. [DOI] [PubMed] [Google Scholar]
- 76.Canakis A, Law R, Baron T. An updated review on ablative treatment of pancreatic cystic lesions. Gastrointest Endosc 2020; 91: 520–526. [DOI] [PubMed] [Google Scholar]
- 77.Moyer MT, Maranki JL, DeWitt JM. EUS-guided pancreatic cyst ablation: a clinical and technical review. Curr Gastroenterol Rep 2019; 21: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Overbeek KA, Kamps A, van Riet PA, et al. Pancreatic cyst surveillance imposes low psychological burden. Pancreatology 2019; 19: 1061–1066. [DOI] [PubMed] [Google Scholar]
- 79.Jais B, Rebours V, Malleo G, et al. Serous cystic neoplasm of the pancreas: a multinational study of 2622 patients under the auspices of the International Association of Pancreatology and European Pancreatic Club (European Study Group on Cystic Tumors of the Pancreas). Gut 2016; 65: 305–312. [DOI] [PubMed] [Google Scholar]
- 80.Reid MD, Choi HJ, Memis B, et al. Serous neoplasms of the pancreas: a clinicopathologic analysis of 193 cases and literature review with new insights on macrocystic and solid variants and critical reappraisal of so-called ‘serous cystadenocarcinoma’. Am J Surg Pathol 2015; 39: 1597–1610. [DOI] [PubMed] [Google Scholar]
- 81.Goh BK, Tan YM, Cheow PC, et al. Cystic lesions of the pancreas: an appraisal of an aggressive resectional policy adopted at a single institution during 15 years. Am J Surg 2006; 192: 148–154. [DOI] [PubMed] [Google Scholar]
- 82.Shin SH, Han DJ, Park KT, et al. Validating a simple scoring system to predict malignancy and invasiveness of intraductal papillary mucinous neoplasms of the pancreas. World J Surg 2010; 34: 776–783. [DOI] [PubMed] [Google Scholar]
- 83.Ingkakul T, Sadakari Y, Ienaga J, et al. Predictors of the presence of concomitant invasive ductal carcinoma in intraductal papillary mucinous neoplasm of the pancreas. Ann Surg 2010; 251: 70–75. [DOI] [PubMed] [Google Scholar]
- 84.Morales-Oyarvide V, Mino-Kenudson M, Ferrone CR, et al. Acute pancreatitis in intraductal papillary mucinous neoplasms: a common predictor of malignant intestinal subtype. Surgery 2015; 158: 1219–1225. [DOI] [PubMed] [Google Scholar]
- 85.Chari ST, Kelly K, Hollingsworth MA, et al. Early detection of sporadic pancreatic cancer: summative review. Pancreas 2015; 44: 693–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leal JN, Kingham TP, D’Angelica MI, et al. Intraductal papillary mucinous neoplasms and the risk of diabetes mellitus in patients undergoing resection versus observation. J Gastrointest Surg 2015; 19: 1974–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anand N, Sampath K, Wu BU. Cyst features and risk of malignancy in intraductal papillary mucinous neoplasms of the pancreas: a meta-analysis. Clin Gastroenterol Hepatol 2013; 11: 913–921; quiz e59–e60. [DOI] [PubMed] [Google Scholar]
- 88.Kim KW, Park SH, Pyo J, et al. Imaging features to distinguish malignant and benign branch-duct type intraductal papillary mucinous neoplasms of the pancreas: a meta-analysis. Ann Surg 2014; 259: 72–81. [DOI] [PubMed] [Google Scholar]
- 89.Kim TH, Song TJ, Hwang JH, et al. Predictors of malignancy in pure branch duct type intraductal papillary mucinous neoplasm of the pancreas: a nationwide multicenter study. Pancreatology 2015; 15: 405–410. [DOI] [PubMed] [Google Scholar]
- 90.Wang W, Zhang L, Chen L, et al. Serum carcinoembryonic antigen and carbohydrate antigen 19-9 for prediction of malignancy and invasiveness in intraductal papillary mucinous neoplasms of the pancreas: a meta-analysis. Biomed Rep 2015; 3: 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim JR, Jang JY, Kang MJ, et al. Clinical implication of serum carcinoembryonic antigen and carbohydrate antigen 19-9 for the prediction of malignancy in intraductal papillary mucinous neoplasm of pancreas. J Hepatobiliary Pancreat Sci 2015; 22: 699–707. [DOI] [PubMed] [Google Scholar]
- 92.Valsangkar NP, Morales-Oyarvide V, Thayer SP, et al. 851 resected cystic tumors of the pancreas: a 33-year experience at the Massachusetts General Hospital. Surgery 2012; 152(3 Suppl. 1): S4–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Singhi AD, McGrath K, Brand RE, et al. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut 2018; 67: 2131–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rift CV, Melchior LC, Scheie D, et al. Molecular heterogeneity of pancreatic intraductal papillary mucinous neoplasms and implications for novel endoscopic tissue sampling strategies. J Clin Pathol. Epub ahead of print 26 May 2021. DOI: 10.1136/jclinpath-2021-207598. [DOI] [PubMed] [Google Scholar]
- 95.Laquière AE, Lagarde A, Napoléon B, et al. Genomic profile concordance between pancreatic cyst fluid and neoplastic tissue. World J Gastroenterol 2019; 25: 5530–5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Haeberle L, Schramm M, Goering W, et al. Molecular analysis of cyst fluids improves the diagnostic accuracy of pre-operative assessment of pancreatic cystic lesions. Sci Rep 2021; 11: 2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schmitz D, Kazdal D, Allgäuer M, et al. KRAS/GNAS-testing by highly sensitive deep targeted next generation sequencing improves the endoscopic ultrasound-guided workup of suspected mucinous neoplasms of the pancreas. Genes Chromosomes Cancer 2021; 60: 489–497. [DOI] [PubMed] [Google Scholar]
- 98.McCarty TR, Paleti S, Rustagi T. Molecular analysis of EUS-acquired pancreatic cyst fluid for KRAS and GNAS mutations for diagnosis of intraductal papillary mucinous neoplasia and mucinous cystic lesions: a systematic review and meta-analysis. Gastrointest Endosc 2021; 93: 1019–1033. [DOI] [PubMed] [Google Scholar]
- 99.Park WG, Wu M, Bowen R, et al. Metabolomic-derived novel cyst fluid biomarkers for pancreatic cysts: glucose and kynurenine. Gastrointest Endosc 2013; 78: 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Faias S, Pereira L, Roque R, et al. Excellent accuracy of glucose level in cystic fluid for diagnosis of pancreatic mucinous cysts. Dig Dis Sci 2020; 65: 2071–2078. [DOI] [PubMed] [Google Scholar]
- 101.McCarty TR, Garg R, Rustagi T. Pancreatic cyst fluid glucose in differentiating mucinous from nonmucinous pancreatic cysts: a systematic review and meta-analysis. Gastrointest Endosc. Epub ahead of print 6 May 2021. DOI: 10.1016/j.gie.2021.04.025. [DOI] [PubMed] [Google Scholar]
- 102.Kovacevic B, Kalaitzakis E, Klausen P, et al. EUS-guided through-the-needle microbiopsy of pancreatic cysts: technical aspects (with video). Endosc Ultrasound 2020; 9: 220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hashimoto R, Lee JG, Chang KJ, et al. Endoscopic ultrasound-through-the-needle biopsy in pancreatic cystic lesions: a large single center experience. World J Gastrointest Endosc 2019; 11: 531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang D, Trindade AJ, Yachimski P, et al. Histologic analysis of endoscopic ultrasound-guided through the needle microforceps biopsies accurately identifies mucinous pancreas cysts. Clin Gastroenterol Hepatol 2019; 17: 1587–1596. [DOI] [PubMed] [Google Scholar]
- 105.Durkin C, Krishna SG. Advanced diagnostics for pancreatic cysts: confocal endomicroscopy and molecular analysis. World J Gastroenterol 2019; 25: 2734–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kadayifci A, Atar M, Basar O, et al. Needle-based confocal laser endomicroscopy for evaluation of cystic neoplasms of the pancreas. Dig Dis Sci 2017; 62: 1346–1353. [DOI] [PubMed] [Google Scholar]
- 107.Napoleon B, Palazzo M, Lemaistre AI, et al. Needle-based confocal laser endomicroscopy of pancreatic cystic lesions: a prospective multicenter validation study in patients with definite diagnosis. Endoscopy 2019; 51: 825–835. [DOI] [PubMed] [Google Scholar]
- 108.Machicado JD, Chao WL, Carlyn DE, et al. High performance in risk stratification of intraductal papillary mucinous neoplasms by confocal laser endomicroscopy image analysis with convolutional neural networks (with video). Gastrointest Endosc 2021; 94: 78–87. [DOI] [PubMed] [Google Scholar]
- 109.Krishna SG, Hart PA, DeWitt JM, et al. EUS-guided confocal laser endomicroscopy: prediction of dysplasia in intraductal papillary mucinous neoplasms (with video). Gastrointest Endosc 2020; 91: 551–563. [DOI] [PubMed] [Google Scholar]
- 110.Oyama H, Tada M, Takagi K, et al. Long-term risk of malignancy in branch-duct intraductal papillary mucinous neoplasms. Gastroenterology 2020; 158: 226–237e5. [DOI] [PubMed] [Google Scholar]
- 111.Kayal M, Luk L, Hecht EM, et al. Long-term surveillance and timeline of progression of presumed low-risk intraductal papillary mucinous neoplasms. AJR Am J Roentgenol 2017; 209: 320–326. [DOI] [PubMed] [Google Scholar]
- 112.Kwong WT, Hunt GC, Fehmi SM, et al. Low rates of malignancy and mortality in asymptomatic patients with suspected neoplastic pancreatic cysts beyond 5 years of surveillance. Clin Gastroenterol Hepatol 2016; 14: 865–871. [DOI] [PubMed] [Google Scholar]
- 113.Crippa S, Pezzilli R, Bissolati M, et al. Active surveillance beyond 5 years is required for presumed branch-duct intraductal papillary mucinous neoplasms undergoing non-operative management. Am J Gastroenterol 2017; 112: 1153–1161. [DOI] [PubMed] [Google Scholar]
- 114.Lekkerkerker SJ, Besselink MG, Busch OR, et al. Comparing 3 guidelines on the management of surgically removed pancreatic cysts with regard to pathological outcome. Gastrointest Endosc 2017; 85: 1025–1031. [DOI] [PubMed] [Google Scholar]
- 115.Lobo JM, Scheiman JM, Zaydfudim VM, et al. Clinical and economic outcomes of patients undergoing guideline-directed management of pancreatic cysts. Am J Gastroenterol 2020; 115: 1689–1697. [DOI] [PubMed] [Google Scholar]