Abstract
Background
Decades of effectiveness research has established the benefits of using patient decision aids (PtDAs), yet broad clinical implementation has not yet occurred. Evidence to date is mainly derived from highly controlled settings; if clinicians and health care organizations are expected to embed PtDAs as a means to support person-centered care, we need to better understand what this might look like outside of a research setting.
Aim
This review was conducted in response to the IPDAS Collaboration’s evidence update process, which informs their published standards for PtDA quality and effectiveness. The aim was to develop context-specific program theories that explain why and how PtDAs are successfully implemented in routine healthcare settings.
Methods
Rapid realist review methodology was used to identify articles that could contribute to theory development. We engaged key experts and stakeholders to identify key sources; this was supplemented by electronic database (Medline and CINAHL), gray literature, and forward/backward search strategies. Initial theories were refined to develop realist context-mechanism-outcome configurations, and these were mapped to the Consolidated Framework for Implementation Research.
Results
We developed 8 refined theories, using data from 23 implementation studies (29 articles), to describe the mechanisms by which PtDAs become successfully implemented into routine clinical settings. Recommended implementation strategies derived from the program theory include 1) co-production of PtDA content and processes (or local adaptation), 2) training the entire team, 3) preparing and prompting patients to engage, 4) senior-level buy-in, and 5) measuring to improve.
Conclusions
We recommend key strategies that organizations and individuals intending to embed PtDAs routinely can use as a practical guide. Further work is needed to understand the importance of context in the success of different implementation studies.
Keywords: implementation, patient decision aids, rapid realist review, realist methods, shared decision making
Decades of effectiveness research has firmly established the patient-level benefits of using patient decision aids (PtDAs).1,2 More work is needed to assess the true impact of routine PtDA implementation on health care users and providers, but the promising benefits and lack of harms identified by controlled studies has led to strong international policy support for more person-centered health care systems underpinned, in part, by increasing implementation of PtDAs.3–9 However, broad clinical implementation has not yet occurred, and there is a notable intention-behavior gap when PtDAs are used outside experimental studies in routine clinical settings.10
PtDAs support patients’ participation in shared decision making (SDM) with health care professionals by making options explicit, providing evidence-based information about the associated benefits/harms, and helping patients to consider what matters most to them in relation to the possible outcomes.1 Formats vary (e.g., paper based, DVD, website), and distribution methods can be tailored to the condition and setting, with PtDAs being delivered either as part of the clinical pathway (e.g., made available to patients before or during consultation) or via direct-to-consumer approaches (e.g., for population-level cancer screening programs, access provided via screening invitations). Various studies have examined and described key factors that influence successful implementation of SDM more broadly.11–15 Interventions studied include PtDAs and other approaches that encourage SDM behaviors, including patient activation materials and clinician SDM skills training.
The International Patient Decision Aid Standards (IPDAS) Collaboration review published in 201316 explored the success levels of different implementation strategies and included findings from controlled trial settings. Key barriers identified in the 2013 review included health care professionals’ (HCPs) attitudes toward SDM, lack of understanding in how to use PtDAs and undertake SDM, HCPs’ lack of trust in PtDA content, lack of clarity among HCPs regarding the purpose of PtDAs in relation to other information available for patients, HCPs believing that patients do not want decisional responsibility, competing clinical demands, and the time it would take to distribute and use the PtDA. Key facilitators included system-level approaches (e.g., systematic identification of patients ahead of appointments via electronic health records and distribution methods that did not rely on HCPs to initiate access), SDM and/or PtDA training and skills development, and dedicated clinical leadership (e.g., clinical champion).
Despite their benefits and various policies mandating their dissemination and use,3–9 widespread adoption of PtDAs has not occurred, and significant gaps exist in understanding factors contributing to adoption, implementation, and sustainability of these interventions in routine clinical settings. Strong foundational research has examined the implementation of SDM in health care, typically through large-scale demonstration projects (e.g., Informed Medical Decisions Foundation/Healthwise),17,18 and excellent examples of local adoption also exist (e.g., Dartmouth Hitchcok Medical Center, Lebanon, NH, USA).19 The literature listing barriers and facilitators of PtDA dissemination and implementation, as perceived by HCPs and patients, is also well established.16 However, despite the valuable learning, much of it is derived from highly controlled settings, which might not be representative of day-to-day processes and resources (human or financial) in routine clinical settings. Further, although lists of barriers and facilitators are useful markers to guide efforts to embed PtDAs, they provide less insight into why and how these factors influence implementation and can overlook the relations between different factors.20 PtDAs are not “magic bullets” that will always deliver the intended benefits to patients; their usefulness will ultimately depend on context and implementation.21 If clinical teams and organizations are being encouraged or mandated (e.g., clinical guidelines) by national health agencies to embed PtDA as a means to support person-centered care, we need to better understand what this might look like outside of a research setting, which contexts are likely to be more successful, and which might face additional challenges.
This current review was conducted in response to the IPDAS Collaboration’s evidence update process, which informs their published standards for PtDA quality and effectiveness.22–24 It updates the theory and evidence provided in the 2013 review16,25 through the sole inclusion of real-world data, exclusion of data from highly controlled settings, an understanding of the contexts that enable or hinder implementation, and the mechanisms (i.e., changes in people’s reasonings and actions) through which implementation is achieved. The main aim of this current review is to develop context-specific program theories that explain why and how PtDAs are successfully implemented in routine health care settings, providing a framework that will be useful to various stakeholders committed to embedding these tools routinely.
Method
We used rapid realist review (RRR) methodology26 and the RAMESES publication standards for realist reviews.27 RRR methodology moves beyond traditional reviews by allowing researchers to answer questions about why interventions in complex social contexts, such as routine health care, work or do not work.28 We chose this method as it allowed us to look beyond the overall success of a PtDA intervention to generate explanations about what works for whom, in what contexts, to what extent, and, most importantly, how and why?28
The resulting knowledge synthesis highlighted possible interventions (I) that could be implemented in a specific context (C) that in turn interact with various mechanisms (M) and produce outcomes (O) of interest,28 in this case, implementation of PtDAs in routine health care settings (see Box 1 for definitions of specific terminology). Two reviewers (N.J.-W. and T.v.d.W.) conducted a scoping exercise of existing literature examining barriers and facilitators to implementing PtDAs and SDM9,11–13,16,25,29,30 to agree on the review questions and scope and to generate initial theories. The a priori proposal was reviewed and approved by the IPDAS Steering Committee and registered with PROSPERO (CRD42019153334).
Box 1.
Glossary of Key Terms and Abbreviations
| Context (C) | Preexisting conditions outside the control of the intervention developers which influence the success or failure of the intervention (ref Pawson and Tiley 200831) |
| Mechanism (M) | Peoples’ reaction(s) to the implementation of the intervention; how does it change their reasoning and actions? (ref Pawson and Tiley 200831) |
| Outcome (O) | Intended and unintended consequences of the intervention as a result of a mechanism operating within a context (ref Pawson and Tiley 200831) |
| Intervention (I) | Features of the intervention resource (ref Pawson and Tiley 200831) |
| Implementation | The constellation of processes intended to get an intervention into use within an organization |
| Patient decision aid (PtDA) | Interventions that support patients to make decisions, by making decisions explicit, providing information about options and associated benefits/harms, and helping clarify congruence between decisions and personal values (ref Stacey 20171) |
We followed the key stages of an RRR: identifying scope/research questions, identifying literature for inclusion, quality appraisal, data extraction, and data synthesis.26 Our specific foci were to engage key experts and stakeholders to streamline the review process, produce useful results for those planning to implement PtDAs, and to create a set of recommendations for the IPDAS Collaboration’s updated evidence document for PtDA implementation. We convened a review team (named co-authors, led by N.J.-W. and T.v.d.W.) identified via the IPDAS Collaboration’s call for evidence update chapter authors to support the review process in the areas of literature identification, data extraction, and theory development. Typically, RRRs involve consultation with a broader expert panel to identify literature and corroborate theory development; however, as the review team consisted of a number of key international experts in the field of PtDA development, evaluation, and implementation, representing a range of disciplines and backgrounds, the review team also fulfilled this role.
Identifying and Selecting Literature for Inclusion
The review team identified an initial list of potential articles that could contribute to theory development and a list of known organizations and individuals involved in the implementation of PtDAs. The lead authors (N.J.-W./T.v.d.W.) screened articles to determine if they could likely contribute to understanding what facilitates and/or hinders PtDA implementation in routine clinical settings. Articles were included if the study reported implementation of a PtDA (as defined by the IPDAS Collaboration)1 in a routine health care setting (defined as daily situations without significant additional resources, in which clinicians and/or providers had been encouraged to integrate the PtDA into usual care routines) and if PtDA and dissemination/implementation strategies were described. Articles were excluded if the study used an intervention not classified as a PtDA1 (e.g., education resource, information leaflet), if the PtDA supported decisions about health insurance/provider options, or if the PtDA was implemented in highly controlled settings, such as randomized controlled trials or process evaluations conducted as a sibling study assessing implementation in a controlled research setting. Although secondary analysis of experimental studies has its relevance, we chose to exclude sibling studies associated with experimental studies that focus on measuring the efficacy or effectiveness of PtDAs. These studies likely bypassed routine clinical procedures to enlarge the effect of the PtDA, thus being less representative of everyday clinical settings, and would have limited bearing on our program theory. Studies exploring routine implementation of SDM outside controlled settings are relatively new, and our aim was to build on the 2013 review; thus, articles were restricted to a 10-y period (2009–2019). There were no restrictions regarding PtDA format (e.g., web based, paper based), type of decision, healthcare settings, or population/participants.
Using the initial set of articles, a combination of free-text and MeSH headings related to “decision aids,”“shared decision making,” and “implementation” were used to develop a Medline search strategy, which was adapted for use in CINAHL (see Supplementary File 1 for the Medline search strategy). Relevant websites (e.g., databases of funders who support PtDA implementation programs), policy documents, and known individuals and organizations were consulted to determine whether any unpublished works relating to the review questions were available. Citations were exported to EndNote; titles and abstracts of all papers identified via electronic searches were screened (by T.v.d.W.) to determine if they could answer the review question. Potentially relevant articles were obtained, and full texts were screened (N.J.-W. and T.v.d.W.) against our inclusion/exclusion criteria (noted above). Reference lists of included studies were consulted for forward and backward searching, and a clear audit trail of all data sources was maintained.
Data Extraction
A data extraction team was convened from members of the broader review team, and a data extraction template was developed, piloted, and streamlined to increase emphasis on context-mechanism-outcome (CMO) configurations. In the final version, qualitative, quantitative, and contextual data that could answer our review questions were extracted under the following broad categories: study/participant characteristics, PtDA characteristics, dissemination and implementation, implementation evaluation data (e.g., reach, dose, feasibility), and data supporting emerging theories about what works, how, and in what circumstances, for implementation of PtDAs (if-then statements). N.J.-W. coordinated extractions completed by the data extraction team members, checked the accuracy and consistency of data extracted, and consulted with individuals when necessary to clarify information or resolve discrepancies.
Data Synthesis
Explanatory data in the results sections of included studies relating to “what works in implementing PtDAs?” were initially extracted as “if-then” statements that described links between elements of contexts, mechanisms, and outcomes. As the synthesis progressed, comparable if-then statements were grouped by N.J.-W., while ensuring linkage to the original data and source of the individual if-then statements. We applied Pawson’s reasoning processes31 to generate refined CMO configurations based on the grouped if-then statements (see Supplementary File 2 for example process of theory development). Realist reviewers typically make use of existing theories to make sense of the evidence generated during their review. We chose the Consolidated Framework for Implementation Research (CFIR)32 to help us interpret the findings emerging from the data, as it is designed to guide systematic assessment of multilevel implementation contexts to identify factors that might influence intervention implementation and effectiveness. It is composed of 5 major domains, each made up of several constructs (see Box 2 for domain descriptions). The initial draft of CMOs was presented back to the review team, who were asked to assess validity, relevance to the research questions, and importance of the inferences made. Feedback from the review team was used to refine the program theory (3 iterations), exclude theories viewed as less important and relevant, and inform further data searches for elements that were perceived as missing, based on prior knowledge and experience.
Results
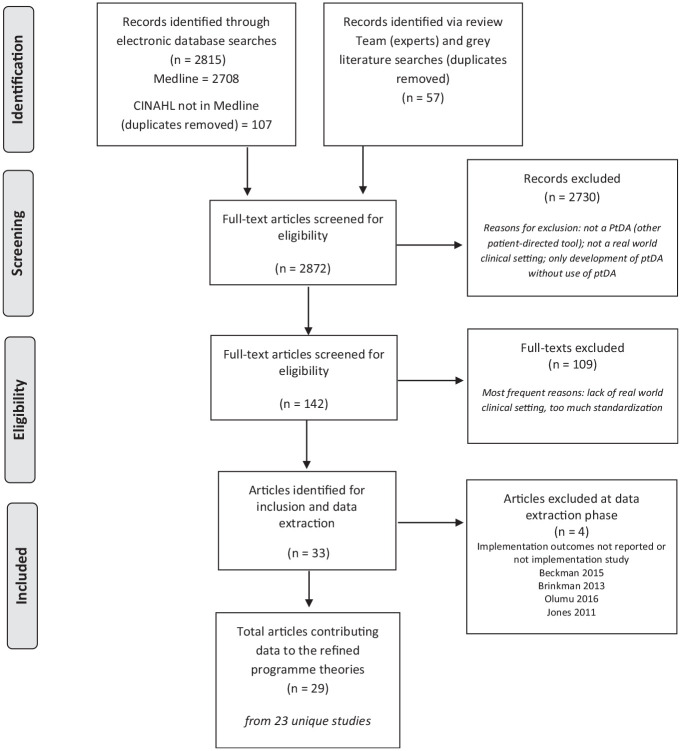
A total of 29 articles from 23 distinct studies contributed data to the developing theories. Figure 1 outlines the review process, including data sources and exclusions, and Table 1 presents the key characteristics of included studies. Most studies were from the United States (n = 14/23) and used a mixed-methods approach (n = 19/23). Seven studies specifically stated that they were underpinned by quality improvement methodology. PtDA delivery varied between the studies, including distribution to patients before the decision-making consultation (n = 11), use during the decision-making consultation (n = 6), or distribution to providers (n = 6). A variety of PtDA formats was used (e.g., video, web based, paper based) across a range of health and behavioral contexts (e.g., cancer, mental health, maternity, family planning, orthopedics) for a range of treatment or management decisions (see Supplementary File 3 for details) in various different settings (e.g., community based, primary care, secondary/specialist care). Implementation strategies differed across the studies, ranging from motivated clinicians embedding PtDAs into their clinics with limited additional support and resources to structured implementation programs using quality improvement methodology with direct and continuous support from implementation teams with expertise in these methods. Full details of study type, setting, PtDA characteristics, and implementation strategy can be found in Table 1. The review team consisted of 18 international SDM experts representing 9 countries: United States (n = 5), Canada (n = 4), United Kingdom (n = 3), Australia (n = 1), Chile (n = 1), Denmark (n = 1), France (n = 1), Germany (n = 1), The Netherlands (n = 1). The team represents a range of professional backgrounds: health services research (n = 7), medical (n = 4), psychology (n = 2), nursing (n = 2), epidemiology (n = 1), public health (n = 1), allied health professions (n = 1). Data extraction was conducted by 15 members of the review team and 2 additional researchers linked with review team members. All members contributed to theory development and refinement.
Figure 1.
Data searches and sources of included articles.
Table 1.
Characteristics of Included Studies (n = 23 from 29 Articles), Grouped according to the Method of PtDA Distribution
| Author, Year, Country, Linked Studies | Study Aim(s), Study Type, Setting | PtDA Characteristics, Intended Distribution and Use | Implementation Strategy and Duration | Process Outcome Data | Supports Theory Number |
|---|---|---|---|---|---|
| PtDAs First Distributed to Patients before Decision-Making Consultation | |||||
| Belkora et al., 2012, United States33 | To examine the reach and impact of 5 PtDAs routinely distributed
to breast cancer patients as part of a SDM demonstration
project. Case series (quantitative). Large multidisciplinary breast cancer center. |
Five video-based breast cancer PtDAs covering different decision points (ductal carcinoma in situ; early-stage surgery; breast reconstruction; adjuvant therapy; metastatic cancer). Decision Services personnel calls patient prior to appointment offering decision support materials/services, including appropriate PtDA. If required, PtDA mailed to patient within 24 h. Accompanied by before-and-after knowledge survey. | Decision Services already integrated into the Breast Cancer
Centre and oversaw routine distribution of the suite of PtDAs as
part of routine care, while measuring process and outcome
data. Thirty-six month implementation phase. |
A total of 61% (1098/1800) of new breast cancer patients received PtDA at home address, after call for consent. Reach attained 70% in the final year of implementation. | 7 |
| Berry et al., 2019, United States34 | To assess various implementation strategies for the Personal
Patient Profile–Prostate decision aid (P3P), by measuring
referral and access rates to P3P and analyzing feedback from
clinical staff and providers. Hybrid type 1 effectiveness-implementation trial (mixed methods). Secondary care (urology and multidisciplinary clinics in large hospitals). |
Personalized web-based PtDA for localized prostate cancer. Distribution methods varied depending on clinic (e.g., independent tool or integrated into existing educational resources), but all involved distributing link to the PtDA after disclosure of positive biopsy but before full options review visit. Patient has option to print and take PtDA report to decision-making consultation or email to clinic to be used during the consultation. iPads provided at each site before consultation for those men without internet access. | Conducted as part of hybrid type 1 effectiveness-implementation
trial. Six largest-volume urology and multidisciplinary clinics
participated in prior randomized effectiveness study selected.
Planning session convened at each site to develop workflow for
implementation. Physician staff received presentation about
study aims/publications; face-to-face nurse/staff meetings
reflected on current practice, considered various approaches
that would fit best; web trainings conducted with clinic staff
(including coordinators and nurses); staff provided with
instruction sheets and flyers for PtDA
referrals. Approximately 6-mo implementation phase. |
A total of 51% (252/495) of patients were informed about PtDA;
43% (107/252) accessed the PtDA. Invitation via personal e-mail/telephone contact resulted in 82%–87% PtDA access rates. Written invitations resulted in only 0% to 14% access rates. |
3a, 3b, 3c, 4a, 5a, 7 |
| Brackett and Kearing, 2015, United States35 | To facilitate SDM during preventative visits by using a
web-based survey system to offer colorectal cancer and prostate
cancer screening decision aids to appropriately identified
patients prior to the visit. Observational (quantitative). Academic general medical practice. |
PtDA (content not the same, different developers). Eligibility to receive PtDA determined prior to appointment. Patients offered choice of video or print version. If accepted, mailed to patient before appointment. Also completed knowledge and personal value questions. Knowledge provided in written report for patient; this report and a preference report fed forward to clinician available at decision-making consultation. | One academic general medical practice. Limited information
provided about implementation strategy. Thirty-eight month implementation phase (January 2008–March 2011). |
A total of 15% (552/3587) of patients that had not previously received a PtDA requested the PtDA after digital invitation. Patients could choose between video or written PtDA; 74% choose the written format. The most common reason to decline PtDA was the patient’s belief that they already knew enough to make the decision. | 1, 7 |
| Dharod et al., 2019, United States36 | Determine the feasibility of a digital health strategy for lung
cancer screening delivered via a patient
portal. Single-arm pragmatic trial. Large academic health care system (4 hospitals and 70+ community-based clinics). |
Interactive website with personalized risk-benefit information (mPATH-Lung). Electronic health record algorithm developed to identify eligible patients. Invitation to view PtDA sent via patient portal. After viewing PtDA, patients who note “yes” or “maybe” to the question “would you like to receive screening?” scheduled follow-up in-person SDM visit with nurse practitioner. | Conducted as part of a single-arm pragmatic trial in large
academic health system, including 4 hospitals and 70
community-based clinics. Informed consent waived by review board
as embedded as part of routine care. Limited information about
implementation strategy. Four-month implementation phase (November 2016–February 2017). |
A total of 40% (400/1000) of current or former smokers visited the web-based PtDA after digital message sent via patient portal. Median number of days between reading the message and PtDA = 0.4 (range 0–75). | 5a, 7 |
| Dontje et al., 2013, United States37 Holmes-Rovner et al., 201138 |
To develop and evaluate the feasibility of multifaceted SDM
interventions to prompt SDM in primary care about angiography in
stable coronary artery disease. Pilot cohort study (quantitative and qualitative). Academic clinics (family and internal medicine). |
Paper-based and DVD PtDA (“Treatment Choices for Coronary Artery Disease”). Eligible patients mailed PtDA and asked to review before they attended a 90-min group educational visit (where they then completed a 1-page treatment encounter planning guide). Patient instructed to take the PtDA and completed treatment encounter planning guide to their appointment (SDM discussion). | Two academic clinics. Unclear why clinics were selected.
Provider training included 1) clinical content in grand rounds,
2) provider training in communication skills and clinical
evidence review in the form of a 90-min workshop that could be
delivered as continuing medical or nursing education or as a
noon-time conference. Recruitment 3 mo. Implementation phase not reported. |
A total of 17% (43/247) patients were invited to review a PtDA. PtDA sent following signed consent. Actual use of the PtDA was not reported. Self-selection of participants, who were the more engaged and motivated in their care, seems to have occurred. | 1 |
| Krist et al., 2017, United States39 | To examine whether patients and clinicians will use a novel
health information technology (decision module) and its impact
on care across 3 cancer screening
decisions. Observational cohort study (quantitative). Twelve primary care practices (breast, colorectal, and prostate cancer). |
Three online PtDAs, informed decision-making modules for the following decisions: when to start breast cancer screening, how to be screened for colorectal cancer, and not being screened for prostate cancer. Guides patients through 7 steps 1) sent to patient via patient portal prior to consultation; 2) assesses personal preferences, knowledge, and needs and patients’ readiness to make a decision; 3) provides personalized education material tailored to preferences and decision stage; 4) allows patients to share their preferences and decision needs with their clinician; 5) provides prompt to patient and HCP to use the information to make a decision; 6) guides patients to make a choice, which can include deferring the decision; and 7) invites patients and clinicians to provide input after the encounter. | Twelve primary care practices that used electronic health
records. PtDA delivery integrated into central patient portal
(MyPreventiveCare). No further information provided regarding
selection of primary care practices. Eight-month implementation phase (January–August 2015). |
At the time of the study, 55,453 patients (34.5% of practice population) had a portal account; 23,546 used the portal during the study period (some evidence that users were more likely to be older or have comorbidities and were less likely to be Hispanic or African American). | 5a, 7 |
| Lin et al., 2013, United States40 | To explore processes for distributing PtDAs to patients in
clinical settings and to identify barriers and facilitators to
implementation. Qualitative and quantitative data sources (collected as part of a prior implementation project). Primary care practices. |
Clinics could choose from choice of 16 PtDAs (could choose multiple topics). Fourteen of 16 were booklet and DVD; 2 of 16 were booklet only. PtDA delivery was adapted by each clinic depending on needs/clinic workflow (i.e., distributed by physician/medical assistants in person, solicitation of patient interest at point of care, direct mailing). Designed for use inside and outside the consultation. | Physician and staff champion established in 5 primary care
clinics. Physician champion provided information about project
to each clinic’s leadership team. Both champions responsible for
promoting the program. Project team members who collaborated
with clinics to agree on PtDA topics tailor decision aid
distribution methods to clinic workflow. Project team attended
bimonthly meetings with project team and engaged in social
marketing efforts. January 2010 to June 2012 (29-month implementation phase) |
Overall rate of distribution of PtDA was 10%. The longitudinal data show a decrease in distribution of PtDA instead of a sustainable increase. | 3a, 3b, 3c, 4a, 4b, 6, 7 |
| Savelberg et al., 2019a, The Netherlands41 Savelberg-Pasmans, 2019b42 |
To explore experiences (including perceived barriers and
facilitators), issues, and concerns of early-adopter
professionals with regard to SDM, and the specific lessons on
implementation of a breast cancer PtDA within an oncological
clinical pathway. Observational prospective process evaluation (mixed-methods). Secondary care (hospital setting). |
Personalized web-based PtDA for early-stage breast cancer decisions (curative surgery, chemotherapy, reconstructive surgery). Patient receives personalized written prescription from clinician during diagnostic consultation (with relevant decision points and log-in code); patient views PtDA at home before decision-making consultation. | Seven breast cancer teams (consisting of at least 1 surgeon and
1 nurse) with positive attitudes toward SDM and willingness to
improve SDM process were recruited. Meeting arranged with each
team (all team members) prior to implementation with focus on
tailoring implementation, covering 1) recommendations on how to
present PtDA to patient, 2) minor adjustments to pathway, and 3)
watching 10-min lecture and 5-min motivational video on SDM
(skills/role modeling). Twenty-four month implementation phase (June 2015–June 2017). |
A total of 91% (77/85) of patients received access to the PtDA at first consultation; 73% (56/77) of patients logged on to web based PtDA. PtDA delivery started in tumor board: tumor board letter reported 2 treatment options 58% (50/85) and indication for PtDA 34% (29/85). | 3b, 4a, 4b, 5b, 6, 8 |
| Sepucha et al., 2017, United States43 Mangla et al., 201844 |
Sepucha et al. 2017: evaluation of the impact of a quality
improvement project to increase use of orthopedic PtDAs and
examine whether PtDAs increase SDM in routine
care. Quality improvement methodology. Tertiary referral center. Mangla et al. 2018: to use quality improvement methodology to test methods of PtDA delivery to increase use of 4 orthopedic PtDAs (as listed above). Quality improvement methodology. Secondary care (large orthopedic hospital department) and 18 primary care practices. |
Four PtDAs for orthopedic treatment/management options: 1) Treatment Choices for Knee Osteoarthritis (42-min DVD; 38-page booklet); 2) Treatment Choices for Hip Osteoarthritis (44-min DVD; 40-page booklet); Treatment Choices for Herniated Disk (44-min DVD; 40-page booklet); 4) Treatment Choices for Spinal Stenosis (44-min DVD; 40-page booklet). Sent to patient ahead of the consultation if feasible. Process of determining eligibility for PtDA and subsequent distribution process differed depending on condition (e.g., automated via electronic medical system and sent before consultation or by specialist and handed to patient during consultation). | Single academic hospital serving a tertiary referral center.
Clinicians and staff at this site were already aware of and able
to order PtDAs via the electronic medical record, which were
delivered after the consultation. This quality improvement
program aimed to increase the use of the PtDA by working with
primary care clinicians, specialists, and clinic staff to design
a more reliable process to identify eligible patients and to
send them the PtDA in advance of the visit (e.g., at the time of
referral from a primary care physician or at the time of
scheduling a visit for new patients. When a PtDA is ordered, it
automatically placed a note in the medical record documenting
that it was sent. Process could be adapted if needed (e.g.,
process for arthroplasty and spine services differed). One-time
bonus for targets reached (viewing/ordering PtDA). Integrated
into quality improvement programs with dedicated lead and
clinical champions. Eighteen-month implementation phase (2013–2014). |
A total of 65% (303/469) of patients were identified by surgeons as eligible to be sent home with PtDA, indicated by automatic not in patient record; 62% (188/303) of these patients reported reading the entire PtDA. | 6, 7 |
| Stacey et al., 2015, Canada45 | Evaluate a sustainable approach for implementing a PtDA for
adults with cystic fibrosis (CF) considering referral for lung
transplantation. Prospective pragmatic observational study. Eighteen adult CF clinics within 8 different provincial health care systems in Canada. |
Printed copies and web-based version of the PtDA. Sent to patients ahead of consultation to discuss possible referral. Patient asked to complete PtDA on their own, which produces a 1-page summary report. This is shared and discussed with the clinician during the appointment and also filed in the patient’s medical records. | Health care professionals involved in the care of adults with CF
invited to participate in implementation study. Of 23 CF
clinics, 18 participated. Various implementation interventions,
including 1) a 5-h knowledge/skills workshop (participants
received monetary honorarium), 2) Ottawa Decision Support
Tutorial (online) offered to those who could not attend
workshop, 3) access to the PtDA (paper and online), and 4)
conference calls every 3 mo in the first year, then twice a year
(reinforce learning and provide support). Guided by the
“Knowledge to Action” framework. Project duration: 24 mo. |
Across 15 clinics, 18 of 62 CF patients used PtDA (29%) at baseline. After initiating implementation interventions, 15 clinics reported that PtDA was used by 58 of 68 patients (85%) in year 1 and 54 of 59 (92%) in year 2. | 7 |
| Stacey et al., 2018, Canada46 | Compare 2 strategies for implementing PtDAs in clinical pathways
for men with localized prostate cancer. Comparative case study (mixed methods). Secondary care, 2 academic teaching hospitals. |
Case 1: Video and booklet version of PtDA containing information
about prostate cancer treatment (including benefits and harms),
values clarification exercise, and video patient testimonials.
Also given decision quality and SURE questionnaire. Handed to
patient during initial appointment (biopsy result); urologist
met patient and instructed nurse to provide the PtDA to the
patient for the next in-person appointment; clinician also
received results of decision quality and SURE questionnaire
(also put on file). Nurse (some trained in decision coaching)
available by phone and next appointment. Case 2A: PtDA similar to version used in case 1 but adapted into a PowerPoint presentation and sent via email (did not include video patient testimonials). Patient called by nurse with biopsy results and then sent PtDA via email (or mailed version used in case 1 if they did not have email access). Asked to review ahead of in-person appointment. Trained nurse reviewed PtDA with patient and answered any questions; appointment then scheduled with urologist if patient’s preferences was to discuss options. |
Guided by the “Knowledge to Action” Framework. Nurses offered 3.5-h workshop using Interprofessional Shared Decision Making Model. Open to all health care professionals, but only nurses, social workers, and policy makers attended. Case 1: 24 mo (January 2011–December 2012) Case 2: 24 mo (January 2014–December 2015) |
Case 1: 158/688 men (23%) received the PtDA. Consistent pattern
of PtDA use over a 2-y period. Case 2A: 265/270 men (98%) received PtDA. Consistent pattern of use over 2 y, but decline in volume used over time. |
7 |
| PtDA first used during decision-making consultation | |||||
| Bonfils et al., 2018, United States (47) | To explore the implementation process of an SDM intervention,
CommonGround, which uses peer specialists and a computerized
decision support center to promote
SDM. Mixed-methods. Four treatment teams in a large community mental health center. |
Computer-based program for people with mental illness housed in the Decision Support Center: access facilitated by “peer specialists” (coaching). Completed prior to decision-making consultation with psychiatric provider. Provider can view “health report” that is produced and can updated with agreed treatment plan. | Four teams chosen due to previous successful implementation
program with different intervention. Target programs agreed with
the teams. Visits to established implementation sites arranged.
Implementation coach (fully trained in CommonGround) assigned to
team, monthly conference calls during early implementation. All
staff trained and new sessions provided for new
staff/refreshers. Implementation overseen by leadership team,
including 3 fidelity visits. Twenty-two month implementation phase (May 2013–March 2015). |
A total of 64% (107/167) of clients used the PtDA at least once by filling in preconsultation appointment goals. Completion was 74% for ACT team clients and 56% for outpatient clients. After 2 y, the center ceased using the PtDA. | 1, 2, 3c, 4a, 7 |
| Brinkman et al., 2017, United States48 | Develop and reliably implement a decision aid to facilitate SDM
between clinicians, patients with juvenile idiopathic arthritis,
and their families around medication choices for treatment of
inflammatory arthritis. Quality improvement methodology. Primary care sites (rheumatology). |
Paper-based medication choice cards for juvenile idiopathic arthritis. Distributed to patient (and family member) during medication change discussion consultations. | Overseen by the project improvement team (quality improvement
consultant, clinician researchers). Train-the-trainer workshop
was held to train key clinician champions on the use of the
medication decision cards so that they could train other
clinicians at their sites on correct use. A supporting video was
also developed and shared with the sites. Each site developed
process maps to identify how the DA would fit their process.
Teams also did iterative plan-do study-act cycles to identify
implementation processes. Six-month implementation phase (March–August 2014). |
In 35% of visits in which drug use was discussed, the PtDA (medication choice cards) was used. The PtDA was used as intended (parent was asked to pick the first card to discuss) in 68% of visits where the PtDA cards were used. | 4b |
| Dahl Steffensen et al., 2018, Denmark14 | To report key lessons on the setup of a Center for Shared
Decision-Making at the Patient’s Cancer Hospital in Vejle
(Denmark). Case study report. Specialized cancer center in large public hospital. |
Generic PtDA developed by clinicians and School of Design. The PtDA template was developed to adhere to the certification and quality criteria set by IPDAS. Various paper-based versions tested for the following settings (using generic PtDA framework): adjuvant breast cancer, diagnostic setting of suspicion of lung cancer, genetic testing, ovarian cancer, colorectal cancer, and herniated disc. Generic preparation sheet viewed by patients before the clinical encounter and PtDA introduced during encounter. | Two-day training program in SDM offered to all clinicians by
department leaders. Supported by strong leadership, commitment
from hospital CEO and CMO. PtDA platform offered in web-based
system; health care providers log in and use platform to build
and develop PtDAs tailored to their specific needs, based on the
adjustable template (“build your own PtDA”). Duration of first implementation phase: 3 y. |
No data on actual use. The Center has moved into the next phase and started a systematic SDM implementation program across all hospitals in the region of Southern Denmark (2019). |
3a, 3c, 4a, 5a, 5b, 6 |
| Johnson et al., 2010, Indonesia/Mexico/Nicaragua49 | To describe the development and testing of a family planning
counseling tool and to discuss challenges and requisites for
shifting to SDM from the extremes of decision making dominated
by the provider, on one hand, or unaided by the provider, on the
other hand. Mixed-methods (pre and post design). Various contexts within health care settings where family planning is discussed (e.g., maternity hospitals, primary/public health clinics). |
Dual-purpose PtDA (patient facing) and “job aid” (clinician facing). Double-sided flip chart presenting contraception options, used during a counseling session between client and provider. Adapted to local language. | Implemented in 3 family-planning teams: Indonesia, Mexico,
Nicaragua. Two- to 4-day training workshop delivered to
providers ahead of implementation in routine
care. Implementation phase: 4 mo in Nicaragua, 1 mo in both Mexico and Indonesia. |
No clear data reported on actual use of the 2-sided flipchart PtDA. A total of 357 videotaped consultations showed total consultation time 550 s. Mean number of seconds of eye contact on PtDA: patient = 187, provider = 170. | 5b, 6 |
| Joseph-Williams et al., 2017, United Kingdom13 Lloyd et al., 201350 THF 2013 |
To understand what works and what does not work in implementing
SDM in routine NHS settings. Quality improvement methodology (mixed methods). Eight primary care practices and 7 secondary care hospital teams (head and neck cancer, pediatric ENT, renal, maternity, urology, and 2 breast cancer teams). Across 2 large university local health boards/trusts in the United Kingdom. |
Various paper-based brief PtDAs (1–4 pages) across a range of conditions, distributed to and used with patients during consultations. Patient given PtDA to use after consultation (when decision was not being made in the same consultation). | Implementation overseen by university-based team. Four dedicated
facilitators supported teams to develop their own
interventions/measures/implementation strategies and conduct
PDSA cycles. Key organizational leaders (e.g., medical
director), clinical champions, and patients part of leadership
team. Eighteen-month implementation program (August 2010–February 2012). All teams designed tools/interventions to use with PtDA as part of the program, and actual implementation phases varied across the teams. All teams attended SDM training as part of the program. |
No data on actual use. | 1, 2, 3a, 3b, 3c, 4a, 4b, 5a, 5b, 6, 7, 8 |
| Munro et al., 2019, United States51 | To explore the feasibility and acceptability of 2 interventions
for facilitating SDM about contraceptive methods with a
particular focus on factors that influenced their implementation
by clinical and administrative staff. Qualitative study (embedded within a 2 × 2 factorial cluster randomized controlled trial). Twelve clinics in which contraceptive counseling takes place. |
Two types of intervention designed to support female
contraceptive options: Patient targeted: “activation” materials delivered in the clinic, before appointment (video on tablet computer and paper-based card reinforcing questions to ask during consultation). Provider-targeted: set of 7 one-page paper-based PtDAs on contraceptive methods (tear pads). Used by HCP during patient visit. Short SDM/PtDA training video and written guidance. Not all groups received PtDAs. |
Twelve intervention arm clinics (patient targeted; provider
targeted; both) of a total 16 total clinics in trial. Each
clinic identified a senior staff member to liaise with research
team and facilitate implementation. The research team provided a
group orientation on the trial. After randomization, an
orientation on the intervention was provided, during which the
teams worked collaboratively to develop their own implementation
strategy, considering patient workflow and routinely used
patient/counseling materials. Short SDM/PtDA training video and
written guidance on how to use PtDAs provided prior to
implementation in 8 of 12 intervention arms. Duration not reported. |
No data on actual use. | 3a, 3b, 4a, 4b, 5a, 5b, 6, 8 |
| PtDA distributed to providers | |||||
| Feibelmann et al., 2011, United States52 | To trial a structured approach to disseminating breast cancer
PtDAs to community breast cancer sites and to explore the
factors associated with successful, sustained implementation of
PtDAs by the providers at these sites. Longitudinal study data examined, and cross-sectional mail/telephone survey. Cancer centers, hospitals, private practices, and patient resource centers. |
Videos/DVDs and accompanying booklet for 5 different breast cancer decisions (ductal carcinoma in situ, early-stage surgery, breast reconstruction, adjuvant therapy, metastatic cancer). Designed to be handed out by provider during consultation, for patient to watch/read following consultation. Limited further information on distribution/intended use. | Sites contacted to ascertain interest; 93 of 195 signed
agreements to distribute, 57 of 93 distributed PtDA to at least
1 patient. Sites identified 1 contact person. Limited further
information regarding implementation planning, adaption into
clinic workflows, etc. Duration not reported. |
A total of 61% (57/93) of US hospitals/practices that signed agreements to adopt PtDAs reported distribution of PtDA to at least 1 patient; 49% (46/93) reported still using the PtDA 1 y later. | 3b, 3c, 4a, 4b, 5a, 8 |
| Giguere et al., 2014, Canada53 | To measure the value and intention to use decision boxes
(Dboxes) in practice and to describe barriers and facilitators
of their use. Observational quality improvement study (mixed methods with sequential explanatory design). Six primary care practices. |
Eight different Dboxes (written in French and English) covering the following health care decisions: 1) cholinesterase inhibitors (ChEIs) to reduce the symptoms of Alzheimer’s disease, 2) acetylsalicylic acid (ASA) for primary prevention of cardiovascular disease, 3) fecal occult blood test (FOBT) to screen for colorectal cancer; 4) serum integrated test to screen women for fetal trisomy 21 (prenatal), 5) statins for primary prevention of cardiovascular disease (statins), 6) BRCA1/2 gene mutation test to evaluate the risks of breast and ovarian cancer (BRCA), 7) bisphosphonates to prevent osteoporotic fractures in postmenopausal women (osteo), and 8) prostate-specific antigen (PSA) test to screen men for prostate cancer. Delivered to clinician via email before consultation to help clinician recognize equipoise and the need to share decision with patient and to support provision of information about risks and benefits of options. | Eight different Dboxes were distributed to clinicians (1 each week for 8 wk of the implementation study). Limited information regarding agreed implementation. | No data on actual use. PtDA (Dboxes on tests or drugs) were intended to be reviewed by clinicians before consultation. Forty percent (190/472) of clinicians reported intention to use the PtDA. | 3c, 4b, 5b, 7 |
| Hsu et al., 2013, United States54 Hsu et al. 201655 |
Hsu 2013: To identify factors that promote and impede
integrating PtDAs into clinical practice in a large health care
delivery system. Qualitative case study methodology. Speciality care: orthopedics, cardiology, urology, women’s health, general surgery, neurosurgery. Hsu 2016: build on Hsu 2013 to understand how differences in provider attitudes across specialities may affect PtDA implementation and how provider attitudes shift after PtDA implementation. Qualitative case study methodology. Speciality care: orthopedics and cardiology. |
DVD and booklet format. Patient could also view PtDA via patient
portal. Hsu 2013: 12 PtDAs (35–55 min) for several elective surgery procedures: orthopedics (hip osteoarthritis, knee osteoarthritis), cardiology (coronary heart disease), urology (benign prostatic hyperplasia, prostate cancer), women’s health (uterine fibroids, uterine bleeding), general surgery (early stage breast cancer, breast reconstruction, ductal carcinoma in situ), and neurosurgery (spinal stenosis, herniated disk). Hsu 2016: Three PtDAs for the following elective surgical procedures: orthopedics (hip osteoarthritis, knee osteoarthritis), cardiology (coronary heart disease). Designed for patient to view/read before consultation with specialist (some exceptions because of the nature of the condition, e.g., abnormal uterine bleeding). |
System-wide implementation across Group Health’s Western
Washington Group Practice Division (serving 366,000 members)
across 6 speciality service lines. Implementation evaluation
conducted by Group Health Research Institute, nonproprietary,
public interest arm of Group Health. PtDAs provided free of charge for first 2 y of demonstration project. Twenty-four-month implementation phase. |
In the 24-mo implementation period, 9827 PtDAs were distributed to patients, via US mail, free of charge. The PtDAs could be ordered via electronic health record or patient portal. | 2, 6 |
| MacDonald-Wilson, 2017, United States56 | To investigate the implementation of decision support practices
by community mental health centers and the impact of decision
support on organizational- and individual-level
outcomes. Quality improvement methodology (mixed methods). Fifty-two community mental health centers. |
Total number of PtDA topics unclear, but sites asked to choose 1 PtDA to implement from a range of topics (e.g., residential rehabilitation, psychiatric rehabilitation, outpatient drug and alcohol services program). Limited further information on format/intended use but appears to be an online library of PtDAs that include decisional balance worksheets. Access given by provider. | Implementation at 52 community mental health centers within
network of parent organization, who volunteered to take part in
initiative. Each site asked to select their own intervention and
develop own implementation plans using PDSA cycles. Learning
collaborative approach established. Parent organization
supported all aspects of implementation (staff training,
implementation of intervention, information gathering/analysis).
Quality improvement team established at each site, including 1
member of senior leadership, a quality clinician, direct service
worker, and person in recovery. Trained learning collaborative
facilitators provided support throughout. Twelve-month implementation phase. |
During the 12-mo implementation period, 52% (27/53) of agencies reached the milestone that 80% of the staff used a PtDA with at least 1 patient each month. | 3c, 8 |
| Scalia et al., 2017, United States57 | To use normalization process theory to explore how and why 2
separate health care organizations in the United States had
spontaneously adopted Option Grid™ PtDAs (for total of 8 health
care issues) in routine clinical practice and investigate
factors that facilitated routine use. Case study (semistructured interviews). Two large sites (1 New York, 1 Minneapolis), offering a range of medical and dental services, including family practice, internal medicine, pediatrics, obstetrics and gynecology, and nurse midwifery. |
Option Grid decision aids (paper based, 1 page) for patients considering the following issues: hypercholesterolemia, antibiotics for pharyngitis, sciatica, knee pain, PSA test, Dupuytren’s contracture, carpal tunnel syndrome, trigger finger. Designed for use in consultation; clinician hands PtDA to patient, and patient takes home. | Included sites were chosen as they independently identified and
chose to embed the PtDAs into existing workflows and had been
and routinely implementing Option Grids for at least a 1-y
period beforehand. CapitalCare site in New York (primary care
organization with 65 physicians across 10 sites) used a total of
5 Option Grid PtDAs and collected quantitative data via the
electronic health record. HealthPartners Speciality Center in
Minnesota (speciality center providing orthopedic care with more
than 600 physicians), used 3 Option Grids (Dupuytren’s disease,
carpal tunnel syndrome, and trigger finger); the lead hand
surgeon had been an editor for these Option Grids that were used
during routine implementation. Unclear how many of the
clinicians at each site were involved in routine implementation,
but 23 interviews conducted with nurses, physicians, hospital
staff, and stakeholders across both sites. Not implementation study per se. But both sites had been routinely using relevant Option Grids for at least 1 y before the study. |
Number of sites using Option Grid (total number of times
decision aid given to patients). Capital Care (10 sites): High cholesterol: 6 (887) Pharyngitis: 2 (163) Sciatica: 3 (80) Knee pain: 1 (41) PSA: 1 (32) HealthPartners: Dupuytren’s contracture: 2 (100) Carpal tunnel: 2 (200) Trigger finger: 2 (200) estimated |
3c, 4a, 4b, 6, 7 |
| Scalia et al., 2018, Poland58 | To study the use of Option Grid PtDAs in a sample of clinics in
Warsaw, Poland, and measure their impact on SDM. Mixed methods (pre and post design). Three large private health care clinics (patients with heartburn, osteoarthritis of the knee, or considering statins). |
Option Grid decision aids (paper based, 1 page) for patients who had heartburn, osteoarthritis of the knee, or were considering statins. Designed for use in consultation. Clinician hands patient PtDA, and patient takes home. Translated from English to Polish. | Clinicians employed at 3 large private clinics were invited by
medical director to participate (selected on basis of high
patient volume). Thirteen clinicians participated across 3 sites
(5 gastroenterologists, 3 orthopedic surgeons, 3 family doctors,
and 2 cardiologists). Pre–post design; following baseline, all
clinicians underwent 1-h training intervention (to increase
understanding of SDM skills/model, familiarize clinicians with
Option Grids and CollaboRATE measurement instrument, and
increase confidence in skills to use Option Grid with patients)
and were given access to relevant Option Grids. Duration not reported. |
Approximately 700 physicians were involved in 2 settings. Total number of PtDAs given to patients in 2 years = 1700. In one setting, 6 of 10 sites used PtDA; 4 sites did not. Physicians reported being selective in their use of the Option Grid, making judgments based on patient characteristics. | 6 |
PtDA, patient decision aids; IPDAS, International Patient Decision Aid Standards; CEO, chief executive officer; CMO, chief medical officer; SDM, shared decision making; NHS, National Health Service; ENT, ear, nose, and throat; HCP, health care professional.
Program Theories: What Works in Implementing PtDAs into Routine Clinical Settings?
A total of 124 explanatory if-then statements were extracted from the included articles. Using the CFIR32 to help understand factors that might influence intervention implementation and effectiveness, these statements were refined into 8 program theories (CMO configurations). The program theories are described below, organized under relevant domains of the CFIR,32 and are summarized in Table 2 with supporting data (“I” is used in the results to denote features of the implementation strategy, e.g., skills training session, automated electronic delivery of PtDA). None of our theories mapped to domain II of the CFIR. Domains are not mutually exclusive, and some CMO configurations could map to more than 1 of the CFIR domains; however, for brevity and clarity, we mapped the 8 program theories to the most relevant domain. Because of the limited number of included studies, CMOs have been presented generically, with limited contextual reference to specific diseases or decisions, with the exception of theory 2, which is specific to crisis-driven and life-threatening situations.
Table 2.
Implementing PtDAs in Routine Clinical Settings Program Theories: Context-Mechanism-Outcome Configurations Identified from 29 Articles (23 Studies)
| Context (C)–Mechanism (M)–Outcome (O) Configurations (I = Intervention) | Source Article |
|---|---|
| I – Intervention characteristics | |
| Theory 1: PtDA complexity: simple tools for busy settings | |
| PtDAs are being implemented in busy health care systems with established processes/interventions (clinical and nonclinical) (C). When more complex PtDAs are selected (e.g., increased number of additional PtDA-related tasks; technical knowledge/support required; potential for technical/access issues; increased length of PtDA; unable to easily view PtDA without additional resources, e.g., computer program), HCPs feel that the PtDA competes with existing practice and is more difficult to integrate into their existing system (M), making them less likely to use the PtDA (O). | Bonfils 2018; Brackett 2015; Dontje 2013; Lloyd 2013; THF 2013 |
| III – Inner setting | |
| Theory 2: Crisis-driven and life-threatening situations: urgent care needs prioritized over decision support needs | |
| If the PtDA is implemented in a setting that is crisis driven or deals with life-threatening issues and diagnoses, which sometimes evoke a strong emotional response from the patient (C), HCPs believed that the immediate and urgent needs of the patient are more important (e.g., safety/reassurance/emotional support) than decision-making needs (M), and they were less likely to use the PtDA as prescribed (O). | Bonfils 2018; Hsu 2013; Hsu 2016; Lloyd 2013; THF 2013 |
| Theory 3: Bringing the whole team on board: establishing a common goal, senior-level buy-in, and distributing PtDA tasks appropriately | |
| 3a: Making sure administrative staff also understand PtDA purpose and intended use | |
| When PtDAs were delivered in settings in which the entire health care team (including all administrative and clinical staff) have been introduced to the PtDA (C), via staff briefing sessions or training (I), administrative staff understood the purpose and intent of the PtDA (M), which meant that they were more supportive and motivated to take part in PtDA coordination/distribution tasks (O). | Berry 2019; Dahl Steffensen 2018; Lin 2013; Munro 2019; Stacey 2018; THF 2013 |
| 3b: Distributing PtDA tasks to appropriate team members | |
| PtDAs are typically implemented in multidisciplinary teams that include various clinical, support, and administrative staff (C). When appropriate PtDA tasks are distributed and delegated to the appropriate individuals across the whole team (I), greater coherence exists among the team about the PtDA purpose and intent; individuals are motivated by the distribution of tasks (e.g., “I’m not in this by myself”), particularly when senior clinical staff engage; they understand how their task fits with the broader process; and they take ownership over their task (M), making the PtDA more likely to be distributed and used as planned (O). | Berry 2019; Feilbelman 2012; Munro 2019; Lin 2013; Mangla 2017; Savelberg 2019; Stacey 2018; THF 2013 |
| 3c: Dedicated and ongoing clinical leadership | |
| HCPs work in ways that align with the expectations and priorities set by the clinical leadership (C). A consistent clinical leader, “champion(s),” or leadership team) (I) plays an important role in continued buy-in through ongoing training for new staff, promoting positive attitudes toward the approach, presenting feedback on PtDA outcomes, supporting reflection and refinement of PtDA processes, and ensuring the approach aligns with key priorities of the health care organization (M). Lack of continued clinical leadership, or staff turnover of the clinical champion leading the work, can be detrimental to motivation and the skill set needed to use the PtDA (M) and lead to discontinued use (O). | Berry 2019; Bonfils 2018; Dahl Steffensen 2018; Feibelman 2017; Giguere 2014; Joseph-Williams 2017; Lin 2013; Lloyd 2013; MacDonald-Wilson 2017; Scalia 2017; Stacey 2018; THF 2013 |
| IV: Characteristics of individuals | |
| Theory 4: Activating and supporting HCPs to deliver PtDAs: HCPs aware, trained, and motivated to change practice | |
| 4a: Raising awareness of PtDA purpose and intended use | |
| When PtDAs are implemented in teams that do not understand the purpose of the PtDA or its intended use (C), they are less likely to use the PtDA as intended (O), because they do not understand the benefits for patients nor the PtDA role in supporting patient decision making (M). Introductory training sessions were provided to all team members about the purpose of the PtDA and its benefits (for patients and HCPs [I], helps teams decide how to integrate PtDAs into existing work practices [M], which in turn makes it more likely that the PtDA will be adopted in routine care [O]). | Berry 2019; Bonfils 2018; Dahl Steffensen 2018; Feibelman 2017; Lin 2013; Lloyd 2013; Munro 2019; Savelberg 2019; Scalia 2017; THF 2013 |
| 4b: Supporting SDM skills development | |
| When HCPs lack knowledge of SDM skills (C), they will be less likely to use the PtDA (O), as they do not have confidence in their SDM/PtDA delivery skills and/or they do understand how SDM differs from usual practice and therefore do not understand why the PtDA needs to be used (M). SDM skills workshops delivered prior to PtDA implementation (I) provided opportunity for HCPs to practice and develop confidence in SDM/PtDA delivery skills, helping HCPs to understand how SDM differs from current practice and thus the importance of PtDAs (M), which results in increased use and proficiency in using PtDAs with patients (O). | Brinkman 2017; Feibelman 2017; Giguere 2014; Joseph-Williams 2017; Lin 2013; Lloyd 2013; Mangla 2018; Munro 2019; Savelberg 2019; Scalia 2017; Stacey 2015; THF 2013 |
| Theory 5: Preparing and encouraging patients to use PtDAs: explicit invitations from clinical team to use PtDA before and during decision-making consultations | |
| 5a: Explicit invitations to use PtDA before decision-making consultations | |
| Many patients have no experience receiving or using a PtDA (C). When the clinical team sends explicit invitations (explaining the purpose/process of using PtDA and encouraging patients to engage with PtDA before the decision-making consultation [I]), patients better understand the relevance and purpose of the PtDA, they perceive that their role in the decision-making process is valid and desired, and they are reminded to use the PtDA (M), thus making them more likely to actively engage with the PtDA and use it to help inform their decision with a HCP (O). | Berry 2019; Dahl Steffensen 2018; Dharod 2019; Feibelman 2017; Joseph-Williams 2017; Krist 2017; Munro 2019; THF 2013 |
| 5b: Explicit invitations to use PtDA during decision-making consultations | |
| Significant power imbalances exist in some consultations, with many patients believing they cannot participate in SDM (C). When HCPs provide an explicit invitation during the decision-making consultation to further discuss the PtDA, preferably accompanied by handover of the PtDA (or duplicate copy, if delivered before consultation), (I) patients will feel that their contribution is valued and sought by the HCP and understand the relevance of the PtDA in the decision-making discussion (M), thus making them more likely to share their preferences, ask questions, and engage in decision making (thus using the PtDA for its intended purpose) (O). | Dahl Steffensen 2018; Giguere 2014; Johnson 2010; Joseph-Williams 2017; Munro 2019; Savelberg 2019 |
| V: Process | |
| Theory 6: Collaborative PtDA development and implementation planning: early and meaningful involvement of clinical teams and providers | |
| HCPs and providers have preexisting approaches/processes to communicate options to patients (C). Early involvement of (or ideally, initiation by) clinical teams/providers in the development of the PtDA content/implementation planning (I) creates a sense of ownership, increases buy-in, helps to legitimize content, and ensures the PtDA (content and delivery) is consistent with current practice (M), making it more likely to be integrated into routine care (O). | Dahl Steffensen 2018; Hsu 2016; Hsu 2013; Johnson 2010; Joseph-Williams 2017; Lin 2013; Lloyd 2013; Munro 2019; Savelberg 2019; Scalia 2017; Scalia 2018; Sepucha 2017; THF 2013 |
| Theory 7: Earlier distribution of PtDAs: systematizing delivery of PtDAs to all eligible patients before decision-making consultations | |
| In clinical environments with limited staff resources, short appointment times, or short time frames between diagnosis and decision discussion (C), preidentifying eligible patients and systematizing (ideally via IT systems) the timely delivery, completion, and return of the PtDA to clinicians prior to decision-making consultations (I) decreases clinicians’ concerns about time to coordinate and do SDM and prompts PtDA/SDM use during consultations (M), improving reach and integration of the PtDA (O). | Belkora 2012; Berry 2019; Bonfils 2018; Brackett 2015; Dharod 2019; Giguere 2014; Krist 2017; Lin 2013; Mangla 2018; Scalia 2017; Sepucha 2017; Stacey 2015; Stacey 2018; THF 2013 |
| Theory 8: Linking with “learning health care systems”: using measurement to show how PtDA outcomes link with and improve key organizational priorities | |
| When organizational priorities align with PtDA outcomes and a “learning health care system” (e.g., quality improvement practices/teams) exists within an organization (C), PtDAs should be implemented alongside routinely collected measures that the organization values (I). These measures demonstrate the improvements that result from using PtDAs and also demonstrate to clinical teams that the use of PtDAs is valued by the organization, which makes PtDAs more likely to be integrated into routine care (O). | Feibelman 2017; Joseph-Williams 2017; Lloyd 2013; MacDonald-Wilson 2017; Munro 2019; Savelberg 2019; THF 2013 |
PtDA, patient decision aids; HCP, health care professional; SDM, shared decision making; IT, information technology.
I. Intervention Characteristics
Theory 1: PtDA complexity: simple tools for busy settings
PtDAs are being implemented in busy health care systems with established processes/interventions (clinical and nonclinical) (C). When more complex PtDAs are selected (I; see Table 2 for examples), HCPs feel that the PtDA competes with existing practice and is more difficult to integrate into their existing system (M), making them less likely to use the PtDA (O).
Box 2.
Definitions of the 5 Major Domains of the Consolidated Framework for Implementation Research32
| I: Intervention Characteristics |
|---|
| Features of an intervention that might influence implementation; 8 constructs: intervention source, evidence strength/quality, relative advantage, adaptability, triability, complexity, design quality, cost |
| II: Outer Setting |
| Features of the external context that might influence implementation (economic, political and social context within which the organization resides); 4 constructs: patient needs and resources, cosmopolitanism, peer pressure, external policies and incentives |
| III: Inner Setting |
| Features of the implementing organization that might influence implementation (e.g., structural, political, cultural contexts through which implementation will proceed); 12 constructs: structural, networks/communications, culture, tension for change, compatibility, relative priority, organizational incentives and rewards, goals and feedback, learning climate, leadership engagement, available resources, access to information/ knowledge |
| IV: Characteristics of Individuals |
| Characteristics of individuals involved in implementation that might influence implementation; 5 constructs: knowledge and beliefs about intervention, self-efficacy, individual stage of change, individual identification with organization |
| V: Process |
| Strategies or tactics that might influence implementation; 4 constructs: planning, engaging, executing, reflecting and evaluating |
Five articles contribute data to the theory that less complex tools are more likely to be integrated into routine care.35,37,47,50,59 When PtDAs were perceived as more complex by the clinical team, especially those PtDAs that required technical knowledge and support, and required an increased number of PtDA-related tasks and personnel time, the teams felt that they competed with existing practice, were too resource intensive, and were more difficult to embed.35,37,47 Some HCPs reported that the shorter and less complex tools (e.g., brief in-consultation paper-based tools) were preferable as they fit better with existing practices and required limited additional resources.35,59,50
III: Inner Setting
Theory 2: Crisis-driven and life-threatening situations—urgent care needs prioritized over decision support needs
If the PtDA is implemented in a setting that is crisis driven or deals with life-threatening issues diagnoses, which sometimes evoke a strong emotional response from the patient (C), HCPs believed that the immediate and urgent needs of the patient are more important (e.g., safety/reassurance/emotional support) than decision-making needs (M), and they were less likely to use the PtDA as prescribed (O).
Five articles support the theory that PtDAs are less likely to be embedded by HCPs in teams that are typically crisis driven or deal with life-threatening issues.47,54,55,59,50 When exploring implementation of a PtDA within a community mental health setting, Bonfils et al.47 found that staff would often need to prioritize immediate patient needs over PtDA distribution; for example, “we’re a crisis-driven clinic and you could use this [resource], and you could use that [resource], but then they’re like ‘well they don’t have a house,’ so some of that stuff gets in the way.” Life-threatening situations54 also present challenging contexts to embed PtDAs, where HCPs tend to prioritize supporting the immediate health care needs, and sometimes the more emotional needs, of the patient.
Theory 3: Bringing the whole team on board: establishing a common goal, senior-level buy-in, and distributing PtDA tasks appropriately
3a: Making sure administrative staff also understand the PtDA purpose and intended use
When PtDAs were delivered in settings where the entire health care team (including all administrative and clinical staff) have been introduced to the PtDA (C), via staff briefing sessions or training (I), administrative staff understood the purpose and intent of the PtDA (M), which meant that they were more supportive and motivated to take part in PtDA coordination/distribution tasks (O).
Six articles provide data for this theory.14,34,40,46,51,59 Various studies reported that PtDA integration was more successful when all members of the clinical team had been introduced to the PtDA and not only the HCPs who would be using the tool. When administrative staff understood the purpose of the PtDA and how it fit into the patient pathway, they were more supportive of its use and motivated to support the distribution processes as part of their administrative role. Joseph-Williams et al.13 reported the “shared understanding” that emerged when all team members were involved and how reception staff played a key role in introducing the concept of choice at the very start of the patient journey as well as in distributing materials. Other studies also found that administrative staff played a critical role in integrating the PtDA into workflows40,51; they were responsible for 73% of PtDA distribution in the study by Lin et al.40 Berry et al.34 further highlighted the importance of “coherence” about purpose and use across the entire team (clinical and administrative); when the administrative staff members knew very little about why a PtDA was important or being used, this acted as a barrier to implementation.
3b: Distributing PtDA tasks to appropriate team members
PtDAs are typically implemented in interprofessional teams that include various clinical, support, and administrative staff (C). When appropriate PtDA tasks are distributed and delegated to the appropriate individuals across the whole team (I), greater coherence exists among the team about the PtDA purpose and intent, individuals are motivated by the distribution of tasks (e.g., “I’m not in this by myself”), particularly when senior clinical staff engage, they understand how their task fits with the broader process, and they take ownership over their task (M), making the PtDA more likely to be distributed and used as planned (O).
Eight articles support the theory that distributing PtDA tasks among a multidisciplinary team (clinical and nonclinical) is more likely to lead to the PtDA being distributed and used as planned.34,40,41,44,46,51,52,59 Lin et al.40 reported how a “team-based practice model,” in which clinic staff were empowered to distribute PtDAs, was more successful than a model that relied on physicians alone; however, they also noted that this model can only support, not substitute, HCP involvement in patient engagement. Whole-team involvement, particularly senior clinical staff, led to perceptions such as “I’m not in this by myself” and “this won’t be seen as my ‘little’ project,”13 which motivated individual team members to continue use of PtDAs. Conversely, Fiebleman et al.52 showed that when service physicians were not supportive of the PtDA, the remaining staff were less likely to use it. Omission of certain team members from the process (e.g., nurses not involved after use of PtDA)41 or inappropriate allocation of PtDA tasks to the wrong team member (e.g., reliance on physicians to order PtDAs)44 can lead to reduced fidelity in the way the PtDA is used and the subsequent SDM discussion and reduced distribution.
3c: Dedicated and ongoing clinical leadership
HCPs work in ways that align with the expectations and priorities set by the clinical leadership (C). A consistent clinical leader (“champion” or leadership team) (I) plays an important role in continued buy-in through ongoing training for new staff, promoting positive attitudes toward the approach, presenting feedback on PtDA outcomes, supporting reflection and refinement of PtDA processes, and ensuring the approach aligns with key priorities of the health care organization (M). Lack of continued clinical leadership or staff turnover of the clinical champion leading the work can be detrimental to motivation and the skill set needed to use the PtDA (M) and lead to discontinued use (O).
Twelve articles support this theory.13,14,34,40,46,47,50,52,53,56,57,59 Leadership from senior clinicians and managers plays an important role in determining whether teams use and continue to use the PtDA. Several studies found that a clinical lead, or “champion” played a significant role in making training available, prioritizing and keeping SDM and the use of the PtDA high on the agenda, conveying seriousness of intent, and ensuring evaluation data were being fed back to the team13,14,46,56—all which results in greater motivation and an improved skill set among the team, making it more likely that the PtDA use will be sustained. As one member of the obstetrics team said in the study by Joseph-Williams et al13: “once you use the big names, the well-respected consultants, people sit up and listen . . . that’s needed.” Scalia et al.58 reported how a champion orthopedic surgeon influenced colleagues by playing a significant role in PtDA development and demonstrating the benefit of using the tool. On the other hand, Berry et al.34 found that even when a designated lead was appointed, the absence of a clinical lead who is physically present in the clinic and seeing patients acted as a barrier to PtDA use.
IV: Characteristics of Individuals
Theory 4: Activating and supporting HCPs to deliver PtDAs: HCPs aware, trained, and motivated to change practice
4a: Awareness of PtDA purpose and intended use
When PtDAs are implemented in teams that do not understand the purpose of the PtDA or its intended use (C), they are less likely to use the PtDA as intended (O), because they do not understand the benefits for patients nor the PtDA role in supporting patient decision making (M). Introductory training sessions provided to all team members about the purpose of the PtDA and its benefits (for patients and HCPs [I]), helps teams decide how to integrate PtDAs into existing work practices (M), which in turn makes it more likely that the PtDA will be adopted in routine care (O).
Ten articles contributed data to support this theory.14,34,40,41,47,51,50,52,57,59 Implementation is unlikely to occur when teams are not familiar with PtDAs or lack awareness of the PtDA’s purpose and intended use. This was an important barrier to routine implementation. For example, one staff member in the study by Bonfils et al.47 of a mental health PtDA noted, “I think it’s underutilized because people don’t understand the richness of it . . . I don’t think they realize how much is on there [the PtDA].” When team members lack knowledge on why or how the PtDA should be used, they do not understand the benefits for patients or the role it plays in the decision-making process, which results in the PtDA being underused or misused.40,41,47 Conversely, when they are clear about the purpose and intended use, PtDA adoption is higher.50–52,59
4b: Supporting SDM skills development
When HCPs lack knowledge of SDM skills (C), they will be less likely to use the PtDA (O), as they do not have confidence in their SDM/PtDA delivery skills and/or they do not understand how SDM differs from usual practice and therefore do not understand why the PtDA needs to be used (M). SDM skills workshops delivered prior to PtDA implementation (I) provided opportunity for HCPs to practice and develop confidence in SDM/PtDA delivery skills, helping HCPs to understand how SDM differs from current practice and thus the importance of PtDAs (M), which results in increased use and proficiency in using PtDAs with patients (O).
Twelve articles contributed to this theory.13,40,41,44,45,48,50–53,57,59 PtDAs are sometimes implemented in settings where the HCPs lack knowledge of SDM skills and therefore do not understand how SDM differs from their current practice and thus the additional benefit of using PtDAs (over other educational resources). For instance, Joseph-Williams et al.13 found that when asked about SDM approaches, many HCPs report “we do it already.” On the other hand, if HCPs lack knowledge of SDM skills, they often lack confidence in their SDM/PtDA delivery skills, thus holding back from enacting the skills. SDM training workshops that incorporate methods for practicing skills (e.g., role-play scenarios) can help HCPs better understand how SDM builds on existing good health care communication practices, that SDM is an approach rather than another communication model, and enhance SDM skills. Lloyd et al.50 reported a significant change in attitudes among HCPs following the workshops; for example, as one nurse stated, “Initially when we started, like many of us, I thought ‘we do that anyway.’ I think the biggest difference is, well actually, we didn’t do it well.” These training opportunities encourage HCPs to reflect on their current practice and understand and agree on the role PtDAs can play in that, making it more likely that the PtDA will be used as intended.
Theory 5: Preparing and encouraging patients to use PtDAs—explicit invitations from clinical team to use PtDA before and during decision-making consultations
5a: Explicit invitations to use PtDA before decision-making consultations
Many patients have no experience of receiving or using a PtDA (C). When the clinical team sends explicit invitations (explaining the purpose/process of using PtDA and encouraging patients to engage with PtDA) to patients before the decision-making consultation (I), patients better understand the relevance and purpose of the PtDA, they perceive that their role in the decision-making process is valid and desired, and they are reminded to use the PtDA (M), thus making them more likely to actively engage with the PtDA and use it to help inform their decision with a HCP (O).
Eight articles provide support for this theory.13,14,34,36,39,51,52,59 Many patients are unfamiliar with PtDAs and have no experience of using them to support their health care decisions. When explicit invitations to engage with the PtDA are sent to patients before the decision-making consultation, this acts as a prompt for the patient and increases the likelihood that they will use the PtDA.34 For instance, Berry et al.34 found that PtDA use increased from 0% to 14% in sites that provided written material suggesting access, compared with 82% to 87% in sites in which patient care coordinators or physicians provided direct email or telephone invitations to engage. Both Dharod et al.36 and Krist et al.39 found that digital delivery of reminders and PtDAs via patient portals was a successful strategy to increase usage. Invitations that explain the purpose of the PtDA, and SDM more broadly, better prepare and “activate” patients as they help them to understand the relevance of the PtDA and their own role in decision making, resulting in increased engagement with the PtDA and in SDM discussions during consultations.13,14,52
5b: Explicit invitations to use PtDA during decision-making consultations
Significant power imbalances exist in some consultations, with many patients believing they cannot participate in SDM (C). When HCPs provide an explicit invitation during the decision-making consultation to further discuss the PtDA, preferably accompanied by handover of the PtDA (or duplicate copy, if delivered before consultation), (I) patients will feel that their contribution is valued and sought by the HCP and understand the relevance of the PtDA in the decision-making discussion (M), thus making them more likely to share their preferences, ask questions, and engage in decision making (thus using the PtDA for its intended purpose) (O).
Six articles support the theory that explicit invitations from HCPs for the patient to engage with the PtDA during decision-making consultations is important to ensuring the PtDA is used in the way intended (e.g., helping patients to understand their options and encouraging them to share their preferences with the HCP, ask questions, and engage in the decision-making process).13,14,41,49,51,53 Patients in the study by Joseph-Williams et al.13 reported that this explicit encouragement from HCPs during consultations provided “permission” for them to share their preferences and become involved, and the handover of the PtDA during the consultation meant it was used in the way intended: to guide questions for the clinician and to prompt them to share their preferences. Conversely, when opportunities to share preferences were not provided after receipt of a PtDA, it was not self-evident to patients that they could and should express their preferences.41
V: Process
Theory 6: Collaborative PtDA development and implementation planning: early and meaningful involvement of the clinical team and providers
HCPs and providers have preexisting approaches/processes to communicate options to patients (C). Early involvement of (or ideally, initiation by) clinical teams/providers in the development of the PtDA content/implementation planning (I) creates a sense of ownership, increases buy-in, helps to legitimize content, ensures the PtDA (content and delivery) supports current practice, and ensures that pathway redesign is considered with the PtDA in mind (M), making it more likely to be integrated into routine care (O).
Thirteen articles support this theory, which indicates that early involvement of those affected by change or the intended knowledge users was important in integrating a new PtDA into routine care.13,14,41,43,49,51,54,55,57–60 PtDAs were distributed in teams where HCPs had preestablished ways of communicating treatment and management options to patients, whether that be via verbal communication or existing educational resources, which HCPs often believed to be effective. When the team/providers collaboratively contributed to plans regarding PtDA focus, content, design, and proposed use from inception, this led to greater ownership and buy-in to the new approach,14,50,59 sometimes helping HCPs to understand how their existing approaches might not fully support SDM50,59; greater trust and legitimacy in the content of the tool50,59; and development of a tool that best fit within their setting, addressing concerns head on.49,50,51,55,59 The opportunity to adapt care pathways meant that the PtDA could fit better with ongoing and simultaneous processes. For example, one breast cancer clinic felt that their current “one-stop-shop,” whereby a patient received diagnosis and was asked to make a decision, would not support PtDA use. Instead, they adapted the pathway so that the patient could take the PtDA home following diagnosis and then discuss at a follow-up appointment.59 Conversely, lack of involvement of those affected by change resulted in less positive attitudes.54 These mechanisms were important in helping teams to adapt existing practices to integrate PtDAs into routine care.
Theory 7: Earlier distribution of PtDAs: systematizing delivery of PtDAs to eligible patients before decision-making consultations
In clinical environments with limited staff resources, short appointment times, or short time frames between diagnosis and decision discussion (C), preidentifying eligible patients and systematizing (ideally via information technology systems) the timely delivery, completion, and return of the PtDA to clinicians prior to decision-making consultations (I) decreases clinicians’ concerns about time to coordinate and do SDM and prompts PtDA/SDM use during consultations (M), improving reach and integration of the PtDA (O).
Fourteen articles contributed data to this theory.33–36,39,40,43–47,53,57,59 PtDAs are typically being implemented in settings characterized by limited staff resources, multiple competing demands and priorities, short appointment times, and, sometimes, short time frames within which to deliver the PtDA, so that it is feasible for the patient to use as intended and relevant. When teams were able to embed processes that could preidentify eligible patients and standardize (ideally automate) the delivery of the PtDA before the decision-making consultations, this resulted in improved reach of the PtDA to the right patient at the right time.34,39 Processes that support and standardize the 2-way delivery of information (i.e., returning patient preferences/questions to the HCP prior to the decision-making consultation) act as a prompt for the HCP to use the PtDA with the patient, meaning HCPs are more likely to integrate it into their consultation. Online delivery of PtDAs prior to the consultation also helps to overcome time limitations (i.e., if there is limited time available between identifying eligible patients and the decision-making consultation taking place).33,46 HCPs also perceived such processes would alleviate concerns regarding the time it would take to deliver the PtDA (e.g., “if you really want to use these kinds of Dboxes, and you want to make it work, I think you could organize it so you would have a pre-visit”).53
Theory 8: Linking with “learning health care systems”: using measurement to show how PtDA outcomes link with and improve key organizational priorities
When organizational priorities align with PtDA outcomes and a “learning health care system” (e.g., quality improvement practices/teams) exists within an organization (C), PtDAs should be implemented alongside routinely collected measures that the organization values (I). These measures demonstrate the improvements that result from using PtDAs and also demonstrate to clinical teams that the use of PtDAs are valued by the organization (M), which makes PtDAs more likely to be integrated into routine care (O).
Seven articles contribute data to this theory.13,41,50,51,52,56,59 Some studies were implementing PtDAs in contexts in which organizational priorities were aligned with principles of SDM and PtDA outcomes, and a learning health care system existed, whereby ‘science, informatics, incentives and culture are aligned for continuous improvement and innovation, with best practices seamlessly embedded in the delivery process and new knowledge captured as an integral by-product of the delivery experience.”18 When such a context exists, integration of routinely collected measures alongside the PtDA encouraged PtDA use and made it more likely to be sustained in routine care. Clinical team members frequently wanted to know whether SDM/PtDA use aligned with existing organizational priorities; as a head and neck consultant commented in the study by Lloyd et al.,50“if you haven’t got Board buy-in, if you haven’t got support from that level of management, then everything is an uphill struggle.” By implementing measures alongside PtDAs (tying in with existing routinely collected data or developing new measurements), the teams felt that an important part of PtDA use was to drive change and improvements, which motivated them to sustain implementation.13,41,50,56,59 The fact that these measures aligned with key organizational priorities showed HCPs that SDM was important, and they started to view it as something the organization does, rather than as another initiative being imposed on them and competing with other demands.13
Discussion
Principal Findings
We developed 8 refined theories using data from 23 implementation studies to describe the mechanisms by which PtDAs become successfully implemented into routine clinical settings and the implementation strategies and contexts that enable these mechanisms. The combined program theory shows that PtDAs are more successfully embedded in health care contexts that make it clear that SDM is an organizational priority, take ownership of PtDA implementation by appointing accountable leadership, coproduce PtDA implementation strategies with end-users, engage and inform the entire team (clinical and administrative) about the PtDAs’ purpose and intended use, recognize the significance every team member plays in PtDA implementation and distributes tasks appropriately, provides adequate SDM skills training for those delivering PtDAs, uses simpler tools that integrate into clinic workflows, and prepares and prompts patients to engage in the SDM process so they understand their role in the process and feel comfortable with having open and honest discussions. Using this program theory as a basis, we recommend key strategies that organizations and individuals intending to embed PtDAs routinely can use as a practical guide (see Table 3).
Table 3.
Strategies for Routine PtDA Implementation, Derived from Our Program Theory of Successful PtDA Implementation in Routine Health Care Settings
| What Does It Involve? | How Does It Help? | Examples of Studies |
|---|---|---|
| 1. Coproduction of PtDA content and processes or adapting existing PtDAs to a local level—designing tools and processes that fit everyone | ||
| Early and meaningful involvement of the intended knowledge
users in PtDA design and implementation planning, via a
coproductive approach. It moves beyond
seeking the input of HCPs/patients on PtDA design/content
(without consulting other core team members, e.g.,
administrative staff) or views on feasibility of intended
PtDA use after several versions of the PtDA have already
been developed. Meaningful involvement from the beginning of the design process: What is the problem our patients face in making decisions that are right for them? How can a PtDA help to address that problem (or is there a better way to promote SDM with these patients)? How can the PtDA be used and integrated into the care pathway, and what changes would we need to make? How can appropriate PtDA tasks be delegated to appropriate staff across the entire team? Views all end-users as equitable—recognizes the skills, abilities, time, and other qualities that they can bring to the PtDA design and implementation planning process61 and would involve everyone from patients and carers, HCPs, administrative staff, to health care managers. |
•Increases ownership, trust, and buy-in to the
PtDA •Leads to equitable division of tasks to appropriate team members •Allows teams to develop a tool that best fit within their setting, which addresses the needs of all end-users |
Dahl Steffensen 2018; Hsu 2016; Hsu 2013; Johnson 2010; Joseph-Williams 2017; Lin 2013; Lloyd 2013; Munro 2019; Savelberg 2019; Scalia 2017; Scalia 2018; Sepucha 2017; THF 2013 |
| 2. Training the entire team—explaining purpose, increasing understanding, developing skills | ||
| Training sessions delivered before PtDA implementation are
essential. SDM/PtDA training has typically focused on upskilling and improving knowledge for HCPs. Our findings highlight the importance of training for the entire team. Administrative staff are not just passive distributors of the tool; they play critical roles in preidentifying eligible patients, successfully integrating PtDAs into workflows, and championing PtDA use. Quite often, they will be the first person to introduce the concept of “choice” to patients, preparing them for PtDA use at the very start of their health care journey. However, they can only achieve this if training is delivered to the entire team and there is whole-team coherence regarding PtDA purpose and intended use. |
•Understanding of how PtDA is intended to be used and how it
fits in the patient pathway •All staff championing PtDA use •Improved integration of PtDA into workflows •Coherence on expected PtDA benefits •Reflection on existing practice and greater clarity of how SDM differs and where PtDAs fit in that process •Improved confidence in SDM/PtDA delivery |
Berry 2019; Bonfils 2018; Brinkman 2017; Dahl Steffensen 2018; Feibelman 2017; Giguere 2014; Joseph-Williams 2017; Lin 2013; Lloyd 2013; Mangla 2018; Munro 2019; Savelberg 2019; Scalia 2017; Stacey 2015; THF 2013 |
| 3. Preparing and prompting the patient to engage with the PtDA—a key 2-step approach | ||
| A 2-step process of preparing patients to engage
with the PtDA followed by a prompt to
engage with the PtDA is important in ensuring
PtDAs are used in the way intended (i.e., to inform patients
and support them to share their preferences with the
HCP). First step (preparing) involves sending an invitation to the patient before the consultation (with an accompanying PtDA, if feasible), informing them of the PtDA purpose and encouraging use. When patients are not prepared, they are less likely to understand the purpose of the PtDA and less likely to engage with it when presented to them during a consultation. Second step (prompting) involves an explicit reminder from the HCP during the consultation to engage with the PtDA (ideally accompanied by a duplicate copy if PtDA was distributed beforehand). When patients are not prompted, they are less likely to share their preferences, even if they had used the PtDA as intended prior to the consultation. Systematizing or automating invitation and PtDA delivery can help, but it is not always feasible (e.g., general practice) or desirable (e.g., sensitive and significant diagnosis) to preidentify patients ahead of their consultations. However, it is still possible to create a culture of preparedness and “permission” for involvement by distilling messages that patient involvement is valued and actively sought, e.g., through the use of general patient activation campaigns.9,59,62–64 |
Preparing •Increases patients’ understanding of the PtDA’s purpose, relevance, and their own role in the decision-making process •Reinforces that patient input is valued and desired •Reminder for patient to use (if sent before consultation) or engage with the PtDA (if delivered during consultation). •Prompting •“Permission” (as perceived by patients) for patients to share their preference •Further validates input in decision-making process •Encourages open and honest discussion of patient preferences |
Berry 2019; Dahl Steffensen 2018; Dharod 2019; Feibelman 2017; Giguere 2014; Johnson 2010; Joseph-Williams 2017; Krist 2017; Munro 2019; Savelberg 2019; THF 2013 |
| 4. Senior-level buy-in: “it’s what we do around here” | ||
| Demonstrable leadership from senior clinicians and managers
is important for successful PtDA implementation and also
sustainability of implementation. Although whole-team
engagement is important (see points 1 and 2 above), it is
important to identify a core leadership team early on, or at
least a “clinical champion,” who will take on the
responsibility for driving the work forward and maintaining
the impetus garnered during the earlier phases of
implementation. It is not intended as a top-down authoritative strategy, in which clinical teams are being told what to do by senior team members. It is intended as a facilitative and motivational strategy that supports the team, demonstrating that they are “in it together,” all contributing to a common goal, and have the necessary support to do so. |
•Provision of adequate training so team has necessary skill
set to use PtDAs •Ensuring PtDAs are prioritized and remain a priority in the team •Ensuring linkage between organizational priorities and PtDA outcomes •Facilitating feedback on PtDA outcomes and associated improvements for the team and their patients (see also point 5 below). •Conveys seriousness of intent; creates a sense that SDM / PtDAs is “what we do around here” |
Berry 2019; Bonfils 2018; Dahl Steffensen 2018; Feibelman 2017; Giguere 2014; Joseph-Williams 2017; Lin 2013; Lloyd 2013; MacDonald-Wilson 2017; Scalia 2017; Stacey 2018; THF 2013 |
| 5. Measuring to improve—linking PtDAs with routinely collected data to demonstrate improvement | ||
| Linking PtDA outcomes with measures that the organization
values is important for successful and sustained
implementation. When an organization can see the
improvements that result from using PtDAs, they are more
likely to become integrated into routine clinical care.
Ideally, a “learning health care system” will be in place,
which will support this. Implementation planners should work to understand the key priorities (at the patient, team, organizational, or national guideline/policy level) and link these with key reported PtDA benefits. Further, they should identify the data that are already being routinely collected and use this where possible, e.g., Patient Reported Experience Measures (PREMS) or Patient Reported Outcome Measures (PROMS). Specifically, designed measures can also be helpful in early stages of implementation; see Coulter (2018, pp. 24–27)9 for examples of both routine and special measures used in various countries. |
•When PtDA outcomes are linked with measures that are valued by the organization, it shows clinical teams that PtDAs are an important driver for change and improvement, making them more likely to be valued and embedded. | Feibelman 2017; Joseph-Williams 2017; Lloyd 2013; MacDonald-Wilson 2017; Munro 2019; Savelberg 2019; THF 2013 |
PtDA, patient decision aid; HCP, health care professional.
The fifth key strategy, “measuring to improve,” will not be sufficient if it focuses solely on measuring the uptake of PtDAs by patients, as this may result in tokenistic use of PtDAs and a focus on distribution rather than actual and meaningful engagement with the tool. Only a few benchmarks for PtDA uptake have been reported in the included studies (e.g., “80% of the staff uses a PtDA with at least 1 individual each month”), and so we lack insight into reasonable benchmarks for PtDA use. Most studies reported actual PtDA distribution (see Table 1); very few focused on how many patients actually used the PtDA. An additional challenge of focusing on uptake is that it fails to take into account legitimate reasons for the PtDA not being distributed. For example, it might be that the HCP has already informed the patient at an earlier consultation, or they have sought an alternative source of information given the patient’s low health literacy. Likewise, patients might also have several good reasons for not using the PtDA that has been offered to them.
Most of the contributing factors we identified relate to the “inner setting” (e.g., how the organization and the team view SDM and PtDAs, appropriate division of work, dedicated leadership), “characteristics of individuals” (e.g., do the team have the necessary skills and self-efficacy to use PtDAs, and are patients aware of their role and feel comfortable being involved?), and “process” CFIR domains (e.g., collaborative development and planning, earlier distribution of PtDA via automated systems).32 Other than complexity of PtDAs (theory 1), few CMOs mapped to the “intervention characteristics” domain. This might indicate that PtDAs as interventions are relatively well accepted in routine clinical settings, and thus, challenges with implementation are less likely to do with the tool itself but more the processes we use to embed the tools (e.g., timing of delivery, collaborative agreement on how they are introduced) and who they are used by (e.g., do they have the skills to introduce and use the PtDA effectively?). No CMOs mapped to the “outer setting” domain, or the external context within which the organization resides. This most likely reflects the recent emergence of such guidelines and processes and awareness of these, rather than them not playing a significant role in successful PtDA implementation. Indeed, elsewhere, the emergence of national governance and guidelines has acted as a key driver for SDM implementation (e.g., several NICE guidelines in the United Kingdom now recommend SDM supported by PtDAs).65 However, another core construct of the “outer setting” domain, “patient needs and resources,” has not been adequately addressed by this review. This signifies that the focus should move from describing “needs assessments” during the development of PtDA, and we should seek to understand patients’ needs and resources in relation to implementation (e.g., delivery modes, readiness for implementation, time, etc.).
Comparison with Other Literature
This review builds on and aligns with a body of work examining SDM implementation.6,30,66–69 More specifically, several contextual factors identified in this review align with factors described in the 2013 review.16 Adequately trained clinicians with the skills and confidence to deliver PtDAs proved to be significant facilitators in both reviews (theory 4), as did processes of systematizing earlier delivery of PtDAs to patients before health care consultations, when feasible (theory 7). The previous review also reported how distrust in content and lack of clarity of the PtDA’s purpose in relation to other sources of information acted as barriers. This review builds on these ideas by showing how contexts that use a collaborative and co-produced approach to PtDA development and implementation can overcome these barriers; by increasing a sense of ownership and buy-in, legitimizing content, and ensuring the PtDA content and delivery can fit with current practice (theory 6). Competing demands, time pressures, and poor teamwork also featured as barriers in the 2013 review.16 We build on these themes by showing how a whole-team approach, with appropriate distribution of PtDA tasks between clinical and administrative staff (theory 3), can help to overcome the dissociation of ownership of PtDA tasks and result in more successful integration of the tool. Further, we show how contexts with adequate and ongoing leadership for PtDA implementation help to overcome the tendency for busy clinicians to relegate the priority of PtDA distribution, by demonstrating how PtDA outcomes align with organizational priorities, monitoring progress and improvement, and providing motivation and the skill set needed to use the PtDA (theory 3c).
Significant contributions of the current review not covered previously include the important role of both preparing (either explicitly via earlier distribution of PtDA or implicitly through organizational messaging that patient involvement is valued) and prompting patients to engage with PtDAs (theory 5). This moves beyond the preparedness and engagement of clinicians covered previously16 and focuses more on the engagement of the main end user, the patient. The current review also highlights the importance of simpler PtDAs for busy and time-limited settings, likely demonstrating further support for brief in-consultation tools (theory 1), the challenge of balancing PtDA tasks with more immediate patient needs in crisis-driven and life-threatening situations (theory 2), and the importance of learning health care system contexts and linking PtDA outcomes with organizational priorities, thus improving integration (theory 8).
Waldron et al.70 have recently published a program theory of SDM. Although the focus of our realist review is on the implementation of one specific intervention (i.e., PtDAs) that can support the SDM process, there are some consistent observations across the 2 program theories. Notably, both reviews demonstrate that contexts that provide system-level support such as training, senior-level leadership, and organizational support are facilitative (theories 3c, 4, 8). We also found that self-efficacy and recognition of the decision were important mechanisms in play when contexts that provided adequate training (theory 4) and preparation and patient engagement opportunities (theory 5) existed. The perception of time mechanism identified by Waldron et al.70 also featured as a prominent mechanism in our theories and can be alleviated somewhat by whole-team approaches (theory 3) and systematization of PtDA delivery before clinical consultations (theory 7).
Strengths and Limitations
The RRR allowed us to understand the mechanisms by which PtDAs become routinely implemented into routine clinical settings and to draw on the expertise of a large international and multidisciplinary team of experts. Inclusivity of this review approach does remain an issue because of the rapid nature,26 but supplementary electronic searches did not identify significant additional papers for consideration, and the large author group was in agreement that no key studies have been missed. Given that exclusive implementation studies in this field are still relatively sparse, our exclusion of highly controlled trials and associated sibling studies might result in data that could contribute to our theories being missed. However, our inclusion of real-world implementation studies should make these findings more relevant to policy makers, organizations, and HCPs looking to implement PtDA in routine settings. Despite this, we acknowledge that these implementation studies typically involved willing volunteers, who were selected, for example, for their commitment to embed person-centered care approaches or prior success in a trial setting. As such, although the core strategies identified are likely to be valid in new implementation settings, their level of success might vary depending on preexisting attitudes and behaviors. Most of the included studies were also from high-income countries with well-established and well-resourced health care services, and so routine implementation in lower- or middle-income countries might look different. We were limited by the number of overall studies and the number of different contexts that examined PtDA implementation, and this impeded our ability to make more specific recommendations of which strategies worked best in which settings (e.g., in line with theory 3). We have limited or no information about what implementation looks like in emergency, pediatric, or end-of-life settings, for example, or for surrogate decision-making processes. We also have limited understanding of supporting patients with low health literacy and a general lack of inclusivity in PtDA approaches. As more implementation studies are conducted, researchers should pay attention to report the contexts and mechanism supportive of implementation; it would then be prudent to assess what works and does not work in those settings, to broaden our understanding of appropriate strategies that can be tailored according to specific contexts.
Practice Implications
Globally, health organizations are developing policies that encourage or mandate person-centered health care approaches when patients are faced with decisions about their health and care. Despite these efforts, limited guidance exists regarding the types of strategies that are likely to be the most effective in routine health care settings. Through this program theory development, we have been able to recommend key strategies that can support successful integration of PtDAs into routine clinical settings (Table 3). Building on existing work,16 this framework emphasizes the importance of training for entire teams, of better preparing patients to engage with SDM and PtDAs, and of linking PtDA outcomes with key organizational priorities and data collection (e.g., PROMS and PREMS). The strategies chosen by PtDA implementers should still ultimately depend on context and the key barriers anticipated in each setting; for example, the simplicity of PtDA design and delivery method would be more significant in settings with very limited time/human resources or limited flexibility in pathway design. This review was also not able to explore the added benefit of the more complex and harder-to-achieve strategies (e.g., strategy 1, co-production of interventions) over relatively more straightforward strategies (e.g., strategy 2, skills training for teams). Unit we have further outcome data reported by implementation studies, considerations of the feasibility and effort versus expected benefit are still needed when choosing strategies. We also fully acknowledge that PtDAs are only one means to improve SDM and that true SDM implementation would require a multifaceted user-centered plan, with interventions/approaches targeting attitudes, knowledge, skills, and self-efficacy of all end users, while also addressing key meso- and macro-level barriers.13,29 However, the proposed strategies may inform an initial framework and then be supplemented by more specific strategies depending on context and to also address the broader SDM goals.
Conclusions
The goal for this review was to identify why and how PtDAs become successfully implemented in routine health care settings. This study presents a program theory derived from implementation studies across a range of routine health care settings and recommended strategies that could be used as a practical guide by organizations and individuals attempting to embed PtDAs routinely. Further work is needed to understand the importance of context in the success of different implementation studies, as these studies become available.
Supplemental Material
Supplemental material, sj-doc-1-mdm-10.1177_0272989X20978208 for What Works in Implementing Patient Decision Aids in Routine Clinical Settings? A Rapid Realist Review and Update from the International Patient Decision Aid Standards Collaboration by Natalie Joseph-Williams, Purva Abhyankar, Laura Boland, Paulina Bravo, Alison T. Brenner, Suzanne Brodney, Angela Coulter, Anik Giguere, Aubri Hoffman, Mirjam Körner, Aisha Langford, France Légaré, Daniel Matlock, Nora Moumjid, Sarah Munro, Karina Dahl Steffensen, Christine Stirling and Trudy van der Weijden in Medical Decision Making
Supplemental material, sj-doc-2-mdm-10.1177_0272989X20978208 for What Works in Implementing Patient Decision Aids in Routine Clinical Settings? A Rapid Realist Review and Update from the International Patient Decision Aid Standards Collaboration by Natalie Joseph-Williams, Purva Abhyankar, Laura Boland, Paulina Bravo, Alison T. Brenner, Suzanne Brodney, Angela Coulter, Anik Giguere, Aubri Hoffman, Mirjam Körner, Aisha Langford, France Légaré, Daniel Matlock, Nora Moumjid, Sarah Munro, Karina Dahl Steffensen, Christine Stirling and Trudy van der Weijden in Medical Decision Making
Supplemental material, sj-doc-3-mdm-10.1177_0272989X20978208 for What Works in Implementing Patient Decision Aids in Routine Clinical Settings? A Rapid Realist Review and Update from the International Patient Decision Aid Standards Collaboration by Natalie Joseph-Williams, Purva Abhyankar, Laura Boland, Paulina Bravo, Alison T. Brenner, Suzanne Brodney, Angela Coulter, Anik Giguere, Aubri Hoffman, Mirjam Körner, Aisha Langford, France Légaré, Daniel Matlock, Nora Moumjid, Sarah Munro, Karina Dahl Steffensen, Christine Stirling and Trudy van der Weijden in Medical Decision Making
Acknowledgments
We are grateful for the assistance of the information specialist, Gregor Franssen Maastricht University, who supported the development of the electronic search strategies and to Hélène Elidor and Inheldia Cossou-Gbet (Université Laval) for their input into the data extraction phase. We also thank Dr. Freya Davies and Dr. Alison Cooper (Cardiff University) for their guidance on the realist review methodology.
Footnotes
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Brodney is currently supported in part by a grant from Healthwise, Inc., a nonprofit organization, to Massachusetts General Hospital.
The grant funding agreement ensured the author’s independence in designing this study, interpreting the data, writing, and publishing the report. All other authors declare that there are no conflicts of interest. The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Natalie Joseph-Williams  https://orcid.org/0000-0002-8944-2969
https://orcid.org/0000-0002-8944-2969
Laura Boland  https://orcid.org/0000-0001-8680-9844
https://orcid.org/0000-0001-8680-9844
Suzanne Brodney  https://orcid.org/0000-0002-4405-0726
https://orcid.org/0000-0002-4405-0726
Angela Coulter  https://orcid.org/0000-0002-6308-8375
https://orcid.org/0000-0002-6308-8375
Aubri Hoffman  https://orcid.org/0000-0003-4803-8668
https://orcid.org/0000-0003-4803-8668
Aisha Langford  https://orcid.org/0000-0003-1758-691X
https://orcid.org/0000-0003-1758-691X
France Légaré https://orcid.org/0000-0002-2296-6696
https://orcid.org/0000-0002-2296-6696
Daniel Matlock  https://orcid.org/0000-0001-9597-9642
https://orcid.org/0000-0001-9597-9642
Supplemental Material: Supplementary material for this article is available on the Medical Decision Making Web site at http://journals.sagepub.com/home/mdm.
Contributor Information
Natalie Joseph-Williams, School of Medicine, Cardiff University, Cardiff, UK.
Purva Abhyankar, Faculty of Health Sciences and Sport, University of Stirling, Stirling, UK.
Laura Boland, The Ottawa Hospital Research Institute, School of Health Sciences, Ottawa, Canada and Western University, School of Health Studies, London, ON, Canada.
Paulina Bravo, School of Nursing, Pontificia Universidad Católica de Chile, Santiago, Chile.
Alison T. Brenner, Division of General Medicine and Clinical Epidemiology, University of North Carolina Medical School, Chapel Hill, NC, USA, and Lineberger Comprehensive Cancer Center, University of North Carolina, Chapel Hill, NC, USA
Suzanne Brodney, Informed Medical Decisions Program, Massachusetts General Hospital, Boston, MA, USA.
Angela Coulter, Coulter & Coulter Ltd, Oxford, UK.
Anik Giguère, Department of Family Medicine and Emergency Medicine, Laval University, Quebec, Canada.
Aubri Hoffman, Department of Gynaecologic Oncology & Reproductive Medicine, The University of Texas MD Anderson Cancer Center, University of Texas, Houston, TX, USA.
Mirjam Körner, Medical Psychology and Medical Sociology, Medical Faculty, Albert-Ludwigs University, Freiburg, Germany.
Aisha Langford, Department of Population Health, NYU Grossman School of Medicine, New York, NY, USA.
France Légaré, Department of Family Medicine and Emergency Medicine, Université of Laval, Quebec, Canada.
Daniel Matlock, Department of Medicine, School of Medicine, University of Colorado, Aurora, CO, USA.
Nora Moumjid, Claude Bernard Lyon 1 University, Léon Bérard Cancer Centre, Lyon, Rhone-Alpes, France.
Sarah Munro, Department of Obstetrics and Gynaecology, University of British Columbia, Vancouver, BC, Canada.
Karina Dahl Steffensen, Center for Shared Decision Making, Region of Southern Denmark and Department of Clinical Oncology, Vejle/Lillebaelt University Hospital of Southern Denmark, Vejle, Denmark and Institute of Regional Health Research, Faculty of Health Sciences, University of Southern Denmark, Odense, Denmark.
Christine Stirling, School of Nursing, University of Tasmania, Hobart, Tasmania, Australia.
Trudy van der Weijden, CAPHRI Care and Public Health Research Institute, Department of Family Medicine, Faculty Health, Medicine and Life Sciences, Maastricht University, Maastricht, Limburg, The Netherlands.
References
- 1.Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scalia P, Durand M-A, Berkowitz JL, et al. The impact and utility of encounter patient decision aids: systematic review, meta-analysis and narrative synthesis. Patient Educ Couns. 2019;102(5):817–41. [DOI] [PubMed] [Google Scholar]
- 3.Calderwood C, Smith G, Baird A, et al. Realising realistic medicine: chief medical officer’s annual report 2015-2016. NHS Scotland; 2017. [Google Scholar]
- 4.Bradley P, Wilson A, Buss P, et al. Achieving prudent healthcare in NHS Wales. Welsh Government; 2014. [Google Scholar]
- 5.Secretary of State for Health. Equity and excellence: liberating the NHS. Stationary Office; 2010. [Google Scholar]
- 6.Härter M, Moumjid N, Cornuz J, Elwyn G, van der Weijden T.Shared decision making in 2017: international accomplishments in policy, research and implementation. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen. 2017;123–124:1–5. [DOI] [PubMed] [Google Scholar]
- 7.Knoepke CE, Allen LA, Kramer DB, Matlock DD. Medicare mandates for shared decision making in cardiovascular device placement. Circ Cardiovasc Qual Outcomes. 2019;12(7):e004899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The National Danish Multidisciplinary Cancer Groups. The National Danish Multidisciplinary Group Strategy 2020-2022. Aarhus, Denmark. 2020. Available from: https://www.dmcg.dk/siteassets/om-dmcg.dk/nyheder/dmcg-strategi/dmcg_strategi_2020-2022_final.pdf
- 9.Coulter A. National Strategies for Implementing Shared Decision Making. Gütersloh (Germany): Bertelsmann Stiftung; 2018. Available from: https://www.bertelsmann-stiftung.de/en/publications/publication/did/national-strategies-for-implementing-shared-decision-making-engl/ [Google Scholar]
- 10.Stacey D, Suwalska V, Boland L, Lewis KB, Presseau J, Thomson R. Are patient decision aids used in clinical practice after rigorous evaluation? A survey of trial authors. Med Decis Making. 2019;39(7):0272989X1986819. [DOI] [PubMed] [Google Scholar]
- 11.Fisher KA, Tan ASL, Matlock DD, Saver B, Mazor KM, Pieterse AH. Keeping the patient in the center: common challenges in the practice of shared decision making. Patient Educ Couns. 2018;101(12):2195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Veenendaal H, van der Weijden T, Ubbink DT, Stiggelbout AM, van Mierlo LA, Hilders CGJM. Accelerating implementation of shared decision-making in the Netherlands: an exploratory investigation. Patient Educ Couns. 2018;101(12):2097–104. [DOI] [PubMed] [Google Scholar]
- 13.Joseph-Williams N, Lloyd A, Edwards A, et al. Implementing shared decision making in the NHS: lessons from the MAGIC programme. BMJ. 2017;357:j1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahl Steffensen K, Vinter M, Cruger D, et al. Lessons in integrating shared decision-making into cancer care. J Oncol Pract. 2018;14(4):229–35. [DOI] [PubMed] [Google Scholar]
- 15.Légaré F, Adekpedjou R, Stacey D, et al. Interventions for increasing the use of shared decision making by healthcare professionals. Cochrane Database Syst Rev. 2018;7:CD006732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elwyn G, Scholl I, Tietbohl C, et al. “Many miles to go . . .”: a systematic review of the implementation of patient decision support interventions into routine clinical practice. BMC Med Inform Decis Making. 2013;13(suppl 2):S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barry MJ, Edgman-Levitan S. Shared decision making-pinnacle of patient-centered care. N Engl J Med. 2012;366(9):780–1. [DOI] [PubMed] [Google Scholar]
- 18.Friedberg MW, Van Busum K, Wexler R, Bowen M, Schneider EC. A demonstration of shared decision making in primary care highlights barriers to adoption and potential remedies. Health Aff. 2013;32(2):268–75. [DOI] [PubMed] [Google Scholar]
- 19.Dartmouth-Hitchcock. Center for shared decision making. Available from: https://med.dartmouth-hitchcock.org/csdm_toolkits.html. Accessed August 26, 2020.
- 20.Nilsen P. Making sense of implementation theories, models and frameworks. Implement Sci. 2015;10:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pawson R, Greenhalgh T, Harvey G, Walshe K. Realist review—a new method of systematic review designed for complex policy interventions. J Health Serv Res Policy. 2005;10(suppl 1):21–34. [DOI] [PubMed] [Google Scholar]
- 22.Elwyn G, O’Connor A, Stacey D, Volk R, Edwards A, Coulter A, et al. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ. 2006;333(7565):417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elwyn G, O’Connor AM, Bennett C, et al. Assessing the quality of decision support technologies using the International Patient Decision Aid Standards instrument (IPDASi). PLoS One. 2009;4(3):e4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.International Patient Decision Aids Standards Collaboration. Resources. Accessed April 21 2020. Available from: http://ipdas.ohri.ca/resources.html
- 25.Elwyn G, Scholl I, Tietbohl C, et al. The implementation of patient decision support interventions into routine clinical practice: a systematic review. 2013. Available from: http://ipdas.ohri.ca/IPDAS-Implementation.pdf. Accessed August 26, 2020. [DOI] [PMC free article] [PubMed]
- 26.Saul JE, Willis CD, Bitz J, Best A. A time-responsive tool for informing policy making: rapid realist review. Implement Sci. 2013;8:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong G, Greenhalgh T, Westhorp G, Buckingham J, Pawson R. RAMESES publication standards: realist syntheses. BMC Med. 2013;11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pawson R. Evidence-Based Policy: A Realist Perspective. London: SAGE; 2006. [Google Scholar]
- 29.Dahl Steffensen K, Hjelholt Baker V, Vinter MM. Implementing shared decision making in Denmark: first steps and future focus areas. Z Evid Fortbild Qual Gesundhwes. 2017;123:36–40. [DOI] [PubMed] [Google Scholar]
- 30.Légaré F, Ratté S, Gravel K, Graham ID. Barriers and facilitators to implementing shared decision-making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns. 2008;73(3):526–35. [DOI] [PubMed] [Google Scholar]
- 31.Pawson R, Tilley N. Realistic Evaluation. London: SAGE; 2008. [Google Scholar]
- 32.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belkora JK, Volz S, Teng AE, Moore DH, Loth MK, Sepucha KR. Impact of decision aids in a sustained implementation at a breast care center. Patient Educ Couns. 2012;86(2):195–204. [DOI] [PubMed] [Google Scholar]
- 34.Berry DL, Hong F, Halpenny B, et al. Evaluating clinical implementation approaches for prostate cancer decision support. Urol Pract. 2019;6(2):93–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brackett CD, Kearing S. Use of a web-based survey to facilitate shared decision making for patients eligible for cancer screening. Patient. 2015;8(2):171–7. [DOI] [PubMed] [Google Scholar]
- 36.Dharod A, Bellinger C, Foley K, Case LD, Miller D. The reach and feasibility of an interactive lung cancer screening decision aid delivered by patient portal. Appl Clin Inform. 2019;10(1):19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dontje K, Kelly-Blake K, Olomu A, et al. Nurse-led group visits support shared decision making in stable coronary artery disease. J Cardiovasc Nurs. 2013;28(3):269–76. [DOI] [PubMed] [Google Scholar]
- 38.Holmes-Rovner M, Kelly-Blake K, Dwamena F, et al. Shared decision making guidance reminders in practice (SDM-GRIP). Patient Educ Couns. 2011;85(2):219–24. [DOI] [PubMed] [Google Scholar]
- 39.Krist AH, Woolf SH, Hochheimer C, et al. Harnessing information technology to inform patients facing routine decisions: cancer screening as a test case. Ann Fam Med. 2017;15(3):217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin GA, Halley M, Rendle KAS, et al. An effort to spread decision aids in five California primary care practices yielded low distribution, highlighting hurdles. Health Aff. 2013;32(2):311–20. [DOI] [PubMed] [Google Scholar]
- 41.Savelberg W, Boersma LJ, Smidt M, Goossens MFJ, Hermanns R, van der Weijden T. Does lack of deeper understanding of shared decision making explains the suboptimal performance on crucial parts of it? An example from breast cancer care. Eur J Oncol Nurs. 2019;38:92–7. [DOI] [PubMed] [Google Scholar]
- 42.Savelberg-Pasmans W. Implementation of shared decision making in breast cancer care: towards placing the next pieces of the puzzle. PhD Thesis, Maastricht University; 2019. [Google Scholar]
- 43.Sepucha K, Atlas SJ, Chang Y, et al. Patient decision aids improve decision quality and patient experience and reduce surgical rates in routine orthopaedic care. J Bone Jt Surg Am. 2017;99(15):1253–60. [DOI] [PubMed] [Google Scholar]
- 44.Mangla M, Cha TD, Dorrwachter JM, et al. Increasing the use of patient decision AIDS in orthopaedic care: results of a quality improvement project. BMJ Qual Saf. 2018;27(5):347–54. [DOI] [PubMed] [Google Scholar]
- 45.Stacey D, Vandemheen KL, Hennessey R, et al. Implementation of a cystic fibrosis lung transplant referral patient decision aid in routine clinical practice: an observational study. Implement Sci. 2015;10(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stacey D, Taljaard M, Breau RH, et al. A patient decision aid for men with localized prostate cancer. Cancer Nurs. 2018;1:E10–21. [DOI] [PubMed] [Google Scholar]
- 47.Bonfils KA, Dreison KC, Luther L, et al. Implementing commonground in a community mental health center: lessons in a computerized decision support system. Psychiatr Rehabil J. 2018;41(3):216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brinkman WB, Lipstein EA, Taylor J, et al. Design and implementation of a decision aid for juvenile idiopathic arthritis medication choices. Pediatr Rheumatol. 2017;15(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnson SL, Kim YM, Church K. Towards client-centered counseling: development and testing of the WHO Decision-Making Tool. Patient Educ Couns. 2010;81(3):355–61. [DOI] [PubMed] [Google Scholar]
- 50.Lloyd A, Joseph-Williams N, Edwards A, Rix A, Elwyn G. Patchy “coherence”: using normalization process theory to evaluate a multi-faceted shared decision making implementation program (MAGIC). Implement Sci. 2013;8(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Munro S, Manski R, Donnelly K, et al. Investigation of factors influencing the implementation of two shared decision-making interventions in contraceptive care: a qualitative interview study among clinical and administrative staff. Implement Sci. 2019;14:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feibelmann S, Yang TS, Uzogara EE, Sepucha K. What does it take to have sustained use of decision aids? A programme evaluation for the Breast Cancer Initiative. Health Expect. 2011;14(suppl 1):85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giguere AMC, Labrecque M, Haynes RB, et al. Evidence summaries (decision boxes) to prepare clinicians for shared decision-making with patients: a mixed methods implementation study. Implement Sci. 2014;9:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hsu C, Liss DT, Westbrook EO, Arterburn D. Incorporating patient decision aids into standard clinical practice in an integrated delivery system. Med Decis Making. 2013;33(1):85–97. [DOI] [PubMed] [Google Scholar]
- 55.Hsu C, Liss DT, Frosch DL, Westbrook EO, Arterburn D. Exploring provider reactions to decision aid distribution and shared decision making: lessons from two specialties. Med Decis Making. 2016;37(1):113–26. [DOI] [PubMed] [Google Scholar]
- 56.MacDonald-Wilson KL. A successful implementation strategy to support adoption of decision making in mental health services. Community Ment Health. 2017;53:251–6. [DOI] [PubMed] [Google Scholar]
- 57.Scalia P, Elwyn G, Durand M-A. “Provoking conversations”: case studies of organizations where Option Grid™ decision aids have become ‘normalized’. BMC Med Inform Decis Making. 2017;17(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scalia P, Elwyn G, Barr P, et al. Exploring the use of Option Grid™ patient decision aids in a sample of clinics in Poland. Z Evid Fortbild Qual Gesundhwes. 2018;134:1–8. [DOI] [PubMed] [Google Scholar]
- 59.The Health Foundation. Implementing shared decision making: clinical teams’ experiences of implementing decision making as part of the MAGIC programme. 2013. Available from: www.health.org.uk/publications/the-magic-programme-evaluation/
- 60.Graham ID, Logan J, Bennett CL, et al. Physicians’ intentions and use of three patient decision aids. BMC Med Inform Decis Making. 2007;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Slay J, Robinson B. In This Together: Building Knowledge about Co-production. London: New Economics Foundation; 2011. [Google Scholar]
- 62.NHS Wales 1000 Lives Improvement Campaign. Making choices together—ask 3 questions. 2019. Available from: http://www.1000livesplus.wales.nhs.uk/making-choices-together. Accessed August 26, 2020.
- 63.Universitetssykehuset Nord-Norge. Hva er samvalg? 2017. Available from: https://www.youtube.com/watch?v=_FFlQa7SazM. Accessed August 26, 2020.
- 64.FIMDM. Shared decision making animated short. 2011. Available from: https://www.youtube.com/watch?v=XPm5iEDEI8Y. Accessed August 26, 2020.
- 65.National Institute for Health and Care Excellence. Shared decision making. Available from: https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/nice-guidelines/shared-decision-making [PubMed]
- 66.Gravel K, Légaré F, Graham ID. Barriers and facilitators to implementing shared decision-making in clinical practice: a systematic review of health professionals’ perceptions. Implement Sci. 2006;1:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Joseph-Williams N, Elwyn G, Edwards A. Knowledge is not power for patients: a systematic review and thematic synthesis of patient-reported barriers and facilitators to shared decision making. Patient Educ Couns. 2014;94(3):291–309. [DOI] [PubMed] [Google Scholar]
- 68.Wirrmann E, Askham J. Implementing patient decision aids in urology—final report. 2006. Picker Institute. Available from: https://www.researchgate.net/profile/Erica_Gadsby/publication/265433761_Implementing_Patient_Decision_Aids_in_Urology/links/54b509500cf26833efd05a52/Implementing-Patient-Decision-Aids-in-Urology.pdf. Accessed August 26, 2020. [Google Scholar]
- 69.O’Connor AM, Graham ID, Visser A. Implementing shared decision making in diverse health care systems: the role of patient decision aids. Patient Educ Couns. 2005;57(3):247–9. [DOI] [PubMed] [Google Scholar]
- 70.Waldron T, Carr T, McMullen L, Westhorp G, Duncan V, Neufeld S M, et al. Development of a program theory for shared decision-making: a realist synthesis. BMC Health Services Research 2020;20: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-doc-1-mdm-10.1177_0272989X20978208 for What Works in Implementing Patient Decision Aids in Routine Clinical Settings? A Rapid Realist Review and Update from the International Patient Decision Aid Standards Collaboration by Natalie Joseph-Williams, Purva Abhyankar, Laura Boland, Paulina Bravo, Alison T. Brenner, Suzanne Brodney, Angela Coulter, Anik Giguere, Aubri Hoffman, Mirjam Körner, Aisha Langford, France Légaré, Daniel Matlock, Nora Moumjid, Sarah Munro, Karina Dahl Steffensen, Christine Stirling and Trudy van der Weijden in Medical Decision Making
Supplemental material, sj-doc-2-mdm-10.1177_0272989X20978208 for What Works in Implementing Patient Decision Aids in Routine Clinical Settings? A Rapid Realist Review and Update from the International Patient Decision Aid Standards Collaboration by Natalie Joseph-Williams, Purva Abhyankar, Laura Boland, Paulina Bravo, Alison T. Brenner, Suzanne Brodney, Angela Coulter, Anik Giguere, Aubri Hoffman, Mirjam Körner, Aisha Langford, France Légaré, Daniel Matlock, Nora Moumjid, Sarah Munro, Karina Dahl Steffensen, Christine Stirling and Trudy van der Weijden in Medical Decision Making
Supplemental material, sj-doc-3-mdm-10.1177_0272989X20978208 for What Works in Implementing Patient Decision Aids in Routine Clinical Settings? A Rapid Realist Review and Update from the International Patient Decision Aid Standards Collaboration by Natalie Joseph-Williams, Purva Abhyankar, Laura Boland, Paulina Bravo, Alison T. Brenner, Suzanne Brodney, Angela Coulter, Anik Giguere, Aubri Hoffman, Mirjam Körner, Aisha Langford, France Légaré, Daniel Matlock, Nora Moumjid, Sarah Munro, Karina Dahl Steffensen, Christine Stirling and Trudy van der Weijden in Medical Decision Making