Abstract
Wearable technology refers to any sensor worn on the person, making continuous and remote monitoring available to many people with chronic disease, including multiple sclerosis (MS). Daily monitoring seems an ideal solution either as an outcome measure or as an adjunct to support rater-based monitoring in both clinical and research settings. There has been an increase in solutions that are available, yet there is little consensus on the most appropriate solution to use in either MS research or clinical practice. We completed a scoping review (using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) guidelines) to summarise the wearable solutions available in MS, to identify those approaches that could potentially be utilised in clinical trials, by evaluating the following: scalability, cost, patient adaptability and accuracy. We identified 35 unique products that measure gait, cognition, upper limb function, activity, mood and fatigue, with most of these solutions being phone applications.
Keywords: Multiple sclerosis, wearable technology, mHealth, biosensors, remote sensing technology, mobile applications
Introduction
Assessment of new treatments in multiple sclerosis (MS) studies necessitates effective, reliable and validated outcome measures. Most clinical MS outcome measures are rater dependent and are applied episodically.1 Clinical trials in MS require sensitive outcome measures, that can detect small changes in disability or functional improvement on a frequent basis, which can then reliably reflect long-term changes.2
With the advances in technology over the last few decades, it is now possible to explore methods of accurate, sensitive and objective continuous remote monitoring.3,4 The World Health Organization (WHO) defines mHealth as a medical and public health practice supported by mobile devices, such as mobile phones.5 Wearable technology is defined as incorporating a microprocessor and an Internet connection. Wearable technology, otherwise often referred to as wearables, are mobile technology solutions that can be worn by the person, as accessories or even embedded in clothing, and often include passive or active tracking capabilities which can be used to assess health and well-being.6 Our definition of wearable technology encompasses mHealth solutions because the growth of mobile networks (3G, 4G and 5G) has enabled the development of wearables. Wearables evolved from fitness activity trackers to wristwatches and the more robust mobile applications including Bluetooth headsets, smartwatches and smartphones. Common examples of these devices include the Fitbit® activity band and smartphone applications like MapMyRun®. Advancements in wearable technology and phone applications (‘apps’) enable continuous patient-based monitoring and provide feedback on daily life. The results of daily monitoring using wearable technology could be used either as an outcome measure or as an adjunct to support rater-based assessments. There has been an increase in solutions that are available for those who are diagnosed with chronic illness, especially in regard to neurological disease.7 Yet, there is little consensus on the most appropriate solution to use in either MS research or clinical practice.
Studies have shown that patients, caregivers and health care professionals find value in using such devices, especially when they are less invasive in day-to-day situations and provide real-time feedback.8,9 Currently, there are two randomised controlled trials (RCTs)10,11 that have utilised wearable technology in people with multiple sclerosis (PwMS); however, there are several more RCTs currently on-going that utilise wearable technology, for example, the MD3001 (SPI2) trial.12
The advantages and disadvantages of wearable technology are summarised in Table 1 above.
Table 1.
Potential advantages and disadvantages of wearable technology in trials.
| Advantages | Disadvantages |
|---|---|
| Continuous or frequent monitoring | Cost of device |
| Remote monitoring ability | Secure data storage |
| Less invasive | Local skin irritation |
| Decreased travel burden for participants | Troubleshooting device |
| Feedback to the participant | Charging and battery life of devices |
| Ease of use | Software upgrade incompatibility |
Software upgrades could be one of the biggest limitations to wearables produced by commercial entities. The upgrade also applies to device algorithms as manufacturers attempt to improve both parameter estimation and user satisfaction. This leads to a change in not only the appearance and behaviour of the device but also the algorithms used in logging and reporting the data.13 Maintaining a solution through a trial without a software update is difficult, and if an update is done, it could significantly change any wearable-related outcomes.
The rationale for conducting this scoping review was to understand each solution and its utility in MS. This review was commissioned by the UK MS Society on behalf of the Outcome Measures Working Group (part of the Expert Consortium on Progression in MS Clinical Trials, a UK MS Society initiative). We reviewed wearable technology solutions with a particular interest in their potential to detect changes in function for PwMS in a more reliable and accurate manner, and their suitability for use in a UK-wide multi-centre platform trial.
Objective
The objective was to identify all validated wearable solutions for PwMS and determine suitability for use in a UK-wide multi-centre platform trial by considering the following factors: reproducibility in MS populations, feasibility (including cost), patient adaptability and prior use in an RCT.
Methods
We used a scoping review approach which aims to map the key concepts underpinning a research area, especially where an area has not been reviewed comprehensively before (Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR)).14
Our search strategy utilised subject heading searches: ‘Multiple Sclerosis’ and ‘wearable electronic devices’, as well as keywords ‘wearable technology’ and ‘electronic devices’. The literature search was conducted using MEDLINE (via PubMed) and Embase (via OVID) databases. This search included articles published from database inception to 30 May 2019. Additional searches looked at authors who have frequently published with different devices as well as forward and backward citation tracking of included papers. The scoping review followed the PRISMA-ScR guidelines.15
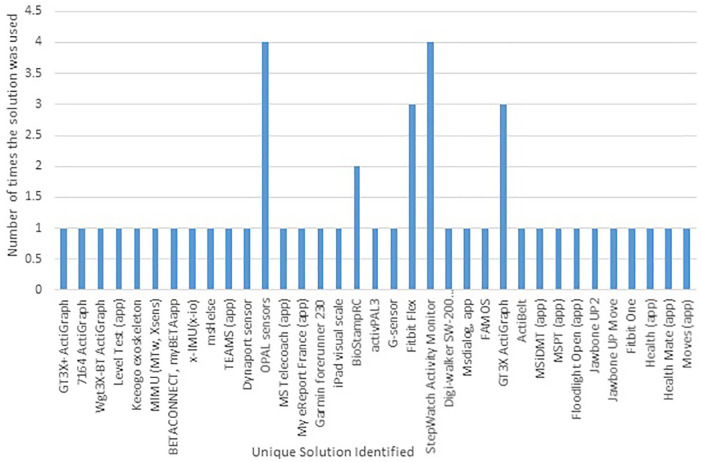
When examining suitability of a wearable device for use in a UK-wide multi-centre platform trial, we considered several factors: reproducibility (defined as the number of studies examining the solution in MS), feasibility (including cost), patient adaptability and prior use in an RCT. The results of this are shown in Figure 2 and Table 2.
Figure 2.
Unique devices and frequency of appearance.
Table 2.
Summary of unique solutions and their general characteristics.
| Wearable in use | Solution type | Cohort (n) | Type of study | Purpose | Functional area of assessment | Outcome | Test environment | Additional requirements for solution |
|---|---|---|---|---|---|---|---|---|
| ActiGraph wGT3X-BT10 | Hardware | 80 MS | RCT | Used to monitor activity levels in a trial to see effect of core exercise on PwMS | Activity level lower limb | Core exercises helped PwMS increase activity levels | At home | Not available |
| My eReport France (private app)11 | Software | 180 MS | RCT | Mobile phone application for drug adverse reaction reporting by patients with RRMS in an RCT | Self-management | Study not completed and results not yet available | At home | Apple iPhone, iPad or iTouch and requires iOS 7.1 or
later Android OS 3.0 and up |
| BETACONNECT, myBETAapp16 | Hardware, software | >100 MS | Interventional | Bayer produced app to assist the BETACONNECT auto injector and to help self-manage symptoms and dose | Injection/self-management | Shown to be an effective patient self-management tool for disease-modifying therapy alongside auto injector | At home | BETACONNECT auto injector IPhone iOS 9.0 or later Android 4.4 and up |
| Fitbit Flex17 | Hardware | 95 MS | Interventional | To assess continuous step count activity remotely among PwMS for 1 year and determine how average daily step count is associated with other measures of MS disability | Lower limb | Appears to be feasible to monitor for 1 year. It showed a decrease in average daily step count correlated to worsening of standard ambulatory measures | At home | IPhone iOS 12.2 or higher Android OS 7.0 or higher |
| Keeogo exoskeleton18 | Hardware | 29 MS | Interventional | An exoskeleton for lower extremity to enable PwMS to benefit from exercise and physical activity | Lower limb | Lower extremity exoskeleton, exercise-mediated benefit to PwMS that improves unassisted gait endurance and stair climbing | At home and in controlled clinic setting | Nil |
| TEAMS (private app)19 | Software | 10 MS | Observational | A tailored telerehabilitation app: Participant-Centered Development and Usability Study | Activity level | App that allows for PwMS to follow an exercise programme and showed good usability. Currently being studied in an RCT | At home | Android iOS phone or tablet Adjustable floor stand |
| StepWatch Activity Monitor20 | Hardware | 64 MS | Observational | Understanding walking activity in MS: step count, walking intensity and uninterrupted walking activity duration related to degree of disability | Activity level | Results showed everyday walking in PwMS was not high and that PwMS rarely walk for more than 6 minutes uninterrupted | At home | Nil |
| Digi-walker SW-200 pedometer, Jawbone UP2, Jawbone UP Move, Fitbit Flex and Fitbit One AND Health app, Health Mate, Moves21 | Hardware, hardware, hardware, hardware, software, software, software | 45 MS | Observational | This study examined the accuracy and precision of common smartphone applications and motion sensors for measuring steps taken by MS patients while walking on a treadmill | Activity level | Fitbit One was the best and most precise solutions for measuring steps but more research is needed before inclusion in clinical research | In controlled clinic setting | Additional software required on a smart device |
| StepWatch Activity Monitor, GT3X+ ActiGraph22 | Hardware, hardware | 63 MS | Observational | Accuracy of StepWatch and ActiGraph accelerometers for measuring steps taken among persons with MS | Activity level | Results showed that the StepWatch was a more accurate choice of accelerometer, especially in those with higher disability levels | In controlled clinic setting | ActiLife 6 software |
| 7164 and GT3X ActiGraph23 | Hardware | 41 MS, 41 HC | Observational | Comparison of ActiGraph activity monitors in persons with MS and controls | Activity level | There was enough discrepancy between the two models to show they are not interchangeable under free-living conditions | At home and in controlled clinic setting | Personal computer with USB 2.0 connection |
| StepWatch Step Activity Monitor (SAM)24 | Hardware | 9 MS | Observational | Validity of the StepWatch SAM: preliminary findings for use in persons with Parkinson disease and MS | Activity level | Results showed that the StepWatch accurately measured step count for those with MS | In controlled clinic setting | Data are downloaded to a host computer via a docking station |
| ActiBelt25 | Hardware | 30 MS | Observational | Creating a robust and autonomous measurement device for long-term monitoring of patient activity | Activity level | Preliminary results are promising but the algorithm needs to be modified to allow for more sensitivity | In controlled clinic setting | Nil |
| Private app26 | Software | 22 MS, 17 HC | Observational | This study illustrated some of the novel approaches that smartphones provide to monitor MS patients in their natural setting | Activity level Cognition Fatigue |
Results show the feasibility of and barriers to deploying a smartphone platform to gather passive and active data. Overall positive data show smartphone platform may therefore enable large-scale studies of PwMS | At home | Android HTC 4G Smartphone |
| MS TeleCoach (Private app)27 | Software | 75 MS | Observational | Assess feasibility of the MS TeleCoach offering telemonitoring of fatigue, telecoaching of physical activity and energy management over a 12-week period | Activity level Fatigue |
Intervention was well accepted and shows use of MS TeleCoach as a self-management tool in PwMS suffering from mild disability and moderate to severe fatigue appeared to be feasible | At home | Not available |
| activPAL328 | Hardware | 20 MS | Observational | Validity of the activPAL3 activity monitor in people moderately affected by MS | Activity level Lower limb |
Good for moderately affected PwMS – slow walking cadence produced large inaccuracies | In controlled clinic setting | ActivPAL Professional Software version 7.2.23 |
| Fitbit Flex, GT3X ActiGraph29 | Hardware, hardware | 99 MS | Observational | Continuous daily assessment of MS disability using remote step count monitoring | Activity level Lower limb |
Evaluated in an RMS and PMS cohort and shown to support remote step count monitoring as an exploratory outcome in MS trials | At home and in controlled clinic setting | Fitbit flex software |
| Phone app (private)30 | Software | 5 MS | Observational | Self-management phone app for PwMS to capture data about symptoms, physical activities, mood and goals | Activity level Mood |
Created a low-fidelity prototype and the high-fidelity prototype is being built to be further evaluated | At home | Not available |
| Floodlight Open31 | Software | 76 MS, 25 HC | Observational | Testing feasibility of remote active testing and passive monitoring using smartphones over 24 weeks. These tests included Cognition (SDMT), Upper Limb (Pinching test and Draw a Shape), Gait and Posture (25FW, 5UTT), Daily mood questionnaires and symptom tracker | Balance Cognition Lower limb Upper limb QoL |
Floodlight Open showed strong correlations to the SDMT, 9HPT and 25FW. Older version of the app (FLOODLIGHT) also included the MSIS-29 which had a good correlation; however, this module is unavailable for the Floodlight Open model | At home and in controlled clinic setting | Apple: Requires iOS 11.0 or later. Compatible with iPhone, iPad,
and iPod touch Android: Requires OS 7.0 and up |
| MSPT32 | Software | 51 MS, 49 HC | Observational | The Multiple Sclerosis Performance Test and iPad-based disability assessment tool. Includes five performance modules: Walking speed to simulate walking speed test (WST), balance test, Manual Dexterity Test for upper limb function, Processing Speed Test to simulate the SDMT, low-contrast letter acuity test designed to simulate the Sloan LCLA test | Balance Cognition Lower limb Upper limb Visual |
Well accepted by patients and strong correlations to their respective standard neurological test. Currently being tested for use in a clinical setting | In controlled clinic setting | Apple iPad (no other details) |
| MSiDMT (private)33 | Software | 40 MS | Observational | iPad-based SDMT test, testing correlation of the iPad solution to the SDMT | Cognition | There was a strong correlation between the MSiDMT and the SDMT and it was well accepted by PwMS | In controlled clinic | Apple iPad (no other details) |
| Level Test (private app)34 | Software | 29 MS | Observational | Smartphone embedded sensors to measure various neurological functions using gamification | Cognition | Measured outcomes showed moderate correlations with various clinical scales and components of neurological examination. Certain level tests discriminated MS from HC | In controlled clinic setting | Android 8.1 Oreo OS and up |
| Activity monitor (private)35 | Hardware | 21 MS | Observational | Examine the impact of MS disability on physical activity behaviours involving ambulation | Lower limb | The activity monitor showed that daily step strongly related to gait and balance measures. EDSS and MSWS scores also strongly related to daily step count | At home and in controlled clinic setting | Nil |
| MIMU (MTw, Xsens)36 | Hardware | 20 HC and 10 MS | Observational | Using sensors to try and detect gait characteristics while ascending stairs | Lower limb | Detected MS-specific gait patterns that would not be picked up otherwise | In controlled clinic setting | Parameters computed from MIMU signals using MATLAB |
| x-IMU(x-io)37 | Hardware | 17 MS, 23 HC | Observational | Quantifying mobility impairment in MS, with a thigh-derived inertial sensor metric to assess the sit-to stand and stand-to-sit transitions in the Timed Up and Go task | Lower limb | Shown to be effective at differentiating between HC and MS | In controlled clinic setting | Micro SD cards to transfer data to a computer Binary file converter software |
| Dynaport sensor, OPAL sensors38 | Hardware, hardware | 14 MS | Observational | Using sensors to quantify gait characteristics and gait deficits from prolonged daily living measurements | Lower limb | Validated a method to quantify walking in real life in PwMS | At home and in controlled clinic setting | Nil |
| OPAL sensors39 | Hardware | 12 MS, 12 HC | Observational | Using sensors, to quantify head and pelvis movement patterns that occur in PwMS with disability and determine how these secondary gait compensations impact on gait stability | Lower limb | Efficient way to screen for excessive compensatory movements and provides information that impacts mobility, stride time, gait stability. Good for identifying high risk of falls | In controlled clinic setting | Custom software in MATLAB and developer protocols |
| Garmin forerunner 23040 | Hardware | 73 MS | Observational | Evaluate the agreement between patients’ and neurologists’ estimates of maximum walking ability and patients’ mean maximum walking ability measured in daily life using GPS smartwatch | Lower limb | Confirmed patient estimate of distance walked is poor and shows that remote monitoring is a good way forward | At home | Not available |
| BioStampRC41 | Hardware | 30 MS | Observational | A machine learning approach for gait speed estimation using skin-mounted wearable sensors | Lower limb | Results support the use of wearable accelerometer arrays for estimating walking speed in normal subjects and their extension to MS patient cohorts with gait impairment | In controlled clinic setting | BioStampRC Investigator Application |
| OPAL sensors42 | Hardware | 52 MS, 21 HC | Observational | Validation study of the Instrumented Push and Release Test to quantify postural responses in persons with MS | Lower limb | Demonstrated strong agreement and correlation between sensor-based metrics and gold standard laboratory measurements. Several metrics were shown to be different between PwMS and HC | In controlled clinic setting | Custom MATLAB algorithm |
| BioStampRC, GT3X ActiGraph43 | Hardware | 45 MS, 15 HC | Observational | Monitoring gait in MS with novel wearable motion sensors | Lower limb | Results showed that the BioStamp is the most accurate device | In controlled clinic setting | Custom-developed MATLAB algorithm |
| G-sensor44 | Hardware | 105 MS, 47 HC | Observational | This study aims to verify the feasibility of using wearable accelerometers in an ambulatory environment to assess spatiotemporal parameters of gait in PwMS, as well as the correlation of objective data with patient-reported outcomes | Lower limb | Wearable accelerometers are a useful tool for assessing gait performance in PwMS in a clinical setting, especially in mild to moderate disability | In controlled clinic setting | BTS Bioengineering G-Studio® computer software |
| OPAL sensors45 | Hardware | 5 MS, 13 HC | Observational | To determine whether gyroscopic corrections improve wearable sensor data prior to measuring dynamic sway in the gait of PwMS | Lower limb | The visualisation of asymmetric pelvic sway in PwMS illustrates the potential to better understand their mobility impairments for reducing fall risk | In controlled clinic setting | Custom software |
| MSdialog, app46 | Software | 76 MS | Observational | A web- and mobile-based software application, captures data on self-administration of subcutaneous interferon β-1a, clinical outcomes and patient-reported outcomes in patients with MS outside the clinic | Self-management | Well accepted by users and user retention was high over 6 weeks. PwMS found it easy to use and superior to previous methods for tracking health | At home | MSdialog web-based software and optional phone- and tablet-based application |
| FAMOS (private)47 | Hardware | 17 MS, 9 HC | Observational | A wireless body measurement system to study fatigue in MS | Fatigue | Preliminary results show significant differences between fatigued PwMS and HCs. This provides a new approach to study fatigue in MS but needs to be validated through larger clinical trials | At home | Custom LabVIEW software |
| iPad visual scale (private)48 | Software | 52 MS, 52 HC | Observational | To explore the reliability and feasibility of electronic visual analogue scales in PwMS and health individuals | Fatigue QoL |
Reliable and useful for PwMS to register fatigue, pain, anxiety and QOL | In controlled clinic setting | Smartphone (Huawei Ascend G6) Tablet (Samsung Galaxy Tab S 10.5) |
| Phone app (private)49 | Software | 76 MS, 19 HC | Observational | Two smartphone tests of fine finger movements to see if correlated with neurological examination | Upper limb | Good correlation with 9HPT and captured richer data than traditional measures | At home and in controlled clinic setting | Android 8.1 Oreo OS and up |
MS: multiple sclerosis; HC: healthy control; RMS: relapsing multiple sclerosis; RRMS: relapsing remitting multiple sclerosis; PMS: progressive multiple sclerosis; PwMS: people with multiple sclerosis; RCT: randomised controlled trial; SDMT: Single Digit Modality Test; 25FW: 25 Foot Walk; 5UTT: 5 U-Turn Test; MSIS-29: Multiple Sclerosis Impact Scale; WST: Walking Speed Test; EDSS: Expanded Disability Status Scale; MSWS: Multiple Sclerosis Walking Scale; QoL: quality of life; 9HPT: 9-Hole Peg Test; Sloan LCLA: Sloan Low-Contrast Letter Acuity; GPS: Global Positioning System.
Study eligibility criteria
The inclusion criteria were defined as (1) primary research studies that used wearable technology in a cohort of PwMS of all ages (adult or paediatric), (2) studies from any geographical location and (3) reported in the English language. Exclusion criteria were defined as (1) wearable solutions intended for another health condition, (2) non-wearable solutions, (3) non-primary research such as narrative reviews and (4) abstracts that did not have full-text available.
After screening titles and abstracts, duplicates were removed and the full text of each paper was assessed for eligibility according to the criteria stated above.
Data extraction
The data to be extracted for each article were determined in consultation with the second author (G.P.) and a data extraction form was created. Descriptive characteristics were extracted where available for (1) wearable device, (2) cohort, (3) type of study, (4) purpose, (5) functional area of assessment and (6) outcome.
Developers of the wearable technology were separated into the following categories:
Health care–related agency: Hospitals, clinics or government organisations directly related to health care
Pharmaceutical company: entities with commercial purposes to research, develop, market or distribute drugs in the context of health care
Educational organisation: any educational organisations such as universities, colleges, libraries or schools not directly related to health care
Small and medium enterprises: start-ups, software developing companies or any other private organisation that identified themselves as an enterprise and not individuals
Results
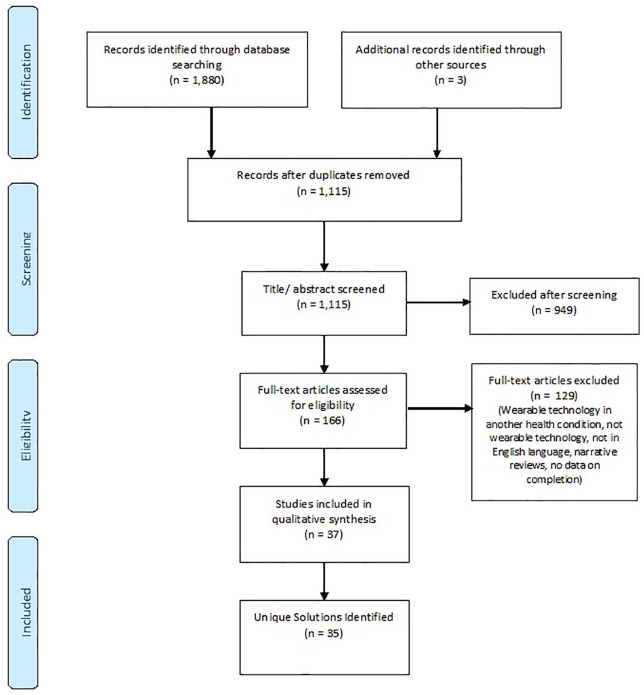
The searches yielded 1880 potentially relevant articles. Removing duplicates and applying the eligibility criteria resulted in a total of 35 unique MS wearable technology solutions, which included 3 unique solutions that were yielded from conferences and scientific meetings. Figure 1 describes the PRISMA flow diagram.
Figure 1.
PRISMA flow diagram.53
The list of the included wearable technology solutions and the frequency with which they appeared in validated studies is shown in Figure 2. A majority of the solutions that were used in studies in PwMS were applications (apps), accelerometers and activity monitors. The older studies predominantly focused on measuring activity, walking or gait since activity monitors, accelerometers and gyroscopes were the most readily available and advanced technology at the time. This result is not unexpected because the nature of MS disease progression would require sensors focused on assessments based on activity and function, both easily derived from accelerometers. Within the solutions available for monitoring activity levels, there is great variability between the outcomes available. Certain solutions provide a basic step count for example, Fitbit, whereas others provide additional metrics such as stride length and gait characteristics. It is important to determine the key outcome measures of interest to best guide which wearable to use. Included are four unique app solutions for cognition, which were all created more recently, as apps are becoming an easier wearable technology to develop and deploy. There were a handful of wearables that focused on fatigue, mood, quality of life (QoL) and self-management.
A summary of the general characteristics of the unique wearable technology solutions found is shown in Table 2.
There is significant variability in the per-unit cost of each product, and the decision as to which wearable to use depends largely on the study budget and outcomes of interest. Costs may vary significantly when using a physical wearable sensor compared to a smart-device application. Aside from per-unit cost, other considerations include repair or replacement of faulty devices, annual maintenance charges, software package costs, return of devices, charging capabilities and collection of data (postage vs. remote upload). Physical wearable sensors risk being ‘phased out’ and being replaced with newer models that have not been tested in an MS population. Applications may alleviate this problem by sending out software updates, which the user can download. Users could however face problems if this update exceeds the smart device support capability.
There does seem to be a shift towards developing more validated wearable technology solutions for MS and focusing on health care adoption to make sure that dissemination of the solutions is more successful and reaches a wider population. This is seen by the increase in the number of publications related to the subject of wearable technology in MS. In addition, as a result of an increased number of solutions being validated, wearable technologies are now becoming more utilised in RCTs. At the time of writing this review, we had identified two RCTs that utilise wearable technology; however, we are aware of at least one other RCT which was published using wearable technology in MS.50 We believe that this study did not appear in our literature search, because the term ‘wearables’ or ‘mHealth’ is not used in the paper nor is it referred to in the keywords or subject headings. We acknowledge that our search terms may have excluded other studies involving wearable technology, in MS. We are also aware of several other RCTs that are currently being run, that employ wearable technology such as the SPI2 and TEAMS studies.51,52
While doing this review, we identified 10 (27%) solutions that we classed as ‘private’ solutions as they had been created, tested and not available to the public, as shown in Table 2. This seems to have happened for various reasons, including not having the necessary resources to further validate or improve the solution or not having enough resources to gain regulatory approval. All the identified solutions that are private and unavailable were created by health care or educational organisations. Ideally, independent validation prior to clinical or research use seems appropriate; however, this may not be feasible due to on-going costs. When comparing this to the solutions created or funded by pharmaceutical companies, for example, the FLOODLIGHT app and the MSPT, it was shown that 98% of the solutions created by pharmaceutical companies were successfully implemented and disseminated, as they had enough resources to manage the on-going cost and effort required to gain regulatory approval and market the products.
Wearables are gaining importance in MS; however, there are many lessons to be learnt from its use in other chronic neurological disorders, such as Parkinson’s disease.54 While the promise of unsupervised assessments is alluring, and could save time and cost, using these devices in clinical and research settings is far from seamless due to several issues. Many wearable devices have not been validated or approved for clinical use in people with Parkinson’s disease. In addition, as gold standards are variable and sometimes scarce, unsupervised patient monitoring also brings new challenges (e.g. using a patients’ diary to validate devices capturing motor fluctuations).55,56
Limiting factors to consider when developing wearable technology are adherence and usability by both the participant and the researcher. PwMS have shown a high level of acceptance when using smartphone applications (apps), although this may wear off as the disease progresses due to increased disability (e.g. decreased hand dexterity).8 Factors to consider when designing a solution are convenience, placement of the wearable device, appearance of the sensor and feedback of results to the PwMS. Patient feedback is extremely important in keeping PwMS interested and engaged in their own health. Many solutions have opted for patient-friendly readouts, while more complex data and parameters are available to the respective clinicians and researchers.57 With regard to usability by the researcher, issues to consider include troubleshooting hardware and software, technical support and ease of implementation with the participant.
Another limiting factor, from a UK perspective, was the approach from regulatory bodies. Stricter guidelines determined what was seen as a medical device or classed as an observational tool. New guidelines which have recently come into effect, such as the new Evidence Standards Framework by the National Institute for Health and Care Excellence (NICE) in March 2019 and the Regulation (EU) 2017/745 by the European Parliament (May 2020), will also work to create stricter guidance for patient safety, security monitoring and data security. Many solutions that are available elsewhere have struggled to implement themselves outside of research in the United Kingdom due to these guidelines.
Limitations
Although we used a detailed process to search and document the currently validated solutions in MS, there were several limitations. The nature of this scoping report was web search based and, thus, relied on university subscription to journals to access the papers, although only 5 of 1115 titles screened could not be accessed in this way. The inclusion of only English language papers may also be considered a limitation.
Conclusion
In the coming years, we can expect to see more sensitive and comprehensive measures being developed, with the idea of using wearable solutions perhaps as the gold standard to measure outcome measures in studies and clinical practice. However, at present, guidelines on what wearable technology should be used in clinical practice and research are absent, and this is an area that will require considerable attention and stringency.
While doing the review, we came across many unvalidated solutions available for PwMS across a range of outcome measures, most of them being phone or iPad solutions. We classed a solution as ‘unvalidated’ if there was an inability to demonstrate test–retest reliability and/or failure to demonstrate difference between healthy controls and PwMS. In comparison, the validated solutions are rather limited, but are most advanced particularly in measurements of gait (characteristics) and balance. These solutions often provide the greatest accuracy and acceptance rate, as gait is one of the earliest outcome measures explored in MS wearable solutions.
Also with the advances in mobile technology, more solutions are focusing on utilising common wearables such as smartphones, smartwatches and tablets, to increase accessibility and minimise costs to the user. Looking forward, there is also a change occurring from single-measure solutions to multi-measure and multi-sensor solutions, such as the Floodlight Open app, which utilises multiple sensors within a smartphone to remotely measure gait, cognition and upper limb function.
As development in wearable technology in MS is still on-going, we can expect to see newer solutions focusing on other areas with technology advancements that allow for more upper body and cognitive measures. There is a dearth of validated solutions available for fatigue, mood and pain.
The future of wearable technology in MS therefore looks promising with the potential to become a primary, co-primary or adjunctive monitoring tool in research and clinical practice.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: S.A. does not have any disclosures. G.P. has received consultancy sums from the MS Society and the Eastern Academic Health Science Network and paid employment from the National Institute for Health Research (NIHR) East of England Applied Research Collaboration. E.G. does not have any disclosures. F.B. is a board member for Neurology, Brain, Radiology and Multiple Sclerosis Journal (MSJ) and a section editor for Neuroradiology. He has also received personal fees from Springer, Bayer, Biogen, IXICO Ltd, GeNeuro and Roche as well as grants from Novartis, TEVA, Merck, Biogen, IMI-EU, GE Healthcare, UK MS Society, Dutch Foundation MS Research, NWO and NIHR, outside the submitted work. In the last 3 years, J.C. has received support from the Efficacy and Evaluation (EME) Programme, a Medical Research Council (MRC) and NIHR partnership and the Health Technology Assessment (HTA) Programme (NIHR), the UK MS Society, the US National MS Society and the Rosetrees Trust. He has been a local principal investigator for a trial in MS funded by the Canadian MS society. A local principal investigator for commercial trials funded by Actelion, Biogen, Novartis and Roche; has received an investigator grant from Novartis; and has taken part in advisory boards/consultancy for Azadyne, Biogen, Celgene, MedDay, Merck and Roche.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This study was supported by the MS Society and the Expert Consortium for Progression in MS Clinical Trials: Outcome Measures Working Group.
ORCID iDs: Sarah Alexander  https://orcid.org/0000-0002-3680-1120
https://orcid.org/0000-0002-3680-1120
Frederik Barkhof  https://orcid.org/0000-0003-3543-3706
https://orcid.org/0000-0003-3543-3706
Contributor Information
Sarah Alexander, Queen Square MS Centre and Department of Neuroinflammation, UCL Queen Square Institute of Neurology, Faculty of Brain Sciences, University College London, London, UK.
Guy Peryer, School of Health Sciences, University of East Anglia, Norwich, UK.
Emma Gray, The Multiple Sclerosis Society, London, UK.
Frederik Barkhof, Queen Square MS Centre and Department of Neuroinflammation, UCL Queen Square Institute of Neurology, Faculty of Brain Sciences, University College London, London, UK/Centre for Medical Image Computing (CMIC), Department of Medical Physics and Biomedical Engineering, University College London, London, UK/National Institute for Health Research (NIHR), Biomedical Research Centre, University College London Hospitals (UCLH), London, UK/Department of Radiology and Nuclear Medicine, VU University Medical Centre, Amsterdam, The Netherlands.
Jeremy Chataway, Queen Square MS Centre and Department of Neuroinflammation, UCL Queen Square Institute of Neurology, Faculty of Brain Sciences, University College London, London, UK/National Institute for Health Research (NIHR), Biomedical Research Centre, University College London Hospitals (UCLH), London, UK/MRC CTU at UCL, Institute of Clinical Trials and Methodology, University College London, London, UK.
References
- 1.Schwid SR, Goodman AD, Apatoff BR, et al. Are quantitative functional measures more sensitive to worsening MS than traditional measures? Neurology 2000; 55(12): 1901–1903, http://www.ncbi.nlm.nih.gov/pubmed/11134392 (accessed 4 September 2019). [DOI] [PubMed] [Google Scholar]
- 2.Goldman MD, Motl RW, Rudick RA.Possible clinical outcome measures for clinical trials in patients with multiple sclerosis. Ther Adv Neurol Disord 2010; 3(4): 229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malasinghe LP, Ramzan N, Dahal K.Remote patient monitoring: A comprehensive study. J Ambient Intell Humaniz Comput 2019; 10(1): 57–76. [Google Scholar]
- 4.Motl RW, McAuley E, Snook EM.Physical activity and multiple sclerosis: A meta-analysis. Mult Scler 2005; 11(4): 459–463. [DOI] [PubMed] [Google Scholar]
- 5.Galvez OM.Based on the findings of the second global survey on eHealth Global Observatory for eHealth series-Volume 3 mHealth New horizons for health through mobile technologies, 2011, http://www.who.int/about/ (accessed 16 May 2020).
- 6.Patel S, Park H, Bonato P, et al. A review of wearable sensors and systems with application in rehabilitation. J Neuroeng Rehabil 2012; 9(1): 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nasi G, Cucciniello M, Guerrazzi C.The role of mobile technologies in health care processes: The case of cancer supportive care. J Med Internet Res 2015; 17(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Apolinario-Hagen J, Menzel M, Hennemann S, et al. Acceptance of mobile health apps for disease management among people with multiple sclerosis: Web-based survey study. JMIR Form Res 2018; 2(2): e11977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marrie RA, Leung S, Tyry T, et al. Use of eHealth and mHealth technology by persons with multiple sclerosis. Mult Scler Relat Disord 2019; 27: 13–19. [DOI] [PubMed] [Google Scholar]
- 10.Arntzen EC, Straume B, Odeh F, et al. Group-based, individualized, comprehensive core stability and balance intervention provides immediate and long-term improvements in walking in individuals with multiple sclerosis: A randomized controlled trial. Physiother Res Int 2020; 25(1): e1798. [DOI] [PubMed] [Google Scholar]
- 11.Defer G, Le Caignec F, Fedrizzi S, et al. Dedicated mobile application for drug adverse reaction reporting by patients with relapsing remitting multiple sclerosis (Vigip-SEP study): Study protocol for a randomized controlled trial. Trials 2018; 19(1): 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tourbah A, Lebrun-Frenay C, Edan G, et al. MD1003 (high-dose biotin) for the treatment of progressive multiple sclerosis: A randomised, double-blind, placebo-controlled study. Mult Scler 2016; 22(13): 1719–1731, http://www.ncbi.nlm.nih.gov/pubmed/26699811 (accessed 20 February 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woolley SI, Collins T, Mitchell J, et al. Investigation of wearable health tracker version updates. BMJ Health Care Inform 2019; 26(1): e100083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med 2018; 169(7): 467–473. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses : The PRISMA statement All use subject to JSTOR Terms and Conditions REPORTING items Preferred for systematic reviews reporting meta-analyses: The PRISMA statement 2009; 339(7716): 332–336. [Google Scholar]
- 16.Limmroth V, Bartzokis I, Bonmann E, et al. The BETACONNECTTM system: MS therapy goes digital. Neurodegener Dis Manag 2018; 8(6): 399–410. [DOI] [PubMed] [Google Scholar]
- 17.Block VJ, Bove R, Zhao C, et al. Association of continuous assessment of step count by remote monitoring with disability progression among adults with multiple sclerosis. JAMA Netw Open 2019; 2(3): e190570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGibbon CA, Sexton A, Jayaraman A, et al. Evaluation of the Keeogo exoskeleton for assisting ambulatory activities in people with multiple sclerosis: An open-label, randomized, cross-over trial. J Neuroeng Rehabil 2018; 15(1): 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thirumalai M, Rimmer JH, Johnson G, et al. TEAMS (Tele-Exercise and Multiple Sclerosis), a tailored telerehabilitation mhealth app: Participant-centered development and usability study. JMIR mHealth uHealth; 2018; 6(5): e10181, http://mhealth.jmir.org/2018/5/e10181/ (accessed 23 May 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neven A, Vanderstraeten A, Janssens D, et al. Understanding walking activity in multiple sclerosis: Step count, walking intensity and uninterrupted walking activity duration related to degree of disability. Neurol Sci 2016; 37(9): 1483–1490. [DOI] [PubMed] [Google Scholar]
- 21.Balto JM, Kinnett-Hopkins DL, Motl RW.Accuracy and precision of smartphone applications and commercially available motion sensors in multiple sclerosis. Mult Scler J – Exp Transl Clin 2016; 2. DOI: 10.1177/2055217316634754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandroff BM, Motl RW, Pilutti LA, et al. Accuracy of StepWatchTM and ActiGraph accelerometers for measuring steps taken among persons with multiple sclerosis. PLoS ONE 2014; 9(4): e93511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandroff BM, Motl RW.Comparison of ActiGraph activity monitors in persons with multiple sclerosis and controls. Disabil Rehabil 2013; 35(9): 725–731. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt AL, Pennypacker ML, Thrush AH, et al. Validity of the stepwatch step activity monitor: Preliminary findings for use in persons with parkinson disease and multiple sclerosis. J Geriatr Phys Ther 2011; 34(1): 41–45. [DOI] [PubMed] [Google Scholar]
- 25.Daumer M, Thaler K, Kruis E, et al. Steps towards a miniaturized, robust and autonomous measurement device for the long-term monitoring of patient activity: ActiBelt. Biomed Tech 2007; 52(1): 149–155. [DOI] [PubMed] [Google Scholar]
- 26.Bove R, White CC, Giovannoni G, et al. Evaluating more naturalistic outcome measures. Neurol Neuroimmunol Neuroinflamm 2015; 2(6): e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D’hooghe M, Van Gassen G, Kos D, et al. Improving fatigue in multiple sclerosis by smartphone-supported energy management: The MS TeleCoach feasibility study. Mult Scler Relat Disord 2018; 22: 90–96. [DOI] [PubMed] [Google Scholar]
- 28.Coulter EH, Miller L, McCorkell S, et al. Validity of the activPAL3 activity monitor in people moderately affected by Multiple Sclerosis. Med Eng Phys 2017; 45: 78–82. [DOI] [PubMed] [Google Scholar]
- 29.Block VJ, Lizée A, Crabtree-Hartman E, et al. Continuous daily assessment of multiple sclerosis disability using remote step count monitoring. J Neurol 2017; 264(2):316–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tonheim AN, Babic A.Assessing information needs for a personal multiple sclerosis application. Stud Heal Technol Inform 2018; 247: 486–490. [PubMed] [Google Scholar]
- 31.Montalban X, Mulero P, Midaglia L, et al. FLOODLIGHT: Smartphone-based self-monitoring is accepted by patients and provides meaningful, continuous digital outcomes augmenting conventional in-clinic multiple sclerosis measures.In: ECTRIMS, 2018, p. 228468, https://onlinelibrary.ectrimscongress.eu/ectrims/2018/ectrims-2018/228468/xavier.montalban.floodlight.smartphone-based.selfmonitoring.is.accepted.by.html?f=listing%3D0%2Abrowseby%3D8%2Asortby%3D1%2Asearch%3Dfloodlight (accessed 23 May 2020).
- 32.Rudick RA, Miller D, Bethoux F, et al. The multiple sclerosis performance test (MSPT): An iPad-based disability assessment tool. J Vis Exp 2014; 88: e51318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Middleton RM.MSiDMT: Development of a consistent electronic cognitive scoring for the UKMS Register.In: ECTRIMS, 2019, p. P813, https://onlinelibrary.ectrims-congress.eu/ectrims/2019/stockholm/279173/rod.middleton.msidmt.development.of.a.consistent.electronic.cognitive.scoring.html?f=listing%3D3%2Abrowseby%3D8%2Asortby%3D1%2Amedia%3D1 (accessed 23 May 2020).
- 34.Boukhvalova AK, Fan O, Weideman AM, et al. Smartphone level test measures disability in several neurological domains for patients with multiple sclerosis. Front Neurol 2019; 10. DOI: 10.3389/fneur.2019.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cavanaugh JT, Gappmaier VO, Dibble LE, et al. Ambulatory activity in individuals with multiple sclerosis. J Neurol Phys Ther 2011; 35(1): 26–33, http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=01253086-201103000-00005 (accessed 23 May 2020). [DOI] [PubMed] [Google Scholar]
- 36.Carpinella I, Gervasoni E, Anastasi D, et al. Instrumental assessment of stair ascent in people with multiple sclerosis, stroke, and Parkinson’s disease: A wearable-sensor-based approach. IEEE Trans Neural Syst Rehabil Eng 2018; 26(12): 2324–2332. [DOI] [PubMed] [Google Scholar]
- 37.Witchel HJ, Oberndorfer C, Needham R, et al. Thighderived inertial sensor metrics to assess the sit-tostand and stand-to-sit transitions in the timed up and go (TUG) task for quantifying mobility impairment in multiple sclerosis. Front Neurol 2018; 9: 684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Storm FA, Nair KPS, Clarke AJ, et al. Free-living and laboratory gait characteristics in patients with multiple sclerosis. PLoS ONE 2018; 13(5): e0196463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Psarakis M, Greene DA, Cole MH, et al. Wearable technology reveals gait compensations, unstable walking patterns and fatigue in people with multiple sclerosis. Physiol Meas 2018; 39(7): 0750. [DOI] [PubMed] [Google Scholar]
- 40.Dalla-Costa G, Radaelli M, Maida S, et al. Smart watch, smarter EDSS: Improving disability assessment in multiple sclerosis clinical practice. J Neurol Sci 2017; 383: 166–168. [DOI] [PubMed] [Google Scholar]
- 41.McGinnis RS, Mahadevan N, Moon Y, et al. A machine learning approach for gait speed estimation using skin-mounted wearable sensors: From healthy controls to individuals with multiple sclerosis. PLoS ONE 2017; 12(6): e0178366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El-Gohary M, Peterson D, Gera G, et al. Validity of the instrumented push and release test to quantify postural responses in persons with multiple sclerosis. Arch Phys Med Rehabil 2017; 98(7): 1325–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moon Y, McGinnis RS, Seagers K, et al. Monitoring gait in multiple sclerosis with novel wearable motion sensors. PLoS ONE 2017; 12(2): e0171346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pau M, Caggiari S, Mura A, et al. Clinical assessment of gait in individuals with multiple sclerosis using wearable inertial sensors: Comparison with patient-based measure. Mult Scler Relat Disord 2016; 10: 187–191. [DOI] [PubMed] [Google Scholar]
- 45.Brodie MAD, Psarakis M, Hoang P.Gyroscopic corrections improve wearable sensor data prior to measuring dynamic sway in the gait of people with Multiple Sclerosis. Comput Methods Biomech Biomed Engin 2016; 19(12): 1339–1346. [DOI] [PubMed] [Google Scholar]
- 46.Greiner P, Sawka A, Imison E.Patient and physician perspectives on MSdialog, an electronic PRO diary in multiple sclerosis. Patient 2015; 8(6): 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu F, Bilberg A, Stenager E, et al. A wireless body measurement system to study fatigue in multiple sclerosis. Physiol Meas 2012; 33: 2033–2048. [DOI] [PubMed] [Google Scholar]
- 48.Kos D, Raeymaekers J, Van Remoortel A, et al. Electronic visual analogue scales for pain, fatigue, anxiety and quality of life in people with multiple sclerosis using smartphone and tablet: A reliability and feasibility study. Clin Rehabil 2017; 31(9): 1215–1225. [DOI] [PubMed] [Google Scholar]
- 49.Boukhvalova AK, Kowalczyk E, Harris T, et al. Identifying and quantifying neurological disability via smartphone. Front Neurol 2018; 9: 740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pilutti LA, Dlugonski D, Sandroff BM, et al.Randomized controlled trial of a behavioral intervention targeting symptoms and physical activity in multiple sclerosis. Mult Scler J 2014; 20(5): 594–601. [DOI] [PubMed] [Google Scholar]
- 51.Rimmer JH, Thirumalai M, Young HJ, et al. Rationale and design of the tele-exercise and multiple sclerosis (TEAMS) study: A comparative effectiveness trial between a clinicand home-based telerehabilitation intervention for adults with multiple sclerosis (MS) living in the deep south. Contemp Clin Trials 2018; 71: 186–193. [DOI] [PubMed] [Google Scholar]
- 52.MedDay Pharmaceuticals SA. Effect of MD1003 in Progressive Multiple Sclerosis (SPI2) - Full Text View. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02936037 (accessed 2 April 2020).
- 53.Mohr D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and metaanalyses: The PRISMA statement. PubMed - NCBI, https://www.ncbi.nlm.nih.gov/pubmed/19621072 (accessed 5 November 2019). [PMC free article] [PubMed]
- 54.Warmerdam E, Hausdorff JM, Atrsaei A, et al. Long-term unsupervised mobility assessment in movement disorders. Lancet Neurol 2020; 19(5): 462–470. [DOI] [PubMed] [Google Scholar]
- 55.Noah B, Keller MS, Mosadeghi S, et al. Impact of remote patient monitoring on clinical outcomes: An updated meta-analysis of randomized controlled trials. npj Digit Med 2018; 1(1): 20172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Piwek L, Ellis DA, Andrews S, et al. The rise of consumer health wearables: Promises and barriers. PLoS Med 2016; 13(2): e1001953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dash S, Shakyawar SK, Sharma M, et al. Big data in healthcare: Management, analysis and future prospects. J Big Data 2019; 6(1). DOI: 10.1186/s40537-019-0217-0. [DOI] [Google Scholar]