Abstract
Considering its small size relative to the rest of the body, the mammalian brain has a disproportionately high energy requirement. This energy is supplied to the brain mainly in the form of glucose through the principal cerebral glucose transporter, Glut1. Inactivation of even a single copy of the Glut1 gene, SLC2A1, has dire consequences for the brain, starving cerebral neurons of energy and triggering the debilitating neurodevelopmental disorder, Glut1 deficiency syndrome (Glut1 DS). Considering the monogenic nature of Glut1 DS, the disease serves as an excellent paradigm to study the larger family of brain energy failure syndromes. Here we review how studies of Glut1 DS are proving instructive to the brain’s energy needs, focusing first on the requirements, both spatial and temporal of the transporter, second, on proposed mechanisms linking low Glut1 to brain dysfunction and, finally on efforts to treat the disease and thus restore nutritional support to the brain. These studies promise not only to inform mechanisms and treatments for the relatively rare Glut1 DS but also the myriad other conditions involving the Glut1 protein.
Keywords: Neuropathology, Murine
Introduction
The human brain accounts for a mere 2% of the weight of the adult individual. Yet it requires a full 25% of the energy consumed by the individual.1,2 This energy, delivered to the brain primarily in the form of the nutrient glucose, must traverse the blood-brain barrier (BBB) through glucose transporter1 (Glut1), the principal cerebral hexose transporter. It is therefore not surprising that levels of the transporter profoundly affect brain function and, consequently, the general health of the individual.3,4 Indeed, homozygous loss of SLC2A1, the Glut1 gene, is embryonic lethal.5 Heterozygous SLC2A1 mutations – haploinsufficiency – do not affect viability. However, haploinsufficiency does result in marked paucity of cerebral glucose, severe depletion of brain energy reserves and a condition known as neuroglycopenia. The net effect is embodied in the infantile-onset neurodevelopmental disorder, Glut1 deficiency syndrome (Glut1 DS).6,7
The classic Glut1 DS disease phenotype is characterized by a pediatric-onset epileptic encephalopathy that responds poorly to antiepileptic drugs, developmental delay, and a complex movement disorder that combines elements of dystonia, ataxia, and spasticity. An expanded disease phenotype is now recognized and includes exercise-induced dyskinesia and hemolytic anemia.8-10 Yet, all patients afflicted with Glut1 DS exhibit hypoglycorrhachia – low (<60 mg/dL or 3.3 mmol/L) levels of glucose in cerebrospinal fluid (CSF), accompanied by CSF lactate levels in the low to low-normal range (<9 mg/dL or 0.5 mmol/L).11 Initially considered exceptionally rare with fewer than 500 patients worldwide, Glut1 DS is now recognized to be significantly under-reported.12 Thus, for instance, Glut1 mutations reportedly underlie ~1% of idiopathic generalized epilepsies (IGEs) and 10% of absence epilepsies;13,14 prevalence of epilepsies over a lifetime is estimated to be ~7.6 per 1000 individuals, 15%–20% of which are reportedly IGEs.15,16 Based on these figures it is thought that Glut1 DS patients number between 3400 and 4500 in the US and ~105,000 worldwide.
Notwithstanding a growing appreciation of the prevalence of Glut1 DS and concomitant awareness of the phenotype associated with the disorder, there is a limited understanding of the mechanisms linking low Glut1 to brain dysfunction. Additionally, relatively little is known about the molecular and cellular consequences of how low brain glucose, indeed brain energy deprivation in general, affects cerebral development, brain circuitry, and cognition. These aspects of brain development and function are broadly relevant, impacting health issues stemming from nutritional deprivation in impoverished nations to neurodegenerative disorders such as Alzheimer’s disease (AD); numerous reports have found AD brains to not only be hypometabolic but also deficient in Glut1.17-21 Yet, investigating the molecular and cellular correlates of brain energy deprivation in multifactorial conditions such as malnourishment and AD can be challenging. One solution is to study them in monogenic conditions. Glut1 DS represents a particularly useful paradigm in this regard. Here we review recent studies that have employed cell and animal models of Glut1 DS to cast light on the consequences of depriving cells and tissues of the energy substrate glucose. We focus specifically on the disease consequences of depleting cerebral levels of this nutrient – by inactivating Glut1 in select brain cells, examine the evidence provided for mechanisms linking the energy deprivation to disease and conclude by summarizing recent advances in the quest to thwart brain dysfunction resulting from glucose paucity by restoring Glut1 to model mice.
Evidence for the cellular sites of action of Glut1
The main energy substrate for the brain is glucose. Yet, brain neurons are thought to mostly rely on lactate to fuel their energy requirements.22 This lactate is generated in brain astrocytes which are predominantly glycolytic. Indeed, like endothelial cells (ECs), astrocytes are richly endowed with the Glut1 protein which localizes in high concentrations in astrocytic end feet.23 Low Glut1 could therefore potentially result in low cerebral glucose levels and brain energy deprivation owing to paucity of the transporter in brain endothelia, astrocytes or both. To determine the extent to which each cell contributes to brain dysfunction in Glut1 DS, investigators induced Glut1 haploinsufficiency selectively in one or the other.24 While it is still not clear if and to what extent cerebral glucose levels decrease in vivo upon selectively inactivating astrocytic Glut1, studies did conclude that such inactivation was not sufficient to arrest brain angiogenesis – a characteristic pathology of the Glut1 DS brain. Yet, glycolytic flux in cultured Glut1-deficient astrocytes was significantly reduced and, accordingly, lactate generated by the cells fell.25 Whether these defects evolve into an overt phenotype remains to be determined.
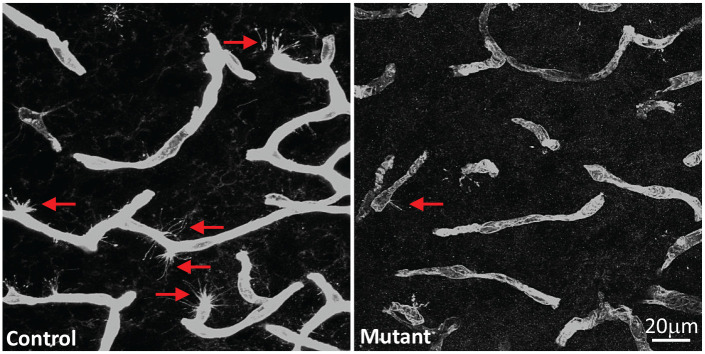
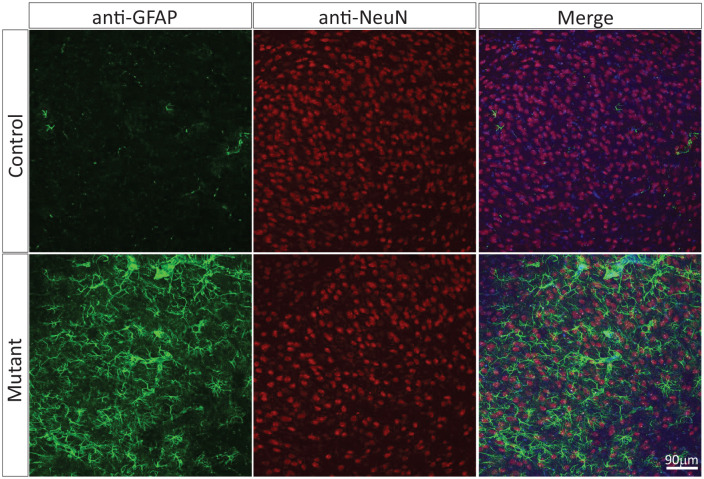
The contribution of reduced Glut1 in brain ECs to brain energy deprivation, cerebral pathology and overt disease is much clearer and based on 2 new studies.26,27 In the more recent investigation conducted by our own group, selectively inducing haploinsufficiency in brain endothelia was sufficient to trigger more or less the entire gamut of cellular and behavioral phenotypes associated with the systemic reduction of Glut1 seen in human Glut1 DS.25 Moreover, these studies identified the cellular origin – endothelial tip cells – of the diminutive Glut1 DS brain microvasculature that had been reported. Endothelial tip cells which are highly glycolytic and critically important for brain angiogenesis28 appear to be devastated by a 50% reduction of Glut1. Indeed, not only did the study find greatly reduced numbers of these cells in mice selectively haploinsufficient for Glut1 in ECs but also showed that they are far less elaborate, lacking the profusion of lamellipodia characteristically observed in wild-type endothelial tip cells during the process of cerebral angiogenesis (Figure 1). Interestingly, EC-specific Glut1 haploinsufficiency was also shown to non cell-autonomously trigger the profound neuroinflammation and loss of neurons that we and others have observed in the Glut1 DS brain (Figure 2). Whether the neuron loss is neurodegenerative or a consequence of a neurodevelopmental defect awaits investigation. Still, it is thought to be mediated by a significant reduction in cerebral levels of the neurotrophin, brain-derived neurotrophic factor (BDNF). Loss of cerebral BDNF was detected in constitutively haploinsufficient Glut1 mice as well as in mutants selectively depleted of the transporter in ECs suggesting that altered levels of the neurotrophic factor are, at least in part, a non cell-autonomous effect of low Glut1 in ECs.27
Figure 1.
Cell-autonomous effects of Glut1 on brain ECs. Depicted are representative sections of the microvasculature from the thalami of a control, 2-week old mouse wild-type for the Slc2a1 gene and an age-matched mutant engineered to be haploinsufficient for the gene specifically in ECs.
Note the greater number of endothelial tip cells each with a profusion of lamellipodia (arrows) in the control mouse. Sections were stained with an antibody against Glut1.
Figure 2.
Neuroinflammation and neuronal loss is a non cell-autonomous effect of low Glut1 in ECs. Depicted are representative thalamic sections of a control adult mouse wild-type for the Slc2a1 gene and an age-matched mutant engineered to be haploinsufficient for the gene specifically in ECs.
Note profound astrocytosis and fewer NeuN-positive puncta representing neurons in the mutant sample.
That Glut1 in brain ECs is central to ensuring that the brain is adequately supplied with glucose and thus primed for proper function was independently concluded in an earlier study.26 Yet, the finer points of that study including the mechanistic insights proffered for Glut1 DS are confounded by the perplexing decision to completely ablate Glut1 and/or its functions in the cells and model mice employed for the experiments. Complete loss of Glut1 has never been reported in humans and is presumed, based on studies of pre-clinical models, incapable of resulting in viable offspring.5 In contrast, brain energy failure and the disease phenotype evoked in Glut1 DS result from heterozygous SLC2A1 mutations. Extrapolating findings in Glut1-ablated models to infer changes in the haploinsufficient state is therefore unwise and can mislead the field about bona fide disease mechanisms. The discrepant models employed in the Veys et al and Tang et al studies likely explain why the studies failed to agree on purported pathways including potential roles for AMP kinase and p53 in Glut1 DS. Nevertheless, there was concordance on a number of major findings. Of particular significance, given the relevance to brain disease phenotypes, is agreement on the effect of Glut1 on the integrity of the blood-brain barrier (BBB).
An initial study examining the BBB in a Glut1 DS mouse model concluded that haploinsufficiency of the transporter in brain ECs is profoundly damaging to this structure.24 Model mice were found to exhibit massive (>10-fold over wild-type) extravasation of serum proteins into the neuropil as early as 2 weeks of age. These observations were accompanied by findings of greatly reduced levels of BBB tight-junction proteins including occludin, zona occludens-1 and claudin-5 at this early stage of the disease – results that were construed as evidence of EC damage that eventually caused neuronal dysfunction/loss. The overall findings and their implications vis-à-vis a role for Glut1 in barriergenesis appeared consistent with the results of a study conducted using a Glut1 deficient zebrafish model, albeit one in which levels of the transporter were reduced by ~90%; such low levels have not been reported in patients and are not disease-relevant. A multi-pronged approach to independently confirm BBB damage in the Glut1 haploinsufficient mutant initially used to suggest defects of the barrier proved unsuccessful.25 The study not only failed to observe extravasation of a variety of proteins, some as small as 900 daltons, but was also unable to detect any alteration in the expression of genes coding for BBB tight-junction proteins. Importantly, claims of damage to the BBB in the Glut1 haploinsufficient state were also refuted by data collected in human patients; the patients (N = 44) ranging in age from 6 months to 10 years did not have significantly higher levels of serum proteins in CSF, contrary to expectations if the barrier was leaky.25
Evidence for an intact and functioning BBB despite low Glut1 was bolstered by 2 independent studies. Veys et al26 repeated many of the experiments conducted by Winkler et al24 in an attempt to confirm the latter’s findings. In contrast to the results reported by Winkler et al but consistent with the study of Tang and colleagues, the investigation of Veys et al failed to observe BBB damage – despite the use of models practically fully ablated of EC-derived Glut1.26 A second study that investigated the role of Glut1 at another blood-CNS barrier, the blood-retinal barrier (BRB), arrived at a similar conclusion.29 Depletion of Glut1 by up to 70% in the retinal pigment epithelium (RPE) which constitutes the BRB failed to have any effect on its barrier properties. The aggregate findings suggest that while Glut1 haploinsufficiency affects brain angiogenesis, it does not have an adverse effect on the integrity of the BBB. Assertions of Glut1-associated BBB damage are therefore puzzling and hard to reconcile with the consensus.
A role for Glut1 in brain development and function is clear. However, the transporter is expressed in multiple locations outside the brain, notably the retinal vasculature. Here, Glut1 is expressed at high levels in the luminal and abluminal faces of choroidal vessels and on the basal and apical membranes of the RPE.30,31 In these structures, Glut1 serves as conduit for glucose required by photoreceptors. Low Glut1 and concomitant glucose deprivation could therefore also affect the retinal vasculature and vision. Studies have demonstrated that this is indeed the case. Targeted depletion of Glut1 in the RPE or in all ECs did not affect the integrity of the BRB, but was reported to trigger Müller glial cell activation, affect retinal angiogenesis and reduce photoreceptor viability.26,29 Moreover, in a patient with Glut1 DS, vision defects and a paucity of perimacular vessels were detected.32 Thus it is clear that while endothelial Glut1 deficiency manifests primarily as a brain disorder, haploinsufficiency of the transporter can also affect other organ systems that rely on glucose to fuel their energy requirements.
Glut1 is especially important early in life
The classic Glut1 DS disease phenotype becomes apparent in infancy, suggestive of a critical early postnatal need for the transporter and glucose to ensure proper brain development and function. This early postnatal requirement for the protein has been empirically determined in 2 important studies that employed model mice.25,27 In the first of these studies, the temporal requirements for Glut1 were assessed by restoring the protein to Glut1 DS mutant mice at 3 different stages – early, middle and late – of the disease.25 Relative therapeutic outcome served as an indicator of when Glut1 is critically needed. Consistent with the pediatric-onset nature of Glut1 DS, this study unambiguously showed that Glut1 is particularly important during early postnatal life. Thus, Glut1 repletion at postnatal day 2 (PND2) (neonatal life) was most effective at preventing disease. In contrast, waiting until the mutants reached adulthood to augment the protein delivered little benefit, despite robust increases in brain Glut1 and CSF glucose levels. Restoring Glut1 to juvenile Glut1 DS mutant mice was partially effective. These observations of diminishing benefit with progressively delayed Glut1 restoration is almost certainly related to the natural course and timing of cerebral angiogenesis and brain circuit establishment witnessed during the early postnatal period of life.33 Once the early postnatal window during which these processes unfold closes, any damage sustained by the brain because of energy deprivation is essentially immutable. In support of this view is evidence from the study of 2 patients with neuroglycopenia, the origin of which was either chronic childhood hypoglycemia or heterozygous mutation of the SLC2A1 gene (Glut1 DS). Interestingly, PET images of the brains of the 2 individuals, captured in adulthood, were almost identical, notwithstanding the fact that normoglycemia had been restored and maintained in the individual without Glut1 DS.34 In light of the combined observations in patients34 and model mice,25 the results of another study35 demonstrating benefit from late Glut1 repletion is intriguing. The results of this last finding remain to be fully explained but, if true, raise much optimism for ameliorating disease in the fully symptomatic Glut1 DS patient.
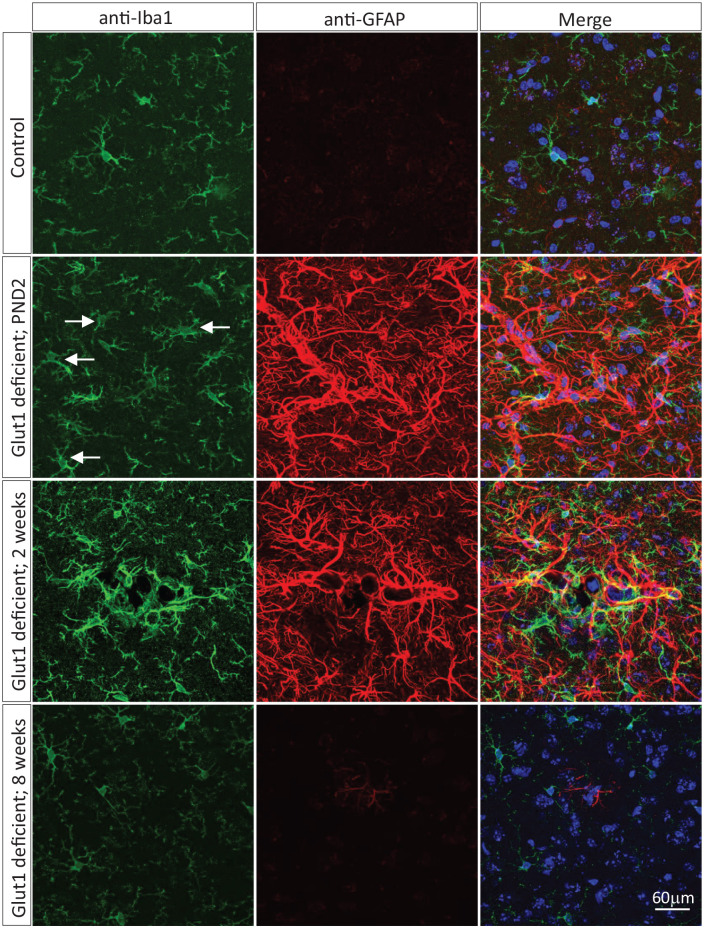
A second study that explored the age-dependent consequences of brain glucose deprivation using Glut1 DS as a disease paradigm did so by inducing systemic Glut1 haploinsufficiency in progressively older wild-type animals.27 Thus, mice were made heterozygous for murine Slc2a1 as neonates (PND2), juveniles (2 weeks of age) or adults (8 weeks of age). The relative severity of disease that was triggered in the 3 cohorts of mice was used to infer the temporal requirements for the transporter. Consistent with the outcome of the Glut1 repletion studies, the depletion experiments showed that early haploinsufficiency induced more pronounced brain pathology and more severe disease. Thus, inactivating Glut1 in neonates evoked a Glut1 DS phenotype that was equivalent in severity to disease observed in constitutively haploinsufficient mutants. In contrast, depleting the transporter in mature mice resulted in few, if any, disease symptoms – despite efficient reduction of Glut1 levels and correspondingly robust induction of hypoglycorrhachia.27 Intriguingly, in a separate study, acute, EC-restricted Glut1 depletion in adult mice was lethal to approximately half the mutants.36 One explanation for the contrasting result is the manner – EC-restricted rather than systemic – in which the Glut1 depletion was effected. Still, congruent with the data generated on neonatal and adult mice,25 controlled depletion of Glut1 in juveniles, a stage of life when cerebral angiogenesis is ongoing and brain circuits being established, produced an intermediate effect. As an illustration of the correlation between timing of Glut1 depletion and severity of the disease phenotype, we depict the extent to which gliosis was observed in mice made haploinsufficient at PND2, 2 weeks of age and 8 weeks of age (Figure 3). While these studies underscore the especially urgent need for Glut1 and cerebral glucose early in life, they also reveal the role of the transporter in the long-term maintenance of brain health. Indeed, longitudinal analyses of mice depleted of Glut1 during adulthood, and devoid of symptoms at 5 months of age, demonstrated that chronic neuroglycopenia is not entirely benign. By 12 months of age, brains from such mice developed considerable gliosis.27 The overall observations parallel findings in another pediatric neurological disorder, spinal muscular atrophy (SMA), caused by a deficiency of the SMN protein.37-39 Studies on SMA revealed a particularly high requirement for SMN during early postnatal life when neuromuscular junctions (NMJs) mature. Following this period, the requirement for SMN dwindles but rebounds locally in subsets of motor neurons as a maintenance factor to restore NMJs damaged in injury or turned over during the aging process. Fully understanding such requirements for a disease-associated protein, irrespective of disease, has important clinical implications in the quest to develop and implement effective therapies.
Figure 3.
Graded neuroinflammation with delayed induction of Glut1 haploinsufficiency. Representative thalamic sections from a control adult mouse wild-type for the Slc2a1 gene and age-matched mutants engineered to be haploinsufficient for the gene during neonatal life, juvenile life or adulthood. Swollen, amoeboid-shaped, activated microglia (arrows) and reactive astrocytes stained respectively for Iba1 and GFAP are more obvious in mutants made Glut1 haploinsufficient early in life.
Mechanistic insights into Glut1 DS
The molecular mediators of low Glut1 have mostly been revealed through analyses of cell culture models. For instance, abrogation of Glut1 function was reported to decrease cellular ATP levels and activate the cellular energy sensor, phospho-AMP kinase (p-AMPK).26 Such activation was also reported to suppress protein synthesis, result in reduced levels of the downstream targets of p-AMPK, and trigger significant increases in the cell growth-arrest factors, p53 and Cdkn1a. Not surprisingly, ablation of Glut1 activity also resulted in reduced levels of all major glycolytic intermediates in the cell model employed for the analysis. While these findings are undoubtedly instructive from a basic biology standpoint, it is unclear to what extent they are of relevance to and reflective of the mechanistic underpinnings of Glut1 DS. In this regard it is worth reiterating that Glut1 DS involves haploinsufficiency of the SLC2A1 gene and, consequently, Glut1 levels of ~50%. In contrast, the study alluded to above elected to completely ablate (<10% of WT levels) transporter activity to levels never seen in Glut1 DS patients or mutant mice that faithfully model the human disease. Indeed, an independent study failed to find increased p-AMPK in brain tissue of Glut1 DS model mice.27 Surprisingly, that study also found evidence of decreased p53 activity. Resolving these contrasting findings will require additional investigation. Still, both studies observed a profound increase in the neuroinflammatory response under conditions of low Glut1. Perturbed expression of pro-inflammatory genes was detected within ECs, and the gliosis that was observed did not require Glut1 knockdown in glial populations suggesting that low Glut1 in ECs is sufficient to elicit gliosis. The extent to which the neuroinflammatory response is adaptive or damaging to neurons remains to be determined as does the precise physiological relevance of reduced BDNF in the Glut1 DS brain.27 BDNF is known to modulate neuronal differentiation and survival.40 Interestingly, it is also reported to mediate angiogenesis.41 Low levels of the neurotrophin could therefore have a dual effect in Glut1 DS, arresting brain angiogenesis on the one hand and acting directly on cerebral neurons to affect their differentiation and viability. Precisely when BDNF levels within the Glut1 DS brain fall and if diminished concentrations of this neurotrophin, its upstream effector, lactate and neuroinflammation in general conspire to define the temporal requirements for Glut1 are yet to be determined. One way to begin addressing some of these questions would be to restore BDNF and/or suppress glial activation in Glut1 DS model mice to ascertain how such manipulations might alter the onset of disease. Thus it is clear that notwithstanding the new insights into Glut1 DS mechanisms, much remains to be investigated to define precisely how reduced levels of the transporter alter brain function in the human disease.
Current and emerging therapeutic strategies for Glut1 DS
The genetic cause of Glut1 DS was described over 2 decades ago.4 Yet, there is no truly effective therapy that addresses the origin – low Glut1 – of the disease. The current standard of care involves treating patients with high-fat ketogenic diets.42,43 Such diets supply the brain with the ketone bodies β-hydroxybutyrate and α-ketoglutarate, which enter the brain through the monocarboxylate transporter 1 (MCT1) and serve as alternate, albeit imperfect fuels for cerebral activity. Ketone bodies can be broken down into acetyl-CoA and fed into the tricarboxylic acid (TCA) cycle. Still, while ketogenic diets mitigate seizure activity in Glut1 DS patients, their effects on cognitive and motor dysfunction in the disease are variable and modest at best.44 Besides, long-term use of ketogenic diets is not without concern and known to be associated with reductions in bone density, cardiovascular complications and, in certain instances, coma and dealth.45-47 An alternative to the ketogenic diet that was considered for the treatment of Glut1 DS is triheptanoin. This odd-chain (C7) triglyceride can be metabolized into both acetyl-CoA as well as propionyl-CoA and thus function as an anaplerotic agent to replenish TCA cycle intermediates. However, triheptanoin failed to achieve clinical efficacy in one commercially funded clinical trial.48 A number of small molecules have also been prescribed for Glut1 DS. These compounds and biological enhancers of Glut1 activity are described elsewhere12 and, for brevity, will not be considered here.
One appealing avenue for the treatment of Glut1 DS relies on repletion of the transporter using viral vector technology. In this regard, adeno-associated viral (AAV) vectors have emerged as an exciting delivery vehicle and have been successfully employed to treat spinal muscular atrophy.49 Accordingly, we and others have pursued this approach as a means to treat the human condition. These studies have resulted in promising outcomes in model mice.25,35 Indeed, treating Glut1 DS mutants with AAV9-Glut1 raised levels of the transporter in the brain, prevented hypoglycorrhachia, facilitated normal brain growth, stabilized motor performance and restored the cerebral microvasculature to its wild-type size and complexity.25 These findings raise considerable optimism for the treatment of the human patient but, as alluded to previously, have also focused attention on the importance of early treatment. Translating these initial findings into a viable treatment is ongoing.
Conclusions
Depleting the brain of its energy reserves is clearly detrimental to the health of the individual. However, the precise molecular and cellular consequences of brain glucose deprivation remain to be fully defined. Studies of brain energy deprivation in Glut1 DS are beginning to cast useful light on the pathology associated with the larger family of cerebral energy failure syndromes. Moreover, efforts to replenish Glut1 in Glut1 DS could prove useful beyond the treatment of this rare disorder. At least 2 other conditions, retinitis pigmentosa and Alzheimer’s disease are known to involve low Glut1 levels.50-53 Insights into brain energy deprivation from the study of Glut1 DS combined with efforts to develop Glut1-based therapeutic strategies for this relatively rare neurodevelopmental disorder could therefore become more generally relevant to treating the extended family of disorders involving the transporter.
Acknowledgments
We thank Serge Przedborski for his unstinting support of our research program. We are also indebted to Darryl C. De Vivo for his sage advice, his guidance and for helping shepherd our Glut1 DS projects these many years.
Footnotes
Funding: The author disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors gratefully acknowledge support from the Glut1 Deficiency and Hope for Children Research Foundations. Studies on Glut1 DS in the Monani lab were funded through grants from the University of Pennsylvania Orphan Disease Center. U.R.M. is supported by the Pediatric Neurology Professorship in the Department of Neurology.
Declaration Of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: M.T. and U.R.M. are listed as inventors on a provisional patent that describes the use of Glut1 repletion to treat Glut1 DS.
Author Contributions: M.T. and U.R.M. conceived of the layout and organization of the review. M.T. generated the data featured in the article and U.R.M. wrote the initial draft. Both authors read, amended and approved the final manuscript.
ORCID iD: Umrao R Monani  https://orcid.org/0000-0002-1101-2483
https://orcid.org/0000-0002-1101-2483
References
- 1.Raichle ME, Gusnard DA. Appraising the brain's energy budget. Proc Natl Acad Sci U S A. 2002;99(16):10237-10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magistretti PJ, Allaman I. Brain energy metabolism. In: Neuroscience in the 21st Century: From Basic to Clinical. Springer; 2013:1591-1620. [Google Scholar]
- 3.De Vivo DC, Trifiletti RR, Jacobson RI, Ronen GM, Behmand RA, Harik SI. Defective glucose transport across the blood-brain barrier as a cause of persistent hypoglycorrhachia, seizures, and developmental delay. N Engl J Med. 1991;325(10):703-709. [DOI] [PubMed] [Google Scholar]
- 4.Seidner G, Alvarez MG, Yeh JI, et al. GLUT-1 deficiency syndrome caused by haploinsufficiency of the blood-brain barrier hexose carrier. Nat Genet. 1998;18(2):188-191. [DOI] [PubMed] [Google Scholar]
- 5.Wang D, Pascual JM, Yang H, et al. A mouse model for Glut-1 haploinsufficiency. Hum Mol Genet. 2006;15(7):1169-1179. [DOI] [PubMed] [Google Scholar]
- 6.Wang D, Pascual JM, De Vivo D. Glucose transporter type 1 deficiency syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. GeneReviews®. University of Washington; 2002. [Google Scholar]
- 7.Wang D, Pascual JM, Yang H, et al. Glut-1 deficiency syndrome: clinical, genetic, and therapeutic aspects. Ann Neurol. 2005;57(1):111-118. [DOI] [PubMed] [Google Scholar]
- 8.Brockmann K. The expanding phenotype of GLUT1-deficiency syndrome. Brain Dev. 2009;31(7):545-552. [DOI] [PubMed] [Google Scholar]
- 9.Suls A, Dedeken P, Goffin K, et al. Paroxysmal exercise-induced dyskinesia and epilepsy is due to mutations in SLC2A1, encoding the glucose transporter GLUT1. Brain. 2008;131(Pt 7):1831-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weber YG, Storch A, Wuttke TV, et al. GLUT1 mutations are a cause of paroxysmal exertion-induced dyskinesias and induce hemolytic anemia by a cation leak. J Clin Invest. 2008;118(6):2157-2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leen WG, Wevers RA, Kamsteeg EJ, Scheffer H, Verbeek MM, Willemsen MA. Cerebrospinal fluid analysis in the workup of GLUT1 deficiency syndrome: a systematic review. JAMA Neurol. 2013;70(11):1440-1444. [DOI] [PubMed] [Google Scholar]
- 12.Tang M, Park SH, De Vivo DC, Monani UR. Therapeutic strategies for glucose transporter 1 deficiency syndrome. Ann Clin Transl Neurol. 2019;6(9):1923-1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arsov T, Mullen SA, Rogers S, et al. Glucose transporter 1 deficiency in the idiopathic generalized epilepsies. Ann Neurol. 2012;72(5):807-815. [DOI] [PubMed] [Google Scholar]
- 14.Arsov T, Mullen SA, Damiano JA, et al. Early onset absence epilepsy: 1 in 10 cases is caused by GLUT1 deficiency. Epilepsia. 2012;53(12):e204-e207. [DOI] [PubMed] [Google Scholar]
- 15.Fiest KM, Sauro KM, Wiebe S, et al. Prevalence and incidence of epilepsy: a systematic review and meta-analysis of international studies [published correction appears in Neurology. 2017 Aug 8;89(6):642]. Neurology. 2017;88(3):296-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jallon P, Latour P. Epidemiology of idiopathic generalized epilepsies. Epilepsia. 2005;46(Suppl 9):10-14. [DOI] [PubMed] [Google Scholar]
- 17.Foster NL, Heidebrink JL, Clark CM, et al. FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer's disease. Brain. 2007;130(Pt 10):2616-2635. [DOI] [PubMed] [Google Scholar]
- 18.Friedland RP, Budinger TF, Ganz E, et al. Regional cerebral metabolic alterations in dementia of the Alzheimer type: positron emission tomography with [18F]fluorodeoxyglucose. J Comput Assist Tomogr. 1983;7(4):590-598. [DOI] [PubMed] [Google Scholar]
- 19.Herholz K, Carter SF, Jones M. Positron emission tomography imaging in dementia. Br J Radiol. 2007;80:S160-S167. [DOI] [PubMed] [Google Scholar]
- 20.Jagust W, Reed B, Mungas D, Ellis W, Decarli C. What does fluorodeoxyglucose PET imaging add to a clinical diagnosis of dementia? Neurology. 2007;69(9):871-877. [DOI] [PubMed] [Google Scholar]
- 21.Dumurgier J, Paquet C, Peoc'h K, et al. CSF Aβ₁₋₄₂ levels and glucose metabolism in Alzheimer's disease. J Alzheimers Dis. 2011;27(4):845-851. [DOI] [PubMed] [Google Scholar]
- 22.Bélanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14(6):724-738. [DOI] [PubMed] [Google Scholar]
- 23.Morgello S, Uson RR, Schwartz EJ, Haber RS. The human blood-brain barrier glucose transporter (GLUT1) is a glucose transporter of gray matter astrocytes. Glia. 1995;14(1):43-54. [DOI] [PubMed] [Google Scholar]
- 24.Winkler EA, Nishida Y, Sagare AP, et al. GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat Neurosci. 2015;18(4):521-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang M, Gao G, Rueda CB, et al. Brain microvasculature defects and Glut1 deficiency syndrome averted by early repletion of the glucose transporter-1 protein. Nat Commun. 2017;8:14152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veys K, Fan Z, Ghobrial M, et al. Role of the GLUT1 glucose transporter in postnatal CNS angiogenesis and blood-brain barrier integrity. Circ Res. 2020;127(4):466-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang M, Park SH, Petri S, et al. An early endothelial cell-specific requirement for Glut1 is revealed in Glut1 deficiency syndrome model mice. JCI Insight. 2020;2020:145789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146(6):873-887. [DOI] [PubMed] [Google Scholar]
- 29.Swarup A, Samuels IS, Bell BA, et al. Modulating GLUT1 expression in retinal pigment epithelium decreases glucose levels in the retina: impact on photoreceptors and Müller glial cells. Am J Physiol Cell Physiol. 2019;316(1):C121-C133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumagai AK. Glucose transport in brain and retina: implications in the management and complications of diabetes. Diabetes Metab Res Rev. 1999;15(4):261-273. [DOI] [PubMed] [Google Scholar]
- 31.Takata K. Glucose transporters in the transepithelial transport of glucose. J Electron Microsc (Tokyo). 1996;45(4):275-284. [DOI] [PubMed] [Google Scholar]
- 32.Henry M, Kitchens J, Pascual JM, Maldonado RS. GLUT1 deficiency: retinal detrimental effects of gliovascular modulation. Neurol Genet. 2020;6(4):e472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harb R, Whiteus C, Freitas C, Grutzendler J. In vivo imaging of cerebral microvascular plasticity from birth to death. J Cereb Blood Flow Metab. 2013;33(1):146-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pascual JM, Wang D, Hinton V, et al. Brain glucose supply and the syndrome of infantile neuroglycopenia. Arch Neurol. 2007;64(4):507-513. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura S, Osaka H, Muramatsu SI, et al. Gene therapy for a mouse model of glucose transporter-1 deficiency syndrome. Mol Genet Metab Rep. 2017;10:67-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jais A, Solas M, Backes H, et al. Myeloid-cell-derived VEGF maintains brain glucose uptake and limits cognitive impairment in obesity. Cell. 2016;165(4):882-895. [DOI] [PubMed] [Google Scholar]
- 37.Lutz CM, Kariya S, Patruni S, et al. Postsymptomatic restoration of SMN rescues the disease phenotype in a mouse model of severe spinal muscular atrophy. J Clin Invest. 2011;121(8):3029-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kariya S, Obis T, Garone C, et al. Requirement of enhanced survival motoneuron protein imposed during neuromuscular junction maturation. J Clin Invest. 2014;124(2):785-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monani UR, De Vivo DC. Neurodegeneration in spinal muscular atrophy: from disease phenotype and animal models to therapeutic strategies and beyond. Future Neurol. 2014;9(1):49-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4(4):299-309. [DOI] [PubMed] [Google Scholar]
- 41.Kermani P, Hempstead B. Brain-derived neurotrophic factor: a newly described mediator of angiogenesis. Trends Cardiovasc Med. 2007;17(4):140-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nordli DR Jr, De, Vivo DC. The ketogenic diet revisited: back to the future. Epilepsia. 1997;38(7):743-749. [DOI] [PubMed] [Google Scholar]
- 43.Klepper J, Leiendecker B. Glut1 deficiency syndrome and novel ketogenic diets. J Child Neurol. 2013;28(8):1045-1048. [DOI] [PubMed] [Google Scholar]
- 44.Bekker YAC, Lambrechts DA, Verhoeven JS, et al. Failure of ketogenic diet therapy in GLUT1 deficiency syndrome. Eur J Paediatr Neurol. 2019;23(3):404-409. [DOI] [PubMed] [Google Scholar]
- 45.Hahn TJ, Halstead LR, DeVivo DC. Disordered mineral metabolism produced by ketogenic diet therapy. Calcif Tissue Int. 1979;28(1):17-22. [DOI] [PubMed] [Google Scholar]
- 46.DeVivo DC, Pagliara AS, Prensky AL. Ketotic hypoglycemia and the ketogenic diet. Neurology. 1973;23(6):640-649. [DOI] [PubMed] [Google Scholar]
- 47.DeVivo DC, Haymond MW, Leckie MP, Bussman YL, McDougal DB, Jr, Pagliara AS. The clinical and biochemical implications of pyruvate carboxylase deficiency. J Clin Endocrinol Metab. 1977;45(6):1281-1296. [DOI] [PubMed] [Google Scholar]
- 48.Globe Newswire, Ultragenyx Pharmaceutical Inc. Ultragenyx announces negative topline results from phase 3 study of UX007 in patients with Glut1 DS with disabling movement disorders. 2018. Accessed June 5, 2019. Available http://ir.ultragenyx.com/news-releases/news-release-details/ultragenyx-announces-negative-topline-results-phase-3-study
- 49.Mendell JR, Al-Zaidy S, Shell R, et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377(18):1713-1722. [DOI] [PubMed] [Google Scholar]
- 50.Aït-Ali N, Fridlich R, Millet-Puel G, et al. Rod-derived cone viability factor promotes cone survival by stimulating aerobic glycolysis. Cell. 2015;161(4):817-832. [DOI] [PubMed] [Google Scholar]
- 51.Mooradian AD, Chung HC, Shah GN. GLUT-1 expression in the cerebra of patients with Alzheimer’s disease. Neurobiol Aging. 1997;18(5):469-474. [DOI] [PubMed] [Google Scholar]
- 52.Kalaria RN, Harik SI. Reduced glucose transporter at the blood-brain barrier and in cerebral cortex in Alzheimer disease. J Neurochem. 1989;53(4):1083-1088. [DOI] [PubMed] [Google Scholar]
- 53.Horwood N, Davies DC. Immunolabelling of hippocampal microvessel glucose transporter protein is reduced in Alzheimer’s disease. Virchows Arch. 1994;425(1):69-72. [DOI] [PubMed] [Google Scholar]