Abstract
Purpose
HIV-associated autonomic neuropathy (HIV-AN) is common and may be associated with both sympathetic and parasympathetic dysfunction. Sympathetic nervous system (SNS) dysfunction occurs on a continuum of hyper-to hypo-adrenergic function, and may be a mediator between psychological stress and chronic inflammation. We sought to describe patterns of SNS dysfunction in people living with HIV, and to determine whether SNS dysfunction is associated with markers of systemic inflammation (focusing on IL-6 and TNF-α) and pain and anxiety.
Methods
Forty-seven people with well-controlled HIV and without confounding medical conditions or medications completed the Medical Outcomes Survey (MOS-HIV), quantification of a panel of 41 plasma cytokines/chemokines, and a standardized, non-invasive autonomic reflex screen (ARS). Adrenergic baroreflex sensitivity (BRSA) was calculated from the ARS as a measure of SNS function.
Results
Pain (46%) and anxiety (52%) were commonly reported on the MOS-HIV. BRSA was reduced in 30% of participants and elevated in 9% with the latter occurring only in participants with normal to mild HIV-AN. BRSA was significantly associated with IL-6, but not with TNF-α, pain or anxiety. Exploratory analyses also revealed positive associations of BRSA with numerous other cytokines with no significant inverse associations.
Conclusion
Higher BRSA, indicative of a more hyperadrenergic state, can be part of the spectrum of early HIV-AN, and may be associated with elevations in multiple cytokines including IL-6. These associations do not appear to be driven by stressors such as pain or anxiety.
Keywords: HIV, Autonomic, Sympathetic, Inflammation, Cytokines, IL-6
Highlights
-
•
Sympathetic dysfunction occurs as part of HIV-associated autonomic neuropathy.
-
•
Hypoadrenergic feature predominate, with some hyperadrenergic features early on.
-
•
Higher adrenergic baroreflex sensitivity is associated with increased IL-6.
-
•
This association is not influenced by pain or anxiety.
-
•
Adrenergic baroreflex sensitivity is also associated with many other cytokines.
1. Introduction
Autonomic neuropathy is common among people living with HIV, usually occurring as part of the spectrum of HIV-associated neuropathies (Robinson-Papp et al., 2012, 2013; Robinson-Papp and Sharma, 2013). Similar to other forms of autonomic dysfunction, HIV-associated autonomic neuropathy (HIV-AN) often leads to impairment of both the parasympathetic (i.e. vagal) and sympathetic nervous systems (SNS). With regard to the parasympathetic nervous system (PNS), we have previously shown that vagal dysfunction (VD) in HIV is associated with small intestinal bacterial overgrowth (SIBO) which in turn is associated with elevation of the pro-inflammatory cytokine IL-6 (Robinson-Papp et al., 2018). The mechanisms underlying these associations are unknown but may be related to alterations in gastrointestinal (GI) motility and the GI microbiome (Deloose et al., 2012; Balzan et al., 2007; Brenchley et al., 2006). However, there are additional non-GI-dependent pathways by which HIV-AN might contribute to systemic inflammation, and which have not yet been explored. In pre-clinical models, the SNS and PNS work in concert to influence inflammation (via a mechanism sometimes referred to as the cholinergic anti-inflammatory pathway) (Martelli et al., 2014). For example, in rodent models, vagal nerve stimulation (VNS) results in reduced systemic TNF-α response to LPS, but depends on the sympathetic splenic nerves as necessary intermediaries (Pavlov et al., 2006; Borovikova et al., 2000; Wang et al., 2003; Rosas-Ballina et al., 2008; Huston et al., 2006).
Human studies have examined associations between autonomic dysfunction and inflammation in various disease states, typically focusing on either vagal or SNS function. Studies focused on vagal function have been more uniform in methodology, using markers of heart rate variability (HRV) as a reflection of vagal function in associational studies, or using VNS in interventional research. For example, studies in sepsis, heart failure and coronary artery disease have demonstrated an association of lower HRV with poorer clinical outcomes and higher plasma IL-6 and other cytokines (Tateishi et al., 2007; Cedillo et al., 2015; Aronson et al., 2001; Janszky et al., 2004). In interventional work, VNS has been studied as a treatment for autoimmune conditions including Crohn’s disease, rheumatoid arthritis and Sjogren’s syndrome (Bonaz et al., 2016; Koopman et al., 2016; Johnson and Wilson, 2018).
Human studies focusing on the SNS are somewhat more complex because: 1) SNS dysfunction can involve hyper- and/or hypoadrenergic activity, 2) SNS activity is influenced by psychological stressors, and 3) there are methodologic challenges in quantifying SNS function. A hyperadrenergic state has been described in early diabetic autonomic neuropathy prior to development of more overt autonomic failure (Jacob et al., 2003). Similarly, postural orthostatic tachycardia syndrome (POTS) can have hyperadrenergic features which have sometimes been attributed to denervation hypersensitivity (Mar and Raj, 2020). Increased SNS activity has also been reported in a variety of psychiatric illnesses and states of chronic stress including post-traumatic stress disorder (PTSD), anxiety, chronic pain and early life adversity (Evans et al., 2019; Mondelli and Vernon, 2019; Fonkoue et al., 2020; Zamuner et al., 2015). With regard to quantification of SNS function, direct measurement can be performed using microneurography to record muscle sympathetic nerve activity (MSNA). However this can be technically challenging and MSNA also displays a significant floor effect in that the response becomes unobtainable in moderate to advanced autonomic disease (Carter, 2019; Donadio et al., 2010). SNS function can be approximated non-invasively by measuring blood pressure changes during the baroreflex, elicited by stimuli including the Valsalva maneuver (VM), tilt table testing or pharmacologic agents (Rudas et al., 1999; Low, 1993). One validated baroreflex-based measure of SNS function is adrenergic baroreflex sensitivity (BRSA), a continuous variable defined as the rate of systolic blood pressure recovery (mmHg/second) following release of the VM (Schrezenmaier et al., 2007; Huang et al., 2007).
Given that reduced vagal function is associated with inflammation and the SNS is an integral part of the pathway, one might hypothesize that a decline in SNS function in human disease would also be pro-inflammatory. However, many studies demonstrate the opposite. Studies measuring SNS function using MSNA found positive correlations with C-reactive protein (CRP) in patients with rheumatoid arthritis and PTSD (Adlan et al., 2017; Park et al., 2017). Other authors using analyses of heart rate and blood pressure variability (including BRSA) as surrogates for SNS function reported positive correlations with IL-6 in healthy volunteers and patients with chronic obstructive pulmonary disease and POTS (Bernstein et al., 2009; Chhabra et al., 2015; Okamoto et al., 2015).
The current study was inspired by these factors, namely, the high prevalence of HIV-AN and chronic stressors in people living with HIV, previous evidence of an indirect (i.e. GI-mediated) link between HIV-AN and elevated IL-6, the mixed hyper- and hypo-adrenergic features of early autonomic disease, and the association of SNS activity with inflammation in other disease states. The goals of the study were twofold. First we sought to describe patterns of SNS function (including hypo- and hyper-adrenergic features) in people living with HIV with normal autonomic function and early HIV-AN, using a standardized non-invasive autonomic reflex screen (ARS). Second we sought to determine whether BRSA, as a non-invasive marker of SNS function, was associated with markers of systemic inflammation (focusing on IL-6 and TNF-α) and pain and anxiety, controlling for potentially confounding effects from VD and SIBO.
2. Methods
2.1. Participants
This is a secondary data analysis of participants recruited to two other studies of HIV-AN, which have been described previously (Robinson-papp et al., 2018; Robinson-Papp et al., 2019). One was a cross-sectional observational study examining relationships between VD, SIBO, and plasma inflammatory markers (Robinson-papp et al., 2018). The second was an interventional study of the effect of a peripherally active acetylcholinesterase inhibitor, pyridostigmine, on SIBO (Robinson-Papp et al., 2019). The inclusion/exclusion criteria for both studies were the same and has been described previously (Robinson-papp et al., 2018; Robinson-Papp et al., 2019). Briefly, participants were adults (≥18 years) living with HIV and treated with combined antiretroviral therapy (CART) for at least 3 months, with HIV-1 plasma RNA load of ≤100 copies/ml. Confounders for autonomic and/or GI dysfunction (e.g. diabetes, interfering medications) or contraindication to any of the procedures were exclusionary. In addition, we sought to enrich the sample for participants with HIV-AN and so specifically sought out older participants and those with known peripheral neuropathy, since our prior work has shown HIV-AN to be more prevalent in these groups (Robinson-Papp et al., 2013).
2.2. Testing procedures
All procedures were performed in accordance with a protocol approved by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai (ISMMS). All participants provided written informed consent. All the data for this study were drawn from the baseline/cross-sectional visit which was performed over three days and was the same for both studies. Relevant procedures included a standardized autonomic reflex screen (ARS), glucose breath testing (GBT) for SIBO, blood draw for cytokine quantification, and administration of the Medical Outcomes Survey for HIV (MOS-HIV) (Wu et al., 1997). Nicotine use was prohibited for 24 h prior to all testing procedures. For the ARS, caffeine use was prohibited on the morning of testing. Briefly, the ARS consisted of: quantitative sudomotor axon reflex testing (QSART), heart rate response to deep breathing (HRDB), VM, and tilt table (Low, 1993; Novak, 2011). For GBT, participants are prescribed a special diet (no slowly-digesting foods) for the day prior and are fasting for 12 h prior. Participants exhale into a standardized apparatus at baseline, and then ingest a standardized glucose solution. Repeat breath samples are then collected every 20 min for 180 min. Samples were analyzed by an external CLIA-certified laboratory (Aerodiagnostics, Lexington, MA), using gas chromatography (QuinTron Micro Analyzer, QuinTron Instrument Company, Inc.), with results expressed as parts per million (ppm) of methane and hydrogen (Erdogan et al., 2015; Rezaie et al., 2017). Blood samples for cytokine quantification were processed within 1 h of draw time and stored as frozen plasma according to standard laboratory protocol. Plasma samples were then analyzed by our institution’s Human Immune Monitoring Center, using a bead-based ELISA method by Milliplex xMAP technology (Millipore, Billerica, MA) with a Luminex 200 system (Luminex Corporation, Austin, TX). We used the premixed 41 plex human cytokine/chemokine panel which includes: EGF, Eotaxin, FGF-2, FLT-3L, Fractalkine, G-CSF, GM-CSF, GRO, IFNα2, IFNγ, IL-1α, IL-1β, IL-1RA, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12P40, IL-12P70, IL-13, IL-15, IL-17A, IP-10, MCP-1, MCP-3, MDC, MIP-1α, MIP-1β, PDGF-AA, PDGF-AB/BB, RANTES, sCD40L, TGF-α, TNFα, TNFβ, VEGF). Results are expressed as mean fluorescence intensity (MFI); laboratory quality assurance procedures have been described previously (Robinson-papp et al., 2018).
2.3. Study variables
Overall autonomic dysfunction was quantified using data from the ARS and age and gender adjusted norms to calculate the Composite Autonomic Severity Score (CASS) which includes sudomotor, cardiovagal, and adrenergic sub-scores (Low, 1993). The sudomotor and cardiovagal sub-scores each range from 0 to 3; the adrenergic sub-score ranges from 0 to 4. For each sub-score zero is normal, and increasing scores indicate declining function; as in prior work we defined HIV-AN as CASS ≥3 (Robinson-Papp et al., 2013). Hyperadrenergic function is not captured by the CASS and so we also calculated BRSA from the VM data as previously described (Schrezenmaier et al., 2007; Huang et al., 2007; Robinson-Papp et al., 2015). Since our main interest here was SNS function we considered the SNS markers in greater detail, focusing on BRSA, but also considering the following which comprise the CASS adrenergic sub-score: mean arterial pressure (MAP) and pulse pressure (PP) decline during phase 2 of the VM (with cut-off values of 20 mmHg for MAP decline and <0.5 for the ratio of lowest PP to baseline PP), failure of blood pressure to return to baseline during phase 2 of the VM, lack of a phase 4 blood pressure overshoot following VM and orthostatic hypotension on tilt table testing. To quantify SIBO we used the increase in summated methane and hydrogen breath content. From the MOS-HIV we focused on items reflective of chronic psychological stress which could be confounders for increased SNS activity. These were questions 2 and 8a which query pain and anxiety over the past 4 weeks (how much bodily pain have you had; how much of the time have you been a very nervous person). From the cytokine panel we focused on IL-6 and TNF-α as we have in prior work (Robinson-papp et al., 2018; Robinson-Papp et al., 2019). However the other cytokines were included in exploratory analyses.
2.4. Statistical analyses
Descriptive statistics including frequencies and measures of center were performed for demographic and autonomic variables. We examined relationships between continuous normally distributed markers of SNS function using Pearson’s correlation coefficient; Spearman’s rank correlation coefficient was substituted if the assumption of normality was violated. Association of BRSA (a continuous, non-normally distributed variable) with binary markers of SNS function (e.g. the presence or absence of phase 4 overshoot) was performed using the Mann-Whitney U test. In exploratory analyses, Spearman’s rank correlation was also used to assess the association between BRSA and each of the cytokines/chemokines in our 41-analyte panel. To account for multiple testing in this analysis, statistical significance was assessed using the Benjamini-Hochberg method for p-value adjustment (Benjamini and Hochberg, 1995); we also present the more conservative Bonferroni-corrected type I error rate for reference. All analyses were performed using SAS 9.4 (Cary, NC) or R 3.6.1 (Team, 2019).
3. Results
3.1. Participant characteristics
A total of 76 participants were screened for the parent studies; all patients (n = 47) with completed baseline ARS and cytokine panels were included in the current analysis. As previously described, reasons for exclusion from the parent studies included uncontrolled HIV, prohibited co-morbid medical conditions (e.g. diabetes) or medications/substances, and loss to follow-up prior to completion of testing procedures (Robinson-papp et al., 2018; Robinson-Papp et al., 2019). Participants were diverse with regards to race/ethnicity and were predominantly middle-aged to older men with longstanding HIV who had experienced significant immune reconstitution (Table 1). Participants commonly experienced anxiety and pain. On the MOS-HIV, 52% of participants endorsed being “a very nervous person” at some point over the past 4 weeks and 46% reported moderate to severe pain. As might be expected, pain and anxiety were correlated with one another (rho = 0.364, p = 0.013). Consistent with our recruitment strategy, a range of autonomic function was observed (Table 1) with 26% of participants being normal and the remainder meeting criteria for mild to moderate HIV-AN. The percentages of participants with a score of ≥1 in individual CASS sub-scores (sudomotor 87%, adrenergic 77%, vagal 43%) was quite similar to our prior work (82%, 75%, 37%) (Robinson-Papp et al., 2013). The majority of participants (83%) displayed a deficits in more than one CASS sub-score.
Table 1.
Sample characteristics (n = 47).
| Age, yearsb | 57.5 (6.4) |
| Sex | |
| Male | 72% |
| Female | 28% |
| Race/ethnicity | |
| African-American | 47% |
| Hispanic/Latino | 30% |
| White | 21% |
| Other/Unknown | 2% |
| Current CD4+ count (cells/mm3)a | 606 (464–833) |
| Nadir CD4+ count (cells/mm3)a | 204 (110–423) |
| Self-reported year of HIV diagnosis, yearsa | 1995 (1991–2000) |
| Pain in the past 4 weeksc: | |
| None | 19% |
| Very mild | 23% |
| Mild | 11% |
| Moderate | 26% |
| Severe | 17% |
| Very Severe | 2% |
| Anxiety in the past 4 weeksc: | |
| All of the time | 2% |
| Most of the time | 4% |
| A good bit of the time | 9% |
| Some of the time | 6% |
| A little of the time | 30% |
| None of the time | 47% |
| Total Composite Autonomic Severity Score (CASS) | |
| Normal (0–2) | 26% |
| Mild (3) | 36% |
| Moderate (4–5) | 38% |
| Abnormal CASS sub-score | |
| Vagal sub-score ≥1 | 42% |
| Adrenergic sub-score ≥1 | 77% |
| Sudomotor sub-score ≥1 | 87% |
| Total CASS ≥3 | 75% |
Values are median (interquartile range).
Values are mean ± standard deviation.
Assessed with the MOS-HIV.
3.2. Patterns of SNS dysfunction
The specific findings that contributed to the abnormal CASS adrenergic sub-score are summarized in Table 2, along with BRSA. These included a high percentage of participants with larger than expected decline in pulse pressure during phase 2 of the VM, and lack of return of BP to baseline in phase 2, although only one participant had a MAP decline in excess of the 20 mmHg threshold. With regard to BRSA, in addition to the 30% of participants with values below the normal range, 9% had values above the normal range indicating a possible hyperadrenergic state (using values proposed by Huang and colleagues). (Huang et al., 2007).
Table 2.
Frequency of cardiovascular markers of reduced sympathetic nervous system (SNS).
| Marker | Phase of VM | Percent of participants with abnormality |
|---|---|---|
| Minimum PP during VM <50% of baseline | 2 | 64% |
| Blood pressure does not return to baseline during VM | 2 | 60% |
| MAP decline during VM > 20 mmHg | 2 | 2% |
| Reduced BRSA | 3 | 30% |
| Phase 4 of VM absent | 4 | 21% |
| Orthostatic hypotension on tilt | N/A | 13% |
PP = pulse pressure; VM = Valsalva maneuver; MAP = mean arterial pressure; BRSA = adrenergic baroreflex sensitivity.
Markers of SNS dysfunction derived from the same parts of the ARS tended to be associated with one another, whereas markers from different portions of the testing were not. For example, measures derived from phase 2 of the VM (MAP and PP decline) were correlated with one another (rho = 0.37, p = 0.011). Measures of SNS function derived from the post-release phases of the VM (phases 3 and 4) were also associated with one another; median BRSA was lower in participants in whom the phase 4 overshoot was absent (p = 0.007; median of 20.4 vs. 5.8). In contrast, markers from phase 2 of the VM were not associated with those from phases 3 and 4, and none of the VM-derived markers were associated with markers of SNS function derived from other tests, namely orthostatic hypotension on tilt and sudomotor sympathetic function as quantified by the CASS sudomotor sub-score (p > 0.05 for all).
Markers of hypo- and hyperadrenergic function sometimes coexisted in the same participants. For example, three participants with excessive blood pressure drop during VM displayed elevated BRSA, and four patients with orthostatic hypotension had BRSA in the normal range. The exception to this variability occurred in the patients with more severe HIV-AN. Among the 7 participants with a CASS of 5, the highest observed in this study, median BRSA was marginally lower than in other groups (U = 75, p = 0.06, median of 8.8 vs. 18.2), suggesting that with more severe HIV-AN a hypoadrenergic state becomes the norm.
3.3. SNS function, inflammatory markers, and potential confounders
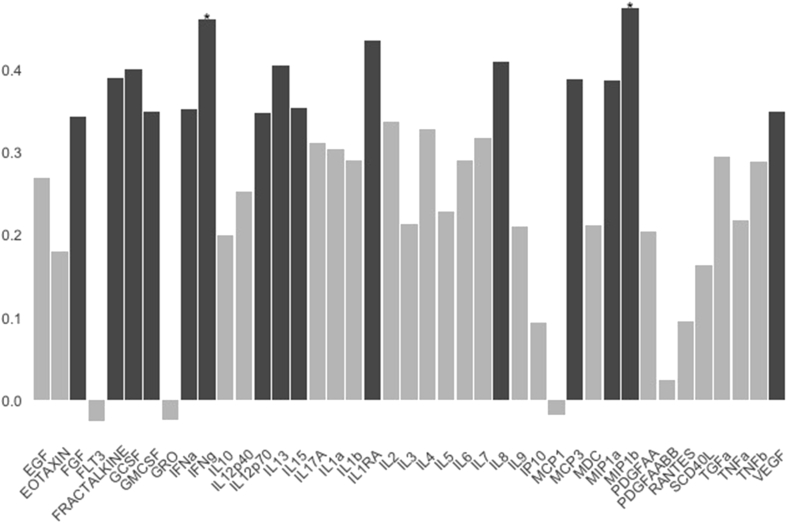
BRSA was not associated with pain, anxiety, age, VD or SIBO severity (p > 0.2 for all). In correlational analyses, BRSA was significantly associated with IL-6 (rho = 0.291, p = 0.048) but not TNF-α (rho = 0.217, p = 0.143). However, comparison of the group of participants with normal/elevated BRSA to those with reduced BRSA revealed that TNF-α was lower in the group with reduced BRSA: 140 (129–169) vs 172 (141–200); (units are MFI, data presented are median (interquartile range), p = 0.03). Finally, in exploratory analyses, we sought to understand whether our findings were specific to our chosen markers of SNS function and inflammation, namely BRSA and IL-6 and TNF-α, or if similar relationships would be observed for other markers of SNS function (e.g. CASS sudomotor sub-score and the markers listed in Table 2) and the other cytokines/chemokines included in our panel. We found no associations between IL-6 or TNF-α with any of the other SNS function markers. However BRSA was associated with numerous other cytokines. Of the 39 other cytokines/chemokines in the panel (see Fig. 1), BRSA was significantly positively correlated with 22 without correction for multiple comparisons; this number was reduced to 14 significant correlations using the Benjamini-Hochberg method for p-value adjustment. There were no significant inverse correlations. The two strongest correlates of BRSA, IFNγ and MIB1b (also known as CCL4), remained significant even under the more conservative Bonferroni correction. IFNγ is an inhibitor of viral replication which, interestingly, has been implicated as a mediator of sympathetic denervation of lymph nodes in the simian immunodeficiency virus (SIV) model (Sloan et al., 2008). MIB1b/CCL4 is an inhibitor of HIV-1 entry into cells (Hudspeth et al., 2012).
Fig. 1.
Figure legend. Results of an exploratory analysis in which bivariate Spearman’s rank correlations were performed between adrenergic baroreflex sensitivity (BRSA) and each of the 41 cytokines in our multiplex panel. Black bars indicate statistically significant correlations using a Benjamini-Hochberg adjustment for multiple testing, whereas asterisks indicate correlations that were statistically significant using the more conservative Bonferroni correction.
4. Discussion
Autonomic dysfunction has been described in HIV since the 1990s (Freeman et al., 1990; Craddock et al., 1987; Villa et al., 1992), although it has been relatively understudied compared to other neurologic complications of HIV. In addition to our own work, studies in the CART-era have documented mild abnormalities in cardiovascular autonomic reflexes and an association of HIV-AN with medical co-morbidities (Mittal et al., 2004; Sakhuja et al., 2007; Lebech et al., 2007; Chow et al., 2013; Askgaard et al., 2011). More recently, an analysis of data from the Multicenter AIDS Cohort Study (MACS) confirmed lower heart rate variability (consistent with cardiovascular autonomic dysfunction) in men with HIV compared to HIV-negative controls. The study also found that the QT variability index, a composite marker which encompasses both arrhythmia risk and cardiovascular autonomic dysfunction, was associated with elevated IL-6 and other inflammatory markers (Heravi et al., 2020). Another recent study focused on the stress response found that participants with HIV and history of intravenous drug use (IDU) had a significantly greater and more prolonged norepinephrine response to stress compared to those without infection or IDU history (Ownby et al., 2019).
In this study we sought to describe patterns of SNS dysfunction in people living with HIV with normal autonomic function and early HIV-AN, and to determine whether BRSA, as a non-invasive measure of adrenergic function, was associated with pain, anxiety and markers of systemic inflammation. We found that the overall distribution of adrenergic, cardiovagal and sudomotor deficits was very similar to our prior work, supporting the reproducibility of these findings (Robinson-Papp et al., 2013). With regard to patterns of SNS dysfunction, we found that hypoadrenergic features rarely occurred in isolation but rather in combination with signs of dysfunction in other parts of the autonomic nervous system. However the specific combinations of abnormalities observed were heterogeneous, suggesting a patchy evolution of deficits in HIV-AN. Some participants with normal or mildly abnormal autonomic function displayed evidence of a co-existing hyperadrenergic state, whereas this was not evident in those with more severe HIV-AN. In correlational analyses BRSA was significantly associated with IL-6 but not TNF-α, although median TNF-α was lower in the group of participants with reduced BRSA. Moreover exploratory analyses revealed positive associations of BRSA with numerous other cytokines (some of which reached moderate strength) and no significant inverse associations.
Our findings add to a growing body of literature demonstrating links between the SNS and inflammation in acute and chronic conditions, in pre-clinical models and human studies (Bellinger and Lorton, 2018). For acute conditions, like experimental sepsis, data from animal studies demonstrate that activity in the sympathetic splenic nerves is an essential controller of the inflammatory response (Rosas-Ballina et al., 2008; Huston et al., 2006; Bellinger and Lorton, 2018). This finding has been recapitulated in humans. In a study of healthy volunteers, controlled hyperventilation resulting in SNS activation dampened the production of IL-6, IL-8 and TNF-α in response to LPS injection (Kox et al., 2014). In contrast, most human studies in chronic disease show an inverse association between markers of SNS function and inflammatory biomarkers (Adlan et al., 2017; Park et al., 2017; Bernstein et al., 2009; Chhabra et al., 2015; Okamoto et al., 2015). Taken together these studies suggest that the relationship between SNS activity and inflammation is context and timing dependent, with the SNS serving an anti-inflammatory role acutely but becoming pro-inflammatory with chronic hyperactivity (Bellinger and Lorton, 2018). Mechanistically, it has been suggested that this transition may be mediated by downregulation and desensitization of immune cell β2-adrenergic receptors with chronic stimulation and switching from canonical to non-canonical signaling pathways (Bellinger and Lorton, 2018). Questions of the directionality of the relationship between the SNS and inflammatory mediators have also been raised. In multiple system atrophy, a neurodegenerative disorder characterized by autonomic failure (both SNS and vagal), IL-6 and TNF-α are elevated, however this has been interpreted as possible evidence for an inflammatory etiology of the disease (Kaufman et al., 2013). Similarly autoimmune diseases are considered risk factors for peripheral neuropathies (Bortoluzzi et al., 2019), and are associated with sympathetic neuropathy in the spleen and affected joints in animal models (Pongratz and Straub, 2013).
To our knowledge, this is the first study to examine these processes in HIV. The variability in SNS function observed in our participants with normal autonomic function or milder HIV-AN, combined with the variety of potential mechanisms at play, raises the question of what factors other than HIV-AN might also contribute to variance in BRSA and by extension to levels of inflammatory biomarkers. We excluded participants with interfering medications or substances of abuse, or confounding medical conditions. However we did not consider psychosocial factors in our enrollment criteria, and mental health issues including prior trauma and ongoing stress represent an important unmeasured variable in this work. We did administer the MOS-HIV which inquires about pain and anxiety, but more in depth questioning may have revealed psychosocial attributes which could explain some of the variance in BRSA. Another limitation of this study is small sample size resulting in reduced power; this resulted in some cases of marginal statistical significance and could have contributed to type 2 (false negative) errors. A specific example of this is the variability in whether BRSA was statistically significantly associated with IL-6 versus TNF-α depending on the analytic technique used. Another limitation is the fact that this is a secondary analysis of data which was not collected with this specific purpose in mind (although the primary focus of study, VD and inflammation, was closely aligned). Additionally we did not have HIV-negative controls, and so it is unknown to what extent the results are directly related to HIV. Moreover this was a purposive sample designed to select participants more likely to have HIV-AN. Thus the results will need to be replicated in a larger, more representative group of people living with HIV and appropriate controls without HIV. Finally this is an associational study which does not provide information about causality or underlying mechanism; these are other issues which should be explored in future work.
In summary, this study demonstrates that there is significant heterogeneity in SNS activity in people living with HIV, and provides preliminary support for an association between increased SNS activity and higher levels of a range of inflammatory cytokines in this population. Larger studies including HIV-uninfected controls, longitudinal follow-up, and more in depth psychosocial assessments are warranted to confirm the reproducibility of these findings and to understand their underlying mechanisms.
Funding
This work was funded by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK): R21DK105917, R01DK122853.
Ethics approval
All procedures described herein were conducted in accordance with a protocol approved by the Icahn School of Medicine at Mount Sinai Institutional Review Board.
Consent to participate
All participants provided written informed consent.
Consent for publication
Not applicable.
Availability of data and material
Source data available upon request.
Code availability
SAS statistical coding available upon request.
Authors’ contributions
Jessica Robinson-Papp: obtaining funding, design of the study, supervision of data collection, preliminary data analysis, composition of the manuscript. Varuna Astha: data analysis, review/editing of manuscript; Alexandra Nmashie: data collection (clinical data), review/editing of manuscript; Seunghee Kim-Schulze: data collection (cytokine analysis), review/editing of manuscript; Jacinta Murray: data collection (laboratory support for cytokine analysis), review/editing of manuscript; Mary Catherine George: design of the study, supervision of data collection, regulatory oversight, review/editing of manuscript.Sandeep K. Sharma: data collection (clinical data), review/editing of manuscript. Bridget R. Mueller: conceptualization of analysis, review/editing of manuscript; Steven A. Lawrence: data analysis, generation of the figure, review/editing of manuscript. Susan Morgello: obtaining funding, design of the study, supervision of data collection (laboratory support for cytokine analysis), review/editing of manuscript. Emma K.T. Benn: supervision of data analysis, review/editing of manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper..
References
- Adlan A.M. Increased sympathetic nerve activity and reduced cardiac baroreflex sensitivity in rheumatoid arthritis. J Physiol. 2017;595(3):967–981. doi: 10.1113/JP272944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson D., Mittleman M.A., Burger A.J. Interleukin-6 levels are inversely correlated with heart rate variability in patients with decompensated heart failure. J. Cardiovasc. Electrophysiol. 2001;12(3):294–300. doi: 10.1046/j.1540-8167.2001.00294.x. [DOI] [PubMed] [Google Scholar]
- Askgaard G. Decreased heart rate variability in HIV positive patients receiving antiretroviral therapy: importance of blood glucose and cholesterol. PloS One. 2011;6(5) doi: 10.1371/journal.pone.0020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzan S. Bacterial translocation: overview of mechanisms and clinical impact. J. Gastroenterol. Hepatol. 2007;22(4):464–471. doi: 10.1111/j.1440-1746.2007.04933.x. [DOI] [PubMed] [Google Scholar]
- Bellinger D.L., Lorton D. Sympathetic nerve hyperactivity in the spleen: causal for nonpathogenic-driven chronic immune-mediated inflammatory diseases (IMIDs)? Int. J. Mol. Sci. 2018;19(4) doi: 10.3390/ijms19041188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Roy. Stat. Soc. B. 1995;57(1):289–300. [Google Scholar]
- Bernstein I.M. The relationship of plasma volume, sympathetic tone, and proinflammatory cytokines in young healthy nonpregnant women. Reprod. Sci. 2009;16(10):980–985. doi: 10.1177/1933719109338876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaz B. Chronic vagus nerve stimulation in Crohn’s disease: a 6-month follow-up pilot study. Neuro Gastroenterol. Motil. 2016;28(6):948–953. doi: 10.1111/nmo.12792. [DOI] [PubMed] [Google Scholar]
- Borovikova L.V. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- Bortoluzzi A. Peripheral nervous system involvement in systemic lupus erythematosus: a review of the evidence. Clin. Exp. Rheumatol. 2019;37(1):146–155. [PubMed] [Google Scholar]
- Brenchley J.M. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Carter J.R. Microneurography and sympathetic nerve activity: a decade-by-decade journey across 50 years. J. Neurophysiol. 2019;121(4):1183–1194. doi: 10.1152/jn.00570.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedillo J.L. Usefulness of α7 nicotinic receptor messenger RNA levels in peripheral blood mononuclear cells as a marker for cholinergic antiinflammatory pathway activity in septic patients: results of a pilot study. J. Infect. Dis. 2015;211(1):146–155. doi: 10.1093/infdis/jiu425. [DOI] [PubMed] [Google Scholar]
- Chhabra S.K. Cardiac sympathetic dominance and systemic inflammation in COPD. COPD. 2015;12(5):552–559. doi: 10.3109/15412555.2014.974743. [DOI] [PubMed] [Google Scholar]
- Chow D. Rates of autonomic dysfunction in HIV patients receiving antiretroviral therapy. J. Neurovirol. 2013;19(5):511–512. doi: 10.1007/s13365-013-0198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock C. Cardiorespiratory arrest and autonomic neuropathy in AIDS. Lancet (London, England) 1987;2(8549):16–18. doi: 10.1016/s0140-6736(87)93054-6. [DOI] [PubMed] [Google Scholar]
- Deloose E. The migrating motor complex: control mechanisms and its role in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2012;9(5):271–285. doi: 10.1038/nrgastro.2012.57. [DOI] [PubMed] [Google Scholar]
- Donadio V. Autonomic innervation in multiple system atrophy and pure autonomic failure. J. Neurol. Neurosurg. Psychiatry. 2010;81(12):1327–1335. doi: 10.1136/jnnp.2009.198135. [DOI] [PubMed] [Google Scholar]
- Erdogan A. Small intestinal bacterial overgrowth: duodenal aspiration vs glucose breath test. Neuro Gastroenterol. Motil. 2015;27(4):481–489. doi: 10.1111/nmo.12516. [DOI] [PubMed] [Google Scholar]
- Evans T.C., Rodriguez A.M., Britton J.C. Sympathetic and self-reported threat reactivity in social anxiety: modulation by threat certainty and avoidance behavior. J. Psychopathol. Behav. Assess. 2019;41(4):627–638. [Google Scholar]
- Fonkoue I.T. Symptom severity impacts sympathetic dysregulation and inflammation in post-traumatic stress disorder (PTSD) Brain Behav. Immun. 2020;83:260–269. doi: 10.1016/j.bbi.2019.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman R. Autonomic function and human immunodeficiency virus infection. Neurology. 1990;40(4):575–580. doi: 10.1212/wnl.40.4.575. [DOI] [PubMed] [Google Scholar]
- Heravi A.S. HIV infection is associated with variability in ventricular repolarization. Circulation. 2020;141(3):176–187. doi: 10.1161/CIRCULATIONAHA.119.043042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.C. Effect of age on adrenergic and vagal baroreflex sensitivity in normal subjects. Muscle Nerve. 2007;36(5):637–642. doi: 10.1002/mus.20853. [DOI] [PubMed] [Google Scholar]
- Hudspeth K. Engagement of NKp30 on Vdelta1 T cells induces the production of CCL3, CCL4, and CCL5 and suppresses HIV-1 replication. Blood. 2012;119(17):4013–4016. doi: 10.1182/blood-2011-11-390153. [DOI] [PubMed] [Google Scholar]
- Huston J.M. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J. Exp. Med. 2006;203(7):1623–1628. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob G., Costa F., Biaggioni I. Spectrum of autonomic cardiovascular neuropathy in diabetes. Diabetes Care. 2003;26(7):2174–2180. doi: 10.2337/diacare.26.7.2174. [DOI] [PubMed] [Google Scholar]
- Janszky I. Inflammatory markers and heart rate variability in women with coronary heart disease. J. Intern. Med. 2004;256(5):421–428. doi: 10.1111/j.1365-2796.2004.01403.x. [DOI] [PubMed] [Google Scholar]
- Johnson R.L., Wilson C.G. A review of vagus nerve stimulation as a therapeutic intervention. J. Inflamm. Res. 2018;11:203–213. doi: 10.2147/JIR.S163248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman E. Proinflammatory cytokines are elevated in serum of patients with multiple system atrophy. PloS One. 2013;8(4) doi: 10.1371/journal.pone.0062354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman F.A. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc. Natl. Acad. Sci. U. S. A. 2016;113(29):8284–8289. doi: 10.1073/pnas.1605635113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kox M. Voluntary activation of the sympathetic nervous system and attenuation of the innate immune response in humans. Proc. Natl. Acad. Sci. U. S. A. 2014;111(20):7379–7384. doi: 10.1073/pnas.1322174111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebech A.-M. Autonomic dysfunction in HIV patients on antiretroviral therapy: studies of heart rate variability. Clin. Physiol. Funct. Imag. 2007;27(6):363–367. doi: 10.1111/j.1475-097X.2007.00760.x. [DOI] [PubMed] [Google Scholar]
- Low P.A. Composite autonomic scoring scale for laboratory quantification of generalized autonomic failure. Mayo Clinic proceedings.Mayo Clinic. 1993;68(8):748–752. doi: 10.1016/s0025-6196(12)60631-4. [DOI] [PubMed] [Google Scholar]
- Mar P.L., Raj S.R. Postural orthostatic tachycardia syndrome: mechanisms and new therapies. Annu. Rev. Med. 2020;71(1):235–248. doi: 10.1146/annurev-med-041818-011630. [DOI] [PubMed] [Google Scholar]
- Martelli D., McKinley M.J., McAllen R.M. The cholinergic anti-inflammatory pathway: a critical review. Auton. Neurosci.: Basic & Clinical. 2014;182:65–69. doi: 10.1016/j.autneu.2013.12.007. [DOI] [PubMed] [Google Scholar]
- Mittal C.M. Heart rate variability in human immunodeficiency virus-positive individuals. Int. J. Cardiol. 2004;94(1):1–6. doi: 10.1016/j.ijcard.2003.02.002. [DOI] [PubMed] [Google Scholar]
- Mondelli V., Vernon A.C. From early adversities to immune activation in psychiatric disorders: the role of the sympathetic nervous system. Clin. Exp. Immunol. 2019;197(3):319–328. doi: 10.1111/cei.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak P. Quantitative autonomic testing. JoVE. 2011;(53) doi: 10.3791/2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto L.E. Sympathetic activation is associated with increased IL-6, but not CRP in the absence of obesity: lessons from postural tachycardia syndrome and obesity. Am. J. Physiol. Heart Circ. Physiol. 2015;309(12):H2098–H2107. doi: 10.1152/ajpheart.00409.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ownby R.L. HIV-1 infection, injecting drug use, and neuroendocrine response to psychological stress. J hiv aids. 2019;5(2) doi: 10.16966/2380-5536.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. Baroreflex dysfunction and augmented sympathetic nerve responses during mental stress in veterans with post-traumatic stress disorder. J Physiol. 2017;595(14):4893–4908. doi: 10.1113/JP274269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov V.A. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc. Natl. Acad. Sci. Unit. States Am. 2006;103(13):5219–5223. doi: 10.1073/pnas.0600506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongratz G., Straub R.H. Role of peripheral nerve fibres in acute and chronic inflammation in arthritis. Nat. Rev. Rheumatol. 2013;9(2):117–126. doi: 10.1038/nrrheum.2012.181. [DOI] [PubMed] [Google Scholar]
- Rezaie A. Hydrogen and methane-based breath testing in gastrointestinal disorders: the north American consensus. Am. J. Gastroenterol. 2017;112(5):775–784. doi: 10.1038/ajg.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-Papp J., Sharma S.K. Autonomic neuropathy in HIV is unrecognized and associated with medical morbidity. AIDS Patient Care STDS. 2013;27(10):539–543. doi: 10.1089/apc.2013.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-Papp J. Autonomic neuropathy in HIV: a case report and review of potential symptoms in an advanced-stage, HIV cohort. WJA. 2012;2(3):265–269. [Google Scholar]
- Robinson-Papp J. Autonomic dysfunction is common in HIV and associated with distal symmetric polyneuropathy. J. Neurovirol. 2013;19(2):172–180. doi: 10.1007/s13365-013-0160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-Papp J. Optimizing measures of HIV-associated neuropathy. Muscle Nerve. 2015;51(1):56–64. doi: 10.1002/mus.24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-papp J. Vagal dysfunction and small intestinal bacterial overgrowth: novel pathways to chronic inflammation in HIV. Aids. 2018;32(9):1147–1156. doi: 10.1097/QAD.0000000000001802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-Papp J. The effect of pyridostigmine on small intestinal bacterial overgrowth (SIBO) and plasma inflammatory biomarkers in HIV-associated autonomic neuropathies. J. Neurovirol. 2019;25(4):551–559. doi: 10.1007/s13365-019-00756-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc. Natl. Acad. Sci. Unit. States Am. 2008;105(31):11008–11013. doi: 10.1073/pnas.0803237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudas L. Human sympathetic and vagal baroreflex responses to sequential nitroprusside and phenylephrine. Am. J. Physiol. 1999;276(5 Pt 2):H1691–H1698. doi: 10.1152/ajpheart.1999.276.5.h1691. [DOI] [PubMed] [Google Scholar]
- Sakhuja A. Heart rate variability and autonomic function tests in HIV positive individuals in India. Clin. Auton. Res.: Official Journal of the Clinical Autonomic Research Society. 2007;17(3):193–196. doi: 10.1007/s10286-007-0412-5. [DOI] [PubMed] [Google Scholar]
- Schrezenmaier C. Adrenergic and vagal baroreflex sensitivity in autonomic failure. Arch. Neurol. 2007;64(3):381–386. doi: 10.1001/archneur.64.3.381. [DOI] [PubMed] [Google Scholar]
- Sloan E.K. SIV infection decreases sympathetic innervation of primate lymph nodes: the role of neurotrophins. Brain Behav. Immun. 2008;22(2):185–194. doi: 10.1016/j.bbi.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi Y. Depressed heart rate variability is associated with high IL-6 blood level and decline in the blood pressure in septic patients. Shock (Augusta, Ga.) 2007;28(5):549–553. doi: 10.1097/shk.0b013e3180638d1. [DOI] [PubMed] [Google Scholar]
- Team R.C.R. R Foundation for Statistical Computing; Vienna, Austria.: 2019. A Language and Environment for Statistical Computing. [Google Scholar]
- Villa A., Foresti V., Confalonieri F. Autonomic nervous system dysfunction associated with HIV infection in intravenous heroin users. AIDS (London, England) 1992;6(1):85–89. doi: 10.1097/00002030-199201000-00011. [DOI] [PubMed] [Google Scholar]
- Wang H. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421(6921):384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- Wu A.W. vol. 6. 1997. Evidence for reliability, validity and usefulness of the medical outcomes study HIV health Survey (MOS-HIV) pp. 481–493. (Quality of Life Research: an International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation). 6. [DOI] [PubMed] [Google Scholar]
- Zamuner A.R. Relationship between sympathetic activity and pain intensity in fibromyalgia. Clin. Exp. Rheumatol. 2015;33(1):S53–S57. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Source data available upon request.