Abstract
Background:
In India, owing to multiple factors, such as insufficient laboratory capacity, poor reporting systems due to limited access to healthcare facilities and limited disease surveillance programmes, the actual disease burden of meningitis is unknown and may be largely under-reported.
Objective:
A systematic literature review was performed to describe: (a) the prevalence of meningitis; and (b) its etiological pathogen across different regions, age groups and patients with comorbidities.
Method:
A systematic literature search was conducted between 1990 and 2020 using PubMed and Google Scholar databases to identify Indian studies reporting the prevalence rates and etiology of meningitis. A total of 51 studies were included in the final analysis.
Results:
A total of 38 studies reported prevalence of meningitis and 21 studies reported data on the etiology of meningitis in India. These studies included mixed patient populations: (a) pyogenic meningitis; (b) meningitis in sick or hospitalized patients with tuberculosis, acute febrile encephalopathy syndrome, septicaemia, invasive pneumococcal disease or respiratory compromise; and (c) meningitis patients with comorbidities. The prevalence of confirmed bacterial meningitis in the pediatric population (0–14 years) ranged between 0.5% and 61.8%. A total of seven studies reported the prevalence of meningitis in patients of all age groups (0–75 years), with prevalence ranging between 8.68% and 78.85%. Cryptococcal meningitis was predominant in patients with positive HIV/AIDS, with a prevalence ranging between 2.09% and 53.1%. Streptococcus pneumoniae was found to be the predominant pathogen causing meningitis across different regions of India, with a frequency ranging from 4% to 61.8% in patients of all age groups.
Conclusion:
This systematic literature review displayed the possible range of frequency of bacterial meningitis pathogens across a wide variety of age groups in different regions of India. Further studies are warranted to monitor meningitis cases, which may facilitate the development of prevention and treatment strategies in India.
Keywords: disease burden, etiology, India, meningitis, systematic review
Introduction
Meningitis, an inflammatory condition involving the meninges and the spinal cord, is a devastating disease with a high mortality rate.1,2 Even in the survivors, meningitis is associated with significant long-term sequelae, and thus is regarded as a major global public health challenge. Outbreaks of meningitis have been considered as a global threat that warrant immediate attention.2 Meningitis can be caused by several different pathogens including bacteria, viruses, fungi and other parasites; the highest global burden reported for meningitis has been caused by bacteria.3,4 Cryptococcal meningitis caused by fungi is a common meningitis with increasing significance among adult patients with positive HIV.4 Besides infectious causes, non-infectious factors, such as certain medications, malignancy and autoimmune diseases, may also cause meningitis.2
As per the 2016 Global Burden of Disease (GBD) Study, the incident cases of meningitis have increased globally from 2.50 million (2.19–2.91) in 1990 to 2.82 million (2.46–3.31) in 2016.1,2 Over 1.2 million people are affected by bacterial meningitis each year across the globe.5 In the United States of America, an estimated 4100 cases of bacterial meningitis were reported annually between 2003 and 2007.6 In Europe, the yearly incidence of bacterial meningitis is 2.6–6.0 cases per 100,000.7 With a much higher disease burden,8,9 resource-poor countries may exhibit even a 10-fold higher incidence of meningitis versus resource-rich countries.7,8 According to the World Health Organization, approximately 290,000 patients lost their lives due to meningitis in 2015.2 The meningitis-associated mortality rate varies based on region, country, age, pathogen, etc., and may climb up to 70% without treatment.5 Despite attenuation in the overall meningitis-related mortality rate by 21% from 1990 to 2016, the overall burden of meningitis remains high. Besides a high mortality rate, potentially life-threatening infections, such as bacterial meningitis, may cause significantly high disability in the survivors.10–12 One in five survivors of bacterial meningitis may be left with permanent sequelae and prolonged neurological sequelae, such as cranial nerve palsies, hydrocephalus, seizures, hemiparesis and visual and hearing impairment. This may have a detrimental impact on the quality of life of the survivors.5,10 Out of the 5.6 million disability-adjusted life years globally caused by meningitis, 98% of the cases occur in low-income and middle-income countries.12
Before the development of immunization programmes, bacterial meningitis was predominantly caused by Streptococcus pneumoniae, Neisseria meningitidis and Hemophilus influenzae type b (Hib).13,14 Meningitis ranks among the top 10 causes of death in children younger than 14 years in high-income countries.12 The mortality rate associated with meningitis caused by S. pneumoniae and N. meningitidis was 30% and 7%, respectively, in high-resourced countries.15 Post-introduction of Hib and S. pneumoniae vaccines, high-income nations have witnessed a significant reduction in bacterial meningitis caused by these bacteria. However, the scenario is different in low and middle-income countries (LMICs), where bacterial meningitis continues to be a major concern. Inadequate vaccine coverage or lack of availability of these vaccines in the national immunization programmes are some of the factors responsible for this menace.13 The detrimental impact of meningitis in terms of mortality and morbidity is more profound among children under 5 years of age, especially in settings that lack proper implementation of vaccination as per national immunization programmes for H. influenzae type b, S. pneumoniae and N. meningitidis.13 Efforts to reduce mortality associated with meningitis lags substantially compared to that for other vaccine-preventable diseases (VPDs) such as measles, tetanus and diarrheal diseases.2
Cryptococcal meningitis is predominantly observed in adults with HIV. Unlike high-income countries, despite the widespread availability of antiretroviral therapy (ART), the prevalence of cryptococcal infection remains unaltered in LMICs. The estimation of regional as well as the national burden of cryptococcal meningitis is essential to devise optimal preventive and management strategies.4
In India, as a result of multiple factors, such as inadequate laboratory capacity, poor reporting due to the lack of access to the healthcare system and limited disease surveillance programmes, the actual disease burden of meningitis is unknown and may be largely underestimated.16 Considering the wide variability in the prevalence and causative pathogens of bacterial meningitis across regions, a comprehensive differentiation between them is essential for optimum management.14 The accurate estimation of meningitis disease burden and its causative pathogens in Indian settings may assist policy-makers to consider the inclusion of necessary vaccines under the national immunization programme.13 This will also help in the uniform expansion and distribution of these vaccines in the remote areas of the country. With that outlook, a comprehensive literature search was performed to describe: (a) the disease burden of meningitis; and (b) its etiological pathogens across different regions, age groups and patients with comorbidities.
Methods
This systematic literature review was conducted per the preferred recording items for systematic reviews and meta-analysis (PRISMA).17 Publications on meningitis disease burden and the etiology of meningitis in India were considered eligible for inclusion based on the eligibility criteria.
Eligibility criteria for studies
All human studies published from 1 January 1990 to 30 September 2020 in the English language that evaluated Indian patients of all age groups, and reported the prevalence of meningitis and the etiology for meningitis were eligible for inclusion.
Exclusion criteria for studies
Non-consecutive case series, series with incomplete reporting of results, case reports, newsletters, editorials, non-Indian data, and publications before 1990 were excluded from the list. Review articles and other publications citing data from more than one study were excluded from the final review. However, those articles were used to identify individual studies that had not already been identified in the literature search.
Measurements
The primary outcome of this study was to report the prevalence of different types of meningitis; i.e. bacterial, viral, or fungal in India. The secondary outcome was to determine the etiological pathogens across different regions, age groups and patients with comorbidities in India. Comorbidity was defined as the presence of one or more additional underlying medical conditions co-occurring with the primary condition (meningitis).
Search strategy
A systematic literature search was conducted using PubMed and Google Scholar database using search terms, such as ‘meningitis AND epidemiology AND India,’ ‘meningitis AND prevalence AND India,’ ‘meningitis AND disease burden AND India,’ for identifying epidemiological studies. The literature search was further extended using search engines, such as ‘pneumococcal meningitis AND India,’ ‘Hemophilus influenzae AND meningitis AND India,’ ‘viral meningitis AND India,’ ‘meningitis AND Streptococcus pneumoniae AND India,’ ‘meningitis AND group B Streptococcus agalactiae AND India,’ ‘meningitis AND Staphylococcus aureus AND India,’ ‘meningitis AND Escherichia coli AND India,’ ‘meningitis AND Listeria monocytogenes AND India,’ ‘meningitis AND Neisseria meningitidis AND India,’ to identify relevant articles. The search was performed after applying constant filters based on these additional search criteria: article types – clinical study, clinical trial, phase III, phase IV, comparative study, controlled clinical trial, evaluation study, meta-analysis, multicenter study, observational study, randomized controlled trial; language – English; publication date – 1 January 1990 to 30 September 2020; species – humans. A bibliographic search was also performed while assessing the full-text articles.
Data extraction
Literature searches were performed and retrieved publications were evaluated for eligibility by two independent reviewers in a two-phase screening process according to the predefined eligibility criteria. Data were extracted from the final list of publications that were considered relevant for this systematic literature review. The study characteristics extracted included authors’ details, year of publication, the title of the study, the place of study and type of study. Any disagreements among reviewers were resolved by discussion.
Our study did not require an ethical board approval because it did not contain human or animal trials.
Results
Literature selection
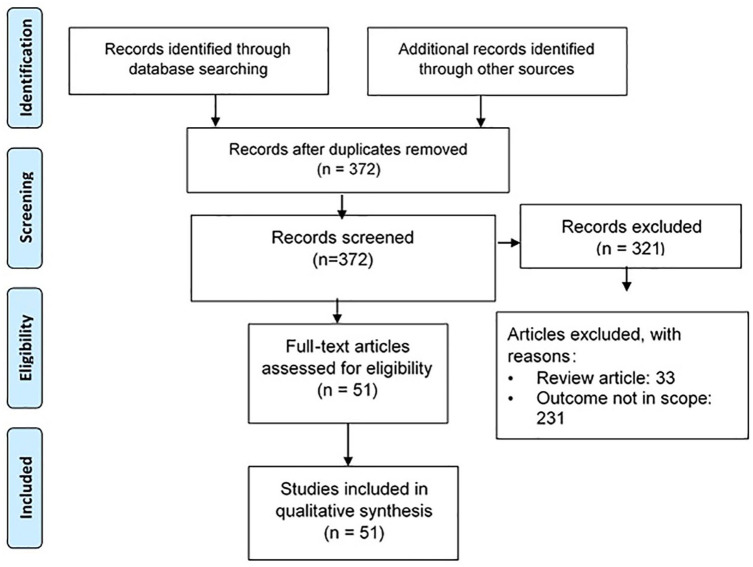
The primary literature searches in PubMed and Google Scholar yielded 706 relevant articles. Ten more articles were added after a bibliographic search. After the removal of 344 duplicate titles and abstracts, 372 articles (200 articles for epidemiology and 172 articles for meningitis etiology) were screened. Following a precise screening and review of the articles, a total of 321 studies were excluded and the remaining 51 studies were included in the systematic review. Figure 1 represents the flowchart of articles included in the analysis.
Figure 1.
Flowchart for study inclusion.
Findings of the analysis
Out of the total 716 unique records screened, a total of 51 studies reported data on either disease burden or etiology or both across different regions and different age-group populations.
Epidemiology/burden of meningitis in India across different regions, age groups and in patients with comorbidities
Out of the total 51 studies included, a total of 38 studies reported the prevalence of meningitis in India. These studies included mixed patient population: (a) pyogenic meningitis; (b) meningitis in sick or hospitalized patients with tuberculosis, acute febrile encephalopathy syndrome, septicaemia or invasive pneumococcal disease (IPD); and (c) meningitis patients with comorbidities. Table 1 shows the prevalence of meningitis in individual studies. A total of 18 studies conducted across various regions of India reported data exclusively on the pediatric population.13,18–34 Among these, a total of seven studies were conducted in northern India,18–24 whereas a total of four, two and four studies were conducted in the southern region,13,25–27 western region28,29 and multiple cities/states30–33 of India, respectively. One study was conducted in north-east India.34 The age of the study participants ranged from one day to 14 years. Two studies reported hospital-acquired meningitis in neonates/newborns, with a prevalence of 5.41% and 10%, respectively.18,22 Studies conducted in multiple states demonstrated the prevalence of confirmed bacterial meningitis in the range of 0.5% (15 cases out of 2904 study participants) to 35% (78 cases out of 226 study participants).30–33 The prevalence of bacterial meningitis ranges from 3% (four cases out of 147 study participants) to 21.81% (484 cases out of 2219 study participants) in north India.18–24 This included two studies that exclusively reported tubercular meningitis in pediatric patients, with a prevalence of 7.9% (12 cases out of 151 patients with acute febrile encephalopathy syndrome) and 20.97% (13 cases out of 62 patients with tuberculosis), respectively.19,21 In the western and southern regions, the prevalence of meningitis ranged from 12% to 20.25%28,29 and 8.28% to 38%,13,25–27 respectively. Three studies included patients with IPD from the southern region, multiple states and north-east India, with a reported prevalence of pneumococcal meningitis of 21.81%,27 35%33 and 61.8%,34 respectively. The data for patients belonging to all age groups were reviewed from seven studies.35–41 Unlike the majority of the studies that reported the predominance of bacterial meningitis, Yerramilli et al.,37 in an observational study, reported a high prevalence of aseptic/viral meningitis (39%). The majority of the studies reported bacterial meningitis. In a prospective surveillance study, conducted in 1037 adult subjects with a suspected invasive bacterial infection, 34.3% of the patients were diagnosed with meningitis.42 In a prospective, laboratory-based study, comprising 408 adults with IPD, the prevalence of meningitis was reported to be 24.3%.43
Table 1.
Epidemiological studies on meningitis.
| Author name | Study period/duration | Study place | Study region | Study participants | Age group | Overall prevalence | Bacterial/ fungal/viral | |
|---|---|---|---|---|---|---|---|---|
| Pediatric population | ||||||||
| 1 | Goel et al.18 | 2010–2011 | Delhi | North | 591 neonates | 0–28 days | Hospital-associated meningitis: 5.41% | Bacterial |
| 2 | Gupta et al.19 | NS | New Delhi | North | 62 children with TB | Median age: 7.73 years (0–12 years) | Tubercular meningitis: 20.97% (13 of the 62 cases) | Bacterial |
| 3 | Kapil and Bagga20 | 1986–1992 | Delhi | North | 3025 children admitted in ICU | Median age: 32.4 months (1 day–14 years) | 8.6% (261 children) | Bacterial |
| 4 | Karmakar et al.21 | 2004–2005 | Delhi | North | 151 patients with acute febrile encephalopathy syndrome | 3.21 ± 2.9 years | • Pyogenic meningitis: 33.8% (51
patients) • Tubercular meningitis: 7.9% (12 patients) |
Bacterial |
| 5 | Kumar et al.22 | NS/1 year | Delhi | North | 300 new-borns with sign and symptoms of sepsis | Newborns (age NR) | Prevalence of healthcare-associated meningitis: 10% (30 patients) | Bacterial |
| 6 | Saluja23 | NS/18 months | Delhi | North | 200 cases of febrile seizure | 6–18 months | 3% | Bacterial |
| 7 | Jhamb et al.24 | 2005–2006 | Delhi | North | 100 hospitalized children with group A meningococcal infection | Not mentioned | 67% | Bacterial |
| 8 | Minz et al.25 | 1997–1999 | Vellore | South | 97 suspected meningitis cases | <5 years | 18.56% | Bacterial |
| 9 | Das et al.26 | 1994–1995 | Pondicherry | South | 65 neonates with septicaemia | Neonates (age NR) | 38% (17 neonates) | Bacterial |
| 10 | Jayaraman et al.13 | 2012–2013 | Chennai | South | 3104 suspected meningitis | 1–59 months | 8.28% (257 cases) | Bacterial |
| 11 | Shah et al.27 | January–December 2006 | Bangalore | South | 2219 children with pneumococcal disease | 0–5 years | 21.81% (484 cases) | Bacterial |
| 12 | Debnath et al.28 | NS/1-year | Pune | West | 79 suspected cases of meningitis | Median age: 2.7 years (0–12 years) | 20.25% (16 cases) | Bacterial |
| 13 | Jain et al.29 | 2010–2012 | Pune | West | 223 children with suspected TB | 31 months | TB meningitis: 4.04% (9 cases) | Bacterial |
| 14 | Hazarika et al.30 | 2008–2009 | Shillong | North-East | children with IPD | 8.48 ± 5.09 year | Meningococcal meningitis: 61.8% | Bacterial |
| 15 | Kabra et al.31 | 1990/1 year | New Delhi Calcutta Rajasthan |
Multiple states | 56,338 admissions (Calcutta: 3400; Jaipur: 11134; Delhi KSCH:20307; Delhi AIIMS: 3348; Calcutta: 2904; Jodhpur 5245) | 1–12 years | 1.5% (852 cases; range: 0.5% to 2.6%) For prevalence of 0.8% in Calcutta (28 cases out of 3400 admissions) For prevalence of 2.6% in Jaipur (287 cases out of 11134 admissions) For prevalence of 1.9% in Delhi KSCH (394 cases out of 20301) For prevalence of 1.4% in Delhi AIIMS (48 cases out of 3348 admissions) For prevalence of 0.5% in Calcutta (15 cases out of 2904 admissions) For prevalence of 1.1% in Jodhpur (80 cases out of 5245 admissions) |
Bacterial |
| 16 | Chauhan et al.32 | NS/1 year | Shimla, Himachal Pradesh | Multiple states | 250 CSF samples | 1–59 months | 32.4% (81 cases) | Bacterial |
| 17 | Ramchandran et al.33 | 2008–2010 | Chennai New Delhi Vellore Lucknow |
Multiple states | 708 cases with abnormal CSF finding | 1–23 months | 12.57% (89 cases) | Bacterial |
| 18 | Manoharan et al.34 | 2011–2015 | New
Delhi Chennai Pune Kerala Bengaluru, Karnataka Mumbai, Maharashtra Hyderabad, Telangana |
Multiple states | 4377 enrolled patients, 361 children with pneumococcal
disease Among 361 patients with culture proven pneumococcal disease, all clinical data were known for 226 patients |
<5 years | Pneumococcal meningitis: 35% (78 cases) | Bacterial |
| All-age patient group | ||||||||
| 19 | Mishra et al.35 | 2011–2012 | Lucknow | North | 235 critically ill neurological patients 76 patients with CNS infection |
Median age: 37.5 (4–75 years) | • TB meningitis: 47% • Pyogenic meningitis: 11% • Fungal meningitis: 5% |
Bacterial and fungal |
| 20 | Bhagawati et al.36 | 2009–2010 | Assam | North-East | 316 CSF samples | 0–70 years | 16.14% | Bacterial and fungal |
| 21 | Yerramilli et al.37 | 2012–2014 | Hyderabad | South | 147 cases of meningitis | • Viral/aseptic meningitis: 39% • Tuberculous meningitis: 28% • Pyogenic meningitis: 28% • Fungal meningitis: 3% (4 cases) |
Bacterial, viral, and fungal | |
| 22 | Nair et al.38 | 2005–2006 | New Delhi | North | 380 suspected cases of meningococcal disease | <5–>45 years | 8.68% (33 cases) | Bacterial |
| 23 | Verghese et al.39 | 2008–2016 | Vellore, Tamil Nadu | South | 830 invasive pneumococcal isolates | All age groups | 20.1% (167 cases) | Bacterial |
| 24 | Srinivas et al.40 | 2001–2007 | Bangalore | South | 18,092 patients who underwent neurosurgery 415 patients with infection following a neurosurgery |
NR | Post-operative meningitis: 2.2% | |
| 25 | Gangane and Doddamani41 | 2010–2012 | Karnataka | South | 304 CSF samples with suspected meningitis 104 cases of confirmed meningitis |
0–>60 years | Bacterial meningitis: 78.85% Fungal meningitis: 9.62% Acid-fast bacilli meningitis: 10.58% |
Bacterial and fungal |
| Adult population | ||||||||
| 26 | Thomas et al.42 | 1993–2008 | Vellore | South | 1037 patients with suspected invasive bacterial infection | >18 years | Meningitis: 34.3% | Bacterial |
| 27 | Jayaraman et al.43 | 2007–2017 | Vellore | South | 408 adults with IPD | >18 years | Meningitis: 24.3% | Bacterial |
| Patients with comorbidities | ||||||||
| 28 | Kadam et al.44 | 2011–2012 | Pune, Maharashtra | West | 208 HIV patients | 40 years | Cryptococcal meningitis: 19% | Fungal |
| 29 | Satpute et al.45 | 1996–2005 | Pune | West | 1922 samples clinically diagnosed with cryptococcosis and 475 of them were HIV positive | NR | Cryptococcal meningitis in HIV patients: 53.1% (252/475) | Fungal |
| 30 | Jaiswal et al.46 | 1992–2001 | Indore | West | 483 AIDs cases | NR | Fungal meningitis: 3.1% (15 cases) • Cryptococcal meningitis: 2.48% (12 cases) • Candida meningitis: 0.62% (3 cases) |
Fungal |
| 31 | Wadia et al.47 | NS | Pune | West | 1527 HIV-positive subjects 457 HIV-positive subjects with neurological complications |
Prevalence of meningitis: 17.88% • Cryptococcal meningitis: 67.44% • Tubercular meningitis: 18.60% |
Fungal and bacterial | |
| 32 | Wadhwa et al.48 | 2005–2006 | New Delhi | North | 17 HIV-positive patients with CNS involvement | NR | • Cryptococcal meningitis: 29.4% • Tubercular meningitis: 52.9% |
Fungal and bacterial |
| 33 | Mathur and Devesh49 | 2006–2011 | Jaipur, Rajasthan | North | Total study patients: 40 20 HIV patients with neurological complications |
25–45 years | • Cryptococcal meningitis: 20% • Tubercular meningitis: 20% • Pyogenic meningitis: 5% • Candida meningitis: 5% |
Fungal and Bacterial |
| 34 | Lakshmi et al.50 | 1997–2005 | Hyderabad | South | 359 CSF samples of HIV-reactive cases suspected for cryptococcal meningitis | NR | Cryptococcal meningitis: 2.09% | Fungal |
| 35 | Kumaraswamy et al.51 | 1996–2001 | Chennai | South | 594 patients with HIV | Median age: 32 years (18–72 years) | Cryptococcal meningitis: 4.7% | Fungal |
| 36 | Teja et al.52 | NS/11 years | Hyderabad | South | 1606 hospitalized patients with HIV and neurological manifestation | 36 years | Meningitis: 39.4% • Tubercular: 25.06% • Cryptococcal: 10.95% • Pyogenic: 1.95% |
Bacterial and fungal |
| 37 | Indira et al.53 | 2011–2012 | Chennai, Tamil Nadu Mangalore, Karnataka |
South | 37 PLHIV-DM 37 PLHIV |
47 years | Cryptococcal meningitis: • PLHIV-DM: 19% • PLHIV: 16% |
Fungal |
| 38 | Sakhuja et al.54 | 1980–1999 | Chandigarh | North | 79 patients with CNS infection following renal transplantation | 33.16 ± 3.4 years | • Cryptococcal meningitis: 15.19% • Bacterial meningitis: 8.86% |
Fungal and bacterial |
CNS, central nervous system; CSF, cerebrospinal fluid; DM, diabetes mellitus; IPD, invasive pneumococcal disease; NR, not reported; NS, not specified; PLHIV-DM, people living with HIV and DM; TB, tuberculosis.
The systematic review included a total of 10 studies that demonstrated the prevalence of meningitis in AIDS/HIV-positive patients.44–53 The prevalence of meningitis, especially cryptococcal meningitis is high in this patient population, ranging from 2.09% to 53.1%. One of these studies included HIV patients with diabetes mellitus (DM) and the prevalence of cryptococcal meningitis in this patient population was 19%, which was higher than patients with HIV but no DM (16%).53 A total of five studies reported mixed-pathogen meningitis in patients with comorbidities.47–49,52,54 One retrospective study included 792 renal allograft recipients with neurological complications; 10% (79 patients) of them had central nervous system (CNS) complications, and among them 15.19% of the patients (12 patients) were diagnosed with cryptococcal meningitis.54
Etiology
Out of the total 51 studies included, 21 studies reported data on the etiology of meningitis. These studies included mixed patient populations: (a) pyogenic meningitis; and (b) meningitis in sick patients with fever, tuberculosis hydrocephalus or respiratory compromise. Of the total 21 studies,13,28,32,33,36,40,41,46,55–67 12 studies reported data on the pediatric population,13,28,32,33,55–62 whereas eight publications reported data on patients belonging to all age groups including neonates, older children, adolescents and adults.36,40,41,63–67 Table 2 highlights all the studies that reported the etiology of meningitis. The commonest cause of meningitis in these studies was S. pneumoniae, across different regions of India, with a frequency (in percentage) ranging from 12% to 82.9% in children and 4% to 61.8% in patients of all age groups. Out of the 12 studies in the pediatric population, a total of eight studies reported S. pneumoniae as the causative pathogen.13,32,33,55–57,59,61 The second commonest causative pathogen in the pediatric population was H. influenzae, predominantly type b, reported in nine studies,13,32,33,55–59,61 with a frequency ranging from 4.5% to 70%. In one study, conducted in 25 children with aseptic meningitis, enterovirus was found to be the causative pathogen in 56% of the cases.60 One of the studies found group B Streptococcus as a causative pathogen in more than 49% of the children aged between one and 59 months.32 In a retrospective study in 100 children with group A meningococcal infection, 67% of children had meningococcal meningitis.24
Table 2.
Etiological factors/pathogens responsible for meningitis.
| Author | Study period/duration | Region | Patient age | No. of patients with meningitis | S. pneumoniae | H. influenzae | N. meningitidis | S. aureus | GBS | E. coli | K. pneumoniae | Pseudomonas | Others | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pediatric population | ||||||||||||||
| 1 | Chinchankar et al.55 | NS/2 years | West | 1 month–5 years | 54 patients with ABM | 39% | 26% | 1.85% | 33.15% | |||||
| 2 | Debnath et al.28 | NS/21 years | West | Median age: 2.7 years | 16 suspected cases of meningitis | 6.25% | 31.20% | • CoNS: 12.5%• Nonfermenters: 25%• Streptococcus pyogenes: 18.8%• Citrobacter freundii: 6.25% | ||||||
| 3 | Vashishth et al.56 | 2009–2010 | North | 41.5 (±26.9) months | 67 patients with ABM | 25.37% | 4.50% | 3% | 3.30% | 7.50% | 3.30% | 3.30% | ||
| 4 | Chauhan et al.32 | NS/1 year | North | 1–59 months | 81 ABM cases | 23.40% | 6.10% | 1.20% | 7.40% | 49.30% | 6.10% | 6.10% | ||
| 5 | Sankar et al.57 | 2002–2003 | North | 2 months–12 years | 24 children with ABM | 41.67% | 29.17% | 20.83% | 8.33% | |||||
| 6 | Pulickal et al.58 | NS/18 months | South | NR | 80 children with ABM | 5% | 10% | 7.50% | CoNS: 9% | |||||
| 7 | Sahai et al.59 | 1994–1996 | South | 1 month–12 years | 100 children with ABM | 12% | 17% | Salmonella: 6% | ||||||
| 8 | Satish et al.60 | 2002 | South | 0–12 years | 25 children with aseptic meningitis | Enterovirus: 56% | ||||||||
| 9 | Shameem et al.61 | NS/48 months | South | 1 month–3 years | 199 cases of meningitis | 44.70% | 25.60% | 6.00% | 5.50% | 9.50% | 3% | 4% | 1.50% | |
| 10 | Jayaraman et al.13 | 2012–2013 | South | 1–59 months | 257 cases of meningitis | 82.90% | 14.40% | 2.70% | ||||||
| 11 | Devi et al.62 | 2013–2015 | East | 67 cases of neonatal meningitis | 3% | 1.50% | 12% | 9% | • baumannii:
18% • Enterococcus spp: 15% • CoNS: 12% • Cronobacter sakazakii: 1.5% • Roseomonas cervicalis: 1.5% |
|||||
| 12 | Ramchandran et al.33 | 2008–2010 | Multiple states | 1–23 months | 89 cases of bacterial meningitis | 13% | 70% | 3.37%* | 8% | 1.12% | • Enterobacter spp.:
2.25% • Citrobacter freundii: 1.12% • Group D Salmonella: 1.12% |
|||
| All age groups | ||||||||||||||
| 13 | Mirdha et al.63 | 1988–1989 | North | 1 month–60 years | 18 cases of bacterial meningitis | 16.67% | 11.11% | 22.22% | 11.11% | 5.50% | • Staph. Albus:
11.11% • Moraxella spp: 5.5% • Propionibacterium acnes: 5.5% |
|||
| 14 | Raza et al.64 | 2015–2017 | North | NR | 400 CSF samples of suspected HAM | 6.25%^ | 8.33% | 27.08% | 10.42% | 8.33% | • baumannii:
25% • Enterococcus faecium: 6.25% • CoNS: 4.16% |
|||
| 15 | Mital et al.65 | NS | North | 15–70 years | 50 cases of pyogenic meningitis | 31.03% | 27.59% | 41.38% | ||||||
| 29 culture positive cases of bacterial meningitis | ||||||||||||||
| 16 | Pandit et al.66 | 2002/10 months | South | 2–65 years | 21 positive CSF samples for bacterial meningitis | 9.52% | 19.04% | 4.76% | 4.76% | 4.76% | • Mycobacterium tuberculosis:
33.33% • Listeria monocytogenes: 4.76% |
|||
| 17 | Srinivas et al.40 | 2001–2007 | South | NR | 415 cases of post-operative meningitis | 8.67% | 12.53% | 15.42% | • NFGNB: 26.98% • CoNS+ S. epidermidis: 8.19% • MSSA: 6.75% • MRSA: 6.50% • MR-CoNS: 3.86% • Enterococcus spp.: 2.17% • Others: 2.65% |
|||||
| 18 | Mani et al.67 | 1996–2005 | South | NR | 284 cases of meningitis | 61.80% | 1.80% | 1% | 1.80% | • Gram-negative bacilli:
4.9% • Streptococcus spp.: 2.3% |
||||
| 19 | Gangane and Doddamani41 | 2010–2012 | South | NR | 308 CSF samples were collected from clinically diagnosed cases of bacterial meningitis. | 31.70% | 3.60% | 2.40% | 7.30% | 12.10% | 20.7% | 9.70% | Bacterial sample
(n = 82) Proteus: 6.09% Acinetobacter: 2.4% Enterococci: 2.4% Citrobacter freundii: 1.2%Fungal sample (n = 10) Cryptococcus: 70% Candida albicans: 30% |
|
| 20 | Bhagawati et al.36 | 2009–2010 | East | Newborn to >45 years | 51 cases of meningitis | 4% | 4% | 29.41% | 11.76% | 15.70% | 5.88% | • Acinetobacter spp.:
7.84% • Cryptococcus neoformans: 7.84% • Candida albicans: 5.88% • Listeria spp.: 4% • Citrobacter spp.: 4% |
||
| Patients with comorbidities | ||||||||||||||
| 21 | Jaiswal et al.46 | 1992–2001 | South | NR | 15 AIDS patients with meningitis | • Cryptococcus spp: 80%
(12) • Candida: 20% (3) |
||||||||
Two from groups A, C, Y or W135, one isolate that was either Neisseria meningitidis group B or Escherichia coli K1;
Community-acquired cases. Others are hospital-acquired meningitis cases; NS: Not specified.
A. baumannii, Acinetobacter baumannii; ABM, acute bacterial meningitis; CoNS, coagulase negative Staphylococci; E. coli, Escherichia coli; GBS, Group B Streptococcus; H. influenzae, Hemophilus influenzae; HAM, hospital-acquired meningitis; K. pneumoniae, Klebsiella pneumoniae; MR-CoNS, Methicillin-resistant coagulase negative Staphylococci; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; NFGNB, non-lactose fermenting Gram-negative bacillus; NR, not reported; S. aureus, Staphylococcus aureus; S. pneumoniae, Streptococcus pneumoniae.
In studies reporting data on patients belonging to all age groups, apart from S. pneumoniae, Staphylococcus aureus (seven studies; frequency range 1.8–41.38%),36,41,63–67 N. meningitidis (five studies; frequency range 1–27.59%),36,41,63,65,67 Klebsiella pneumoniae (five studies; frequency range 5.5–20.7%),36,40,41,63,64,66 Escherichia coli (five studies; frequency range 4.76–27.08%),36,40,41,64,66 H. influenzae (four studies; frequency range 1.8–19.4%),41,63,66,67 etc. were the other causative pathogens. Table 3 lists all the major etiological bacteria responsible for causing meningitis.
Table 3.
Major bacteria responsible for causing meningitis in the included studies.
| S. pneumoniae | |||||
|---|---|---|---|---|---|
| Author | Study period/duration | Region | Study details | Age group | Frequency |
| Chinchankar et al.55 | NS/2 years | West | 54 patients with ABM. Chief presentation was high fever,
refusal of feeds, altered sensorium and
seizures. • 26% of patients had meningeal signs. • 39% patients developed acute neurological complications during the hospital course. |
1 month–5 years | 39% |
| Vashishtha et al.56 | 2009–2010 | North | 67 patients with ABM Fever (91%), altered sensorium (62.7%), vomiting (50.8%), seizures (47.8%) and refusal of feeds (23.9%) were the most presenting features. |
41.5 (±26.9) months | 25.37% |
| Chauhan et al.32 | NS/1 year | North | 81 ABM cases. Observed complications included seizures (23.4%), increased intracranial pressure (20.9%), infarct on imaging (4.9%), coma (4.9%), and respiratory compromise leading to ventilator support (3.7%) | 1–59 months | 23.40% |
| Sankar et al.57 | 2002–2003 | North | 24 children with ABM | 2 months–12 years | 41.67% |
| Sahai et al.59 | 1994–1996 | South | 100 children with ABM | 1 month–12 years | 12% |
| Shameem et al.61 | NS/48 months | South | 199 cases of meningitis | 1 month–3 years | 44.70% |
| Jayaraman et al.13 | 2012–2013 | South | 257 cases of meningitis Male: female ratio in confirmed meningitis patients was 1.3:1 |
1–59 months | 82.90% |
| Ramchandran et al.33 | 2008–2010 | Multiple states | 89 cases of bacterial meningitis | 1–23 months | 13% |
| Mirdha et al.63 | 1988–1989 | North | 18 cases of bacterial meningitis | 1 month–60 years | 16.67% |
| Raza et al.64 | 2015–2017 | North | 400 CSF samples of suspected HAM | NR | 6.25%^ |
| Mital et al.65 | NS | North | 50 cases of pyogenic meningitis, 29 culture positive cases
of bacterial meningitis Male: female ratio of 5:1 |
15–70 years | 31.03% |
| Pandit et al.66 | 2002/10 months | South | 21 positive CSF samples for bacterial meningitis | 2–65 years | 9.52% |
| Mani et al.67 | 1996–2005 | South | CSF samples from 385 clinically suspected cases of pyogenic meningitis were analysed. Proof of infective agent was studied in 284 cases of meningitis | NR | 61.80% |
| Gangane and Doddamani41 | 2010–2012 | South | 308 CSF samples were collected from clinically diagnosed cases of bacterial meningitis. Out of 308 samples 92 samples were culture positive (30.6%). Out of which 82 were bacterial culture,10 fungal cultures and 11 smears were AFB positive. | NR | 31.70% |
| Bhagawati et al.36 | 2009–2010 | East | 51 cases of meningitis | Newborn to >45 years | 4% |
| H. influenzae | |||||
| Chinchankar et al.55 | NS/2 years | West | 54 patients with ABM. Chief presentation was high fever,
refusal of feeds, altered sensorium and
seizures. • 26% of patients had meningeal signs. • 39% patients developed acute neurological complications during the hospital course. |
1 month–5 years | 26% (Hib) |
| Vashishtha56 | 2009–2010 | North | 67 patients with ABM Fever (91%), altered sensorium (62.7%), vomiting (50.8%), seizures (47.8%) and refusal of feeds (23.9%) were the most presenting features. |
41.5 (±26.9) months | 4.50% (Hib) |
| Chauhan et al.32 | NS/1 year | North | 81 ABM cases. Observed complications included seizures (23.4%), increased intracranial pressure (20.9%), infarct on imaging (4.9%), coma (4.9%), and respiratory compromise leading to ventilator support (3.7%) | 1–59 months | 6.10% (Hib) |
| Sankar et al.57 | 2002–2003 | North | 24 children with ABM | 2 months–12 years | 29.17% (Hib) |
| Pulickal et al.58 | NS/18 months | South | 80 children with ABM | NR | 5% (Hib) |
| Sahai et al.59 | 1994–1996 | South | 100 children with ABM | 1 month–12 years | 17% (Hib) |
| Shameem et al.61 | NS/48 months | South | 199 cases of meningitis | 1 month–3 years | 25.60% (Hib) |
| Jayaraman et al.13 | 2012–2013 | South | 257 cases of meningitis Male: female ratio in confirmed meningitis patients was 1.3:1 |
1–59 months | 14.40% (Hib) |
| Ramchandran et al.33 | 2008–2010 | Multiple states | 89 cases of bacterial meningitis | 1–23 months | 70% (Hib) |
| Mirdha et al.63 | 1988–1989 | North | 18 cases of bacterial meningitis | 1 month–60 years | 11.11% |
| Pandit et al.66 | 2002/10 months | South | 21 positive CSF samples for bacterial meningitis | 2–65 years | 19.04% |
| Mani et al.67 | 1996–2005 | South | CSF samples from 385 clinically suspected cases of pyogenic meningitis were analysed. Proof of infective agent was studied in 284 cases of meningitis | NR | 1.80% |
| Gangane and Doddamani41 | 2010–2012 | South | 308 CSF samples were collected from clinically diagnosed cases of bacterial meningitis. Out of 308 samples 92 samples were culture positive (30.6%). Out of which 82 were bacterial culture,10 fungal cultures and 11 smears were AFB positive. | NR | 3.60% |
| K. pneumoniae | |||||
| Debnath et al.28 | NS/21 years | West | 79 suspected cases of meningitis with majority of cases
(74.7%) under-five. 16 suspected cases of
meningitis. Male to female ratio was 1.82:1 |
Median age: 2.7 years | 31.2% |
| Vashishtha et al.56 | 2009–2010 | North | 67 patients with ABM Fever (91%), altered sensorium (62.7%), vomiting (50.8%), seizures (47.8%) and refusal of feeds (23.9%) were the most presenting features. |
41.5 (±26.9) months | 3.30% |
| Chauhan et al.32 | NS/1 year | North | 81 ABM cases. Observed complications included seizures (23.4%), increased intracranial pressure (20.9%), infarct on imaging (4.9%), coma (4.9%), and respiratory compromise leading to ventilator support (3.7%) | 1–59 months | 6.10% |
| Shameem et al.61 | NS/48 months | South | 199 cases of meningitis | 1 month–3 years | 4% |
| Devi et al.62 | 2013–2015 | East | 67 cases of neonatal meningitis | 12% | |
| Mirdha et al.63 | 1988–1989 | North | 18 cases of bacterial meningitis | 1 month–60 years | 5.50% |
| Raza et al.64 | 2015–2017 | North | 400 CSF samples of suspected HAM | NR | 10.42% |
| Srinivas et al.40 | 2001–2007 | South | 415 cases of post-operative meningitis (underwent neurosurgical procedures). The overall incidence of meningitis was 2.2%. The incidence of meningitis was high (7.7%) in patients who had a pre-existing infection like post-pyogenic meningitis ortuberculosis hydrocephalus. | NR | 12.53% |
| Gangane and Doddamani41 | 2010–2012 | South | 308 CSF samples were collected from clinically diagnosed cases of bacterial meningitis. Out of 308 samples 92 samples were culture positive (30.6%). Out of which 82 were bacterial culture,10 fungal cultures and 11 smears were AFB positive. | NR | 20.7% |
| Bhagawati et al.36 | 2009–2010 | East | 51 cases of meningitis | Newborn to >45 years | 15.70% |
| P. aeruginosa | |||||
| Vashishtha et al.56 | 2009–2010 | North | 67 patients with ABM Fever (91%), altered sensorium (62.7%), vomiting (50.8%), seizures (47.8%) and refusal of feeds (23.9%) were the most presenting features. |
41.5 (±26.9) months | 3.30% |
| Pulickal et al.58 | NS/18 months | South | 80 children with ABM | NR | 7.50% |
| Shameem et al.61 | NS/48 months | South | 199 cases of meningitis | 1 month–3 years | 1.50% |
| Devi et al.62 | 2013–2015 | East | 67 cases of neonatal meningitis | 9% | |
| Ramchandran et al.33 | 2008–2010 | Multiple states | 89 cases of bacterial meningitis | 1–23 months | 1.12% |
| Raza et al.64 | 2015–2017 | North | 400 CSF samples of suspected HAM | NR | 8.33% |
| Pandit et al.66 | 2002/10 months | South | 21 positive CSF samples for bacterial meningitis | 2–65 years | 4.76% |
| Srinivas40 | 2001–2007 | South | 415 cases of post-operative meningitis (underwent neurosurgical procedures). The overall incidence of meningitis was 2.2%. The incidence of meningitis was high (7.7%) in patients who had a pre-existing infection like post-pyogenic meningitis ortuberculosis hydrocephalus. | NR | 15.42% |
| Gangane and Doddamani41 | 2010–2012 | South | 308 CSF samples were collected from clinically diagnosed cases of bacterial meningitis. Out of 308 samples 92 samples were culture positive (30.6%). Out of which 82 were bacterial culture,10 fungal cultures and 11 smears were AFB positive. | NR | 9.70% |
| Bhagawati et al.36 | 2009–2010 | East | 51 cases of meningitis | Newborn to >45 years | 5.88% |
| S. aureus | |||||
| Vashishtha et al.56 | 2009–2010 | North | 67 patients with ABM Fever (91%), altered sensorium (62.7%), vomiting (50.8%), seizures (47.8%) and refusal of feeds (23.9%) were the most presenting features. |
41.5 (±26.9) months | 3.30% |
| Chauhan et al.32 | NS/1 year | North | 81 ABM cases. Observed complications included seizures (23.4%), increased intracranial pressure (20.9%), infarct on imaging (4.9%), coma (4.9%), and respiratory compromise leading to ventilator support (3.7%) | 1–59 months | 7.40% |
| Sankar et al.57 | 2002–2003 | North | 24 children with ABM | 2 months–12 years | 20.83% |
| Pulickal et al.58 | NS/18 months | South | 80 children with ABM | NR | 10% |
| Shameem et al.61 | NS/48 months | South | 199 cases of meningitis | 1 month–3 years | 5.50% |
| Mirdha et al.63 | 1988–1989 | North | 18 cases of bacterial meningitis | 1 month–60 years | 11.11% |
| Raza et al.64 | 2015–2017 | North | 400 CSF samples of suspected HAM | NR | 8.33% |
| Mital et al.65 | NS | North | 50 cases of pyogenic meningitis, 29 culture positive cases
of bacterial meningitis Male: female ratio of 5:1 |
15–70 years | 41.38% |
| Pandit et al.66 | 2002/10 months | South | 21 positive CSF samples for bacterial meningitis | 2–65 years | 4.76% |
| Mani et al.67 | 1996–2005 | South | CSF samples from 385 clinically suspected cases of pyogenic meningitis were analysed. Proof of infective agent was studied in 284 cases of meningitis | NR | 1.80% |
| Gangane and Doddamani41 | 2010–2012 | South | 308 CSF samples were collected from clinically diagnosed cases of bacterial meningitis. Out of 308 samples 92 samples were culture positive (30.6%). Out of which 82 were bacterial culture,10 fungal cultures and 11 smears were AFB positive. | NR | 7.30% |
| Bhagawati et al.36 | 2009–2010 | East | 51 cases of meningitis | Newborn to >45 years | 29.41% |
| E. coli | |||||
| Debnath et al.28 | NS/21 years | West | 79 suspected cases of meningitis with majority of cases
(74.7%) under-five. 16 suspected cases of
meningitis. Male to female ratio was 1.82:1 |
Median age: 2.7 years | 6.25% |
| Vashishtha et al.56 | 2009–2010 | North | 67 patients with ABM Fever (91%), altered sensorium (62.7%), vomiting (50.8%), seizures (47.8%) and refusal of feeds (23.9%) were the most presenting features. |
41.5 (±26.9) months | 7.50% |
| Chauhan et al.32 | NS/1 year | North | 81 ABM cases. Observed complications included seizures (23.4%), increased intracranial pressure (20.9%), infarct on imaging (4.9%), coma (4.9%), and respiratory compromise leading to ventilator support (3.7%) | 1–59 months | 6.10% |
| Shameem et al.61 | NS/48 months | South | 199 cases of meningitis | 1 month–3 years | 3% |
| Devi et al.62 | 2013–2015 | East | 67 cases of neonatal meningitis | 1.50% | |
| Raza et al.64 | 2015–2017 | North | 400 CSF samples of suspected HAM | NR | 27.08% |
| Pandit et al.66 | 2002/10 months | South | 21 positive CSF samples for bacterial meningitis | 2–65 years | 4.76% |
| Srinivas et al.40 | 2001–2007 | South | 415 cases of post-operative meningitis (underwent neurosurgical procedures). The overall incidence of meningitis was 2.2%. The incidence of meningitis was high (7.7%) in patients who had a pre-existing infection like post-pyogenic meningitis or tuberculosis hydrocephalus. | NR | 8.67% |
| Gangane and Doddamani41 | 2010–2012 | South | 308 CSF samples were collected from clinically diagnosed cases of bacterial meningitis. Out of 308 samples 92 samples were culture positive (30.6%). Out of which 82 were bacterial culture,10 fungal cultures and 11 smears were AFB positive. | NR | 12.10% |
| Bhagawati et al.36 | 2009–2010 | East | 51 cases of meningitis | Newborn to >45 years | 11.76% |
| N. meningitidis | |||||
| Chinchankar et al.55 | NS/2 years | West | 54 patients with ABM. Chief presentation was high fever,
refusal of feeds, altered sensorium and
seizures. • 26% of patients had meningeal signs. • 39% patients developed acute neurological complications during the hospital course. |
1 month–5 years | 1.85% |
| Vashishtha et al.56 | 2009–2010 | North | 67 patients with ABM Fever (91%), altered sensorium (62.7%), vomiting (50.8%), seizures (47.8%) and refusal of feeds (23.9%) were the most presenting features. |
41.5 (±26.9) months | 3% |
| Chauhan et al.32 | NS/1 year | North | 81 ABM cases. Observed complications included seizures (23.4%), increased intracranial pressure (20.9%), infarct on imaging (4.9%), coma (4.9%), and respiratory compromise leading to ventilator support (3.7%) | 1–59 months | 1.20% |
| Shameem et al.61 | NS/48 months | South | 199 cases of meningitis | 1 month–3 years | 6.00% |
| Jayaraman et al.13 | 2012–2013 | South | 257 cases of meningitis Male: female ratio in confirmed meningitis patients was 1.3:1 |
1–59 months | 2.70% |
| Devi et al.62 | 2013–2015 | East | 67 cases of neonatal meningitis | 2.70% | |
| Ramchandran et al.33 | 2008–2010 | Multiple states | 89 cases of bacterial meningitis | 1–23 months | 3%* |
| Mirdha et al.63 | 1988–1989 | North | 18 cases of bacterial meningitis | 1 month–60 years | 22.22% |
| Mital et al.65 | NS | North | 50 cases of pyogenic meningitis, 29 culture positive cases
of bacterial meningitis Male: female ratio of 5:1 |
15–70 years | 27.59% |
| Mani et al.67 | 1996–2005 | South | CSF samples from 385 clinically suspected cases of pyrogenic meningitis were analysed. Proof of infective agent was studied in 284 cases of meningitis | NR | 1% |
| Gangane and Doddamani41 | 2010–2012 | South | 308 CSF samples were collected from clinically diagnosed
cases of bacterial meningitis. Out of 308 samples 92 samples
were culture positive (30.6%). Out of which 82 were
bacterial culture,10 fungal cultures and 11 smears
were AFB positive. |
NR | 2.40% |
| Bhagawati et al.36 | 2009–2010 | East | 51 cases of meningitis | Newborn to >45 years | 4% |
Community-acquired cases.
Two from groups A, C, Y or W135, one isolate that was either Neisseria meningitidis group B or Escherichia coli K1.
ABM, acute bacterial meningitis; E. coli, Escherichia coli; NR, not reported; HAM, hospital-acquired meningitis; CSF, cerebrospinal fluid; H. influenzae, Hemophilus influenzae; Hib, Hemophilus influenzae type b; NS, not specified; P. aeruginosa, Pseudomonas aeruginosa; S. aureus, Staphylococcus aureus; S. pneumoniae, Streptococcus pneumoniae; K. pneumoniae, Klebsiella pneumoniae.
Discussion
This systematic literature review provides an overview of the meningitis disease burden (1990–2020) and the etiology of meningitis across different age groups and regions in India. To our knowledge, this is the first systematic review of meningitis in India that focused on all forms of meningitis instead of limiting only to bacterial, viral or fungal meningitis. For this review, we considered published results from studies that were randomized controlled, cross-sectional, prospective cohort or observational. Non-consecutive case series, series with incomplete reporting of results, case reports, newsletters, editorials and non-Indian data were excluded in the systematic literature review.
Owing to the immense heterogeneity of the articles and study design, analyzing the true prevalence of meningitis was highly challenging. A total of 38 studies reported the prevalence of meningitis and 21 studies reported data on the etiology of meningitis in India. These studies included mixed patient populations: (a) pyogenic meningitis; (b) meningitis in sick or hospitalized patients with tuberculosis, acute febrile encephalopathy syndrome, septicaemia, invasive pneumococcal disease or respiratory compromise; and (c) meningitis patients with comorbidities. In the current review, the prevalence of confirmed bacterial meningitis in the pediatric population suspected of meningitis ranged between 3% and 61.8%, whereas in studies reporting patients of all age groups, the prevalence ranged between 8.68% and 78.85%. This sheds light on a crucial aspect that meningitis is not a disease confined to children and can equally affect the adult population.
This systematic literature review demonstrated that S. pneumoniae is the predominant pathogen that causes bacterial meningitis in the pediatric population as well as in patients belonging to all age groups across different regions of India. H. influenzae is the second major pathogen that causes meningitis in the pediatric population. Our report on the predominance of S. pneumoniae in the pediatric population is in concordance with global systematic review and meta-analysis data that demonstrated S. pneumoniae as the most predominant pathogen responsible for 25.1–41.2% of all bacterial meningitis cases.14 A systematic review comprising 23 studies involving 36,714 South-East Asian children demonstrated S. pneumoniae as the predominant pathogen for causing invasive bacterial disease including meningitis, with a frequency of 12.8% and 28% in population-based and retrospective hospital-based studies, respectively.68 Another systematic review and meta-analysis, comprising 21 studies, reported S. pneumoniae as the predominant causative pathogen for meningitis in adults and children, responsible for causing 25% of the total bacterial meningitis cases.69
Unlike global meta-analyses where N. meningitidis was found to be the second most prevalent pathogen responsible for bacterial meningitis,14 in the current systematic review analysis, it was observed that N. meningitidis is the seventh predominant pathogen that causes bacterial meningitis in India. In developed countries, meningococcal meningitis is one of the leading causes of bacterial meningitis.70 Out of 13 serogroups of meningococci, five serogroups (A, B, C, W135 and Y) are mostly responsible for invasive diseases.70,71 While in Europe, including England, and the US the predominance of serogroup B and serogroup Y, respectively, was observed, in parts of Asia, including India, the predominance of meningococcal A serogroup was observed.71 However, immunization targeting multiple serogroups may be the optimum strategy to confer protection against this disease.70
In this current literature review, viral meningitis was reported in only two studies, with a prevalence of 39% in one study37 and 56% in a study on children suspected of aseptic meningitis.60 In the pediatric study, most of the affected children were boys. A similar trend was observed in a meta-analysis reporting nation-wide data from five countries.72 A wide range of viruses are responsible for the occurrence of meningitis, yet there are limited treatment options, which limits its effective management. Intravenous acyclovir is widely used for the treatment of viral meningitis due to its associated benefits, such as the low risk of complications and mortality rate.73 No vaccination is available for viral meningitis (except treatment for meningitis caused by the herpes virus).74 Extensive research is warranted in this field to reduce the burden of viral meningitis.
The current literature review has demonstrated the high prevalence of fungal meningitis in the adult patient population ranging between 3% and 9.62%. Cryptococcal meningitis was predominant in patients with HIV/AIDS, with a prevalence ranging between 2.09% and 53.1%. According to the Joint United Nations Program on AIDS/HIV (UNAIDS) 2014 country-level estimates of the global incidence and cases of HIV infection data, the annual global burden of cryptococcal meningitis in HIV-positive patients was 223,100 [95% confidence interval (CI) 150,600–282,400] with 73% of cases reported from sub-Saharan Africa, followed by 19% of total global cases from Asia.4 Despite being responsible for 15% of AIDS-related global mortality, cryptococcal meningitis remains a neglected condition. Cryptococcal meningitis may cause the failure of HIV treatment strategies.4 However, the adoption of available preventive strategies may confer survival benefits and aid in attenuating the potentially fatal CNS infection in HIV patients.4,75 Rapid and accurate diagnoses is also essential to initiate an appropriate therapy.75 Robust screening of cryptococcal antigenemia along with pre-emptive fluconazole therapy needs to be an integral part of the routine HIV care package.4
As S. pneumoniae is the predominant pathogen responsible for bacterial meningitis globally as well as in India; we would like to emphasize the burden of pneumococcal meningitis and the required preventive action in India. Bacterial meningitis continues to be a significant cause of morbidity and mortality.9,76 S. pneumoniae and N. meningitidis are the two predominant pathogens responsible in these cases, with the former being accountable for two-thirds of cases in Europe and the US. Globally, bacterial meningitis continues to be a significant cause of morbidity and mortality.9,76 S. pneumoniae and N. meningitidis are the two predominant pathogens responsible in these cases, with the former being accountable for two-thirds of cases in Europe and the US.77
In the past two decades, post-implementation of vaccination strategies, an enormous change in the epidemiology of bacterial meningitis has been observed in the US. Owing to the administration of routine H. influenzae vaccines, a massive eradication of Hib-caused meningitis was evident among infants and children.78,79 Post-implementation of pneumococcal serotype vaccine, a similar declining trend was witnessed in terms of the incidence of pneumococcal meningitis among the pediatric population in the US.80 However, in the current scenario, bacterial meningitis predominantly affects adults more than children and infants,78 and S. pneumoniae is the predominant causative pathogen for bacterial meningitis in the US.69,78,81 In high-income countries, despite immense advancement in medical science, pneumococcal meningitis continues to be a serious disease among adults, with a high fatality rate and neurological sequelae ranging from 16% to 37% and 30% to 52%, respectively.15,82–85
Unlike developed countries, bacterial meningitis continues to be a matter of grave concern in LMICs. An inadequate level of vaccine coverage or unavailability of these vaccines in the respective national immunization programmes could be the attributing factors for this continual menace. Not only in the pediatric population, but pneumococcal diseases are also associated with a high mortality rate among older adults in India.42,43 Invasive pneumococcal disease demonstrated a 26% rise in mortality among adults aged over 66 years from 1993 to 2008 in India.42 Recently, a study published by Nandi et al.86 evaluated the associations of Hib vaccination on cognitive functioning and schooling attainment outcomes in Indian children (age groups 11–12 and 14–15 years). The study found that Hib-vaccinated children scored higher on the English test (p < 0.001) and mathematics test (p < 0.001), and attained more schooling grades (p < 0.05) than Hib-unvaccinated children, indicating cognitive benefits.86 India has witnessed a declining trend in Hib-caused meningitis in children.86 The Hib vaccine has been privately available in India since 1997.86 In 2011, the government of India introduced Hib immunization as part of the pentavalent vaccine in combination with vaccines for diphtheria, tetanus, pertussis and hepatitis B.86 This has led to S. pneumoniae being the most common causative pathogen for bacterial meningitis.13 Choosing an appropriate antibiotic is key to the optimum management of bacterial meningitis.5,13 However, emerging multidrug resistance in S. pneumoniae has raised a global concern, including in India,13,43 and has had a significant impact on its management. This signifies the necessity for the implementation of vaccination programmes for the prevention of the disease.5,13
Vaccines are the cornerstone of prevention and control of bacterial, especially pneumococcal, meningitis.5 For adults, two vaccines, i.e. pneumococcal conjugate vaccine 13 (PCV13) and pneumococcal polysaccharide vaccine 23 (PPSV23), may confer protection against pneumococcal disease including meningitis. PCV13 and PPSV23 protect against 13 serotypes and 23 serotypes of pneumococcal bacteria, respectively.87 PPSV23 has been available in the Indian market since the early 1980s but is not too effective in infants.88 In the mid-2010s, PCV13 was launched in India.89 In India, pneumococcal conjugate vaccine for adults is recommended for all healthy and at-risk adults (>50 years old) by the Indian Association of Occupational Health,90 the Indian Medical Association91 and the Association of Physicians in India.92 Sadly, despite being recommended by several regulatory bodies, the routine use of adult pneumococcal vaccines is limited due to the paucity of data on the pneumococcal disease burden and its serotype distribution.13 In India, currently two meningococcal conjugate vaccines (MCVs) are licensed: (a) quadrivalent meningococcal polysaccharide-protein conjugate vaccine (A, C, W135,Y) licensed in 2012 for use among persons aged 2–55 years; and (b) meningococcal group A conjugate vaccine licensed in 2009 for individuals 1–29 years of age.93 Therefore, the estimation of bacterial meningitis disease burden along with its predominant etiological pathogen is indispensable in a setting such as India, where an adult vaccination programme is yet to be established and implemented as a national immunization programme for broader public health benefit. Considering the high mortality rate and associated complications in the survivors of bacterial, viral, or fungal meningitis, there is a need for active disease surveillance systems that help in the accurate estimation of the disease burden. Furthermore, the development and implementation of locally available guidelines are warranted for the optimum management of meningitis in India.
Limitations of the study
The study limitations include the lack of quality assessment of included studies, the potential for publication bias related to high variability among the studies selected and study period (1990–2020; present data not included). India is a large heterogeneous country with limited surveillance for meningitis in spite of considerable disease burden. Owing to the lack of diagnostic and treatment facilities; there is a lack of reporting of the study population. The study populations in most of the included studies were poorly defined, which further complicated the review analysis. Considering the wide heterogeneity of the patient population as well as the inclusion criteria (most of the studies included patients with suspected meningitis) in the selected published articles, concluding the true meningitis burden in India was challenging. However, we tried to minimize the concerns through our precise adherence to the PRISMA model in selecting studies for inclusion. Also, due to the heterogeneity of the studies and mixed population groups, obtaining a pooled analysis was not feasible.
Further studies are required to monitor meningitis cases to facilitate preventive measures such as the implementation of vaccine programmes for tackling the meningitis disease burden in the Indian subcontinent.
Conclusion
This systematic literature review displayed the possible range of frequency of bacterial meningitis pathogens across a wide variety of age groups in different regions of India. A predominance in the frequency of bacterial meningitis caused by different etiological pathogens, especially S. pneumoniae, was evident in this analysis. Continual robust monitoring is essential to estimate the disease burden along with the distribution and variety of pneumococcal serotypes to assess further the emerging antibiotic resistance pattern in India. Wider implementation of adult immunization programmes may hold substantial potential for reducing the pneumococcal meningitis burden in India. Further studies are warranted to monitor meningitis cases, which may facilitate the further development of prevention and treatment strategies in India.
Supplemental Material
Supplemental material, sj-doc-1-tai-10.1177_20499361211046453 for A systematic literature review on the prevalence and etiology of meningitis among critically ill and hospitalized patients in India by Canna J. Ghia and Gautam S. Rambhad in Therapeutic Advances in Infectious Disease
Footnotes
Authorship: Both the authors have contributed equally to the conception, design, analysis and interpretation of data; drafted the article or revised it critically for important intellectual content; approved the version to be published; are in agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of interest statement: The authors declare that there is no conflict of interest. The authors are employees of Pfizer India.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This systematic literature review was funded by Pfizer Limited.
ORCID iD: Canna J. Ghia  https://orcid.org/0000-0001-9839-3209
https://orcid.org/0000-0001-9839-3209
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Canna J. Ghia, Medical and Scientific Affairs, Pfizer Limited, Mumbai, Maharashtra 400051, India.
Gautam S. Rambhad, Medical and Scientific Affairs, Pfizer Limited, Mumbai, Maharashtra, India
References
- 1.Zunt JR, Kassebaum NJ, Blake N. Global, regional, and national burden of meningitis, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2018; 17: 1061–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Defeating meningitis by 2030: baseline situation analysis. https://www.who.int/immunization/sage/meetings/2019/april/2_DEFEATING_MENINGITIS_BY_2030_baseline_situation_analysis.pdf (2019, accessed 16 November 2020).
- 3.World Health Organization. Meningitis. https://www.who.int/health-topics/meningitis#tab=tab_1 (accessed 16 November 2020).
- 4.Rajasingham R, Smith RM, Park BJ, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 2017; 17: 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Chapter 2: Epidemiology of meningitis caused by Neisseria meningitidis, Streptococcus pneumoniae, and Hemophilus influenzae. https://www.cdc.gov/meningitis/lab-manual/chpt02-epi.pdf (accessed 16 November 2020).
- 6.Thigpen MC, Whitney CG, Messonnier NE, et al. Bacterial meningitis in the United States, 1998–2007. N Engl J Med 2011; 364: 2016–2025. [DOI] [PubMed] [Google Scholar]
- 7.Van de Beek D, Farrar JJ, de Gans J, et al. Adjunctive dexamethasone in bacterial meningitis: a meta-analysis of individual patient data. Lancet Neurol 2010; 9: 254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scarborough M, Thwaites GE. The diagnosis and management of acute bacterial meningitis in resource-poor settings. Lancet Neurol 2008; 7: 637–648. [DOI] [PubMed] [Google Scholar]
- 9.Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev 2010; 23: 467–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tebruegge M, Curtis N. Epidemiology, etiology, pathogenesis, and diagnosis of recurrent bacterial meningitis. Clin Microbiol Rev 2008; 21: 519–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van de Beek D. Progress and challenges in bacterial meningitis. Lancet 2012; 380: 1623–1624. [DOI] [PubMed] [Google Scholar]
- 12.McIntyre PB, O’Brien KL, Greenwood B, et al. Effect of vaccines on bacterial meningitis worldwide. Lancet 2012; 380: 1703–1711. [DOI] [PubMed] [Google Scholar]
- 13.Jayaraman Y, Veeraraghavan B, Purushothaman GKC, et al. Burden of bacterial meningitis in India: preliminary data from a hospital-based sentinel surveillance network. PLoS One 2018; 13: e0197198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oordt-Speets AM, Bolijn R, van Hoorn RC, et al. Global etiology of bacterial meningitis: a systematic review and meta-analysis. PLoS One 2018; 13: e0198772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van de Beek D, de Gans J, Spanjaard L, et al. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med 2004; 351: 1849–1859. [DOI] [PubMed] [Google Scholar]
- 16.Dutta AK, Swaminathan S, Abitbol V. A comprehensive review of meningococcal disease burden in India. Infect Dis Ther 2020; 9: 537–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151: 264–269. [DOI] [PubMed] [Google Scholar]
- 18.Goel K, Randhawa VS, Saili A, et al. Healthcare-associated meningitis in neonates – a prospective study of epidemiology, etiology and risk factors. EC Microbiol 2017; 6: 14–23. [Google Scholar]
- 19.Gupta N, Kashyap B, Dewan P, et al. Clinical spectrum of pediatric tuberculosis: a microbiological correlation from a tertiary care center. J Trop Peadiatr 2018; 65: 130–138. [DOI] [PubMed] [Google Scholar]
- 20.Kapil D, Bagga A. The profile and outcome of patients admitted to a pediatric intensive care unit. Indian J Pediatr 1993; 60: 5–10. [DOI] [PubMed] [Google Scholar]
- 21.Karmakar SA, Aneja S, Khare S, et al. A study of acute febrile encephalopathy with special reference to viral etiology. Indian J Pediatr 2008; 75: 801–805. [DOI] [PubMed] [Google Scholar]
- 22.Kumar S, Shankar B, Arya S, et al. Healthcare associated infections in neonatal intensive care unit and its correlation with environmental surveillance. J Infect Public Health 2018; 11: 275–279. [DOI] [PubMed] [Google Scholar]
- 23.Saluja C. Prevalence of meningitis in the first episode of febrile seizure in children aged between six to eighteen months. Joint Event on 29th International Conference on Pediatrics and Primary Care and 15th International Conference on Clinical Dermatology, 23–24 September 2019, Barcelona, Spain. Qual Prim Care 2019; 27: 40. [Google Scholar]
- 24.Jhamb U, Chawla S, Khanna B. Clinical profile of group A meningococcal outbreak in Delhi. Indian Pediatr 2009; 46: 794–796. [PubMed] [Google Scholar]
- 25.Minz S, Balraj V, Lalitha M, et al. Incidence of Hemophilus influenzae type B meningitis in India. Indian J Med Res 2008; 128: 57–64. [PubMed] [Google Scholar]
- 26.Das GC, Mahadevan S, Srinivasan S. Meningitis in home delivered neonates in Pondicherry, South India. J Trop Pediatr 1998; 44: 56–57. [DOI] [PubMed] [Google Scholar]
- 27.Shah AS, Nisarga R, Kumar KLR, et al. Establishment of population-based surveillance for invasive pneumococcal disease in Bangalore, India. Indian J Med Sci 2009; 63: 498–507. [PubMed] [Google Scholar]
- 28.Debnath DJ, Wanjpe A, Kakrani V. Epidemiological study of acute bacterial meningitis in admitted children below twelve years of age in a tertiary care teaching hospital in Pune, India. Med J DY Patil Univ 2012; 5: 28–30. [Google Scholar]
- 29.Jain SK, Ordonez A, Kinikar A, et al. Pediatric tuberculosis in young children in India: a prospective study. Biomed Res Int 2013; 2013: 783698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hazarika DR, Deka NM, Khyriem AB, et al. Invasive meningococcal infection: analysis of 110 cases from a tertiary care centre in North East India. Indian J Pediatr 2013; 80: 359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kabra SK, Kumar P, Verma IC, et al. Bacterial meningitis in India: an IJP survey. Indian J Pediatr 1991; 58: 505–511. [DOI] [PubMed] [Google Scholar]
- 32.Chauhan D, Mokta K, Kanga A, et al. Epidemiology, clinical profile and role of rapid tests in the diagnosis of acute bacterial meningitis in children (aged 1–-59 months). Neurol India 2018; 66: 1045–1049. [DOI] [PubMed] [Google Scholar]
- 33.Ramachandran P, Fitzwater SP, Aneja S, et al. Prospective multi-centre sentinel surveillance for Hemophilus influenzae type B and other bacterial meningitis in Indian children. Indian J Med Res 2013; 137: 712–720. [PMC free article] [PubMed] [Google Scholar]
- 34.Manoharan A, Manchanda V, Balasubramanian S, et al. Invasive pneumococcal disease in children aged younger than 5 years in India: a surveillance study. Lancet Infect Dis 2017; 17: 305–312. [DOI] [PubMed] [Google Scholar]
- 35.Mishra UK, Kalita J, Bhoi SK. Spectrum and outcome predictors of central nervous system infections in a neurological critical care unit in India: a retrospective review. Trans R Soc Trop Med Hyg 2014; 108: 141–146. [DOI] [PubMed] [Google Scholar]
- 36.Bhagawati G, Barkataki D, Hazarika NK. Study on isolates of acute meningitis in a tertiary care centre in Assam. Int J Public Health 2014; 4: 446–450. [Google Scholar]
- 37.Yerramilli A, Mangapati P, Prabhakar S, et al. A study on the clinical outcomes and management of meningitis at a tertiary care centre. Neurol India 2017; 65: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 38.Nair D, Dawar R, Deb M, et al. Outbreak of meningococcal disease in and around New Delhi, India, 2005–2006: a report from a tertiary care hospital. Epidemiol Infect 2009; 137: 570–576. [DOI] [PubMed] [Google Scholar]
- 39.Verghese VP, Veeraraghavan B, Jayaraman R, et al. Increasing incidence of penicillin- and cefotaxime-resistant Streptococcus pneumoniae causing meningitis in India: time for revision of treatment guidelines? Indian J Med Microbiol 2017; 35: 228–236. [DOI] [PubMed] [Google Scholar]
- 40.Srinivas D, Veena Kumari HB, Somanna S, et al. The incidence of postoperative meningitis in neurosurgery: an institutional experience. Neurol India 2011; 59: 195–198. [DOI] [PubMed] [Google Scholar]
- 41.Gangane R, Doddamani PK. Bacteriological profile of bacterial meningitis at tertiary care hospital in North Karnataka. Int J Pharm Bio Sci 2013; 4: 1356–1361. [Google Scholar]
- 42.Thomas K, Kesavan LM, Veeraraghavan B, et al. Invasive pneumococcal disease associated with high case fatality in India. J Clin Epidemiol 2013; 66: 36–43. [DOI] [PubMed] [Google Scholar]
- 43.Jayaraman R, Varghese R, Kumar JL, et al. Invasive pneumococcal disease in Indian adults: 11 years’ experience. J Microbiol Immunol Infect 2019; 52: 736–742. [DOI] [PubMed] [Google Scholar]
- 44.Kadam D, Chandanwale A, Bhardwaj R, et al. High prevalence of cryptococcal antigenaemia amongst asymptomatic advanced HIV patients in Pune, India. Indian J Med Microbiol 2017; 35: 105–108. [DOI] [PubMed] [Google Scholar]
- 45.Satpute MG, Telang MV, Litake GM, et al. Prevalence of cryptococcal meningitis at a tertiary care centre in Western India (1996–2005). J Med Microbiol 2006; 55: 1301–1302. [DOI] [PubMed] [Google Scholar]
- 46.Jaiswal SPB, Hemwani N, Sharma N, et al. Prevalence of fungal meningitis among HIV positive and negative subjects in Indore (MP State). Indian J Med Sci 2002; 56: 325–329. [PubMed] [Google Scholar]
- 47.Wadia RS, Pujari SN, Kothari S. Neurological manifestations of HIV disease. J Assoc Physicians India 2001; 49: 343–348. [PubMed] [Google Scholar]
- 48.Wadhwa A, Kaur R, Bhalla P. Profile of central nervous system disease in HIV/AIDS patients with special reference to cryptococcal infections. Neurologist 2008; 14: 247–251. [DOI] [PubMed] [Google Scholar]
- 49.Mathur AD, Devesh S. A comparative study of CSF viral RNA loads between HIV positive patients with neurological manifestations and neurologically asymptomatic HIV patients. J Assoc Physicians India 2017; 65: 14–17. [PubMed] [Google Scholar]
- 50.Lakshmi V, Sudha T, Teja VD, et al. Prevalence of central nervous system cryptococcosis in human immunodeficiency virus reactive hospitalized patients. Indian J Med Microbiol 2007; 25: 146–149. [DOI] [PubMed] [Google Scholar]
- 51.Kumaraswamy N, Solomon S, Flanigan TP, et al. Natural history of human immunodeficiency virus disease in southern India. Clin Infect Dis 2003; 36: 79–85. [DOI] [PubMed] [Google Scholar]
- 52.Teja VD, Talasila SR, Vemu L. Neurologic manifestations of HIV infection: an Indian hospital-based study. AIDS Read 2005; 15: 139–145. [PubMed] [Google Scholar]
- 53.Indira P, Kumar PM, Shalini S, et al. Opportunistic infections among people living with HIV (PLHIV) with diabetes mellitus (DM) attending a tertiary care hospital in coastal city of South India. PLoS One 2015; 10: e0136280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sakhuja V, Sud K, Kalra OP, et al. Central nervous system complications in renal transplant recipients in a tropical environment. J Neurol Sci 2001; 183: 89–93. [DOI] [PubMed] [Google Scholar]
- 55.Chinchankar N, Mane M, Bhabe S, et al. Diagnosis and outcome of acute bacterial meningitis in early childhood. Indian Pediatr 2002; 39: 914–921. [PubMed] [Google Scholar]
- 56.Vashishtha VM, Garg A, John TJ. Etiology of acute bacterial meningitis in hospitalized children in Western Uttar Pradesh. Indian Pediatr 2011; 48: 986. [PubMed] [Google Scholar]
- 57.Sankar G, Singhi P, Bansal A, et al. Role of dexamethasone and oral glycerol in reducing hearing and neurological sequelae in children with bacterial meningitis. Indian Pediatr 2007; 44: 649–656. [PubMed] [Google Scholar]
- 58.Pulickal AS, Mathew AM, Xavier D. Patterns and outcome of acute bacterial meningitis in a south Indian tertiary level hospital. Indian J Public Health 2005; 49: 254–255. [PubMed] [Google Scholar]
- 59.Sahai S, Mahadevan S, Srinivasan S, et al. Childhood bacterial meningitis in Pondicherry, South India. Indian J Pedlatr 2001; 68: 839–841. [DOI] [PubMed] [Google Scholar]
- 60.Sathish N, Scot JX, Shaji RV, et al. An outbreak of echovirus meningitis in children. Indian Pediatr 2004; 41: 384–388. [PubMed] [Google Scholar]
- 61.Shameem S, Kumar CSV, Neelagund YF. Bacterial meningitis – rapid diagnosis and microbial profile: a multicentered study. J Commun Dis 2008; 40: 111–120. [PubMed] [Google Scholar]
- 62.Devi U, Bora R, Malik V, et al. Bacterial aetiology of neonatal meningitis: a study from north-east India. Indian J Med Res 2017; 145: 138–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mirdha BR, Gupta U, Bhujwala RA. Latex agglutination test: an adjunct to the laboratory diagnosis of pyogenic bacterial meningitis. Indian J Pediatr 1991; 58: 521–524. [DOI] [PubMed] [Google Scholar]
- 64.Raza MS, Das BK, Goyal V, et al. Emerging multidrug resistance isolates of hospital-acquired bacterial meningitis in a tertiary care centre in North India. J Med Microbiol 2019; 68: 1585–1590. [DOI] [PubMed] [Google Scholar]
- 65.Mital HS, Mehrotra T, Dutt S, et al. Pyogenic meningitis. J Assoc Physicians India 1990; 38: 779–780. [PubMed] [Google Scholar]
- 66.Pandit L, Kumar S, Karunasagar I, et al. Diagnosis of partially treated culture-negative bacterial meningitis using 16S rRNA universal primers and restriction endonuclease digestion. J Med Microbiol 2005; 54: 539–542. [DOI] [PubMed] [Google Scholar]
- 67.Mani R, Pradhan S, Nagarathna S, et al. Bacteriological profile of community acquired acute bacterial meningitis: a ten-year retrospective study in a tertiary neuro care centre in South India. Indian J Med Microbiol 2007; 25: 108–114. [DOI] [PubMed] [Google Scholar]
- 68.Jaiswal N, Singh M, Thumburu KK, et al. Burden of invasive pneumococcal disease in children aged 1 month to 12 years living in South Asia: a systematic review. PLoS One 2014; 9: e96282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tabatabaei SR, Shamshiri AR, Nasiri MJ, et al. Pneumococcal meningitis in Iran: a systematic review and meta-analysis. J Acute Dis 2019; 8: 99–105. [Google Scholar]
- 70.Pellegrino P, Perrone V, Radice S, et al. Immunogenicity of meningococcal quadrivalent (serogroup A, C, W135 and Y) tetanus toxoid conjugate vaccine: systematic review and meta-analysis. Pharmacol Res 2015; 92: 31–39. [DOI] [PubMed] [Google Scholar]
- 71.McGill F, Heyderman RS, Panagiotou S, et al. Acute bacterial meningitis in adults. Lancet 2016; 388: 3036–3047. [DOI] [PubMed] [Google Scholar]
- 72.Peer V, Schwartz N, Green MS. Consistent, excess viral meningitis incidence rates in young males: a multi-country, multi-year, meta-analysis of national data. The importance of sex as a biological variable. EClinicalMedicine 2019; 15: 62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sigfrid L, Perfect C, Rojek A, et al. A systematic review of clinical guidelines on the management of acute, community-acquired CNS infections. BMC Med 2019; 17: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.University of Michigan. Meningitis and vaccination. https://uhs.umich.edu/meningitis#:~:text=There%20is%20no%20vaccination%20or,of%20brain%20damage%20and%20death (accessed 24 November 2020).
- 75.Veltman JA, Bristow CC, Klausner JD. Meningitis in HIV-positive patients in sub-Saharan Africa: a review. J Int AIDS Soc 2014; 17: 19184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van de Beek D, de Gans J, Tunkel AR, et al. Community-acquired bacterial meningitis in adults. N Engl J Med 2006; 354: 44–53. [DOI] [PubMed] [Google Scholar]
- 77.Mook-Kanamori BB, Geldhoff M, van der Poll T, et al. Pathogenesis and pathophysiology of pneumococcal meningitis. Clin Microbiol Rev 2011; 2: 557–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Short WR, Tunkel AR. Changing epidemiology of bacterial meningitis in the United States. Curr Infect Dis Rep 2000; 2: 327–331. [DOI] [PubMed] [Google Scholar]
- 79.Weisfelt M, de Gans J, van der Poll T, et al. Pneumococcal meningitis in adults: new approaches to management and prevention. Lancet Neurol 2006; 5: 332–342. [DOI] [PubMed] [Google Scholar]
- 80.Janowskia AB, Newland JG. From the microbiome to the central nervous system, an update on the epidemiology and pathogenesis of bacterial meningitis in childhood. F1000Res 2017; 6: F1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stanek RJ, Mufson MA. A 20-year epidemiological study of pneumococcal meningitis. Clin Infect Dis 1999; 28: 1265–1272. [DOI] [PubMed] [Google Scholar]
- 82.Kastenbauer S, Pfister HW. Pneumococcal meningitis in adults: spectrum of complications and prognostic factors in a series of 87 cases. Brain 2003; 126: 1015–1025. [DOI] [PubMed] [Google Scholar]
- 83.van de Beek D. Cognitive impairment in adults with good recovery after bacterial meningitis. J Infect Dis 2002; 186: 1047–1052. [DOI] [PubMed] [Google Scholar]
- 84.Weisfelt M. Dexamethasone and long-term outcome in adults with bacterial meningitis. Ann Neurol 2006; 60: 456–468. [DOI] [PubMed] [Google Scholar]
- 85.Weisfelt M, van de Beek D, Spanjaard L, et al. Clinical features, complications, and outcome in adults with pneumococcal meningitis: a prospective case series. Lancet Neurol 2006; 5: 123–129. [DOI] [PubMed] [Google Scholar]
- 86.Nandi A, Deolalikar AB, Bloom DE, et al. Haemophilus influenzae type B vaccination and anthropometric, cognitive, and schooling outcomes among Indian children. Ann N Y Acad Sci 2019; 1449: 70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Centers for Disease Control and Prevention. Adults: protect yourself with pneumococcal vaccine. https://www.cdc.gov/features/adult-pneumococcal/index.html (accessed 18 November 2020).
- 88.Ministry of Health & Family Welfare, Government of India. National Operational Guidelines – introduction of pneumococcal conjugate vaccine. https://nhm.gov.in/New_Updates_2018/NHM_Components/Immunization/Guildelines_for_immunization/Operational_Guidelines_for_PCV_introduction.pdf (accessed 18 August 2021).
- 89.Verma R, Khanna P. Pneumococcal conjugate vaccine: a newer vaccine available in India. Hum Vaccin Immunother 2012; 8: 1317–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Indian Medical Association. Guidebook on adult immunization in occupational health settings healthy worker: key to productivity and sustainability. http://occuconindia.com/img/iaoh_book.pdf (accessed 10 August 2020).
- 91.Wankhedkar R, Tandon RN, Monga VK. Life course immunization guidebook: a quick reference guide. Publication of Indian Medical Association. http://www.ima-india.org/ima/pdfdata/IMA_LifeCourse_Immunization_Guide_2018_DEC21.pdf (2018, accessed 19 November 2019). [Google Scholar]
- 92.Dhar R. Review of guidelines for the use of vaccines to prevent community-acquired pneumonia in Indian adults. JAPI 2016; 64: 45–51.27762515 [Google Scholar]
- 93.Vashishtha VM, Kalra A, et al.; Indian Academy of Pediatrics, Advisory Committee on Vaccines and Immunization Practices (ACVIP). Indian Academy of Pediatrics (IAP) recommended immunization schedule for children aged 0 through 18 years, India, 2013 and updates on immunization. Indian Pediatr 2013; 50: 1095–1108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-doc-1-tai-10.1177_20499361211046453 for A systematic literature review on the prevalence and etiology of meningitis among critically ill and hospitalized patients in India by Canna J. Ghia and Gautam S. Rambhad in Therapeutic Advances in Infectious Disease