Abstract
Introduction
The sequence of chemotherapy and pembrolizumab may affect antitumor immune response and efficacy of immunotherapy.
Methods
This multicenter, randomized, phase 2 trial was designed to evaluate the efficacy of two sequences of chemotherapy and pembrolizumab in patients with stage 4 NSCLC. Both arms were considered investigational, and the study used a “pick a winner” design. The primary end point was objective response rate by independent radiologic review after eight cycles (24 wk). Patients were randomized 1:1 to arm A (chemotherapy for four cycles followed by pembrolizumab for four cycles) or arm B (pembrolizumab for four cycles followed by chemotherapy for four cycles). Patients in both arms without disease progression after the initial eight cycles continued pembrolizumab until disease progression, unacceptable toxicity, or a maximum of 2 years.
Results
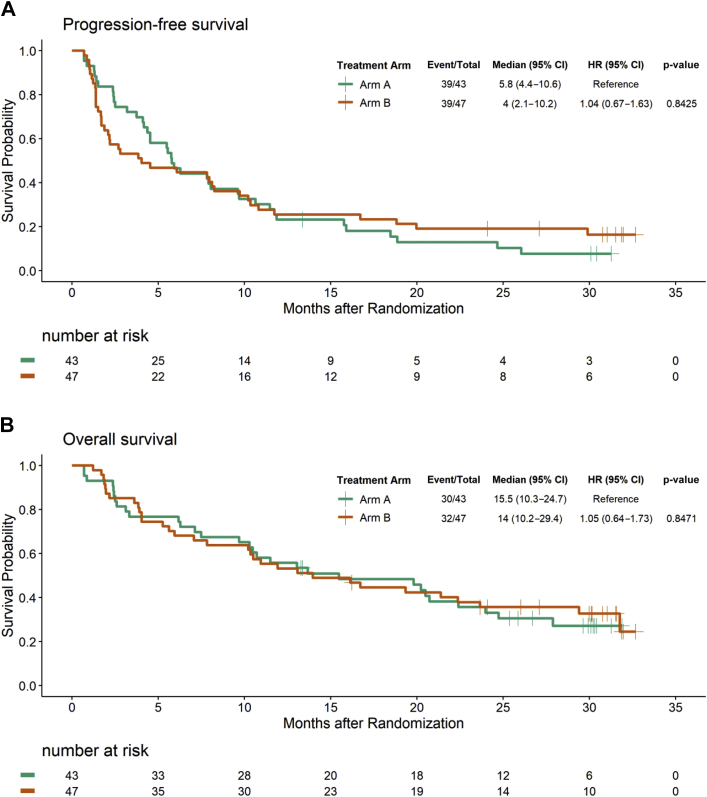
From March 2016 to July 2018, a total of 90 eligible patients were randomized (43 patients to arm A and 47 patients to arm B). The objective response rate at 24 weeks in arms A and B was 39.5 % (95 % confidence interval [CI]: 24.9%–54.1 %) and 40.4 % (95 % CI: 26.4%–54.5 %), respectively (p = 0.93). The progression-free survival in arms A and B was as follows: hazard ratio of B versus A equals to 1.06, 95 % CI: 0.68–1.66, p value equals to 0.84, and median progression-free survival of 5.8 months and 4 months, respectively. The overall survival was as follows: hazard ratio of B versus A equals to 1.04, 95 % CI: 0.63–1.74, p value equals to 0.85, and median overall survival of 15.5 months and 14 months, respectively.
Conclusions
Additional evaluation of either sequence in a phase 3 trial is not warranted.
Keywords: Clinical trial, Immunotherapy, Metastatic non–small cell lung cancer, Immunotherapy and chemotherapy sequencing
Introduction
Lung cancer is the leading cause of cancer-related mortality in the United States and a leading cause of cancer mortality globally.1,2 Most patients have NSCLC and locally advanced or metastatic disease at the time of diagnosis.3,4 Historically, platinum-based, double-agent chemotherapy was the standard therapy for patients with metastatic NSCLC, and most patients succumbed to their disease within one or two years of diagnosis.5 The development of targeted therapies for patients with specific molecular alterations dramatically improved outcomes. More recently, immune checkpoint inhibitors (ICIs) alone and in combination with chemotherapy have further improved outcomes in patients with advanced NSCLC. The recent therapeutic improvements for NSCLC have improved population-level mortality and substantially improved survival after diagnosis.6
At the time this trial was designed, pembrolizumab had been found to have preliminary activity but was not a standard-of-care therapy, and the use of tumor programmed death-ligand 1 (PD-L1) expression was not established for selection of patients for single-agent pembrolizumab.7 Subsequently, a phase 3 trial revealed superior efficacy of pembrolizumab compared with platinum-based chemotherapy in patients with PD-L1 expression greater than or equal to 50 %, and another phase 3 trial revealed superior efficacy in patients with PD-L1 expression greater than or equal to 1 %.8,9 Subsequent phase 3 trials revealed the superior efficacy of chemotherapy and pembrolizumab compared with chemotherapy alone in patients regardless of PD-L1 tumor expression.10,11
Retrospective studies reported a higher-than-expected response rate to chemotherapy after ICI, suggesting the sequencing of ICI and chemotherapy may affect efficacy.12,13 The timing and administration may influence the efficacy of ICI through increased neoantigen presentation caused by the cytotoxic chemotherapy or alteration of the immune antitumor response.14 On the basis of the clinical and preclinical data, we sought to prospectively evaluate whether the sequence of administration of platinum-based chemotherapy and pembrolizumab affected the outcomes of patients with advanced NSCLC in a phase 2 trial. At the time this trial was developed, there was interest in use in the immune Response Evaluation Criteria for Solid Tumors (iRECIST) in addition to the standard RECIST, so the study evaluated outcomes by both criteria.15,16
Materials and Methods
Patients
Patients were required to have stage 4 NSCLC (American Joint Committee on Cancer seventh edition criteria) and be treatment naive. Patients with tumors with an EGFR mutation or ALK rearrangement who experienced disease progression with the appropriate tyrosine kinase inhibitor in first-line setting were eligible. Molecular testing was performed before trial enrollment and according to standard of care. Patients were required to have Eastern Cooperative Oncology Group performance status of 0 or 1 and measurable disease by RECIST 1.1.15,17 Patients were also required to have adequate renal, hepatic, and hematologic function according to laboratory parameters. In addition, patients were required to have a core or excisional biopsy specimen obtained within 42 days of study enrollment, and the biopsy was performed before the patient received any chemotherapy or immunotherapy for NSCLC. Patients were not required to have tumor testing for PD-L1 expression of for enrollment. Patients with an underlying autoimmune disease requiring systemic therapy or history of steroid use or other immunosuppressive therapy, interstitial lung disease, or noninfectious pneumonitis requiring steroids were excluded. Furthermore, those with previous treatment with anti–programmed cell death protein-1/PD-L1 or anti–CTLA-4 therapies were excluded.
The institutional review boards of all the participating centers approved the study, and this trial was conducted in accordance with Good Clinical Practice guidelines and the provisions of the Declaration of Helsinki. Patients were required to provide written informed consent before any study-related procedures.
This was an investigator-initiated trial, performed through the Alliance Foundation Trials (AFT) and registered in clinicaltrials.gov (NCT 02591615). AFT is a nonprofit research organization that develops and conducts investigator-initiated trials, working closely with scientific investigators of the Alliance for Clinical Trials in Oncology, institutional member networks, and pharmaceutical partners. The AFT operational structure and funding sources are separate from the National Cancer Institute-funded clinical trial program. AFT provides data and safety monitoring, database and statistical support, and clinical trial site selection and monitoring.
Study Therapy and Assessments
Patients were assigned to arm A (platinum-based chemotherapy for four cycles followed by pembrolizumab for four cycles) or arm B (pembrolizumab for four cycles followed by platinum-based chemotherapy for four cycles). After eight cycles, patients in both arms without disease progression or unacceptable adverse events (AEs) received “maintenance” pembrolizumab. The platinum-based chemotherapy was selected on the basis of histology. Patients with nonsquamous histology received carboplatin area under the curve of 6 and pemetrexed 500 mg/m2 every 3 weeks. Patients with squamous histology received carboplatin area under the curve of 6 and paclitaxel 200 mg/m2 every three weeks. These patients also received pembrolizumab 200 mg every 3 weeks. Parameters for dose adjustment and delays related to AEs were included in the protocol.
Treatment was continued until disease progression, unacceptable AEs, or withdrawal of consent. Patients who experienced disease progression or unacceptable toxicity as assessed by the treating physician were discontinued from the study therapy, regardless of the reason, and did not proceed to the next treatment phase, and participation in the study-related radiographic imaging ceased at the time of study therapy discontinuation. Poststudy therapy was at the discretion to the treating physician, and the rate, type, and efficacy of poststudy therapy were not collected.
Radiographic imaging to evaluate disease status was performed every two cycles (6 wk) for the first eight cycles and then every three cycles (9 wk). AEs were assessed using the National Cancer Institute Common Terminology for Adverse Events version 4.0, and AEs were attributed as a reasonable possibility of a relationship or not a reasonable possibility of a relationship to study therapy.18
Study Design and Statistical Analysis
Patients were randomized 1:1 to arm A or arm B, and patients were stratified on the basis of history of tobacco use (never, previous, current) and histology (squamous, nonsquamous). The primary end point was objective response rate (ORR) after eight cycles (24 wk) by independent radiological review using RECIST 1.1, and secondary end points were ORR by investigator, progression-free survival (PFS), safety, and overall survival (OS). Additional secondary end points were ORR and PFS using iRECIST by an independent radiological review committee.16 All randomized patients who initiated study therapy were included in the primary analysis. PFS was defined as time from randomization to disease progression by RECIST 1.1 or death (whichever occurred first), and OS was defined as time from randomization to death of all causes. The independent radiological review was performed after completion of the study.
This trial was designed using a “pick a winner” design to select the more promising of the two experimental regimens to investigate in a larger comparative phase 2/3 trial.19 The treatment regimen with the best ORR per RECIST 1.1 would be selected for further investigation, without restriction on the minimal magnitude of the difference in ORR or a minimal ORR. The ORR with platinum-based chemotherapy was estimated to be 30 %, and the response rate for single-agent pembrolizumab was estimated to be 20 %, on the basis of the data available at the time the study was designed.7 For the purpose of sample size determination, we assume that the ORR is 30 % and 45 % for the less promising arm and the more promising arm, respectively. A sample size of 45 each arm can achieve a conclusive decision with at least 91 % probability on the basis of the observed ORRs. ORR, hazard ratio, and their confidence intervals are estimated. The survival distribution is characterized by Kaplan-Meier method, and the comparison of survival distribution is done with log-rank test for descriptive purpose. All p values reported are two sided for a descriptive purpose without adjustment for multiplicity. All statistical analyses were performed in the Statistical Analysis System 9.4 (SAS Inc., Cary, NC) by the study statisticians. All analyses were on the basis of the study database frozen on June 25, 2020.
The AFT Data and Safety Monitoring Board reviewed the study every six months and provided independent recommendations to investigators regarding the continuation, termination, or modification of the trial. The AFT Data and Safety Monitoring Board reviewed protocol compliance, safety, accrual, and severe AEs and released the data to the study team after all patients completed the protocol treatments.
Results
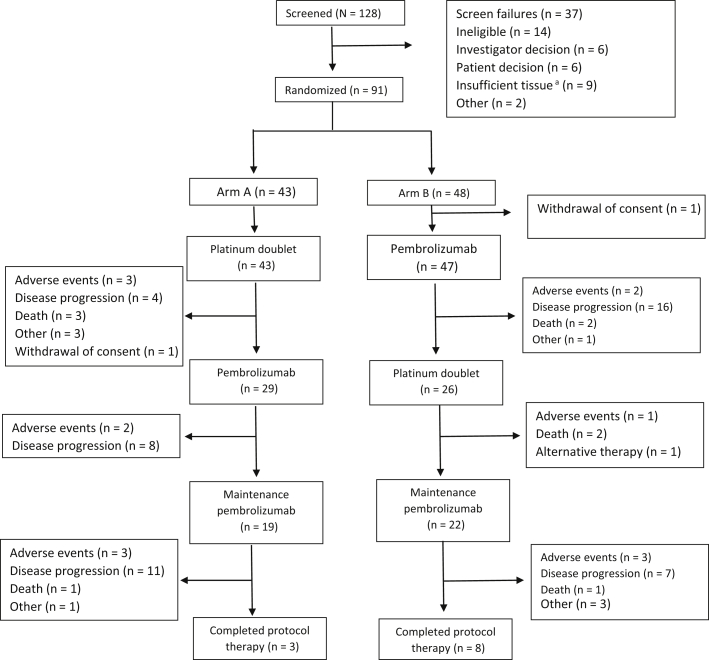
From March 2016 to July 2018, a total of 128 patients were screened, 91 patients were enrolled, and 90 patients were eligible and initiated the study therapy; one patient was assigned to arm B and withdrew consent before starting study therapy (Fig. 1). The median age was 68 years (range: 40–83 y). Most were of male sex (53 %), had a performance status of 1 (70 %), had a history of tobacco use or were currently using tobacco (92 %), and had nonsquamous histology (79 %) (Table 1). One patient with an EGFR mutation was enrolled in arm A, and no patient with ALK rearrangement was enrolled.
Figure 1.
Patient disposition. aPatients were required to have core or excisional biopsy of tumor lesion within 6 weeks of study enrollment.
Table 1.
Patient Characteristics
| Patient Characteristics | Arm A (n = 43) | Arm B (n = 47) | p Value |
|---|---|---|---|
| Median age (range) | 69 y (62–75) | 67 y (60–72) | 0.23 |
| Gender, n (%) | |||
| Female | 19 (44) | 23 (49) | 0.65 |
| Male | 24 (56) | 24 (51) | |
| ECOG performance status, n (%) | |||
| 0 | 14 (33) | 13 (28) | 0.62 |
| 1 | 29 (67) | 34 (72) | |
| History of tobacco use, n (%) | |||
| Current | 9 (21) | 14 (30) | 0.57 |
| Previous | 31 (72) | 29 (62) | |
| Never | 3 (7) | 4 (8) | |
| Histology, n (%) | |||
| Nonsquamous | 34 (79) | 37 (79) | 0.97 |
| Squamous | 9 (21) | 10 (21) | |
| EGFR or ALK status,a n (%) | |||
| EGFR mutation positive | 1 (2) | 0 | |
| ALK rearrangement | 0 | 0 |
ECOG, Eastern Cooperative Oncology Group.
EGFR and ALK testing performed per standard of care before trial enrollment. Data available on EGFR testing for 71 patients and on ALK testing for 63 patients.
Of the 43 patients who initiated platinum-based chemotherapy in arm A, 29 patients (67 %) initiated second-stage therapy with pembrolizumab and 19 patients (44 %) initiated the maintenance pembrolizumab (Fig. 1). Of the 47 patients who initiated pembrolizumab in arm B, 26 patients (55 %) initiated second-stage therapy with platinum-based chemotherapy and 22 patients (46 %) initiated maintenance pembrolizumab. The number of patients who completed both four cycles of platinum doublet chemotherapy and four cycles of single-agent pembrolizumab in arms A and B was 19 and 22, respectively.
The ORR by independent radiological review using RECIST in arms A and B after eight cycles of therapy (at 24 wk) was 39.5 % (95 % confidence interval [CI]: 24.9%–54.1 %) and 40.4 % (95 % CI: 26.4%–54.5 %), respectively (p = 0.93). The ORR in arms A and B by independent radiologic review committee using iRECIST, a secondary end point, was 18.6 % (95 % CI: 7%–30.2 %) and 29.8 % (95 % CI: 16.7–42.9 %), respectively (p = 0.22). The ORR as observed with platinum-based chemotherapy as initial therapy in arm A was 23.3 % (95 % CI: 10.6–35.9) and that with single-agent pembrolizumab as initial therapy in arm B was 27.7 % (95 % CI: 14.9–40.4).
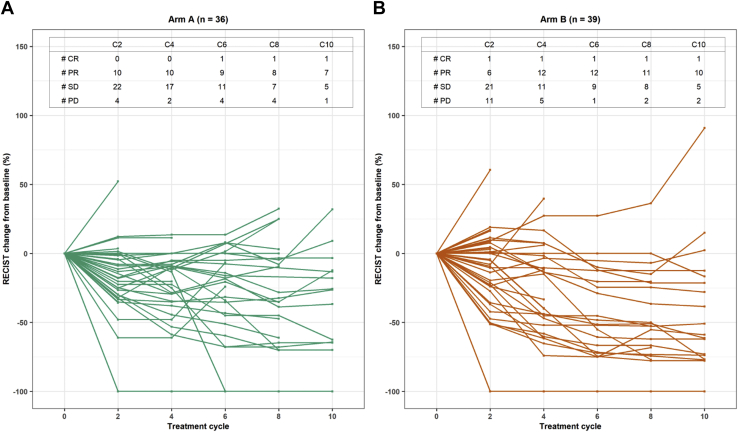
The median follow-up for all patients was 13.5 months (range: 0.69–32.7 mo). The PFS in arms A and B was as follows: hazard ratio (HR) B versus A = 1.04, 95 % CI: 0.67–1.64, log-rank test p = 0.84, and median PFS of 5.8 months and 4 months, respectively (Fig. 2A). The PFS by independent radiologic review committee using iRECIST in arms A and B was as follows: HR B versus A of 1.04, 95 % CI: 0.64–1.66, log-rank test p = 0.88, and median PFS of 7.9 months and 8 months, respectively (Supplementary Table 1). The spider plot for PFS by cycle is presented in Fig. 3. The median follow-up for 28 patients who are alive was 30.1 months (range: 13.3–32.7 mo). The OS in arms A and B was as follows: HR of B versus A = 1.05, 95 % CI: 0.64–1.73, log-rank p = 0.85, and median of 15.5 months and 14 months, respectively (Fig. 2B).
Figure 2.
Kaplan-Meier curves for (A) progression-free survival and (B) overall survival. CI, confidence interval; HR, hazard ratio.
Figure 3.
Spider plot of progression-free survival by cycle in arms A and B. In arm A: data unavailable on seven patients owing to death (n = 2), discontinued study therapy owing to adverse event (n = 2), patient noncompliance (n = 1) before first study imaging; imaging not available (n = 1), no measurable disease per response evaluation criteria in solid tumors (n = 1). In arm B: data unavailable on eight patients owing to disease progression (n = 5), discontinued study therapy owing to adverse event (n = 2), or patient death (n = 1) before first study imaging. #, number; CR, complete response; PD, progressive disease; PR, partial response; RECIST, Response Evaluation Criteria for Solid Tumors; SD, stable disease.
The median number of treatment cycles in arms A and B was 8 (range: 1–39) and 6 (range: 1–43), respectively. The prevalence of grade greater than or equal to 3 AEs, hematologic grade greater than or equal to 3 AEs, and nonhematologic grade greater than or equal to 3 AEs are presented in Table 2. Eight patients (18 %) in arm A and six patients (13 %) in arm B discontinued treatment owing to AE (Fig. 1). The specific grade greater than or equal to 2 AEs (regardless of attribution) is presented on Supplementary Table 1. The prevalence of grade 5 events in arms A and B was 2.3 % (n = 1) and 4.4 % (n = 2), respectively. In arm A, one patient died from neutropenic sepsis, and in arm B, one patient died from sepsis and one patient died from a myocardial infarction, which was determined to be possibly related to the study therapy.
Table 2.
Summary of Grade Greater Than or Equal to Three Adverse Events (Regardless of Attribution)
| Adverse Event | Arm A (n = 43) | Arm B (n = 47) |
|---|---|---|
| Total events, n (%) | ||
| Grade 3 event | 21 (48.8) | 21 (46.7) |
| Grade 4 event | 3 (7.0) | 4 (8.9) |
| Grade 5 event | 6 (14.0) | 5 (11.1) |
| Hematologic adverse events, n (%) | ||
| Grade 3 event | 9 (20.9) | 6 (14.0) |
| Grade 4 event | 3 (7.0) | 1 (2.3) |
| Grade 5 event | 0 (0) | 0 (0) |
| Nonhematologic events, n (%) | ||
| Grade 3 event | 20 (46.5) | 20 (46.5) |
| Grade 4 event | 1 (2.3) | 3 (7.0) |
| Grade 5 event | 6 (14.0) | 5 (11.6) |
Discussion
The purpose of this phase 2 trial was to evaluate if the activity of one of these two treatment sequences was superior and whether a phase 3 trial should be pursued. The results of the trial do not support a larger trial because neither sequence is likely to result in a clinically relevant improvement in patient outcomes. Furthering complicating the issue, in the interval between the time this trial was designed and completed, the treatment landscape has significantly changed. Specifically the development of chemotherapy and pembrolizumab combinations and better understanding of the patients who are most likely to benefit from single-agent pembrolizumab on the basis of PD-L1 expression levels.8,10,20 Currently, most patients receive immunotherapy as part of first-line therapy. The treatment sequence investigated in arm A is rarely used, and most patients who currently receive single-agent pembrolizumab as initial therapy, as in arm B, have a PD-L1 expression of greater than or equal to 50 % rather than unselected patients as in our study. These changes in the treatment landscape have reduced the importance of investigating different sequences of chemotherapy and immunotherapy.
This trial has several weaknesses. We would have included a concurrent chemotherapy and pembrolizumab arm to compare the two sequential approaches to the concurrent approach in a single trial. At the time this trial was developed, PD-L1 testing was not standard, and many different assays and scoring systems were in development. An imbalance of patients with a high expression (PD-L1 ≥ 50 %) or low expression (PD-L1 < 1 %) according to the standard assay may have contributed to the results. The interpretation of any subset analysis from this trial on the basis of PD-L1 expression would be limited by the small sample size and a potential imbalance in prognostic factors. The sample size is too small to evaluate the concordance between RECIST and iRECIST, and so these results are observational.
An important observation is that a substantial proportion of patients who initiated study therapy did not proceed to the second phase of the study, and an even smaller proportion initiated the maintenance pembrolizumab. The most common reason patients did not proceed from the first to the second phase was a PFS event. The ORR observed after four cycles of single-agent pembrolizumab was 27.7 % (95 % CI: 14.9–40.4) in this study, and the ORR observed with single-agent pembrolizumab when this trial was designed was 19.4 % (95 % CI: 16.0–23.2).7 The ORR observed after four cycles of platinum-based, double-agent chemotherapy was 23.3 % (95 % CI: 10.6–35.9), which is consistent with that in previous studies.21,22 This suggests that the therapies were performed similar to the estimates of activity when we designed the trial. The rate of early disease progression during the initial phase reduced the number of patients who received the second phase of the study therapy and our ability to evaluate the impact of the sequence of therapies.
The National Cancer Institute National Clinical Trials Network is performing a phase 3 trial to investigate the optimal therapy at time of disease progression in stage 4 NSCLC with nonsquamous histology with PD-L1 tumor proportion score of greater than or equal to 1 % (NCT03793179).23 This phase 3 trial includes the following two investigational arms: (1) single-agent pembrolizumab and at the time of disease progression treatment with carboplatin and pemetrexed alone and (2) single-agent pembrolizumab and at the time of disease progression continuation of pembrolizumab and addition of carboplatin and pemetrexed to the standard arm of carboplatin. Each investigational arm is compared with the standard arm of carboplatin, pemetrexed, and pembrolizumab followed by pemetrexed and pembrolizumab as used in KEYNOTE-189.10 This trial should provide information on whether continuation of pembrolizumab and adding chemotherapy at the time of disease progression provides benefit than the use of chemotherapy alone and the activity of chemotherapy after first-line, single-agent pembrolizumab.
Our trial was not designed to evaluate whether the response to chemotherapy was higher after previous immunotherapy. Nevertheless, this trial reveals the challenges in evaluating the question because a significant proportion of patients experienced disease progression or had unacceptable adverse effects during the first phase. The patients who proceeded to the second phase of therapy most likely had better prognostic features, more indolent disease, or more treatment-responsive disease. Given the difficulty we had in evaluating this clinical question in a clinical trial, retrospective studies reporting higher response rates with chemotherapy after immunotherapy should be interpreted cautiously.
After initiating our trial, other chemotherapy regimens and ICI combinations have been found to have benefit in first-line setting ICI.24, 25, 26, 27, 28 The multiple treatment options available have significantly improved the care of patients with metastatic NSCLC. Importantly, many patients do not respond or experience disease progression after initial response. Thus, the optimal therapy at the time of disease progression and the sequencing of available therapies remain clinically relevant questions.
CRediT Authorship Contribution Statement
Thomas A. Hensing, Everett E. Vokes: Conceptualization, Methods, Writing (original, review, and editing).
Xiaofei Wang: Methodology, Formal analysis, Writing (original, review, and editing), Supervision.
Thomas E. Stinchcombe: Investigation, Writing (original, review, and editing), Supervision.
Junheng Gao: Formal analysis, Writing (original, review, and editing).
Michael V. Knopp: Investigation, Resources, Data curation, Writing (original, review, and editing).
Mark Watson: Investigation, Resources (original, review, and editing).
Arkadiusz Z. Dudek, Stephen L. Graziano, Jyoti D. Patel, Bryan A. Faller: Investigation, Writing (original, review, and editing).
Konstantin H. Dragnev: Resources, Investigation, Writing (original, review, and editing).
David Kozono: Investigation, Project administration, Writing (original, review, and editing).
Acknowledgments
Support from research reported in this publication was provided by Merck & Co. (https://acknowledgments.alliancefound.org).
Footnotes
Disclosure: Dr. Stinchcombe reports receiving personal fees from Takeda, AstraZeneca, Genentech/Roche, Foundation Medicine, Pfizer, EMD Serono, Novartis, Daiichi Sankyo, Lilly, Medtronic, Puma, and Biotechnology and grants to institution from Genentech/Roche, Blueprint Medicines, AstraZeneca, Takeda, Advaxis, and Regeneron outside of the submitted work. Dr. Dudek reports receiving grants from the National Cancer Institute, during the conduct of the study, and grants from Merck and Eli Lilly, outside of the submitted work. Dr. Patel reports receiving personal fees from Takeda, AstraZeneca, and AbbVie and grants from Bristol-Myers Squibb outside of the submitted work. Dr. Faller reports receiving nonfinancial support from Celgene, Janssen Biotech, Genentech, Novartis, Amgen, Merck, EMD Serono, AstraZeneca, Tersera, Boehringer Ingelheim, Bayer Healthcare, Genzyme Corp., Aurobindo Pharma, E.R. Squibb, Lilly USA, Pfizer Inc., Takeda Pharmaceuticals, Rigel Pharmaceuticals, Incyte Corp., Puma Biotech, Agios, Pharmacyclics, Lexicon Pharmaceuticals, Regeneron Healthcare, AbbVie Inc., Verastem, Inc., Gilead, Taiho Oncology, CSL Behring, Atellas, Sirtex Medical, Teva Pharma, and Janssen Pharma outside of the submitted work. Dr. Dragnev reports receiving support from the Alliance Foundation for Trials during the conduct of the study and grants to institution from Merck, Eli Lilly, Roche/Genentech, G1 Therapeutics, Novartis, and Io Therapeutics outside of the submitted work. Dr. Vokes reports receiving personal fees from AbbVie, Amgen, AstraZeneca, BioNTech, Bristol-Myers Squibb, Celgene, Eli Lilly, EMD Serono, Genentech, GlaxoSmithKline, Merck, Novartis, and Regeneron outside of the submitted work. The remaining authors declare no conflict of interest.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2021.100208.
Supplementary Data
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Govindan R., Page N., Morgensztern D. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24:4539–4544. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 4.National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: lung and bronchus cancer. https://seer.cancer.gov/statfacts/html/lungb.html
- 5.Schiller J.H., Harrington D., Belani C.P. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 6.Howlader N., Forjaz G., Mooradian M.J. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383:640–649. doi: 10.1056/NEJMoa1916623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garon E.B., Rizvi N.A., Hui R. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 8.Reck M., Rodríguez-Abreu D., Robinson A.G. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37:537–546. doi: 10.1200/JCO.18.00149. [DOI] [PubMed] [Google Scholar]
- 9.Mok T.S.K., Wu Y.L., Kudaba I. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 10.Gadgeel S., Rodríguez-Abreu D., Speranza G. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol. 2020;38:1505–1517. doi: 10.1200/JCO.19.03136. [DOI] [PubMed] [Google Scholar]
- 11.Paz-Ares L., Luft A., Vicente D. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379:2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 12.Park S.E., Lee S.H., Ahn J.S., Ahn M.J., Park K., Sun J.M. Increased response rates to salvage chemotherapy administered after PD-1/PD-L1 inhibitors in patients with non-small cell lung cancer. J Thorac Oncol. 2018;13:106–111. doi: 10.1016/j.jtho.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Schvartsman G., Peng S.A., Bis G. Response rates to single-agent chemotherapy after exposure to immune checkpoint inhibitors in advanced non-small cell lung cancer. Lung Cancer. 2017;112:90–95. doi: 10.1016/j.lungcan.2017.07.034. [DOI] [PubMed] [Google Scholar]
- 14.Champiat S., Ileana E., Giaccone G. Incorporating immune-checkpoint inhibitors into systemic therapy of NSCLC. J Thorac Oncol. 2014;9:144–153. doi: 10.1097/JTO.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 15.Eisenhauer E.A., Therasse P., Bogaerts J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Seymour L., Bogaerts J., Perrone A. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143–e152. doi: 10.1016/S1470-2045(17)30074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldstraw P., Crowley J., Chansky K. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 18.National Cancer Institute, DCTD Division of Cancer Treatment & Diagnosis CTEP Cancer Therapy Evaluation Program. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_40-
- 19.Simon R., Wittes R.E., Ellenberg S.S. Randomized phase II clinical trials. Cancer Treat Rep. 1985;69:1375–1381. [PubMed] [Google Scholar]
- 20.Paz-Ares L., Vicente D., Tafreshi A. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol. 2020;15:1657–1669. doi: 10.1016/j.jtho.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 21.Sandler A., Gray R., Perry M.C. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 22.Scagliotti G.V., Parikh P., von Pawel J. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 23.ClinicalTrials.gov Testing the timing of pembrolizumab alone or with chemotherapy as first line treatment and maintenance in non-small cell lung cancer. https://clinicaltrials.gov/ct2/show/NCT03793179
- 24.Socinski M.A., Jotte R.M., Cappuzzo F. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 25.Herbst R.S., Giaccone G., de Marinis F. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383:1328–1339. doi: 10.1056/NEJMoa1917346. [DOI] [PubMed] [Google Scholar]
- 26.Hellmann M.D., Paz-Ares L., Bernabe Caro R. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381:2020–2031. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 27.West H., McCleod M., Hussein M. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:924–937. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 28.Paz-Ares L., Ciuleanu T.E., Cobo M. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:198–211. doi: 10.1016/S1470-2045(20)30641-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.