Abstract
Introduction
The phase 3 RELAY global study (NCT02411448) revealed significant improvement in progression-free survival (PFS) with ramucirumab plus erlotinib (RAM + ERL) compared with placebo plus ERL (PL + ERL) in untreated EGFR-mutated metastatic NSCLC (hazard ratio [HR] = 0.59 [95% confidence interval (CI): 0.46–0.76, p < 0.0001]). This prespecified analysis evaluates efficacy, safety, and postprogression EGFR T790M rates of RELAY patients enrolled in Japan.
Methods
Patients were randomized (1:1) to oral ERL (150 mg/d) plus intravenous RAM (10 mg/kg) or PL every 2 weeks. End points included PFS (primary), safety (secondary), and biomarker analyses (exploratory). Plasma samples collected at baseline and poststudy treatment discontinuation were evaluated for EGFR T790M mutations by next-generation sequencing.
Results
The Japanese subset included 211 of 449 (47.0%) RELAY patients (RAM + ERL, n = 106; PL + ERL, n = 105). Median PFS was 19.4 versus 11.2 months for RAM + ERL versus PL + ERL treatment (HR = 0.610 [0.431–0.864]) in the Japanese intent-to-treat population, 16.6 versus 12.5 months (HR = 0.701 [0.424–1.159]) in the EGFR exon 19 deletion subgroup, and 19.4 versus 10.9 months (HR = 0.514 [0.317–0.835]) in the EGFR exon 21 L858R subgroup, respectively. Adverse events of grade 3 or above with RAM + ERL included hypertension (24.8%, all grade 3) and dermatitis acneiform (23.8%). Postprogression treatment-emergent T790M rates were similar between arms (RAM + ERL: 47%, 9 of 19 patients; PL + ERL: 50%, 20 of 40 patients).
Conclusions
Clinically meaningful efficacy was observed with RAM + ERL versus PL + ERL in the RELAY Japanese subset, with no new safety concerns. Postprogression T790M rates were similar across treatment arms, indicating the addition of RAM did not affect the ERL-associated EGFR T790M rates at disease progression.
Keywords: Circulating tumor DNA, EGFR, Non–small cell lung cancer, Japan, Ramucirumab
Introduction
Activating mutations in the EGFR gene are important drivers in NSCLC, making EGFR-mutated NSCLC amenable to precision medicine.1,2 EGFR tyrosine kinase inhibitors (TKIs), the standard first-line treatment for EGFR-mutated NSCLC,3,4 have different affinities for specific EGFR mutations.5,6 The clinical benefit with EGFR TKIs is greater in patients with exon 19 deletions (ex19dels) versus an exon 21 L858R (ex21.L858R) point mutation.7,8 The prevalence of EGFR mutations is higher in Asian and Japanese populations (approximately 40%) than in White populations (approximately 20%).9 In contrast to White patients, ex21.L858R is more common than ex19del in Japanese patients.10
Many patients with NSCLC who receive first-line EGFR TKIs acquire the EGFR T790M resistance mutation.11 Osimertinib is the only targeted treatment against EGFR T790M and is approved in Japan for first- and second-line treatment of patients with EGFR-mutated NSCLC.3,4 Nevertheless, the mechanisms of resistance to osimertinib are heterogeneous and mostly not targetable with approved small-molecule inhibitors; thus, the only remaining treatment options at the time of progression are chemotherapy-based regimens or immunotherapy.3,12,13 Chemotherapy efficacy is limited by its toxicity, and immunotherapy has not been found to be effective in EGFR-mutated NSCLC. Therefore, treatment strategies that enhance EGFR TKI efficacy and prolong the chemotherapy-free period are desired.
The EGFR and vascular endothelial growth factor pathways are interconnected, and dual inhibition reduces angiogenesis and abrogates tumor resistance to EGFR TKIs.14,15 Targeting both pathways is therefore a viable strategy to improve outcomes in EGFR-mutated NSCLC.16,17 RELAY was a global, phase 3, randomized, double-blind, placebo-controlled study investigating the efficacy and safety of the addition of ramucirumab (RAM), a human IgG1 vascular endothelial growth factor receptor 2 antagonist, to erlotinib (ERL) in treatment-naive EGFR-mutated metastatic NSCLC.18 Progression-free survival (PFS) was superior with RAM plus ERL versus placebo plus ERL (median PFS = 19.4 versus 12.4 mo; hazard ratio [HR] = 0.59 [95% confidence interval (CI): 0.46–0.76, p < 0.0001]), and the safety profile was consistent with the known safety profiles of RAM and ERL monotherapy.18, 19, 20 EGFR T790M mutation rates after disease progression were similar between treatment arms, indicating subsequent treatment with a T790M-targeting drug remains possible.18
Treatment selection after progression on EGFR TKI therapy depends on the mechanism of resistance. The challenge of obtaining tumor biopsies during and after treatment can be overcome using plasma samples containing circulating tumor DNA.21 Blood samples are less invasive than tissue biopsies, and high concordance between tumor and circulating tumor DNA has been observed using digital polymerase chain reaction and next-generation sequencing (NGS) for T790M analysis and driver mutation detection.22,23
Because the prevalence of EGFR-mutated NSCLC in patients from Japan is relatively high and the RELAY Japanese population constituted almost half of the overall RELAY population, we conducted subgroup analyses of clinical efficacy, safety, and postprogression T790M rates in the RELAY Japanese population.
Materials and Methods
Study Design and Participants
RELAY was a global, phase 3, randomized, double-blind, placebo-controlled study in patients with untreated EGFR-mutated metastatic NSCLC (www.clinicaltrials.gov: NCT02411448).18 The RELAY study protocol was approved by the ethics review board of each site, and the study was conducted in accordance with the Declaration of Helsinki, The Council of International Organizations of Medical Sciences International Ethical Guidelines, Good Clinical Practice guidelines, and local guidelines. All patients provided written informed consent. Eligibility criteria have been previously published and are briefly described in Supplementary Materials.18,24
Randomization and Treatment Protocol
Patients were randomized (1:1) to RAM (10 mg/kg intravenously every 2 wk) plus ERL (150 mg orally once daily) (RAM + ERL) or placebo (PL) plus ERL (PL + ERL). Study drugs were assigned using the Interactive Web Response System. Masking of patients, investigators, and all clinical study personnel to the assigned treatment will continue until the prespecified number of 300 overall survival (OS) events is reached. Patients received study treatment until disease progression, unacceptable toxicity, noncompliance, or investigator or patient decision.
Outcome Measures
The primary end point for the randomized phase 3 portion of RELAY was PFS (time from randomization to disease progression or death from any cause) as evaluated by investigators according to Response Evaluation Criteria in Solid Tumours version 1.1 (RECIST v1.1). Secondary end points included OS, objective response rate (ORR), disease control rate (DCR), duration of response (DoR), and safety.18 Prespecified exploratory end points included PFS2 (time from randomization to second disease progression or death from any cause, whichever occurred first) and biomarker analyses. Liquid biopsy samples from baseline and poststudy treatment discontinuation (30-d follow-up) were analyzed for treatment-emergent EGFR T790M by Guardant360 (Guardant Health, Redwood City, CA) NGS. Time to chemotherapy treatment (TTCT; time from randomization to start of chemotherapy or death) was a post hoc exploratory end point.18
Statistical Analysis
Details of the RELAY statistical analyses have been published.18 The analyses in this report were exploratory, and multiplicity was not adjusted. Therefore, only point estimates and CIs are reported, without p values. The data cutoff date was January 23, 2019. Efficacy end points were evaluated in the Japanese intent-to-treat (ITT) population (all randomized patients enrolled in Japan; prespecified analysis). PFS, PFS2, and DoR (for patients achieving a best overall response of partial or complete response) were analyzed using the Kaplan-Meier method and compared using the unstratified log-rank test. ORR and DCR were calculated as defined by RECIST v1.1 and compared using the Cochran-Mantel-Haenszel test. HRs and 95% CIs were estimated using unstratified Cox proportional hazards models, which, where applicable, also included an interaction term with treatment for evaluating predictive relationships. Safety end points were evaluated in the Japanese safety population (all patients enrolled in Japan who were treated with ≥1 dose of study treatment) as previously described.18,24
Guardant360 NGS T790M analysis subpopulations comprised Japanese ITT patients who had disease progression by data cutoff and NGS results at baseline and at 30-day follow-up (population 1) or NGS results at 30-day follow-up containing an EGFR-activating mutation (population 2), indicating the liquid biopsy contained tumor-shed DNA. T790M mutation rates, defined as the proportion of patients with T790M mutation, and associated Wilson score 95% CIs were determined, and the differences in T790M mutation rate between treatment arms at each time point were evaluated using Fisher’s exact test.
Results
Patient Disposition and Baseline Demographics
The Japanese ITT population comprised 211 (47.0% of the RELAY global population) patients (RAM + ERL: 106 patients; PL + ERL: 105 patients; Supplementary Fig. 1) enrolled at 41 sites. One patient in the RAM + ERL arm was randomized but never treated. Median duration of follow-up was 22.4 months (range: 0.4–35.0). At the time of data cutoff, 31 of 106 patients (29.2%) in the RAM + ERL arm and 18 of 105 (17.1%) in the PL + ERL arm were still on study treatment. The most common reasons for discontinuation of all study treatment were progressive disease (RAM + ERL: 48 of 106 [45.3%]; PL + ERL: 62 of 105 [59.0%]) and adverse events (AEs) (RAM + ERL: 17 of 106 [16.0%]; PL + ERL: 22 of 105 [21.0%]). Baseline characteristics were mostly balanced across treatment arms (Table 1).
Table 1.
Demographic and Clinical Characteristics of Patients at Baseline (Japanese ITT Population)
| Characteristicsa | RAM + ERL (N = 106) | PL + ERL (N = 105) |
|---|---|---|
| Sex | ||
| Female | 72 (67.9) | 68 (64.8) |
| Age, y | ||
| Median | 67 | 66 |
| Min–max | 41–86 | 35–83 |
| Race | ||
| Asian | 106 (100) | 105 (100) |
| Smoking history | ||
| Ever | 28 (26.4) | 34 (32.4) |
| Never | 63 (59.4) | 64 (61.0) |
| Unknown or missing | 15 (14.2) | 7 (6.7) |
| ECOG performance status | ||
| 0 | 59 (55.7) | 60 (57.1) |
| 1 | 47 (44.3) | 45 (42.9) |
| Disease classification | ||
| Primary metastatic | 95 (89.6) | 94 (89.5) |
| Recurrent metastatic | 11 (10.4) | 11 (10.5) |
| EGFR mutation typeb | ||
| Ex19del | 49 (46.2) | 51 (48.6) |
| Ex21.L858R | 56 (52.8) | 54 (51.4) |
| EGFR testing methodc | ||
| therascreen and cobas | 40 (37.7) | 42 (40.0) |
| Other PCR and sequencing-based methods | 65 (61.3) | 63 (60.0) |
ECOG, Eastern Cooperative Oncology Group; ERL, erlotinib; ex19del, exon 19 deletion; ex21.L858R, exon 21 L858R; ITT, intent-to-treat; Max, maximum; Min, minimum; PCR, polymerase chain reaction; PL, placebo; RAM, ramucirumab.
Except where otherwise indicated, data are n (%).
In the RAM + ERL arm, 1 patient was classified as other.
In the RAM + ERL arm, 1 patient was classified as missing.
Efficacy
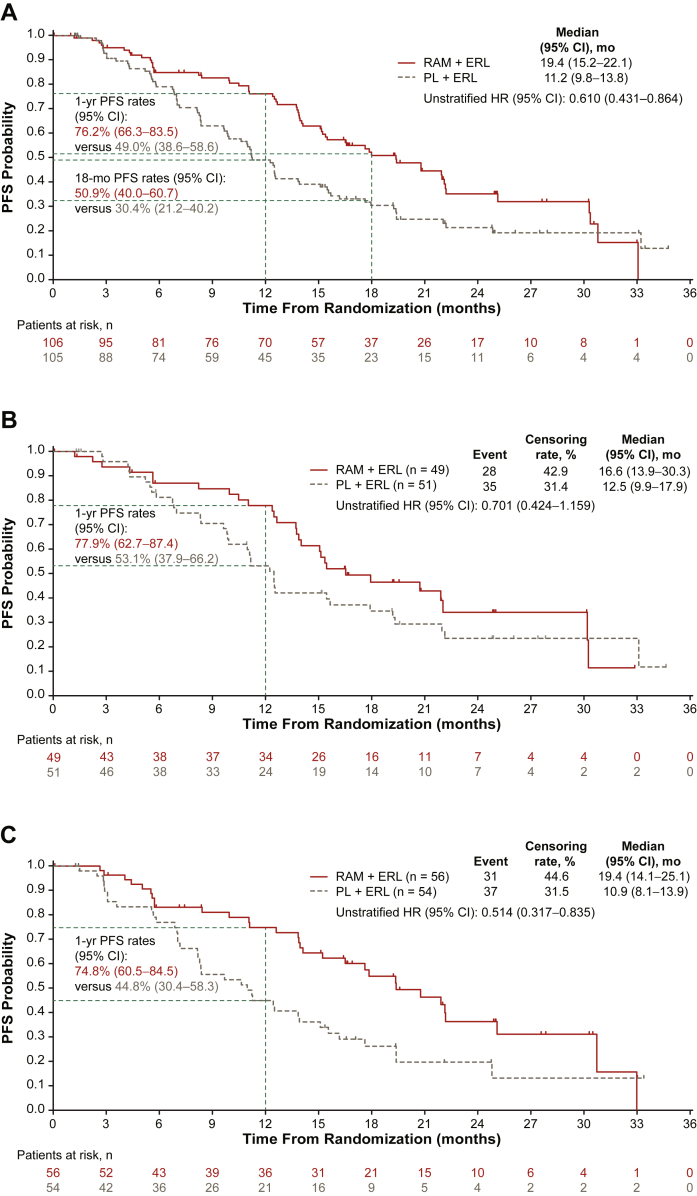
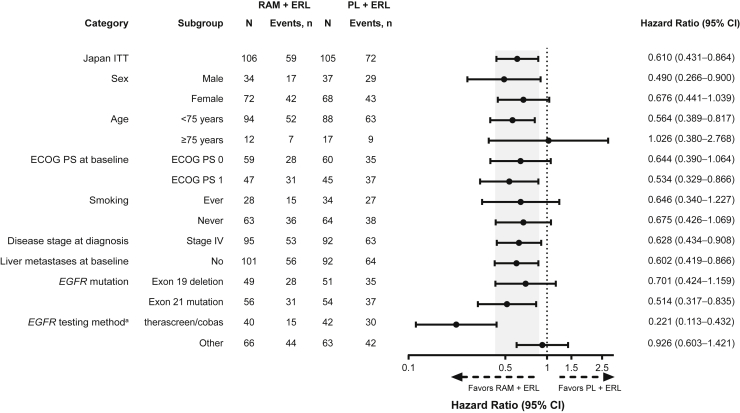
In the Japanese ITT population, PFS (investigator-assessed) was longer in the RAM + ERL arm versus the PL + ERL arm (Fig. 1A). Median (95% CI) PFS was 19.4 (15.2–22.1) versus 11.2 (9.8–13.8) months (HR = 0.610 [95% CI: 0.431–0.864]). The 1-year and 18-month PFS rates were greater in the RAM + ERL arm versus the PL + ERL arm (Fig. 1A). PFS was improved in the RAM + ERL arm versus the PL + ERL arm in patients with ex19del (16.6 [95% CI: 13.9–30.3] versus 12.5 [95% CI: 9.9–17.9] mo; HR = 0.701 [95% CI: 0.424–1.159]) (Fig. 1B) and in patients with ex21.L858R (19.4 [95% CI: 14.1–25.1] versus 10.9 [95% CI: 8.1–13.9] mo; HR = 0.514 [95% CI: 0.317–0.835]) (Fig. 1C). The PFS benefit of RAM + ERL compared with PL + ERL was consistent across most prespecified patient subgroups (including sex, smoking status, and EGFR mutation type), although there is currently no clear explanation for the different HRs for EGFR testing method (Fig. 2).
Figure 1.
Kaplan-Meier plot of investigator-assessed PFS (Japanese ITT population). (A) All patients in the Japanese ITT population. (B) Patients with EGFR exon 19 deletion at baseline. (C) Patients with EGFR exon 21 L858R mutation at baseline. CI, confidence interval; ERL, erlotinib; HR, hazard ratio; ITT, intent-to-treat; mo, month; PFS, progression-free survival; PL, placebo; RAM, ramucirumab; yr, year.
Figure 2.
Investigator-assessed PFS subgroup analysis (Japanese ITT population). The following categories with events less than 10 in either treatment arm are not revealed: unknown smoking history, a disease stage of “other” at diagnosis, and liver metastases at baseline. Shaded area represents the 95% CI for the Japan ITT population. a96% agreement between preplanned confirmatory central EGFR testing (therascreen assay) and local laboratory results (therascreen, cobas, or other PCR and sequencing-based methods) was observed. CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; ERL, erlotinib; ITT, intent-to-treat; PCR, polymerase chain reaction; PFS, progression-free survival; PL, placebo; RAM, ramucirumab.
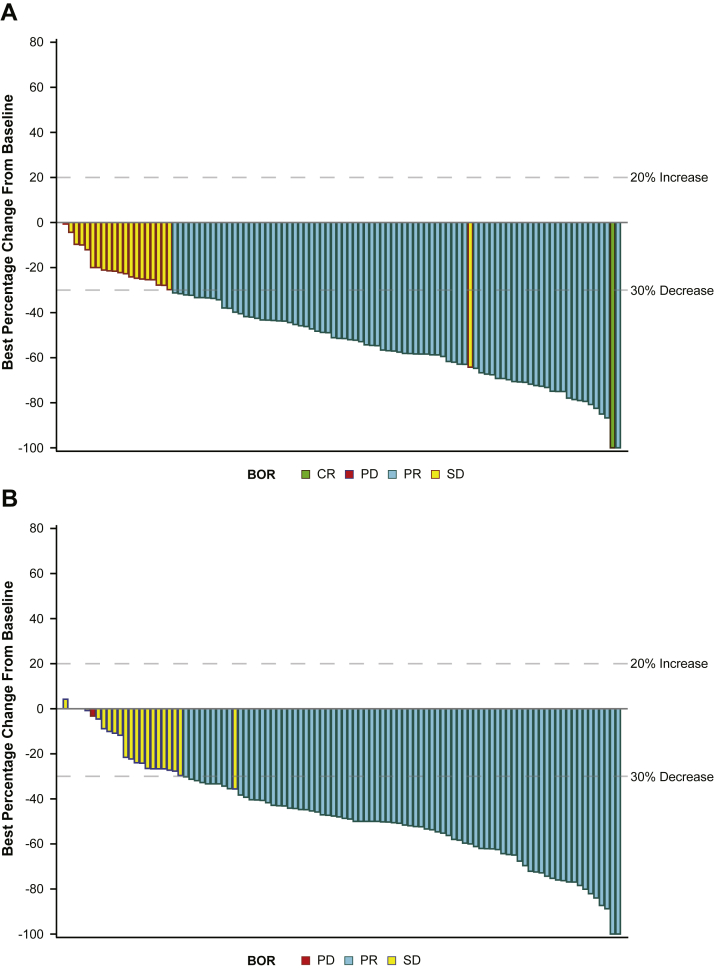
The ORR, DCR, and pattern of best overall response were similar between treatment arms (Table 2 and Fig. 3). In patients who responded, median DoR was longer with RAM + ERL versus PL + ERL (18.0 [95% CI: 12.9–20.9] versus 11.1 [95% CI: 9.0–12.6] mo; HR = 0.585 [0.402–0.850]) (Table 2). At data cutoff, PFS2 and OS data were immature (censoring rates were 67.0% and 86.8% in the RAM + ERL arm and 54.3% and 78.1% in the PL + ERL arm, respectively). HRs (95% CI) for PFS2 and OS were 0.664 (0.430–1.028) (Supplementary Fig. 2) and 0.604 (95% CI: 0.311–1.174), respectively (Table 2). Patients in the RAM + ERL arm had a longer TTCT or death than patients in the PL + ERL arm (median 32.3 versus 23.7 mo; HR = 0.552 [95% CI: 0.363–0.838]) (Supplementary Fig. 3).
Table 2.
Secondary Efficacy End Points (Japanese ITT Population)
| Variables | RAM + ERL (N = 106) | PL + ERL (N = 105) |
|---|---|---|
| Best overall response, n (%) | ||
| Complete response | 1 (0.9) | 0 |
| Partial response | 80 (75.5) | 79 (75.2) |
| Stable disease | 20 (18.9) | 22 (21.0) |
| Progressive disease | 1 (0.9) | 1 (1.0) |
| Not assessable | 4 (3.8) | 3 (2.9) |
| Objective response rate, n | 81 | 79 |
| % (95% CI) | 76.4 (68.3–84.5) | 75.2 (67.0–83.5) |
| Disease control rate, n | 101 | 101 |
| % (95% CI) | 95.3 (91.2–99.3) | 96.2 (92.5–99.9) |
| Duration of responsea | ||
| Events | 50 (61.7) | 62 (78.5) |
| Median (95% CI), mo | 18.0 (12.9–20.9) | 11.1 (9.0–12.6) |
| Unstratified HR (95% CI) | 0.585 (0.402–0.850) | |
| Interim overall survival | ||
| Events | 14 | 23 |
| Censoring rate, % | 86.8 | 78.1 |
| Median (95% CI), mo | Not reached | Not reached |
| Unstratified HR (95% CI) | 0.604 (0.311–1.174) | |
| Survival rate, % (95% CI) | ||
| 12 mo | 95.1 (88.7–98.0) | 94.3 (87.7–97.4) |
| 18 mo | 92.0 (84.6–95.9) | 88.4 (80.4–93.2) |
| Progression-free survival 2b | ||
| Events | 35 | 48 |
| Censoring rate, % | 67.0 | 54.3 |
| Median (95% CI), mo | 33.1 (26.48–NA) | 28.0 (21.82–NA) |
| Unstratified HR (95% CI) | 0.664 (0.430–1.028) | |
CI, confidence interval; ERL, erlotinib; HR, hazard ratio; ITT, intent-to-treat; NA, not available; PL, placebo; RAM, ramucirumab.
In patients who responded (RAM + ERL: n = 81; PL + ERL: n = 79).
Time from randomization to second disease progression (defined as objective radiologic or symptomatic progression after start of additional systemic anticancer treatment) or death from any cause, whichever occurred first.
Figure 3.
Maximum percentage change from baseline in the sum of all target tumors (Japanese ITT population). (A) RAM + ERL treatment arm. (B) PL + ERL treatment arm. BOR, best overall response; CR, complete response; ERL, erlotinib; ITT, intent-to-treat; PD, progressive disease; PL, placebo; PR, partial response; RAM, ramucirumab; SD, stable disease.
Occurrence of Central Nervous System Metastases
A total of 8 of 211 Japanese patients (3.8%) reported central nervous system metastases as a site of disease progression (RAM + ERL: 2 of 106 patients, 1.9%; PL + ERL: 6 of 105 patients, 5.7%).
Treatment Exposure
In the RAM + ERL arm, median (minimum–maximum) duration of exposure (censored analysis excluding 31 patients still on treatment) to RAM was 12.4 months (0.5–33.8+) and to ERL was 15.2 (0.1–33.8+) months; median relative dose intensity for the Japan ITT population was 93.8% and 85.7%, respectively. In the PL + ERL arm, median (minimum–maximum) duration of exposure (censored analysis excluding 18 patients still on treatment) to PL was 8.3 months (0.5–35.4+) and to ERL was 10.7 (0.8–35.5+) months; median relative dose intensity for the Japan ITT population was 95.6% and 79.3%, respectively.
Duration of RAM exposure (censored analysis excluding patients still on treatment) was similar by age group (<75 y; ≥75 y) with dose adjustments in both treatment arms. Duration of ERL exposure was longer for patients younger than 75 years versus patients 75 years or older in the PL + ERL arm (Supplementary Table 1 and Supplementary Fig. 4). Median relative dose intensity of RAM was high and comparable between age groups; median relative dose intensity was lower for ERL (Supplementary Table 1).
In the RAM + ERL arm, RAM dose adjustments were required for 78 of 105 patients (74.3%); ERL dose adjustments were required for 81 of 105 patients (77.1%) (Supplementary Table 2). In the PL + ERL arm, PL dose adjustments were required for 64 of 105 patients (61.0%); ERL dose adjustments were required for 71 of 105 patients (67.6%) (Supplementary Table 2). Dose adjustments for RAM or PL were mainly dose delays (RAM + ERL: 71 of 105 [67.6%]; PL + ERL: 62 of 105 [59.0%]) primarily owing to an AE, most often increased blood bilirubin and alanine aminotransferase. Dose adjustments for ERL were mainly dose omissions (RAM + ERL: 71 of 105 [67.6%]; PL + ERL: 67 of 105 [63.8%]) and dose reductions (RAM + ERL: 56 of 105 [53.3%]; PL + ERL: 53 of 105 [50.5%]). Almost all dose adjustments were due to an AE, most often dermatitis acneiform.
Postdiscontinuation Therapy
Subsequent anticancer therapy after study treatment discontinuation was at the investigator’s discretion. With 29.2% versus 17.1% of patients in the RAM + ERL and PL + ERL arms, respectively, remaining on the study treatment at data cutoff, 63 of 106 patients (59.4%) in the RAM + ERL arm and 78 of 105 patients (74.3%) in the PL + ERL arm received 1 or more subsequent lines of anticancer therapy (Supplementary Table 3). The most common first subsequent line of treatment after study treatment discontinuation was an EGFR TKI (RAM + ERL: 51 of 63 patients [81.0%]; PL + ERL: 58 of 78 patients [74.4%]), with 61.9% (RAM + ERL: 39 of 63 patients) and 41.0% (PL + ERL: 32 of 78 patients) receiving ERL versus 9.5% (RAM + ERL: 6 of 63 patients) and 14.1% (PL + ERL: 11 of 78 patients) receiving osimertinib. Chemotherapy was received by 10 of 63 patients (15.9%) and 18 of 78 patients (23.1%) in the RAM + ERL and PL + ERL arms, respectively. A second subsequent line of therapy after study treatment discontinuation was received by 41 of 106 patients (38.7%) in the RAM + ERL arm and 47 of 105 patients (44.8%) in the PL + ERL arm. EGFR TKI treatment was received by 24 of 41 patients (58.5%) and 15 of 47 patients (31.9%) in the RAM + ERL and PL + ERL arms, respectively. Osimertinib was the most common EGFR TKI (RAM + ERL: 17 of 41 patients [41.5%]; PL + ERL: 13 of 47 patients [27.7%]). Chemotherapy was received by 17 of 41 patients (41.5%) and 31 of 47 patients (66.0%) in the RAM + ERL and PL + ERL arms, respectively.
Safety
All patients in the Japanese safety population reported at least 1 treatment-emergent AE (TEAE). The most common any-grade TEAEs were dermatitis acneiform (RAM + ERL: 91.4%; PL + ERL: 90.5%) and diarrhea (RAM + ERL: 74.3%; PL + ERL: 73.3%) (Table 3). TEAEs of grade 3 or above were reported by 81 of 105 patients (77.1%) in the RAM + ERL arm and 64 of 105 patients (61.0%) in the PL + ERL arm. Most common TEAEs of grade 3 or above were hypertension (24.8%, all grade 3) and dermatitis acneiform (23.8%) in the RAM + ERL arm and increased alanine aminotransferase (14.3%) and dermatitis acneiform (10.5%) in the PL + ERL arm (Table 3). AEs of special interest for antiangiogenic agents included any-grade bleeding or hemorrhage (62.9% versus 39.0%), hypertension (52.4% versus 16.2%), and proteinuria (45.7% versus 10.5%) in the RAM + ERL versus PL + ERL arm, respectively (Table 3). Interstitial lung disease (ILD) was reported in both arms (RAM + ERL: 1 patient, grade 3; PL + ERL: 3 patients, grades 1, 3, and 5; the patient with grade 5 ILD died from ILD after study treatment discontinuation). The incidence of treatment-emergent serious AEs was similar across treatment arms: 25 of 105 patients (23.8%) for RAM + ERL versus 28 of 105 patients (26.7%) for PL + ERL. No patient in the Japanese safety population died while on study treatment.
Table 3.
TEAEs and AEs of Special Interest for RAM (Japanese Safety Population)
| Event | RAM + ERL (N = 105) |
PL + ERL (N = 105) |
||
|---|---|---|---|---|
| Any Grade | Grade ≥3 | Any Grade | Grade ≥3 | |
| ≥1 TEAE | 105 (100.0) | 81 (77.1) | 105 (100.0) | 64 (61.0) |
| TEAEs occurring in >40% of patients treated with RAM + ERL | ||||
| Dermatitis acneiform | 96 (91.4) | 25 (23.8) | 95 (90.5) | 11 (10.5) |
| Diarrhea | 78 (74.3) | 7 (6.7) | 77 (73.3) | 2 (1.9) |
| Paronychia | 73 (69.5) | 7 (6.7) | 66 (62.9) | 5 (4.8) |
| Increased ALT | 68 (64.8) | 14 (13.3) | 50 (47.6) | 15 (14.3) |
| Increased AST | 66 (62.9) | 6 (5.7) | 45 (42.9) | 8 (7.6) |
| Stomatitis | 59 (56.2) | 1 (1.0) | 41 (39.0) | 2 (1.9) |
| Hypertension | 55 (52.4) | 26 (24.8) | 17 (16.2) | 5 (4.8) |
| Dry skin | 48 (45.7) | 1 (1.0) | 46 (43.8) | 3 (2.9) |
| Proteinuria | 47 (44.8) | 3 (2.9) | 11 (10.5) | 0 (0.0) |
| Epistaxis | 47 (44.8) | 0 (0.0) | 19 (18.1) | 0 (0.0) |
| Alopecia | 45 (42.9) | 0 (0.0) | 18 (17.1) | 0 (0.0) |
| AEs of special interesta | ||||
| Bleeding or hemorrhage | 66 (62.9) | 1 (1.0) | 41 (39.0) | 2 (1.9) |
| Epistaxis | 47 (44.8) | 0 (0.0) | 19 (18.1) | 0 (0.0) |
| Hematuria | 8 (7.6) | 0 (0.0) | 6 (5.7) | 2 (1.9) |
| Purpura | 8 (7.6) | 0 (0.0) | 5 (4.8) | 0 (0.0) |
| Gingival bleeding | 7 (6.7) | 0 (0.0) | 1 (1.0) | 0 (0.0) |
| GI hemorrhageb | 12 (11.4) | 1 (1.0) | 4 (3.8) | 0 (0.0) |
| Hypertension | 55 (52.4) | 26 (24.8) | 17 (16.2) | 5 (4.8) |
| Proteinuriac | 48 (45.7) | 3 (2.9) | 11 (10.5) | 0 (0.0) |
| Liver injury or liver failure and liver infection | 86 (81.9) | 20 (19.0) | 78 (74.3) | 22 (21.0) |
| Increased ALT | 68 (64.8) | 14 (13.3) | 50 (47.6) | 15 (14.3) |
| Increased AST | 66 (62.9) | 6 (5.7) | 45 (42.9) | 8 (7.6) |
| Increased blood bilirubin | 40 (38.1) | 2 (1.9) | 43 (41.0) | 0 (0.0) |
| Increased GGT | 7 (6.7) | 2 (1.9) | 6 (5.7) | 0 (0.0) |
| Abnormal hepatic function | 7 (6.7) | 4 (3.8) | 6 (5.7) | 3 (2.9) |
| Infusion-related reactions | 2 (1.9) | 0 (0.0) | 1 (1.0) | 0 (0.0) |
| Other TEAE of interest | ||||
| ILD | 1 (1.0) | 1 (1.0) | 3 (2.9) | 2 (1.9) |
| Pneumonitis | 0 (0.0) | 0 (0.0) | 2 (1.9) | 0 (0.0) |
Note: Data are n (%).
AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ERL, erlotinib; GGT, gamma-glutamyltransferase; GI, gastrointestinal; ILD, interstitial lung disease; PL, placebo; RAM, ramucirumab; TEAE, treatment-emergent adverse event; VEGF, vascular endothelial growth factor.
AEs of special interest are prespecified selected AEs of clinical interest that have been associated with other antiangiogenic agents, in a similar pharmacologic class as ramucirumab, that inhibit the VEGF signaling pathway.
Includes the preferred terms hemorrhoidal hemorrhage, anal hemorrhage, small intestinal hemorrhage, lower gastrointestinal hemorrhage, and hematochezia.
Includes the preferred terms proteinuria and protein urine present.
The frequency of TEAEs of grade 3 or above in patients aged 75 years or older was higher than in patients younger than 75 years (RAM + ERL: 10 of 12 patients [83.3%] versus 71 of 93 patients [76.3%]; PL + ERL: 12 of 17 patients [70.6%] versus 52 of 88 patients [59.1%]) (Supplementary Table 4). Common TEAEs of any grade with RAM + ERL treatment were similar between age groups but occurred less frequently in patients aged 75 years or older, except for any-grade proteinuria, which occurred in 50.0% of patients aged 75 years or older and 44.1% in those younger than 75 years (Supplementary Table 4). Discontinuation of all study treatment owing to an AE or serious AE occurred in 3 of 12 patients (25.0%) and 2 of 12 patients (16.7%) aged 75 years or older, respectively, in the RAM + ERL arm, and 14 of 93 patients (15.1%) and 3 of 93 patients (3.2%) in the PL + ERL arm.
Treatment-Emergent T790M Rates
Postprogression T790M rates for Japanese patients were similar in the RAM + ERL and PL + ERL arms (population 1: RAM + ERL: 25% [95% CI: 14–41], 9 of 36 patients; PL + ERL: 34% [95% CI: 23–47], 20 of 59 patients; population 2: RAM + ERL: 47% [95% CI: 27–68], 9 of 19 patients; PL + ERL: 50% [95% CI: 35–65], 20 of 40 patients) (Supplementary Fig. 5). Postprogression T790M rates were evaluated according to the number of treatment cycles received before the 30-day follow-up visit (Supplementary Fig. 6).
Discussion
In this prespecified subset analysis of RELAY Japanese patients with previously untreated metastatic EGFR-mutated NSCLC, the safety and clinically meaningful efficacy of RAM + ERL versus PL + ERL was confirmed. The primary efficacy end point (PFS) was improved with RAM + ERL versus PL + ERL (19.4 versus 11.2 mo; HR = 0.610 [95% CI: 0.431–0.864]). The PFS benefit was consistent for ex19del and ex21.L858R and other prespecified subgroups in the Japanese population. Clinical benefit with RAM + ERL was confirmed in secondary (DoR) and exploratory (TTCT) end points, with a manageable safety profile. This subset analysis suggests that RAM + ERL is an effective and safe treatment option for EGFR-mutated NSCLC in Japanese patients.
Despite initial benefits, early generation EGFR TKIs are associated with the development of treatment resistance and loss of clinical benefit. Osimertinib has efficacy and tolerability advantages over these earlier EGFR TKIs. In the phase 3 double-blind FLAURA study in patients with EGFR-mutated NSCLC, median PFS was significantly improved with first-line osimertinib versus gefitinib or ERL therapy (18.9 versus 10.2 mo; HR = 0.46 [95% CI: 0.37–0.57], p < 0.001),25 and PFS in the Japanese subset population was 19.1 months with osimertinib versus 13.8 months with gefitinib (HR = 0.61 [95% CI: 0.38–0.99]).26 Nevertheless, the mechanisms of resistance after first-line osimertinib are heterogeneous and mostly nontargetable. Thus, chemotherapy-based regimens are the only option once patients progress on osimertinib. RELAY was the first global, phase 3, randomized, double-blind, PL-controlled study to reveal improved median PFS with antiangiogenic plus EGFR TKI combination therapy,18,24 confirming the results obtained in the randomized phase 2 (JO25567) and phase 3 (NEJ026) Japanese open-label studies of bevacizumab plus ERL.17,27 In this RELAY Japanese subset, PFS was longer and TTCT was delayed for patients receiving RAM + ERL compared with patients receiving PL + ERL. Furthermore, the rates of postprogression T790M acquisition were similar across treatment arms (population 2: 47% [RAM + ERL] versus 50% [PL + ERL]) and similar to the RELAY global and East Asian populations.18,24 The cumulative incidence of T790M across increasing numbers of treatment cycles received before progression suggests that RAM + ERL might delay the emergence of this resistance mechanism; however, the sample size was limited. T790M is a resistance mechanism for which effective targeted treatment, that is, osimertinib, is available. In the phase 3 AURA3 study, median PFS was significantly longer with osimertinib than with platinum therapy plus pemetrexed (10.1 versus 4.4 mo, respectively; HR = 0.30, 95% CI: 0.23–0.41, p < 0.001) in patients with T790M-positive advanced NSCLC in whom disease had progressed during first-line EGFR TKI therapy.28 These results suggest that for patients with NSCLC who acquire the T790M mutation, treatment sequence could comprise first-line EGFR TKI plus antiangiogenic therapy, second-line osimertinib, and then chemotherapy. The results of the RELAY global study, and the East Asian and Japanese subset analyses, reveal that antiangiogenic plus EGFR TKI combination therapy is a viable first-line treatment strategy for patients with NSCLC with EGFR mutations.18,24
Preclinical studies revealed that EGFR-activating mutations have differential sensitivity to EGFR TKIs.5,6 In a meta-analysis of 12 trials, patients with NSCLC with ex19del mutation had longer PFS and OS, and higher response rates, with EGFR TKI therapy than patients with ex21.L858R mutation.8 In the RELAY global ITT population, PFS benefit with RAM + ERL treatment was similar for both EGFR mutation subgroups (median [95% CI] PFS: ex21.L858R: 19.4 [14.1–21.9] mo; HR [95% CI]: 0.62 [0.44–0.87]; ex19del, 19.6 [15.1–22.2] mo; HR: 0.65 [0.47–0.90]).18 A PFS benefit for ex21.L858R patients with RAM + ERL treatment was also observed in the RELAY Japanese population (median [95% CI] PFS: ex21.L858R: 19.4 [14.1–25.1] mo; unstratified HR = 0.514 [0.317–0.835]; ex19del: 16.6 [13.9–30.3] mo; unstratified HR = 0.701 [0.424–1.159]).
Japanese patients are at risk of developing ILD, a known complication of EGFR TKI therapy.29 The incidence rate of ILD or pneumonitis with EGFR TKI treatment in Japanese patients with NSCLC has been reported to be 12.3% with osimertinib and 1.8% with gefitinib.26 In NEJ026, no ILD events were reported with bevacizumab plus ERL versus 4% of patients with ERL monotherapy.27 The incidence of ILD or pneumonitis with RAM + ERL in both the RELAY global and Japanese populations was low (1.0%–1.8%) versus PL + ERL (2.7%–4.8%).18 This is an important finding for Japanese patients because EGFR TKI treatment cannot continue in patients who develop ILD.
Because the Japanese subset analysis was not powered to reveal differences between RAM + ERL and PL + ERL, the results need to be interpreted with caution, although the results do align with those of the global RELAY study.18 In addition, the exploratory analysis of EGFR T790M rates was limited to patients with both a baseline and a 30-day follow-up NGS sample, or to those with an EGFR-activating mutation detected in the postprogression 30-day follow-up sample, which further reduced the sample size.
In conclusion, RELAY revealed a favorable benefit-risk profile for RAM + ERL in the first-line treatment of Japanese patients with EGFR-mutated NSCLC, consistent with the global RELAY population. Liquid biopsy analyses indicated that the addition of RAM did not affect the ERL-associated EGFR T790M rates at disease progression.
Acknowledgments
This study was sponsored by Eli Lilly and Company, manufacturer and licensee of ramucirumab. Eli Lilly and Company was involved in the study design, data collection, data analysis, and preparation of the manuscript, and in the decision to submit the manuscript for publication. The authors thank all the study participants. Medical writing assistance was provided by Rebecca Lew, PhD, CMPP, and Prudence Stanford, PhD, of ProScribe—Envision Pharma Group, and was funded by Eli Lilly and Company. ProScribe’s services complied with international guidelines for Good Publication Practice (GPP3).
Data Sharing Statement
Eli Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
Footnotes
Cite this article as: Nishio K, Seto T, Nishio M, et al. Ramucirumab plus erlotinib versus placebo plus erlotinib in patients with untreated metastatic EGFR-mutated NSCLC: RELAY Japanese subset. JTO Clin Res Rep. 2;6:100171.
Disclosure: Prof. K. Nishio reports receiving grants and personal fees from Otsuka Pharmaceutical Co., Ltd., Life Technologies Japan, Nippon Boehringer Ingelheim, and Eli Lilly Japan; grants from Ignyta, Inc., and Chugai Pharmaceutical Co., Ltd.; and personal fees from Eisai Co., Ltd., Pfizer, Novartis Pharma, Merck Sharp & Dohme, Ono Pharmaceutical Co., Ltd., Bristol-Myers Squibb, SymBio Pharmaceuticals, Solasia Pharma, Yakult Honsha, Roche Diagnostics, AstraZeneca, Sanofi, Guardant Health, Inc., Astellas Pharma, Inc., Takeda Pharmaceutical Co., Ltd., and Kobayashi Pharmaceutical, outside of the submitted work. Dr. Seto is an employee of Precision Medicine Asia and reports receiving lecture fees, honoraria, or other fees from AstraZeneca, Chugai Pharmaceutical Co., Ltd., Eli Lilly Japan, Merck Sharp & Dohme, Pfizer Japan, and Taiho Pharmaceutical, and grants from AbbVie Inc., AstraZeneca, Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo, Eli Lilly Japan K.K., Kissei Pharmaceutical Co., Ltd., LOXO Oncology, Inc., Merck Sharp & Dohme, Nippon Boehringer Ingelheim Co., Ltd., Novartis, Pfizer Japan Inc., and Takeda Pharmaceutical Company Limited. Dr. M. Nishio reports receiving grants and personal fees from Ono Pharmaceutical Co., Ltd., Bristol-Myers Squibb, Pfizer, Chugai Pharmaceutical Co., Ltd., Eli Lilly and Company, Taiho Pharmaceutical, AstraZeneca, Merck Sharp & Dohme, Novartis, Daiichi Sankyo, and Takeda Pharmaceutical Co., Ltd., and personal fees from Boehringer Ingelheim, Merck Biopharma, Teijin Pharma Limited, and AbbVie, outside of the submitted work. Prof. Dr. Reck reports receiving honoraria for lectures and consultancy from Amgen, Inc., AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly and Company, Merck, Mirati, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Samsung, outside of the submitted work. Prof. Garon reports receiving grants from Eli Lilly and Company during the conduct of the study and, outside of the submitted work, grants from Novartis, AstraZeneca, Bristol-Myers Squibb Company, Dynavax, EMD Serono, Genentech, Inc., Iovance Biotherapeutics, Merck & Co., Inc., Mirati, Neon, and Novartis. Dr. Sakai reports receiving personal fees from Roche Diagnostics, Bio-Rad, AstraZeneca, and Chugai Pharmaceutical Co., Ltd., outside of the submitted work. Dr. Goto reports receiving grants and personal fees from Chugai Pharmaceutical Co., Ltd. and Eli Lilly Japan K.K. during the conduct of the study and, outside of the submitted work, personal fees from Guardant Health, Inc. and Otsuka Pharmaceutical Co., Ltd., grants from Amgen Inc., Astellas Pharma Inc., Eisai Co., Ltd., Ignyta, Inc., Loxo Oncology, Inc., Medical & Biological Laboratories Co., Ltd., Merck Serono Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., and Sysmex Corporation, and grants and personal fees from Takeda Pharmaceutical Co., Ltd., Amgen Astellas BioPharma K.K., AstraZeneca K.K., Boehringer Ingelheim Japan, Inc., Bristol-Myers Squibb K.K., Daiichi Sankyo Co., Ltd., Janssen Pharmaceutical K.K., Kyowa Hakko Kirin Co., Ltd., Merck Biopharma Co., Ltd., Merck Sharp & Dohme K.K., Nippon Kayaku Co., Ltd., Novartis Pharma K.K., Ono Pharmaceutical Co., Ltd., Pfizer, Inc., Taiho Pharmaceutical Co., Ltd., Thermo Fisher Scientific K.K., Xcoo, Inc., and Takeda Pharmaceutical Co., Ltd. Dr. Kato reports receiving grants and personal fees from AbbVie, Amgen Inc., AstraZeneca, Bristol-Myers Squibb, Chugai, Eli Lilly, Merck Biopharma, Merck Sharp & Dohme, Novartis, Ono Pharmaceutical Co., Ltd., Pfizer, and Taiho; personal fees from Boehringer Ingelheim, Daiichi Sankyo, F. Hoffman-La Roche, Nippon Kayaku, Nitto Denko, Shionogi & Co., Sumitomo Dainippon, and Takeda Pharmaceutical Co., Ltd.; and grants from Astellas Pharma Inc., Kyorin, Kyowa-Kirin, and Regeneron Pharmaceuticals Inc., outside of the submitted work. Dr. Nakanishi reports receiving grants and personal fees from Chugai Pharmaceutical Co., Ltd. and personal fees from Eli Lilly and Company, AstraZeneca, Boehringer Ingelheim Japan, Merck Sharp & Dohme, Ono Pharmaceutical Co., Ltd., and Pfizer outside of the submitted work. Dr. Takahashi reports receiving grants from Japan Agency for Medical Research and Development during the conduct of the study and, outside of the submitted work, grants and personal fees from AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Ono Pharmaceutical Co., Ltd., Merck Sharp & Dohme K.K., and Pfizer Japan Inc., and personal fees from Nippon Boehringer Ingelheim Co., Ltd. and Roche Diagnostics K.K. Dr. Yamamoto reports receiving grants and personal fees from Merck Sharp & Dohme K.K., AstraZeneca, Ono Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Boehringer Ingelheim, Novartis, and Pfizer Inc.; personal fees from Thermo Fisher Scientific, Bristol-Myers Squibb, Life Technologies Japan Ltd., Nippon Kayaku, and Merck Biopharma; and grants from Astellas Pharma Inc., Tsumura & Co., Shionogi & Co., Ltd., AbbVie G.K., Amgen Inc., Kyorin Pharmaceutical Co., Ltd., Eisai Co., Ltd., Terumo Corporation, Toppan Printing Co., Ltd., and Tosoh outside of the submitted work. Prof. Kiura reports receiving personal fees from AstraZeneca K.K., Eli Lilly Japan K.K., and Novartis International AG; grants and personal fees from Daiichi Sankyo Co., Ltd., Pfizer Japan Inc., Boehringer Ingelheim Co., Ltd., Taiho Pharmaceutical Co. Ltd., Ono Pharmaceutical Co., Ltd., Merck Sharp & Dohme K.K., Chugai Pharmaceutical Co., Ltd., and Bristol-Myers Squibb K.K.; and grants from Teijin Pharma Limited, Shionogi & Co., Ltd., Nippon Kayaku Co., Ltd., Kyorin Pharmaceutical Co., Ltd., Merck Biopharma Co., Ltd., and Takeda Pharmaceutical Company Limited, outside of the submitted work. Dr. Ohe reports receiving grants and personal fees from Eli Lilly during the conduct of the study and, outside of the submitted work, grants and personal fees from AstraZeneca, Amgen Inc., Bristol-Myers Squibb, Chugai, Kyorin, Merck Sharp & Dohme, Nippon Kayaku, Ono Pharmaceutical Co., Ltd., Taiho, Takeda Pharmaceutical Co., Ltd., and Pfizer; personal fees from Boehringer Ingelheim, Celtrion, and Novartis; and grants from Janssen, Kissei Pharmaceutical Co., Ltd., and Ignyta, Inc. Dr. Tamura reports receiving personal fees from Eli Lilly, Chugai, Ono Pharmaceutical Co., Ltd., Nippon Kayaku, Taiho Pharmaceutical, Boehringer Ingelheim, Merck Sharp & Dohme, and CMIC Shift Zero, outside of the submitted work. Dr. Visseren-Grul, Mrs. Frimodt-Moller, R. Hozak, Dr. Wijayawardana, Mrs. Zimmermann, Dr. Homma, and Dr. Enatsu are employees and minor shareholders of Eli Lilly and Company. Prof. Nakagawa reports receiving grants and personal fees from AstraZeneca K.K., Astellas Pharma Inc., Merck Sharp & Dohme K.K., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., Bristol-Myers Squibb Company, Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., and Merck Serono Co., Ltd./Merck Biopharma Co., Ltd.; grants, personal fees, and other fees from Ono Pharmaceutical Co., Ltd., Pfizer Japan Inc., and Eli Lilly Japan K.K. during the conduct of the study and, outside of the submitted work, personal fees from Clinical Trial Co., Ltd., Medicus Shuppan, Publishers Co., Ltd., Care Net, Inc., Reno Medical K.K., Medical Review Co., Ltd., Roche Diagnostics K.K., Bayer Yakuhin, Ltd., Medical Mobile Communications Co., Ltd., 3H Clinical Trial Inc., Nichi-Iko Pharmaceutical Co., Ltd., Nanzando Co., Ltd., Yodosha Co., Ltd., Nikkei Business Publications, Inc., Thermo Fisher Scientific K.K., Yomiuri Telecasting Corporation, and Nippon Kayaku Co., Ltd.; personal fees and other fees from Kyorin Pharmaceutical Co., Ltd.; grants from inVentiv Health Japan, ICON Japan K.K., Gritsone Oncology Inc., Parexel International Corporation, Kissei Pharmaceutical Co., Ltd., EPS Corporation, Syneos Health, Pfizer R&D Japan G.K., A2 Healthcare Corporation, Quintiles Inc./IQVIA Services JAPAN K.K., EP-CRSU Co., Ltd., Linical Co., Ltd., Eisai Co., Ltd., CMIC Shift Zero K.K., Kyowa Hakko Kirin Co., Ltd., Bayer Yakuhin, Ltd., EPS International Co., Ltd., and Otsuka Pharmaceutical Co., Ltd.; grants and personal fees from Taiho Pharmaceutical Co., Ltd., SymBio Pharmaceuticals Ltd., and AbbVie Inc.; and grants, personal fees, and other fees from Takeda Pharmaceutical Co., Ltd.
Cite this article as: Nishio K, Seto T, Nishio M, et al. Ramucirumab plus erlotinib versus placebo plus erlotinib in patients with untreated metastatic EGFR-mutated NSCLC: RELAY Japanese subset. JTO Clin Res Rep. 2;6:100171.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2021.100171.
Supplementary Data
References
- 1.Liu X., Wang P., Zhang C., Ma Z. Epidermal growth factor receptor (EGFR): a rising star in the era of precision medicine of lung cancer. Oncotarget. 2017;8:50209–50220. doi: 10.18632/oncotarget.16854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saito M., Shiraishi K., Kunitoh H., Takenoshita S., Yokota J., Kohno T. Gene aberrations for precision medicine against lung adenocarcinoma. Cancer Sci. 2016;107:713–720. doi: 10.1111/cas.12941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akamatsu H., Ninomiya K., Kenmotsu H. The Japanese Lung Cancer Society Guideline for non-small cell lung cancer, stage IV. Int J Clin Oncol. 2019;24:731–770. doi: 10.1007/s10147-019-01431-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Y.L., Planchard D., Lu S. Pan-Asian adapted Clinical Practice Guidelines for the management of patients with metastatic non-small-cell lung cancer: a CSCO-ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019;30:171–210. doi: 10.1093/annonc/mdy554. [DOI] [PubMed] [Google Scholar]
- 5.Carey K.D., Garton A.J., Romero M.S. Kinetic analysis of epidermal growth factor receptor somatic mutant proteins shows increased sensitivity to the epidermal growth factor receptor tyrosine kinase inhibitor, erlotinib. Cancer Res. 2006;66:8163–8171. doi: 10.1158/0008-5472.CAN-06-0453. [DOI] [PubMed] [Google Scholar]
- 6.Gilmer T.M., Cable L., Alligood K. Impact of common epidermal growth factor receptor and HER2 variants on receptor activity and inhibition by lapatinib. Cancer Res. 2008;68:571–579. doi: 10.1158/0008-5472.CAN-07-2404. [DOI] [PubMed] [Google Scholar]
- 7.Li W.Q., Cui J.W. Non-small cell lung cancer patients with ex19del or exon 21 L858R mutation: distinct mechanisms, different efficacies to treatments. J Cancer Res Clin Oncol. 2020;146:2329–2338. doi: 10.1007/s00432-020-03296-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheng M., Wang F., Zhao Y. Comparison of clinical outcomes of patients with non-small-cell lung cancer harbouring epidermal growth factor receptor exon 19 or exon 21 mutations after tyrosine kinase inhibitors treatment: a meta-analysis. Eur J Clin Pharmacol. 2016;72:1–11. doi: 10.1007/s00228-015-1966-0. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y.L., Yuan J.Q., Wang K.F. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget. 2016;7:78985–78993. doi: 10.18632/oncotarget.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham R.P., Treece A.L., Lindeman N.I. Worldwide frequency of commonly detected EGFR mutations. Arch Pathol Lab Med. 2018;142:163–167. doi: 10.5858/arpa.2016-0579-CP. [DOI] [PubMed] [Google Scholar]
- 11.Wu S.G., Shih J.Y. Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol Cancer. 2018;17:38. doi: 10.1186/s12943-018-0777-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ettinger D.S., Wood D.E., Aggarwal C. NCCN guidelines insights: non-small cell lung cancer, version 1.2020. J Natl Compr Canc Netw. 2019;17:1464–1472. doi: 10.6004/jnccn.2019.0059. [DOI] [PubMed] [Google Scholar]
- 13.Planchard D., Popat S., Kerr K. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv192–iv237. doi: 10.1093/annonc/mdy275. [DOI] [PubMed] [Google Scholar]
- 14.Byers L.A., Heymach J.V. Dual targeting of the vascular endothelial growth factor and epidermal growth factor receptor pathways: rationale and clinical applications for non-small-cell lung cancer. Clin Lung Cancer. 2007;8(suppl 2):S79–S85. doi: 10.3816/clc.2007.s.006. [DOI] [PubMed] [Google Scholar]
- 15.Viloria-Petit A., Crombet T., Jothy S. Acquired resistance to the antitumor effect of epidermal growth factor receptor-blocking antibodies in vivo: a role for altered tumor angiogenesis. Cancer Res. 2001;61:5090–5101. [PubMed] [Google Scholar]
- 16.Naumov G.N., Nilsson M.B., Cascone T. Combined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistance. Clin Cancer Res. 2009;15:3484–3494. doi: 10.1158/1078-0432.CCR-08-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seto T., Kato T., Nishio M. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol. 2014;15:1236–1244. doi: 10.1016/S1470-2045(14)70381-X. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa K., Garon E.B., Seto T. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:1655–1669. doi: 10.1016/S1470-2045(19)30634-5. [DOI] [PubMed] [Google Scholar]
- 19.CYRAMZA. (Ramucirumab) [package insert] Eli Lilly and Company; Indianapolis, IN: 2020. [Google Scholar]
- 20.TARCEVA® (Erlotinib) [package insert] Astellas Pharma US, Inc and Genentech, Inc; South San Francisco, CA: 2016. [Google Scholar]
- 21.Wu Z., Yang Z., Dai Y., Zhu Q., Chen L.-A. Update on liquid biopsy in clinical management of non-small cell lung cancer. Onco Targets Ther. 2019;12:5097–5109. doi: 10.2147/OTT.S203070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishii H., Azuma K., Sakai K. Digital PCR analysis of plasma cell-free DNA for non-invasive detection of drug resistance mechanisms in EGFR mutant NSCLC: correlation with paired tumor samples. Oncotarget. 2015;6:30850–30858. doi: 10.18632/oncotarget.5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu S., Lou F., Wu Y. Circulating tumor DNA identified by targeted sequencing in advanced-stage non-small cell lung cancer patients. Cancer Lett. 2016;370:324–331. doi: 10.1016/j.canlet.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishio M., Seto T., Reck M. Ramucirumab or placebo plus erlotinib in EGFR-mutated, metastatic non-small-cell lung cancer: East Asian subset of RELAY. Cancer Sci. 2020;111:4510–4525. doi: 10.1111/cas.14655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soria J.C., Ohe Y., Vansteenkiste J. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 26.Ohe Y., Imamura F., Nogami N. Osimertinib versus standard-of-care EGFR-TKI as first-line treatment for EGFRm advanced NSCLC: FLAURA Japanese subset. Jpn J Clin Oncol. 2019;49:29–36. doi: 10.1093/jjco/hyy179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saito H., Fukuhara T., Furuya N. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20:625–635. doi: 10.1016/S1470-2045(19)30035-X. [DOI] [PubMed] [Google Scholar]
- 28.Mok T.S., Wu Y.L., Ahn M.J. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoneda K.Y., Hardin K.A., Gandara D.R., Shelton D.K. Interstitial lung disease associated with epidermal growth factor receptor tyrosine kinase inhibitor therapy in non-small-cell lung carcinoma. Clin Lung Cancer. 2006;8(suppl 1):S31–S35. doi: 10.3816/clc.2006.s.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.