Abstract
Introduction
The programmed death-ligand 1 inhibitor atezolizumab improves progression-free survival (PFS) and overall survival (OS) for patients with previously treated advanced NSCLC. Preclinical studies indicate that targeting CD38-positive cells with daratumumab may synergistically enhance atezolizumab’s antitumor activity by increasing the effector T-cell activity.
Methods
This phase 1b-2 study included a safety run-in (one cycle of daratumumab plus atezolizumab) and randomized phases (daratumumab plus atezolizumab versus atezolizumab alone). The primary objective of the randomized phase was to compare overall response rates. The secondary objectives included evaluations of safety, clinical benefit rate (stable disease or better), PFS, OS, and pharmacokinetics.
Results
In total, 99 patients were enrolled (safety run-in, n = 7; randomized, n = 46 per arm). In the randomized phase, the overall response rate was 4.3% for daratumumab plus atezolizumab and 13.0% for atezolizumab alone (OR: 0.30; 95% confidence interval: 0.03–1.92). The respective clinical benefit rates were 52.2% and 43.5%. No improvements were observed in the median PFS or median OS for combination therapy. The study was terminated because of the limited efficacy of daratumumab plus atezolizumab.
Conclusions
Daratumumab plus atezolizumab therapy did not improve efficacy versus atezolizumab monotherapy for patients with previously treated advanced NSCLC.
Keywords: Daratumumab, Atezolizumab, Non‒small cell lung cancer, Combination immunotherapy
Introduction
NSCLC therapy options depend on tumor histology, sensitizing mutations, and biomarker expression (e.g., programmed death-ligand 1 [PD-L1]).1, 2, 3 Inhibitors of PD-L1 and the programmed cell death protein 1 (PD-1) receptor are salvage therapy in immunotherapy-naive patients with NSCLC. For frontline therapy, the current standard of care includes pembrolizumab (a PD-1 inhibitor) as monotherapy or combined with chemotherapy.1, 2, 3
Atezolizumab is an anti–PD-L1 monoclonal antibody that promotes T-cell activation and restoration of antitumor surveillance.4 In the phase 3 OAK trial, atezolizumab monotherapy improved the overall survival (OS) for patients with previously treated advanced NSCLC versus docetaxel, regardless of PD-L1 expression level.5,6 The greatest OS benefit occurred in patients with tumors having high PD-L1 expression (tumor cell [TC] 3, immune cell [IC] 3).6
Daratumumab, an anti-CD38 antibody, is approved as monotherapy or combined with standard-of-care therapies for patients with newly diagnosed or relapsed/refractory multiple myeloma.7, 8, 9 CD38 is an IC marker expressed on multiple IC populations, including normal lymphoid cells, myeloid cells, and some nonhematopoietic tissues.10 CD38 has roles as an ectoenzyme in the production of adenosine using nicotinamide adenine dinucleotide–plus as a substrate and is a cell surface receptor.10 Preclinical studies revealed that CD38 may play a role in tumorigenesis of solid tumors, and the combined blockade of CD38 plus PD-(L)1 enhances antitumor responses by reducing acquired resistance to anti‒PD-L1 therapies.11, 12, 13 These data provide the rationale to study daratumumab plus PD-L1 inhibition in NSCLC.
Here, we describe the results from a randomized, open-label, multicenter, phase 1b-2 study evaluating the safety and efficacy of daratumumab plus atezolizumab compared with atezolizumab alone in patients with previously treated advanced or metastatic NSCLC.
Materials and Methods
Trial Design and Patients
In this study (NCT03023423), eligible patients were 18 years of age or older with histologically or cytologically confirmed advanced or metastatic previously treated NSCLC and had known PD-L1 tumor expression status at screening. Patients were immunotherapy-naive and must have received at least two cycles of standard platinum-based therapy for stage IIIb or IV NSCLC with disease progression on or after therapy (patients with less than two cycles of platinum-based therapy were allowed if they were intolerant to the therapy and had documented disease progression). Patients with known genetic alterations (ALK, EGFR, or ROS1) were excluded. Additional eligibility criteria are in the Supplementary Data. Stratification factors included tumor PD-L1 status per the SP142 assay (PD-L1–negative [TC0, IC0] versus other), histology (squamous versus nonsquamous), and previous lines of therapy (one versus more than one). Each patient provided written informed consent.
Treatments
The safety run-in phase comprised a single arm; the patients received 21-day cycles of daratumumab (16 mg/kg intravenously [IV] given once weekly during cycles 1 through 3, then on d 1 of each cycle thereafter) plus atezolizumab (1200 mg IV on d 2 of cycle 1, and on d 1 of every cycle thereafter). In the randomized (1:1) phase, the patients received either daratumumab plus atezolizumab using the safety run-in dose regimen, or atezolizumab alone (atezolizumab 1200 mg IV on d 1 of 21-day cycles). Patients receiving daratumumab also received preinfusion and postinfusion medication (including corticosteroids) to prevent infusion-related reactions (IRRs) (see the Supplementary Data for additional treatment information).
Objectives and Assessments
The primary objective of the randomized phase was to compare the overall response rate (ORR), defined by Response Evaluation Criteria in Solid Tumors version 1.1. The key secondary objectives included evaluations of the following: (1) safety; (2) duration of response; (3) clinical benefit rate (CBR) (the proportion of patients who achieved a complete response, partial response [PR], or stable disease with a duration ≥16 wk); (4) progression-free survival (PFS); (5) OS; (6) pharmacokinetics; and (7) immunogenicity. The biomarker evaluation and statistical analysis methods are in the Supplementary Data.
Results
Patients and Treatment
Seven patients were enrolled in the safety run-in, and 46 patients each were randomized to daratumumab plus atezolizumab or atezolizumab alone (Supplementary Fig. 1). In total, eight patients in the atezolizumab group crossed over to the daratumumab plus atezolizumab group after progression, as permitted by the protocol. There was no evidence of tumor response after crossover. In addition, poststudy therapy was received by 12 patients in the daratumumab plus atezolizumab group and seven patients in the atezolizumab group (Supplementary Table 1). Baseline characteristics between arms were similar, with exceptions such as age, sex, smoking status, Eastern Cooperative Oncology Group performance status, presence of brain metastases, and median times from initial diagnosis and diagnosis of metastatic disease to the first dose of study drug; this potentially suggests that patients in the daratumumab plus atezolizumab group may have poorer prognoses (Table 1). Similar numbers of patients in each arm were PD-L1‒negative (TC0 and IC0), but the daratumumab plus atezolizumab group had more patients with negative PD-L1 TCs (Supplementary Table 2) and fewer patients with high PD-L1 expression (TC3, any IC) (Table 1).
Table 1.
Baseline Patient Characteristics
| Characteristic | Phase 1b |
Phase 2 |
|
|---|---|---|---|
| Safety Run-In: Daratumumab Plus Atezolizumab (n = 7) | Daratumumab Plus Atezolizumab (n = 46) | Atezolizumab (n = 46) | |
| Median age, y (range) | 66.0 (44–69) | 65.5 (38–85) | 61.0 (30–81) |
| Age ≥65 y, n (%) | 4 (57.1) | 25 (54.3) | 17 (37.0) |
| Male, n (%) | 4 (57.1) | 38 (82.6) | 28 (60.9) |
| Race, n (%) | |||
| White | 7 (100.0) | 41 (89.1) | 35 (76.1) |
| Not reported | 0 | 5 (10.9) | 11 (23.9) |
| Ethnicity, n (%) | |||
| Not Hispanic or Latino | 7 (100.0) | 40 (87.0) | 36 (78.3) |
| Hispanic or Latino | 0 | 0 | 1 (2.2) |
| Unknown | 0 | 1 (2.2) | 0 |
| Not reported | 0 | 5 (10.9) | 9 (19.6) |
| Baseline ECOG PS score, n (%) | |||
| 0 | 3 (42.9) | 12 (26.1) | 19 (41.3) |
| 1 | 4 (57.1) | 33 (71.7) | 27 (58.7) |
| Current smoker, n (%) | 1 (14.3) | 17 (37.0) | 10 (21.7) |
| Previous lines of therapy, n (%) | |||
| 1 | 6 (85.7) | 32 (69.6) | 30 (65.2) |
| ≥2 | 1 (14.3) | 14 (30.4) | 16 (34.8) |
| Histology at diagnosis, n (%) | |||
| Squamous | 1 (14.3) | 10 (21.7) | 13 (28.3) |
| Nonsquamous/adenocarcinoma | 6 (85.7) | 34 (73.9) | 32 (69.6) |
| Nonsquamous/large-cell carcinoma | 0 | 2 (4.3) | 1 (2.2) |
| Cancer stage at screening | |||
| IIIB | 0 | 4 (8.7) | 2 (4.3) |
| IV | 7 (100.0) | 42 (91.3) | 44 (95.7) |
| Median time since initial diagnosis,a mo (range) | 7.0 (3–30) | 12.8 (4–69) | 11.3 (3–133) |
| Median time since diagnosis of metastatic disease,a mo (range) | 4.0 (1–30) | 11.0 (0–69) | 8.4 (2–40) |
| Location of metastasis, n (%) | |||
| Brain | 1 (14.3) | 4 (8.7) | 11 (23.9) |
| Liver | 2 (28.6) | 7 (15.2) | 5 (10.9) |
| Baseline PD-L1 status,b n (%) | |||
| N | 7 | 46 | 45 |
| Negative (TC0 and IC0) | 5 (71.4) | 23 (50.0) | 22 (48.9) |
| Low (all others) | 2 (28.6) | 19 (41.3) | 16 (35.6) |
| High (TC3, any IC) | 0 | 4 (8.7) | 7 (15.6) |
| Baseline CD38 staining subgroup, n (%) | |||
| ≥1% TCs | 5 (71.4) | 30 (65.2) | 34 (73.9) |
| Other | 2 (28.6) | 16 (34.8) | 12 (26.1) |
ECOG PS, Eastern Cooperative Oncology Group performance status; IC, immune cell; PD-L1, programmed death-ligand 1; TC, tumor cell.
N = 44 each for both randomized groups.
PD-L1 status on the basis of the SP142 assay; one patient in the atezolizumab group did not have data for PD-L1 status.
After a planned data monitoring committee review on May 23, 2018, enrollment and ongoing combination therapy were halted owing to a lack of observed improved efficacy in the daratumumab plus atezolizumab group. At data cutoff (May 17, 2018), the median duration of follow-up among randomized patients was 7.26 months (range: 0.2–11.8).
Efficacy
In the daratumumab plus atezolizumab group, the ORR was 4.3% (95% confidence interval [CI]: 0.5–14.8) versus 13.0% (95% CI: 4.9–26.3) in the atezolizumab group (OR: 0.30; 95% CI: 0.03–1.92; Table 2). All responses were PRs and ongoing at study closure. The CBRs were 52.2% versus 43.5%, respectively (OR: 1.41; 95% CI: 0.58–3.36; Table 2). A reduction in tumor size from baseline occurred in 16 patients in the daratumumab plus atezolizumab group and 14 in the atezolizumab group, with reductions greater than 30% occurring for three and six patients, respectively (Fig. 1).
Table 2.
Summary of Overall Best Responses, Investigator-Assessed Per RECIST Version 1.1 (ITT Population)
| Response Category | Daratumumab Plus Atezolizumab (n = 46), n (%, 95% CI) | Atezolizumab (n = 46), n (%, 95% CI) | OR (95% CI)a |
|---|---|---|---|
| CR | 0 (NE–NE) | 0 (NE–NE) | — |
| PR | 2 (4.3, 0.5–14.8) | 6 (13.0, 4.9–26.3) | — |
| SD | 22 (47.8, 32.9–63.1) | 14 (30.4, 17.7–45.8) | — |
| PD | 15 (32.6, 19.5–48.0) | 21 (45.7, 30.9–61.0) | — |
| NE | 7 (15.2, 6.3–28.9) | 5 (10.9, 3.6–23.6) | — |
| Overall response (CR+PR) | 2 (4.3, 0.5–14.8) | 6 (13.0, 4.9–26.3) | 0.30 (0.03–1.92) |
| CBR (CR+PR+SD) | 24 (52.2, 36.9–67.1) | 20 (43.5, 28.9–58.9) | 1.41 (0.58–3.36) |
CBR, clinical benefit rate; CI, confidence interval; CR, complete response; IC, immune cell; ITT, intent-to-treat; NE, not estimable; PD, progressive disease; PD-L1, programmed death-ligand 1; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease; TC, tumor cell.
A Mantel-Haenszel estimate of the common OR stratified by PD-L1 expression status (IC0 and TC0 versus others), histology (squamous versus nonsquamous), and the number of previous lines of therapy received (one or greater than one).
Figure 1.
Best percentage change from baseline in the sum of diameters for all target lesions in the ITT population among evaluable patients (daratumumab plus atezolizumab, n = 38; atezolizumab, n = 41). PD-L1 status is illustrated for each patient (PD-L1 expression by the SP142 assay: black, high [TC3, any IC]; dark gray, low [all not in the negative or high group]; light gray, negative [TC0 and IC0]; and white, NE). Bars corresponding to patients who achieved the best response of PR by investigator assessment are indicated. IC, immune cell; ITT, intent-to-treat; NE, not estimable; PD-L1, programmed death-ligand 1; PR, partial response; TC, tumor cell.
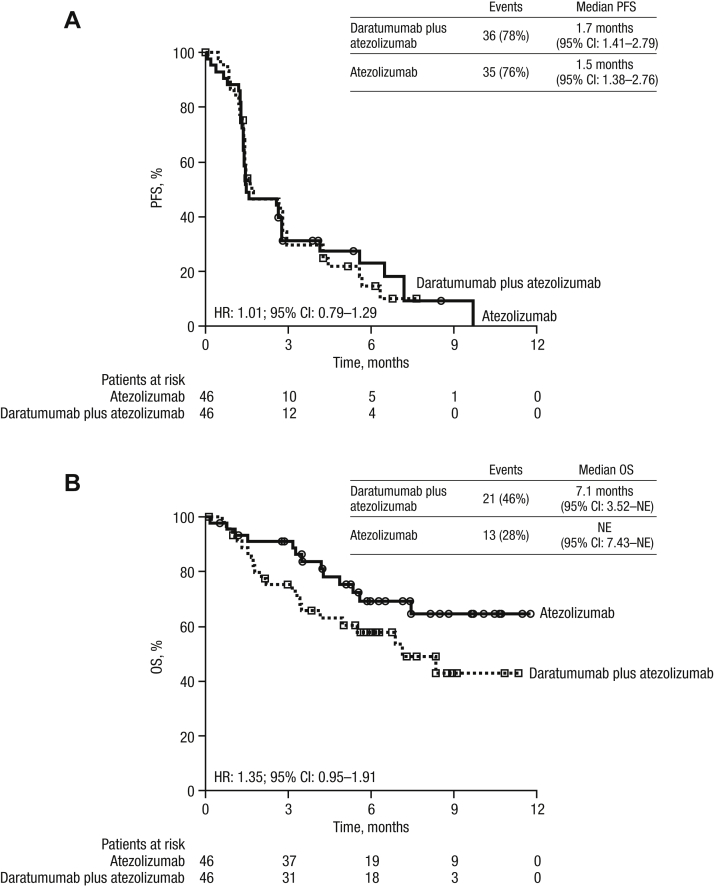
At clinical cutoff, the median PFS for the daratumumab plus atezolizumab group was 1.7 months (95% CI: 1.41–2.79) versus 1.5 months (95% CI: 1.38–2.76) for the atezolizumab group (hazard ratio: 1.01; 95% CI: 0.79–1.29; Fig. 2A).
Figure 2.
PFS and OS. Kaplan-Meier estimates and HRs are illustrated for the ITT population for (A) PFS and (B) OS. CI, confidence interval; HR, hazard ratio; ITT, intent-to-treat; NE, not estimable; OS, overall survival; PFS, progression-free survival.
The median OS was 7.1 months (95% CI: 3.52–not estimable) for the daratumumab plus atezolizumab group and was not reached in the atezolizumab group (hazard ratio: 1.35; 95% CI: 0.95–1.91; Fig. 2B). Survival curves separated early, with estimated 3-month survival rates at 75.1% for daratumumab plus atezolizumab and 91.1% for the atezolizumab group.
Safety
No dose-limiting toxicities were reported in the safety run-in. Adverse events (AEs) occurring in at least 10% of randomized patients are reported in Table 3. When grouped by system organ class, those occurring more often in the daratumumab plus atezolizumab group were related to respiratory, thoracic, and mediastinal disorders, infections and infestations, and vascular disorders (Table 3).
Table 3.
Most Common (≥10%) AEs by Preferred Term and System Organ Class (Safety Population)
| AE | Phase 1b |
Phase 2 |
||||
|---|---|---|---|---|---|---|
| Safety Run-In: Daratumumab Plus Atezolizumab (n = 7) |
Daratumumab Plus Atezolizumab (n = 44) |
Atezolizumab (n = 44) |
||||
| Any Grade | Grade 3 or 4 | Any Grade | Grade 3 or 4a | Any Grade | Grade 3 or 4b | |
| AEs, n (%) | 7 (100.0) | 4 (57.1) | 44 (100.0) | 25 (56.8) | 42 (95.5) | 17 (38.6) |
| AEs occurring in ≥10% of patients, n (%) | ||||||
| Dyspnea | 5 (71.4) | 0 | 16 (36.4) | 7 (15.9) | 9 (20.5) | 1 (2.3) |
| Hypertension | 4 (57.1) | 1 (14.3) | 4 (9.1) | 1 (2.3) | 0 | 0 |
| Asthenia | 3 (42.9) | 0 | 14 (31.8) | 0 | 15 (34.1) | 0 |
| Fatigue | 3 (42.9) | 0 | 9 (20.5) | 0 | 7 (15.9) | 2 (4.5) |
| Nausea | 3 (42.9) | 1 (14.3) | 7 (15.9) | 0 | 9 (20.5) | 0 |
| Decreased appetite | 2 (28.6) | 0 | 11 (25.0) | 0 | 7 (15.9) | 0 |
| Abdominal pain | 2 (28.6) | 0 | 7 (15.9) | 1 (2.3) | 2 (4.5) | 0 |
| Pyrexia | 2 (28.6) | 0 | 4 (9.1) | 0 | 5 (11.4) | 0 |
| Constipation | 2 (28.6) | 0 | 4 (9.1) | 0 | 5 (11.4) | 0 |
| Cough | 1 (14.3) | 0 | 16 (36.4) | 1 (2.3) | 10 (22.7) | 0 |
| Anemia | 1 (14.3) | 1 (14.3) | 7 (15.9) | 2 (4.5) | 6 (13.6) | 1 (2.3) |
| Vomiting | 1 (14.3) | 1 (14.3) | 6 (13.6) | 0 | 3 (6.8) | 0 |
| Hemoptysis | 0 | 0 | 7 (15.9) | 2 (4.5) | 5 (11.4) | 1 (2.3) |
| Respiratory tract infection | 0 | 0 | 7 (15.9) | 3 (6.8) | 1 (2.3) | 1 (2.3) |
| Respiratory, thoracic, and mediastinal disorders | 5 (71.4) | 1 (14.3) | 31 (70.5) | 13 (29.5) | 18 (40.9) | 6 (13.6) |
| Infections and infestations | 0 | 0 | 15 (34.1) | 6 (13.6) | 8 (18.2) | 5 (11.4) |
| Metabolism and nutrition disorders | 2 (28.6) | 0 | 13 (29.5) | 2 (4.5) | 9 (20.5) | 1 (2.3) |
| Vascular disorders | 5 (71.4) | 1 (14.3) | 8 (18.2) | 1 (2.3) | 0 | 0 |
Note: The safety population included all patients who received greater than or equal to one dose of study drug.
AE, adverse event.
Grade 5 AEs were reported in three patients (6.8%) (hemoptysis, cerebrovascular accident, and sudden death in one patient [2.3%] each); none were related to the study treatment.
A single grade 5 AE was reported in one patient (2.3%) (cardiac failure) and was not related to study treatment.
More patients in the daratumumab plus atezolizumab group had at least one grade 3 or 4 AE; however, both groups had similar rates of treatment-related grade 3 or 4 events. Serious AEs occurred in 21 patients (47.7%) in the daratumumab plus atezolizumab group and 15 patients (34.1%) in the atezolizumab group; most were attributed to respiratory complications from progressive disease and were not associated with the study drug. AEs leading to death occurred in three patients (6.8%) in the daratumumab plus atezolizumab group and in one patient in the atezolizumab group; none in either group were treatment-related.
IRRs, frequently observed with daratumumab therapy, occurred in 28 patients (63.6%) receiving daratumumab plus atezolizumab and none receiving atezolizumab alone. Nearly all occurred during the first infusion, which is typical of daratumumab therapy. Immune-mediated AEs were infrequent and reported at equal rates (three patients [6.8%] per group).
Biomarker Analysis
At screening, over half the patients had greater than or equal to 1% of TCs expressing CD38 in the daratumumab plus atezolizumab and atezolizumab groups (65.2% and 73.9% of patients, respectively; Table 1). However, CD38 expression levels were generally low (average H-scores, 26.1 and 28.3, respectively). There was no association between response to therapy and baseline CD38 expression (Supplementary Fig. 2) or PD-L1 expression (Supplementary Fig. 3). There was a modest correlation between PD-L1 and CD38 expression among the PR group; however, the number of patients was small (n = 8).
Natural killer (NK) cells and plasma cells from patients with myeloma express high levels of CD38; these cells decrease on daratumumab treatment14 and are pharmacodynamic biomarkers of daratumumab activity. Daratumumab plus atezolizumab therapy rapidly reduced NK cell levels in whole blood (Supplementary Fig. 4A) and intratumoral plasma cell levels measured by CD138 density (Supplementary Fig. 4B).
Discussion
The daratumumab plus atezolizumab group had fewer patients having overall response compared with the atezolizumab group, although the number of responders was low in each group. Despite the small number of responders, similar PFS and CBRs suggested comparable antitumor activity in both treatment arms, as exhibited by the similar appearance of each group’s waterfall plot illustrating a change in tumor size from baseline.
An imbalance of early deaths between the treatment groups led to a lower median OS in the daratumumab plus atezolizumab group, but these deaths primarily occurred in nonresponding patients and were not a result of drug-related toxicity. The difference in survival may reflect an imbalance in baseline characteristics between the treatment groups, with poorer predictive and prognostic characteristics among patients in the daratumumab plus atezolizumab group.
The incidences and severity of AEs were imbalance between the study groups, with the largest discrepancies occurring for AEs likely associated with disease progression for NSCLC (respiratory disorders and infections) rather than drug toxicity, as these AEs were reported as unrelated to study treatment. More importantly, the types and frequencies of immune-mediated AEs in this study were comparable to previous reports and occurred equally across treatment groups.5,6
NK cell levels, a known pharmacodynamic biomarker of daratumumab activity, were reduced in peripheral blood, and the reduction was sustained throughout daratumumab plus atezolizumab therapy. We found, for the first time, that daratumumab could penetrate solid tumors, as evidenced by a decrease in intrastromal plasma cells. In addition to ICs, TCs expressed CD38 in most patients; however, baseline CD38 expression did not correlate with response to therapy.
In conclusion, the hypothesis of improved antitumoral activity with daratumumab and atezolizumab in previously treated advanced or metastatic NSCLC was not confirmed in this study. It is possible that coadministration of high-dose steroids to mitigate daratumumab-related IRRs blunted robust immune responses, as there are reports that administration of high-dose steroids may interfere with the efficacy of PD-(L)1 inhibitors.15,16 However, the similar waterfall plots between the groups in this study suggest a possibility of no such impact. Whereas the study reported a lower ORR for daratumumab plus atezolizumab compared with atezolizumab monotherapy, other efficacy end points were similar between treatment groups, and the imbalance of death events did not reflect increased toxicity in the combination regimen.
Acknowledgments
This study was funded by Janssen Research & Development. The authors thank the patients who volunteered to participate in the trial, their families, and the staff members at the trial sites who cared for them. The authors thank Dr. Paz-Ares of Hospital Universitario 12 de Octubre, Centro Nacional de Investigaciones Oncológicas, and Universidad Complutense, Madrid, Spain, for his contributions to the study and critical review of the manuscript. The authors also thank Dr. Chu of Janssen Research & Development for his assistance with immunohistochemistry analyses. Editorial assistance was provided by Drs. Majerczyk and Kolesar of MedErgy and they were funded by Janssen Global Services. The authors participated in trial design, data review, analysis, interpretation, and manuscript writing. All authors had full access to all data on request and had final responsibility for the decision to submit for publication.
Footnotes
Disclosure: Ramalingam served on an advisory board and received honoraria from Amgen, AbbVie, Bristol Myers Squibb, Genentech, Eli Lilly, Takeda, and Loxo Oncology; and received research support from AstraZeneca, Merck, and Tesaro. Horn received personal fees from AstraZeneca, AbbVie, Merck, Incyte, Genentech-Roche, Xcovery, EMD Serono, Pfizer, and Tesaro; and received research support from Xcovery and Bristol Myers Squibb. Reck received honoraria for consultancy and lectures from AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Celgene, Merck, Merck Sharp & Dohme, Novartis, Pfizer, and Roche. Thayu, Lorenzini, Arias, Moy, Hutnick, Knoblauch, Feng, and Kane are employees of Janssen. Hutnick and Knoblauch also hold stock in Johnson and Johnson. The remaining authors declare no conflict of interest.
To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2020.100104.
Supplementary Data
References
- 1.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): non-small cell lung cancer version 1.2020. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf [DOI] [PMC free article] [PubMed]
- 2.Planchard D., Popat S., Kerr K. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(suppl 4):iv192–iv237. doi: 10.1093/annonc/mdy275. [DOI] [PubMed] [Google Scholar]
- 3.Planchard D., Popat S., Kerr K. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:863–870. doi: 10.1093/annonc/mdy474. [DOI] [PubMed] [Google Scholar]
- 4.Hendriks L., Besse B. New windows open for immunotherapy in lung cancer. Nature. 2018;558:376–377. doi: 10.1038/d41586-018-05312-9. [DOI] [PubMed] [Google Scholar]
- 5.Rittmeyer A., Barlesi F., Waterkamp D. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial [published correction appears in Lancet. 2017;389:e5] Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fehrenbacher L., von Pawel J., Park K. Updated efficacy analysis including secondary population results for OAK: a randomized phase III study of atezolizumab versus docetaxel in patients with previously treated advanced non-small cell lung cancer. J Thorac Oncol. 2018;13:1156–1170. doi: 10.1016/j.jtho.2018.04.039. [DOI] [PubMed] [Google Scholar]
- 7.Moreau P., San Miguel J., Sonneveld P. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):iv52–iv61. doi: 10.1093/annonc/mdx096. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): multiple myeloma version 2.2020. https://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf. Accessed November 19, 2019.
- 9.DARZALEX® (Daratumumab) injection, for intravenous use [package insert] Janssen Biotech, Inc.; Horsham, PA: 2019. [Google Scholar]
- 10.Konen J.M., Fradette J.J., Gibbons D.L. The good, the bad and the unknown of CD38 in the metabolic microenvironment and immune cell functionality of solid tumors. Cells. 2020;9:52. doi: 10.3390/cells9010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bu X., Kato J., Hong J.A. CD38 knockout suppresses tumorigenesis in mice and clonogenic growth of human lung cancer cells. Carcinogenesis. 2018;39:242–251. doi: 10.1093/carcin/bgx137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L., Diao L., Yang Y. CD38-mediated immunosuppression as a mechanism of tumor cell escape from PD-1/PD-L1 blockade. Cancer Discov. 2018;8:1156–1175. doi: 10.1158/2159-8290.CD-17-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verma V., Shrimali R.K., Ahmad S. PD-1 blockade in subprimed CD8 cells induces dysfunctional PD-1+CD38hi cells and anti-PD-1 resistance [published correction appears in Nat Immunol. 2019;20:1555] Nat Immunol. 2019;20:1231–1243. doi: 10.1038/s41590-019-0441-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krejcik J., Casneuf T., Nijhof I.S. Daratumumab depletes CD38+ immune-regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016;128:384–394. doi: 10.1182/blood-2015-12-687749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ricciuti B., Dahlberg S.E., Adeni A., Sholl L.M., Nishino M., Awad M.M. Immune checkpoint inhibitor outcomes for patients with non-small-cell lung cancer receiving baseline corticosteroids for palliative versus nonpalliative indications. J Clin Oncol. 2019;37:1927–1934. doi: 10.1200/JCO.19.00189. [DOI] [PubMed] [Google Scholar]
- 16.Arbour K.C., Mezquita L., Long N. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. 2018;36:2872–2878. doi: 10.1200/JCO.2018.79.0006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.