Abstract
Peripheral inflammation has been found associated with psychiatric disorders. However, results are inconclusive as to its role in common mental disorders (CMDs), i.e., depression, anxiety, insomnia and stress-related disorders. Further, some research suggests that cognitive behavior therapy (CBT) could reduce inflammatory markers in CMDs. In the present study, we measured pro-inflammatory cytokines (tumor necrosis factor alpha [TNF-α], interleukin-6 [IL-6] and IL-8) pre- and post-treatment in two clinical trials (N = 367) investigating CBT for patients with CMDs in primary care. We hypothesized that higher levels of these cytokines would be associated with more severe psychiatric symptoms (i.e., symptoms of depression, stress and anxiety). We also hypothesized that level of cytokines would decrease after CBT and that the reduced levels would correlate with a reduction in symptoms. Results showed that in men, higher levels of TNF-α were associated with more severe psychiatric symptoms. Further, age moderated the association between TNF-α, as well as IL-6, and stress, and exploratory stratified analyses revealed significant associations in subgroups. No other significant associations between cytokines and psychiatric symptoms were found. None of the cytokines were reduced following CBT, and the marked improvements in psychiatric symptoms after treatment were not associated with changes in cytokines. In conclusion, although inflammation might be of relevance in subgroups, it seems to be of limited importance for clinical improvements across mild to moderate CMDs.
Keywords: Cytokines, Inflammation, Common mental disorders, Cognitive behavior therapy
Highlights
-
•
Immune dysregulation has been implicated in the etiology of common mental disorders.
-
•
Pro-inflammatory cytokines were measured pre and post cognitive behavior therapy.
-
•
Baseline associations between cytokines and symptoms were only found in subgroups.
-
•
Cytokine levels (TNF-α, IL-6 and IL-8) were not reduced after treatment.
-
•
Symptom improvement was not accompanied by reductions in cytokine levels.
1. Introduction
Common mental disorders (CMDs), i.e., depression, anxiety disorders, insomnia, and stress-related disorders are highly prevalent (Casey, 2014; Kessler et al., 2005; Morin and Benca, 2012) and associated with significant suffering, reduced quality of life and impaired functioning (Ormel et al., 2008; Saarni et al., 2007). Several lines of research have suggested that a dysregulation of the immune system, particularly the pro-inflammatory cytokines, play an important role in the pathogenesis of these disorders. Indeed, pro-inflammatory cytokines can impact the brain through vagal and humoral routes (Dantzer, 2009) to induce a so called “sickness response”. This response is viewed as a motivational state that promotes recovery and includes loss of appetite, sleepiness, withdrawal from social activities and fatigue (Dantzer and Kelley, 2007). In experimental research, where administration of lipopolysaccharide (endotoxin) are used to induce a transient inflammation, healthy participants display symptoms of depressed mood, loss of motivation, anxiety, fatigue and social withdrawal (Schedlowski et al., 2014). Thus, experimental induction of inflammation has been shown to produce symptoms that are hallmarks of CMDs. Moreover, communication between the immune system and the brain is bidirectional, i.e., change in stress or threat perception also causes alterations of the immune system. The stress response includes activation of the sympathetic nervous system and the hypothalamic–pituitary–adrenal axis, resulting in secretion of norepinephrine, epinephrine and cortisol, and the immune system expresses receptors for, and is regulated by, these neurotransmitters and hormones (Dhabhar, 2014). Consequently, changes in reactions to stressors and perceived threat could lead to changes in the immune system.
Research results vary as to whether elevated pro-inflammatory cytokines are present in the CMDs. The evidence is most convincing regarding depression where meta-analyses have found increased levels of inflammatory markers, including elevated levels of pro-inflammatory cytokines interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), as well as c-reactive protein (CRP), in depressed subjects as compared to controls (Dowlati et al., 2010; Haapakoski et al., 2015). Most studies have compared inflammatory markers in depressed and healthy subjects (Raison et al., 2006), but there are also studies that have reported correlations between higher inflammatory markers and more severe depressive symptoms (see e.g., Miller et al., 2002; Thomas et al., 2005). The source of this chronic low-grade inflammation is not clear but could include for example psychosocial stress, physical inactivity, poor diet, smoking, obesity, altered gut permeability, disturbed sleep, and vitamin D deficiency (Berk et al., 2013).
Although less studied, elevated levels of inflammatory markers have been found in obsessive-compulsive disorder (Gray and Bloch, 2012), generalized anxiety disorder (Vieira et al., 2010), post-traumatic stress disorder (Renna et al., 2018), sleep disturbance (Irwin, 2015), and burnout (Grossi et al., 2003) relative to healthy controls. Importantly, better understanding of the pathophysiology of CMDs could help in the development of more efficacious and personalized treatment approaches.
Cognitive behavior therapy (CBT) has substantial empirical support in the treatment of CMDs, especially regarding depression, anxiety disorders and insomnia (Cuijpers et al., 2013; Öst, 2008; Trauer et al., 2015). Regarding stress-related symptoms, CBT produces larger effects than other interventions for occupational stress (Bhui et al., 2012), but the evidence base of CBT for patients with a diagnosis of adjustment disorder or clinical burnout is uncertain (Perski et al., 2017) although promising results are emerging (Lindsäter et al., 2018; Persson Asplund et al., 2018). Importantly, the biological underpinnings of the symptom improvements seen in CBT across disorders are little understood. Reducing chronic low-grade inflammation could be one important physiological change induced by CBT.
Only a few studies have investigated the potentially anti-inflammatory effects of CBT for CMDs (without comorbid medical conditions) and results are inconsistent between studies. For example, among studies investigating inflammatory markers in CBT for depression, IL-6 decreased significantly between pre- and post-treatment in three studies (Dahl et al., 2016; Gazal et al., 2013; Moreira et al., 2015), while two studies found no such change (Euteneuer et al., 2017; Keri et al., 2014). Further, results are ambiguous regarding whether a reduction in inflammation is associated with improvements in depression (Gazal et al., 2013; Keri et al., 2014; Moreira et al., 2015). So far one study has investigated the effect of CBT on markers of inflammation in CMDs broadly, including depression, anxiety and/or stress-related disorders (Memon et al., 2017). No changes in inflammatory markers were found after treatment, which may be related to sample size and analyzed inflammatory markers (IL-8 and CRP).
The aim of the present study was to investigate (a) the association between levels of pro-inflammatory cytokines and psychiatric symptom severity in a large sample of primary care patients with CMDs, taking potential moderators into account, (b) change in pro-inflammatory cytokines and psychiatric symptoms, respectively, following CBT for CMDs, and (c) if changes in symptoms correlate with changes in inflammation. We hypothesized that:
-
(1)
Higher levels of pro-inflammatory cytokines (TNF-α, IL-6 and IL-8) would be associated with more severe psychiatric symptoms (i.e., level of depression, stress and anxiety).
-
(2)
Pro-inflammatory cytokines and psychiatric symptoms would be reduced after CBT.
-
(3)
Reduced psychiatric symptoms would correlate with lowered levels of pro-inflammatory cytokines.
2. Methods
2.1. Design
The study sample originated from two clinical trials of CBT for CMDs (Salomonsson et al., 2017, 2018). The trials were preregistered at ClinicalTrials.gov (identifiers NCT01636791 and NCT01667822). Participants were recruited consecutively from four primary care clinics in Stockholm, Sweden. In clinical trial 1 (Salomonsson et al., 2017), patients (n = 211) on sick leave were randomized to one of three conditions: (a) disorder-specific CBT, (b) a return-to-work intervention that included CBT-based psychoeducation or (c) a combination of the two. Depending on principal disorder and treatment allocation, treatment lasted for between 8 and 25 weeks. Clinical trial 2 (Salomonsson et al., 2018) was a two-phased trial. All patients (n = 396) were, in phase one, treated with guided self-help CBT according to their principal disorder for nine weeks. Patients not in remission after phase one were randomized to continued self-help CBT or face-to-face CBT in phase two for an additional 11 weeks. Consequently, patients in both clinical trials, regardless of treatment allocation, received CBT in some form. All types of CBT (hereafter referred to as CBT) were associated with marked reductions of psychiatric symptoms and between-group effects were generally small, which further justified that patients were analyzed as a single group (Salomonsson et al., 2017, 2018). The clinical trials, including blood sampling for cytokine analysis, were approved by the Regional Ethics Review Board in Stockholm, Sweden, and all patients provided informed consent.
2.2. Procedure
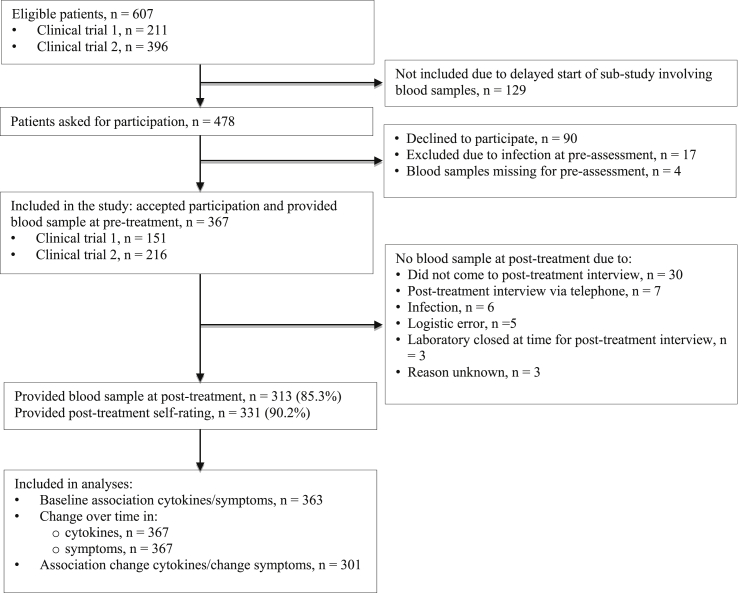
Patients were assessed with the Mini International Neuropsychiatric Interview (MINI) (Sheehan et al., 1998) with additional questions to assess criteria for exhaustion disorder (ICD-code 43.8; The Swedish National Board of Health and Welfare, 2003). Exhaustion disorder is by many considered to be a diagnostic equivalent to clinical burnout (Grossi et al., 2015), a stress-related condition with severe symptoms of physical and mental exhaustion developed as a consequence of prolonged exposure to non-traumatic stressors (for diagnostic criteria, see e.g., Beser et al., 2014). In clinical trial 1, patients were required to fulfill diagnostic criteria for their primary CMD to be considered for inclusion (see Salomonsson et al., 2017 for details). In clinical trial 2, subclinical CMDs were also allowed, i.e., patients presenting with marked symptoms but not fulfilling all criteria for a diagnosis, were also included (see Salomonsson et al., 2018 for details). Treatment took place at the respective primary care clinics. Licensed psychologists conducted both assessment and treatment. All participants in clinical trial 1 or 2 that provided a pre-treatment blood sample, and were not suffering from an infection at the time, were included in the present study. Fig. 1 displays the flow of patients through the study. Because the blood sampling procedures were established after inclusion to the clinical trials had commenced, the first 129 included patients were not asked for participation in this study.
Fig. 1.
Flow chart of participants in the study.
2.3. Patients
The study population (N = 367) is described in Table 1. A vast majority of patients fulfilled full diagnostic criteria for their primary CMD (94%). Further, a majority of patients (55%) fulfilled criteria for more than one CMD. The most prevalent disorder in the sample, counting both principal and comorbid disorders, was depression (n = 131).
Table 1.
Sample characteristics at baseline.
| Variable | Patients (n = 367) |
|---|---|
| Women, n (%) | 281 (76.6%) |
| Age | |
| Mean (SD) | 39.7 (10.8) |
| Range | 18–64 |
| Principal disorder/problem area | |
| Exhaustion disorder, n (%) | 108 (29.4%) |
| Adjustment disorder, n (%) | 66 (18.0%) |
| Depression, n (%) | 59 (16.1%) |
| Generalized anxiety disorder, n (%) | 49 (13.4%) |
| Social anxiety disorder, n (%) | 39 (10.6%) |
| Insomnia, n (%) | 18 (4.9%) |
| Panic disorder, with or without agoraphobia, n (%) | 18 (4.9%) |
| Post-traumatic stress syndrome, n (%) | 5 (1.4%) |
| Obsessive-compulsive disorder, n (%) | 5 (1.4%) |
| Fulfilling full criteria for the principal disorder | 344 (93.7%) |
| Number of patients with comorbid disorder(s) | 202 (55.0%) |
| Education, highest finished, n (%) | |
| Compulsory school, 9 years | 22 (6.0%) |
| Secondary school, 2–3 years | 130 (35.4%) |
| College/university | 215 (58.6%) |
| Psychotropic medication at pre-treatment assessment | |
| Number of patients on any psychotropic medication | 146 (39.8%) |
| Antidepressantsa, n (%) | 59 (16.1%) |
| Anxiety reducing/calminga, n (%) | 43 (11.7%) |
| Sleep medicationa, n (%) | 80 (21.8%) |
| Pain medicationa, n (%) | 11 (3%) |
| Patient recruitment | |
| Clinical trial 1 | 151 (41.1%) |
| Clinical trial 2 | 216 (58.9%) |
Patients can be represented in more than one category of psychotropic medication.
2.4. Measures
2.4.1. Blood sampling and cytokine analyses
A psychologist accompanied patients to the laboratory in connection to pre- and post-treatment assessments. The psychologist made sure that patients were well rested before the venipuncture (e.g., slowly walked the patient to the laboratory after session). Because assessment and treatment took place within the regular primary care context, it was not feasible to allocate blood sampling to a pre-defined time schedule but the time for the sample was registered. At each assessment point, 7 ml of blood was drawn into an EDTA-tube. After centrifugation (3500 RPM, 10 min), plasma was transferred to two separate cryotubes via pipette for initial storage in a freezer at −20 °C. After transportation from each primary care clinic, aliquots were stored in a −80 °C freezer until assays were performed. Plasma levels of three pro-inflammatory cytokines, i.e., TNF-α, IL-6 and IL-8, were analyzed. The cytokines were assessed using manual enzyme-linked immunosorbent assays (ELISA), detection thresholds were 0.5 pg/ml for TNF-α, 0.156 pg/ml for IL-6 and 1 pg/ml for IL-8. The intra-assay coefficient of variability (CV) was 3.1%–8.7% for TNF-α, 6.9%–7.8% for IL-6, and 3.7%–7.3% for IL-8. The inter-assay CV was 7.4%–10.4% for TNF-α, 6.5%–9.6% for IL-6, and 8.0%–9.4% for IL-8. All samples were analyzed in duplicates and the mean value was used. Six blood samples (0.5%) at pre-treatment (3 TNF-α; 2 IL-6 and 1 IL-8) and nine samples (0.9%) at post-treatment (7 TNF-α; 1 IL-6 and 1 IL-8) were below the detection threshold. For these samples, the detection threshold value for the respective cytokine was imputed.
2.4.2. Psychiatric symptoms and psychotropic medication
Self-rated measures of psychiatric symptoms were completed by patients pre- and post-treatment using an online platform. Symptoms of depression were assessed using the Montgomery Åsberg Depression Rating Scale Self-rated (MADRS-S; Svanborg and Åsberg, 1994). MADRS-S measures nine depressive symptoms and the total score range is 0–54. Symptoms of stress were assessed using the Perceived Stress Scale (PSS; Cohen et al., 1983). The PSS consists of 14 items, summed total score ranging from 0-56. Symptoms of anxiety were assessed using the Hospital Anxiety and Depression Scale (HADS; Zigmond and Snaith, 1983), Anxiety subscale (HADS-A). This subscale consists of 7 items with a total score ranging from 0-21. Use of psychotropic medication and changes during treatment were inquired by the assessing psychologists pre- and post-treatment.
2.5. Statistical analyses
We analyzed data using Stata (Version 13.1, StataCorp, College Station, Texas). In all analyses (linear regressions, mixed effects models and Spearman rank correlations) bootstrapping was applied because the cytokine values were not normally distributed. Bias corrected confidence intervals were calculated for all analyses.
For the baseline association between cytokines and psychiatric symptoms (hypothesis 1) we used linear regression analyses with bootstrapping. Cytokines were analyzed as independent variables and psychiatric symptoms as dependent variables. We analyzed five variables (sex, age, psychotropic medication, education and time of day for blood sampling) as both confounders and moderators. Age was centered to allow useful interpretations of coefficients. Use of psychotropic medication was dichotomized, i.e., taking or not taking any kind of psychotropic medication. Confounders were included in the model if they changed the regression coefficient with more than 10% from the crude estimate. Moderators were kept in the model if they generated a significant interaction term with the explanatory variable (i.e., cytokines). To investigate associations in subgroups, we conducted stratified analyses. For age, exploratory stratified analyses were conducted by dividing patients in two samples around the mean age of 39.7 years.
For analyses of change over time (hypothesis 2) we used mixed effects regression models with bootstrapping (implemented on the individual level). Time (pre- and post-treatment) was the independent variable and cytokine levels as well as psychiatric symptoms were the dependent variables. Random intercept was included in all models. We tested sex and age as moderators in all analyses. In addition, we analyzed time for blood sampling as a potential confounder in analyses of change over time in cytokines (same 10% decision criteria for inclusion as described above). All models were analyzed using restricted maximum likelihood (REML), meaning that all available data were used for parameter estimates (Enders, 2011). Thus, patients with missing data at post-treatment also contributed to models. Further, data were analyzed based on the intention-to-treat principle, that is, patients were included in analyses irrespective of whether they had completed treatment or not.
Finally, we used Spearman rank correlation coefficients with bootstrapping for analyzing the association between change in cytokines (delta cytokine, i.e., cytokine pre minus cytokine post) and change in psychiatric symptoms (delta symptoms, i.e., symptom pre minus symptom post) (hypothesis 3). Patients that contributed with pre- and posttreatment data on both cytokines and psychiatric symptoms were included in these analyses.
3. Results
There was no difference in average time of day for blood samples between pre (M = 12:06) and post (M = 12:09) measures, t = −0.251; df = 291; p = .802. Of the 337 patients that provided information on psychotropic medication at both pre- and post-treatment, 28 (8.3%) had initiated or increased medication and 37 (11.0%) terminated or decreased their medication. Observed values for the cytokines (i.e., TNF-α, IL-6 and IL-8), psychiatric symptoms (i.e., MADRS-S, PSS and HADS-A), as well as data completeness (i.e., percentage of each measure completed by patients at post-treatment) are shown in Table 2.
Table 2.
Observed values of plasma levels of pro-inflammatory cytokines and psychiatric symptoms at pre- and post-treatment, and data completeness.
| Measure | Pre-treatment (n = 367) |
Post-treatment |
|||
|---|---|---|---|---|---|
| Mean (SD) |
Median (25%–75%) |
n (%) |
Mean (SD) |
Median (25%–75%) |
|
| TNF-α (pg/ml) |
1.4 (0.7) |
1.3 (0.9–1.8) |
313 (85.3) |
1.5 (1.1) |
1.3 (0.9–1.7) |
| Il-6 (pg/ml) |
1.0 (0.9) |
0.8 (0.6–1.2) |
313 (85.3) |
1.1 (1.0) |
0.8 (0.6–1.3) |
| IL-8 (pg/ml) |
4.5 (2.5) |
4.2 (3.0–5.5) |
312 (85.0) |
4.5 (3.7) |
3.9 (2.9–5.3) |
| MADRS-S | 19.1 (7.8) |
19 (14–25) |
331 (90.2) |
10.1 (8.2) |
9 (4–14) |
| PSS | 35.0 (8.0) |
36 (31–41) |
331 (90.2) |
23.7 (10.2) |
23 (16–31) |
| HADS-A | 11.7 (4.1) |
12 (9–14) |
330 (89.9) |
7.6 (4.6) |
7 (4–11) |
Note. There were no missing data at pre-treatment. TNF-α = tumor necrosis factor alpha; IL-6 = interleukin-6; IL-8 = interleukin-8; MADRS-S = Montgomery Åsberg Depression Rating Scale Self-rated; PSS = Perceived Stress Scale; HADS-A = Hospital Anxiety and Depression Scale, Anxiety subscale.
3.1. Associations between cytokines and psychiatric symptom severity at baseline
Associations between cytokines and psychiatric symptom severity are displayed in Table 3. As sex and/or age were included as moderators in some of the models, the regression coefficients in Table 3 are interpreted as follows: “b sex” represents the interaction effect of sex and cytokines on psychiatric symptoms and “b age” represents the interaction effect of age and cytokines on psychiatric symptoms. Thus, for models including these moderators, the “b” represents the regression coefficient for men and for average aged patients, respectively (as woman was coded as 1 and age was centered). Correspondingly, for models not including moderators, the “b” represents the association between cytokines and symptoms in the overall sample. As displayed in Table 3, sex was a significant moderator for the associations between TNF-α and all symptom measures. Further, age moderated the association between TNF-α, as well as IL-6, and stress symptoms.
Table 3.
Regression coefficients (b) and bootstrapped based 95% confidence intervals (CI) for the associations between cytokines and psychiatric symptoms (with respective confounders and moderators included for each association), n = 363 in all models.
| MADRS-Sa |
PSSb |
HADS-Ac |
|||||
|---|---|---|---|---|---|---|---|
| b [95% CI] | b sex [95% CI] | b [95% CI] | b sex [95% CI] | b age [95% CI] | b [95% CI] | b sex [95% CI] | |
| TNF-α | 3.88 [1.52, 6.29] | −4.22 [-7.01, -1.39] | 4.15 [1.62, 6.74] | −3.36 [-6.06, -0.36] | −0.09 [-0.18, -0.01] | 2.03 [0.49, 3.37] | −1.93 [-3.35, -0.18] |
| IL-6 | 0.01 [-1.08,1.09] | – | 0.52 [-0.40, 1.9] | – | −0.08 [-0.21, -0.01] | 0.17 [-0.40, 0.70] | – |
| IL-8 | −0.01 [-0.32, 0.29] | – | −0.02 [-0.28, 0.30] | – | – | 0.03 [-0.12, 0.23] | – |
Note. Bootstrapping with 2000 iterations and bias corrected confidence intervals (CI) were used. Significant CIs are in bold for clarity. MADRS-S = Montgomery Åsberg Depression Rating Scale Self-rated; PSS = Perceived Stress Scale; HADS-A = Hospital Anxiety and Depression Scale, Anxiety subscale; b sex = interaction term for sex (woman = 1) and cytokine; b age = interaction term for age (centered values) and cytokine; TNF-α = tumor necrosis factor alpha; IL-6 = interleukin 6; IL-8 = interleukin 8.
MADRS-S/TNF-α relation adjusted for age, psychotropic medication and time of day for blood sampling; MADRS-S/IL-6 and MADRS-S/IL-8 relations adjusted for age, psychotropic medication, education and time for blood sampling.
PSS/TNF-α relation adjusted for education and time for blood sampling; PSS/IL-6 relation adjusted for age, education and time of day for blood sampling; PSS/IL-8 relation adjusted for sex, age and time of day for blood sampling.
HADS-A/TNF-α relation adjusted for age, psychotropic medication and time of day for blood sampling; HADS-A/IL-6 relation adjusted for age and education; HADS-A/IL-8 relation adjusted for sex, age, psychotropic medication, education and time of day for blood sampling.
There was a significant association between higher levels of TNF-α and higher ratings on all measures of psychiatric symptoms in men. For example, an increase of 1 pg/ml in TNF-α predicts an increase of almost 4 points on the measure of depression (i.e., MADRS-S) for men. The combination of the regression coefficient (“b”) and the interaction term for sex (“b sex”) apply for the relationship between TNF-a and psychiatric symptoms in women. The associations between TNF-α and psychiatric symptoms in women were non-significant: depression (b = −0.34; 95% CI [-1.65, 1.15]), stress (b = 0.78; 95% CI [-0.50, 2.04]) and anxiety (b = 0.10; 95% CI [-0.51, 0.79]). However, the regression model for the association between TNF-α and stress also included age as a significant moderator. As this model contained two moderators, follow-up stratified analyses were performed in men and women separately. Results showed that for men, higher levels of TNF-α were significantly associated with higher stress levels (b = 4.30; 95% CI [1.66, 7.38]) and age did not significantly moderate this association (b age = 0.01; 95% CI [-0.24, 0.28]). For women, there was no significant overall association between TNF-α and stress level (b = 0.89; 95% CI [-0.37, 2.25]). However, as age was a significant moderator for this association in women (b age = −0.11; 95% CI [ −0.22, −0.02]), we conducted further stratified analyses by dividing patients in two samples around the mean age of 39.7 years. Results showed that higher levels of TNF-α were significantly associated with higher stress levels in younger women (b = 2.86; 95% CI [0.95, 4.62]) but with lower stress levels in older women (b = −1.44; 95% CI [-2.97, −0.20]).
Associations between IL-6 and severity of depression as well as anxiety were non-significant. The regression model for the association between IL-6 and stress included age as a significant moderator. The negative coefficient indicates a decreased association between IL-6 and stress with older age (i.e., an increased association with younger age). We conducted follow-up stratified analyses of patients divided in two samples around the mean age of 39.7 years. There was a significant association between higher levels of IL-6 and more symptoms of stress, irrespective of sex, in younger patients (b = 1.53; 95% CI [0.37, 3.96]), but the association was non-significant in older patients (b = −0.49; 95% CI [-1.63, 0.98]). Regarding IL-8, we found no significant associations with psychiatric symptoms (see Table 3).
3.2. Change over time in cytokine levels and psychiatric symptoms
The main effects of time, i.e., the change in cytokine levels and psychiatric symptoms between baseline and post-treatment, are displayed in Table 4. Sex and age did not moderate the association between time and any outcomes (cytokines or psychiatric symptoms). Cytokine levels did not change from pre-to post-treatment but there were significant improvements in all symptom measures.
Table 4.
Mixed effects analyses for change in cytokine levels and psychiatric symptoms over time. Intercepts represent modeled baseline values and b-coefficients represent average change between baseline and post with 95% confidence intervals (CI), n = 367 in all models.
| Measure | Intercept | b | SE | 95% CI |
|---|---|---|---|---|
| TNF-αa | 1.66 | 0.04 | 0.06 | [-0.05, 0.22] |
| IL-6 | 1.04 | 0.10 | 0.05 | [-0.00, 0.20] |
| IL-8a | 3.37 | −0.07 | 0.13 | [-0.28, 0.26] |
| MADRS-S | 19.13 | −9.10 | 0.45 | [-9.96, -8.21] |
| PSS | 34.99 | −11.22 | 0.55 | [-12.34, -10.18] |
| HADS-A | 11.71 | −4.14 | 0.22 | [-4.57, -3.71] |
Note. Bootstrapping with 2000 iterations and bias corrected confidence intervals (CI) were used. Significant CIs are in bold for clarity. TNF-α = tumor necrosis factor alpha; IL-6 = interleukin-6; IL-8 = interleukin-8; MADRS-S = Montgomery Åsberg Depression Rating Scale Self-rated; PSS = Perceived Stress Scale; HADS-A = Hospital Anxiety and Depression Scale, Anxiety subscale.
Adjusted for time of day for blood sampling.
3.3. Association between change in inflammation and change in symptoms
There were no significant associations between change in cytokine levels (delta-cytokine) and change in psychiatric symptoms (delta-symptom), see Table 5. Due to the consistent correlations between higher levels of TNF-α and symptom severity at baseline in men, we conducted further exploratory analyses of the association between change in TNF-α and change in symptoms (depression, stress and anxiety) in men only. These exploratory analyses were non-significant (not shown).
Table 5.
Association between change (pre to post) in cytokines and psychiatric symptoms using Spearman’s rank correlation test with 95% confidence intervals (CI).
| ΔMADRS-S |
ΔPSS |
ΔHADS-A |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Rho | 95% CI | n | Rho | 95% CI | n | Rho | 95% CI | |
| ΔTNF-α | 301 | −0.06 | [-0.17, 0.06] | 301 | −0.05 | [-0.17, 0.06] | 300 | −0.07 | [-0.18, 004] |
| ΔIL-6 | 301 | 0.00 | [-0.11, 0.13] | 301 | −0.01 | [-0.13, 0.10] | 300 | −0.06 | [-0.18, 0.04] |
| ΔIL-8 | 300 | 0.02 | [-0.10, 0.12] | 300 | 0.04 | [-0.07, 0.15] | 299 | 0.01 | [-0.11, 0.12] |
Note. Bootstrapping with 2000 iterations and bias corrected confidence intervals (CI) were used. MADRS-S = Montgomery Åsberg Depression Rating Scale Self-rated; PSS = Perceived Stress Scale; HADS-A = Hospital Anxiety and Depression Scale, Anxiety subscale; TNF-α = tumor necrosis factor alpha; IL-6 = interleukin-6; IL-8 = interleukin-8.
4. Discussion
In this sample of primary care patients with CMDs, we found partial support for hypothesis 1 as higher levels of circulating TNF-α were related to psychiatric symptom severity (i.e., depression, stress and anxiety) in men. Further, age moderated the association between TNF-α, as well as IL-6, and stress. Exploratory stratified analyses revealed that higher levels of TNF-α were associated with higher stress levels in younger women, higher levels of TNF-α were associated with lower stress levels in older women, and higher levels of IL-6 were associated with higher stress levels in younger patients (irrespective of sex). No other significant associations between cytokines and psychiatric symptoms were observed. Contrary to hypotheses 2 and 3, pro-inflammatory cytokines were not reduced after CBT and the substantial reductions in psychiatric symptoms after treatment were not associated with reductions in cytokines.
The lack of consistent associations between inflammatory markers and CMD symptom severity might be understood partly by research findings on depression specifically. Recent reviews (Felger and Lotrich, 2013; Raison and Miller, 2011) suggest that there seems to be a subtype of depression that includes elevated pro-inflammatory cytokines, i.e., not all depressions are associated with this elevation. Support for this notion can be found in studies showing that increased inflammation characterizes a subgroup of depressed patients that do not respond to conventional anti-depressants (Pariante, 2017). Also, some data suggest that in treatment resistant depression, anti-inflammatory medication (with a TNF-α antagonist) may be effective in reducing depressive symptoms in patients with high baseline inflammation (Raison et al., 2013).
Some evidence point to sex differences in immune responses (see e.g., Fish, 2008). However, results are ambiguous regarding whether men and women display different pro-inflammatory responses to stress or differ in emotional responses to peripheral inflammation (Bekhbat and Neigh, 2017; Lasselin et al., 2018). In the present study, TNF-α was found associated with all measures of psychiatric symptoms in men, which is in line with some studies showing that inflammatory markers are more consistently linked to anxiety (Liukkonen et al., 2011) and depression (Ramsey et al., 2016) in men compared to women.
We also found age to moderate the association between both TNF-α and IL-6, and symptoms of stress. However, results of stratified follow-up analyses were sprawling: although younger age was found related with stronger positive associations between inflammatory markers and stress levels, a negative association was found in older women. It is possible that the present findings could be explained by differences between younger and older patients in unmeasured confounders, such as diet, smoking and exercise. It should also be noted that the exploratory analyses were based on an arbitrary mean age split.
The results in the present study do not suggest that CBT has anti-inflammatory effects in mild to moderate CMDs. These findings are in line with Memon et al. (2017), who also investigated CMDs in primary care and did not find any reductions in inflammatory markers (IL-8 and CRP) after treatment. Previous research shows inconsistent results regarding the effects of CBT on immune markers. In a review by Lopresti (2017), 23 studies were identified as investigating the anti-inflammatory effects of CBT for depression and other conditions. Reductions in at least one inflammatory marker after treatment were found in 14 studies, while nine of the included studies found no reductions post-treatment. For example, Keri et al. (2014) investigated 16 sessions of CBT for patients with major depression. At baseline, depressed patients exhibited significantly higher levels of IL-6 than healthy controls, but levels were not significantly reduced in the depressed patients after CBT, in spite of reductions in depressed mood (Keri et al., 2014). In another study of CBT for depression, patients were randomized to either traditional CBT, CBT emphasizing exercise (due to the anti-inflammatory effects of physical activity), or waiting list (Euteneuer et al., 2017). No significant difference between groups was found regarding reductions in IL-6 over the treatment period. However, anti-inflammatory cytokine IL-10 was significantly higher in the CBT exercise group than in the other groups post-treatment.
Given the lack of change in cytokine levels after treatment it was not surprising that we found no association between change in symptoms and change in cytokine levels. A meta-analysis of pharmacological treatment for depressed patients (Hannestad et al., 2011) concluded that antidepressants, specifically serotonin reuptake inhibitors (SSRIs), may lower inflammatory markers (TNF-α, IL-6 and IL-1β), but that remission of depression is not dependent on a reduction in pro-inflammatory cytokines. Further, the lack of a clear association between inflammation and symptoms across the study group at baseline in the present study makes the absence of linked reductions over time somewhat expected.
To our knowledge, this is one of the first studies to investigate pro-inflammatory cytokines in CBT for a broad sample of patients with CMDs. There are several strengths to this study, including the to date largest sample size, the inclusion of common mental disorders broadly, the well-validated measures of psychiatric symptoms and the analytic approach using bootstrapping and bias corrected confidence intervals.
There are also several limitations. First, patients in this study were assessed and treated according to the standard scheduling of sessions in the primary care units, i.e., approximately between 08.00 a.m. and 17.00 p.m., and therefore blood sampling took place throughout the working day. Circadian variations in the immune system (Nilsonne et al., 2016; Scheiermann et al., 2013) may have introduced systematic bias in our results. Indeed, time of blood sampling was found a confounder in most analyses, and the statistical adjustments may not be sufficient to eliminate this possible bias. Second, the lack of an untreated control condition in this study precludes comparisons with the natural course of inflammatory markers in patients with CMDs. However, consecutive recruitment and treatment of patients throughout the year at least limited the impact of potential seasonal effects on cytokines. Third, the present sample consisted of patients with mild to moderate psychiatric symptoms, as compared to some of the previous studies including patients with more severe symptoms, which might have affected both baseline levels of cytokines and the potential for change over time (e.g., due to floor effects). Fourth, psychotropic medication can have an effect on pro-inflammatory cytokines (Hannestad et al., 2011). To handle this possible bias in the baseline analyses, we adjusted regression models when called for. Concerning changes in medication during treatment (reported by patients at post-treatment assessment), less than 9% of patients initiated or increased medication, while 11% terminated or reduced their medication use. However, more detailed information regarding the actual use throughout treatment would have been preferable. Lastly, potentially important confounders (e.g., body mass index, diet, smoking, alcohol use and exercise) were not measured in the present study, which could have affected the results.
4.1. Future studies
If inflammation only contributes to psychiatric symptoms in a subgroup of patients (Raison and Miller, 2011), then it would be reasonable to compare CBT to a control condition regarding the association between decreased inflammation and symptom reduction only in patients with elevated inflammatory activity at baseline. To complete the overall picture, measurement of anti-inflammatory cytokines should also be included in future investigations.
5. Conclusions
Elevated inflammatory markers might be of relevance for psychiatric symptom severity in subgroups, but our results do not support an overall association across patients with mild to moderate CMDs. Moreover, if there are anti-inflammatory effects of psychological treatment, these effects are likely small and of limited importance for clinical improvement in this patient group.
Funding
This study was funded by Karolinska Institutet, research grants from Stockholm County Council, Sweden’s Innovation Agency (Vinnova), Stockholm Stress Center and Osher Center for Integrative Medicine, none of which had any role in the design, execution or publication of the study.
Declaration of competing interest
The authors declare that there is no conflict of interest.
Acknowledgments
We thank Paraskevi Peristera for advice on the statistical analyses. Author contributions: ML, FS, AA and EHL designed the study, oversaw data collection, performed statistical analyses and drafted the manuscript. SS, EL, BL and GK designed the study, participated in data collection and edited the manuscript. All authors approved the final manuscript.
References
- Bekhbat M., Neigh G.N. Sex differences in the neuro-immune consequences of stress: focus on depression and anxiety. Brain Behav. Immun. 2017 doi: 10.1016/j.bbi.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk M., Williams L.J., Jacka F.N., O’Neil A., Pasco J.A., Moylan S.…Maes M. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11:200. doi: 10.1186/1741-7015-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beser A., Sorjonen K., Wahlberg K., Peterson U., Nygren A., Asberg M. Construction and evaluation of a self rating scale for stress-induced exhaustion disorder, the Karolinska Exhaustion Disorder Scale. Scand. J. Psychol. 2014;55(1):72–82. doi: 10.1111/sjop.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhui K.S., Dinos S., Stansfeld S.A., White P.D. A synthesis of the evidence for managing stress at work: a review of the reviews reporting on anxiety, depression, and absenteeism. J. Environ. Publ. Health. 2012:515874. doi: 10.1155/2012/515874. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey P. Adjustment disorder: new developments. Curr. Psychiatr. Rep. 2014;16(6):451. doi: 10.1007/s11920-014-0451-2. [DOI] [PubMed] [Google Scholar]
- Cohen S., Kamarck T., Mermelstein R. A global measure of perceived stress. J. Health Soc. Behav. 1983;24(4):385–396. doi: 10.2307/2136404. [DOI] [PubMed] [Google Scholar]
- Cuijpers P., Berking M., Andersson G., Quigley L., Kleiboer A., Dobson K.S. A meta-analysis of cognitive-behavioural therapy for adult depression, alone and in comparison with other treatments. Canadian Journal of Psychiatry. Rev. Canad. Psychiatr. 2013;58(7):376–385. doi: 10.1177/070674371305800702. [DOI] [PubMed] [Google Scholar]
- Dahl J., Ormstad H., Aass H.C., Sandvik L., Malt U.F., Andreassen O.A. Recovery from major depressive disorder episode after non-pharmacological treatment is associated with normalized cytokine levels. Acta Psychiatr. Scand. 2016;134(1):40–47. doi: 10.1111/acps.12576. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine, sickness behavior, and depression. Immunol. Allergy Clin. 2009;29(2):247–264. doi: 10.1016/j.iac.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R., Kelley K.W. Twenty years of research on cytokine-induced sickness behavior. Brain Behav. Immun. 2007;21(2):153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar F.S. Effects of stress on immune function: the good, the bad, and the beautiful. Immunol. Res. 2014;58(2–3):193–210. doi: 10.1007/s12026-014-8517-0. [DOI] [PubMed] [Google Scholar]
- Dowlati Y., Herrmann N., Swardfager W., Liu H., Sham L., Reim E.K., Lanctot K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatr. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Enders C.K. Analyzing longitudinal data with missing values. Rehabil. Psychol. 2011;56(4):267–288. doi: 10.1037/a0025579. [DOI] [PubMed] [Google Scholar]
- Euteneuer F., Dannehl K., Del Rey A., Engler H., Schedlowski M., Rief W. Immunological effects of behavioral activation with exercise in major depression: an exploratory randomized controlled trial. Transl. Psychiatry. 2017;7(5):e1132. doi: 10.1038/tp.2017.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger J.C., Lotrich F.E. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229. doi: 10.1016/j.neuroscience.2013.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish E.N. The X-files in immunity: sex-based differences predispose immune responses. Nat. Rev. Immunol. 2008;8(9):737–744. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazal M., Souza L.D., Fucolo B.A., Wiener C.D., Silva R.A., Pinheiro R.T.…Kaster M.P. The impact of cognitive behavioral therapy on IL-6 levels in unmedicated women experiencing the first episode of depression: a pilot study. Psychiatr. Res. 2013;209(3):742–745. doi: 10.1016/j.psychres.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Gray S.M., Bloch M.H. Systematic review of proinflammatory cytokines in obsessive-compulsive disorder. Curr. Psychiatr. Rep. 2012;14(3):220–228. doi: 10.1007/s11920-012-0272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossi G., Perski A., Evengård B., Blomkvist V., Orth-Gomér K. Physiological correlates of burnout among women. J. Psychosom. Res. 2003;55(4):309–316. doi: 10.1016/s0022-3999(02)00633-5. [DOI] [PubMed] [Google Scholar]
- Grossi G., Perski A., Osika W., Savic I. Stress-related exhaustion disorder--clinical manifestation of burnout? A review of assessment methods, sleep impairments, cognitive disturbances, and neuro-biological and physiological changes in clinical burnout. Scand. J. Psychol. 2015;56(6):626–636. doi: 10.1111/sjop.12251. [DOI] [PubMed] [Google Scholar]
- Haapakoski R., Mathieu J., Ebmeier K.P., Alenius H., Kivimaki M. Cumulative meta-analysis of interleukins 6 and 1beta, tumour necrosis factor alpha and C-reactive protein in patients with major depressive disorder. Brain Behav. Immun. 2015;49:206–215. doi: 10.1016/j.bbi.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannestad J., DellaGioia N., Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology. 2011;36(12):2452–2459. doi: 10.1038/npp.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin M.R. Why sleep is important for health: a psychoneuroimmunology perspective. Annu. Rev. Psychol. 2015;66:143–172. doi: 10.1146/annurev-psych-010213-115205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keri S., Szabo C., Kelemen O. Expression of Toll-Like Receptors in peripheral blood mononuclear cells and response to cognitive-behavioral therapy in major depressive disorder. Brain Behav. Immun. 2014;40:235–243. doi: 10.1016/j.bbi.2014.03.020. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatr. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Lasselin J., Lekander M., Axelsson J., Karshikoff B. Sex differences in how inflammation affects behavior: what we can learn from experimental inflammatory models in humans. Front. Neuroendocrinol. 2018 doi: 10.1016/j.yfrne.2018.06.005. [DOI] [PubMed] [Google Scholar]
- Lindsäter E., Axelsson E., Salomonsson S., Santoft F., Ejeby K., Ljotsson B.…Hedman-Lagerlöf E. Internet-based cognitive behavioral therapy for chronic stress: a randomized controlled trial. Psychother. Psychosom. 2018;1–10 doi: 10.1159/000490742. [DOI] [PubMed] [Google Scholar]
- Liukkonen T., Rasanen P., Jokelainen J., Leinonen M., Jarvelin M.R., Meyer-Rochow V.B., Timonen M. The association between anxiety and C-reactive protein (CRP) levels: results from the Northern Finland 1966 birth cohort study. Eur. Psychiatr. 2011;26(6):363–369. doi: 10.1016/j.eurpsy.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Lopresti A.L. Cognitive behaviour therapy and inflammation: a systematic review of its relationship and the potential implications for the treatment of depression. Aust. N. Z. J. Psychiatr. 2017;51(6):565–582. doi: 10.1177/0004867417701996. [DOI] [PubMed] [Google Scholar]
- Memon A.A., Sundquist K., Ahmad A., Wang X., Hedelius A., Sundquist J. Role of IL-8, CRP and epidermal growth factor in depression and anxiety patients treated with mindfulness-based therapy or cognitive behavioral therapy in primary health care. Psychiatr. Res. 2017;254:311–316. doi: 10.1016/j.psychres.2017.05.012. [DOI] [PubMed] [Google Scholar]
- Miller G.E., Stetler C.A., Carney R.M., Freedland K.E., Banks W.A. Clinical depression and inflammatory risk markers for coronary heart disease. Am. J. Cardiol. 2002;90(12):1279–1283. doi: 10.1016/S0002-9149(02)02863-1. [DOI] [PubMed] [Google Scholar]
- Moreira F.P., Cardoso Tde A., Mondin T.C., Souza L.D., Silva R., Jansen K.…Wiener C.D. The effect of proinflammatory cytokines in Cognitive Behavioral Therapy. J. Neuroimmunol. 2015;285:143–146. doi: 10.1016/j.jneuroim.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Morin C.M., Benca R. Chronic insomnia. Lancet. 2012;379(9821):1129–1141. doi: 10.1016/s0140-6736(11)60750-2. [DOI] [PubMed] [Google Scholar]
- Nilsonne G., Lekander M., Akerstedt T., Axelsson J., Ingre M. Diurnal variation of circulating interleukin-6 in humans: a meta-analysis. PloS One. 2016;11(11) doi: 10.1371/journal.pone.0165799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormel J., Petukhova M., Chatterji S., Aguilar-Gaxiola S., Alonso J., Angermeyer M.C.…Kessler R.C. Disability and treatment of specific mental and physical disorders across the world. Br. J. Psychiatry. 2008;192(5):368–375. doi: 10.1192/bjp.bp.107.039107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öst L.G. Cognitive behavior therapy for anxiety disorders: 40 years of progress. Nord. J. Psychiatr. 2008;62(Suppl. 47):5–10. doi: 10.1080/08039480802315590. [DOI] [PubMed] [Google Scholar]
- Pariante C.M. Why are depressed patients inflamed? A reflection on 20 years of research on depression, glucocorticoid resistance and inflammation. Eur. Neuropsychopharmacol. 2017;27(6):554–559. doi: 10.1016/j.euroneuro.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Perski O., Grossi G., Perski A., Niemi M. A systematic review and meta-analysis of tertiary interventions in clinical burnout. Scand. J. Psychol. 2017;58(6):551–561. doi: 10.1111/sjop.12398. [DOI] [PubMed] [Google Scholar]
- Persson Asplund R., Dagoo J., Fjellstrom I., Niemi L., Hansson K., Zeraati F.…Andersson G. Internet-based stress management for distressed managers: results from a randomised controlled trial. Occup. Environ. Med. 2018;75(2):105–113. doi: 10.1136/oemed-2017-104458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison C.L., Capuron L., Miller A.H. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison C.L., Miller A.H. Is depression an inflammatory disorder? Curr. Psychiatr. Rep. 2011;13(6):467–475. doi: 10.1007/s11920-011-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison C.L., Rutherford R.E., Woolwine B.J., Shuo C., Schettler P., Drake D.F.…Miller A.H. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatr. 2013;70(1):31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey J.M., Cooper J.D., Bot M., Guest P.C., Lamers F., Weickert C.S.…Bahn S. Sex differences in serum markers of major depressive disorder in The Netherlands study of depression and anxiety (NESDA) PloS One. 2016;11(5) doi: 10.1371/journal.pone.0156624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renna M.E., O’Toole M.S., Spaeth P.E., Lekander M., Mennin D.S. The association between anxiety, traumatic stress, and obsessive-compulsive disorders and chronic inflammation: a systematic review and meta-analysis. Depress. Anxiety. 2018;35(11):1081–1094. doi: 10.1002/da.22790. [DOI] [PubMed] [Google Scholar]
- Saarni S.I., Suvisaari J., Sintonen H., Pirkola S., Koskinen S., Aromaa A., Lonnqvist J. Impact of psychiatric disorders on health-related quality of life: general population survey. Br. J. Psychiatry. 2007;190:326–332. doi: 10.1192/bjp.bp.106.025106. [DOI] [PubMed] [Google Scholar]
- Salomonsson S., Santoft F., Lindsäter E., Ejeby K., Ljotsson B., Öst L.G.…Hedman-Lagerlöf E. Cognitive-behavioural therapy and return-to-work intervention for patients on sick leave due to common mental disorders: a randomised controlled trial. Occup. Environ. Med. 2017;74(12):905–912. doi: 10.1136/oemed-2017-104342. [DOI] [PubMed] [Google Scholar]
- Salomonsson S., Santoft F., Lindsäter E., Ejeby K., Ljotsson B., Öst L.G.…Hedman-Lagerlöf E. Stepped care in primary care - guided self-help and face-to-face cognitive behavioural therapy for common mental disorders: a randomized controlled trial. Psychol. Med. 2018;48(10):1644–1654. doi: 10.1017/S0033291717003129. [DOI] [PubMed] [Google Scholar]
- Schedlowski M., Engler H., Grigoleit J.S. Endotoxin-induced experimental systemic inflammation in humans: a model to disentangle immune-to-brain communication. Brain Behav. Immun. 2014;35:1–8. doi: 10.1016/j.bbi.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Scheiermann C., Kunisaki Y., Frenette P.S. Circadian control of the immune system. Nat. Rev. Immunol. 2013;13(3):190–198. doi: 10.1038/nri3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E.…Dunbar G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatr. 1998;59(Suppl. 20):22–33. [PubMed] [Google Scholar]
- Svanborg P., Åsberg M. A new self-rating scale for depression and anxiety states based on the Comprehensive Psychopathological Rating Scale. Acta Psychiatr. Scand. 1994;89(1):21–28. doi: 10.1111/j.1600-0447.1994.tb01480.x. [DOI] [PubMed] [Google Scholar]
- The Swedish National Board of Health and Welfare . National Board of Health and Welfare; Stockholm: 2003. Utmattningssyndrom. Stressrelaterad Psykisk Ohälsa. [Exhaustion Syndrome. Stress Related Mental Illness] (in Swedish) [Google Scholar]
- Thomas A.J., Davis S., Morris C., Jackson E., Harrison R., O’Brien J.T. Increase in interleukin-1beta in late-life depression. Am. J. Psychiatr. 2005;162(1):175–177. doi: 10.1176/appi.ajp.162.1.175. [DOI] [PubMed] [Google Scholar]
- Trauer J.M., Qian M.Y., Doyle J.S., Rajaratnam S.M., Cunnington D. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann. Intern. Med. 2015;163(3):191–204. doi: 10.7326/M14-2841. [DOI] [PubMed] [Google Scholar]
- Vieira M.M.M., Ferreira T.B., Pacheco P.A.F., Barros P.O., Almeida C.R.M., Araújo-Lima C.F.…Linhares U.C. Enhanced Th17 phenotype in individuals with generalized anxiety disorder. J. Neuroimmunol. 2010;229(1–2):212–218. doi: 10.1016/j.jneuroim.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]