Abstract
Introduction
Blood-based next-generation sequencing assays of circulating tumor DNA (ctDNA) have the ability to detect tumor-associated mutations in patients with SCLC. We sought to characterize the relationship between ctDNA mean variant allele frequency (VAF) and radiographic total-body tumor volume (TV) in patients with SCLC.
Methods
We identified matched blood draws and computed tomography (CT) or positron emission tomography (PET) scans within a prospective SCLC blood banking cohort. We sequenced plasma using our previously developed 14-gene SCLC-specific ctDNA assay. Three-dimensional TV was determined from PET and CT scans using MIM software and reviewed by radiation oncologists. Univariate association and multivariate regression analyses were performed to evaluate the association between mean VAF and total-body TV.
Results
We analyzed 75 matched blood draws and CT or PET scans from 25 unique patients with SCLC. Univariate analysis revealed a positive association between mean VAF and total-body TV (Spearman’s ρ = 0.292, p < 0.01), and when considering only treatment-naive and pretreatment patients (n = 11), there was an increase in the magnitude of association (ρ = 0.618, p = 0.048). The relationship remained significant when adjusting for treatment status and bone metastases (p = 0.046). In the subgroup of patients with TP53 variants, univariate analysis revealed a significant association (ρ = 0.762, p = 0.037) only when considering treatment-naive and pretreatment patients (n = 8).
Conclusions
We observed a positive association between mean VAF and total-body TV in patients with SCLC, suggesting mean VAF may represent a dynamic biomarker of tumor burden that could be followed to monitor disease status.
Keywords: Circulating tumor DNA (ctDNA), Small cell lung cancer (SCLC), Variant allele frequency (VAF), Total-body tumor volume (TV)
Introduction
Despite recent advances, the 5-year survival rate for lung cancer remains 16%.1 Among lung cancer types, SCLC is the most aggressive form, accounting for nearly 15% of all lung cancers and causing approximately 30,000 deaths annually in the United States.2
Circulating tumor DNA (ctDNA) is a tumor-derived fragment of DNA detectable in the bloodstream in which canonical SCLC mutations can be detected.3, 4, 5, 6, 7, 8 Studies have revealed that ctDNA variant allele frequency (VAF) correlates with total-body tumor volume (TV) in patients with breast cancer, colon cancer, and NSCLC.9 For example, Abbosh et al.9 found that each 10 cm3 of TV predicted a plasma VAF of 0.1% in patients with NSCLC. The relationship between radiographic total-body TV and ctDNA VAF in patients with SCLC has not been reported.
Here, we characterize the relationship between ctDNA mean VAF and radiographic total-body TV derived from a three-dimensional volumetric analysis of standard-of-care computed tomography (CT) and positron emission tomography (PET) scans.
Materials and Methods
Patient Selection
Patients with SCLC treated at Vanderbilt Ingram Cancer Center were prospectively identified and consented using an Institutional Review Board (IRB #030763)–approved protocol. A cohort of 25 unique patients with SCLC who had blood draws within 16 days of a standard-of-care CT or PET scan were included in the analysis (initial next-generation sequencing results of this analysis from which VAF was derived were previously reported independent of volumetric analyses).10 The sequencing panel included all coding exons of BRAF, KIT, NOTCH1-4, PIK3CA, PTEN, RB1, and TP53 and assessed copy number variation in FGFR1, MYC, MYCL1, and MYCN. Table 1 describes the patient characteristics.
Table 1.
Baseline Demographics of Patients With SCLC
| Descriptor | Overall (n = 25) |
|---|---|
| Age, y, median (range) | 68 (43–83) |
| Sex, n (%) | |
| Female | 13 (52) |
| Male | 12 (48) |
| Race, n (%) | |
| White | 25 (100) |
| Smoking history, n (%) | |
| Yes | 24 (96) |
| No | 1 (4) |
| TNM stage at diagnosis, n (%) | |
| IIIA | 4 (15) |
| IIIB | 3 (12) |
| IIIC | 2 (9) |
| IVA | 5 (20) |
| IVB | 11 (44) |
| Limited vs. extensive stage at diagnosis, n (%) | |
| Limited | 10 (40) |
| Extensive | 15 (60) |
Radiographic Total-Body TV
All patients with a CT or PET scan within 16 days of a blood draw were included. DICOM images were imported into MIM version 6.8.8 (Cleveland, OH) for gross tumor segmentation and total-body TV calculation. Gross areas of tumor involvement were identified and segmented using available imaging data including radiologist impression and clinical judgment. Segmentations were independently verified by Vanderbilt Ingram Cancer Center radiation oncologists who were blinded to individual patient clinical and ctDNA data. If PET was available for segmentation, fluorodeoxyglucose-avid areas without clear CT correlate were excluded. Lung nodules less than 2 mm in size, consolidative densities judged to be atelectatic or inflammatory, and lesions deemed to be benign by interpreting radiologists were excluded. Total-body TV was calculated as Boolean sum of all segmented TVs. Among 80 scans for 25 patients, five scans from five different patients were excluded owing to technical errors preventing scan import and analysis.
Statistical Analysis
The VAF for a given locus was calculated by dividing the number of variant allele DNA fragments by the number of wild-type plus variant DNA fragments to yield a percentage describing the prevalence of that particular variant allele. Mean VAF for a given blood draw was calculated by averaging the VAF for all variants detected on that blood draw. To evaluate the association between mean VAF and total-body TV, Spearman’s correlations were reported. To account for correlations among multiple blood samples collected from the same individual, linear mixed-effects regression analyses were conducted. We performed separate analyses for mean VAF of all variants identified and of TP53 variants only, as this is the most often mutated gene in SCLC tumors. Multivariate analysis of all variants was adjusted for TNM stage at diagnosis, presence of bone metastasis at time of scan, and treatment status (defined categorically as on or off). For both the all-variant and TP53 variant-only groups, we separately analyzed the subgroup of patients who had blood collected when they were treatment-naive or at the time of a change in therapy after any line of progression (pretreatment). All statistical inferences were assessed using a two-sided 5% significance level, and all summary statistics and models were generated using R version 3.6 statistical software.
Results
We analyzed 75 concordant scans and blood draws from 25 patients with SCLC. Median age of patients was 68 years (mean: 67.8, range: 43–83), 52% were female, and 100% were white (Table 1). The median interval between imaging and blood collection was 1 day (mean: 3.5, range: 0–16) (Table 2). A compiled listing of all blood draws with time points, analyses, and TV measurements is provided in Supplementary Table 1.
Table 2.
Sample Characteristics and Covariates
| Descriptor | Overall (n = 75) |
|---|---|
| Median interval between scan and blood collection, d (range) | 1 (0–16) |
| Bone metastases at time of scan, n (%) | |
| Present | 24 (32) |
| Absent | 51 (68) |
| Treatment-naive or pretreatment sample,a n (%) | |
| Yes | 13 (17) |
| No | 62 (83) |
| Treatment status at time of blood collection, n (%) | |
| On treatment | 27 (36) |
| Chemotherapy alone | 12 (16) |
| Chemotherapy with concurrent radiation | 2 (3) |
| Immunotherapy | 9 (12) |
| Other | 4 (5) |
| Off treatment | 48 (64) |
In the treatment-naive and pretreatment analyses, n = 11 as two samples were excluded to ensure one sample per patient.
Mean VAF of All Variants and Total-Body TV
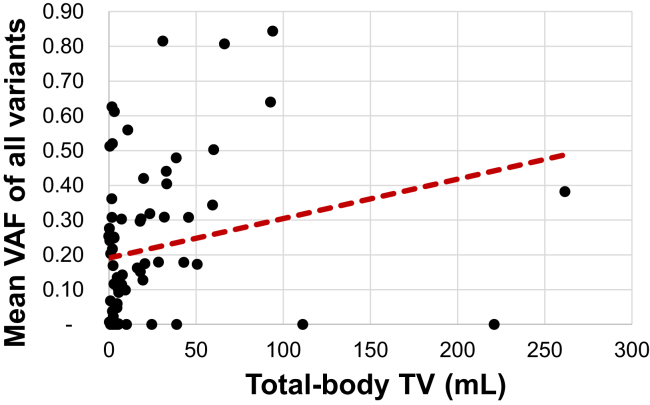
Mean total-body TV of 75 analyzable scans is 22.54 mL and mean VAF of all variants is 21.65%. Univariate analysis revealed a positive association between mean VAF of all variants and total-body TV (Spearman’s ρ = 0.292, p < 0.01) (Fig. 1). A listing of the clinical details of the analyzed samples is provided in Supplementary Table 2. To better account for multiple samples from a single patient, we applied a linear mixed-effects model which also revealed a positive association, with mean VAF increasing 1.7% for each fold increase in TV (95% confidence interval [CI]: 0.1%–3%, p = 0.037). The relationship between mean VAF of all variants and total-body TV remained significant when adjusting for treatment status and bone metastases (p = 0.046), but not when adjusting for treatment status and TNM stage (p = 0.085).
Figure 1.
Mean VAF of all variants and total-body TV. Spearman’s plot of total-body TV versus mean VAF of all variants. TV, tumor volume; VAF, variant allele frequency.
Mean VAF of All Variants and Total-Body TV Among Only Treatment-Naive and Pretreatment Samples
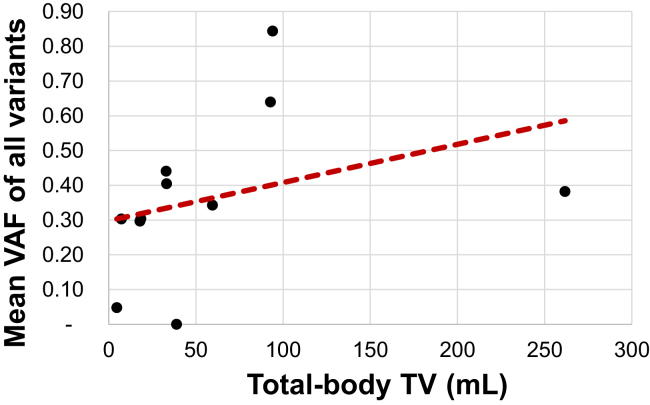
When considering only the 11 treatment-naive and pretreatment samples each from a unique patient, the mean total-body TV was 60.4 mL and mean VAF of all variants was 36.42% (Supplementary Table 2). Univariate analysis revealed that the association between total-body TV and mean VAF increased in magnitude compared with the all-sample analysis above and remained significant (ρ = 0.618, p = 0.048) (Fig. 2).
Figure 2.
Mean VAF of all variants and total-body TV among only treatment-naive and pretreatment samples. Spearman’s plot of total-body TV versus mean VAF of all variants among only treatment-naive and pretreatment samples. TV, tumor volume; VAF, variant allele frequency.
Mean TP53 VAF and Total-Body TV
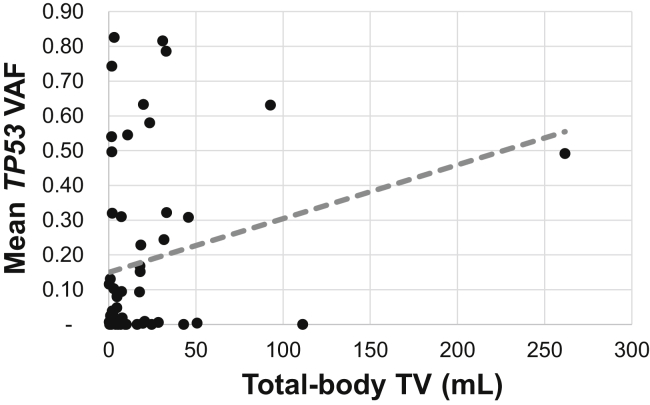
When considering only the 56 samples with TP53 variants, mean total-body TV was 19.50 mL and mean TP53 VAF was 17.98% (Supplementary Table 2). We did not observe a statistically significant association between mean VAF and total-body TV (ρ = 0.184, p = 0.175) among these samples in the univariate analysis (Fig. 3). However, the mixed-effects analysis revealed a statistically significant association, with mean VAF increasing 3.9% for each fold increase in TV (95% CI: 1%–6.8%, p = 0.011). This association remained significant after adjusting for treatment status and presence of bone metastases, with VAF increasing 3.7% for each fold increase in TV (95% CI: 0.7%–6.7%, p = 0.021). Similar results were obtained when adjusting for treatment status and TNM stage, with VAF increasing 3.5% for each fold increase in TV (95% CI: 0.5%–6.5%, p = 0.028).
Figure 3.
Mean TP53 VAF and total-body TV. Spearman’s plot of total-body TV versus mean TP53 VAF. TV, tumor volume; VAF, variant allele frequency.
Mean TP53 VAF and Total-Body TV Among Only Treatment-Naive and Pretreatment Samples
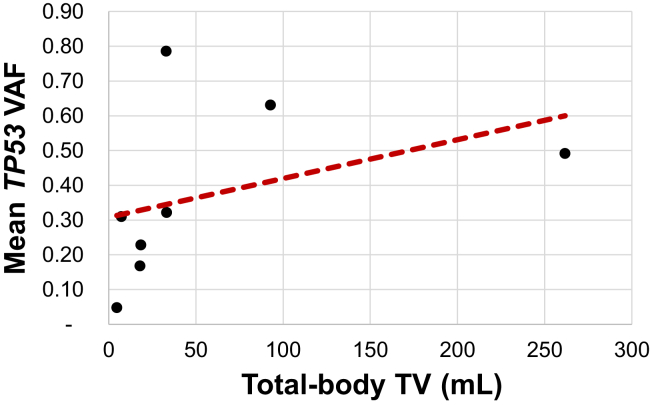
Among the eight treatment-naive and pretreatment samples from the eight patients with TP53 variants, mean total-body TV was 58.63 mL and mean TP53 VAF was 37.32% (Supplementary Table 2). Univariate analysis revealed a significant positive association between mean TP53 VAF and total-body TV (ρ = 0.762, p = 0.037) (Fig. 4).
Figure 4.
Mean TP53 VAF and total-body TV among only treatment-naive and pretreatment samples. Spearman’s plot of total-body TV versus mean TP53 VAF among only treatment-naive and pretreatment samples. TV, tumor volume; VAF, variant allele frequency.
Discussion
In our study, mean VAF (of all variants and TP53 variants only) was positively correlated with three-dimensional total-body TV in patients with SCLC. For both the all-variant and TP53-only groups, the strength of association increased when considering only treatment-naive and pretreatment samples, suggesting therapy may alter tumor biology in a manner that affects ctDNA concentration. Similar results in the all-variant and TP53-only analyses emphasize that the association between mean VAF and total-body TV is likely driven by TP53 mutations, which are present in most SCLC tumors. Our multivariate models suggest that the strongest predictor of mean VAF besides total-body TV is TNM stage; on or off treatment status and bone metastases have smaller effects on the association between mean VAF and TV than did TNM stage.
This study has several limitations, including the small number of treatment-naive and pretreatment samples and the use of a racially homogenous population from a single institution. In addition, we were unable to account for differences in types of systemic therapy (i.e., conventional chemotherapy versus immunotherapy versus targeted therapy) or for the effects of radiation therapy owing to the small number of patients in these subgroups (Table 2). Finally, confirmation of the intrapatient reliability of this correlation is needed in future studies. In this study, we were limited by variability in the timing of blood draws and their relationship to radiographic imaging. We have provided an example of what this intrapatient, longitudinal correlation may illustrate with one patient example in Supplementary Figure 1.
These results suggest that mean VAF may provide a useful snapshot of total-body tumor burden and represent a dynamic biomarker that could be followed to monitor disease status in patients with SCLC, both on and off treatment, during disease surveillance.
Acknowledgments
Dr. Iams was supported by the National Cancer Institute (NCI) Vanderbilt Clinical Oncology Research Career Development Award (VCORCDP) 2K12CA090625-17 and an American Society of Clinical Oncology/Conquer Cancer Foundation Young Investigator Award. SCLC studies in the Lovly laboratory and effort for Drs. Lovly, Kopparapu, Almodovar, and Yan were supported by the Lung Cancer Foundation of America/International Association for the Study of Lung Cancer Lori Monroe Scholarship, NCI R01CA227833, UG1CA233259, U54CA217450-01, U01CA224276-01, and a Vanderbilt Ingram Cancer Center Young Ambassadors Award. Dr. Lovly was also supported by P30-CA086485. Dr. Massion was supported by UO1CA152662 and UO1CA186145. The authors first and foremost thank the patients and their families. The authors also thank all the members of the Lovly Laboratory (authors Drs. Kopparapu, Yan, Brandon Williams, Yunkai Zhang, Huan Qiao, Henry Henderson, and Portia Thomas) and the Cancer Systems Biology Consortium U54 Research Team at Vanderbilt for valuable project discussions. Drs. Iams, Kopparapu, Yan, Lim, Kluwe, and Lovly performed the experiments. Drs. Smith, Balar, Kopparapu, Almodovar, Yan, Bertucci, Shaffer, Hodsdon, Garg, and Hosseini designed the experiments. Drs. Smith, Balar, Lakhani, Kluwe, Iams, Zhao, Bertucci, Shaffer, Horn, Garg, Hosseini, and Lovly generated and analyzed the data. Drs. Horn and York provided direct patient care. Drs. Smith, Balar, Iams, and Lovly wrote the manuscript. Drs. Bertucci, Shaffer, Horn, Garg, Hosseini, Lim, and Zhao performed statistical analysis. Drs. Smith, Balar, Lakhani, Kluwe, Zhao, Kopparapu, Almodovar, Muterspaugh, Yan, York, Horn, Antic, Bertucci, Shaffer, Horn, Garg, Hosseini, Lim, Osmundson, Massion, Lovly, and Iams reviewed the data and final manuscript.
Footnotes
Drs. Smith and Balar contributed equally to this work.
Disclosure: Dr. Lovly is a consultant/advisory board member for Foundation Medicine, Pfizer, Novartis, AstraZeneca, Genoptix, Sequenom, Ariad, Takeda, Blueprints Medicine, Cepheid, Achilles, Genentech, Eli Lilly, and Syros and reports receiving commercial research grants from Xcovery and Novartis. Dr. Horn is a consultant for Astra Zeneca, EMD Serono, Genentech-Roche, Tesaro, Pfizer, Incyte, AbbVie, Bristol-Myers Squibb, Merck, and Xcovery. Dr. Horn has received research support from Xcovery, Bristol-Myers Squibb, and Boehringer Ingelheim. Dr. Iams reports receiving consulting fees for Genentech, Outcomes Insights, and Defined Health and clinical trial funding from EMD Serono. Drs. Bertucci, Shaffer, Hodsdon, Garg, Hosseini, and Lim are employees and shareholders of Resolution Biosciences. The remaining authors declare no conflict of interest.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2020.100110.
Supplementary Data
References
- 1.American Cancer Society. www.cancer.org. Accessed May 6, 2020.
- 2.Bernhardt E.B., Jalal S.I. Small cell lung cancer. Cancer Treat Res. 2016;170:301–322. doi: 10.1007/978-3-319-40389-2_14. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-Cuesta L., Perdomo S., Avogbe P.H. Identification of circulating tumor DNA for the early detection of small-cell lung cancer. EBioMedicine. 2016;10:117–123. doi: 10.1016/j.ebiom.2016.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du M., Thompson J., Fisher H., Zhang P., Huang C.C., Wang L. Genomic alterations of plasma cell-free DNAs in small cell lung cancer and their clinical relevance. Lung Cancer. 2018;120:113–121. doi: 10.1016/j.lungcan.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Nong J., Gong Y., Guan Y. Circulating tumor DNA analysis depicts subclonal architecture and genomic evolution of small cell lung cancer [published correction appears in Nat Commun. 2019;10:552] Nat Commun. 2018;9:3114. doi: 10.1038/s41467-018-05327-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Board R.E., Williams V.S., Knight L. Isolation and extraction of circulating tumor DNA from patients with small cell lung cancer. Ann N Y Acad Sci. 2008;1137:98–107. doi: 10.1196/annals.1448.020. [DOI] [PubMed] [Google Scholar]
- 7.Devarakonda S., Sankararaman S., Herzog B.H. Circulating tumor DNA profiling in small-cell lung cancer identifies potentially targetable alterations. Clin Cancer Res. 2019;25:6119–6126. doi: 10.1158/1078-0432.CCR-19-0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morgensztern D., Devarakonda S.H., Masood A. Circulating cell-free tumor DNA (cfDNA) testing in small cell lung cancer. J Clin Oncol. 2016;34(suppl 15) e23077–e23077. [Google Scholar]
- 9.Abbosh C., Birkbak N.J., Wilson G.A. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution [published correction appears in Nature. 2017;:] Nature. 2017;545:446–451. doi: 10.1038/nature22364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Almodovar K., Iams W.T., Meador C.B. Longitudinal cell-free DNA analysis in patients with small cell lung cancer reveals dynamic insights into treatment efficacy and disease relapse. J Thorac Oncol. 2018;13:112–123. doi: 10.1016/j.jtho.2017.09.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.