Abstract
Introduction
Systemic treatment with chemotherapy is warranted for patients with extensive-stage SCLC (ES-SCLC). The objective of this study was to determine whether racial and other healthcare disparities exist in receipt of chemotherapy for ES-SCLC.
Methods
Utilizing the National Cancer Database, 148,961 patients diagnosed to have stage IV SCLC from 2004 to 2016 were identified. Adjusted ORs with 95% confidence intervals (95% CIs) were computed for receipt of chemotherapy using multivariate logistic regression modeling. Cox regression modeling was used to perform overall survival analysis, and adjusted hazard ratios were calculated.
Results
A total of 82,592 patients were included, among which chemotherapy was not administered to 6557 (7.9%). Higher education, recent year of diagnosis, and treatment at more than one facility were associated with increased odds of receiving chemotherapy. Factors associated with a decreased likelihood of receiving chemotherapy were increasing age, race, nonprivate insurance, and comorbidities. On multivariate analysis, black patients had lower odds of receiving chemotherapy compared with white patients (adjusted OR, 0.85; 95% CI: 0.77–0.93, p = 0.0004). Furthermore, black patients had better survival compared with white patients (adjusted hazard ratio, 0.91; 95% CI: 0.89–0.94, p = 0.91). The 1-year survival (median survival) for black and white patients was 31.7% (8.3 mo) and 28.6% (8 mo), respectively.
Conclusions
Black patients with ES-SCLC were less likely to receive chemotherapy, as were elderly, uninsured, and those with nonprivate insurance. Further studies are required to address underlying reasons for lack of chemotherapy receipt in black patients with ES-SCLC and guide appropriate interventions to mitigate disparities.
Keywords: Small cell lung cancer, Racial disparities, Outcomes, Chemotherapy
Introduction
SCLC is a poorly differentiated neuroendocrine tumor that accounts for approximately 15% of the neoplasms of the lung. It is characterized by rapid doubling time and early development of metastases.1,2 Two-thirds of all cases of SCLC are extensive-stage SCLC (ES-SCLC),1 and the treatment approach has been mostly focused on systemic therapy with etoposide with platinum-based regimen having response rates of 60% to 65%.3,4 Most recently, immunotherapy was added to this regimen in the front-line setting revealing improvement in response rates and overall survival (OS).5,6 Given the propensity of this cancer to rapidly progress, current recommendations and practices favor initiating treatment as soon as possible.7
Nevertheless, there is evidence that guideline-concordant treatment may not be provided to all patients with lung cancer in the United States.10, 11, 12, 8, 9 An analysis of patients with ES-SCLC revealed that the black race was associated with lower doses of consolidative thoracic radiation therapy (TRT).13 There are limited data regarding racial and other potential disparities in SCLC, especially in patients with ES-SCLC. However, these have been widely reported in patients with NSCLC. Earlier studies suggested that patients less likely to receive treatment for NSCLC include black and Hispanic,14, 15, 16 older patients, men, and patients with lower socioeconomic status.17,18 Black patients were less likely to receive surgical treatment for early-stage NSCLC than white patients.19, 20, 21, 22, 23, 24 In addition, older patients with lung cancer, even after adjusting for comorbidity, were less likely to receive treatment.8,23,24 Although a previous study revealed that men had a lower probability of receiving surgical treatment, no significant sex differences were reported in the likelihood of receiving chemotherapy and RT.25
As there are limited data regarding potential disparities in SCLC, we sought to determine whether racial disparities exist in the receipt of chemotherapy in patients with ES-SCLC and evaluate factors associated with receipt of chemotherapy for this deadly disease.
Materials and Methods
Study Design and Data Source
This retrospective study was exempt from review by the institutional review board. We queried the U.S. National Cancer Database (NCDB) for all patients with a diagnosis of SCLC between 2004 and 2016. Informed consent was not required as the data were derived from a deidentified file. NCDB is a hospital-based nationwide, comprehensive clinical surveillance resource oncology data set that currently captures approximately 70% of all newly diagnosed malignancies in the United States.
Patient Selection
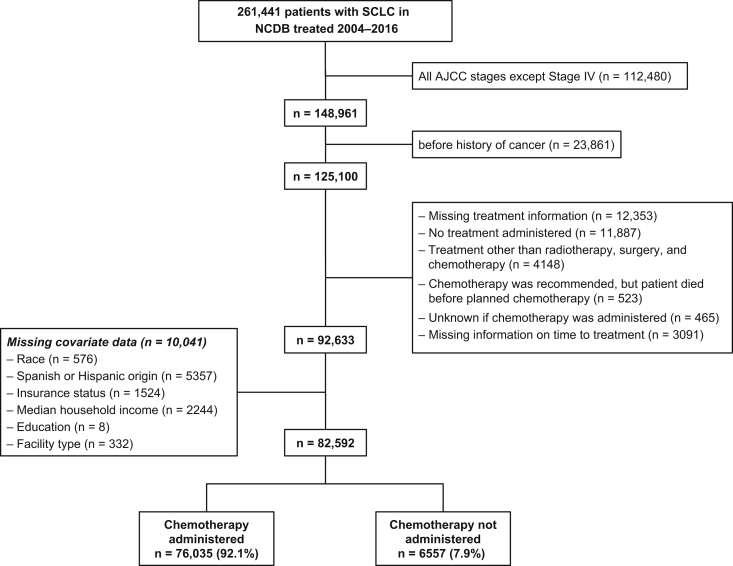
Using the International Classification of Diseases for Oncology (third edition), we identified 261,441 patients diagnosed to have SCLC between 2004 and 2016. Of these, 148,961 patients had ES-SCLC (American Joint Committee on Cancer stage IV disease). Patients with a history of cancer (n = 23,861), missing information on treatment (n = 12,353), no treatment (n = 11,887), and primary treatment other than radiotherapy, surgery, or chemotherapy (n = 4148) were excluded from the analysis. Patients in whom chemotherapy could not be administered or chemotherapy information was missing were also excluded. Finally, the exclusion of patients missing a priori selected covariates (n = 10,041) left a total of 82,592 patients with ES-SCLC in our study (Fig. 1).
Figure 1.
Patient cohort included in the analysis. AJCC, American Joint Committee on Cancer; NCDB, National Cancer Database.
Study Variables
The following demographic variables were obtained from the database: patient age at diagnosis (y), sex, race (white, black, and other), Hispanic origin, insurance status (private, Medicare, Medicaid, other government insurance, or uninsured), median household income (<$30,000, $30,000–$34,999, $35,000–$45,999, and ≥$46,000), and education level recorded as percent with no high school degree (≥29%, 20%–28.9%, 14%–19.9%, or <14%). The zip code of patient residence determined median household income and education level. Information on the year of diagnosis and Charlson-Deyo Comorbidity Score (CCS) (0, 1, or ≥2) was also collected.
Treatment factors included time to start of the first treatment, treatment facility type (community program, comprehensive community program, academic and research program, and integrated network cancer program), and treatment at more than one facility.
Treatment Groups
The study population was categorized into the following two groups: chemotherapy administered and chemotherapy not administered. Patients who received chemotherapy comprised of the following subgroups: chemotherapy only, chemotherapy followed by RT, RT followed by chemotherapy, chemotherapy with surgery, and chemotherapy with RT and surgery. Patients who did not receive chemotherapy comprised of the following subgroups: RT only, surgery only, and surgery with RT. For analysis of survival, we excluded patients who received surgery as part of their first-course treatment.
Statistical Analysis
Baseline characteristics were presented as frequency (percentage) for categorical data and as median (interquartile range [IQR]) for continuous data. A chi-square test was used to evaluate differences in categorical variables by chemotherapy administration. For continuous variables, the nonparametric Wilcoxon-Mann-Whitney test or Kruskal-Wallis test was used to assess differences in the distribution. The primary outcome of interest was chemotherapy administration. The analysis was performed on a full patient cohort from 2004 to 2016. Logistic regression modeling was used to compute the odds of chemotherapy administration by patient and clinicopathologic characteristics. Crude (OR) and adjusted ORs (aORs) with 95% confidence interval (95% CI) were calculated. A sensitivity analysis was performed in which the cohort was stratified into early (2004–2009) and late (2010–2016) time frames, to assess the impact of the implementation of the Patient Protection and Affordable Care Act (ACA) in 2010.
In patients with available survival data (diagnosis years of 2004–2015), OS, clinicopathologic characteristics, and treatment were analyzed. Median OS and 1-year survival rates were estimated using the Kaplan-Meier method, and the log-rank test was used to determine statistical significance. Cox proportional hazards regression modeling was used to compute crude and adjusted hazard ratios (aHRs) with 95% CI.
Statistical computations were performed on SAS 9.4 system (SAS Institute, Cary, NC) or GraphPad Prism software (version 3.0, GraphPad Software). All tests were two-sided, and a p value of less than 0.05 was considered statistically significant.
Results
A total of 82,592 patients with stage IV ES-SCLC were included in the analysis. The median age of the study population was 65 years (IQR: 58–72). Most patients were white (n = 74,807; 90.6%), non-Hispanic (n = 80,710; 97.7%), and had no comorbidities (n = 46,191; 55.9%). Chemotherapy was administered to 76,035 patients (92.1%), whereas 6557 (7.9%) did not receive chemotherapy. Full distributions are described in Table 1.
Table 1.
Patient and Clinicopathologic Characteristics by Chemotherapy Administration
| Characteristic | All Patients |
Chemotherapy |
No Chemotherapy |
p |
|---|---|---|---|---|
| N = 82,592 | N = 76,035 | N = 6557 | ||
| Age, y (median, IQR) | 65 (58–72) | 65 (58–72) | 68 (61–76) | <0.0001 |
| Age, y | <0.0001 | |||
| 40 to <65 | 38,831 (47.0) | 36,492 (94.0) | 2339 (6.0) | |
| 65 to <75 | 28,951 (35.1) | 26,688 (92.2) | 2263 (7.8) | |
| 75 to <80 | 8972 (10.9) | 8014 (89.3) | 958 (10.7) | |
| ≥80 | 5838 (7.1) | 4841 (82.9) | 997 (17.1) | |
| Sex | 0.856 | |||
| Male | 42,663 (51.7) | 39,283 (92.1) | 3380 (7.9) | |
| Female | 39,929 (48.3) | 36,752 (92.0) | 3177 (8.0) | |
| Race | <0.0001 | |||
| White | 74,807 (90.6) | 68,989 (92.2) | 5818 (7.8) | |
| Black | 6416 (7.8) | 5805 (90.5) | 611 (9.5) | |
| Other | 1369 (1.7) | 1241 (90.7) | 128 (9.4) | |
| Hispanic origin | 0.960 | |||
| Non-Hispanic | 80,710 (97.7) | 74,303 (92.1) | 6407 (7.9) | |
| Hispanic | 1882 (2.3) | 1732 (92.0) | 150 (8.0) | |
| Insurance status | <0.0001 | |||
| Private | 25,670 (31.1) | 24,227 (94.4) | 1443 (5.6) | |
| Medicare | 43,984 (53.3) | 39,966 (90.9) | 4018 (9.1) | |
| Medicaid | 7678 (9.3) | 7109 (92.6) | 569 (7.4) | |
| Other government insurance | 1350 (1.6) | 1169 (86.6) | 181 (13.4) | |
| Uninsured | 3910 (4.7) | 3564 (91.2) | 346 (8.9) | |
| Median household income, $ | 0.038 | |||
| <30,000 | 13,144 (15.9) | 12,027 (91.5) | 1117 (8.5) | |
| 30,000–34,999 | 17,461 (21.1) | 16,057 (92.0) | 1404 (8.0) | |
| 35,000–45,999 | 24,989 (30.3) | 23,030 (92.2) | 1959 (7.8) | |
| ≥46,000 | 26,998 (32.7) | 24,921 (92.3) | 2077 (7.7) | |
| Education (% with no HS degree) | <0.0001 | |||
| ≥29 | 16,059 (19.4) | 14,590 (90.9) | 1469 (9.2) | |
| 20–28.9 | 22,646 (27.4) | 20,889 (92.2) | 1757 (7.8) | |
| 14–19.9 | 20,834 (25.2) | 19,209 (92.2) | 1625 (7.8) | |
| <14 | 23,053 (27.9) | 21,347 (92.6) | 1706 (7.4) | |
| Year of diagnosis | <0.0001 | |||
| 2004–2006 | 14,661 (17.8) | 13,169 (89.8) | 1492 (10.2) | |
| 2007–2009 | 17,063 (20.7) | 15,643 (91.7) | 1420 (8.3) | |
| 2010–2012 | 20,747 (25.1) | 19,256 (92.8) | 1491 (7.2) | |
| 2013–2016 | 30,121 (36.5) | 27,967 (92.9) | 2154 (7.2) | |
| CCS | <0.0001 | |||
| 0 | 46,191 (55.9) | 42,613 (92.3) | 3578 (7.8) | |
| 1 | 24,057 (29.1) | 22,192 (92.3) | 1865 (7.8) | |
| ≥2 | 12,344 (15.0) | 11,230 (91.0) | 1114 (9.0) | |
| Facility type | 0.451 | |||
| CP | 10,646 (12.9) | 9796 (92.0) | 850 (8.0) | |
| Comprehensive CP | 38,225 (46.3) | 35,238 (92.2) | 2987 (7.8) | |
| Academic and research program | 22,143 (26.8) | 20,381 (92.0) | 1762 (8.0) | |
| Integrated Network Cancer Program | 11,578 (14.0) | 10,620 (91.7) | 958 (8.3) | |
| Treatment at >1 facility | <0.0001 | |||
| No | 74,146 (89.8) | 68,166 (91.9) | 5980 (8.1) | |
| Yes | 8446 (10.2) | 7869 (93.2) | 577 (6.8) |
Note: Values are given in number (%) unless indicated otherwise.
CCS, Charlson-Deyo Comorbidity Score; CP, community program; HS, high school; IQR, interquartile range.
Clinicopathologic Characteristics by Chemotherapy Receipt
Patients who received chemotherapy were younger (median age: 65 y; IQR: 58–72) than patients who did not (median age: 68 y; IQR: 61–76; p < 0.0001). When analyzed on the basis of age distribution, only 82.9% of patients aged greater than or equal to 80 years received chemotherapy compared with 94.0% of patients aged 40 to less than 65 years (p < 0.0001). Black race (90.5%), other government insurance (86.6%), and diagnosis years of 2004 to 2006 (89.8%) were other factors with the lowest rates of receiving chemotherapy in their respective categories. Sex, Hispanic origin, and treatment facility type did not correlate with chemotherapy administration. The full results are presented in Table 1.
Clinicopathologic and Treatment Characteristics by Race
Black patients were younger, with 52.5% being 40 to 65 years of age (compared with 46.7% and 40.5% in white patients and patients from other race groups, respectively), had more females (49.3, 48.5%, and 33.2% in black patients, white patients, and patients from other race groups, respectively), and were more often treated at academic and research centers (43.3%, 25.2%, and 38.9% in black, white, and patients from other race groups, respectively) (Table 2). Furthermore, black patients were more likely to be uninsured (7.4%, 4.5%, and 5.2% in black patients, white patients, and patients from other race groups, respectively) and had fewer private insurance holders (23.7%, 31.8%, and 27.3% in black patients, white patients, and patients from other race groups, respectively). Finally, time to start of the first treatment (IQR) was 13 days (6–24), 14 (6–28) days, and 15 days (2–27) for white patients, black patients, and patients from other race groups, respectively (p < 0.0001).
Table 2.
Patient and Clinicopathologic Characteristics by Race
| Characteristic | White |
Black |
Other |
p |
|---|---|---|---|---|
| N = 74,807 | N = 6416 | N = 1369 | ||
| Age, y (median, IQR) | 65 (58–72) | 64 (57–71) | 67 (59–73) | <0.0001 |
| Time to treatment, d | 13 (6–24) | 14 (6–28) | 15 (2–27) | <0.0001 |
| Age, y | <0.0001 | |||
| 40 to <65 | 34,907 (46.7) | 3369 (52.5) | 555 (40.5) | |
| 65 to <75 | 26,386 (35.3) | 2052 (32.0) | 513 (37.5) | |
| 75 to <80 | 8188 (11.0) | 603 (9.4) | 181 (13.2) | |
| ≥80 | 5326 (7.1) | 392 (6.1) | 120 (8.8) | |
| Sex | <0.0001 | |||
| Male | 38,494 (51.5) | 3254 (50.7) | 915 (66.8) | |
| Female | 36,313 (48.5) | 3162 (49.3) | 454 (33.2) | |
| Hispanic origin | <0.0001 | |||
| Non-Hispanic | 73,074 (97.7) | 6364 (99.2) | 1272 (92.9) | |
| Hispanic | 1733 (2.3) | 52 (0.81) | 97 (7.1) | |
| Insurance status | <0.0001 | |||
| Private | 23,775 (31.8) | 1521 (23.7) | 374 (27.3) | |
| Medicare | 40,097 (53.6) | 3222 (50.2) | 665 (48.6) | |
| Medicaid | 6369 (8.5) | 1091 (17.0) | 218 (15.9) | |
| Other government insurance | 1200 (1.6) | 109 (1.7) | 41 (3.0) | |
| Uninsured | 3366 (4.5) | 473 (7.4) | 71 (5.2) | |
| Median household income, $ | <0.0001 | |||
| <30,000 | 10,390 (13.9) | 2543 (39.6) | 211 (15.4) | |
| 30,000–34,999 | 15,812 (21.1) | 1446 (22.5) | 203 (14.8) | |
| 35,000–45,999 | 23,242 (31.1) | 1404 (21.9) | 343 (25.1) | |
| >46,000 | 25,363 (33.9) | 1023 (15.9) | 612 (44.7) | |
| Education (% with no HS degree) | <0.0001 | |||
| ≥29 | 13,027 (17.4) | 2751 (42.9) | 281 (20.5) | |
| 20–28.9 | 20,292 (27.1) | 1997 (31.1) | 357 (26.1) | |
| 14–19.9 | 19,677 (26.3) | 900 (14.0) | 257 (18.8) | |
| <14 | 21,811 (29.2) | 768 (12.0) | 474 (34.6) | |
| Year of diagnosis | <0.0001 | |||
| 2004–2006 | 13,444 (18.0) | 1029 (16.0) | 188 (13.7) | |
| 2007–2009 | 15,508 (20.7) | 1296 (20.2) | 259 (18.9) | |
| 2010–2012 | 18,788 (25.1) | 1623 (25.3) | 336 (24.5) | |
| 2013–2016 | 27,067 (36.2) | 2468 (38.5) | 586 (42.8) | |
| CCS | 0.001 | |||
| 0 | 41,813 (55.9) | 3564 (55.6) | 814 (59.5) | |
| 1 | 21,870 (29.2) | 1809 (28.2) | 378 (27.6) | |
| ≥2 | 11,124 (14.9) | 1043 (16.3) | 177 (12.9) | |
| Facility type | <0.0001 | |||
| CP | 9951 (13.3) | 540 (8.4) | 155 (11.3) | |
| Comprehensive CP | 35,537 (47.5) | 2169 (33.8) | 519 (37.9) | |
| Academic and research program | 18,838 (25.2) | 2773 (43.2) | 532 (38.9) | |
| Integrated Network Cancer Program | 10,481 (14.0) | 934 (14.6) | 163 (11.9) | |
| Treatment at >1 facility | <0.0001 | |||
| No | 67,037 (89.6) | 5858 (91.3) | 1251 (91.4) | |
| Yes | 7770 (10.4) | 558 (8.7) | 118 (8.6) |
Note: Values are given in number (%) unless indicated otherwise.
CCS, Charlson-Deyo Comorbidity Score; CP, community program; HS, high school; IQR, interquartile range.
Factors Predicting Receipt of Chemotherapy
Several factors were associated with receipt of chemotherapy on multivariate analysis (Table 3). Compared with patients aged 40 to less than 65 years, those in the oldest age group (≥80 y) were associated with the lowest adjusted odds of receiving chemotherapy (aOR, 0.30; 95% CI: 0.27–0.33, p ≤ 0.0001). Black patients were found to have lower odds of receiving chemotherapy compared with white patients (aOR, 0.85; 95% CI: 0.77–0.93, p = 0.0004). Patients with higher comorbidity scores (CCS score ≥ 2) were less likely to receive chemotherapy (aOR, 0.90; 95% CI: 0.84–0.97, p = 0.003). Other factors associated with lower odds of receipt of chemotherapy included patients with nonprivate insurance or no insurance. Patients living in zip codes with higher levels of education (fewer percentage of residents with no high school degree) and treatment at more than one facility also had increased odds of receiving chemotherapy (aOR, 1.15; 95% CI: 1.05–1.25, p = 0.003). Patient sex, Hispanic origin, and treatment facility type were not associated with the receipt of chemotherapy. Odds of receiving chemotherapy improved over the years with patients diagnosed in 2013 to 2016 having the highest odds (aOR, 1.50; 95% CI: 1.40–1.61, p < 0.0001), compared with the referent group of 2004 to 2006.
Table 3.
Crude and Adjusted Odds of Receiving Chemotherapy by Patient and Clinicopathologic Characteristics: Analysis on 82,592 Patients Diagnosed From 2004 to 2016
| Variable | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| OR (95% CI), p | ||||||
| Age, y | ||||||
| 40 to <65 | Ref | Ref | ||||
| 65 to <75 | 0.76 | (0.71–0.80) | <0.0001 | 0.72 | (0.67–0.78) | <0.0001 |
| 75 to <80 | 0.54 | (0.50–0.58) | <0.0001 | 0.52 | (0.47–0.57) | <0.0001 |
| ≥80 | 0.31 | (0.29-0.34) | <0.0001 | 0.30 | (0.27–0.33) | <0.0001 |
| Sex | ||||||
| Male | Ref | Ref | ||||
| Female | 1.00 | (0.95–1.05) | 0.856 | 0.98 | (0.93–1.04) | 0.510 |
| Race | ||||||
| White | Ref | Ref | ||||
| Black | 0.80 | (0.73–0.87) | <0.0001 | 0.85 | (0.77–0.93) | 0.0004 |
| Other | 0.82 | (0.68–0.98) | 0.032 | 0.87 | (0.72–1.04) | 0.126 |
| Hispanic origin | ||||||
| Non-Hispanic | Ref | Ref | ||||
| Hispanic | 1.00 | (0.84–1.18) | 0.960 | 1.09 | (0.92–1.29) | 0.335 |
| Insurance status | ||||||
| Private | Ref | Ref | ||||
| Medicare | 0.59 | (0.56–0.63) | <0.0001 | 0.87 | (0.80–0.94) | 0.0004 |
| Medicaid | 0.74 | (0.67–0.82) | <0.0001 | 0.72 | (0.65–0.80) | <0.0001 |
| Other government insurance | 0.39 | (0.33–0.45) | <0.0001 | 0.41 | (0.35–0.48) | <0.0001 |
| Uninsured | 0.61 | (0.54–0.69) | <0.0001 | 0.59 | (0.52–0.66) | <0.0001 |
| Median household income, $ | ||||||
| <30,000 | Ref | Ref | ||||
| 30,000–34,999 | 1.06 | (0.98–1.15) | 0.150 | 0.97 | (0.89–1.06) | 0.465 |
| 35,000–45,999 | 1.09 | (1.01–1.18) | 0.025 | 0.93 | (0.85–1.02) | 0.143 |
| ≥46,000 | 1.11 | (1.03–1.20) | 0.005 | 0.90 | (0.81–1.00) | 0.041 |
| Education (% with no HS degree) | ||||||
| ≥29 | Ref | Ref | ||||
| 20–28.9 | 1.20 | (1.11–1.29) | <0.0001 | 1.22 | (1.12–1.32) | <0.0001 |
| 14–19.9 | 1.19 | (1.11–1.28) | <0.0001 | 1.26 | (1.15–1.38) | <0.0001 |
| <14 | 1.26 | (1.17–1.36) | <0.0001 | 1.40 | (1.27–1.55) | <0.0001 |
| Year of diagnosis | ||||||
| 2004–2006 | Ref | Ref | ||||
| 2007–2009 | 1.25 | (1.16–1.35) | <0.0001 | 1.26 | (1.17–1.37) | <0.0001 |
| 2010–2012 | 1.46 | (1.36–1.58) | <0.0001 | 1.49 | (1.38–1.61) | <0.0001 |
| 2013–2016 | 1.47 | (1.37–1.58) | <0.0001 | 1.50 | (1.40–1.61) | <0.0001 |
| CCS | ||||||
| 0 | Ref | Ref | ||||
| 1 | 1.00 | (0.94–1.06) | 0.976 | 1.03 | (0.98–1.10) | 0.263 |
| ≥2 | 0.85 | (0.79–0.91) | <0.0001 | 0.90 | (0.84–0.97) | 0.003 |
| Facility type | ||||||
| CP | Ref | Ref | ||||
| Comprehensive CP | 1.02 | (0.95–1.11) | 0.564 | 1.03 | (0.95–1.12) | 0.451 |
| Academic and research program | 1.00 | (0.92–1.09) | 0.933 | 0.97 | (0.89–1.06) | 0.551 |
| Integrated Network Cancer Program | 0.96 | (0.87–1.06) | 0.429 | 0.95 | (0.86–1.05) | 0.286 |
| Treatment at >1 facility | ||||||
| No | Ref | Ref | ||||
| Yes | 1.20 | (1.10–1.31) | <0.0001 | 1.15 | (1.05–1.25) | 0.003 |
CCS, Charlson-Deyo Comorbidity Score; CI, confidence interval; CP, community program; HS, high school; Ref, referent.
Chemotherapy Receipt by Year of Diagnosis
We further explored the relationship between patient and clinicopathologic characteristics and chemotherapy administration on the basis of early (2004–2009) versus late (2010–2016) treatment periods. The results of the analysis are presented in Supplementary Table 1. Compared with white patients, black patients had lower odds of receiving chemotherapy in 2004 to 2009 (aOR, 0.80; 95% CI: 0.69–0.92, p = 0.002) than in 2010 to 2016 (aOR, 0.88; 95% CI: 0.78–1.0, p = 0.043). Compared with patients with private insurance, patients with Medicare, Medicaid, or without insurance had lower odds of receiving chemotherapy in 2010 to 2016 than in 2004 to 2009 (Supplementary Table 1), indicating a decreasing trend of receiving chemotherapy for this group in recent years. The impact of age and education on chemotherapy administration remained significant in both early and late treatment groups (p < 0.0001). The CCS score of greater than or equal to 2 and treatment at more than one facility were significantly associated with chemotherapy receipt in the 2010 to 2016 group (p = 0.001 and p < 0.0001 respectively) and not in 2004 to 2009.
Survival Analysis
Survival analysis was performed on 74,501 patients diagnosed between 2004 and 2015. Patients from 2016 were excluded from the analysis as follow-up data were not available for these patients. The median follow-up in this study was 7.8 months, while the median follow-up for surviving patients was 16.5 months. A total of 70,139 deaths were reported during the follow-up period, with a 1-year survival rate of 29.0 and a median survival of 8 months. The results from Kaplan-Meier analysis for all the patient and clinicopathologic characteristics and treatment are presented in Table 4. The 1-year survival (median survival) for white patients, black patients, and patients from other race groups was 28.6% (8 mo), 31.7% (8.3 mo), and 34.0% (8.3 mo), respectively (Fig. 2). Compared with white patients, black patients (aHR, 0.92; 95% CI: 0.90–0.95, p < 0.0001) and patients from other race groups (aHR, 0.86; 95% CI: 0.81–0.91, p < 0.0001) had improved survival on multivariate analysis. Increasing age and nonprivate or no insurance were associated with poor OS. Female sex, Hispanic origin, and income of $35,000 or more were associated with improved survival (Table 4).
Table 4.
Survival Analysis in 74,501 Patients Diagnosed From 2004 to 2015
| Variable | N | Events | 1-Y Survival, % | Median Survival, mo | p | Multivariate HR (95% CI) | p |
|---|---|---|---|---|---|---|---|
| Age, y | |||||||
| 40 to <65 | 35,111 | 32,770 | 33.3 | 8.9 | <0.0001 | Ref | |
| 65 to <75 | 26,008 | 24,534 | 27.7 | 7.7 | 1.10 (1.08–1.13) | <0.0001 | |
| 75 to <80 | 8077 | 7713 | 22.2 | 6.4 | 1.28 (1.24–1.31) | <0.0001 | |
| ≥80 | 5305 | 5122 | 17.0 | 4.9 | 1.45 (1.40–1.50) | <0.0001 | |
| Time to treatment, d | |||||||
| ≤13 | 37,985 | 36,197 | 23.7 | 6.9 | <0.0001 | Ref | |
| >13 | 36,516 | 33,942 | 34.5 | 9.2 | 0.73 (0.72–0.74) | <0.0001 | |
| Sex | |||||||
| Male | 38,475 | 36,458 | 26.1 | 7.5 | <0.0001 | Ref | |
| Female | 36,026 | 33,681 | 32.0 | 8.5 | 0.85 (0.84–0.87) | <0.0001 | |
| Race | |||||||
| White | 67,597 | 63,792 | 28.6 | 8.0 | <0.0001 | Ref | |
| Black | 5709 | 5287 | 31.7 | 8.3 | 0.92 (0.90–0.95) | <0.0001 | |
| Other | 1195 | 1060 | 34.0 | 8.3 | 0.86 (0.81–0.91) | <0.0001 | |
| Hispanic origin | |||||||
| Non-Hispanic | 72,792 | 68,641 | 28.9 | 8.0 | <0.0001 | Ref | |
| Hispanic | 1709 | 1498 | 31.0 | 8.3 | 0.84 (0.80–0.89) | <0.0001 | |
| Insurance status | |||||||
| Private | 23,333 | 21,829 | 34.7 | 9.2 | <0.0001 | Ref | |
| Medicare | 39,516 | 37,479 | 25.6 | 7.2 | 1.10 (1.07–1.12) | <0.0001 | |
| Medicaid | 6824 | 6339 | 29.4 | 8.3 | 1.10 (1.08–1.14) | <0.0001 | |
| Other government insurance | 1171 | 1092 | 28.6 | 7.6 | 1.12 (1.05–1.19) | 0.0003 | |
| Uninsured | 3657 | 3400 | 28.4 | 8.0 | 1.10 (1.07–1.15) | <0.0001 | |
| Median household income, $ | |||||||
| <30,000 | 11,850 | 11,118 | 27.9 | 7.8 | <0.0001 | Ref | |
| 30,000–34,999 | 15,721 | 14,772 | 28.4 | 7.8 | 0.97 (0.95–1.0) | 0.050 | |
| 35,000–45,999 | 22,607 | 21,367 | 28.8 | 8.0 | 0.97 (0.94–1.0) | 0.026 | |
| ≥46,000 | 24,323 | 22,882 | 30.0 | 8.2 | 0.94 (0.91–0.97) | 0.0001 | |
| Education (% with no HS degree) | |||||||
| ≥29 | 14,541 | 13,564 | 28.1 | 7.8 | 0.032 | Ref | |
| 20–28.9 | 20,359 | 19,162 | 29.0 | 8.0 | 1.02 (0.99–1.04) | 0.228 | |
| 14–19.9 | 18,774 | 17,745 | 28.6 | 8.0 | 1.02 (1.0–1.05) | 0.118 | |
| <14 | 20,827 | 19,668 | 29.9 | 8.2 | 1.02 (0.99–1.05) | 0.383 |
Note: In addition to the variables in the table, the multivariate model also adjusted for year of diagnosis, CCS, facility type, treatment at more than one facility, and treatment received.
CCS, Charlson-Deyo Comorbidity Score; CI, confidence interval; HR, hazard ratio; HS, high school.
Figure 2.
Kaplan-Meier curve for overall survival in 74,501 patients with SCLC by race.
Discussion
This study provides a large-scale analysis of real-world data identifying racial and socioeconomic factors affecting systemic therapy delivery and survival in ES-SCLC. In brief, we found that black race, lack of insurance or having nonprivate insurance, lower education, and older age were factors associated with lower odds of receiving systemic treatment for ES-SCLC and that black race was associated with improved survival, whereas white race and lower education were associated with worsened survival. To the best of our knowledge, this is the largest study to date investigating racial and other healthcare disparities in patients with ES-SCLC.
Black patients had lower odds of receiving chemotherapy compared with whites in our cohort. This was similar to what was described in a recent study on both NSCLC and SCLC, which revealed that black patients were less likely to receive guideline-concordant treatment (i.e., chemotherapy) than white patients.11 In addition to racial disparities in the delivery of chemotherapy for patients with ES-SCLC, other studies have reported that black patients are less likely to receive prophylactic cranial irradiation26 and effective doses of consolidative TRT.13 We were not able to assess receipt of prophylactic cranial irradiation or TRT owing to limitations in NCDB coding of RT treatment sites. It is important to note the dearth of research evaluating healthcare disparities in patients with ES-SCLC, thus necessitating this current study. Our results indicate that black patients had higher odds of receiving chemotherapy in the 2010 to 2016 period compared with the 2004 to 2009 period, suggesting a positive impact of the implementation of the Patient Protection and ACA in 2010, although more research is needed to confirm this association. It is important to note that the impact of ACA in the receipt of lung cancer-directed therapy has not been specifically reported. Nevertheless, a recent NCDB study revealed that after the implementation of ACA, the rate of uninsured patients decreased and a higher percentage of patients were diagnosed at an early stage; however, there was no impact on timely treatment in patients with breast, colon, or lung cancer.27
Although black patients had lower odds of receiving chemotherapy, they did not have worse survival compared with other race groups in our study. This is unexpected because receipt of chemotherapy is the most important predictor of survival, as SCLC is highly sensitive to chemotherapy.28 Since the 1990s, there has been a decline in lung cancer mortality overall, with a reduction in the survival disparities between white and black men.29 Moreover, from 2012 to 2016, the lung cancer mortality rate of black women was lower than that of white women (33.3% versus 37.9%). This was not true for men, as white men had a 54.1% mortality rate whereas black men had 63.9%.30 Previous studies revealed that being female may be associated with longer survival.31,32 In addition, in a study on SCLC in older patients, being female and black race were associated with improved survival.33 In our cohort, black patients presented at younger ages and were more likely to be female and treated at academic cancer centers, which are usually associated with better OS. Consistent with our results, a previously published surveillance, epidemiology, and end results analysis revealed that black patients with SCLC presented at younger age compared with white patients.34 We found that several other factors, which have been associated with lower survival, were more often present in black patients such as low income, low educational status, and higher percentage of patients with CCSs greater than 2. It is important to note that 55.9% of the patients had no comorbidities, which likely reflects paucity of the collected data. We speculate that additional factors, such as performance status, which are not captured by NCDB might account for better survival for black patients as our analysis was adjusted for several prognostic factors listed previously.
Conflicting results have been published regarding racial disparities in SCLC and survival. A study using the Kentucky Cancer Registry on both limited-stage SCLC (LS-SCLC) and ES-SCLC found that race did not affect survival.35 In addition, in a study from the U.S. Military Cancer Institute with presumed equal access to care, race was not found to significantly affect survival (p > 0.05).36 Of note, the California Cancer Registry37 found that white patients had worse survival compared with black patients. A similar result was found by a study looking at the Cancer Surveillance Programs of Orange, San Diego, California, and Imperial counties in Southern California.38 In a recent Surveillance, Epidemiology and End Results analysis, SCLC incidence was noted to be down-trending in all racial groups and no significant survival difference was found among white and black patients between the years of 2003 and 2012 (p = 0.4220).2
Socioeconomic factors such as type of health insurance may also affect receipt of chemotherapy and survival. Government health insurance, such as Medicare or Medicaid, was designed to provide healthcare coverage for low-income populations. Nevertheless, it was previously noted to be a barrier to the delivery of combined modality therapy in LS-SCLC.17,18 Compared with the privately insured, Medicare or Medicaid beneficiaries were less likely to receive RT.17 This was not true for chemotherapy delivery, as government health insurance had no effect on chemotherapy administration but was associated with a lower likelihood of RT delivery in LS-SCLC.17 The reason why systemic therapy is not entirely affected by insurance could be related to competitive reimbursement afforded by the 340b Drug Pricing Program.17 In contrast to these studies, patients with nonprivate insurance or without insurance were less likely to receive systemic treatment in our cohort.
There have been contradictory data on the impact of insurance and survival in patients with SCLC. One study found that Medicaid was not associated with a survival benefit compared with being uninsured for patients with SCLC,39 whereas another revealed worse survival for Medicaid patients.17,40 In our cohort, private insurance was associated with the highest OS (9.2 mo), and patients with Medicaid had the second-highest survival (8.3 mo). Interestingly, we found that lower household income was associated with an increased likelihood of receiving chemotherapy, which appears contradictory. A possible explanation is that patients with ES-SCLC often present with a symptomatic disease requiring hospitalization. In such circumstances, inpatient treatment is started and socioeconomic factors that would affect care delivery in the outpatient setting do not affect treatment in the hospital. In our cohort, lower income was associated with worse survival, which is concordant with a previous study on lung cancer.41
In addition to the type of health insurance, the impact of other socioeconomic factors on cancer care in SCLC has also been evaluated, such as education. Our study found that higher education was associated with an increased likelihood of receiving chemotherapy. In previous studies on early-stage SCLC, income and education did not affect the patient’s ability to receive adjuvant chemotherapy42 or surgical resection43; however, data are lacking for ES-SCLC. As to survival, in Swedish patients diagnosed to have LS-SCLC, higher education was associated with a significantly lower risk of death (p = 0.0356).44
Among patients diagnosed with SCLC, approximately 43% are over 70 years of age and 10% are over 80 years old.45 A previous study on patients more than 80 years old revealed that 30% of them did not receive therapy and had decreased survival, with a median OS of 1.3 months. In contrast, those who received both chemotherapy plus local therapy survived the longest regardless of the extent of disease (LS-SCLC or ES-SCLC).46 In terms of receipt of chemotherapy among elderly patients, a study on patients with SCLC treated in the British Columbia Cancer Agency revealed that older age and comorbidity were both associated with lower use of chemoradiation.47 In our cohort, although a higher percentage of patients more than 80 years old received chemotherapy compared with the aforementioned study, increasing age remained an important factor in the receipt of chemotherapy. Similarly, a higher CCS score was associated with lower odds of receiving chemotherapy.
It is known that older patients have a higher incidence of comorbidities and tend to have worse outcomes in general. The poorer OS in elderly patients with SCLC could be related to decreased tolerance or dose limitations of chemotherapy or RT, in addition to noncancer-related causes of death.48 In our study, we found that increasing age and CCS scores greater than 0 were factors associated with poor OS, consistent with published reports.
Irrespective of health disparities, the median survival for patients with ES-SCLC historically was approximately 10 months3,49,50 until recent approval of immunotherapy, which has resulted in approximately 12 months of OS.5,6 In this study, patients who were treated with chemotherapy only (median survival of 7.6 mo) or RT followed by chemotherapy (median survival of 8.1 mo) had lower survival compared with historical data. Median survival for patients who received chemotherapy followed by RT (10.3 mo) was similar to published data, suggesting a potential impact of consolidative TRT.
There were several limitations to our study. The first one is its retrospective nature. Second, we are unable to account for certain clinical variables that might affect treatment decisions and outcomes, namely information on performance status and detailed information on chemotherapy regimens, dosages, and subsequent treatments, which are not fully captured by the NCDB. Furthermore, the impact of patient preferences on receipt of chemotherapy is not recorded by the database. Moreover, treatment guidelines have changed since the time period of the study, with the approval of immunotherapy for front-line treatment of ES-SCLC. In contrast, the strengths of this study include its large sample size and detailed information regarding treatment modalities from approximately 1500 Commission on Cancer-affiliated hospitals, comprising approximately 70% of cancer cases in the United States.11 Furthermore, this study was the first to analyze two different time periods to assess trends in racial disparity in relation to the implementation of ACA.
Although some of our findings correlate with previously published reports, there were contrasting or new findings in our cohort that warrant further investigation. As chemotherapy is the most important predictor of survival in ES-SCLC,28 measures should be taken to address barriers related to racial and other sociodemographic factors that result in a decreased chance of receiving chemotherapy.
This study revealed that black patients with ES-SCLC were less likely to receive chemotherapy, in addition to the elderly, uninsured, and those with nonprivate insurance. The association of race and receipt of chemotherapy persisted in the 2010 to 2016 period, after the implementation of ACA when patients with nonprivate insurance reported even lower odds of obtaining chemotherapy. Further studies are required to address racial and other disparities in the receipt of systemic treatment in ES-SCLC and to guide appropriate interventions to mitigate healthcare inequalities.
Footnotes
Drs. Tapan and Furtado contributed equally to this work.
Disclosure: Dr. Mak reports receiving personal fees from Merck outside of the submitted work. The remaining authors declare no conflict of interest.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2020.100109.
Supplementary Data
References
- 1.Oronsky B., Reid T.R., Oronsky A., Carter C.A. What’s new in SCLC? A review. Neoplasia. 2017;19:842–847. doi: 10.1016/j.neo.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang S., Tang J., Sun T. Survival changes in patients with small cell lung cancer and disparities between different sexes, socioeconomic statuses and ages. Sci Rep. 2017;7:1339. doi: 10.1038/s41598-017-01571-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farago A.F., Keane F.K. Current standards for clinical management of small cell lung cancer. Transl Lung Cancer Res. 2018;7:69–79. doi: 10.21037/tlcr.2018.01.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Socinski M.A., Smit E.F., Lorigan P. Phase III study of pemetrexed plus carboplatin compared with etoposide plus carboplatin in chemotherapy-naive patients with extensive-stage small-cell lung cancer. J Clin Oncol. 2009;27:4787–4792. doi: 10.1200/JCO.2009.23.1548. [DOI] [PubMed] [Google Scholar]
- 5.Horn L., Mansfield A.S., Szczęsna A. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 6.Paz-Ares L., Dvorkin M., Chen Y. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929–1939. doi: 10.1016/S0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network Small cell lung cancer version 3. 2020. https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf [PubMed]
- 8.Wang S., Wong M.L., Hamilton N., Davoren J.B., Jahan T.M., Walter L.C. Impact of age and comorbidity on non-small-cell lung cancer treatment in older veterans. J Clin Oncol. 2012;30:1447–1455. doi: 10.1200/JCO.2011.39.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nadpara P.A., Madhavan S.S., Tworek C., Sambamoorthi U., Hendryx M., Almubarak M. Guideline-concordant lung cancer care and associated health outcomes among elderly patients in the United States. J Geriatr Oncol. 2015;6:101–110. doi: 10.1016/j.jgo.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nadpara P., Madhavan S.S., Tworek C. Guideline-concordant timely lung cancer care and prognosis among elderly patients in the United States: a population-based study. Cancer Epidemiol. 2015;39:1136–1144. doi: 10.1016/j.canep.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blom E.F., Ten Haaf K., Arenberg D.A., de Koning H.J. Disparities in receiving guideline-concordant treatment for lung cancer in the United States. Ann Am Thorac Soc. 2020;17:186–194. doi: 10.1513/AnnalsATS.201901-094OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nadpara P.A., Madhavan S.S., Tworek C. Disparities in lung cancer care and outcomes among elderly in a medically underserved state population—a Cancer Registry-Linked Database study. Popul Health Manag. 2016;19:109–119. doi: 10.1089/pop.2015.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasan S., Renz P., Turrisi A., Colonias A., Finley G., Wegner R.E. Dose escalation and associated predictors of survival with consolidative thoracic radiotherapy in extensive stage small cell lung cancer (SCLC): a National Cancer Database (NCDB) propensity-matched analysis. Lung Cancer. 2018;124:283–290. doi: 10.1016/j.lungcan.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang R., Cheung M.C., Byrne M.M. Do racial or socioeconomic disparities exist in lung cancer treatment? Cancer. 2010;116:2437–2447. doi: 10.1002/cncr.24986. [DOI] [PubMed] [Google Scholar]
- 15.Yorio J.T., Yan J., Xie Y., Gerber D.E. Socioeconomic disparities in lung cancer treatment and outcomes persist within a single academic medical center. Clin Lung Cancer. 2012;13:448–457. doi: 10.1016/j.cllc.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LaPar D.J., Bhamidipati C.M., Harris D.A. Gender, race, and socioeconomic status affects outcomes after lung cancer resections in the United States. Ann Thorac Surg. 2011;92:434–439. doi: 10.1016/j.athoracsur.2011.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pezzi T.A., Schwartz D.L., Mohamed A.S.R. Barriers to combined-modality therapy for limited-stage small cell lung cancer. JAMA Oncol. 2018;4 doi: 10.1001/jamaoncol.2017.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chun S.G., Pezzi T.A., Schwartz D.L. Underutilization of combined-modality therapy in limited-stage small cell lung cancer-reply. JAMA Oncol. 2018;4:1436–1437. doi: 10.1001/jamaoncol.2018.3292. [DOI] [PubMed] [Google Scholar]
- 19.Bach P.B., Cramer L.D., Warren J.L., Begg C.B. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341:1198–1205. doi: 10.1056/NEJM199910143411606. [DOI] [PubMed] [Google Scholar]
- 20.Fry W.A., Menck H.R., Winchester D.P. The National Cancer Data Base report on lung cancer. Cancer. 1996;77:1947–1955. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1947::AID-CNCR27>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 21.Esnaola N.F., Gebregziabher M., Knott K. Underuse of surgical resection for localized, non-small cell lung cancer among whites and African Americans in South Carolina. Ann Thorac Surg. 2008;86:220–227. doi: 10.1016/j.athoracsur.2008.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Check D.K., Albers K.B., Uppal K.M. Examining the role of access to care: racial/ethnic differences in receipt of resection for early-stage non-small cell lung cancer among integrated system members and non-members. Lung Cancer. 2018;125:51–56. doi: 10.1016/j.lungcan.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balekian A.A., Wisnivesky J.P., Gould M.K. Surgical disparities among patients with stage I lung cancer in the National Lung Screening Trial. Chest. 2019;155:44–52. doi: 10.1016/j.chest.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Cykert S., Dilworth-Anderson P., Monroe M.H. Factors associated with decisions to undergo surgery among patients with newly diagnosed early-stage lung cancer. JAMA. 2010;303:2368–2376. doi: 10.1001/jama.2010.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rana R.H., Alam F., Alam K., Gow J. Gender-specific differences in care-seeking behaviour among lung cancer patients: a systematic review. J Cancer Res Clin Oncol. 2020;146:1169–1196. doi: 10.1007/s00432-020-03197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma S., McMillan M.T., Doucette A. Effect of prophylactic cranial irradiation on overall survival in metastatic small-cell lung cancer: a propensity score-matched analysis. Clin Lung Cancer. 2018;19:260–269.e3. doi: 10.1016/j.cllc.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takvorian S.U., Oganisian A., Mamtani R. Association of Medicaid expansion under the Affordable Care Act with insurance status, cancer stage, and timely treatment among patients with breast, colon, and lung cancer. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2019.21653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agra Y., Pelayo M., Sacristan M., Sacristan A., Serra C., Bonfill X. Chemotherapy versus best supportive care for extensive small cell lung cancer. Cochrane Database Syst Rev. 2003;4:CD001990. doi: 10.1002/14651858.CD001990. [DOI] [PubMed] [Google Scholar]
- 29.Singh G.K., Jemal A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950–2014: over six decades of changing patterns and widening inequalities. J Environ Public Health. 2017;2017:2819372. doi: 10.1155/2017/2819372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlaus A.P., Spencer M.S., Zhu X.Y. Light-matter interaction and lasing in lead halide perovskites. Acc Chem Res. 2019;52:2950–2959. doi: 10.1021/acs.accounts.9b00382. [DOI] [PubMed] [Google Scholar]
- 31.Spiegelman D., Maurer L.H., Ware J.H. Prognostic factors in small-cell carcinoma of the lung: an analysis of 1,521 patients. J Clin Oncol. 1989;7:344–354. doi: 10.1200/JCO.1989.7.3.344. [DOI] [PubMed] [Google Scholar]
- 32.Wheatley-Price P., Ma C., Ashcroft L.F. The strength of female sex as a prognostic factor in small-cell lung cancer: a pooled analysis of chemotherapy trials from the Manchester Lung Group and Medical Research Council Clinical Trials Unit. Ann Oncol. 2010;21:232–237. doi: 10.1093/annonc/mdp300. [DOI] [PubMed] [Google Scholar]
- 33.Caprario L.C., Kent D.M., Strauss G.M. Effects of chemotherapy on survival of elderly patients with small-cell lung cancer: analysis of the SEER-Medicare database. J Thorac Oncol. 2013;8:1272–1281. doi: 10.1097/JTO.0b013e3182a007ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eskandar A., Ahmed A., Daughtey M., Kenderian S., Mahdi F., Khan A. Racial and sex differences in presentation and outcomes of small cell lung cancer in the United States: 1973 to 2010. Chest. 2015;147:e164–e165. doi: 10.1378/chest.14-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee K., Kloecker G., Pan J., Rai S., Dunlap N.E. The integration of multimodality care for the treatment of small cell lung cancer in a rural population and its impact on survival. Am J Clin Oncol. 2015;38:448–456. doi: 10.1097/COC.0b013e3182a5346d. [DOI] [PubMed] [Google Scholar]
- 36.Zheng L., Enewold L., Zahm S.H. Lung cancer survival among black and white patients in an equal access health system. Cancer Epidemiol Biomarkers Prev. 2012;21:1841–1847. doi: 10.1158/1055-9965.EPI-12-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lara J.D., Brunson A., Riess J.W., Kelly K., Lara P.N., Jr., Gandara D.R. Clinical predictors of survival in young patients with small cell lung cancer: results from the California Cancer Registry. Lung Cancer. 2017;112:165–168. doi: 10.1016/j.lungcan.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 38.Ou S.H., Ziogas A., Zell J.A. Prognostic factors for survival in extensive stage small cell lung cancer (ED-SCLC): the importance of smoking history, socioeconomic and marital statuses, and ethnicity. J Thorac Oncol. 2009;4:37–43. doi: 10.1097/JTO.0b013e31819140fb. [DOI] [PubMed] [Google Scholar]
- 39.Pezzi T.A., Schwartz D.L., Pisters K.M.W. Association of Medicaid insurance with survival among patients with small cell lung cancer. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slatore C.G., Au D.H., Gould M.K. American Thoracic Society Disparities in Healthcare Group. An official American Thoracic Society systematic review: insurance status and disparities in lung cancer practices and outcomes. Am J Respir Crit Care Med. 2010;182:1195–1205. doi: 10.1164/rccm.2009-038ST. [DOI] [PubMed] [Google Scholar]
- 41.Erhunmwunsee L., Joshi M.B., Conlon D.H., Harpole D.H., Jr. Neighborhood-level socioeconomic determinants impact outcomes in nonsmall cell lung cancer patients in the Southeastern United States. Cancer. 2012;118:5117–5123. doi: 10.1002/cncr.26185. [DOI] [PubMed] [Google Scholar]
- 42.Ahmed Z., Grover P., Kennedy K.F., Masood A., Davis J.R., Subramanian J. Predictors for chemotherapy in early stage small cell lung carcinoma (SCLC): a National Cancer Database (NCDB) analysis. Lung Cancer. 2017;113:85–87. doi: 10.1016/j.lungcan.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 43.Ahmed Z., Kujtan L., Kennedy K.F., Davis J.R., Subramanian J. Disparities in the management of patients with stage I small cell lung carcinoma (SCLC): a surveillance, epidemiology and end results (SEER) analysis. Clin Lung Cancer. 2017;18:e315–e325. doi: 10.1016/j.cllc.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Tendler S., Holmqvist M., Wagenius G., Lewensohn R., Lambe M., De Petris L. Educational level, management and outcomes in small-cell lung cancer (SCLC): a population-based cohort study. Lung Cancer. 2020;139:111–117. doi: 10.1016/j.lungcan.2019.11.008. [DOI] [PubMed] [Google Scholar]
- 45.Owonikoko T.K., Ragin C.C., Belani C.P. Lung cancer in elderly patients: an analysis of the Surveillance, Epidemiology, and End Results database. J Clin Oncol. 2007;25:5570–5577. doi: 10.1200/JCO.2007.12.5435. [DOI] [PubMed] [Google Scholar]
- 46.Schild S.E., Zhao L., Wampfler J.A. Small-cell lung cancer in very elderly (≥ 80 years) patients. Clin Lung Cancer. 2019;20:313–321. doi: 10.1016/j.cllc.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 47.Ludbrook J.J., Truong P.T., MacNeil M.V. Do age and comorbidity impact treatment allocation and outcomes in limited stage small-cell lung cancer? a community-based population analysis. Int J Radiat Oncol Biol Phys. 2003;55:1321–1330. doi: 10.1016/s0360-3016(02)04576-5. [DOI] [PubMed] [Google Scholar]
- 48.Janssen-Heijnen M.L., Maas H.A., Koning C.C., van der Bruggen-Bogaarts B.A., Groen H.J., Wymenga A.N. Tolerance and benefits of treatment for elderly patients with limited small-cell lung cancer. J Geriatr Oncol. 2014;5:71–77. doi: 10.1016/j.jgo.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 49.Pietanza M.C., Byers L.A., Minna J.D., Rudin C.M. Small cell lung cancer: will recent progress lead to improved outcomes? Clin Cancer Res. 2015;21:2244–2255. doi: 10.1158/1078-0432.CCR-14-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rossi A., Di Maio M., Chiodini P. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. J Clin Oncol. 2012;30:1692–1698. doi: 10.1200/JCO.2011.40.4905. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.