We report about a patient with NSCLC with HER2 exon 20 insertion who had a deep central nervous system (CNS) response with first-line poziotinib. A 53-year-old never-smoker woman presented with right shoulder pain and cough. Imaging revealed a right upper lobe (RUL) pulmonary mass, multiple large destructive lytic bone lesions, and brain metastases. Core biopsy from the left acetabular lesion confirmed lung adenocarcinoma. Plasma cell-free DNA hybrid capture comprehensive genomic profiling (CGP) (Guardant Health, Redwood City, CA) revealed an ERBB2 (HER2) A775_G776insYVMA exon 20 insertion mutation with an allele frequency of 13.5% and TP53 C277F of 8.6%. To include the patient in NCT03318939, an ongoing phase II study with poziotinib, the HER2 exon 20 insertion was confirmed using tissue next-generation sequencing Illumina NextSeq (IBM Watson Genomics, Quest Diagnostics, Secaucus, NJ).
The imaging, 4 weeks after starting the first-line poziotinib (16 mg orally daily), revealed a partial response in both the brain and systemic disease using the Response Evaluation Criteria in Solid Tumors version 1.1, including complete resolution of several brain metastases (Fig. 1). In addition, the patient reported improvement in headaches, bone pain, fatigue, cough, cognition, and ambulation.
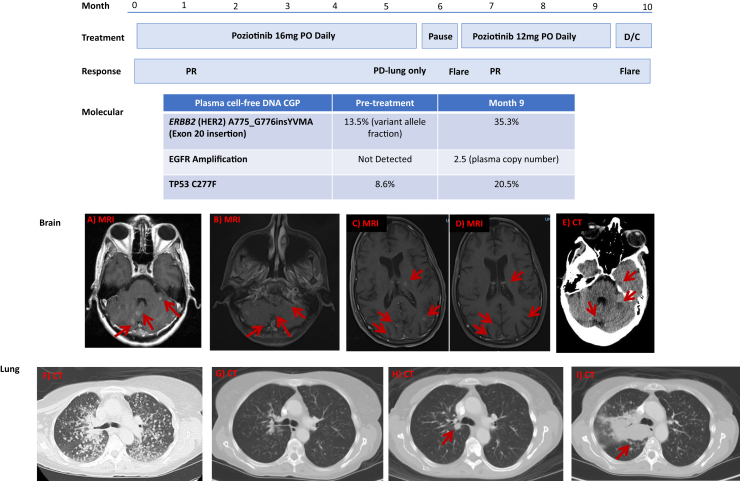
Figure 1.
Chronology of treatment, response, molecular pathology, and imaging. Images A and F reveal the pretreatment baseline scans with extensive bilateral miliary metastases in the lung; B and G, the 4-week post-poziotinib scans; C, the flare 3 weeks after poziotinib discontinuation; D, the treatment response after reinitiation of poziotinib on the EAP; E, the second flare 2 weeks after discontinuation of poziotinib EAP; H, the development of a new RUL nodule 5 months and 19 days after 16 mg PO daily of poziotinib; I, progression of the right lung disease 12 weeks after 12 mg PO daily of poziotinib. CGP, comprehensive genomic profiling; CT, computed tomography; D/C, discontinued; EAP, expanded access protocol; MRI, magnetic resonance imaging; PD, progressive disease; PO, orally; PR, partial response; RUL, right upper lobe.
After 5 months and 19 days of poziotinib use, a new RUL mass with a size of 1.1 cm developed in the patient; therefore, poziotinib was discontinued (Fig. 1). However, brain magnetic resonance imaging indicated ongoing partial response in the CNS. Hence, the patient who was off therapy for 21 days was restarted on poziotinib at a reduced oral dose of 12 mg daily on an expanded access protocol; the reduced dose was chosen to mitigate tyrosine kinase inhibitor–associated toxicities, including diarrhea, rash, and mucositis at a time when the patient’s performance status had deteriorated owing to disease progression. While off therapy, 15 new cerebral metastases developed in the patient. After 4 weeks of restarting poziotinib, these 15 new cerebral lesions resolved, and the RUL lesion decreased in size (Fig. 1). After 12 weeks of reinitiating poziotinib, the RUL mass progressed, but the brain metastases increased only minimally. Poziotinib was discontinued, and repeat plasma cell-free DNA CGP revealed the known HER2 and TP53 mutations and an acquired EGFR amplification (plasma copy number 2.5). The patient expired 3 weeks after the discontinuation of poziotinib, with imaging evidence of multiple hemorrhagic brain metastases (Fig. 1).
Poziotinib is an oral pan-HER inhibitor that irreversibly blocks signaling through the EGFR family of tyrosine kinase receptors, including human EGFR (HER1, ErbB1, EGFR), HER2 (ErbB2), and HER4 (ErbB4).1 Poziotinib has preliminarily engendered a 42% response rate in a phase II study involving heavily pretreated patients with NSCLC with an EGFR or HER2 exon 20 mutation.2,3
Currently, there are no Food and Drug Administration–approved tyrosine kinase inhibitors for patients with NSCLC bearing HER2 exon 20 insertions. This portends an increased risk of CNS metastases and reduced overall survival.4 Several novel agents, including TAK-788, tarloxitinib, pyrotinib, and trastuzumab deruxtecan are currently being tested in this population.
To our knowledge, this is the first documented case illustrating that first-line poziotinib may exert profound CNS response in a case of HER2 exon 20 inserted NSCLC. This case illustrates the potential of poziotinib to affect the CNS disease, the importance of access to CGP, and the flare phenomenon after discontinuation. Clinical studies are underway to determine whether poziotinib is effective in other HER2- or EGFR-mutated solid tumors, including high-grade glioma and breast cancer (NCT04172597).
Acknowledgments
The study was funded by the Spectrum Pharmaceuticals. The patient involved in this study gave her informed consent authorizing the use and disclosure of her health information.
Footnotes
Disclosure: Dr. Tchekmedyian reports receiving personal fees from the Foundation Medicine Virtual Molecular Tumor Board and IntrinsiQ Specialty Solutions outside of the submitted work. Drs. Paxton and Lebel report receiving personal fees (employment and/or stocks) from Spectrum Pharmaceuticals, Inc. outside of the submitted work. Dr. Heymach reports receiving grants and personal fees from AstraZeneca; grants from the National Institutes of Health/National Cancer Institute, American Cancer Society, and Checkmate Pharmaceuticals; personal fees from Bristol-Myers Squibb, GlaxoSmithKline, Kairos Venture Investments, BrightPath Therapeutics, Hengrui Therapeutics, Eli Lilly, and EMD Serono; and grants and personal fees (royalties and patents) from Spectrum Pharmaceuticals, Inc. outside of the submitted work. Ms. Keossayan declares no conflict of interest.
References
- 1.Han J.Y., Lee K.H., Kim S.W. A phase II study of poziotinib in patients with epidermal growth factor receptor (EGFR)-mutant lung adenocarcinoma who have acquired resistance to EGFR-tyrosine kinase inhibitors. Cancer Res Treat. 2017;49:10–19. doi: 10.4143/crt.2016.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robichaux J.P., Elamin Y.Y., Vijayan R.S.K. Pan-cancer landscape and analysis of ERBB2 mutations identifies poziotinib as a clinically active inhibitor and enhancer of T-DM1 activity [published correction appears in Cancer Cell. 2020;37:420] Cancer Cell. 2019;36:444–457.e7. doi: 10.1016/j.ccell.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robichaux J.P., Elamin Y.Y., Tan Z. Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nat Med. 2018;24:638–646. doi: 10.1038/s41591-018-0007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noronha V., Choughule A., Patil V.M. Epidermal growth factor receptor exon 20 mutation in lung cancer: types, incidence, clinical features and impact on treatment. Onco Targets Ther. 2017;10:2903–2908. doi: 10.2147/OTT.S133245. [DOI] [PMC free article] [PubMed] [Google Scholar]