Abstract
Introduction
Kras mutation is the most common driver oncogene present in patients with NSCLC. Recently, the precision medicine for patients with Kras-mutated NSCLC has been under investigation, but the best treatment is still unknown. This study aimed to analyze the clinical characteristics, immune checkpoint inhibitor (ICI) response, and prognostic factors of patients with NSCLC with different Kras mutation subtypes.
Methods
From 2005 to 2018, we collected nonsquamous NSCLC tissue samples for Kras mutation analysis using direct Sanger sequencing or MassARRAY genotyping (Agena Bioscience, San Diego, CA) at the National Taiwan University Hospital. Clinical characteristics, ICI treatment effectiveness, time-to-tumor recurrence (TTR), and overall survival (OS) were analyzed using multivariate Cox models, to estimate adjusted hazard ratios (HRs).
Results
Among 5278 patients with nonsquamous NSCLC, 246 (4.7%) had Kras mutations. The major Kras mutation subtypes were G12C (32.9%), G12D (23.7%), and G12V (18.9%). Patients with Kras-G12C had a higher proportion of male individuals (p = 0.018) and smokers (p < 0.001). Among the 25 patients treated with ICIs, patients with Kras-G12C had a higher response rate (53.8% versus 8.3%, p = 0.030) and longer progression-free survival (4.8 mo versus 2.1 mo, p = 0.028) than those with Kras-non-G12C. For the 85 patients with early-stage NSCLC, those with G12C had shorter TTR (22.8 mo) than those with Kras-non-G12C (97.7 mo, p = 0.004). For the 143 patients with advanced-stage NSCLC, there was a significant difference in OS between patients with Kras-G12C and Kras-non-G12C (7.7 mo versus 6.0 mo, p = 0.018) and patients with Kras-G12V had the shortest OS (5.2 mo). Multivariate analysis revealed association of shorter OS with Kras-G12V (HR = 2.47, p = 0.002), stage IV disease status (HR = 2.69, p = 0.008), and NSCLC—not otherwise specified histology (HR = 3.12, p = 0.002).
Conclusions
Kras-G12C was associated with favorable ICI treatment effectiveness in patients with NSCLC. Kras-G12C mutation was associated with shorter TTR in patients with early-stage NSCLC, and Kras-G12V mutation was associated with shorter OS in patients with advanced-stage NSCLC when comparing with Kras-G12C.
Keywords: Kras mutation, G12C, Lung cancer, Immunotherapy, PD-L1, Concomitant mutation
Introduction
Lung cancer is the leading cause of cancer deaths worldwide. In recent decades, the promising evolution of NSCLC treatment is personalized therapy. Treatment with the corresponding molecular-targeted medication provides a favorable antitumor effectiveness and improves the survival for patients with NSCLC harboring oncogenic driver mutations, such as EGFR, Braf, ALK, and ROS-1 fusion genes.1, 2, 3, 4, 5 However, for personalized target therapy, it is most important to detect the oncogenic driver mutations.
Activating mutations in Kras (KRAS homolog), impairing guanosine triphosphatase activity, play a pivotal role in oncogenic transformation.6,7 Kras mutation is frequently identified in a variety of cancers, including lung, pancreatic, and colorectal.8,9 Kras-mutant lung cancers account for approximately 25% to 35% of NSCLCs, thus representing an enormous cancer burden worldwide.10 The Kras mutation rates of lung adenocarcinoma in non-Asian patients are higher than those in Asian patients.10,11 In addition, Kras mutation occurs more frequently in smokers than in nonsmokers.12,13 Owing to the low incidence of Kras mutations in lung cancer in Eastern Asia, there were only a few Asian studies and they contained small patient numbers. The incidences of variable Kras mutation subtypes, clinical characteristics, and prognostic factors for NSCLC in Asia are not clear.
The prognostic value of Kras mutation in lung cancer is controversial.14, 15, 16 In addition, the different Kras mutation subtypes may affect clinical outcome and prognosis.17 Kras-G12C or G12V mutations have been reported to be associated with shorter progression-free survival (PFS) than other Kras mutation subtypes.17,18 However, some studies did not reveal the predicted value of specific Kras mutation subtypes in their clinical prognosis.19,20
For more than three decades, there were no promising medications targeted at Kras mutations. Therefore, patients with advanced-stage NSCLC with Kras mutations received chemotherapy as the standard treatment. Recently, the first investigation on a Kras-G12C inhibitor, AMG 510, is currently enrolling, in a phase 2 clinical trial. In addition to AMG 510, MRTX 849, a Kras-G12C inhibitor, is being used in phase 1 and 2 clinical trials (NCT 03785249, NCT04330664, NCT04613596). Nevertheless, identifying the clinical characteristics and prognosis of patients with NSCLC with different subtypes of Kras mutations is still very important for clinical researchers to plan future treatment strategy and related clinical trials on Kras mutations.
NSCLC with Kras mutation is associated with not only a high frequency of co-occurring genetic mutations but also increased programmed death-ligand 1 (PD-L1) expression and high tumor mutation burden.16,21, 22, 23 The exploratory analysis of KEYNOTE-042 and KEYNOTE-189 revealed that pembrolizumab, an immune checkpoint inhibitor (ICI), improved the treatment response and prolonged the PFS and overall survival (OS) in Kras-mutant NSCLC compared with chemotherapy only.24,25 Because of the genetic heterogeneity in Kras-mutant NSCLC, the effectiveness of chemotherapy or immunotherapy varies.16,25 Therefore, the optimal therapy was still unclear.
To understand the clinical prognosis and to explore the optimal therapies of nonsquamous NSCLC with Kras mutations, we collected a large cohort of nonsquamous NSCLC tissue samples for Kras mutation analysis and correlated the Kras mutation subtypes with their clinical characteristics, effectiveness of immunotherapy, and prognosis.
Materials and Methods
Patients and Tissue Procurement
From 2005 to 2018, we consecutively collected tissue specimens to detect Kras mutation at the National Taiwan University Hospital (NTUH). Most tissue specimens, which were of NSCLC, included those of surgical excisional tumor, bronchoscopy biopsy, sonography or computed tomography (CT)–guided biopsy, and malignant pleural effusions or ascites. All enrolled patients signed informed consent for future molecular analyses. This study was approved by the Institutional Review Board (REC Nos. 201103013RC and 201111039RIC) of the NTUH Research Ethics Committee. The NTUH provides service to more than 12% of newly diagnosed patients with lung cancer in Taiwan every year. The high proportion is thought to be representative of the population of patients with lung cancer in Taiwan.
The WHO classification of lung tumors was adopted for the diagnosis and histology classification of lung cancer.26 For all patients with lung cancer, a complete lung cancer staging workup was arranged, including CT of the chest and abdomen, brain imaging, and whole-body bone scintigraphy.27 The clinical and demographic characteristics of the patients and imaging reports were recorded. In addition, nonsmokers were defined as patients who had smoked less than 100 cigarettes in their lifetime,28 and all others were categorized as smokers. The patients’ cancer stage, Eastern Cooperative Oncology Group performance status,29 systemic therapy, and treatment response were also recorded.
Response Evaluation of Patients With Nonsquamous NSCLC
The enrolled patients received systemic treatments, including target therapy, chemotherapy, and immunotherapy. Chest radiography every 2 to 4 weeks and CT of the chest (including the liver and adrenal glands) every 2 to 3 months were performed as routine clinical practice and as needed, for the evaluation of the treatment response. The treatment response was evaluated using the Response Evaluation Criteria in Solid Tumors guidelines (version 1.1).30 The objective responses were defined as complete remission, partial response, stable disease, and progressive disease.30 Time-to-tumor recurrence (TTR) was defined as the time from the tumor resection to tumor recurrence (local or metastatic) in patients with early-stage (I–IIIa) disease status. OS was defined as the period from the date of first-line systemic treatment to the date of death. PFS of ICIs was defined as the duration from the initiation of ICI treatment to disease progression or death, whichever occurred first.
Detection of Kras Mutation
The detection of Kras mutations in tissue specimens was by Sanger sequencing or matrix-assisted laser desorption ionization-time-of-flight (MALDI-TOF) mass spectrometry. The process and related methods of the Kras mutation analysis are as described previously.31,32 Briefly, for the Sanger sequence, the reversed-transcriptase polymerase chain reaction (PCR) was used to amplify exons 2 to 3 of the Kras gene by using the following primers: forward, 5-GGCCTGCTGAAAATGACTGA-3, and reverse, 5-TCTTGCTAAGTCCTGAGCCTGTT-3. The Kras reference sequence was based on NM_004985 from the National Center for Biotechnology Information database. PCR amplicons were sequenced with ABI PRISM 3100 (Applied Biosystems, Foster City, CA) in both sense and antisense directions.
After 2011, a MALDI-TOF mass spectrometry was adapted for the gene mutation detection of lung cancer specimens at the NTUH in addition to the detection by Sanger sequencing. First, DNA was extracted from the tumor samples using the QIAmp DNA Mini kit (Qiagen, Valencia, CA), following the manufacturer’s protocol. MALDI-TOF MS was used to detect the genetic alterations of Kras on the basis of modified methods.33 The analysis of the results was performed according to the manufacturer’s protocol for the MassARRAY system.
Histologic Evaluation and Immunohistochemistry Staining
For immunohistochemistry (IHC) staining of PD-L1 expression in tumor tissue, 4-μm-thick sections from each formalin-fixed, paraffin-embedded tissue blocks were dewaxed with xylene and rehydrated in a graded series of ethanol. The following two PD-L1 test methods were adopted: PD-L1 IHC 22C3 pharmDx (Agilent Technologies, Santa Clara, CA) on the DAKO Autostainer Link 48 platform (Agilent Technologies) and the Ventana PD-L1 (SP263) assays (Ventana Medical System, Tucson, AZ) on Ventana BenchMark platforms (Ventana Medical System). The quantification of immunoreactive tumor cells was performed according to the manufacturer’s recommendations. Briefly, tumor cells with partial or complete membranous staining with any staining intensity were considered positive. The ratio between stained tumor cells and all viable tumor cells, defined as tumor proportion score (TPS), was evaluated by experienced pathologists.34
Statistical Analysis
All statistical analyses were performed by IBM SPSS for Windows, version 22.0 (IBM Corp., Armonk, NY). Chi-square test was used for the analysis of all categorical variables. Fisher’s exact test was used when the sampling variability was less than or equal to five. Nonparametric Mann-Whitney U test was used to compare the median ages between the two groups. One-way analysis of variance was used to analyze the differences between patient characteristics among the different subtypes of Kras mutations. Survival curves were plotted using the Kaplan-Meier method and compared between groups using the log-rank test. Multivariate Cox models were used to estimate adjusted hazard ratios (HRs). Two-sided p value of less than 0.05 was considered statistically significant.
Results
Clinical Demographics of Patients With Nonsquamous NSCLC With Kras Mutations
From 2005 to 2018, we consecutively collected tumor tissues from 5278 patients with nonsquamous NSCLC, and 246 patients (4.7%) had tumors with Kras mutations. Of these, 18 patients with double cancers in addition to lung cancers were excluded and 228 patients were enrolled for further analysis.
The clinical and demographic characteristics of the 228 patients are listed in Table 1 and Supplementary Table 1. The median age was 66.0 (range: 31.6–93.3) years. There were 159 male individuals (69.7%) and 144 smokers (63.2%). The disease status of the patients included 85 early-stage (I–IIIa) and 143 advanced-stage (IIIb or IV) disease statuses. The histology subtypes of nonsquamous NSCLC included 203 lung adenocarcinoma, 14 pleomorphic or sarcomatoid, and 11 NSCLC—not otherwise specified (NOS).
Table 1.
Clinical Characteristics of Kras Mutation in Patients With Nonsquamous NSCLC
| Characteristics | All Patients | G12C | Non-G12C | p |
|---|---|---|---|---|
| Total | 228 | 75 (32.9) | 153 (67.1) | |
| Age, median, y (range) | 66.0 (31.6–93.3) | 67.0 (45.5–93.0) | 65.5 (31.6–93.3) | 0.463a |
| Sex | 0.018 | |||
| Female | 69 | 15 (20.0) | 54 (35.3) | |
| Male | 159 | 60 (80.0) | 99 (64.7) | |
| Smoking status | <0.001 | |||
| Nonsmokers | 84 | 14 (18.7) | 70 (45.8) | |
| Smokers | 144 | 61 (81.3) | 83 (54.2) | |
| Stage | 0.376 | |||
| I–IIIa | 85 | 31 (41.3) | 54 (35.3) | |
| IIIb or IV | 143 | 44 (58.7) | 99 (64.7) | |
| Tumor | 0.132 | |||
| Adenocarcinoma | 203 | 71 (94.7) | 132 (86.3) | |
| Pleomorphic or sarcomatoid | 14 | 3 (4.0) | 11 (7.2) | |
| NSCLC-NOS | 11 | 1 (1.3) | 10 (6.5) |
Note: Values are given in number (%) unless indicated otherwise.
NOS, not otherwise specified.
By Mann-Whitney U test.
The Proportion of Patients With Kras Mutations
Of the 228 patients with Kras mutations, the mutation types included 75 G12C (32.9%), 54 G12D (23.7%), 43 G12V (18.9%), 38 G12A or S or R (16.7%), 16 codon G13C or D or S (7.0%), and two Q61H or K (0.9%) (Supplementary Table 1 and Supplementary Fig. 1). Patients with Kras-G12C also had a higher proportion of male individuals (80.0%, p = 0.018) and smokers (81.3%, p < 0.001) than those with Kras-non-G12C (64.7% and 54.2%, respectively) (Table 1). In contrast, patients with Kras-G12C accounted for 42.4% (N = 61) of the 144 smokers. In addition, there was a trend that patients with other Kras mutation subtypes (G13C or D or V, and Q61H or K) had more advanced-stage (IIIb or IV) status compared with those with codon G12 mutations (p = 0.054) (Supplementary Table 1).
PD-L1 Expression and Clinical Outcome of Immunotherapy According to Kras Mutation Subtypes
In the cohort, there were 42 patients who had adequate tissues for PD-L1 IHC staining. The PD-L1 IHC stains revealed 16 patients (38.1%) with TPS of greater than 50%, 13 (31.0%) with TPS of 1% to 49%, and 13 patients (31.0%) with TPS of less than 1%. There were no considerable differences in sex, age, smoking, stage (I–IIIa versus IIIb or IV), tumor histology, or Kras mutation subtypes between PD-L1 positivity (≥1% TPS) and negativity (<1% TPS) (Table 2).
Table 2.
Clinical Characteristics of Patients With Nonsquamous NSCLC Who Had the Results of PD-L1 IHC Stain
| Characteristics | All Patients | PD-L1 Positivea (TPS ≥ 1%) | PD-L1 Negative (TPS < 1%) | p |
|---|---|---|---|---|
| Total | 42 | 29 (69.0) | 13 (31.0) | |
| Age, median, y (range) | 64.1 (38.9–87.5) | 64.3 (38.9–85.8) | 63.9 (50.1–87.5) | 1.000b |
| Sex | 0.422c | |||
| Female | 9 | 5 (17.2) | 4 (30.8) | |
| Male | 33 | 24 (82.8) | 9 (69.2) | |
| Smoking status | 1.000c | |||
| Nonsmokers | 13 | 9 (31.0) | 4 (30.8) | |
| Smokers | 29 | 20 (69.0) | 9 (69.2) | |
| Stage | 0.501 | |||
| I–IIIa | 18 | 11 (37.9) | 7 (53.8) | |
| IIIb or IV | 24 | 18 (62.1) | 6 (46.2) | |
| Tumor | 0.540c | |||
| Adenocarcinoma | 39 | 26 (89.7) | 13 (100.0) | |
| Otherd | 3 | 3 (10.3) | 0 (0.0) | |
| Kras mutation | 0.317 | |||
| G12C | 21 | 13 (44.8) | 8 (61.5) | |
| Non-G12C | 21 | 16 (55.2) | 5 (38.5) |
Note: Values are given in number (%) unless indicated otherwise.
IHC, immunohistochemistry; NOS, not otherwise specified; PD-L1, programmed death-ligand 1; TPS, tumor proportion score.
PD-L1 IHC by DAKO 22C3 and SP263.
By Mann-Whitney U test.
By Fisher’s exact test.
Two NSCLC-NOS and one pleomorphic carcinoma.
There were 27 patients with Kras mutation who had received ICI treatment. Two patients were excluded from the analysis on the treatment effectiveness because of having taken ICIs as a palliative treatment under severe critical conditions. Of the remaining 25 patients, there were 21 male individuals and 18 smokers (Table 3). The Kras mutation subtypes included 13 Kras-G12C and 12 Kras-non-G12C. Of these, 20 patients received ICIs as a single treatment.
Table 3.
Clinical Characteristics of Patients With Nonsquamous NSCLC Who Received ICIs
| Characteristics | All Patients | Responder | Nonresponder | pa |
|---|---|---|---|---|
| Total | 25 | 8 (32.0) | 17 (68.0) | |
| Age, median, y (range) | 63.2 (38.9–85.8) | 60.2 (45.5–80.5) | 63.9 (38.9–85.8) | 0.673b |
| Sex | 1.000 | |||
| Female | 4 | 1 (12.5) | 3 (17.6) | |
| Male | 21 | 7 (87.5) | 14 (82.4) | |
| Smoking status | 1.000 | |||
| Nonsmokers | 7 | 2 (25.0) | 5 (29.4) | |
| Smokers | 18 | 6 (75.0) | 12 (70.6) | |
| Tumor | 0.448 | |||
| Adenocarcinoma | 22 | 8 (100.0) | 14 (82.4) | |
| Pleomorphic or sarcomatoid | 1 | 0 (0.0) | 1 (5.9) | |
| NSCLC-NOS | 2 | 0 (0.0) | 2 (11.8) | |
| PD-L1 TPS, % | 0.225 | |||
| >50 | 12 | 5 (62.5) | 7 (41.2) | |
| 1–49 | 8 | 3 (37.5) | 5 (29.4) | |
| <1 | 5 | 0 (0.0) | 5 (29.4) | |
| Kras mutations | 0.030 | |||
| G12C | 13 | 7 (87.5) | 6 (35.3) | |
| Non-G12C | 12 | 1 (12.5) | 11 (64.7) | |
| ICIs | 0.548 | |||
| Pembrolizumab | 14 | 6 (75.0) | 8 (47.1) | |
| Nivolumab | 2 | 0 (0.0) | 2 (11.8) | |
| Atezolizumab | 5 | 1 (12.5) | 4 (23.5) | |
| Otherc | 4c | 1 (12.5) | 3 (17.6) | |
| Combination therapy | 1.000 | |||
| Combo | 5 | 2 (25.0) | 3 (17.6) | |
| Single use | 20 | 6 (75.0) | 14 (82.4) |
Note: Values are given in number (%) unless indicated otherwise.
ICI, immune checkpoint inhibitor; NOS, not otherwise specified; PD-L1, programmed death-ligand 1; TPS, tumor proportion score.
By Fisher’s exact test.
By Mann-Whitney U test.
One tremelimumab plus durvalumab, one tislelizumab (BGB-A317), and two spartalizumab (PDR001).
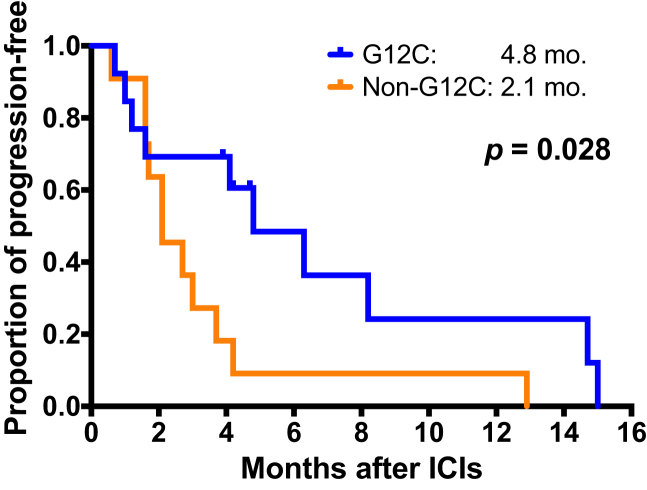
Among the 25 patients treated with ICIs, there were eight responders (32.0%), including seven Kras-G12C and one Kras-non-G12C. Patients with Kras-G12C (53.8%, seven of 13) had higher ICI objective response rate (ORR) than those with Kras-non-G12C (8.3%, one of 12) (p = 0.030). In addition, patients with Kras-G12C had significantly longer PFS (4.8 mo) than those with Kras-non-G12C (2.1 mo, p = 0.028) (Fig. 1).
Figure 1.
Kaplan-Meier survival curve of progression-free survival in patients with nonsquamous NSCLC with Kras mutations who received ICIs. Differences in progression-free survival between patients with Kras-G12C and Kras-non-G12C were statistically significant (G12C [4.8 mo] versus non-G12C [2.1 mo], p = 0.028, by the log-rank test). ICI, immune checkpoint inhibitor.
A total of five (41.7%;,five of 12), three (37.5%; three of eight), and zero (0%; zero of five) ICI responders had tumors with TPS of greater than or equal to 50%, 1% to 49%, and less than 1% of PD-L1 expression, respectively (p = 0.225). There were no marked differences in sex, age, smoking history, PD-L1 IHC, ICIs, or combination treatment between responders and nonresponders.
The Prognosis of the Patients With Early-Stage (Stages I–IIIa) NSCLC
For the 85 patients with early-stage (I–IIIa) NSCLC, the clinical and demographic characteristics are listed in Table 4. The disease status of the patients revealed that 45, 20, and 20 patients had stages I, II, and IIIa, respectively. Of the patients with early-stage NSCLC, Kras-G12C was the major (36.5%, 31 of 85) Kras mutation subtype and associated with a higher proportion of male individuals (77.4%, p = 0.044) and smokers (77.4%, p = 0.002) than those with Kras-non-G12C (Table 4).
Table 4.
Clinical Characteristics of Kras Mutation in Patients With Early-Stage (I–IIIa) and Advanced-Stage (IIIb or IV) Nonsquamous NSCLC
| Characteristics | Early Stage (I–IIIa) |
P | Advanced Stage (IIIb or IV) |
p | ||||
|---|---|---|---|---|---|---|---|---|
| Total | G12C | Non-G12C | Total | G12C | Non-G12C | |||
| Total | 85 | 31 (36.5) | 54 (63.5) | 143 | 44 (30.8) | 99 (69.2) | ||
| Age, median, y (range) | 64.4 (40.5–87.5) | 67.9 (45.5–84.9) | 63.1 (40.5–87.5) | 0.517a | 66.2 (31.6–93.3) | 66.2 (46.7–93.0) | 66.5 (31.6–93.3) | 0.723a |
| Sex | 0.044 | 0.130 | ||||||
| Female | 31 | 7 (22.6) | 24 (44.4) | 38 | 8 (18.2) | 30 (30.3) | ||
| Male | 54 | 24 (77.4) | 30 (55.6) | 105 | 36 (81.8) | 69 (69.7) | ||
| Smoking status | 0.002 | 0.006 | ||||||
| Nonsmokers | 38 | 7 (22.6) | 31 (57.4) | 46 | 7 (15.9) | 39 (39.4) | ||
| Smokers | 47 | 24 (77.4) | 23 (42.6) | 97 | 37 (84.1) | 60 (60.6) | ||
| Stage | 0.246 | |||||||
| I | 45 | 13 (41.9) | 32 (59.3) | |||||
| II | 20 | 8 (25.8) | 12 (22.2) | |||||
| IIIa | 20 | 10 (32.3) | 10 (18.5) | 0.004 | ||||
| IIIb | 16 | 10 (22.7) | 6 (6.1) | |||||
| VI | 127 | 34 (77.3) | 93 (93.9) | |||||
| Histology | 0.742 | 0.192 | ||||||
| Adenocarcinoma | 81 | 30 (96.8) | 51 (94.4) | 122 | 41 (93.2) | 81 (81.8) | ||
| Pleomorphic or sarcomatoid | 3 | 1 (3.2) | 2 (3.7) | 11 | 2 (4.5) | 9 (9.1) | ||
| NSCLC-NOS | 1 | 0 (0.0) | 1 (1.9) | 10 | 1 (2.3) | 9 (9.1) | ||
Note: Values are given in number (%) unless indicated otherwise.
NOS, not otherwise specified.
By Kruskal-Wallis test.
After the surgical intervention for the patients with early-stage lung cancer, 46 patients had tumor recurrence. The recurrence lesions include 25 intrathoracic (19 lung, five mediastinal lymph nodes, and one malignant pleural effusions), 14 extrathoracic (11 brain, one bone, one liver, and one neck lymph node), and seven intrathoracic and extrathoracic. There were no significant differences in recurrence lesion between different Kras mutation subtypes (p = 0.589) (Supplementary Table 2).
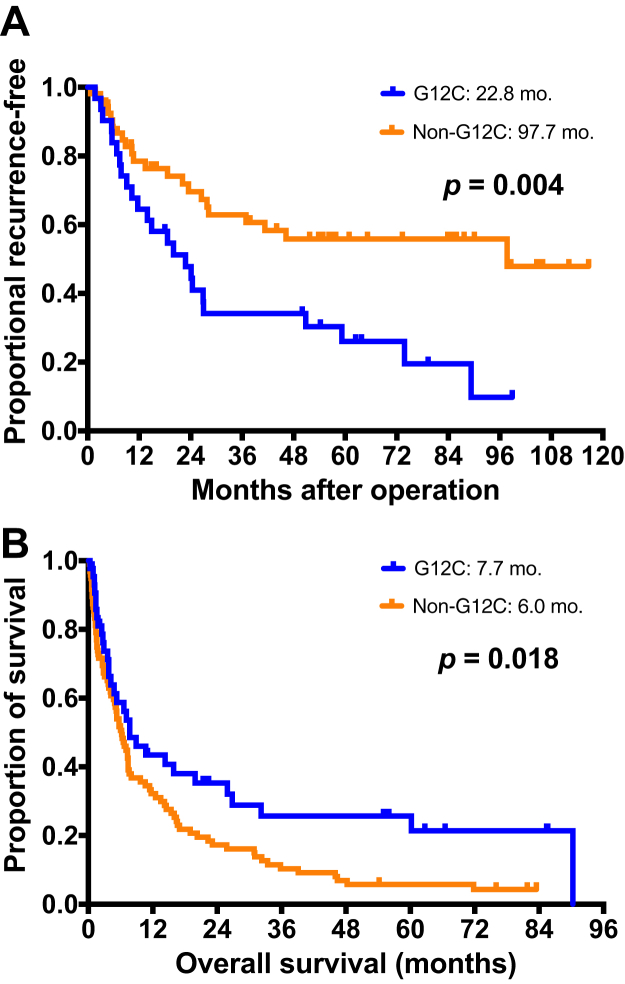
Patients with Kras-G12C had shorter TTR (22.8 mo) than those with Kras-non-G12C (97.7 mo, p = 0.004) (Fig. 2A). For different mutation subtypes of Kras, the difference in TTR among patients with G12C (22.8 mo), G12D (unmatured), G12V (unmatured), and G12A or S or R or other (97.7 mo) Kras mutation patterns was statistically significant (p = 0.036) (Supplementary Fig. 2A).
Figure 2.
Kaplan-Meier survival curve of TTR and overall survival in patients with nonsquamous NSCLC with Kras mutations. (A) The patient with G12C (22.8 mo) had a shorter TTR than those with non-G12C (97.7 mo, p = 0.004). (B) The patients with G12C (7.7 mo) had longer OS than those with non-G12C (6.0 mo, p = 0.018). TTR, time-to-tumor recurrence.
The Prognosis of the Patients With Advanced-Stage (Stages IIIb–IV) NSCLC
For the 143 patients with advanced-stage (IIIb or IV) NSCLC, the clinical and demographic characteristics are listed in Table 4. Among the patients with advanced-stage NSCLC, Kras-G12C was the major (30.8%) Kras mutation subtype. Compared with patients with Kras-non-G12C, the patients with Kras-G12C had a higher proportion of smokers (84.1%, p = 0.006). In addition, Kras-non-G12C was associated with higher proportion of stage IV disease status (93.9%, p = 0.004) (Table 4).
There was a significant difference in OS between patients with Kras-G12C and Kras-non-G12C (7.7 mo versus 6.0 mo, p = 0.018) (Table 5 and Fig. 2B). For different Kras mutation subtypes, the difference in OS among patients with G12C (7.7 mo), G12D (11.7 mo), G12V (5.2 mo), and G12A or S or R or other (5.7 mo) Kras mutation subtypes was significant (p = 0.019) (Supplementary Fig. 2B).
Table 5.
Multivariate Analysis of the Predictive Factors for OS in the Patients With Advanced-Stage (IIIb or IV) Nonsquamous NSCLC With Kras Mutations
| Factors | No. of Patients | OS (mo) | Univariate Analysis |
Multivariate Analysis |
|
|---|---|---|---|---|---|
| P | HR (95% CI) | P | |||
| Sex | |||||
| Female | 38 | 7.3 | 1 | ||
| Male | 105 | 5.7 | 0.150 | 0.96 (0.55–1.68) | 0.887 |
| Smoking history | |||||
| Nonsmokers | 46 | 7.4 | 1 | ||
| Smokers | 97 | 5.3 | 0.039 | 1.69 (0.99–2.90) | 0.057 |
| Stage | |||||
| IIIb | 16 | 32.2 | 1 | ||
| IV | 127 | 5.7 | 0.002 | 2.69 (1.30–5.59) | 0.008 |
| Histology | |||||
| Adenocarcinoma | 122 | 7.4 | 1 | ||
| Pleomorphic or sarcomatoid | 11 | 2.5 | 1.82 (0.90–3.71) | 0.098 | |
| NSCLC-NOS | 10 | 1.3 | <0.001 | 3.12 (1.53–6.36) | 0.002 |
| Kras mutations | |||||
| G12C | 44 | 7.7 | 1 | ||
| G12D | 40 | 11.7 | 1.40 (0.84–2.35) | 0.197 | |
| G12V | 24 | 5.2 | 2.47 (1.39–4.39) | 0.002 | |
| G12A or S or R or others | 35 | 5.7 | 0.019 | 1.54 (0.90–2.63) | 0.119 |
CI, confidence interval; HR, hazard ratio; NOS, not otherwise specified; OS, overall survival.
Patients with stage IIIb disease status had longer OS than those with stage IV disease status (32.2 mo versus 5.7 mo, p = 0.002). Nonsmokers had a longer OS than smokers (7.4 mo versus 5.3 mo, p = 0.039). In addition, there was a significant difference in OS among patients with lung adenocarcinoma (7.4 mo), pleomorphic or sarcomatoid carcinoma (2.5 mo), and NSCLC-NOS (1.3 mo, p < 0.001) (Table 5).
Multivariate analysis was performed using the Cox regression model to determine the potential predictive factors of OS, including sex, smoking, stage, histology classification, and Kras mutation subtypes in patients with advanced-stage (IIIb or IV) NSCLC with Kras mutations (Table 5). Compared with Kras-G12C, Kras-G12V (HR, 2.47, 95% confidence interval [CI]: 1.39–4.39, p = 0.002) was associated with shorter OS. In addition, smokers (HR = 1.69, 95% CI: 0.99–2.90, p = 0.057), stage IV disease status (HR = 2.69, 95% CI: 1.30–5.59, p = 0.008), and NSCLC-NOS histology (HR = 3.12, 95% CI: 1.53–6.36, p = 0.002) were associated with shorter OS.
Concomitant Mutation in Patients With Kras Mutations
Among the 228 patients with Kras mutations, six (2.6%) had tumors with concomitant Kras and EGFR mutations, including three deletions in exon 19, two L858R, and one G719S + E709A (Supplementary Table 3). Of the six patients, four received EGFR tyrosine kinase inhibitor (TKI) treatment (three gefitinib and one erlotinib). The treatment responses were two for partial response and two for progressive disease. In addition, comutation of ALK fusions (three of 120 patients [2.5%] studied) and Braf mutation (one of 201 patients [0.5%] studied) was detected.
Among the 10 patients with concomitant mutations, there were four Kras-G12D, three Kras-G12S, one Kras-G12V, one Kras-G12C, and one Kras-Q61H. All six (100.0%) patients co-concomitant with EGFR mutations had three Kras-G12D and three Kras-G12S, respectively. In contrast, patients with ALK fusion genes did not have concomitant Kras-G12D or S.
Discussion
The Kras mutation is an important oncogenic driver mutation of lung cancer. However, the mutation rates differ between patients in Eastern Asia and Western countries. This study is the largest lung cancer cohort of Kras mutations in Eastern Asia, and 4.7% (246 of 5278) of patients with nonsquamous NSCLC in Taiwan were found harboring tumors with Kras mutations. Kras-G12C was the major subtype. Patients with Kras-G12C mutation had higher response rate and PFS of ICI treatment than those with Kras-non-G12C. In addition, Kras-G12C was associated with shorter TTR in patients with early-stage (I–IIIa) NSCLC. For patients with advanced-stage (IIIb or IV) NSCLC, Kras-G12V mutation was associated with shorter OS compared with Kras-G12C. Patients co-concomitant with EGFR mutations had more Kras-G12D or S.
Patients with Kras mutations have higher tumor mutation burdens and PD-L1 expression,16,23, 24, 25 and both were highly correlated with the response to immunotherapy. According to the subset analysis of KEYNOTE-042 and KEYNOTE-189, pembrolizumab monotherapy or plus chemotherapy both improved the treatment response and prolonged the PFS and OS in Kras-mutant NSCLC compared with chemotherapy only.24,25 In KEYNOTE-042 study, 12 patients with Kras-G12C mutations who received pembrolizumab have a 66.7% of ORR versus a ORR of 23.5% in 17 patients on chemotherapy. This study revealed that the patients with Kras-G12C experienced better treatment benefit from ICIs. Especially, patients with Kras-G12C had higher response rate (53.8%) and longer PFS than those with Kras-non-G12C. The favorable treatment effectiveness of ICIs seems to indicate that ICIs is the treatment choice for patients with Kras-G12C. Further clinical trials are necessary to investigate the treatment effectiveness.
This study revealed a Kras mutation rate of 4.7% in patients with nonsquamous NSCLC in Taiwan, which was significantly lower than that in the Western populations.21,35 The finding is similar to that of studies from other Eastern Asian countries, in which Kras mutation rates have been reported to be less than 10%.36, 37, 38 However, it is difficult to analyze and draw strong conclusions owing to the small sample sizes of the patients with Kras mutations in previous studies,31,36,38 especially for the different mutation subtypes. Geographic (ethnic) differences and methodology in the detection of Kras mutations may also have led to the differences. This study has the largest sample size of the Eastern Asian population studies and could provide promising information to clinicians and for drug development in clinical trials.
Recently, different studies comprehensively evaluated the molecular epidemiology of NSCLC by next-generation sequencing (NGS), and the incidence of Kras mutation in the recent studies was 10% to 20% in East Asians.39, 40, 41 The different detection methods may result in the different incidences of Kras mutation. The detection methods of this study were direct Sanger sequencing or MassARRAY genotyping, which may have lower detection sensitivity than NGS. Therefore, the clinical utility of high-throughput NGS testing is important to identify actionable variants and making treatment strategy in advanced lung cancer.
Most Kras mutation subtypes occur at codons 12 and 13 and less often at codon 61.20 Kras-G12C is the most common subtype in cell lines and different lung adenocarcinoma cohorts, and the incidence of Kras-G12C ranged from 41% to 48%,18,20,35 which were consistent with the results in this study. However, the incidence in previous studies was higher than that in this study (32.9%). The difference may be owing to smoking history. Especially, Kras-G12C was highly associated with heavy smoking and correlated with higher pack-years.35,36 The proportion of smokers in this study was 62.3%, which was significantly lower than that in previous reports.18,20,42 In addition, the patients with Kras-G12D mutations had higher proportion of nonsmokers, and this is consistent with report of El Osta et al.35 The difference in smoking prevalence in different countries results in proportional differences in Kras mutation subtypes. Further studies are required to explore how smoking affects the different subtypes of the Kras mutations.
Kras mutation is a poor prognostic factor of NSCLC.35,43, 44, 45 However, the real clinical impact was still insignificant and controversial.14 Villaruz et al.46 revealed that neither Kras mutation status nor the mutation subtypes were of prognostic value for survival. The Lung Adjuvant Cisplatin Evaluation-BIO collaborate group pooled four clinical trials of adjuvant chemotherapy and found Kras mutation to be of no significant prognostic factor.15 However, Pan et al.47 revealed the negative prognostic value of Kras mutations after pooling 41 studies which included 2374 patients with Kras mutations. In addition, a meta-analysis of Kras mutation in circulating tumor DNA also revealed a negative prognostic value.48 The heterogeneity of patient geographic characteristics, smoking history, disease stage status, and treatment modality in various studies may have resulted in the controversial results.
Furthermore, the impact of the different Kras mutation subtypes on OS was controversial. Scheffler et al.21 evaluated 375 patients with stage IV NSCLC with Kras mutation using NGS and found no significant difference in the OS between patients with different Kras mutation subtypes. Yu et al.20 also reported no apparent differences in the OS on the basis of Kras mutation subtype (codon 12 versus codon 13) for patients harboring metastatic lung adenocarcinoma with Kras mutations. Nadal et al.18 reported that Kras-G12C is associated with worse OS in comparison with Kras wild-type and Kras-non-G12C. Evaluation of EGFR Mutation status for the administration of EGFR-TKIs in non-small cell lung Carcinoma by French Collaborative Thoracic Cancer Intergroup 2 cohort revealed that there was no prognostic value related to the Kras alteration type (transition or transversion) or mutation location (codon 12 versus codon 13) among the 76 erlotinib-treated patients with Kras mutations.19 This study revealed that the patients with Kras-non-G12C had shorter OS than those with Kras-G12C (p = 0.018). Especially, Kras-G12V was a poor prognostic factor for patients with stage IV nonsquamous NSCLC. The different detection methods and different groupings may account for the controversial results.
For surgically resected early-stage lung cancer, Kras-G12C mutation is associated with shorter disease-free survival,18 as also observed in this study. However, Izar et al.49 reported that patients with Kras-G12C or G12V had superior disease-free survival than those with other mutations in patients with resected stage I lung adenocarcinoma. The major differences between the above-mentioned studies and this study are the patient disease stage status and the different groupings. Because of the negative prognostic value of Kras-G12C mutation, more active postoperative monitoring or aggressive adjuvant therapy may be considered necessary for patients with Kras-G12C mutations.
Although most of the oncogenic driver mutations were mutually exclusive in lung cancer, there is growing evidence that Kras-mutant lung cancer is not a homogenous NSCLC subgroup, especially when high-throughput–sensitive methods are used to detect the genetic alteration of lung tumors.21,50 Kras-mutant NSCLC frequently has concomitant mutations.21,50 This study revealed concomitant mutation in patients with Kras mutations, including EGFR, ALK fusions, and Braf. Scheffler et al.21 reported that Kras-mutant NSCLC with concomitant EGFR mutation was 1.2%, which was lower than the incidence of 2.6% of concomitant EGFR mutations in this study. In addition to the small sample size, the other possible reason for this difference may also be that the patients with NSCLC in Eastern Asia had higher EGFR mutation rate than those in Western countries.
The concomitant mutation may have an impact on prognosis in patients with lung cancer with Kras-mutant.21 The Lung Cancer Mutation Consortium Experience revealed that patients with concomitant Kras and EGFR mutation or ALK rearrangement have a favorable clinical outcome. However, patients with concomitant Kras and STK11 mutations have a significantly poor prognosis.35,50 Although this study revealed a response rate of EGFR TKIs of 50%, the sample size was too small to confirm the final treatment efficacy. Increased use of NGS will detect new and more genetic alterations, which is necessary to elucidate their biological impact. Clinical trials are necessary to confirm the treatment effectiveness in concomitant Kras and druggable gene mutations.
The detection of patients with NSCLC harboring oncogenic driver mutations and prescribing of the corresponding molecular-targeted medication provide a favorable prognosis.42 Recently, novel sensitive and high-throughput methods were adopted, for example, NGS or droplet digital PCR. However, for patients with lung cancer harboring Kras mutations, highly sensitive methods increased the detection rate of Kras mutation in lung cancer, but the increased sensitivity of minor subclones detection (<1%) failed to offer prognostic impact.19 It is still necessary to clarify the detection sensitivity of Kras mutation and the clinical relevance in a patient with lung cancer.
There were limitations to this cohort study. First, although this study enrolled a large series of Kras-mutant patients in Taiwan, the enrolled patients were all Asian, known to have low Kras mutation rate. Therefore, it took more than 10 years to enroll an adequate patient number for analysis. The treatment modality and medication have had a rapid evolution in the recent decade, especially immunotherapy; this may have an impact on the prognosis of lung cancer. Second, there were not enough tissue samples left for PD-L1 IHC because multiple testing for gene mutations had been performed for stage IV disease of nonsquamous NSCLC in real-world setting. The effectiveness of immunotherapy for Kras mutation and the relationship between Kras mutation and PD-L1 were unclear before. The proportion of tissues with stage IV disease status for PD-L1 testing was limited, which led to weak statistical power to make conclusion. Third, we did not evaluate the comutation status of other known and unknown driver genes, such as STK-11. In addition, none of the enrolled patients was administered with the treatment targeted at Kras mutation. Recently, AMG 510, a novel small molecular TKI, had regression effects on Kras-G12C tumors and improved the antitumor efficacy of chemotherapy and targeted agents in vitro and in vivo.51 This will have a large effect on the survival prognosis of patients with Kras-mutant lung cancers.
Kras-G12C was associated with favorable ICI treatment effectiveness. ICIs may be the treatment option for patients with NSCLC with Kras-G12C. Kras-G12C mutation was associated with shorter TTR in patients with early-stage NSCLC whereas Kras-G12V mutation was associated with shorter OS in patients with advanced-stage NSCLC compared with Kras-G12C. The patients with NSCLC with different Kras mutation subtypes confer variable prognosis, which serves as a source of prevalence data for the development of clinical trials and precision medicine.
Acknowledgments
This study was supported by the Ministry of Science and Technology, Republic of China (MOST 106-2314-B-002-099-MY3, 109-2628-B-002-029), National Health Research Institutes (NHRI-EX105-10421BI), National Taiwan University Hospital (NTUH), Taipei, Taiwan (106-003689, 107-N4002, 107-CGN-16, and 108-CGN-10), and New Century Health Care Promotion Foundation, Taiwan (2018). The authors thank the second and the third Core Laboratory, Department of Medical Research, NTUH, the Pharmacogenomics Laboratory of the National Core Facility for Biopharmaceuticals, and the next-generation sequencing and microarray core facility of the National Taiwan University Centers of Genomic and Precision Medicine for providing laboratory facilities. The authors thank Editage (www.editage.com) for English language editing. The study was approved by the Institutional Review Board of the NTUH Research Ethics Committee. All patients provided written informed consent for the collection of demographic and clinical outcome data and for molecular analysis before the collection of tissue specimens. Drs. Wu and Shih had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Su, Yu, and Shih contributed to sample preparation and gene detection. Drs. Wu, Shih, Liao, Yu, and Yang contributed to study design and patient specimen collection. Drs. Wu and Shih contributed to data collection, literature search, and drafting of the manuscript. Drs. Liao, Yu, Yang, and Shih contributed to study supervision. All authors approved the final draft of the submitted manuscript.
Footnotes
Disclosure: Dr. Wu has received speaking honoraria from Roche, AstraZeneca, and Pfizer. Dr. Shih has received personal fees for serving in the advisory boards from AstraZeneca, Roche, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Ono Pharmaceutical, Chugai Pharmaceutical, and Bristol-Myers Squibb; speaking honoraria from AstraZeneca, Roche, Boehringer Ingelheim, Eli Lilly, Pfizer, Novartis, Merck Sharp & Dohme, Ono Pharmaceutical, Chugai Pharmaceutical, and Bristol-Myers Squibb; and travel expenses from Roche, Pfizer, Merck Sharp & Dohme, Chugai Pharmaceutical, and Bristol-Myers Squibb. Dr. Yang has received advisory board and speaker honoraria from Boehringer Ingelheim, AstraZeneca, Roche/Genentech, Pfizer, Novartis, Merck Sharp & Dohme, Merck Serono, Clovis Oncology, and Bayer. The remaining authors declare no conflict of interest.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2020.100140
Supplementary Data
References
- 1.Shaw A.T., Engelman J.A. ALK in lung cancer: past, present, and future. J Clin O ncol. 2013;31:1105–1111. doi: 10.1200/JCO.2012.44.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mok T.S., Wu Y.L., Thongprasert S. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 3.Recondo G., Facchinetti F., Olaussen K.A., Besse B., Friboulet L. Making the first move in EGFR-driven or ALK-driven NSCLC: first-generation or next-generation TKI? Nat Rev Clin Oncol. 2018;15:694–708. doi: 10.1038/s41571-018-0081-4. [DOI] [PubMed] [Google Scholar]
- 4.Shaw A.T., Solomon B.J., Chiari R. Lorlatinib in advanced ROS1-positive non-small-cell lung cancer: a multicentre, open-label, single-arm, phase 1–2 trial. Lancet Oncol. 2019;20:1691–1701. doi: 10.1016/S1470-2045(19)30655-2. [DOI] [PubMed] [Google Scholar]
- 5.Singal G., Miller P.G., Agarwala V. Association of patient characteristics and tumor genomics with clinical outcomes among patients with non-small cell lung cancer using a clinicogenomic database [published correction appears in JAMA. 2020;323:480] JAMA. 2019;321:1391–1399. doi: 10.1001/jama.2019.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibbs J.B., Sigal I.S., Poe M., Scolnick E.M. Intrinsic GTPase activity distinguishes normal and oncogenic ras p21 molecules. Proc Natl Acad Sci U S A. 1984;81:5704–5708. doi: 10.1073/pnas.81.18.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mills N.E., Fishman C.L., Rom W.N., Dubin N., Jacobson D.R. Increased prevalence of K-ras oncogene mutations in lung adenocarcinoma. Cancer Res. 1995;55:1444–1447. [PubMed] [Google Scholar]
- 8.Riely G.J., Ladanyi M. KRAS mutations: an old oncogene becomes a new predictive biomarker. J Mol Diagn. 2008;10:493–495. doi: 10.2353/jmoldx.2008.080105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karnoub A.E., Weinberg R.A. Ras oncogenes: split personalities. Nat Rev Mol Cell Biol. 2008;9:517–531. doi: 10.1038/nrm2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saito M., Shiraishi K., Kunitoh H., Takenoshita S., Yokota J., Kohno T. Gene aberrations for precision medicine against lung adenocarcinoma. Cancer Sci. 2016;107:713–720. doi: 10.1111/cas.12941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riely G.J., Marks J., Pao W. KRAS mutations in non-small cell lung cancer. Proc Am Thorac Soc. 2009;6:201–205. doi: 10.1513/pats.200809-107LC. [DOI] [PubMed] [Google Scholar]
- 12.Ricciuti B., Leonardi G.C., Metro G. Targeting the KRAS variant for treatment of non-small cell lung cancer: potential therapeutic applications. Expert Rev Respir Med. 2016;10:53–68. doi: 10.1586/17476348.2016.1115349. [DOI] [PubMed] [Google Scholar]
- 13.Dearden S., Stevens J., Wu Y.L., Blowers D. Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap) Ann Oncol. 2013;24:2371–2376. doi: 10.1093/annonc/mdt205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Román M., Baraibar I., López I. KRAS oncogene in non-small cell lung cancer: clinical perspectives on the treatment of an old target. Mol Cancer. 2018;17:33. doi: 10.1186/s12943-018-0789-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shepherd F.A., Domerg C., Hainaut P. Pooled analysis of the prognostic and predictive effects of KRAS mutation status and KRAS mutation subtype in early-stage resected non-small-cell lung cancer in four trials of adjuvant chemotherapy. J Clin Oncol. 2013;31:2173–2181. doi: 10.1200/JCO.2012.48.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skoulidis F., Heymach J.V. Co-occurring genomic alterations in non-small-cell lung cancer biology and therapy. Nat Rev Cancer. 2019;19:495–509. doi: 10.1038/s41568-019-0179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ihle N.T., Byers L.A., Kim E.S. Effect of KRAS oncogene substitutions on protein behavior: implications for signaling and clinical outcome. J Natl Cancer Inst. 2012;104:228–239. doi: 10.1093/jnci/djr523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nadal E., Chen G., Prensner J.R. KRAS-G12C mutation is associated with poor outcome in surgically resected lung adenocarcinoma. J Thorac Oncol. 2014;9:1513–1522. doi: 10.1097/JTO.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 19.Beau-Faller M., Texier M., Blons H. Clinical relevance of EGFR- or KRAS-mutated subclones in patients with advanced non-small-cell lung cancer receiving erlotinib in a French prospective cohort (IFCT ERMETIC2 cohort—part 2) Clin Lung Cancer. 2019;20:222–230. doi: 10.1016/j.cllc.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Yu H.A., Sima C.S., Shen R. Prognostic impact of KRAS mutation subtypes in 677 patients with metastatic lung adenocarcinomas. J Thorac Oncol. 2015;10:431–437. doi: 10.1097/JTO.0000000000000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scheffler M., Ihle M.A., Hein R. K-ras mutation subtypes in NSCLC and associated co-occurring mutations in other oncogenic pathways. J Thorac Oncol. 2019;14:606–616. doi: 10.1016/j.jtho.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 22.Chen N., Fang W., Lin Z. KRAS mutation-induced upregulation of PD-L1 mediates immune escape in human lung adenocarcinoma. Cancer Immunol Immunother. 2017;66:1175–1187. doi: 10.1007/s00262-017-2005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu C., Zheng S., Jin R. The superior efficacy of anti-PD-1/PD-L1 immunotherapy in KRAS-mutant non-small cell lung cancer that correlates with an inflammatory phenotype and increased immunogenicity. Cancer Lett. 2020;470:95–105. doi: 10.1016/j.canlet.2019.10.027. [DOI] [PubMed] [Google Scholar]
- 24.Gadgeel S., Rodriguez-Abreu D., Felip E. KRAS mutational status and efficacy in KEYNOTE-189: pembrolizumab (pembro) plus chemotherapy (chemo) vs placebo plus chemo as first-line therapy for metastatic non-squamous NSCLC. Ann Oncol. 2019;30:xi64–xi65. [Google Scholar]
- 25.Herbst R.S., Lopes G., Kowalski D.M. LBA4 Association of KRAS mutational status with response to pembrolizumab monotherapy given as first-line therapy for PD-L1-positive advanced non-squamous NSCLC in Keynote-042. Ann Oncol. 2019;30(suppl 11):xi63–xi64. [Google Scholar]
- 26.Travis W.D., Brambilla E., Nicholson A.G. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10:1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 27.Goldstraw P., Crowley J., Chansky K. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours [published correction appears in J Thorac Oncol. 2007;2:985. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention CDC Cigarette smoking among adults--United States, 2006. MMWR Morb Mortal Wkly Rep. 2007;56:1157–1161. [PubMed] [Google Scholar]
- 29.Oken M.M., Creech R.H., Tormey D.C. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 30.Eisenhauer E.A., Therasse P., Bogaerts J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 31.Hsu K.H., Ho C.C., Hsia T.C. Identification of five driver gene mutations in patients with treatment-naive lung adenocarcinoma in Taiwan. PLoS One. 2015;10 doi: 10.1371/journal.pone.0120852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu S.G., Kuo Y.W., Chang Y.L. EML4-ALK translocation predicts better outcome in lung adenocarcinoma patients with wild-type EGFR. J Thorac Oncol. 2012;7:98–104. doi: 10.1097/JTO.0b013e3182370e30. [DOI] [PubMed] [Google Scholar]
- 33.Su K.Y., Chen H.Y., Li K.C. Pretreatment epidermal growth factor receptor (EGFR) T790M mutation predicts shorter EGFR tyrosine kinase inhibitor response duration in patients with non-small-cell lung cancer [published correction appears in J Clin Oncol. 2015;33:2124. J Clin Oncol. 2012;30:433–440. doi: 10.1200/JCO.2011.38.3224. [DOI] [PubMed] [Google Scholar]
- 34.Garon E.B., Rizvi N.A., Hui R. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 35.El Osta B., Behera M., Kim S. Characteristics and outcomes of patients with metastatic KRAS-mutant lung adenocarcinomas: the Lung Cancer Mutation Consortium experience. J Thorac Oncol. 2019;14:876–889. doi: 10.1016/j.jtho.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki M., Shigematsu H., Hiroshima K. Epidermal growth factor receptor expression status in lung cancer correlates with its mutation. Hum Pathol. 2005;36:1127–1134. doi: 10.1016/j.humpath.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 37.Wu C.C., Hsu H.Y., Liu H.P. Reversed mutation rates of KRAS and EGFR genes in adenocarcinoma of the lung in Taiwan and their implications. Cancer. 2008;113:3199–3208. doi: 10.1002/cncr.23925. [DOI] [PubMed] [Google Scholar]
- 38.Bae N.C., Chae M.H., Lee M.H. EGFR, ERBB2, and KRAS mutations in Korean non-small cell lung cancer patients. Cancer Genet Cytogenet. 2007;173:107–113. doi: 10.1016/j.cancergencyto.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 39.Tan A.C., Lai G.G.Y., Tan G.S. Utility of incorporating next-generation sequencing (NGS) in an Asian non-small cell lung cancer (NSCLC) population: incremental yield of actionable alterations and cost-effectiveness analysis. Lung Cancer. 2020;139:207–215. doi: 10.1016/j.lungcan.2019.11.022. [DOI] [PubMed] [Google Scholar]
- 40.Chen J., Yang H., Teo A.S.M. Genomic landscape of lung adenocarcinoma in East Asians. Nat Genet. 2020;52:177–186. doi: 10.1038/s41588-019-0569-6. [DOI] [PubMed] [Google Scholar]
- 41.Tan A.C., Loong H., Ho G.F. Molecular profiling of non-small cell lung cancer (NSCLC) in Asia with targeted next-generation sequencing (NGS): interim analysis of a co-operative group study (ATORG-001) Ann Oncol. 2019;30(suppl 9):ix126. [Google Scholar]
- 42.Kris M.G., Johnson B.E., Berry L.D. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311:1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodenhuis S., van de Wetering M.L., Mooi W.J., Evers S.G., van Zandwijk N., Bos J.L. Mutational activation of the K-ras oncogene. A possible pathogenetic factor in adenocarcinoma of the lung. N Engl J Med. 1987;317:929–935. doi: 10.1056/NEJM198710083171504. [DOI] [PubMed] [Google Scholar]
- 44.Slebos R.J., Kibbelaar R.E., Dalesio O. K-ras oncogene activation as a prognostic marker in adenocarcinoma of the lung. N Engl J Med. 1990;323:561–565. doi: 10.1056/NEJM199008303230902. [DOI] [PubMed] [Google Scholar]
- 45.Mascaux C., Iannino N., Martin B. The role of RAS oncogene in survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer. 2005;92:131–139. doi: 10.1038/sj.bjc.6602258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Villaruz L.C., Socinski M.A., Cunningham D.E. The prognostic and predictive value of KRAS oncogene substitutions in lung adenocarcinoma. Cancer. 2013;119:2268–2274. doi: 10.1002/cncr.28039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pan W., Yang Y., Zhu H., Zhang Y., Zhou R., Sun X. KRAS mutation is a weak, but valid predictor for poor prognosis and treatment outcomes in NSCLC: a meta-analysis of 41 studies. Oncotarget. 2016;7:8373–8388. doi: 10.18632/oncotarget.7080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fan G., Zhang K., Ding J., Li J. Prognostic value of EGFR and KRAS in circulating tumor DNA in patients with advanced non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget. 2017;8:33922–33932. doi: 10.18632/oncotarget.15412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Izar B., Zhou H., Heist R.S. The prognostic impact of KRAS, its codon and amino acid specific mutations, on survival in resected stage I lung adenocarcinoma. J Thorac Oncol. 2014;9:1363–1369. doi: 10.1097/JTO.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 50.Aredo J.V., Padda S.K., Kunder C.A. Impact of KRAS mutation subtype and concurrent pathogenic mutations on non-small cell lung cancer outcomes. Lung Cancer. 2019;133:144–150. doi: 10.1016/j.lungcan.2019.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Canon J., Rex K., Saiki A.Y. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575:217–223. doi: 10.1038/s41586-019-1694-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.