Introduction
Osimertinib was approved for the treatment of patients with NSCLC with EGFR T790M mutation, revealing both progression-free and overall survival benefits in first- and second-line settings.1 However, resistance to osimertinib inevitably develops because of various mechanisms. One of the most common mechanisms is mutation at osimertinib’s primary target residue C797 that impedes binding of the drug to EGFR, particularly in a cis position. Subsequently, several studies have reported mutations at G796R/S, L792 (in trans with C797), and L718Q/V that compromise the efficacy of osimertinib.2 Other than acquired EGFR mutations, aberration on bypass pathways, including human EGFR 2 and MNNG HOS transforming gene (MET) amplifications,2 can induce osimertinib resistance. Big efforts are being made in seeking postosimertinib treatment options, including treatments combining osimertinib with other targeted therapies.3 Here, we report a case of a novel acquired resistance mechanism for osimertinib, TPD52L1-ROS1 rearrangement, that responded to crizotinib in combination with osimertinib in lung adenocarcinoma, in the hope of providing new insights into postosimertinib options (Fig. 1A).
Figure 1.
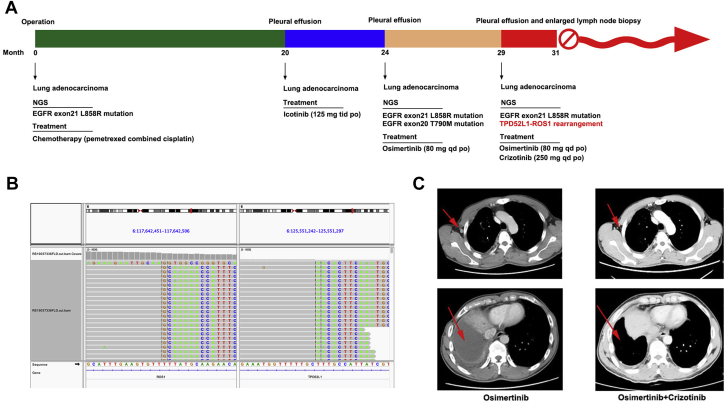
(A) Timeline of the patient receiving various treatments and duration of each treatment. (B) Integrated Genomics Viewer snapshot of paired-end sequencing data revealing a somatic intrachromosomal TPD52L1-ROS1 rearrangement. (C) Computed tomography images of the patient before and after the combined treatment (red arrows indicate the lesions). NGS, next-generation sequencing.
Case Report
A 51-year-old never-smoker Chinese man presented to the hospital in January 2017 with cough that had been persisting for a month. A computed tomography (CT) scan revealed an upper right lung mass (diameter 2.0 cm, marginal spiny). The patient underwent thoracoscopic left lower lobectomy and mediastinal lymph node dissection. Postoperative pathologic diagnosis indicated upper right lung adenocarcinoma, invasion of visceral pleura, and pT2N0M0 stage (pIB). The patient received pemetrexed combined with cisplatin for four cycles after the operation. Molecular profiling was performed by next-generation sequencing (NGS) using genomic DNA extracted from the surgical sample (Burning Rock, Guangzhou, People’s Republic of China) and revealed the driver mutation of EGFR exon 21 L858R. In October 2018, a follow-up CT scan revealed a large amount of pleural effusion at the right side of the thorax, in which malignant lung adenocarcinoma cells were found, indicating the recurrence of lung cancer. Consequently, the patient received oral administration of icotinib (125 mg three times a day) and achieved a partial response. After 4 months, he presented to the hospital with chest congestion owing to massive pleural effusion at the right side of the thorax as revealed by CT. Cytologic tests of the pleural effusion revealed metastatic adenocarcinoma and the coexistence of EGFR exon 21 L858R and EGFR exon 20 T790M mutations detected by NGS. The patient was then treated with osimertinib (80 mg daily) and achieved a partial response for 5 months. In July 2019, the same symptom of pleural-effusion–induced chest congestion was reported again with an enlarged lymph node in the right axilla, both confirmed to be malignant adenocarcinomas, suggesting disease progression and osimertinib resistance. Interestingly, NGS revealed an EGFR exon 21 L858R mutation with a newly emerged MET amplification and TPD52L1-ROS1 rearrangement, whereas T790M was absent (Fig. 1B). Subsequently, the patient received treatment with crizotinib combined with osimertinib to suppress the MET and ROS1 tumors and overcome resistance; this was found to be effective with a substantial decrease of pleural effusion and the disappearance of lymph node enlargement in the right axilla in 2 months (Fig. 1C). Nevertheless, the patient was forced to discontinue the treatment owing to severe gastrointestinal toxicity, such as vomiting and diarrhea.
Discussion
The ROS1 gene was first discovered in 1986, and ROS1 rearrangement as a driver event accounts for 1% to 3% of all lung adenocarcinomas.4 At present, 26 genes have been found to fuse with ROS1 and 13 of them have been reported in NSCLC, including CD74, SLC34A2, GOPC, CCDC6, SDC4, TPM3, EZR, LRIG3, KDEL R2, LIMA1, MSN, CLTC, and TMEM106B.5 TPD52L1, as a very rare ROS1 fusion partner, was previously detected in a newly treated patient with lung adenosquamous cell carcinoma.6 ROS1 fusion (GOPC-ROS1 rearrangement), as an acquired resistance mechanism for osimertinib in lung adenocarcinoma, has been reported in only one previous study.7 The treatment regimen for osimertinib-resistant patients with ROS1 rearrangements is still unclear. In this case, we tried to treat the novel acquired resistance to osimertinib by combining crizotinib with osimertinib. As mentioned earlier, the treatment proved to be effective in controlling tumor progression. Nevertheless, combination therapies might bring severe gastrointestinal toxicity, as presented by this patient.
Acknowledgments
This work was supported by Wu Jieping Medical Foundation of the People’s Republic of China (320.6750.18537). The authors would like to thank the patient for providing written informed consent for publication and all the research staff involved in this case study.
Footnotes
Disclosure: The authors declare no conflict of interest.
References
- 1.Soria J.C., Ohe Y., Vansteenkiste J. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 2.Tang Z.H., Lu J.J. Osimertinib resistance in non-small cell lung cancer: mechanisms and therapeutic strategies. Cancer Lett. 2018;420:242–246. doi: 10.1016/j.canlet.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Romaniello D., Mazzeo L., Mancini M. A combination of approved antibodies overcomes resistance of lung cancer to osimertinib by blocking bypass pathways. Clin Cancer Res. 2018;24:5610–5621. doi: 10.1158/1078-0432.CCR-18-0450. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y.F., Hsieh M.S., Wu S.G. Clinical and the prognostic characteristics of lung adenocarcinoma patients with ROS1 fusion in comparison with other driver mutations in East Asian populations. J Thorac Oncol. 2014;9:1171–1179. doi: 10.1097/JTO.0000000000000232. [DOI] [PubMed] [Google Scholar]
- 5.Uguen A., De Braekeleer M. ROS1 fusions in cancer: a review. Future Oncol. 2016;12:1911–1928. doi: 10.2217/fon-2016-0050. [DOI] [PubMed] [Google Scholar]
- 6.Zhu V.W., Upadhyay D., Schrock A.B., Gowen K., Ali S.M., Ou S.H. TPD52L1-ROS1, a new ROS1 fusion variant in lung adenosquamous cell carcinoma identified by comprehensive genomic profiling. Lung Cancer. 2016;97:48–50. doi: 10.1016/j.lungcan.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Zeng L., Yang N., Zhang Y. GOPC-ROS1 rearrangement as an acquired resistance mechanism to osimertinib and responding to crizotinib combined treatments in lung adenocarcinoma. J Thorac Oncol. 2018;13:e114–e116. doi: 10.1016/j.jtho.2018.02.005. [DOI] [PubMed] [Google Scholar]