Abstract
Introduction
Chronic inflammation is associated with an increased risk of several diseases, including cancer. A complex tumor microenvironment created and maintained by a range of cell types promotes tumor growth, angiogenesis, and metastasis. Inflammasomes, multicomplex cytosolic proteins, generate much of this inflammation, including the activation of the cytokine interleukin (IL)-1β. Inflammation generated by IL-1β is present in several disease states, including atherosclerosis, diabetes, and arthritis. IL-1β is activated when a specific inflammasome, nucleotide-binding domain–like receptor protein 3, induces cleavage of pro–IL-1β into its active form. Nucleotide-binding domain–like receptor protein 3 is up-regulated in lung cancer; IL-1β binds to its receptor and activates signaling pathways, including the MAPK, cyclooxygenase, and nuclear factor–κB pathways, leading to macrophage activation, intratumoral accumulation of immunosuppressive myeloid cells, and tumor growth, invasiveness, metastasis, and angiogenesis. Evidence suggests a role for IL-1β and some of its downstream effectors (e.g., IL-6, IL-8, C-reactive protein, cyclooxygenase-2) as prognostic markers in many malignancies, including lung cancer.
Methods
A phase III cardiovascular study of canakinumab, a human immunoglobulin Gk monoclonal antibody with high affinity and specificity for IL-1β, was conducted in patients who had a myocardial infarction.
Results
A subanalysis of this study found that treatment with canakinumab substantially reduced incident lung cancer and lung cancer mortality in a dose-dependent manner.
Conclusions
A phase III trial is currently recruiting participants to evaluate canakinumab as adjuvant treatment versus placebo in patients with lung cancer. Other studies are investigating combinations of established antineoplastic agents and canakinumab in both early- and advanced-stage NSCLC.
Keywords: Lung cancer, IL-1β, Inflammasome, Canakinumab
Introduction
Chronic inflammation is associated with an increased risk of cancer through promotion of transformation, immune suppression, tumor invasion, and metastasis.1, 2, 3 Tumors promote a constant influx of myelomonocytic cells, including macrophages, angiopoietin-1 receptor-expressing monocytes, and myeloid-derived suppressor cells (MDSCs) that express inflammatory mediators and promote tumor growth, metastasis, and angiogenesis.4,5 Much of this inflammatory response is mediated by inflammasomes, multicomplex cytosolic proteins formed in response to infection- or stress-associated stimuli.2,6,7
Activation of inflammasomes induces the release of pro-inflammatory cytokines such as interleukin (IL)-1β, elevated levels of which correlate with tumor progression in a variety of malignancies.2,7 Indeed, overexpression of IL-1β has been found to increase inflammation-associated tumor invasiveness3,8,9 and stimulate the tumor microenvironment to favor increased cell proliferation and angiogenesis.2 Inhibition of IL-1β signaling has been linked to suppressed tumor progression and enhanced tumor immunity.3
Recent data from the phase III Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS) have revealed that treatment with the IL-1β–specific inhibitor canakinumab substantially reduced incident lung cancer and lung cancer mortality in patients with atherosclerosis and high levels of C-reactive protein (CRP).10 The objective of this review is to present current available data demonstrating the link between IL-1β activity and lung cancer development and provide the basis for targeting IL-1β in lung cancer prevention and therapy.
Interleukin-1 Activation and Signaling
The IL-1 family consists of 11 members, including the two pro-inflammatory cytokines IL-1α and IL-1β, both of which bind to IL-1R1 on the surface of target cells. IL-1α and IL-1β have distinct functional profiles.11,12 IL-1α is constitutively expressed in numerous cell types, including both hematologic and nonhematologic cells.12
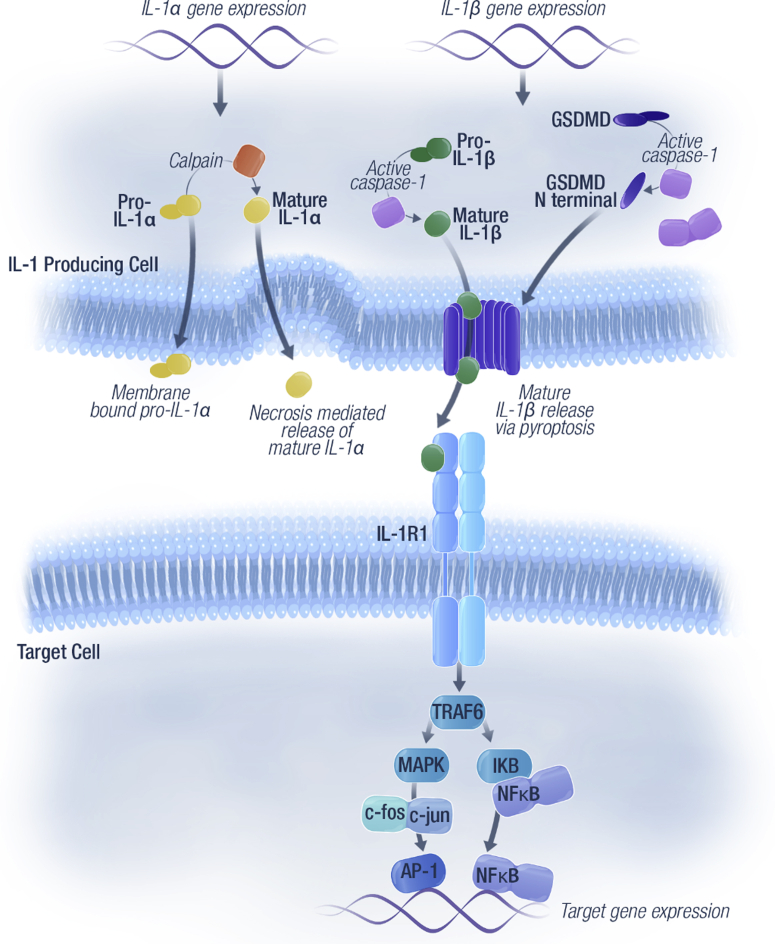
IL-1α typically remains membrane-bound on the cell surface and signals at short distances by autocrine or paracrine signaling with cells expressing IL-1R1. It can also be released from the cell on necrosis, thereby serving as an alarmin (Fig. 1).11,12
Figure 1.
Key components of the IL-1 inflammatory pathway and downstream effects.19,81 Pro-inflammatory cytokines IL-1α and IL-1β bind to IL-1R1 on the surface of target cells. IL-1α can remain membrane-bound or be released from cells on necrosis. Activated NLRP3 inflammasome induces caspase 1–mediated cleavage of pro–IL-1β to mature IL-1β and of the effector molecule gasdermin. The latter causes large pores to form in the cell membrane, leading to a form of cell death called pyroptosis. Mature IL-1β is released from the cell by means of pyroptosis and binds to the IL-1 receptor, which activates signaling pathways (involving MAPK and NF-κB) and causes downstream effects that promote tumor invasion and metastasis. AP-1, activated protein-1; GSDMD, gasdermin D; IL-1β, interleukin 1 beta; IL-1R, IL-1 receptor; NF-κB, nuclear factor–kappa B; NLRP3, nucleotide-binding domain–like receptor protein 3; TRAF6, TNF receptor associated factor 6.
In contrast, IL-1β is produced by a limited number of cells (e.g., monocytes, macrophages, and dendritic cells). IL-1β requires additional processing to exert its biological effects. Active IL-1β is released from immune cells by means of pyroptosis, which is a form of regulated cell death occurring through nucleotide-binding domain–like receptor protein 3 (NLRP3) activation of the inflammasome.13 When activated, NLRP3 induces a caspase 1–mediated inflammatory response, leading to cleavage of pro–IL-1β into its mature active form, which lacks signal sequences for secretion. Therefore, the process of pyroptosis allows the release of active IL-1β when caspase 1–mediated cleavage of the effector molecule gasdermin causes large pores to form in the cell membrane, leading to cell death (Fig. 1).14,15 This secretion of IL-1β allows it to exert its effects on inflammation at the tissue level and also systemically.16 IL-1β plays an important role in the host defense against infections and in tissue homeostasis and repair. IL-1β has emerged as a therapeutic target for a number of inflammatory diseases and more recently, tumor promotion.14,16
The Role of IL-1β in Lung Cancer Development
Promotion of Tumorigenesis and Metastasis
IL-1β is involved in the promotion of tumor growth and metastasis through the induction of growth factors, including vascular endothelial growth factor, prostaglandin E2 (PGE2), and transforming growth factor β.7,17,18 Aberrant IL-1β signaling has been found to drive tumorigenesis through a variety of pathways, as summarized in Table 1.19 IL-1β is a key mediator in the initiation of the inflammatory response in pulmonary diseases, including chronic obstructive pulmonary disease and lung cancer.20,21 Lung inflammation is characterized by macrophage infiltration and increased thickness and fibrosis of the airways, leading to airflow obstruction.22,23
Table 1.
Aberrant IL-1β Signaling in Cancer
| Cell Type | Effect of Aberrant IL-1β Signaling |
|---|---|
| Cancer and stem cells | Promotion of epithelial-to-mesenchymal transition during oncogenesis |
| Endothelial cells | Increases leukocyte adhesions, inflammatory mediator, and prostaglandin production |
| Tumor and stromal cells | Increases production of basement membrane-degrading proteinases involved in cancer invasion and metastasis Induces immune suppression, apoptosis resistance, and angiogenesis by means of induction of COX2–PGE2 pathway in tumor cells |
| Hepatocytes | Increases acute phase C-reactive protein |
| Leukocytes | Increases production of inflammatory cytokines, chemokines, and lipids |
COX2, cyclooxygenase 2; PGE2, prostaglandin E2.
NLRP3 has been associated with increased lung metastasis24 and has been found to regulate protumor activity in lung tumor–derived macrophages.25 Furthermore, NLRP3 is up-regulated in lung adenocarcinoma (ADC) and SCLC; notably, high-grade ADC was associated with increased expression of NLRP3 components compared with low-grade ADC.14 Inflammation can be mediated by the NLRP3 inflammasome even in the lack of immune cells through local production of IL-1β.10,26
Mechanisms of IL-1β-Driven Tumorigenesis
Once released, active IL-1β binds to the IL-1 receptor, leading to the activation of signaling pathways involving MAPK and nuclear factor–κB.27 Downstream effects of these pathways include the release of granulocyte-macrophage colony-stimulating factor, which is involved in the activation of M2 macrophages and MDSCs, leading to their intratumoral recruitment and promotion of tumor invasiveness, immune evasion, and malignant progression.23,28 Tumor cell–derived IL-1β has been found to induce abnormal differentiation of myeloid cells, immune suppression, accumulation of immature myeloid cells in the spleen, and leukocytosis.3 IL-1β also facilitates chemokine release and the expression of leukocyte adhesion molecules on the vascular endothelium, which lead to chemotaxis, angiogenesis, and increased cell adhesiveness.17,29 IL-1β is a potent inducer of the cyclooxygenase 2–PGE2 pathway, leading to immune suppression,30, 31, 32 invasion, epithelial-mesenchymal transition,33,34 apoptosis resistance,35 and angiogenesis.36 In NSCLC cells, IL-1β–mediated activation of the cyclooxygenase 2 pathway down-regulated the microRNA tumor suppressor miR-101, suggesting a role for IL-1β in the development of lung cancer.9 In addition, PGE2-mediated induction of c-Myc and the subsequent elevation in oncomiR-17-92 was found to contribute to apoptosis resistance in NSCLC cells.37
IL-1β polymorphisms that lead to IL-1β up-regulation may influence the level of reactive oxygen and nitrogen species in the lung epithelial microenvironment, which may invoke inflammatory-mediated induction of mutations in tumor suppressor genes such as TP53.20 The single-nucleotide polymorphisms G-1464C (rs1143623) and G-3893A (rs12621220) located in the enhancer region of the IL1B gene have been associated with TP53 mutations, increased levels of IL-1β, and increased risk of NSCLC.20,38 Two other single-nucleotide polymorphisms identified in the promoter region of IL1B, −511 (C > T; rs16944) and −31 (T > C; rs1143627), have also been associated with increased expression of IL-1β and increased risk of lung cancer, including NSCLC.20,38, 39, 40, 41
Long-term exposure to external factors such as asbestos, silica, cigarette smoke, and other inhaled toxins also results in a persistent pulmonary inflammatory response that can drive tumor promotion.23,42,43 Indeed, exposure to tobacco smoke is associated with increased levels of IL-1β,44 although this observation is not consistent across all studies.27,28
Preclinical studies have reported beneficial effects of IL1B knockdown and inhibition. In macrophages obtained from IL-1β–deficient mice, induction of inflammation or angiogenesis was not observed, and local tumor growth and lung metastases were absent in the IL-1β–deficient mice compared with wild-type mice.45 IL-1β inhibition using an anti–IL-1β antibody suppressed tumor progression and enhanced antitumor immunity in mice by limiting inflammation and inducing maturation of MDSCs into M1 macrophages.46
Clinical Agents Modulating the IL-1β Pathway
Several strategies are being used to inhibit IL-1 signaling in human disease, including antibodies directed against IL-1α, IL-1β, and the IL-1 receptor. Anakinra, a recombinant version of the naturally occurring IL-1 receptor antagonist is approved for the treatment of rheumatoid arthritis, cryopyrin-associated periodic syndromes (CAPS),47,48 and Still’s disease.48 It competitively inhibits the binding of IL-1α and IL-1β to the IL-1 receptor type 1 (Fig. 2).47 IL-1 receptor blockade by anakinra decreased tumor proliferation rate and improved median progression-free survival in patients with multiple myeloma (NCT00635154).49 In this setting, the hypothesis is that myeloma plasma cell–derived IL-1β induces marrow stromal cells to produce large amounts of IL-6, thereby promoting the survival and expansion of myeloma cells, highlighting the pleotropic effects of IL-1β.50
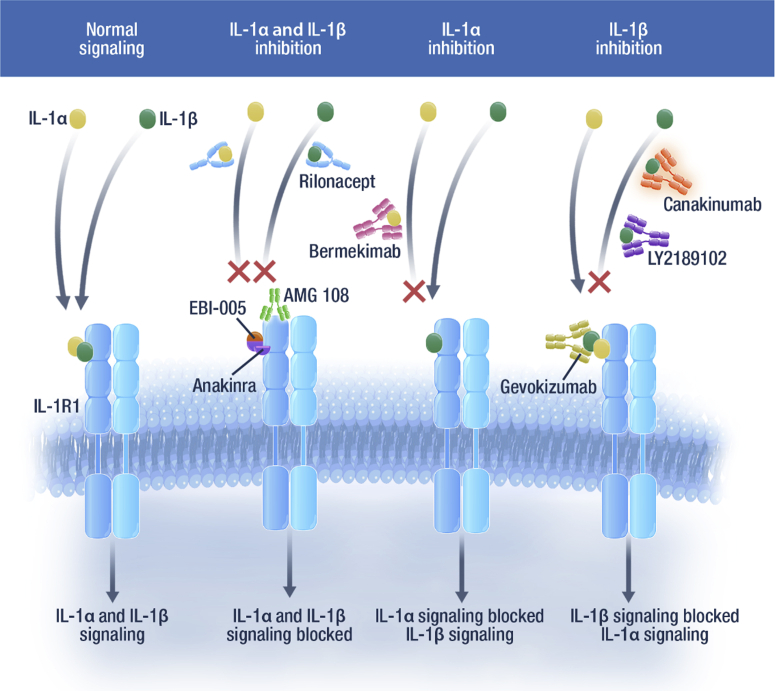
Figure 2.
Biologics that modulate the IL-1 pathway.54,82 The IL-1α and IL-1β signaling pathways can be inhibited by several biologics. Anakinra, a selective IL-1R1 antagonist, and rilonacept, a soluble decoy receptor, can inhibit the activity of both IL-1α and IL-1β. AMG 108 can bind to IL-1R1 to inhibit the activity of IL-1α and IL-1β. Protein chimera EBI-005 inhibits IL-1 signaling by binding to IL-1R1. Bermekimab is a monoclonal antibody that targets IL-1α. Canakinumab is an anti–IL-1β human monoclonal IgG1 antibody. Gevokizumab and LY2189102 are IL-1β neutralizing antibodies. IL-1β, interleukin 1 beta; IL-1α, interleukin 1 alpha; IL-1R, IL-1 receptor; IgG1, immunoglobulin G1.
Since the introduction of anakinra, two additional IL-1 targeted therapies have been approved. Rilonacept, approved in the United States, is a soluble decoy receptor (IL-1 trap) (Fig. 2) that is indicated for the treatment of CAPS, including familial cold autoinflammatory syndrome and Muckle-Wells syndrome, in adults and children 12 years and older.51 Canakinumab, an anti–IL-1β neutralizing monoclonal antibody that blocks binding to the IL-1 receptor (Fig. 2), is indicated for the treatment of several autoinflammatory periodic fever syndromes in adults, adolescents, and children, including CAPS (Muckle-Wells syndrome, neonatal-onset multisystem inflammatory disease, chronic infantile neurologic, cutaneous, articular syndrome, and familial cold autoinflammatory syndrome, familial cold urticaria), tumor necrosis factor receptor associated periodic syndrome, hyperimmunoglobulin D syndrome, mevalonate kinase deficiency, familial Mediterranean fever, and Still’s disease (including systemic juvenile idiopathic arthritis and adult-onset Still’s disease).52,53
The anti–IL-1β monoclonal antibody, gevokizumab, is being evaluated in a clinical trial for metastatic colorectal, gastroesophageal, and renal cancers.54,55 The safety and pharmacokinetics of another IL-1β neutralizing antibody, LY2189102, was evaluated in clinical trials for rheumatoid arthritis.54 AMG 108, a monoclonal antibody that inhibits IL-1α and IL-1β by binding to human IL-1R1, was investigated in patients with osteoarthritis and chronic obstructive pulmonary disease.54 EBI-005, a protein chimera of IL-1β and IL-1 receptor antagonists, modulates the IL-1β pathway by binding IL-1R1 and has been studied in ocular surface inflammatory diseases (Fig. 2).54
IL-1β as a Prognostic Marker in Cancer
A retrospective analysis of tumor tissue from patients with early-stage, surgically treated NSCLC reported that a high level of IL-1β was independently associated with 3-year mortality in NSCLC,56 suggesting a role for IL-1β and its downstream effectors as biomarkers in NSCLC and possibly other malignancies.
IL-6 is a downstream effector cytokine of IL-1β and is involved in inflammation and cancer through promotion of angiogenesis and tumor growth. These effects are mediated through IL-6–induced activation of the signal transducer and activator of transcription 3 and Nuclear Factor–κB pathways, which results in increased tumor cell proliferation, survival, angiogenesis, and invasion.57,58 IL-6 is also highly expressed in lung tumors, and increased serum levels have been associated with lung cancer risk. Data from a case-control study investigating the association between serum IL-6 levels and lung cancer revealed that the levels of IL-6 were increased in patients who had a diagnosis of lung cancer and in patients who developed lung cancer within 2 years of blood sample collection. Serum levels of IL-8 were also increased in patients with lung cancer, with increased levels identified up to 5 years before diagnosis. These findings suggest that IL-6 may be primarily involved in tumor progression, whereas IL-8 may have a role in tumor initiation and promotion.59
CRP, another downstream effector of IL-1β–induced signaling, is a well-known marker of chronic inflammation in several disease states and is widely used as a sensitive inflammatory biomarker in routine clinical practice, with baseline levels potentially indicative of chronic inflammation preceding lung cancer.60,61 In a nested case-control study from the prospective Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial, individuals with baseline circulating CRP levels in the highest quartile had a twofold increased risk of lung cancer compared with those with CRP levels in the lowest quartile (OR = 1.98, 95% CI: 1.35–2.89).62 A prospective population-based study of individuals who were aged 55 years or more also reported that high baseline CRP levels (> 3 mg/L) were associated with increased risk of incident cancer when compared with low levels (< 1 mg/L; hazard ratio [HR] = 1.4, 95% CI: 1.1–1.7), particularly lung cancer (HR = 2.8, 95% CI: 1.6–4.9).61 A study evaluating serum CRP levels in patients with NSCLC reported significantly higher levels in patients with metastatic NSCLC than in those with localized disease (p < 0.01).60 Elevated serum CRP levels may be useful for predicting tumor progression and poor prognosis in patients with NSCLC; high preoperative levels of CRP have been found to predict poor survival in patients undergoing resection for NSCLC.60
This evidence suggests that IL-1β and its downstream effectors (IL-6, IL-8, and CRP) may have a role as biomarkers and prognostic indicators in patients with lung cancer.
IL-1β Inhibition With Canakinumab
Canakinumab is a human immunoglobulin Gκ monoclonal antibody with high affinity and specificity for IL-1β (dissociation equilibrium constant, 35–40 pM).63 Canakinumab has been and is being studied in a large number of patients across various therapeutic areas, including rheumatoid arthritis, CAPS, chronic obstructive pulmonary disease, and atherosclerosis.10,64,65
CANTOS was a large, phase III, randomized, double-blind, placebo-controlled trial of patients with a history of myocardial infarction (MI) with atherosclerosis and was designed to investigate whether canakinumab could prevent recurrent cardiovascular events in patients with persistent pro-inflammatory responses. Adults (N = 10,061) with no previous cancer diagnosis (patients with previous basal cell skin carcinoma were allowed on the study) who had persistent blood high-sensitivity CRP (hsCRP) levels of greater than or equal to 2 mg/L were randomized to one of four treatment arms: canakinumab 50 mg, 150 mg, or 300 mg or placebo. Canakinumab was administered subcutaneously every 3 months. The primary end point was the first occurrence of nonfatal MI, nonfatal stroke, or cardiovascular death; study participants were also followed up prospectively for incident medical events, including cancer. At baseline, 2366 patients (24%) were current smokers, 4753 (47%) were past smokers, and the median hsCRP was 4.2 mg/L. Although primarily designed to evaluate cardiovascular events, an additional analysis was performed to evaluate if IL-1β inhibition with canakinumab may change cancer incidence, including lung cancers in a high-risk population (high hsCRP levels, previous MI, high rate of cigarette smoke exposure).10
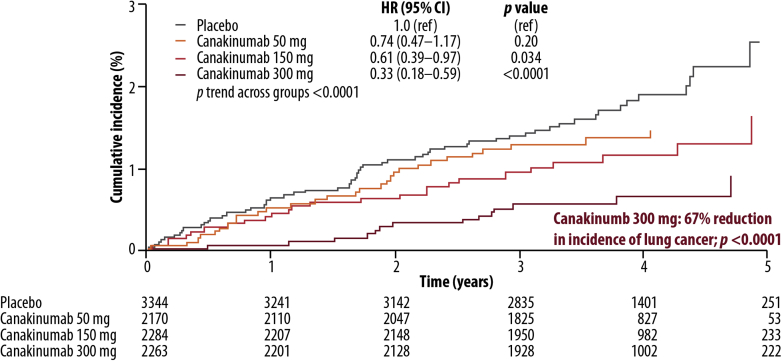
With a median follow-up of 3.7 years, lung cancer mortality was lower in patients treated with canakinumab than in patients in the placebo group (Fig. 3). Baseline median hsCRP level was significantly higher in patients who developed lung cancer than in those who did not (6.0 versus 4.2 mg/L, respectively; p < 0.0001); a similar pattern was seen with IL-6 levels (3.2 versus 2.6 ng/L, respectively; p < 0.0001). Canakinumab treatment resulted in reductions in cancer incidence and mortality rates overall compared with placebo. The rate of total cancer mortality was lower in the pooled canakinumab group than in the placebo group (p = 0.0158), and the treatment effects were dose-dependent, with significant reductions observed with the 300-mg dose (Table 2). The treatment effect on lung cancer incidence was particularly prominent in patients with greater reductions in hsCRP levels (HR = 0.29, p < 0.0001) and IL-6 levels (HR = 0.24, p < 0.0001) at 3 months.10
Figure 3.
Canakinumab Anti-Inflammatory Thrombosis Outcomes Study lung cancer incidence data. The incidence of lung cancer was lower in patients treated with canakinumab than in patients receiving placebo. The effects were dose-dependent, with a relative hazard reduction of 67% (p < 0.0001) for total lung cancer patients in the canakinumab 300-mg group. HR, hazard ratio. Reprinted with permission from Ridker et al.10
Table 2.
Cancer Mortality Rate in CANTOS
| 300-mg Dose Group | Rate Reduction |
|---|---|
| Cancer mortality | 51% (p = 0.0009) |
| Lung cancer incidence | 67% (p < 0.0001) |
| Lung cancer mortality | 77% (p = 0.0002) |
Although no significant benefit was observed in the incidence of other types of cancers, total cancer mortality was significantly reduced in the 300-mg group (Table 2). In the placebo group, lung cancer accounted for 26% of all cancers and 47% of cancer deaths, whereas in the canakinumab groups, lung cancer accounted for 16% of all cancers and 34% of cancer deaths.10
Because IL-1β levels have been linked to tumorigenesis and tumor progression in other cancer types,17 the observation that canakinumab was of benefit in lung cancer is of interest. The specific effects of canakinumab on lung cancer incidence and mortality may be driven by several factors. Most patients in CANTOS were current or past smokers (71%);10 lung cancer is the most common form of cancer worldwide,66 and its prevalence may have allowed for a more robust statistical comparison of treatment groups with placebo.10 In fact, 12.9% of global cancer incidence and 19.4% of global cancer mortality is attributed to lung cancer.66 In addition, screening programs for other highly prevalent cancers such as breast or prostate may have reduced the number of patients entering the study with undiagnosed tumors for these indications. By contrast, NSCLC has been reported to be most usually detected when the disease is already at an advanced stage.67 However, changes in screening methodology may improve this statistic. The National Lung Screening Trial enrolled former or heavy smokers from 2002 to 2004 with the objective of determining whether low-dose computed tomography (CT) or radiography could best detect early-stage lung cancer. Participants were followed until 2009, and the results revealed that low-dose CT resulted in higher rates of early-stage detection and potentially treatable NSCLC.68,69 Because the enrollment period for CANTOS extended from 2011 to 2014 with follow-up until mid-2017, enrollment to the National Lung Screening Trial or adoption of the findings into routine practice did not likely impact enrollment in CANTOS.10 Enrollment also coincided with a 2015 Centers for Medicare and Medicaid Services decision to allow low-dose CT lung cancer screening, but uptake of this screening tool is low in the CANTOS-eligible population.70
Although findings from CANTOS suggest that the use of canakinumab was associated with a reduced incidence of fatal and nonfatal lung cancers in patients with atherosclerosis (who had increased hsCRP and no previous cancer diagnoses), CANTOS was not formally designed with lung cancer as a prespecified end point. In addition, the proportions of occult, pre-existing lung cancers between treatment arms in CANTOS are unknown, as the participants did not undergo CT screening for lung cancer before study entry. The marked separation of the incidence curves for lung cancer and lung cancer mortality with the 300-mg dose during the early period of the trial suggests that canakinumab has an immediate therapeutic effect on established lung tumors, but the lack of baseline scans obfuscates further interpretation.10,71
Prospective Cancer Studies: Opportunities
Single-agent and combination studies of canakinumab in patients with NSCLC are being developed as a result of the findings from CANTOS. A phase III, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of single-agent canakinumab as adjuvant treatment versus placebo in patients with surgically resected NSCLC is currently recruiting (NCT03447769). Approximately 1500 patients will be randomized (1:1) to receive canakinumab 200 mg or placebo every 3 weeks for 18 cycles (≈54 w). The primary end point is disease-free survival, and the key secondary end point is overall survival. Other secondary end points are lung cancer-specific survival, safety, pharmacokinetics, immunogenicity, and patient-reported outcomes. The estimated primary completion date for this study is 2021.72,73
The combination of an anti-inflammatory agent such as canakinumab with established targeted therapies (e.g., EGFR inhibitors) also will be investigated. Preclinical data suggest that IL-1 blockade overcomes erlotinib resistance in head and neck squamous cell carcinoma.74 The complex interplay between immunity and inflammation makes the combination of IL-1β inhibitors with checkpoint inhibitors an attractive clinical research area in both early- and later-line settings. Results from CANTOS indicate that IL-1β inhibition is not sufficient to reduce elevated baseline levels of IL-6 and hsCRP in all patients and that the addition of other anti-inflammatory agents targeting overlapping signaling pathways may be a potential area for clinical exploration. Targeting the programmed cell death 1 (PD-1) protein checkpoint pathway has changed the treatment landscape of advanced NSCLC.75, 76, 77 A phase III study is investigating the effect of pembrolizumab plus platinum-based doublet chemotherapy with or without canakinumab in previously untreated patients with locally advanced or metastatic NSCLC (NCT03631199).78 The activity of combination therapies is also being investigated in a phase Ib study of an anti–PD-1 monoclonal antibody in combination with canakinumab or an anti–IL-17A monoclonal antibody (NCT02900664).79
CANTOS did not generate information on the molecular profile of the lung cancers observed in the study follow-up, and it is therefore unknown whether patients developed tumors that were positive for well-established oncogenic drivers such as EGFR, ALK, BRAF, or MET. In the same phase Ib study, anti–PD-1 therapy in combination with EGFR or MEK inhibitors are also being evaluated in patients with cancer (NCT02900664). The study is currently recruiting, and approximately 432 patients will be randomized with an estimated trial completion date of 2020.79
Patient Selection
With the advent of new agents and multiple combinatorial opportunities, patient selection takes on ever-increasing importance to best position patients to benefit from tailored treatment. Future studies will certainly include important pathologic details such as tumor histology, staging, molecular, and genetic profiling to help identify patient populations who stand to benefit the most from anti–IL-1β therapy. Combinations with radiation or other immunomodulating therapies may also be of interest.
Although elevated baseline levels of hsCRP and IL-6 were associated with a greater risk of lung cancer, not all patients with this profile derived benefit from canakinumab. In an analysis of combined canakinumab doses compared with placebo, the incidence of lung cancer was not significantly different between the two groups, in patients whose serum hsCRP concentrations were higher than the median of 1.8 mg/L at 3 months (p = 0.34).10 The sustained inflammation observed in some patients suggests redundancy between cytokine signaling pathways, whereby inhibition of one pathway is compensated for by another.
It is likely that a panel of biomarkers may be required to provide an overview of the inflammatory state of each patient, improve the predictive value of biomarker selection, and identify patients who are most likely to benefit from IL-1β inhibitor treatment. Outstanding questions remain regarding the overlap of biomarkers for inflammatory and immune status, given the role of IL-1β signaling in immunosuppression and the induction of MDSCs.3,46
Although data from KEYNOTE-001 (NCT01295827), KEYNOTE-010 (NCT01905657), and KEYNOTE-024 (NCT02142738) have reported that approximately 68% of patients with advanced NSCLC had programmed cell death 1 ligand 1 tumor proportional scores of greater than or equal to 1% and 28% had scores of greater than or equal to 50%,80 the proportion of patients with lung cancer who have elevated biomarkers of chronic inflammation and T-cell exhaustion remains unclear.
Conclusions
IL-1β signaling is involved in the inflammatory component of many disease states, including cancer. The role of inflammasomes and IL-1β is an emergent topic in lung cancer; high levels of IL-1β and its downstream effectors (IL-6, IL-8, and CRP) have been associated with lung cancer progression. Although therapies targeting IL-1 have been approved for the treatment of several inflammatory diseases, there are no approved IL-1 targeted therapies for lung cancer. Data from CANTOS have highlighted the possible benefit of IL-1β inhibition with canakinumab in patients with lung cancer. As a result, single-agent and combination studies of canakinumab in patients with NSCLC are underway and may establish IL-1 targeted therapies as new treatment options for patients with lung cancer.
Acknowledgments
This work was supported by Novartis Pharmaceuticals Corporation.
Dubinett was supported by the National Institutes of Health (NIH) National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881, VA Merit Review 1I01CX000345, NIH/National Cancer Institute (NCI) Molecular Characterization Laboratory 5U01CA196408, DOD W81XWH-17-1-0399, NCI Early Detection Research Network 1U01CA214182, and NCI Human Tumor Atlas Network: A Multi-Dimensional Atlas of Pulmonary Premalignancy 1U2CCA233238. Garon was supported by NIH/NCI R01 CA208403. Editorial support for this manuscript was provided by Ann Yeung, PhD, CMPP, and Rohini Roy, PhD (Scientific Pathways Inc., Warren, NJ), and funded by Novartis Pharmaceuticals Corporation.
Footnotes
Disclosure: Dr. Garon reports nonfinancial writing support from Novartis; grants from AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Genentech, Novartis, Merck, Iovance, Mirati, Dynavax, and Neon; and personal fund as an advisory board member and steering committee member for Novartis, Dracen, and EMD Serono. Dr. Yang reports personal fees from Boehringer Ingelheim, Eli Lilly, Bayer, Roche-Genentech, Chugai, Astellas, Merck Sharp & Dohne, Merck Serono, Pfizer, Novartis, Celgene, Merrimack, Yuhan Pharmaceuticals, Bristol-Myers Squibb, Ono Pharmaceuticals, Daiichi Sankyo, Hansoh Pharmaceuticals, Takeda, Blueprint Medicines, AstraZeneca, and G1 Therapeutics; has served on advisory boards for the aforementioned companies; and has received honorariums from Boehringer Ingelheim, Eli Lilly, Bayer, Roche-Genentech, Chugai, Merck Sharp & Dohne, Pfizer, Novartis, Bristol-Myers Squibb, Ono Pharmaceuticals, and AstraZeneca; Dr. Dubinett is an advisory board member for EarlyDx Inc., T-Cure Bioscience Inc., LungLifeAI, and the Johnson & Johnson Lung Cancer Initiative; reports personal fees from the Johnson & Johnson Lung Cancer Initiative; and has a patent Combination Immunotherapy and a patent Vault Immunotherapy issued.
References
- 1.Grivennikov S.I., Greten F.R., Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karki R., Kanneganti T.D. Diverging inflammasome signals in tumorigenesis and potential targeting. Nat Rev Cancer. 2019;19:197–214. doi: 10.1038/s41568-019-0123-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apte R.N., Dotan S., Elkabets M. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 2006;25:387–408. doi: 10.1007/s10555-006-9004-4. [DOI] [PubMed] [Google Scholar]
- 4.Porta C., Larghi P., Rimoldi M. Cellular and molecular pathways linking inflammation and cancer. Immunobiology. 2009;214:761–777. doi: 10.1016/j.imbio.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Altorki N.K., Markowitz G.J., Gao D. The lung microenvironment: an important regulator of tumour growth and metastasis. Nat Rev Cancer. 2019;19:9–31. doi: 10.1038/s41568-018-0081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamkanfi M., Dixit V.M. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Van Gorp H., Lamkanfi M. The emerging roles of inflammasome-dependent cytokines in cancer development. EMBO Rep. 2019;20 doi: 10.15252/embr.201847575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Multhoff G., Molls M., Radons J. Chronic inflammation in cancer development. Front Immunol. 2011;2:98. doi: 10.3389/fimmu.2011.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castro D., Moreira M., Gouveia A.M., Pozza D.H., De Mello R.A. MicroRNAs in lung cancer. Oncotarget. 2017;8:81679–81685. doi: 10.18632/oncotarget.20955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ridker P.M., MacFadyen J.G., Thuren T. Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:1833–1842. doi: 10.1016/S0140-6736(17)32247-X. [DOI] [PubMed] [Google Scholar]
- 11.Schett G., Dayer J.M., Manger B. Interleukin-1 function and role in rheumatic disease. Nat Rev Rheumatol. 2016;12:14–24. doi: 10.1038/nrrheum.2016.166. [DOI] [PubMed] [Google Scholar]
- 12.Di Paolo N.C., Shayakhmetov D.M. Interleukin 1α and the inflammatory process. Nat Immunol. 2016;17:906–913. doi: 10.1038/ni.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green D.R. The coming decade of cell death research: five riddles. Cell. 2019;177:1094–1107. doi: 10.1016/j.cell.2019.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kong H., Wang Y., Zeng X., Wang Z., Wang H., Xie W. Differential expression of inflammasomes in lung cancer cell lines and tissues. Tumour Biol. 2015;36:7501–7513. doi: 10.1007/s13277-015-3473-4. [DOI] [PubMed] [Google Scholar]
- 15.Shi J., Zhao Y., Wang K. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 16.de Mooij C.E.M., Netea M.G., van der Velden W., Blijlevens N.M.A. Targeting the interleukin-1 pathway in patients with hematological disorders. Blood. 2017;129:3155–3164. doi: 10.1182/blood-2016-12-754994. [DOI] [PubMed] [Google Scholar]
- 17.Idris A., Ghazali N.B., Koh D. Interleukin 1β—a potential salivary biomarker for cancer progression? Biomark Cancer. 2015;7:25–29. doi: 10.4137/BIC.S25375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakao S., Kuwano T., Tsutsumi-Miyahara C. Infiltration of COX-2-expressing macrophages is a prerequisite for IL-1 beta-induced neovascularization and tumor growth. J Clin Invest. 2005;115:2979–2991. doi: 10.1172/JCI23298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Libby P. Interleukin-1 beta as a target for atherosclerosis therapy: biological basis of CANTOS and beyond. J Am Coll Cardiol. 2017;70:2278–2289. doi: 10.1016/j.jacc.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landvik N.E., Hart K., Skaug V., Stangeland L.B., Haugen A., Zienolddiny S. A specific interleukin-1B haplotype correlates with high levels of IL1B mRNA in the lung and increased risk of non-small cell lung cancer. Carcinogenesis. 2009;30:1186–1192. doi: 10.1093/carcin/bgp122. [DOI] [PubMed] [Google Scholar]
- 21.Yi G., Liang M., Li M. A large lung gene expression study identifying IL1B as a novel player in airway inflammation in COPD airway epithelial cells. Inflamm Res. 2018;67:539–551. doi: 10.1007/s00011-018-1145-8. [DOI] [PubMed] [Google Scholar]
- 22.Rogliani P., Calzetta L., Ora J., Matera M.G. Canakinumab for the treatment of chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2015;31:15–27. doi: 10.1016/j.pupt.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 23.O’Callaghan D.S., O’Donnell D., O’Connell F., O’Byrne K.J. The role of inflammation in the pathogenesis of non-small cell lung cancer. J Thorac Oncol. 2010;5:2024–2036. doi: 10.1097/jto.0b013e3181f387e4. [DOI] [PubMed] [Google Scholar]
- 24.Karki R., Man S.M., Kanneganti T.D. Inflammasomes and cancer. Cancer Immunol Res. 2017;5:94–99. doi: 10.1158/2326-6066.CIR-16-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terlizzi M., Colarusso C., Popolo A., Pinto A., Sorrentino R. IL-1α and IL-1β-producing macrophages populate lung tumor lesions in mice. Oncotarget. 2016;7:58181–58192. doi: 10.18632/oncotarget.11276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sayan M., Mossman B.T. The NLRP3 inflammasome in pathogenic particle and fibre-associated lung inflammation and diseases. Part Fibre Toxicol. 2016;13:51. doi: 10.1186/s12989-016-0162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong J., Magun B.E., Wood L.J. Lung inflammation caused by inhaled toxicants: a review. Int J Chron Obstruct Pulmon Dis. 2016;11:1391–1401. doi: 10.2147/COPD.S106009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mortaz E., Henricks P.A., Kraneveld A.D., Givi M.E., Garssen J., Folkerts G. Cigarette smoke induces the release of CXCL-8 from human bronchial epithelial cells via TLRs and induction of the inflammasome. Biochim Biophys Acta. 2011;1812:1104–1110. doi: 10.1016/j.bbadis.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Slaats J., Ten Oever J., van de Veerdonk F.L., Netea M.G. IL-1β/IL-6/CRP and IL-18/ferritin: distinct inflammatory programs in infections. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stolina M., Sharma S., Lin Y. Specific inhibition of cyclooxygenase 2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. J Immunol. 2000;164:361–370. doi: 10.4049/jimmunol.164.1.361. [DOI] [PubMed] [Google Scholar]
- 31.Sharma S., Yang S.C., Zhu L. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005;65:5211–5220. doi: 10.1158/0008-5472.CAN-05-0141. [DOI] [PubMed] [Google Scholar]
- 32.Sharma S., Stolina M., Yang S.C. Tumor cyclooxygenase 2-dependent suppression of dendritic cell function. Clin Cancer Res. 2003;9:961–968. [PubMed] [Google Scholar]
- 33.Dohadwala M., Yang S.C., Luo J. Cyclooxygenase-2-dependent regulation of E-cadherin: prostaglandin E(2) induces transcriptional repressors ZEB1 and snail in non-small cell lung cancer. Cancer Res. 2006;66:5338–5345. doi: 10.1158/0008-5472.CAN-05-3635. [DOI] [PubMed] [Google Scholar]
- 34.Dohadwala M., Batra R.K., Luo J. Autocrine/paracrine prostaglandin E2 production by non-small cell lung cancer cells regulates matrix metalloproteinase-2 and CD44 in cyclooxygenase-2-dependent invasion. J Biol Chem. 2002;277:50828–50833. doi: 10.1074/jbc.M210707200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krysan K., Dalwadi H., Sharma S., Pold M., Dubinett S. Cyclooxygenase 2-dependent expression of survivin is critical for apoptosis resistance in non-small cell lung cancer. Cancer Res. 2004;64:6359–6362. doi: 10.1158/0008-5472.CAN-04-1681. [DOI] [PubMed] [Google Scholar]
- 36.Pold M., Zhu L.X., Sharma S. Cyclooxygenase-2-dependent expression of angiogenic CXC chemokines ENA-78/CXC Ligand (CXCL) 5 and interleukin-8/CXCL8 in human non-small cell lung cancer. Cancer Res. 2004;64:1853–1860. doi: 10.1158/0008-5472.can-03-3262. [DOI] [PubMed] [Google Scholar]
- 37.Krysan K., Kusko R., Grogan T. PGE2-driven expression of c-Myc and oncomiR-17-92 contributes to apoptosis resistance in NSCLC. Mol Cancer Res. 2014;12:765–774. doi: 10.1158/1541-7786.MCR-13-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eaton K.D., Romine P.E., Goodman G.E., Thornquist M.D., Barnett M.J., Petersdorf E.W. Inflammatory gene polymorphisms in lung cancer susceptibility. J Thorac Oncol. 2018;13:649–659. doi: 10.1016/j.jtho.2018.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zienolddiny S., Ryberg D., Maggini V., Skaug V., Canzian F., Haugen A. Polymorphisms of the interleukin-1 beta gene are associated with increased risk of non-small cell lung cancer. Int J Cancer. 2004;109:353–356. doi: 10.1002/ijc.11695. [DOI] [PubMed] [Google Scholar]
- 40.Marshall A.L., Christiani D.C. Genetic susceptibility to lung cancer--light at the end of the tunnel? Carcinogenesis. 2013;34:487–502. doi: 10.1093/carcin/bgt016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li C., Wang C. Current evidences on IL1B polymorphisms and lung cancer susceptibility: a meta-analysis. Tumour Biol. 2013;34:3477–3482. doi: 10.1007/s13277-013-0925-6. [DOI] [PubMed] [Google Scholar]
- 42.Lee J.M., Yanagawa J., Peebles K.A., Sharma S., Mao J.T., Dubinett S.M. Inflammation in lung carcinogenesis: new targets for lung cancer chemoprevention and treatment. Crit Rev Oncol Hematol. 2008;66:208–217. doi: 10.1016/j.critrevonc.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takahashi H., Ogata H., Nishigaki R., Broide D.H., Karin M. Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation. Cancer Cell. 2010;17:89–97. doi: 10.1016/j.ccr.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lugade A.A., Bogner P.N., Thatcher T.H., Sime P.J., Phipps R.P., Thanavala Y. Cigarette smoke exposure exacerbates lung inflammation and compromises immunity to bacterial infection. J Immunol. 2014;192:5226–5235. doi: 10.4049/jimmunol.1302584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dinarello C.A. Why not treat human cancer with interleukin-1 blockade? Cancer Metastasis Rev. 2010;29:317–329. doi: 10.1007/s10555-010-9229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carmi Y., Dotan S., Rider P. The role of IL-1beta in the early tumor cell-induced angiogenic response. J Immunol. 2013;190:3500–3509. doi: 10.4049/jimmunol.1202769. [DOI] [PubMed] [Google Scholar]
- 47.Kineret (anakinra) [package insert] Swedish Orphan Biovitrum AB; Stockholm, Sweden: 2018. [Google Scholar]
- 48.Kineret (anakinra) [summary of product characteristics] Swedish Orphan Biovitrum AB; Stockholm, Sweden: 2019. [Google Scholar]
- 49.Lust J.A., Lacy M.Q., Zeldenrust S.R. Induction of a chronic disease state in patients with smoldering or indolent multiple myeloma by targeting interleukin 1{beta}-induced interleukin 6 production and the myeloma proliferative component. Mayo Clin Proc. 2009;84:114–122. doi: 10.4065/84.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dinarello C.A. Targeting the pathogenic role of interleukin 1{beta} in the progression of smoldering/indolent myeloma to active disease. Mayo Clin Proc. 2009;84:105–107. doi: 10.4065/84.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arcalyst (rilonacept) [package insert] Regeneron Pharmaceuticals Inc.; Tarrytown, NY: 2008. [Google Scholar]
- 52.Ilaris (canakinumab) [package insert] Novartis Pharmaceuticals Corporation; East Hanover, NJ: 2012. [Google Scholar]
- 53.Ilaris (canakinumab) [summary of product characteristics] Novartis Pharmaceuticals Corporation; Huningue, France: 2019. [Google Scholar]
- 54.Kaneko N., Kurata M., Yamamoto T., Morikawa S., Masumoto J. The role of interleukin-1 in general pathology. Inflamm Regen. 2019;39:12. doi: 10.1186/s41232-019-0101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.ClinicalTrials.gov Gevokizumab with standard of care anti-cancer therapies for metastatic colorectal, gastroesophageal, and renal cancers. https://clinicaltrials.gov/ct2/show/NCT03798626
- 56.Millares L., Barreiro E., Cortes R. Tumor-associated metabolic and inflammatory responses in early stage non-small cell lung cancer: local patterns and prognostic significance. Lung Cancer. 2018;122:124–130. doi: 10.1016/j.lungcan.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 57.Ochoa C.E., Mirabolfathinejad S.G., Ruiz V.A. Interleukin 6, but not T helper 2 cytokines, promotes lung carcinogenesis. Cancer Prev Res (Phila) 2011;4:51–64. doi: 10.1158/1940-6207.CAPR-10-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dalwadi H., Krysan K., Heuze-Vourc’h N. Cyclooxygenase-2-dependent activation of signal transducer and activator of transcription 3 by interleukin-6 in non-small cell lung cancer. Clin Cancer Res. 2005;11:7674–7682. doi: 10.1158/1078-0432.CCR-05-1205. [DOI] [PubMed] [Google Scholar]
- 59.Pine S.R., Mechanic L.E., Enewold L. Increased levels of circulating interleukin 6, interleukin 8, C-reactive protein, and risk of lung cancer. J Natl Cancer Inst. 2011;103:1112–1122. doi: 10.1093/jnci/djr216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kanoh Y., Abe T., Masuda N., Akahoshi T. Progression of non-small cell lung cancer: diagnostic and prognostic utility of matrix metalloproteinase-2, C-reactive protein and serum amyloid A. Oncol Rep. 2013;29:469–473. doi: 10.3892/or.2012.2123. [DOI] [PubMed] [Google Scholar]
- 61.Siemes C., Visser L.E., Coebergh J.W. C-reactive protein levels, variation in the C-reactive protein gene, and cancer risk: the Rotterdam Study. J Clin Oncol. 2006;24:5216–5222. doi: 10.1200/JCO.2006.07.1381. [DOI] [PubMed] [Google Scholar]
- 62.Chaturvedi A.K., Caporaso N.E., Katki H.A. C-reactive protein and risk of lung cancer. J Clin Oncol. 2010;28:2719–2726. doi: 10.1200/JCO.2009.27.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dhimolea E. Canakinumab. MAbs. 2010;2:3–13. doi: 10.4161/mabs.2.1.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Church L.D., McDermott M.F. Canakinumab, a fully-human mAb against IL-1beta for the potential treatment of inflammatory disorders. Curr Opin Mol Ther. 2009;11:81–89. [PubMed] [Google Scholar]
- 65.Alten R., Gomez-Reino J., Durez P. Efficacy and safety of the human anti-IL-1β monoclonal antibody canakinumab in rheumatoid arthritis: results of a 12-week, phase II, dose-finding study. BMC Musculoskelet Disord. 2011;12:153. doi: 10.1186/1471-2474-12-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ferlay J., Soerjomataram I., Dikshit R. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 67.Walters S., Maringe C., Coleman M.P. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004–2007. Thorax. 2013;68:551–564. doi: 10.1136/thoraxjnl-2012-202297. [DOI] [PubMed] [Google Scholar]
- 68.Church T.R., Black W.C., Aberle D.R. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368:1980–1991. doi: 10.1056/NEJMoa1209120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aberle D.R., DeMello S., Berg C.D. Results of the two incidence screenings in the national lung screening trial. N Engl J Med. 2013;369:920–931. doi: 10.1056/NEJMoa1208962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Centers for Medicare & Medicaid Services Decision memo for screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439N) https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274&bc=AAAAAAAAQAAA&
- 71.Chabner B.A., Nabel C.S. Canakinumab and lung cancer: intriguing, but is it real? Oncologist. 2018;23:637–638. doi: 10.1634/theoncologist.2018-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.ClinicalTrials.gov Study of efficacy and safety of canakinumab as adjuvant therapy in adult subjects with stages AJCC/UICC v. 8 II-IIIA and IIIB (T >5cm N2) completely resected non-small cell lung cancer acronym: CANOPY-A (canopy-A) https://clinicaltrials.gov/ct2/show/NCT03447769
- 73.Paz-Ares L., Ardizzoni A., Ardizzoni A. The CANOPY program: canakinumab in patients (pts) with non-small cell lung cancer (NSCLC) J Clin Oncol. 2019;37(suppl 15) TPS9124–TPS9124. [Google Scholar]
- 74.Stanam A., Gibson-Corley K.N., Love-Homan L., Ihejirika N., Simons A.L. Interleukin-1 blockade overcomes erlotinib resistance in head and neck squamous cell carcinoma. Oncotarget. 2016;7:76087–76100. doi: 10.18632/oncotarget.12590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Keytruda. (pembrolizumab) [package insert] Merck & Co, Inc.; Whitehouse Station, NJ: 2018. [Google Scholar]
- 76.Opdivo. (nivolumab) [package insert] Bristol-Myers Squibb Company; Princeton, NJ: 2018. [Google Scholar]
- 77.Imfinzi. (durvalumab) [package insert] Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2017. [Google Scholar]
- 78.ClinicalTrials.gov Study of efficacy and safety of pembrolizumab plus platinum-based doublet chemotherapy with or without canakinumab in previously untreated locally advanced or metastatic non-squamous and squamous NSCLC subjects (CANOPY-1) https://clinicaltrials.gov/ct2/show/NCT03631199
- 79.ClinicalTrials.gov A study of PDR001 in combination with CJM112, EGF816, Ilaris® (canakinumab) or Mekinist® (trametinib) https://clinicaltrials.gov/ct2/show/NCT02900664
- 80.Aggarwal C., RAD F.E., Felip E. Prevalence of PD-L1 expression in patients with non-small cell lung cancer screened for enrollment in KEYNOTE-001, −010, and −024. Ann Oncol. 2016;27:359–378. [Google Scholar]
- 81.Sborgi L., Ruhl S., Mulvihill E. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016;35:1766–1778. doi: 10.15252/embj.201694696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kurzrock R., Hickish T., Wyrwicz L. Interleukin-1 receptor antagonist levels predict favorable outcome after bermekimab, a first-in-class true human interleukin-1α antibody, in a phase III randomized study of advanced colorectal cancer. Oncoimmunology. 2019;8:1551651. doi: 10.1080/2162402X.2018.1551651. [DOI] [PMC free article] [PubMed] [Google Scholar]