Abstract
The spread of next-generation sequencing enables clinicians to identify rare oncogene alterations, including MET exon 14 skipping mutation, in clinical practice for NSCLC. Several tyrosine kinase inhibitors for MET exon 14 skipping mutation such as capmatinib and tepotinib have elucidated their effectiveness. Only a few reports have suggested their efficacy against central nervous system lesions, especially leptomeningeal metastases. We here report a case of a patient with NSCLC with MET exon 14 skipping mutation and poor performance status salvaged by marked leptomeningeal metastases response to tepotinib. We further provide measures of plasma/cerebrospinal fluid concentrations of tepotinib and its cerebrospinal fluid penetration rate.
Keywords: Tepotinib, MET exon 14 skipping mutation, Leptomeningeal metastases, Non–small cell lung cancer, Case report
Introduction
Next-generation sequencing has been widely used in current clinical practice for NSCLC. We have more opportunities to identify rare oncogene alterations, including MET exon 14 skipping mutation, which accounts for 3% to 4% of NSCLC. Tepotinib and capmatinib, MET tyrosine kinase inhibitors (TKIs), revealed their efficacies,1,2 and they are currently approved for patients with NSCLC with MET exon 14 skipping mutation.
Central nervous system metastases, especially leptomeningeal metastases (LM), have a devastating impact on the prognosis of patients with NSCLC. The efficacy of cytotoxic chemotherapies for LM is quite limited, but TKIs for specific driver oncogene alterations, such as osimertinib and alectinib, have revealed their efficacies for LM in EGFR-mutant and ALK-fusioned NSCLC, respectively.3,4 However, little is known regarding the efficacy of MET TKIs for LM. We here report a case of a patient with NSCLC with MET exon 14 skipping mutation and poor performance status (PS), in whom tepotinib exerted a remarkable response for LM.
Case Presentation
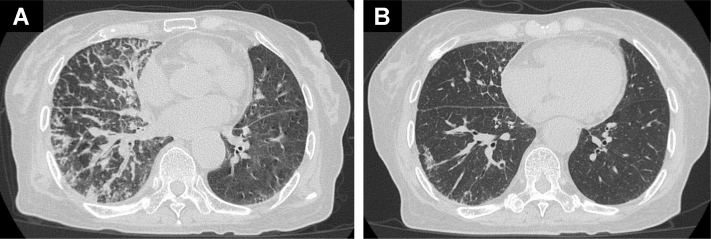
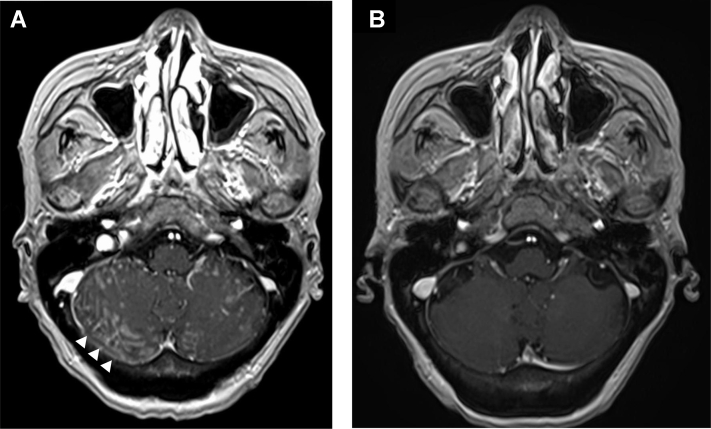
A 77-year-old woman was diagnosed as having metastatic lung adenocarcinoma with a mass on the left upper lobe of the lung, mediastinal lymph node, and multiple bone metastases. The tumor proportion score of programmed death-ligand 1 was more than 90% using the 22C3 antibody. Oncogenic driver alterations such as EGFR mutations, ALK fusions, and ROS1 rearrangements were negative, but MET exon 14 skipping mutation was positive by a multiplex polymerase chain reaction method. She then received pembrolizumab with carboplatin plus pemetrexed and subsequent pembrolizumab with pemetrexed as first-line treatment for 1 year. After progression, docetaxel plus ramucirumab was administered as second-line treatment for 6 months. During the therapy, she experienced loss of appetite and headache. Neck stiffness was observed on physical examination. Chest computed tomography revealed disease progression and pulmonary lymphangitis carcinomatosa (Fig. 1A). Brain magnetic resonance imaging (MRI) revealed high enhancement lesions along the surface and sulcus of the cerebellum, suggesting LM (Fig. 2A). She then received whole-brain radiotherapy (WBRT) at a dose of 30 Gy in 10 fractions. However, her central nervous system–related symptoms worsened and she developed other symptoms, such as stumbling and delirium with PS deterioration from 2 to 3. Owing to progressing general status before tepotinib prescription, it was difficult to perform an invasive examination such as lumbar puncture for cerebrospinal fluid (CSF) cytologic examination. On the basis of neck stiffness, neurologic symptoms, and definitive MRI findings, we diagnosed highly likely LM without CSF cytologic confirmation. Soon after tepotinib approval in Japan, we initiated tepotinib at 500 mg as third-line treatment. After 1 month of initiation of tepotinib, she experienced remarkable clinical improvement with regard to all of her neurologic symptoms, which led to improvement of PS from 3 to 1. The results of her chest computed tomography and brain MRI revealed dramatic response (Figs. 1B and 2B). We investigated plasma and CSF concentration of tepotinib after 2 weeks, 4 weeks, and 8 weeks of tepotinib initiation, using liquid chromatography-tandem mass spectrometry. Plasma/CSF concentrations were as follows: 4945 nM/59.3 nM (2 wk); 3784 nM/53.8 nM (4 wk); and 2869 nM/49.7 nM (8 wk). The CSF penetration rate of tepotinib in each period was estimated at 1.19%, 1.42%, and 1.73%, respectively. The response has continued 5 months after tepotinib initiation.
Figure 1.
(A) Chest CT result before tepotinib initiation. (B) Chest CT result 1 month after tepotinib initiation. CT, computed tomography.
Figure 2.
(A) Brain MRI result revealed high enhancement lesions along the surface and sulcus of the cerebellum (arrowhead) before tepotinib initiation. (B) Brain MRI result 1 month after tepotinib initiation. MRI, magnetic resonance imaging.
Discussion
To the best of our knowledge, this is the first report of NSCLC with MET exon 14 skipping mutation revealing marked LM response to tepotinib and providing measures of plasma/CSF concentrations of tepotinib and its CSF penetration rate. Tepotinib and capmatinib are recently approved MET inhibitors.1,2 There is a case report of leptomeningeal response to capmatinib after failure of crizotinib in a patient with MET exon 14–mutant NSCLC,5 but as for tepotinib, its activity against LM and its CSF penetration rate remains unclear. Our case found that its CSF penetration rate was approximately 1.2% to 1.7%. LM is a devastating situation in advanced cancers, including NSCLC. WBRT does not contribute to the prognosis of patients with NSCLC with LM6 and was not effective in our case either. Furthermore, conventional cytotoxic agents are ineffective for LM and are not indicated for such patients with poor PS. Only molecular-targeted agents have revealed potency against LM. Gefitinib has previously induced this “Lazarus response” of dramatic PS improvement in EGFR-mutant NSCLC.7,8 Thus, examination of driver oncogene alterations is extremely important to salvage patients with LM and poor PS using molecular-targeted agents. Further studies are needed to explore more effective TKIs against LM in patients with driver oncogene alterations.
Conclusions
We experienced a case of lung adenocarcinoma with MET exon 14 skipping mutation responding to tepotinib. The Lazarus response to tepotinib salvaged a patient with LM and poor PS. The case underlines the clinical importance of analyzing rare oncogene alterations to provide a clinical benefit from molecular-targeted agents.
Acknowledgments
The patient involved in this case report gave her informed consent authorizing use and disclosure of her health information.
Footnotes
Disclosure: Dr. Hata received personal fees and grants from Boehringer Ingelheim, Eli Lilly, and AstraZeneca; grants from Merck Sharp & Dohme; and personal fees from Chugai Pharmaceuticals. The remaining authors declare no conflict of interest.
Cite this article as: Ninomaru T, Okada H, Fujishima M, et al. Lazarus response to tepotinib for leptomeningeal metastases in a patient with MET exon 14 skipping mutation–positive lung adenocarcinoma: case report. JTO Clin Res Rep 2021;2:100145
References
- 1.Paik P.K., Felip E., Veillon R. Tepotinib in non-small-cell lung cancer with MET exon 14 skipping mutations. N Engl J Med. 2020;383:931–943. doi: 10.1056/NEJMoa2004407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolf J., Seto T., Han J.Y. Capmatinib in MET exon 14-mutated or MET-amplified non-small-cell lung cancer. N Engl J Med. 2020;383:944–957. doi: 10.1056/NEJMoa2002787. [DOI] [PubMed] [Google Scholar]
- 3.Nanjo S., Hata A., Okuda C. Standard-dose osimertinib for refractory leptomeningeal metastases in T790M-positive EGFR-mutant non-small cell lung cancer. Br J Cancer. 2018;118:32–37. doi: 10.1038/bjc.2017.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gainor J.F., Sherman C.A., Willoughby K. Alectinib salvages CNS relapses in ALK-positive lung cancer patients previously treated with crizotinib and ceritinib. J Thorac Oncol. 2015;10:232–236. doi: 10.1097/JTO.0000000000000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cravero P., Vaz N., Ricciuti B. Leptomeningeal response to capmatinib after progression on crizotinib in a patient with MET exon 14 Mutant non-small cell lung cancer. JTO Clin Res Rep. 2020;1:100072. doi: 10.1016/j.jtocrr.2020.100072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris P.G., Reiner A.S., Szenberg O.R. Leptomeningeal metastasis from non-small cell lung cancer: survival and the impact of whole brain radiotherapy. J Thorac Oncol. 2012;7:382–385. doi: 10.1097/JTO.0b013e3182398e4f. [DOI] [PubMed] [Google Scholar]
- 7.Inoue A., Kobayashi K., Usui K. First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy [published correction appears in J Clin Oncol. 2009;27:3071] J Clin Oncol. 2009;27:1394–1400. doi: 10.1200/JCO.2008.18.7658. [DOI] [PubMed] [Google Scholar]
- 8.Langer C.J. The “Lazarus response” in treatment-naive, poor performance status patients with non-small-cell lung cancer and epidermal growth factor receptor mutation. J Clin Oncol. 2009;27:1350–1354. doi: 10.1200/JCO.2008.20.4859. [DOI] [PubMed] [Google Scholar]