Abstract
The era of precision medicine has resulted in the identification of a number of genomic alterations that can be targeted with novel therapies. In lung adenocarcinomas, a histology structure that accounts for nearly 50% of all cases of lung cancer, and a number of genomic targets have been linked with effective targeted therapies. For patients with advanced-stage lung adenocarcinomas, molecular testing is now a standard part of diagnostic workup; for patients that have specific driver molecular events, targeted therapies have resulted in substantial improvement in efficacy without excessive toxicity.
RET gene fusions are present in approximately 1% to 2% of NSCLC. It is emerging as a new targetable driver for this population. Despite sensitivity to platinum-based chemotherapy and conflicting small reports regarding the efficacy of immune checkpoint inhibitors, there have been limited treatment approaches for this subset of patients. Multiple nonselective RET tyrosine kinase inhibitors exhibited modest anti-RET activity with an increased off-target toxicity profile that often required dose interruption, reduction, or treatment cessation. Recently, novel selective RET inhibitors pralsetinib (BLU-667) and selpercatinib (LOXO-292) have exhibited promising clinical activity with low adverse effect profile in early clinical trials. These new agents are poised to represent a new hope for this special subgroup with unmet needs.
Keywords: Lung cancer, Precision oncology, Pralsetinib, RET fusion, Selpercatinib, Targeted Therapy
Introduction
Targeted therapy has reshaped the care of patients with lung cancer with a specific molecular driver. Sensitizing EGFR 1 and BRAF V600E 2 mutations, ALK,3 ROS1,4, and NTRK 5,6 gene rearrangements have emerged as targetable molecular drivers in patients with lung cancer. The use of novel targeted therapies provides a robust response rate and progression-free survival (PFS). In the case of EGFR-targeted therapy, an improvement in overall survival (OS) has recently been reported with a third-generation tyrosine kinase inhibitor.1 Similarly, for patients with ALK gene rearrangement, second-generation ALK inhibitors are associated with a median PFS of longer tan 2 years.3,7 These agents are also associated with marked anticancer activity against brain metastasis, which is common in these molecular subsets of NSCLC. Consequently, a number of agents are being developed for various targets observed in patients with NSCLC. Among these, promising results have been observed with novel agents targeting RET fusion, MET exon 14 alteration,8 and KRAS G12C mutation9, 10, 11 in lung cancer.
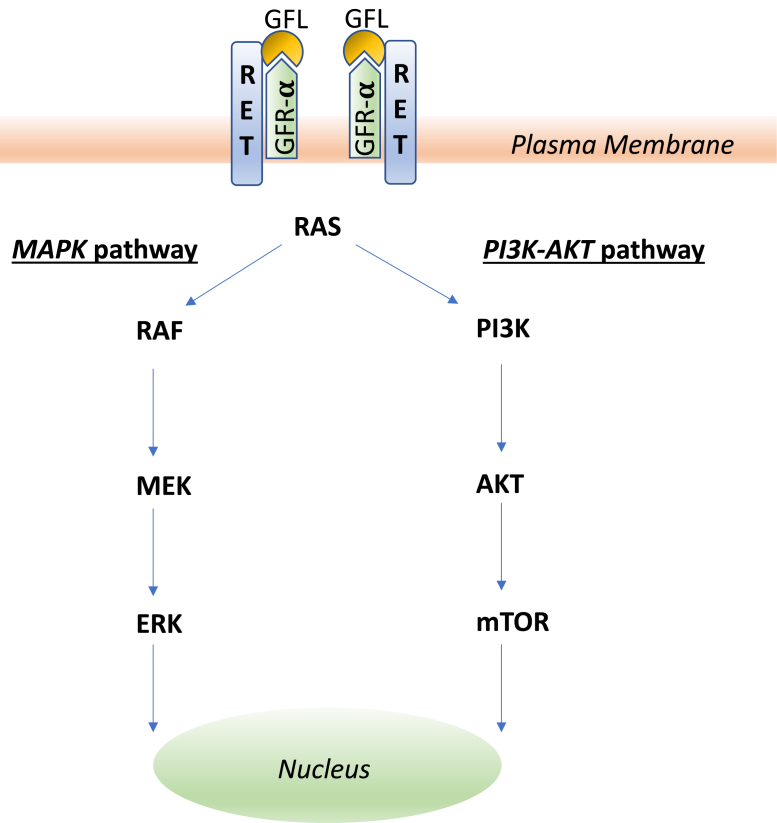
The RET-activating gene was originally identified in 1985.12 It encodes the transmembrane RET kinase; RET is activated when the glial cell line-derived neurotrophic factor family ligands binds to the RET coreceptor, glycosyl-phosphatidylinositol–anchored coreceptor (GFR-α).13 This leads to a signaling cascade that triggers the activation of downstream signals including MAPK and PI3K-AKT pathways (Fig. 1) and promotes cancer initiation and progression.14 In normal cells, RET kinase signaling is well-controlled. In cells with activating alterations of the RET gene, aberrant signaling leads to uncontrolled cell growth that eventually results in malignant transformation.15 RET is activated by two major mechanisms in cancer: RET fusions and RET point mutations. In RET fusions, owing to aberrant DNA repair processes, the RET gene is fused to another unrelated gene. KIF5B and CCDC6 are the most frequently reported RET fusion partners in patients with NSCLCs.16 These fusion partners can encode proteins that contain the coiled-coil domain, which causes RET fusion protein to dimerize allowing for constitutive ligand-independent RET activation. In addition to RET fusions, activating RET point mutations can also lead to constitutive ligand-independent RET signaling.
Figure 1.
RET signaling diagram. GFL, glial cell line-derived neurotrophic factor family ligands; GFR-α, glycosyl-phosphatidylinositol-anchored coreceptor.
RET gene fusions have been reported in 1% to 2% of NSCLC and in 10% to 20% of sporadic papillary thyroid cancer.16, 17, 18, 19, 20 Other cancer types like breast cancer, colorectal cancer, and pancreatic cancer are also known to harbor activating RET fusions at a lower frequency (<1%). In addition, approximately 60% sporadic medullary thyroid cancer (MTC) and greater than 90% of hereditary MTC harbor an activating intracellular or extracellular RET mutation.
Although no therapy that selectively targets RET in NSCLC is currently approved, clinical trials that focus on RET-altered cancers are ongoing. In this review, we discuss the biology, clinical characteristics, emerging treatment options, and mechanisms for acquired resistance for patients with RET-positive NSCLC.
Characteristics of Patients With RET Fusion–Positive NSCLC
The characteristics and outcomes of patients with RET fusion–positive NSCLC were presented by Gautschi et al.16 from the Global Multicenter RET Registry (GLORY), the largest and international registry of 165 patients identified by a global network of thoracic oncologists. RET rearrangements were identified by reverse transcriptase–polymerase chain reaction (RT-PCR), fluorescence in situ hybridization (FISH), or next-generation sequencing (NGS). The median age of patients was 61 years (range, 29–89), and most patients were never-smokers (63%), with lung adenocarcinomas (98%). Most patients had the advanced-stage disease (stage III-IV) (91%). The most frequent rearrangements were KIF5B-RET (72%) and CCDC6-RET (23%). NCOA4 (2%), EPHA4 (1%), and PICALM (1%) were uncommon partners. It is not known whether there are any biological differences in downstream signaling on the basis of the RET fusion partner. Most patients were from the United States and Europe (86%) with a modest representation of Asian patients (16%). A total of 53 patients (32%) with RET-rearranged lung cancers received a RET inhibitor during the course of their therapy. All patients had advanced disease (stage III or IV). Apart from staging (p = 0.004), their clinical characteristics were similar to patients who were not treated with a RET inhibitor.
RET-Directed Therapies in Lung Cancer
RET-positive NSCLC seems to be sensitive to platinum-based chemotherapy. There are limited data on the role of cytotoxic therapy in patients with RET-positive NSCLC. Gautschi et al.16 described a cohort of 108 patients treated with first-line chemotherapy for advanced-stage disease. In this posthoc analysis, the median PFS was 6.6 months (95% confidence interval [CI]: 5.1–9.3), median OS was 23.6 months (95% CI: 13.6–30.8), and the best response rate was 52% (95% CI: 39.8–64.4). Out of the 108 patients, 54 were treated with platinum-doublet chemotherapy. These results were comparable with the outcome with systemic chemotherapy in other oncogene-addicted NSCLC subsets.21,22 Therefore, platinum-based chemotherapy is a rational treatment approach for RET-positive NSCLC; before the emergence of selective RET inhibitors, systemic chemotherapy has remained the standard first-line therapy for this subset of patients. In a multicenter retrospective study of stage IIIB or IV patients with RET fusion–positive NCSLC adenocarcinomas, Shen et al.23 found an increase of median PFS in patients who received pemetrexed-based chemotherapy compared with those who received other regimens in the first-line setting (N = 40; 9.2 versus 5.2 mo; p = 0.007). There was no statistical difference in PFS between patients with KIF5B and non-KIF5B fusions. Drilon et al.24 found that similar patients achieved an overall response rate (ORR) of 45%, and a median PFS of 19 months with pemetrexed-based chemotherapy (N = 18).
The potential efficacy of immune checkpoint inhibitors (ICIs) in this population has not been tested prospectively. With the aim to address immune therapy efficacy in the context of driver mutation in a retrospective study (IMMUNOTARGET registry), Mazieres et al.25 found that 16 patients with RET fusion–positive NSCLC had a lower response rate and shorter PFS than 271 patients with KRAS mutation (6% versus 26% and 2.1 versus 3.2 mo, respectively). In another retrospective study, Hegde et al.26 found that 12 patients positive with RET fusion cancer treated with ICI had a shorter time to progression than 21 patients positive with RET fusion cancer treated with non-ICI (3 versus 8.3 mo, respectively; hazard ratio 1.73 [0.70, 4.26], p = 0.25). In a series of 74 patients with RET fusion–positive NSCLC, Offin et al.27 found that most of these patients (81%) had low programmed death ligand-1 (less than 50%) and a low tumor mutation burden score (median 1.75 mutations/Mb). The median PFS was 3.4 months (95% CI: 2.1–5.6). There was no association between PFS and programmed death ligand-1 or tumor mutation burden. No response to ICI was observed. In contrast, Guisier et al.28 reported that ICIs used beyond the first line was effective in nine patients with RET-positive NSCLC with a response rate of 37.5%, disease control rate (DCR) of 62.5%, median PFS of 7.6 months (95% CI: 2.3–not reached [NR]), and the 12-month OS rate of 88.9% (95% CI: 70.6–100%). The low number of therapies before ICI (median 1), and the local evaluation of tumor response, which might lead to overestimation, could explain why Guisier’s study had better outcomes compared with others. From these limited experiences, it does not seem that immune checkpoint inhibition is an effective therapy for RET-positive NSCLC. Future studies combining chemotherapy and ICI are warranted.
When the first reports of RET fusions in NSCLC emerged in 2012,15,19,29,30 clinical trials were launched with multikinase inhibitors such as cabozantinib,17 vandetanib,31, 32, 33 lenvatinib,34 and sunitinib16 that also inhibit RET. These agents have revealed modest anti-RET activity with an increased off-target toxicity profile that often required dose interruption, reduction, or treatment cessation. The increased toxicity is because of stronger inhibition of other targets such as VEGFR and EGFR inhibition and unfavorable pharmacokinetic profile for use in this setting (Table 1). However, the emergence of a new generation of highly selective RET inhibitors has revealed robust clinical results with favorable toxicity profiles (Table 2).
Table 1.
Summary of Studies With MKI for the Treatment of Patients With RET Fusion–Positive NSCLC
| Author | MKI | N | Detection Method (Tissue) | ORR, % (n; 95% CI) | PFS (Range) | OS (Range) | Grade 3–4 TRAE, % |
|---|---|---|---|---|---|---|---|
| Retrospective study | |||||||
| Gautschi et al.201716 | Cabozantinib | 21 | FISH, PCR, NGS | 37 (7; 16.3–61.5) | 3.6 (1.3–7.0) | 4.9 (1.9–14.3) | Nr |
| Vandetanib | 11 | 18 (2; 2.3–51.8) | 2.9 (1.0–6.4) | 10.2 (2.4–NR) | |||
| Sunitinib | 10 | 22 (2; 2.8–60.0) | 2.2 (0.7–5.0) | 6.8 (1.1– NR) | |||
| Prospective studies—phase 2 | |||||||
| Drilon et al.201617 | Cabozantinib | 26 | FISH, NGS | 28 (7; 12–49) | 5.5 (3.8– 8.4) | 9.9 (8.1–NR) | 69 |
| Lee et al.201731 | Vandetanib | 18 | FISH, PCR, NGS | 18 (3; Nr) | 4.5 | 11.6 | 28 |
| Yoh et al.201732 | Vandetanib | 19 | FISH, PCR | 47 (9; 28–77) | 4.7 (2.8– 8.5) | 11.1 (9.4–NR) | >58 |
| Hida et al.201934 | Lenvatinib | 25 | NGS | 16 (4; 4.5–36.1) | 7.3 (3.6– 10.2) | NR | 92 |
| PROSPECTIVE STUDIES – Phase 1b | |||||||
| Drilon et al.201936 | RXDX-105 | 31 | NGS | 19 (6; 8–38) | Nr | Nr | Nr |
CI, confidence interval; FISH, fluorescence in situ hybridization; MKI, multikinase inhibitor; NGS, next-generation sequencing; NR, not reached; Nr, not reported; ORR, objective response rate; OS, overall survival; PCR, polymerase chain reaction; PFS, progression-free survival; TRAE, treatment-related adverse event.
Table 2.
Summary of Phase 2 Clinical Trials With Selective RET Kinase Inhibitor for the Treatment of Patients with RET Fusion–Positive NSCLC
| Author | RET Inhibitor | N | Platinum Exposed, N (%) | Detection Method (tissue) | ORR, % (95% CI; n) | PFS (Range) | DCR, % | Grade 3–4 TRAE, % |
|---|---|---|---|---|---|---|---|---|
| Gainor et al.201940 | Pralsetinib | 57 | 30 (53) | Nr | 56 (32; -) | Nr | 91 | 28 |
| Drilon et al.201941 | Selpercatinib | 105 | 105 (100) | PCR, NGS | 68 (71; 58–76) | 18.4 (Nr) | 94 | Nr |
CI, confidence interval; DCR, disease control rate; NGS, next-generation sequencing; Nr, not reported; ORR, objective response rate; PFS, progression-free survival; OS, overall survival; PCR, polymerase chain reaction; TRAE, treatment-related adverse event.
Nonselective Multikinase Inhibitors
In the retrospective study of the GLORY registry (Table 1),16 53 patients (32%) with RET-positive advanced NSCLC were treated with one or more multikinase inhibitors: cabozantinib (21 patients), vandetanib (11 patients), sunitinib (10 patients), sorafenib (2 patients), alectinib (2 patients), lenvatinib (2 patients), nintedanib (2 patients), ponatinib (2 patients), and regorafenib (1 patient). The ORR to cabozantinib, vandetanib, and sunitinib were 37%, 18%, and 22%, respectively. Objective responses were also observed with lenvantinib and nintedanib. However, the median PFS was relatively modest at 2.3 months (95% CI: 1.6–5.0), and the median OS was only 6.8 months (95% CI: 3.9–14.3).
Cabozantinib
Cabozantinib inhibits RET, VEGFR2, ROS1, MET, AXL, TIE2, and KIT; it is approved for the treatment of renal cell carcinoma and thyroid cancers. It has also revealed clinical activity in patients with advanced NSCLC.35 It is administered orally at a standard dose of 60 mg/day for renal cancer and 140 mg/day for MTC. Dose reduction is often necessary to manage adverse events associated with cabozantinib.
In a phase 2 clinical trial, Drilon et al.17,31 evaluated the efficacy of cabozantinib in 26 patients with RET fusion–positive NSCLC. The median age was 59 years; most participants were women (58%) and never-smokers (65%); 32% had brain metastases. Among 25 patients evaluable for efficacy, seven had a partial response (PR) (ORR 28%, primary end point). The median PFS was 5.5 months (95% CI: 3.8–8.4), and the median OS was 9.9 months (95% CI: 8.1–NR). Although responses were observed in 20% of patients with KIF5B-RET, none was observed in patients with CCDC6-RET. Dose reduction for cabozantinib was done for 73% of patients; the most common treatment-related adverse events (TRAEs) were grade 1 or 2 in severity. The most common grade 3 TRAE included asymptomatic elevation of serum lipase (15%), the elevation of alanine aminotransferase (ALT) (8%) and aspartate aminotransferase (AST) (8%), thrombocytopenia (4%), and hypophosphatemia (4%). No grade 4 or 5 TRAE was reported. Cabozantinib was discontinued in 8% of patients for retroperitoneal hemorrhage (4%) and thrombocytopenia (4%).
Vandetanib
Vandetanib inhibits RET, EGFR, and VEGFR; it is administered orally at a dose of 300 mg/day. In a phase 2 clinical trial, Lee et al.31 evaluated the efficacy of vandetanib in 18 patients with RET fusion–positive NSCLC (selected by FISH assay) who had received previous platinum-doublet chemotherapy. The median age was 56 years, with a higher proportion of men (67%) and never-smokers (61%). Among 17 evaluable patients, three had PR (ORR 18%, primary end point), eight had stable disease (SD) (DCR 65%); clinical benefit beyond 6 months was observed in eight patients (73%). The median PFS was 4.5 months (6-mo PFS rate 44%), and the median OS was 11.6 months (12-mo OS rate was 33%). The most common TRAE included hypertension (89%), rash (72%), diarrhea (44%), acne (28%), and xerosis (22%). No grade 4 or 5 TRAEs were reported.
In another multicenter phase 2 clinical trial of 19 patients with previously treated RET-rearranged NSCLC (LURET), Yoh et al.32 observed objective responses in nine of the 19 intention-to-treat patients with vandetanib (47%); the median PFS was 4.7 months (95% CI: 2.8–8.5) and the median OS was 11.1 months. RET was detected by RT-PCR and confirmed with the FISH assay. In a subset of six patients with CCDC6-RET fusion, ORR, the median PFS, 12-month OS were more favorable (83%, 8.3 mo, 67%, respectively) than the 10-patient subgroup with KIF5B-RET fusion (20%, 2.9 mo, 42%, respectively).
Lenvatinib
Lenvatinib, an inhibitor of RET, VEGFR, and FGFR, is approved for the treatment of thyroid cancer, renal cell cancer, and hepatocellular carcinoma. It is administered orally at a standard dose of 24 mg/day. In a phase 2 clinical trial, Hida et al.34 evaluated the efficacy of lenvatinib in 25 patients with RET fusion–positive NSCLC. The median age was 63 years; most patients were women (72%), never-smokers (56%), and had received previous chemotherapy (92%). KIF5B-RET was present in 52% of the patients. The ORR was relatively modest at 16% (95% CI: 4.5–36.5). The median PFS was 7.3 months (95% CI: 3.6–10.2), and the median OS was not reached. The response rate, median PFS, and 12-month OS were more favorable in patients (N = 12) with CCDC6-RET fusion (16.7%, 9.1 mo, 66.7%, respectively) than patients (N = 13) with KIF5B-RET fusion (15.4%, 3.6 mo, 40.4%, respectively). Grade 3 and 4 TRAEs were observed in 92% of patients: hypertension (56%), hyponatremia (20%), pneumonia (16%), nausea (12%), vomiting (8%) diarrhea (8%), fatigue (8%), and proteinuria (16%). One patient had a grade 5 TRAE (pneumonia).
RXDX-105
In a phase 1b cohort with 31 RET fusion–positive, RET inhibitor–naive NSCLC,36 RXDX-105, a VEGFR-sparing potent RET and BRAF inhibitor, revealed activity in non–KIF5B-RET (ORR 67%, n = 6 of 9) but not in KIF5 (ORR 0%, n = 0 of 20) RET fusion partner. RXDX-105 had a manageable safety profile. The most common severe grade TRAEs were hypophosphatemia (9%), elevated ALT (8%), maculopapular rash (7%), elevated AST (5%), and diarrhea (5%). No update on this agent's future development plan.
Alectinib
Alectinib is currently used for the treatment of ALK-positive NSCLC; it has a good tolerability profile and high central nervous system activity. In addition to ALK inhibition, alectinib also inhibits RET. The anticancer effects of alectinib in RET-positive NSCLC have been reported in xenograft models. In a small cohort of six patients with RET fusion–positive NSCLC, two objective responses were observed.16,37 The ALERT-lung is an ongoing single-arm, phase 2 trial investigating the efficacy of alectinib in patients with advanced-stage RET-positive NSCLC treated with at least one platinum-based chemotherapy (Clinicaltrials.gov identifier: NCT03445000). Alectinib was also active in resistant RET gatekeeper mutations (RET V804L and V804M).38 A list of ongoing clinical trials targeting RET-positive NSCLC is summarized in Table 3.
Table 3.
Summary of Ongoing Clinical Trials With RET Kinase Inhibitor for the Treatment of Patients With RET Fusion–Positive NSCLCa
| Trials | Status | RET Inhibitor | ClinicalTrials.gov Identifier |
|---|---|---|---|
| AcceleRET lung study of pralsetinib for the first-line RET fusion–positive, metastatic NSCLC (phase 3) | Recruiting | Pralsetinib (BLU-667) | NCT04268550 |
| Phase 1/2 study of the highly selective RET inhibitor, pralsetinib (BLU-667), in patients with thyroid cancer, NSCLC, and other advanced solid tumors | Recruiting | Pralsetinib (BLU-667) | NCT04222972 |
| Phase 1/2 study of the highly selective RET inhibitor, pralsetinib (BLU-667), in patients with thyroid cancer, NSCLC, and other advanced solid tumors | Recruiting | Pralsetinib (BLU-667) | NCT03037385 |
| Targeted treatment for RET fusion–positive advanced NSCLC cancer (a LUNG-MAP treatment trial) | Recruiting | Selpercatinib (LOXO-292) | NCT03037385 |
| Phase 1/2 study of LOXO-292 in patients with advanced solid tumors, RET fusion–positive solid tumors, and MTC | Recruiting | Selpercatinib (LOXO-292) | NCT03157128 |
| A study of Selpercatinib (LY3527723) in participants with advanced or metastatic RET fusion–positive NSCLC (LIBRETTO-431; phase 3) | Recruiting | Selpercatinib (LOXO-292) | NCT04194944 |
| Study of TPX-0046, A RET/SRC inhibitor in adult subjects with advanced solid tumors harboring RET fusions or mutations | Recruiting | TPX-0046 | NCT04161391 |
| ALEctinib for the treatment of pretreated RET-rearranged advanced NSCLC | Recruiting | Alectinib | NCT03445000 |
MTC, medullary thyroid cancer.
Details of this table were accessed on April 28, 2020.
The lower activity seen with nonselective RET inhibitors is possibly owing to high half-maximal inhibitory concentration leading to suboptimal RET kinase inhibition; inhibition of concomitant targets such as VEGFR also contributes to toxicity, limiting their long-term use.
Another reason for the lower efficacy of nonselective RET inhibitors is that most studies included heavily-treated patients. Their tumors were more likely to develop mechanisms of resistance to RET inhibition by multikinase inhibitors that remained not well understood. Preclinical studies38,39 revealed that RET gatekeeper mutations and EGFR/VEGFR pathway activation may drive this resistance that may require combination therapy to improve outcomes. Future trials with tissue sampling and liquid biopsy before and after RET therapy are needed to understand the mechanisms of resistance to RET inhibition. Combination therapies that build on selective RET inhibitors may provide a more tolerable option to improve the clinical outcomes of these patients.
New Selective RET-Targeted Therapies (Table 2)
New selective RET-targeted therapies are summarized in Table 2.
Pralsetinib (BLU-667)
Pralsetinib, a highly selective and potent oral RET inhibitor, is currently under investigation in patients with RET-positive NSCLC. A global phase 1/2 clinical trial (ARROW) is evaluating the safety and efficacy of pralsetinib for the treatment of patients with RET fusion–positive refractory solid tumors.40 In the dose-escalation phase (phase 1), the recommended phase 2 dose (RP2D) was established at 400 mg/day. In the expansion phase (phase 2), patients were enrolled in cohorts on the basis of tumor type, previous therapies, and RET alterations with response rate and safety as primary end points.
The primary analysis of the registrational data included the 79 enrolled patients with RET fusion–positive NSCLC: 21 in the dose-escalation and 58 in the dose-expansion. The most common fusion partners were KIF5B (56%) and CCDC6 (20%). Approximately 40% of the patients had brain metastases. Patients had received a median of two previous treatment regimens for their disease and 41% were previously treated with ICI; nearly 30% had previously received a multikinase inhibitor.
Among 57 patients evaluable for response, the objective response rate was 56% (95% CI: 42%–69%): six patients (19%) had achieved response duration longer than or equal to 6 months; 26% had SD; the overall DCR was 91%. Over 90% of the 32 responding patients remain on treatment (December 2018). Among the 30 platinum-exposed patients treated at RP2D, the response rate was 60% with 18 PR. Objective responses were also noted in the brain of seven of nine patients (78%) with measurable brain disease, providing evidence of activity against brain metastases. Responses did not differ on the basis of previous therapy, fusion partner, or brain metastases.
Pralsetinib was tolerated well with most adverse events of grade 1 or 2 in severity; 28% of the patients experienced greater than or equal to grade 3 toxicity. The most common all-grade TRAE included elevation of AST (22%), hypertension (18%), elevation of ALT (17%), constipation (17%), fatigue (15%), and neutropenia (15%); severe grade hypertension (10%). Only 7% of patients with NSCLC discontinued therapy owing to treatment-related toxicity at the RP2D. Pralsetinib was granted breakthrough therapy designation by the U.S. Food and Drug Administration (FDA) in 2018 after initial data from the ARROW study revealed broad and durable antitumor activity and was well tolerated in patients with RET fusion–positive NSCLC that progressed after platinum-based chemotherapy.
Pralsetinib is to be compared with the combination of platinum and pemetrexed with or without pembrolizumab in LIBRETTO-431, a randomized phase 3 trial in patients with treatment-naive RET fusion–positive NSCLC with PFS as the primary end point (Table 3).
Selpercatinib (LOXO-292)
Selpercatinib is another highly selective RET inhibitor that has revealed promising activity in RET-positive NSCLC. Drilon et al.41 presented data from the clinical trial “LOXO-292 investigated to block RET-altered tumors” (LIBRETTO-001) at the 2019 World Conference on Lung Cancer (2019). In the dose-escalation phase (phase 1), the RP2D was established at 160 mg given orally twice daily. In the expansion phase (phase 2), patients were enrolled in various cohorts on the basis of tumor type, previous therapies, and RET alterations. The primary end point was objective response rate; the duration of response (DOR), PFS, and safety were secondary end points.
The primary analysis of the registrational data included the first 105 enrolled patients with RET fusion–positive NSCLC previously treated with platinum-based therapy. The median age was 61 years, 59% were women, and 35% had brain metastases. The median number of previous systemic therapies was three (1–5). Most patients (55%) had previously received treatment with an ICI; 48% had previously received one or more multikinase inhibitor therapy.
The response rate was 68% (95% CI: 58%–76%); 26% had SD, and DCR was 94%. Only 2% had progressive disease as best response, and the remaining were not evaluable for response. Responses did not differ on the basis of the type or number of previous therapies, or the fusion partner. In the subset of 11 patients with measurable brain metastases at baseline, 10 patients (91%) had an intracranial objective response (95% CI: 59%–100%), with a DCR of 100%. One patient with heavily pretreated brain metastases achieved a clinical response within the first week of selpercatinib for her leptomeningeal metastases followed by a resolution on imaging at week 8.42 With a median follow-up of 8 months, the median DOR, and PFS were 20.3 months (95% CI: 13.8–24) and 18.4 months (95% CI: 12.9–24.9), respectively. In patients (N = 39) who received selpercatinib as first-line therapy, the response rate was 85% (95% CI: 69–91), and 9% had SD (DCR 94%). Median DOR and PFS were not reached (follow-up duration 4.8 and 3.7 mo, respectively). For both groups combined, the most common fusion partners were KIF5B (53%) and CCDC6 (22%).
Selpercatinib was tolerated well; only nine patients (8.5%) discontinued therapy owing to treatment-related toxicity. The most common all-grade treatment-emergent adverse events in the primary analysis set were: dry mouth (32%), diarrhea (31%), hypertension (29%), increased AST (28%), increased ALT (26%), fatigue (24%), constipation (22%), headache (20%), nausea (19%), peripheral edema (19%), and increased creatinine (18%). The most common grade 3 to 4 was hypertension (14%). No other severe grade treatment-emergent adverse event occurred in more than 8% of patients.
Selpercatinib was granted breakthrough therapy designation by the FDA in 2018 after initial data from the clinical trial LIBRETTO-001, which revealed robust antitumor activity against several diverse RET fusions and brain metastases and also strong evidence of durability in a population with an unmet need, which are patients with RET fusion–positive NSCLC.
Selpercatinib will be compared with the first line to platinum-based chemotherapy in AcceleRET lung, a randomized phase 3 trial in patients with RET fusion–positive NSCLC with PFS as the primary end point (Table 3).
Comparing RET inhibitors' safety profile (Table 4), hypertension is reported but less frequently with the selective RET inhibitors compared with the other RET multikinase inhibitors. This is likely owing to the high degree of similarity between RET and VEGR kinases, which renders avoiding VEGFR inhibition challenging. In addition, neutropenia reported with pralsetinib but not with selpercatinib explains that selpercatinib is likely to be selectively cytotoxic to RET-altered cells.
Table 4.
Incidence Comparison of Some Important All-Grade TRAEs Among Different RET Inhibitors
| Cabozantinib (N = 26), % | Vandetanib (N = 18), % | Lenvatinib (N = 25), % | RXDX-105 (N = 31), % | Pralsetinib (N = 57), % | Selpercatinib (N = 105), % | |
|---|---|---|---|---|---|---|
| Nausea | 31 | 6 | 60 | 8 | Nr | 19 |
| Diarrhea | 62 | 44 | 52 | 18 | Nr | 31 |
| Fatigue | 46 | 11 | 36 | 22 | 15 | 24 |
| Hypertension | 19 | 89 | 68 | Nr | 18 | 29 |
| Neutropenia | Nr | Nr | Nr | Nr | 15 | Nr |
| Elevated ALT | 96 | 6 | 20 | 16 | 17 | 26 |
| Elevated AST | 73 | 6 | 24 | 16 | 22 | 28 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; Nr, not reported. TRAE, treatment-related adverse event.
Currently, there are no FDA-approved options for this patient subpopulation. The potential approval of the two selective RET inhibitors on the basis of phase 2 data would represent a major step forward in providing the first effective targeted therapy for patients positive with RET fusion with NSCLC.
Detection of RET Fusion
Given the robust efficacy with selective RET inhibitors, RET rearrangement should now be considered as a targetable driver mutation in NSCLC similar to EGFR mutations and ALK and ROS1 rearrangements. Therefore, it is very important to screen patients with NSCLC for RET rearrangements at the time of diagnosis. Because there are no specific clinical features of this subset of NSCLC, clinical selection cannot be used to determine whether a given patient should be screened for RET. Multiple methods have been used for RET analysis: NGS, FISH, immunohistochemistry, and RT-PCR. NGS is currently the most sensitive method for RET analysis.43 NGS also provides comprehensive molecular profiling to identify upstream gene partners and concurrent genomic aberration that may predict treatment response.17 In addition, it will allow for a better selection of patients for referral to matched clinical trials targeting RET. DNA-based NGS offers a comprehensive tool to detect genomic alterations. However, it may not detect gene fusions in samples with low tumor purity (<20%) or suboptimal DNA quality or quantity. One consideration would be to perform an RNA-based NGS to uncover targetable RET fusions that were not detected by DNA-based NGS.44 RT-PCR is successful to detect most common RET fusion partners but not the less common ones.45 RET FISH has a high positive rate owing to the narrow spacing between the split probe signals. It has a sensitivity of 100% and a suboptimal specificity of 45% to 60% and a false-positive of 39% to 55%.45 Screening for RET using FISH after RT-PCR might have decreased the detection of patients with false-positive in the study by Yoh et al.32; this allowed a better selection of patients and could explain the increased ORR with vandetanib compared with the study by Lee et al.31 RET immunohistochemistry (anti-RET antibody ab134100, Abcam, Cambridge, United Kingdom) does not have strong corroborating evidence to warrant clinical use because of low sensitivity 55% to 65% and variable specificity 40% to 85%.45
Most of the clinical studies included RET with different fusion partners. There is no strong preclinical and clinical data to our knowledge to support the sensitivity of different fusion partners to RET inhibition. Therefore, future studies are needed to determine the predictive and prognostic value of the RET fusion partner.
RET Emergence as a Mechanism of Acquired Resistance to EGFR Inhibition
Few reports reported that an actionable driver oncogene (RET) can develop as a mechanism of acquired resistance to another actionable driver mutation (EGFR) during therapy with EGFR inhibition and that targeting both drivers is a promising therapeutic strategy for these patients. In a comprehensive review, Zhu et al.46 reported the distribution of receptor tyrosine kinase fusions detected in tissue or blood at the time of acquired resistance to EGFR inhibition in NSCLC. RET fusion was detected in 55%, 27%, 40%, and 42% at the time of acquired resistance to first-, second-, and third-generation EGFR inhibition, and also after osimertinib use in T790 mutation, respectively. Most RET fusions were CCDC6 (58%) and NCOA4 (26%).
CCDC6-RET fusion has emerged as a potential acquired mechanism of resistance to osimertinib, an EGFR inhibitor used in metastatic, recurrent NSCLC with EGFR exon 19 deletion, exon 21 L858R, or T790 mutations.47,48 The combination of second-line osimertinib with a selective RET inhibitor, pralsetinib, led to a decrease in cell viability in vitro; it was well tolerated and led to an impressive response with 78% tumor shrinkage at 8 weeks in two patients with EGFR exon 19 deletions: one with CCDC6-RET fusion identified on MGH Solid Fusion Assay 18 months after progression on second-line osimertinib; and another with NCOA4-RET fusion identified on NGS 2 years after progression on the combination afatinib and cetuximab.49 Other reports described the fusion KIF5B-RET found on tissue50 or liquid51 biopsy as a potential mechanism of acquired resistance in patients with NSCLC with EGFR exon 19 deletions who progressed on EGFR inhibition with erlotinib52 or icotinib.51 The patient who progressed after 11 months of treatment with icotinib, had an SD for 2 months with the addition of cabozantinib.
NCOA4-RET is a rare fusion reported as a potential mechanism of resistance to a patient with EGFR mutation L858R NSCLC treated with afatinib for 20 months and responded to the addition of cabozantinib with 7-month of SD.52
Mechanism of Resistance to RET Inhibition
Knowledge regarding the biological mechanisms that mediate acquired resistance to RET inhibitors is emerging; bypass pathways that are not blocked by current RET multikinase inhibitors are likely to be among the most common mechanisms.
Preclinical studies found that activation of the EGFR, VEGFR, and downstream mTOR pathways may drive the resistance to RET inhibition; the combination of everolimus, an mTOR inhibitor, and vandetanib, a RET/VEGFR/EGFR inhibitor, was able to overcome resistance. On the basis of this rationale, the combination has been studied in RET-positive NSCLC.53,54 Among 13 patients, seven objective responses, and a median PFS of 4.4 months (95% CI: 3.4–NR) were observed. In patients with RET fusion–positive by NGS, the response rate was 70%, and the median PFS was 8 months (95% CI: 3.4–NR). No response was seen in the three patients with FISH-positive NGS-negative RET fusion. Severe toxicities included diarrhea (21%), thrombocytopenia (16%), QTc prolongation (5%), and rash (5%). Most patients (89%) required dose modifications after one cycle owing to toxicity.
Solvent mutations sterically hinder the binding of RET inhibitor leading to the loss of RET activity in selective and multikinase RET inhibitors. Preclinical studies reported the emergence of RET G810R solvent mutation as acquired resistance to selective RET inhibitors such as selpercatinib and pralsetinib. However, solvent mutations remained sensitive to another potent and selective next-generation RET/SRC oral kinase inhibitor, TPX-0046, that lacks activity against RET V804M gatekeeper mutation. TPX-0046 is VEGFR2 sparing and is currently being tested in phase 1/2 clinical trial for patients with advanced solid tumors harboring RET fusions or mutations (NCT04161391). Of note, RET V804M mutation has been reported to confer resistance to vandetanib (and RET S904F mutation) and can be overcome with selpercatinib but not TPX-0046.55, 56, 57, 58
Recently, Solomon et al.56 described the first mechanism of “on-target” resistance to selpercatinib with the detection of RET solvent front mutations G810R, G810S, and G810C on circulating tumor DNA few months before the emergence of clinical resistance in five patients who had a dramatic initial response to selpercatinib, three of them with NSCLC. At autopsy, plasma and tumor biopsies confirmed G810 mutations at multiple sites of metastatic disease for the same patient. These important observations will allow for developing strategies to overcome acquired resistance.
Conclusions
With the emergence of selective RET inhibitors, patients with advanced-stage NSCLC should have their tumors tested routinely for RET fusion using NGS. Enrollment of patients with RET fusion–positive metastatic NSCLC in clinical trials should be highly encouraged. Otherwise, given RET fusion–positive NSCLC sensitivity to platinum-based chemotherapy and the low activity of RET multikinase inhibitors, it is reasonable to treat these patients first with platinum-doublet chemotherapy, then consider a RET multikinase inhibitor as their next line of therapy until FDA approval of the selective inhibitors.
It is hoped that the selective RET inhibitors will be available for routine clinical practice in the near future. With a response rate of approximately 60% to 70% and a median PFS of approximately 18 months, RET inhibition should be considered as first-line therapy for patients with RET fusion. Knowledge regarding mechanisms of acquired resistance to RET inhibition is beginning to emerge; this will pave the way for the development of novel approaches to overcome acquired resistance and promote long-term efficacy.
Acknowledgments
This work was supported by National Cancer Institute P50 CA217691 and research grant provided by the Lee Foundation, Atlanta, Georgia. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Footnotes
Disclosure: Dr. El Osta has received research support (to institutions) from Merck, Novartis, Bristol-Myers Squibb, and Xcovery Holdings. Dr. Ramalingam has served on advisory board meetings or consulted for Amgen, AstraZeneca, Bristol-Myers Squibb, Merck, Genentech/Roche, Tesaro, and Takeda; and has received research grants (to institution) from Advaxis, Amgen, AstraZeneca, Bristol-Myers Squibb, Merck, Tesaro, Takeda, and Genmab.
References
- 1.Ramalingam S.S., Vansteenkiste J., Planchard D. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382:41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 2.Planchard D., Smit E.F., Groen H.J.M. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol. 2017;18:1307–1316. doi: 10.1016/S1470-2045(17)30679-4. [DOI] [PubMed] [Google Scholar]
- 3.Peters S., Camidge D.R., Shaw A.T. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377:829–838. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 4.Shaw A.T., Ou S.H., Bang Y.J. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371:1963–1971. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drilon A., Laetsch T.W., Kummar S. Efficacy of Larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378:731–739. doi: 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farago A.F., Le L.P., Zheng Z. Durable clinical response to entrectinib in NTRK1-rearranged non-small cell lung cancer. J Thorac Oncol. 2015;10:1670–1674. doi: 10.1097/01.JTO.0000473485.38553.f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camidge D.R., Kim H.R., Ahn M.J. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N Engl J Med. 2018;379:2027–2039. doi: 10.1056/NEJMoa1810171. [DOI] [PubMed] [Google Scholar]
- 8.Multikinase inhibitor crizotinib is active in MET Exon 14-altered lung cancer. Cancer Discov. 2020;10:337. doi: 10.1158/2159-8290.CD-RW2020-015. [DOI] [PubMed] [Google Scholar]
- 9.U.S. National Library of Medicine, ClinicalTria;s.gov 2018 A phase 1, study evaluating the safety, tolerability, PK, and efficacy of AMG 510 in subjects with solid tumors with a specific KRAS mutation. https://clinicaltrials.gov/ct2/show/NCT03600883
- 10.AMG 510 first to inhibit “Undruggable” KRAS. Cancer Discov. 2019;9:988–989. doi: 10.1158/2159-8290.CD-NB2019-073. [DOI] [PubMed] [Google Scholar]
- 11.Fell J.B., Fischer J.P., Baer B.R. Identification of the clinical development candidate MRTX849, a covalent KRASG12C inhibitor for the treatment of cancer. J Med Chem. 2020;63(13):6679–6693. doi: 10.1021/acs.jmedchem.9b02052. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi M., Ritz J., Cooper G.M. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell. 1985;42:581–588. doi: 10.1016/0092-8674(85)90115-1. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi M. The GDNF/RET signaling pathway and human diseases. Cytokine Growth Factor Rev. 2001;12:361–373. doi: 10.1016/s1359-6101(01)00012-0. [DOI] [PubMed] [Google Scholar]
- 14.Raue F., Frank-Raue K. Thyroid cancer: risk-stratified management and individualized therapy. Clin Cancer Res. 2016;22:5012–5021. doi: 10.1158/1078-0432.CCR-16-0484. [DOI] [PubMed] [Google Scholar]
- 15.Lipson D., Capelletti M., Yelensky R. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat Med. 2012;18:382–384. doi: 10.1038/nm.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gautschi O., Milia J., Filleron T. Targeting RET in patients with RET-rearranged lung cancers: results from the global, multicenter RET registry. J Clin Oncol. 2017;35:1403–1410. doi: 10.1200/JCO.2016.70.9352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drilon A., Rekhtman N., Arcila M. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm trial. Lancet Oncol. 2016;17:1653–1660. doi: 10.1016/S1470-2045(16)30562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bronte G., Ulivi P., Verlicchi A., Cravero P., Delmonte A., Crinò L. Targeting RET-rearranged non-small-cell lung cancer: future prospects. Lung Cancer (Auckl) 2019;10:27–36. doi: 10.2147/LCTT.S192830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kohno T., Ichikawa H., Totoki Y. KIF5B-RET fusions in lung adenocarcinoma. Nat Med. 2012;18:375–377. doi: 10.1038/nm.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang R., Hu H., Pan Y. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J Clin Oncol. 2012;30:4352–4359. doi: 10.1200/JCO.2012.44.1477. [DOI] [PubMed] [Google Scholar]
- 21.Ku G.Y., Haaland B.A., de Lima Lopes G., Jr. Gefitinib vs. chemotherapy as first-line therapy in advanced non-small cell lung cancer: meta-analysis of phase III trials. Lung Cancer. 2011;74:469–473. doi: 10.1016/j.lungcan.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Mok T.S., Wu Y.L., Thongprasert S. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 23.Shen T, Pu X, Wang L, et al. Association between RET fusions and efficacy of pemetrexed-based chemotherapy for patients with advanced NSCLC in China: a multicenter retrospective study. Clin Lung Cancer. https://doi.org/10.1016/j.cllc.2020.02.006, accessed May 28, 2020. [DOI] [PubMed]
- 24.Drilon A., Bergagnini I., Delasos L. Clinical outcomes with pemetrexed-based systemic therapies in RET-rearranged lung cancers. Ann Oncol. 2016;27:1286–1291. doi: 10.1093/annonc/mdw163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazieres J., Drilon A., Lusque A. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol. 2019;30:1321–1328. doi: 10.1093/annonc/mdz167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hegde A, Huang L, Liu S, et al. Responsiveness to immune checkpoint inhibitors in RET dependent cancers. Paper presented at: Proceedings of the American Association for Cancer Research Annual Meeting; March 29–April 3, 2019; Atlanta, GA.
- 27.Offin M., Guo R., Wu S.L. Immunophenotype and response to immunotherapy of RET-rearranged lung cancers. JCO Precis Oncol. 2019;3:1–8. doi: 10.1200/PO.18.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guisier F., Dubos-Arvis C., Vinas F. Efficacy and safety of anti–PD-1 immunotherapy in patients with advanced NSCLC with BRAF, HER2 or MET mutation or RET-translocation: GFPC 01-2018. J Thorac Oncol. 2020;15:628–636. doi: 10.1016/j.jtho.2019.12.129. [DOI] [PubMed] [Google Scholar]
- 29.Takeuchi K., Soda M., Togashi Y. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012;18:378–381. doi: 10.1038/nm.2658. [DOI] [PubMed] [Google Scholar]
- 30.Ju Y.S., Lee W.C., Shin J.Y. A transforming KIF5B and RET gene fusion in lung adenocarcinoma revealed from whole-genome and transcriptome sequencing. Genome Res. 2012;22:436–445. doi: 10.1101/gr.133645.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee S.H., Lee J.K., Ahn M.J. Vandetanib in pretreated patients with advanced non-small cell lung cancer-harboring RET rearrangement: a phase II clinical trial. Ann Oncol. 2017;28:292–297. doi: 10.1093/annonc/mdw559. [DOI] [PubMed] [Google Scholar]
- 32.Yoh K., Seto T., Satouchi M. Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): an open-label, multicentre phase 2 trial. Lancet Respir Med. 2017;5:42–50. doi: 10.1016/S2213-2600(16)30322-8. [DOI] [PubMed] [Google Scholar]
- 33.Falchook G.S., Ordóñez N.G., Bastida C.C. Effect of the RET inhibitor vandetanib in a patient with RET fusion-positive metastatic non-small-cell lung cancer. J Clin Oncol. 2016;34:e141–e144. doi: 10.1200/JCO.2013.50.5016. [DOI] [PubMed] [Google Scholar]
- 34.Hida T., Velcheti V., Reckamp K.L. A phase 2 study of lenvatinib in patients with RET fusion-positive lung adenocarcinoma. Lung Cancer. 2019;138:124–130. doi: 10.1016/j.lungcan.2019.09.011. [DOI] [PubMed] [Google Scholar]
- 35.Neal J.W., Dahlberg S.E., Wakelee H.A. Erlotinib, cabozantinib, or erlotinib plus cabozantinib as second-line or third-line treatment of patients with EGFR wild-type advanced non-small-cell lung cancer (ECOG-ACRIN 1512): a randomised, controlled, open-label, multicentre, phase 2 trial. Lancet Oncol. 2016;17:1661–1671. doi: 10.1016/S1470-2045(16)30561-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drilon A., Fu S., Patel M.R. A phase I/Ib trial of the VEGFR-sparing multikinase RET inhibitor RXDX-105. Cancer Discov. 2019;9:384–395. doi: 10.1158/2159-8290.CD-18-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin J.J., Kennedy E., Sequist L.V. Clinical activity of alectinib in advanced RET-rearranged non-small cell lung cancer. J Thorac Oncol. 2016;11:2027–2032. doi: 10.1016/j.jtho.2016.08.126. [DOI] [PubMed] [Google Scholar]
- 38.Kodama T., Tsukaguchi T., Satoh Y. Alectinib shows potent antitumor activity against RET-rearranged non-small cell lung cancer. Mol Cancer Ther. 2014;13:2910–2918. doi: 10.1158/1535-7163.MCT-14-0274. [DOI] [PubMed] [Google Scholar]
- 39.Huang Q., Schneeberger V.E., Luetteke N. Preclinical modeling of KIF5B-RET fusion lung adenocarcinoma. Mol Cancer Ther. 2016;15:2521–2529. doi: 10.1158/1535-7163.MCT-16-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gainor J.F., Lee D.H., Curigliano G. Clinical activity and tolerability of BLU-667, a highly potent and selective RET inhibitor, in patients (pts) with advanced RET-fusion+ non-small cell lung cancer (NSCLC) J Clin Oncol. 2019;37(suppl 15) 9008–9008. [Google Scholar]
- 41.Drilon A, Oxnard G, Wirth L, et al. Registrational results of Libretto-001: a phase 1/2 trial of LOXO-292 in patients with RET fusion-positive lung cancers. Presented at: The IASLC 2019 World Conference on Lung Cancer of the International Association for the Study of Lung Cancer. September 7–10, 2019; Barcelona Spain.
- 42.Guo R., Schreyer M., Chang J.C. Response to selective RET inhibition with LOXO-292 in a patient with RET fusion-positive lung cancer with leptomeningeal metastases. JCO Precis Oncol. 2019;3:1–6. doi: 10.1200/PO.19.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lindeman N.I., Cagle P.T., Aisner D.L. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Thorac Oncol. 2018;13:323–358. doi: 10.1016/j.jtho.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 44.Ferrara R., Auger N., Auclin E., Besse B. Clinical and translational implications of RET rearrangements in non-small cell lung cancer. J Thorac Oncol. 2018;13:27–45. doi: 10.1016/j.jtho.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 45.Zhu V.W., Klempner S.J., Ou S.I. Receptor tyrosine kinase fusions as an actionable resistance mechanism to EGFR TKIs in EGFR-mutant non-small-cell lung cancer. Trends Cancer. 2019;5:677–692. doi: 10.1016/j.trecan.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 46.Guo Y., Guo R., Cheng H. P2.14-42 emergence of CCDC6-RET fusion with maintained EGFR T790M mutation after resistance to osimertinib in NSCLC: a case report. J Thorac Oncol. 2019;14:S846. [Google Scholar]
- 47.Klempner S.J., Bazhenova L.A., Braiteh F.S. Emergence of RET rearrangement co-existing with activated EGFR mutation in EGFR-mutated NSCLC patients who had progressed on first- or second-generation EGFR TKI. Lung Cancer. 2015;89:357–359. doi: 10.1016/j.lungcan.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 48.Piotrowska Z., Isozaki H., Lennerz J.K. Landscape of acquired resistance to osimertinib in EGFR-mutant NSCLC and clinical validation of combined EGFR and RET inhibition with osimertinib and BLU-667 for acquired RET fusion. Cancer Discov. 2018;8:1529–1539. doi: 10.1158/2159-8290.CD-18-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirai F., Takenoyama M., Taguchi K. Experience with erlotinib in lung adenocarcinoma harboring a coexisting KIF5B-RET fusion gene and EGFR mutation: report of a rare case. J Thorac Oncol. 2014;9:e37–e39. doi: 10.1097/JTO.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 50.Zhu Y.C., Wang W.X., Zhang Q.X. The KIF5B-RET fusion gene mutation as a novel mechanism of acquired EGFR tyrosine kinase inhibitor resistance in lung adenocarcinoma. Clin Lung Cancer. 2019;20:e73–e76. doi: 10.1016/j.cllc.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 51.Schrock A.B., Zhu V.W., Hsieh W.S. Receptor tyrosine kinase fusions and BRAF kinase fusions are rare but actionable resistance mechanisms to EGFR tyrosine kinase inhibitors. J Thorac Oncol. 2018;13:1312–1323. doi: 10.1016/j.jtho.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 52.Subbiah V., Cascone T., Hess K.R. Multi-kinase RET inhibitor vandetanib combined with mTOR inhibitor everolimus in patients with RET rearranged non-small cell lung cancer. J Clin Oncol. 2018;36 9035–9035. [Google Scholar]
- 53.Subbiah V., Berry J., Roxas M. Systemic and CNS activity of the RET inhibitor vandetanib combined with the mTOR inhibitor everolimus in KIF5B-RET re-arranged non-small cell lung cancer with brain metastases. Lung Cancer. 2015;89:76–79. doi: 10.1016/j.lungcan.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Benayed R., Offin M., Mullaney K. High yield of RNA sequencing for targetable kinase fusions in lung adenocarcinomas with no mitogenic driver alteration detected by DNA sequencing and low tumor mutation burden. Clin Cancer Res. 2019;25:4712–4722. doi: 10.1158/1078-0432.CCR-19-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Subbiah V., Yang D., Velcheti V. State-of-the-art strategies for targeting RET-dependent cancers. J Clin Oncol. 2020;38:1209–1221. doi: 10.1200/JCO.19.02551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Solomon B.J., Tan L., Lin J.J. RET solvent front mutations mediate acquired resistance to selective RET inhibition in RET-driven malignancies. J Thorac Oncol. 2020;15:541–549. doi: 10.1016/j.jtho.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Subbiah V., Velcheti V., Tuch B.B. Selective RET kinase inhibition for patients with RET-altered cancers. Ann Oncol. 2018;29:1869–1876. doi: 10.1093/annonc/mdy137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakaoku T., Kohno T., Araki M. A secondary RET mutation in the activation loop conferring resistance to vandetanib. Nat Commun. 2018;9:625. doi: 10.1038/s41467-018-02994-7. [DOI] [PMC free article] [PubMed] [Google Scholar]