Abstract
Optimal management of EGFR-mutated NSCLC with leptomeningeal (LM) disease progression through EGFR tyrosine kinase inhibitor remains unclear. We present a 39-year-old man with EGFR-mutated NSCLC and LM disease progression through osimertinib 80 mg daily, with subsequent durable radiographic and symptomatic response to systemic pemetrexed in combination with osimertinib. This builds on the limited data evaluating LM disease response to systemic pemetrexed and lends further support to consideration of this treatment strategy.
Keywords: Pemetrexed, EGFR mutation, Non–small cell lung cancer, Leptomeningeal disease, Case report
Introduction
Leptomeningeal (LM) disease is a late complication of NSCLC associated with substantial morbidity and a poor prognosis. Although osimertinib and other EGFR tyrosine kinase inhibitors (TKIs) have activity against LM disease, the best treatment approach after LM disease progression through TKI is unclear. Systemic or intrathecal chemotherapy, or dose escalation of osimertinib from 80 mg daily to 160 mg daily is often used, but clinical benefit in this population has not been clearly defined. Retrospective data suggest that systemic pemetrexed has activity against LM disease. We report a case of a patient with EGFR-mutated NSCLC with LM disease who experienced remarkably durable disease control on pemetrexed and osimertinib after disease progression on osimertinib monotherapy.
Case Presentation
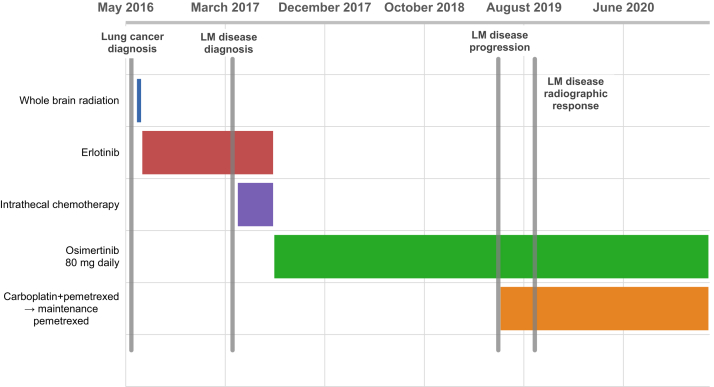
A 39-year-old man with no smoking history developed a left neck mass, cough, dyspnea, and chest pain in late 2015. Initial computed tomography of the head and chest and positron emission tomography scan in June 2016 revealed innumerable metastases throughout the cerebellum and cerebral hemispheres bilaterally, supraclavicular and hilar adenopathy, diffuse pulmonary nodules, and multiple osseous lesions. Biopsy of a right pulmonary mass and mediastinal lymph nodes revealed lung adenocarcinoma, with an EGFR exon 19 deletion on molecular profiling. Given the large burden of parenchymal brain metastases, he completed whole-brain radiation before starting erlotinib in June 2016 (Fig. 1).
Figure 1.
Case timeline. Timeline of all antineoplastic therapies administered from date of initial lung cancer diagnosis to present (February 2021), relative to timing of LM disease development and radiographic response. LM, leptomeningeal.
He experienced good radiographic and symptomatic response to erlotinib until March 2017, when he presented after a syncopal episode. He noted new-onset dizziness, blurred vision, changes in his ability to articulate his thoughts, and full-body stiffness with arthralgias and myalgias in the preceding month. A magnetic resonance imaging (MRI) of the brain revealed numerous new subcentimeter parenchymal metastases bilaterally and extensive LM enhancement along the superior cerebellar folia, brainstem, and fifth, seventh and eighth cranial nerve complexes (Fig. 1). An MRI of the cervical, thoracic, and lumbar spine revealed diffuse LM seeding along the spinal cord and nerve roots. Cerebrospinal fluid (CSF) cytology results revealed rare atypical cells, suggestive of metastatic adenocarcinoma. An EGFR p.T790M mutation was not detected in CSF circulating tumor DNA (ctDNA), although there were low levels of ctDNA. Computed tomography imaging revealed stable systemic disease. He continued erlotinib and started intrathecal methotrexate in April 2017. This was changed to intrathecal liposomal cytarabine after he developed aseptic meningitis attributed to methotrexate. He developed an enlarging left supraclavicular lymph node in July 2017, and a biopsy of this revealed an EGFR p.T790M mutation. He discontinued erlotinib and intrathecal chemotherapy and started osimertinib 80 mg daily in August 2017, with good radiologic and symptomatic response.
In May 2019, he developed near-syncope, back stiffness, and diplopia. An MRI brain revealed new LM enhancement along the cerebellar hemispheres bilaterally, and he started carboplatin and pemetrexed in June 2019. Osimertinib was discontinued but quickly resumed due to a flare of his neurologic symptoms, which improved soon after restarting. He switched to maintenance pemetrexed after four cycles of carboplatin and pemetrexed. A lumbar puncture in August 2019 revealed rare atypical cells concerning for malignancy, which were positive for an EGFR exon 19 deletion, negative for an EGFR p.T790M mutation, and positive for a two- to four-gene MET copy gain on University of Washington OncoPlex Single Gene Mutational Analysis. Subsequently, the patient had complete resolution of neurologic symptoms and his systemic disease has remained stable on imaging since January 2018. In September 2019, an MRI brain revealed radiographic resolution of LM disease and an MRI spine showed decreased diffuse LM disease. The patient remains on osimertinib 80 mg daily and maintenance pemetrexed as of February 2021, with ongoing radiologic and clinical responses (Fig. 2).
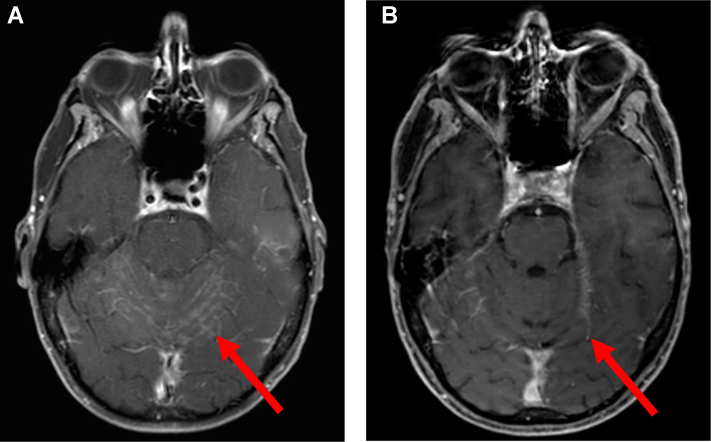
Figure 2.
MRI brain scans before and after starting carboplatin and pemetrexed. An MRI of the brain from June 2019 (A) showed progressive leptomeningeal disease on osimertinib monotherapy, before the start of carboplatin and pemetrexed. The most recent MRI of the brain from December 2020 (B) revealed sustained radiographic response of leptomeningeal disease on osimertinib 80 mg daily and maintenance pemetrexed. MRI, magnetic resonance imaging.
Discussion
We present a case of EGFR-mutated NSCLC with LM disease progression through osimertinib monotherapy, now with a durable response to systemic pemetrexed in combination with osimertinib. We elected to start pemetrexed-based systemic therapy on LM disease progression due to evidence of activity against both parenchymal brain metastases and LM disease in patients with EGFR-mutated NSCLC. Although we initially planned to switch to chemotherapy alone, we quickly resumed osimertinib to address a neurologic symptom flare. The AURA3 trial showed a 31% intracranial response rate to combination platinum and pemetrexed chemotherapy in EGFR-mutated NSCLC.1 Retrospective data also suggest that the combination of osimertinib and chemotherapy after progression through previous EGFR TKI provides effective control of parenchymal brain metastases.2 Most of these patients received a regimen including pemetrexed, although the study did not specifically evaluate the effectiveness of combination osimertinib and pemetrexed-based chemotherapy for brain metastases.
Data on LM disease response to pemetrexed with or without an EGFR TKI are limited. A retrospective review of 110 patients with EGFR-mutated advanced NSCLC treated with EGFR TKI found that median overall survival after LM disease development was longer with receipt of pemetrexed versus no pemetrexed (13.7 versus 4.0 mo, p = 0.008) and with continuation of EGFR TKI relative to discontinuation (16.9 versus 3.0 mo, p < 0.001).3 Another retrospective study of 30 patients with NSCLC and LM disease found that the receipt of a modern systemic treatment regimen including pemetrexed, bevacizumab or a TKI at or during LM diagnosis was associated with a decreased risk of death relative to receipt of a regimen without at least one of those systemic agents (hazard ratio = 0.24, p = 0.007).4 Of the 16 patients who received a modern treatment regimen, nine (56%) had a known or suspected EGFR mutation. A study of six patients with new LM disease and NSCLC previously well controlled on gefitinib, five (83%) of whom had a sensitizing EGFR mutation, reported a 67% cerebral response rate to the combination of cisplatin, pemetrexed, and erlotinib.5 Most patients on these studies were not treated with osimertinib, and their interpretation is limited by small study size, potential selection bias, and heterogeneous treatment approaches.
Conclusions
Our patient’s durable radiographic and symptomatic response to systemic pemetrexed in combination with osimertinib for recurrent LM disease lends further support to use of this treatment strategy for LM disease in EGFR-mutated NSCLC. This case also illustrates the potential value of using CSF ctDNA in evaluating for resistance alterations of LM disease. Prospective studies are needed to better characterize the efficacy of this combination at a population level.
Acknowledgments
The Fred Hutchinson Institutional Review Board does not require informed consent for case reports, but the authors did obtain verbal consent from the patient authorizing use and disclosure of his health information.
Footnotes
Cite this article as: Merkhofer CM, Baik CS. Durable response of leptomeningeal disease with osimertinib and pemetrexed in EGFR-mutated metastatic NSCLC: a case report. JTO Clin Res Rep. 2021;2:100177.
Disclosure: Dr. Baik receives research funding from AbbVie, Celgene, Eli Lilly Oncology, Pfizer, Rain Therapeutics, Spectrum Pharmaceuticals, and Turning Point Therapeutics and personal consulting fees from AstraZeneca, Blueprint Medicines, Daiichi-Sankyo, and Takeda Pharmaceuticals. Dr. Merkhofer declares no conflict of interest.
References
- 1.Wu Y.L., Ahn M.J., Garassino M.C. CNS efficacy of osimertinib in patients with T790M-positive advanced non-small-cell lung cancer: data from a randomized phase III trial (AURA3) J Clin Oncol. 2018;36:2702–2709. doi: 10.1200/JCO.2018.77.9363. [DOI] [PubMed] [Google Scholar]
- 2.White M.N., Piotrowska Z., Stirling K. Combining osimertinib with chemotherapy in EGFR-mutant NSCLC at progression [e-pub ahead of print]. Clin Lung Cancer. https://doi.org/10.1016/j.cllc.2021.01.010 accessed March 2, 2021. [DOI] [PMC free article] [PubMed]
- 3.Choi M., Keam B., Ock C.Y. Pemetrexed in the treatment of leptomeningeal metastasis in patients with EGFR-mutant lung cancer. Clin Lung Cancer. 2019;20:e442–e451. doi: 10.1016/j.cllc.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Riess J.W., Nagpal S., Iv M. Prolonged survival of patients with non-small-cell lung cancer with leptomeningeal carcinomatosis in the modern treatment era. Clin Lung Cancer. 2014;15:202–206. doi: 10.1016/j.cllc.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang H., Yang X., Zhang Y. Erlotinib in combination with pemetrexed/cisplatin for leptomeningeal metastases and cerebrospinal fluid drug concentrations in lung adenocarcinoma patients after gefitinib failure. Target Oncol. 2015;10:135–140. doi: 10.1007/s11523-014-0326-9. [DOI] [PubMed] [Google Scholar]