Abstract
Hypoxia-ischemia (HI) injury is a leading cause of neonatal death and long-term disability, and existing treatment options for HI offer only modest benefit. Early intervention with the drug metformin has been shown to promote functional improvement in numerous rodent models of injury and has pleiotropic cellular effects in the brain. We have previously shown that 1 week of metformin treatment initiated 24 h after HI in neonatal mice resulted in improved motor and cognitive performance, activation of endogenous neural precursor cells (NPCs), and increased oligodendrogenesis. While promising, a limitation to this work is that immediate pharmacological intervention is not always possible in the clinic. Herein, we investigated whether delaying metformin treatment to begin in the subacute phase post-HI would still effectively promote recovery. Male and female C57/BL6 mice received HI injury postnatally, and metformin treatment began 7 days post-HI for up to 4 weeks. Motor and cognitive performance was assessed across time using behavioural tests (cylinder, foot fault, puzzle box). We found that metformin improved motor and cognitive behaviour, decreased inflammation, and increased oligodendrocytes in the motor cortex. Our present findings demonstrate that a clinically relevant subacute metformin treatment paradigm affords the potential to treat neonatal HI, and that improved outcomes occur through modulation of the inflammatory response and oligodendrogenesis.
Keywords: Hypoxia ischemia, Metformin, Inflammation, Oligodendrogenesis, Neonate
Highlights
-
•
Subacute metformin treatment improves functional recovery after neonatal hypoxia ischemia.
-
•
Metformin reduces the number of microglia present in the brain early after injury.
-
•
Metformin increases the number of oligodendrocytes present in the chronic post-injury phase.
-
•
Metformin treatment has therapeutic potential in the treatment of hypoxic ischemic brain damage.
1. Introduction
Neonatal hypoxia-ischemia (HI) is a leading cause of childhood brain injury, and results in profound physical and cognitive consequences including seizures, cerebral palsy, and even death (Kurinczuk et al., 2010). HI occurs when there is an interruption of blood flow to the brain around the time of birth, and can result from fetal asphyxia or perinatal stroke (Gunn and Thoresen, 2019). Consequently, excitotoxicity, inflammation, and oxidative stress leads to the death of neurons and oligodendrocytes and subsequent motor and cognitive impairments (Volpe, 2009). Presently, the standard practice to treat HI is therapeutic hypothermia, which is only successful when administered within the first hours of insult. This treatment offers only modest benefits, leaving many patients with long-term deficits (Adstamongkonkul and Hess, 2017; Wassink et al., 2018). Hence, there is a critical need for novel strategies to improve repair and recovery following HI, especially during the subacute period.
Metformin, a commonly prescribed type II diabetes medication, has been shown to have pleiotropic effects in the central nervous system (CNS) (Dadwal et al., 2015; Jin et al., 2014; Liu et al., 2014; Ruddy et al., 2019; Venna et al., 2014). It has been shown to enhance neural precursor cells (NPCs) in the subependymal zone (SEZ) lining the lateral ventricles and in the dentate gyrus of the hippocampus. Specifically, metformin can activate NPCs within the SEZ, increase their migration into the brain parenchyma, and promote their differentiation into new neurons and oligodendrocytes after injury (Dadwal et al., 2015; Ruddy et al., 2019). Furthermore, metformin has been shown to reduce inflammation in adult and neonatal models of brain damage (Fang et al., 2017; Tao et al., 2018). Acute administration of metformin (starting within 24 h after injury) has been shown to improve motor (Dadwal et al., 2015; Ruddy et al., 2019) and cognitive (Ruddy et al., 2019) function, and reduce brain injury (Fang et al., 2017). Therefore, metformin represents a promising treatment strategy following HI.
With the goal of clinical translation, it is important to take into account factors that more closely reflect clinical realities (Vexler et al., 2006). A key consideration in establishing treatments for HI is timing of the intervention. It is not always feasible to initiate therapies immediately following injury, particularly due to the time of hospital admittance, the need to identify and diagnose a condition, and the time required to stabilize the patient. Moreover, HI injury involves pathophysiological processes that take place over hours and days following the initial insult (Wassink et al., 2018) and which affect the efficacy of a given treatment over time. Hence, it is important to investigate the window of opportunity in which metformin treatment is effective.
Herein, we delayed metformin treatment to the subacute phase following HI injury in order to investigate its efficacy as an intervention during a time at which deleterious pathophysiological processes are largely complete (Wassink et al., 2018). We examined cognitive and motor behaviour, as well as the cellular response to delayed administration. We observed improved functional outcomes, which were concomitant with reduced inflammation, and increased oligodendrogenesis at 7 weeks post-injury. Our results demonstrate positive outcomes similar to those seen with acute treatment and indicate a broader window of opportunity within which metformin administration can improve outcomes following HI.
2. Methods
2.1. Animals
A total of 109 C57/BL6 male and female mice (from a total of 27 litters; bred in-house) were used in this study. Animals were group-housed in standard laboratory conditions under a 12:12 h light:dark cycle with ad libitum access to food and water. All procedures were approved by the University of Toronto Animal Care Committee and were performed in accordance with guidelines from the Canadian Council for Animal Care.
2.2. Surgery and drug administration
Mouse pups received HI injury using a modified Rice-Vannucci model (Rice et al., 1981) on postnatal day (P) 8 as previously described (Dadwal et al., 2015; Ruddy et al., 2019). Briefly, animals were anesthetized with isoflurane, and a ventral midline incision was made on the neck. The left common carotid artery was carefully separated from surrounding tissue and ligated using 6-0 silk sutures then transected, resulting in irreversible ischemia. The wound was treated with bupivacaine and sutured using 6-0 silk, and pups were returned to their mother for 90 min. Pups were then placed into a hypoxia chamber with 8% oxygen for 60 min on a 37 °C heating pad then returned to their home cage. Each litter contained sham controls animals that did not receive the HI procedure.
Metformin (20 mg/kg dissolved in sterile PBS; Sigma-Aldrich, ON) treatment began on P15 and continued for 4 weeks. Administration was achieved via daily subcutaneous injections until mice reached at least 10 g body mass (by P28; in accordance with institutional animal care guidelines), and then subcutaneously via implanted mini osmotic pumps (Alzet, USA) for the remaining time. Vehicle control animals received sterile PBS.
2.3. Functional assessments
Functional assessments took place in a dedicated behavioural testing room, and animals were allowed to acclimate to the room for at least 30 min before testing. Data were analyzed by an experimenter blinded to group.
2.3.1. Cylinder test
The cylinder test was performed at P22 to measure spontaneous forelimb use during exploration. Uninjured animals use both forelimbs equally, while following injury, they rely more heavily on the unimpaired limb. Mice were placed into a clear plastic cylinder and allowed to explore for 8 min while being video recorded. The number of times the mice used each forelimb was analyzed. Ipsilesional forelimb use (relative to the ischemic insult) was determined using the following formula: [(#ipsilesional touches - # contralesional touches)/(# touches total)].
2.3.2. Foot fault
The foot fault test was performed at P42 to assess motor function and coordination while mice traversed a grid. Uninjured animals demonstrate good coordination and slip minimally during this task. Mice were placed on a metal grid (1 cm spaces) suspended 12 inches above a table surface and video recorded while exploring for 3 min. The number of steps and the number of foot slips made with the hindlimbs were counted and the difference in foot slip ratio was calculated as (#contralesional slips/#contralesional steps)-(#ipsilesional slips/#ipsilesional steps).
2.3.3. Puzzle box
The puzzle box test was performed over 3 days beginning on P53, as previously described, to assess performance on short- and long-term memory, and problem-solving (Abdallah et al., 2011; Nusrat et al., 2018; Ruddy et al., 2019). Mice were placed into a box comprised of a brightly lit open area and a dark covered area (goal box), connected by a passageway. Animals were given 3 trials per day, during which they had to solve increasingly difficult tasks to enter the goal box (Table 1). On the first day, animals were acclimated to the task by being placed in the box with the opportunity to move unimpeded from the bright area to the goal box through an open doorway, followed by accessing it through a closed-off doorway (requiring traveling beneath the doorway through the open passageway) for two trials. The following day, animals had to repeat the task from the last two trials the previous day (long-term memory). Following a 2 min inter-trial interval, they were then placed back in the box, this time with bedding blocking the passageway that had to be removed to gain access (problem-solving). Following another 2 min inter-trial interval, they were subjected to the same task (short-term memory). On the last day, animals were presented once more with the same task (long-term memory), followed by a 2 min inter-trial interval and the application of a cardboard plug blocking access to the goal box (problem-solving). This task was repeated once more following a 2 min interval (short-term memory). Animals were excluded from analysis if they did not enter the goal box within the first 3.5 min during any acclimation trial on the first day (n = 1; sham control). Uninjured animals are able to complete this progression of tasks with relatively low latencies, while those with cognitive deficits take longer, or are sometimes unable to solve the tasks.
Table 1.
Summary of puzzle box trials. The test took place over three days, wherein mice had to solve increasingly difficult tasks to access the goal box (Abdallah et al., 2011). The latency to enter the box was recorded.
| Day | Trial | Task | Function |
|---|---|---|---|
| 1 | 1 | Pass through unimpeded doorway over tunnel | Test acclimation |
| 2 | Pass through tunnel; doorway closed off | Test acclimation | |
| 3 | Pass through tunnel; doorway closed off | Test acclimation | |
| 2 | 4 | Pass through tunnel; doorway closed off | Long term memory |
| 5 | Pass through tunnel; bedding impediment | Problem solving | |
| 6 | Pass through tunnel; bedding impediment | Short term memory | |
| 3 | 7 | Pass through tunnel; bedding impediment | Long term memory |
| 8 | Pass through tunnel; cardboard plug impediment | Problem solving | |
| 9 | Pass through tunnel; cardboard plug impediment | Short term memory |
2.4. Neurosphere assay
Following metformin treatment from P15-21, a subset of animals was sacrificed on P22 to quantify stem cell number in vitro using the neurosphere assay. Mice were anesthetized with isoflurane and euthanized by cervical dislocation. The brains were removed and the SEZ was microdissected and incubated in an enzyme solution containing trypsin (1.3 mg/ml), hyaluronidase (0.76 mg/ml), and kynurenic acid (0.12 mg/ml) (Sigma-Adrich, Missouri, USA) for 30 min at 37 °C, then centrifuged (1500 rpm for 5 min). The supernatant was discarded and cells were resuspended in a trypsin inhibitor solution (0.67 mg/ml; Worthington Biochemical Corporation, NJ, USA), triturated and centrifuged. Cells were resuspended in serum-free media (SFM) containing l-glutamine (2 mM, Invitrogen), penicillin/streptavidin (100 U/0.1 mg/ml, Invitrogen), mitogens (epidermal growth factor; EGF, 20 ng/ml; Peprotech, QC; fibroblast growth factor; FGF, 10 ng/ml; Gibco, NY, USA; heparin, 2 μg/ml, Sigma), triturated, centrifuged and resuspended in 1 ml of SFM. The total number of cells was assessed on a hemocytometer and plated at 10 cells/ul in 24 well plates (ThermoFisher, PA) in SFM with growth factors at 37OC and 5% CO2. The number of neurospheres (>80 μm) were counted in 6 wells per animal after 7 days in vitro.
2.5. Immunohistochemistry
At the time of sacrifice, animals were injected with an overdose of tribromomethanol (Avertin; Sigma-Aldrich, Missouri, USA) and perfused with 30 ml of ice-cold 1 X PBS, followed by 4% paraformaldehyde. Brains were removed and post-fixed for 4 h, then transferred to 30% sucrose in PBS until saturation. Tissue was embedded with OCT compound (ThermoFisher, PA) and frozen. Brains were cryosectioned (20 μm) and collected onto SuperfrostPlus slides (ThermoFisher, PA), and stored at −20 °C until use.
At the time of staining, slides were thawed at room temperature. For Ki67 and Dcx staining, slides were placed into citric acid and heated for 15 min at 95°C, cooled and rinsed 3 × 5 min in 1 X PBS. For all staining, slides were incubated with 5% donkey serum in 1 X PBS+0.3% Triton-X [PBS-T; collectively, blocking solution (BS)] for 1 h at room temperature. BS was replaced with primary antibodies to assess markers for microglia/macrophages [1:500 Iba-1 (Wako, Japan)]; cell proliferation [1:1000 mouse Ki67 (BD Biosciences, ON)]; neuroblasts [1:200 mouse doublecortin (Dcx; Santa Cruz, CA)]; and oligodendrocytes and oligodendrocyte precursor cells [1:500 rabbit Olig2 (Millipore, MA)] in BS, and incubated overnight at 4°C. The following day, slides were rinsed 3 × 5 min in 1 X PBS+0.2% Tween, followed by incubation with secondary antibody (1:400 goat-anti-mouse 488, 1:400 goat-anti-mouse 568; 1:400 goat-anti-rabbit 568, Invitrogen, CA) and incubation for 5 min in the presence of 1:10,000 DAPI (Invitrogen, CA). Following a final 3 × 5 min rinse in 1 X PBS, slides were cover slipped using Dako fluorescent mounting media (ThermoFisher, PA).
Imaging was performed using Zeiss microscopes (Axiovert 200 M or spinning disc confocal; Zeiss, Germany). Immunopositive cells were counted from an average of 12 sections per animal spaced 200 μm apart encompassing the motor cortex between AP+1.18 and −0.8 from bregma, an average of 6 sections encompassing the dentate gyrus region of the hippocampus from between AP-1.46 to −2.46, and an average of 7 sections encompassing the SEZ between AP+1.18 and + 0.14 from bregma. The number of positive cells for each marker is reported relative to the average of sham controls.
2.6. Statistical analyses
All statistical analyses were conducted using SPSS (v. 23; IBM) and Prism (v. 6; GraphPad) software. Cylinder and foot fault were analyzed by one-way analysis of variance (ANOVA) followed by Bonferroni post-hoc tests. Puzzle box was analyzed using repeated-measures ANOVA followed by Bonferroni post-hoc tests. Immunohistochemical and neurosphere data were transformed into fold change compared to the average value of respective sham animals and analyzed using two-tailed unpaired t-tests. A p-value of <0.05 was considered significant.
3. Results
3.1. Metformin treatment rescues motor and cognitive deficits
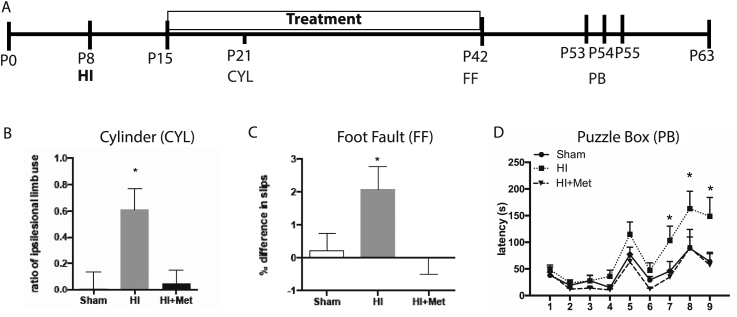
To determine whether delaying metformin treatment to the subacute phase after HI could promote functional recovery, mice received HI injury on P8, and metformin treatment was initiated 1 week later (P15). Periodic behavioural testing was performed throughout the study (Fig. 1A). Motor function was assessed at an early time point (P22) using the cylinder test, which measures spontaneous limb use during exploratory movement. This test revealed a significant deficit in HI animals (ratio of ipsilesional forelimb use in shams = −0.01 ± 0.07; HI = 0.61 ± 0.36; p < 0.001; Fig. 1B), which was rescued in animals who had received metformin (HI + Met = 0.17 ± 0.08; p = 0.094). Motor function was tested at a chronic timepoint (P42) using the foot fault task, which assesses coordinated motor function while traversing a grid. This test revealed a significant deficit in HI animals (% slip difference shams = 0.22 ± 0.52; HI = 2.066 ± 0.71; p = 0.026; p = 0.008; Fig. 1C), which was recovered following metformin treatment (% slip difference 0.00 ± 0.50; p = 0.821).
Fig. 1.
Metformin treatment improves motor and cognitive outcomes following hypoxia-ischemia. (A) Experimental timeline (B) Following HI, there was a significant increase in the ratio of unimpaired limb use at P22 when assessed using the cylinder task. This effect was reversed by metformin treatment. (C) Following HI, animals made significantly more foot slips at PID42 when assessed using the foot fault task. This impairment was rescued in animals that received metformin treatment. (D) Following HI, animals demonstrated an increased latency to perform the puzzle box task on trials 7, 8, and 9. This effect was rescued following metformin treatment. Data are presented as mean ± SEM; n = 14–24/group; ∗p < 0.05.
Neonatal HI results in cognitive deficits that are apparent in adulthood (Muntsant et al., 2019; Ruddy et al., 2019). We assessed cognition using the puzzle box task starting on P53. The puzzle box tests cognitive domains including problem solving and short- and long-term memory (Table 1) (Abdallah et al., 2011). As predicted, the HI injured mice were impaired compared to sham mice, with significant deficits observed on trials 7, 8, and 9 (p = 0.030, 0,003, and 0.0006, respectively) which test long-term memory, problem-solving, and short-term memory, respectively. Strikingly, the HI injured mice that received metformin for 4 weeks were not impaired on these trials (all p > 0.05 compared to shams; Fig. 1D).
Taken together, these results show that delaying metformin treatment to the subacute phase post-HI is effective in improving functional outcome. We next investigated cellular outcomes to determine potential mechanisms of metformin’s action.
3.2. Metformin treatment reduces inflammation following hypoxia ischemia
Metformin has been shown to have anti-inflammatory effects in the adult brain following injury (Tao et al., 2018). In neonates, there is evidence that immediate metformin administration has similar effects 24 h after injury (Fang et al., 2017), but the long-term effects following neonatal ischemia, and following delayed administration, have not been examined. We identified microglia/macrophages, the inflammatory cells of the CNS (Fernández-Arjona et al., 2017; Zhang et al., 2014), using ionized calcium-binding adaptor molecule 1 (Iba-1). These were quantified in the motor cortex and hippocampus, areas that underlie the functional deficits observed in the HI-injured brain (Rumajogee et al., 2016), in subsets of animals at P28, following 1 week of subacute metformin treatment (Fig. 2A). We observed a significant increase in Iba1+ cells in the motor cortex of HI injured brain (shams = 1.00 ± 0.36-fold; HI = 4.48 ± 1.05-fold increase compared to shams; p = 0.009; Fig. 2B and C). HI injured mice that received metformin treatment did not have a significantly different number of Iba1+ cells compared to shams (0.67 ± 0.29-fold decrease compared to shams; p = 0.007; Fig. 2B and C).
Fig. 2.
Metformin treatment reduces inflammation following hypoxia-ischemia. (A) Experimental timeline. The relative number of Iba1+ cells was significantly increased in the motor cortex (B–C) and hippocampus (D–E) following HI at P28 and exhibited a more amoeboid shape (solid arrowheads). There were significantly fewer Iba1+ cells, and Iba1+ cells demonstrated a more ramified morphology following metformin treatment in both brain regions (empty arrowheads). Data are presented as mean ± SEM; n = 3–5/group.
Similar findings were observed in the hippocampus, where HI-injured mice showed increased Iba1+ cells at P28 (sham = 1.00 ± 0.23-fold; 6.9 ± 0.97-fold increase compared to shams; p = 0.002; Fig. 2D and E). Metformin treatment led to significantly fewer Iba1+ cells compared to injured mice, and not significantly different from sham (1.90 ± 2.46-fold increase; p = 0.008).
By day 63, irrespective of the brain region, the number of Iba1+ cells returned to control (uninjured) levels in both HI (treated and untreated) groups (p > 0.05 for all groups; Supplemental Fig. S1A), with no further effects resulting from metformin treatment. These data suggest that the early inflammatory response is reduced by metformin treatment, an effect that was associated with functional improvements, while inflammation is resolved in vehicle-treated mice by 8 weeks post-injury.
3.3. Metformin treatment increases oligodendrogenesis
Previous work, including our own, has shown that metformin increases the size of the neonatal neural stem cell pool when administered in vivo (Dadwal et al., 2015; Ruddy et al., 2019), and exposure in vitro enhances neurogenesis and oligodendrogenesis from neural precursor cells. Notably, this neural stem cell expansion is age-dependent, and not observed in pre-pubescent (juvenile) mice that receive the same duration of metformin treatment (from P15-21) (Ruddy et al., 2019). Herein, we asked whether NPC activation, neurogenesis, and/or oligodendrogenesis were associated with the functional improvements observed in HI injured mice that received delayed metformin treatment.
We first assessed the stem cell pool using the in vitro neurosphere assay. There was no change in the size of the neural stem cell pool in mice that received HI and 7 days of vehicle or metformin treatment (from P15–P22) (Veh = 1.10 ± 0.12-fold versus Metformin = 1.03 ± 0.12-fold change; p = 0.637; Fig. 3A and B). Proliferation in the SEZ was examined using the marker Ki67 at P28, the time at which motor recovery was observed on cylinder test (Fig. 3A). We observed no difference in Ki67+ cells in the SEZ as a result of metformin treatment (sham = 1.00 ± 0.04-fold; HI = 0.917 ± 0.05-fold, HI + Met = 0.862 ± 0.09-fold compared to shams; p = 0.333; Fig. 3C and D). A similar analysis in the hippocampus at P63 (Fig. 3E), at a time when cognitive function had improved, revealed no change in the number of proliferating cells in the dentate gyrus following HI, with or without metformin treatment (sham = 1.00 ± 0.31-fold; HI = 0.66 ± 0.16-fold; HI + Met = 1.94 ± 0.94-fold compared to shams; Fig. 3F and G; p = 0.651).
Fig. 3.
Metformin treatment does not alter proliferation in neurogenic regions. (A) The neurosphere assay revealed no significant differences in the relative size of the stem cell pool following metformin treatment (n = 12/group over 3 independent experiments). (B) Experimental timeline. (C–D) The number of Ki67+ cells (arrowheads) in the subependymal zone (SEZ) were also unaffected by metformin treatment (LV; lateral ventricle). (E) Experimental timeline. (F–G) There was no effect of metformin treatment on Ki67+ cells in the hippocampus. Data are represented as mean ± SEM; n = 3–5/group.
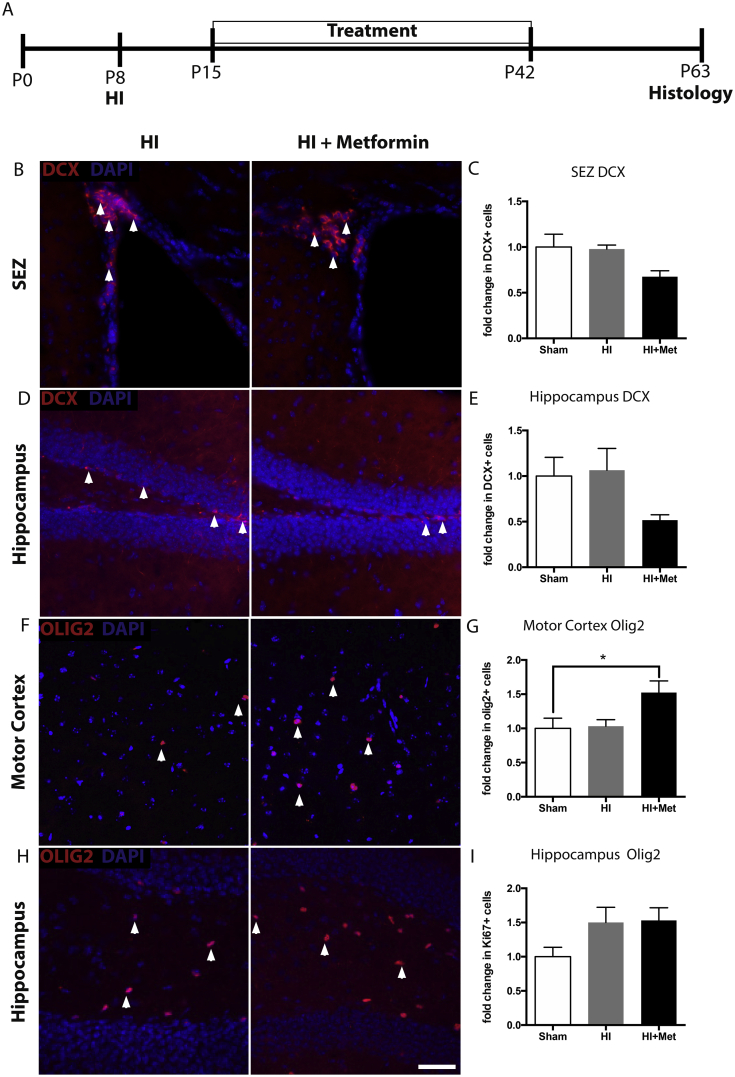
Given the reported pro-neurogenic effects of metformin (Dadwal et al., 2015; Jin et al., 2014; Liu et al., 2014) we assessed potential long-term changes in neurogenesis. We examined the neurogenic regions of the brain at P63, a time when long-term metformin treatment was sufficient to promote functional recovery across domains, using the neuroblast marker Dcx (Fig. 4A). We found no significant difference in Dcx + cells following metformin treatment in the SEZ (sham = 1.00 ± 0.14-fold; HI = 0.91 ± 0.04-fold; HI + Met = 0.63 ± 0.06-fold decrease compared to shams; p = 0.138; Fig. 4B and C), and no difference between groups in the dentate gyrus (sham = 1.00 ± 0.21-fold; HI = 1.07 ± 0.24-fold; HI + Met = 0.52 ± 0.06-fold change compared to shams; p = 0.212; Fig. 4D and E). Based on the observed trend of fewer Dcx + cells in both regions, we hypothesized that metformin treatment may be leading to neuroblast migration into the brain parenchyma. However, virtually all of the Dcx + cells were confined to the neurogenic germinal zones in all groups.
Fig. 4.
Metformin treatment alters neural cell fate in a brain region-specific manner. (A) Experimental timeline. (B–C) Metformin treatment did not significantly reduce the number of neuroblasts (arrowheads) in the subependymal zone. (D–E) No significant reduction in neuroblasts was detected in the hippocampus. (F–G) Metformin treatment significantly increased the number of Olig2+ cells in the motor cortex of metformin-treated animals. (H–I) The number of Olig2+ cells in the hippocampus was unaffected (n = 3–5/group). Data are presented as mean ± SEM; ∗p < 0.05.
Metformin has been shown to promote oligodendrogenesis in the naïve (Wang et al., 2012) and injured brain (Dadwal et al., 2015); therefore we examined the numbers of Olig2-expressing cells in the motor cortex and hippocampus. Interestingly, we found a significant increase in the number of Olig2+ cells in the motor cortex of metformin-treated animals, but not vehicle-treated animals, after HI (sham = 1.00 ± 0.15-fold; HI = 1.027 ± 0.11-fold; HI + Met = 1.45 ± 0.16-fold increase compared to shams; p = 0.034; Fig. 4F and G). There was no difference in the number of Olig2+ cells in the hippocampus (sham = 1.00 ± 0.14-fold; HI = 1.50 ± 0.22-fold; HI + Met = 1.53 ± 0.18-fold compared to shams; p = 0.187; Fig. 4H and I), revealing regionally distinct effects of metformin’s actions.
Metformin administration in naïve animals (20 mg/kg daily from P15-42) did not have a significant effect on Ki67+, Dcx+, or Olig2+ cells present at P63 (Supplemental Figure S1C-G), suggesting that a combination of injury and metformin treatment is necessary for the increased oligodendrogenesis observed.
4. Discussion
HI is a devastating injury that leads to the loss of neurons and oligodendrocytes and results in chronic motor and cognitive impairments. With few treatment options available, alternative therapeutic approaches to treat HI are required. Metformin has gained much attention for its pleiotropic effects in the CNS. We have previously shown that metformin treatment, when initiated as early as 1 day post-HI in neonatal mice, improves recovery (Dadwal et al., 2015; Ruddy et al., 2019). Here, we explored whether there is an extended therapeutic window in which this recovery is possible. Our data indicate that delaying metformin treatment to the subacute stage post-injury results in motor and cognitive recovery. Further, metformin treatment results in a profound reduction in the number of microglia in the injured brain and an increase in oligodendrogenesis in the motor cortex. These findings highlight metformin’s pleiotropic effects and suggest that there are regionally distinct responses that are coincident with the functional recovery observed (Ayoub et al., 2020).
Microglia and infiltrating macrophages become activated following injury and express both pro-inflammatory (e.g.; TNF-α, IL-1β and IL-6) and anti-inflammatory (e.g.; IL-10, TFGβ) cytokines (Fernández-Arjona et al., 2017). The extent and time course of the microglia response is dependent on the type of injury, age and sex (Kerr et al., 2019; Li et al., 2013; Villa et al., 2018; Wofford et al., 2019). After neonatal HI, microglia/macrophages increase in number as early as 6 h post-injury, and remain elevated for at least a week (Hellström Erkenstam et al., 2016; Serdar et al., 2019) with concomitant increases in the release of pro-inflammatory cytokines, creating a hostile environment and further tissue damage (Mifsud et al., 2014). It is postulated that neuroinflammation is more detrimental to pathological progression than the primary injury itself (Patterson and Holahan, 2012; Wofford et al., 2019). We demonstrate that neonatal HI leads to increased numbers of microglia/macrophages for at least 21 days after injury, and that metformin decreases this response. Moreover, the metformin-induced change in the inflammatory response post-injury was correlated with functional recovery in motor tasks at early and late times post-injury. In vitro and in vivo metformin treatment increases phosphorylation of adenosine monophosphate-activated kinase (AMPK) in microglia (Pan et al., 2016). This process inhibits inflammatory detection and microglial activation and leads to a reduction in the release of pro-inflammatory cytokines. As a result, the environment is shifted to a more anti-inflammatory state, conducive to oligodendrogenesis (Wu et al., 2010; Liu et al., 2019).
Recent work has shown that the effect of metformin on the size of the SEZ-derived NPC pool is age-dependent. These cells are responsive to metformin when administered to neonatal (starting at P9), but not juvenile (starting at P18), mice (Ruddy et al., 2019). Herein, we extend these findings and establish that metformin is ineffective at expanding the size of the neural stem cell pool as early at P15. These data are consistent with metformin regulating the differentiation/survival of NPC-derived cells, rather than expansion of the NPC pool.
In the absence of an expansion in the NPC pool, the observed increase in Olig2+ cells in the motor cortex suggests a possible increase in survival of NPCs directed toward an oligodendroglial lineage. Alternatively, the target of metformin’s actions could include oligodendrocyte precursor cells that exist throughout the parenchyma and comprise approximately 5% of the CNS (Dawson et al., 2003). These cells, also known as NG2+ cells, can be inhibited by microglia following injury (Wu et al., 2010), hence the reduction in microglia we observed following metformin treatment may support the proliferation, differentiation, and survival of these cells. Future research involving the lineage tracking of this population will further elucidate whether parenchymal NG2 cells demonstrate a response to metformin treatment.
Our results revealed an increase in the number of oligodendrocytes in the motor cortex, but not the hippocampus. This was in line with the lack of effect on cell proliferation or neuroblast production in this brain region, and is perhaps not surprising given that NPCs in the dentate gyrus do not normally differentiate into oligodendrocytes, in striking contrast to those originating from the SEZ (Braun et al., 2015). Nonetheless, we observed cognitive recovery using the puzzle box task. This task requires the contribution of several areas of the brain, and it is possible this effect was mediated by compensation involving non-hippocampal brain regions. Indeed, we detected an impairment when mice were challenged with a problem-solving task, which involves input from the medial prefrontal cortex (Abdallah et al., 2011).
Our findings are consistent with the cellular basis for the functional recovery being related to oligodendrogenesis. Enhancing oligodendrocyte formation and myelination has been associated with improved functional recovery following ischemic injury in the adult (Sun et al., 2013) and neonatal (Iwai et al., 2010) brain, as well as following cranial irradiation (Ayoub et al., 2020). Oligodendrocytes can provide protection for existing neuronal axons from degradation, and/or remyelinate axons for appropriate signal conductance (Iwai et al., 2010). There is increasing emphasis on therapeutic strategies that target oligodendrocytes (Mifsud et al., 2014), Taken together, our findings show that delaying metformin treatment in a clinically relevant paradigm effectively facilitates functional recovery following HI. Delayed metformin treatment was associated with an altered inflammatory response and increased oligodendrogenesis, further demonstrating the therapeutic potential of metformin treatment following neonatal HI in patients who are in the subacute injury phase. We anticipate that these findings will be important to guide the development and implementation of novel interventions in the clinic involving subacute metformin treatment to accelerate and/or augment post-injury recovery, to ultimately improve pediatric outcomes in a wider patient population.
Funding
This work was supported by the Canadian Institute for Health Research, the Stem Cell Network, and the Ontario Institute of Regenerative Medicine.
Author contributions
CM, JL, and EG designed the study and wrote the manuscript. Surgeries, behaviour testing, data analysis, and manuscript preparation was performed by JL. Histological analyses were performed by JL, TS, and TC. Imaging was performed by JL and EG.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank A. Azimi for assistance with histological analyses, and R. Ruddy for valued insight into the experiments design and interpretation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2020.100119.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Cellular analyses (A) Experimental timeline. (B) The number of Iba1+ cells in the HI-injured brain returned to control levels by P63, and was not further affected by metformin treatment (p = 0.700). (C-G) Metformin treatment in the naïve brain did not alter Ki67 expression in in the (C) SEZ or (D) hippocampus; did not affect doublecortin expression from (E) SEZ or (F) hippocampus, or alter Olig2 expression in the (G) motor cortex or (H) hippocampus when analyzed at P63, 19 days following the termination of treatment. Data are presented as mean ± SEM; ∗p < 0.05. n = 3–7/group.
References
- Abdallah B., Nada M.-B., Fuss J., Trusel M., Galsworthy M.J., Bobsin K., Colacicco G., Deacon R.M.J., Riva M.A., Kellendonk C., Sprengel R., Lipp H.-P., Gass P. The puzzle box as a simple and efficient behavioral test for exploring impairments of general cognition and executive functions in mouse models of schizophrenia. Exp. Neurol. 2011;227:42–52. doi: 10.1016/j.expneurol.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Adstamongkonkul D., Hess D.C. Ischemic Conditioning and neonatal hypoxic ischemic encephalopathy: a literature review. Cond. Med. 2017;1:9–16. [PMC free article] [PubMed] [Google Scholar]
- Ayoub R., Ruddy R., Cox E., Oyefiade A., Derkach D., Laughlin S., Ades-aron B., Shirzadi Z., Fieremans E., MacIntosh B.J., de Medeiros C.B., Skocic J., Bouffet E., Miller F.D., Morshead C.M., Mabbott D.J. Metformin for cognitive and neural recovery in a preclinical model of cranial radiation and in a pilot clinical trial with survivors of a paediatric brain tumour. Nat. Med. 2020 doi: 10.1038/s41591-020-0985-2. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun S.M.G., Pilz G.-A., Machado R.A.C., Becher B., Toni N., Correspondence S.J. Programming hippocampal neural stem/progenitor cells into oligodendrocytes enhances remyelination in the adult brain after injury. Cell Rep. 2015;11:1679–1685. doi: 10.1016/j.celrep.2015.05.024. [DOI] [PubMed] [Google Scholar]
- Dadwal P., Mahmud N., Sinai L., Azimi A., Fatt M., Wondisford F.E., Miller F.D., Morshead C.M. Activating endogenous neural precursor cells using metformin leads to neural repair and functional recovery in a model of childhood brain injury. Stem Cell Rep. 2015;5:166–173. doi: 10.1016/j.stemcr.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M.R.L., Polito A., Levine J.M., Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol. Cell. Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Fang M., Jiang H., Ye L., Cai C., Hu Y., Pan S., Li P., Xiao J., Lin Z. Metformin treatment after the hypoxia-ischemia attenuates brain injury in newborn rats. Oncotarget. 2017;8:75308–75325. doi: 10.18632/oncotarget.20779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Arjona M. del M., Grondona J.M., Granados-Durán P., Fernández-Llebrez P., López-Ávalos M.D. Microglia morphological categorization in a rat model of neuroinflammation by hierarchical cluster and principal components analysis. Front. Cell. Neurosci. 2017;11:235. doi: 10.3389/fncel.2017.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn A.J., Thoresen M. Neonatal encephalopathy and hypoxic-ischemic encephalopathy. Handb. Clin. Neurol. 2019;162:217–237. doi: 10.1016/B978-0-444-64029-1.00010-2. [DOI] [PubMed] [Google Scholar]
- Hellström Erkenstam N., Smith P.L.P., Fleiss B., Nair S., Svedin P., Wang W., Boström M., Gressens P., Hagberg H., Brown K.L., Sävman K., Mallard C. Temporal characterization of microglia/macrophage phenotypes in a mouse model of neonatal hypoxic-ischemic brain injury. Front. Cell. Neurosci. 2016;10:286. doi: 10.3389/fncel.2016.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai M., Stetler R.A., Xing J., Hu X., Gao Y., Zhang W., Chen J., Cao G. Enhanced oligodendrogenesis and recovery of neurological function by erythropoietin after neonatal hypoxic/ischemic brain injury. Stroke. 2010;41:1032–1037. doi: 10.1161/STROKEAHA.109.570325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q., Cheng J., Liu Y., Wu J., Wang X., Wei S., Zhou X., Qin Z., Jia J., Zhen X. Improvement of functional recovery by chronic metformin treatment is associated with enhanced alternative activation of microglia/macrophages and increased angiogenesis and neurogenesis following experimental stroke. Brain Behav. Immun. 2014;40:131–142. doi: 10.1016/j.bbi.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Kerr N., Dietrich D.W., Bramlett H.M., Raval A.P. Sexually dimorphic microglia and ischemic stroke. CNS Neurosci. Ther. 2019;25:1308–1317. doi: 10.1111/cns.13267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurinczuk J.J., White-Koning M., Badawi N. Epidemiology of neonatal encephalopathy and hypoxic–ischaemic encephalopathy. Early Hum. Dev. 2010;86:329–338. doi: 10.1016/J.EARLHUMDEV.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Li K., Tan Y.-H., Light A.R., Fu K.-Y. Different peripheral tissue injury induces differential phenotypic changes of spinal activated microglia. Clin. Dev. Immunol. 2013:1–8. doi: 10.1155/2013/901420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.-Y., Wang X., Liu C., Zhang H.-L. Pharmacological targeting of microglial activation: new therapeutic approach. Front. Cell. Neurosci. 2019;13:514. doi: 10.3389/fncel.2019.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Tang G., Zhang Z., Wang Y., Yang G.-Y. Metformin promotes focal angiogenesis and neurogenesis in mice following middle cerebral artery occlusion. Neurosci. Lett. 2014;579:46–51. doi: 10.1016/j.neulet.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Mifsud G., Zammit C., Muscat R., Di Giovanni G., Valentino M. Oligodendrocyte pathophysiology and treatment strategies in cerebral ischemia. CNS Neurosci. Ther. 2014;20:603–612. doi: 10.1111/cns.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntsant A., Shrivastava K., Recasens M., Giménez-Llort L. Severe perinatal hypoxic-ischemic brain injury induces long-term sensorimotor deficits, anxiety-like behaviors and cognitive impairment in a sex-, age- and task-selective manner in C57BL/6 mice but can be modulated by neonatal handling. Front. Behav. Neurosci. 2019;13:7. doi: 10.3389/fnbeh.2019.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusrat L., Livingston-Thomas J.M., Raguthevan V., Adams K., Vonderwalde I., Corbett D., Morshead C.M. Cyclosporin A-mediated activation of endogenous neural precursor cells promotes cognitive recovery in a mouse model of stroke. Front. Aging Neurosci. 2018;10:93. doi: 10.3389/fnagi.2018.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Sun X., Jiang L., Hu L., Kong H., Han Y., Qian C., Song C., Qian Y., Liu W. Metformin reduces morphine tolerance by inhibiting microglial-mediated neuroinflammation. J. Neuroinflammation. 2016;13:294. doi: 10.1186/s12974-016-0754-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson Z.R., Holahan M.R. Understanding the neuroinflammatory response following concussion to develop treatment strategies. Front. Cell. Neurosci. 2012;6 doi: 10.3389/fncel.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice J.E., Vannucci R.C., Brierley J.B. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann. Neurol. 1981;9:131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- Ruddy R., Adams K., Morshead C. Age- and sex-dependent effects of metformin on neural precursor cells and cognitive recovery in a model of neonatal stroke. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aax1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumajogee P., Bregman T., Miller S.P., Yager j.Y., Fehlings M.G. Rodent hypoxia-ischemia models for cerebral palsy research: a systematic review. Front. Neurol. 2016;7:1–20. doi: 10.3389/fneur.2016.00057. April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serdar M., Kempe K., Rizazad M., Herz J., Bendix I., Felderhoff-Müser U., Sabir H. Early pro-inflammatory microglia activation after inflammation-sensitized hypoxic-ischemic brain injury in neonatal rats. Front. Cell. Neurosci. 2019;13:237. doi: 10.3389/fncel.2019.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Fang Y.Q., Ren H., Chen T., Guo J.J., Yan J., Song S., Zhang L.Y., Liao H. WIN55,212-2 protects oligodendrocyte precursor cells in stroke penumbra following permanent focal cerebral ischemia in rats. Acta Pharmacol. Sin. 2013;34:119–128. doi: 10.1038/aps.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao L., Li D., Liu H., Jiang F., Xu Y., Cao Y., Gao R., Chen G. Neuroprotective effects of metformin on traumatic brain injury in rats associated with NF-κB and MAPK signaling pathway. Brain Res. Bull. 2018;140:154–161. doi: 10.1016/j.brainresbull.2018.04.008. [DOI] [PubMed] [Google Scholar]
- Venna V.R., Li J., Hammond M.D., Mancini N.S., McCullough L.D. Chronic metformin treatment improves post-stroke angiogenesis and recovery after experimental stroke. Eur. J. Neurosci. 2014;39:2129–2138. doi: 10.1111/ejn.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vexler Z.S., Sharp F.R., Feuerstein G.Z., Ashwal S., Thoresen M., Yager J.Y., Ferriero D.M. Translational stroke research in the developing brain. Pediatr. Neurol. 2006;34:459–463. doi: 10.1016/j.pediatrneurol.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Villa A., Gelosa P., Castiglioni L., Cimino M., Rizzi N., Pepe G., Lolli F., Marcello E., Sironi L., Vegeto E., Maggi A. Sex-specific features of microglia from adult mice. Cell Rep. 2018;23:3501–3511. doi: 10.1016/j.celrep.2018.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe J.J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Gallagher D., Devito L.M., Cancino G.I., Tsui D., He L., Keller G.M., Frankland P.W., Kaplan D.R., Miller F.D. Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell. 2012;11:23–35. doi: 10.1016/j.stem.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Wassink G., Davidson J.O., Lear C.A., Juul S.E., Northington F., Bennet L., Gunn A.J. A working model for hypothermic neuroprotection. J. Physiol. 2018;596:5641–5654. doi: 10.1113/JP274928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wofford K.L., Loane D.J., Cullen D.K. Acute drivers of neuroinflammation in traumatic brain injury. Neural Regen. Res. 2019;14:1481. doi: 10.4103/1673-5374.255958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Yoo S., Wilcock D., Lytle J.M., Leung P.Y., Colton C.A., Wrathall J.R. Interaction of NG2+ glial progenitors and microglia/macrophages from the injured spinal cord. Glia. 2010;58:410–422. doi: 10.1002/glia.20932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.M., Lund H., Mia S., Parsa R., Harris R.A. Adoptive transfer of cytokine-induced immunomodulatory adult microglia attenuates experimental autoimmune encephalomyelitis in DBA/1 mice. Glia. 2014;62:804–817. doi: 10.1002/glia.22643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cellular analyses (A) Experimental timeline. (B) The number of Iba1+ cells in the HI-injured brain returned to control levels by P63, and was not further affected by metformin treatment (p = 0.700). (C-G) Metformin treatment in the naïve brain did not alter Ki67 expression in in the (C) SEZ or (D) hippocampus; did not affect doublecortin expression from (E) SEZ or (F) hippocampus, or alter Olig2 expression in the (G) motor cortex or (H) hippocampus when analyzed at P63, 19 days following the termination of treatment. Data are presented as mean ± SEM; ∗p < 0.05. n = 3–7/group.