Abstract
Introduction
YS110, a humanized monoclonal antibody with a high affinity to CD26, exhibited promising antitumor activity and was generally well-tolerated in the phase 1 part of a phase 1 and 2 Japanese trial in patients with malignant pleural mesothelioma (MPM). Here we report the results of the phase 2 part of the study.
Methods
The patients included were aged 20 years and older, had histologically confirmed MPM, were refractory to or intolerant of existing antineoplastic agents, and were not candidates for standard therapy. YS110 6 mg/kg, determined in the phase 1 dose-determination part, was given in 6-weekly cycles (5 × once-weekly infusions, followed by a 1-wk rest).
Results
The study included 31 patients (median age = 68 y, 90.3% men); 64.5% had stage IV MPM, 90.3% had greater than or equal to 20% CD26 expression in tumor tissue, and 38.7% (12 patients) had previously received nivolumab. The 6-month disease control rate was 3.2%. The best overall response was partial response in one patient and stable disease in 14 patients. The median progression-free survival was 2.8 months (both in patients who had and had not previously received nivolumab—groups A and B, respectively). Respective progression-free survival rates at 6 months were 9.1% and 31.6% in groups A and B. The median overall survival was 9.7 months. A total of 30 patients (96.8%) had at least one adverse event. Common treatment-related adverse events were infusion-related reaction (16.1%), hiccups (9.7%), and interstitial lung disease (9.7%). There were no treatment-related deaths.
Conclusions
The 6-month disease control rate did not exceed the predefined threshold, but YS110 revealed modest efficacy in response rate as salvage therapy in difficult-to-treat patients with MPM. YS110 was generally well tolerated.
Keywords: YS110, Malignant mesothelioma, Phase 2, CD26, Japanese
Introduction
Malignant pleural mesothelioma (MPM) is a relatively rare but aggressive malignancy that is generally associated with a very poor prognosis.1,2 Surgery, chemotherapy, and radiation therapy are the anticancer treatment options available for patients with MPM, and multimodal treatment has been noted to be the most effective approach, but only benefits selected patients with favorable disease subtypes. Most patients with MPM present with extensive disease and receive palliative chemotherapy. Guidelines from the National Comprehensive Cancer Network recommend vinorelbine, gemcitabine, and pemetrexed as second-line treatment options if not administered in the first line.3 However, treatment for MPM beyond first-line therapy is still unsatisfactory owing to limited efficacy. The most recent update to the National Comprehensive Cancer Network guidelines also includes the added recommendation to consider using pembrolizumab or nivolumab, alone or in combination with ipilimumab, as subsequent treatment options for patients with MPM.3 In Japan, nivolumab was approved as second-line or later therapy for MPM on the basis of a phase 2 study that reported a promising overall response rate (29.4%), progression-free survival (PFS) (median = 6.1 mo), and overall survival (OS) (median = 17.3 mo) with nivolumab monotherapy in patients with advanced MPM.4 In contrast, pembrolizumab as salvage therapy was superior to chemotherapy in objective response rate, but not in PFS and OS, in a phase 3 study.5 Because treatment options for MPM as second-line or later therapy are still limited, further investigation is warranted to explore active drugs for MPM.
CD26, a 110-kDa type II transmembrane glycoprotein with dipeptidyl peptidase IV activity, is overexpressed in MPM cells but not in benign mesothelial tissue.6,7 CD26 expression is also an important factor associated with improved survival in patients with MPM who receive chemotherapy and was closely linked to cell-cycle regulation, apoptosis, and chemotherapy resistance in in vitro and microarray studies.7 In addition, CD26 antibodies were found to suppress tumor growth by means of ubiquitin-specific protease 22 in a preclinical study.8 These findings indicate that CD26 is a potential therapeutic target for MPM.
YS110, a humanized monoclonal antibody with a high affinity to CD26, has exhibited promising antitumor activity in phase 1 studies involving French9 and Japanese10 patients with MPM. In the Japanese open-label, dose-escalation phase 1 study (part of a phase 1 and 2 study), three patients were enrolled into each dose group of 2, 4, or 6 mg/kg, and each 6-week treatment cycle consisted of weekly YS110 administration for 5 weeks followed by a 1-week rest period. The best overall response was partial response (PR) in one of nine patients and stable disease in four of nine patients, and no patient developed dose-limiting toxicity (maximum dose 6 mg/kg).10 Given the favorable toxicity profile and clinical activity of YS110 seen in the phase 1 part of this study, the phase 2 part of the study was conducted in a larger group of patients to evaluate the efficacy of this new drug for MPM.
Materials and Methods
Study Design and Procedure
This was the phase 2 part of a multicenter, open-label, phase 1 and 2 study (ClinicalTrials.gov: NCT03177668). The study protocol was almost the same as in the previously published phase 1 part.10 Patients included were those aged 20 years and older (no upper limit, unlike the phase 1 part), had histologically confirmed MPM with at least one measurable lesion, were refractory to or intolerant of existing antineoplastic agents, and were not suitable candidates for standard therapy.
Patients received YS110 6 mg/kg in 6-week cycles (once-weekly infusions for 5 wk, 1-wk rest), which was determined in the phase 1 dose-determination part. Study treatment was continued until patients met discontinuation criteria, including progressive disease (PD), the occurrence of intolerable toxicities, or request by the patient or physician to discontinue. Prophylactic agents, d-chlorpheniramine maleate, methylprednisolone, dexamethasone, acetaminophen, and ranitidine hydrochloride were also administered to reduce infusion-related reactions on the basis of the French phase 1 study results.9 Methylprednisolone could be omitted in the second administration of cycle one and subsequent administrations. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice (GCP) guidelines. All patients provided written informed consent. The study protocol was approved by the ethics committees of the participating centers.
Clinical Evaluation
Efficacy outcomes included a 6-month disease control rate (DCR), DCR, overall response rate (ORR), PFS, and OS. These end points were chosen on the basis of a retrospective multicenter survey of second-line chemotherapy in MPM.11 Tumor diameter was measured every 6 weeks using computed tomography. Tumor response was analyzed by the central assessment committee using Response Evaluation Criteria In Solid Tumors criteria modified for MPM.12 The 6-month DCR was defined as the proportion of patients with complete response (CR), PR, or stable disease at 24 weeks. DCR was defined as the percentage of patients with CR, PR, or stable disease as their best overall response. ORR was defined as the percentage of patients with CR or PR as their best overall response. PFS was the time from the first treatment date to PD or death from any cause. OS was the time from the first treatment date to death from any cause. Safety outcomes included adverse events (AEs), laboratory tests, vital signs, and a 12-lead electrocardiogram. AE severity was evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03). CD26 expression rate was analyzed by immunohistochemical staining using a prototype companion diagnostics kit (Nichirei Biosciences Inc., Tokyo, Japan).
Statistical Analysis
A sample size of 30 patients was estimated to confirm that the lower limit of the two-sided 95% confidence interval (CI) of 6-month DCR would exceed the threshold of 23% at a statistical power of 80% or higher. This threshold was defined on the basis of a retrospective multicenter survey of second-line chemotherapy in MPM.11
The efficacy analysis was performed using the full analysis set, and the safety analysis was performed using the safety set. The full analysis set included patients who had measurable lesions at baseline and were treated with the study drug at least once, excluding those with GCP or protocol violations. The safety set included all patients who received at least one dose of the study drug, except those with GCP violations.
PFS and OS were analyzed using the Kaplan-Meier method. To explore further biomarkers that may determine treatment efficacy, subgroup analyses were performed to investigate the association between MPM tissue type (epithelioid, sarcomatoid, or biphasic) or pretreatment and the antitumor efficacy of YS110. For the second subgroup analysis, responses in patients who had (group A) and had not (group B) previously received nivolumab were analyzed. The correlation between CD26 expression levels and tumor shrinkage or PFS was also assessed. All statistical analyses used the Statistical Analysis System version 9.4 (SAS Institute Inc., Cary, NC).
Results
Patient Characteristics
Between June 2018 and March 2019, a total of 38 patients from 12 institutions consented to participate. Seven patients did not receive YS110 because they did not meet the eligibility criteria, and the remaining 31 patients received at least one dose of YS110 (Table 1). The median age was 68 years (range: 55–81). The MPM histologic subtype was epithelioid in 26 patients, biphasic in three patients, and sarcomatoid in two patients. Most patients (64.5%) had stage IV MPM. Most patients were men (90.3%) and had an Eastern Cooperative Oncology Group performance status of 1 (61.3%). In 28 patients (90.3%), CD26 expression in tumor tissue was at least 20%. All patients had previously received platinum-based chemotherapy and 12 patients (38.7%) had received nivolumab (Table 1). The median number of treatment cycles per patient was 2 (range: 1–5). A total of 27 patients continued the study until PD, and four patients discontinued the study because of AEs (n = 2), at patient's request (n = 1), and as per the investigator's judgment (n = 1). At data cutoff on February 7, 2020, a total of 19 patients had died.
Table 1.
Patient Baseline Demographic and Clinical Characteristics
| Characteristic | YS110 6 mg/kg (N = 31) |
Nivolumab Treatment History |
|
|---|---|---|---|
| Previously Treated (n = 12, 38.7%) Group A | Untreated (n = 19, 61.3%) Group B | ||
| Age (y), median (range) | 68 (55–81) | 67 (55–81) | 68 (57–75) |
| Sex, n (%) | |||
| Male | 28 (90.3) | 10 (83.3) | 18 (94.7) |
| Female | 3 (9.7) | 2 (16.7) | 1 (5.3) |
| Tumor histology, n (%) | |||
| Epithelioid | 26 (83.9) | 10 (83.3) | 16 (84.2) |
| Sarcomatoid | 2 (6.5) | 1 (8.3) | 1 (5.3) |
| Biphasic | 3 (9.7) | 1 (8.3) | 2 (10.5) |
| CD26 expression in tumor tissue, % | |||
| Mean ± SD | 71.3 ± 31.44 | 62.9 ± 35.96 | 76.6 ± 27.94 |
| <20%, n (%) | 3 (9.7) | 2 (16.7) | 1 (5.3) |
| ≥20%, n (%) | 28 (90.3) | 10 (83.3) | 18 (94.7) |
| ECOG performance status, n (%) | |||
| 0 | 12 (38.7) | 5 (41.7) | 7 (36.8) |
| 1 | 19 (61.3) | 7 (58.3) | 12 (63.2) |
| Tumor stage (IMIG TMN), n (%) | |||
| II | 3 (9.7) | 0 | 3 (15.8) |
| III | 8 (25.8) | 3 (25.0) | 5 (26.3) |
| IV | 20 (64.5) | 9 (75.0) | 11 (57.9) |
ECOG, Eastern Cooperative Oncology Group; IMIG, International Mesothelioma Interest Group; TMN, tumor-node metastasis.
Efficacy
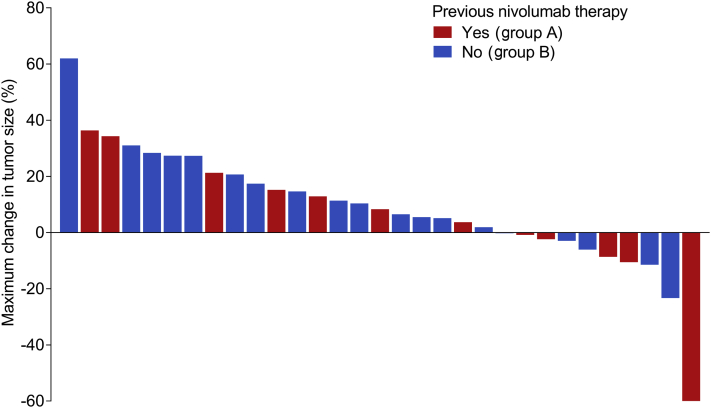
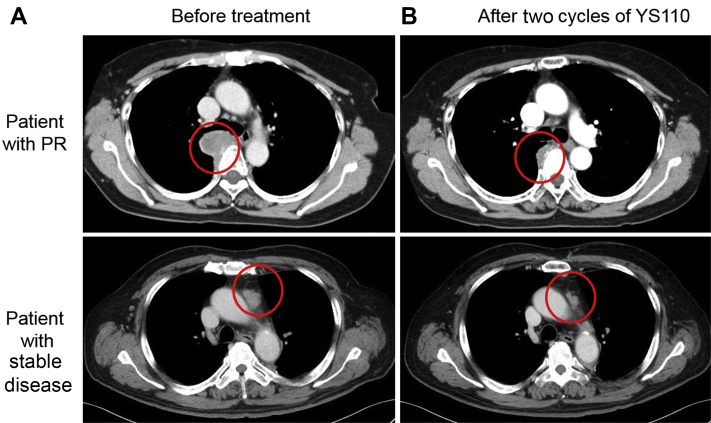
At the data cutoff, the median follow-up time was 9.7 months (interquartile range: 4.8–12.2) and 27 progression events had occurred. Of the 31 patients, the best overall response was PR in one patient, stable disease in 14, PD in 14 patients, and not assessable in two patients. The DCR and ORR were 48.4% and 3.2%, respectively. The patient who achieved PR was a 67-year-old woman with a baseline Eastern Cooperative Oncology Group performance status of 0, epithelioid MPM, and CD26 expression in tumor tissue of 40%. She had previously been treated with triple therapy of pemetrexed, cisplatin, and nivolumab. PR was observed after one cycle and continued for 4.5 months; the maximum tumor shrinkage was 64%. Another patient had a long-lasting stable disease (>24 wk) and the 6-month DCR was 3.2% (1 of 31 patients). A waterfall plot has illustrated that 10 patients had tumor shrinkage and one of 10 patients satisfied the criterion for PR (Fig. 1). Seven patients who exhibited less than 20% tumor increase in Figure 1 were classified as either PD or not assessable because of the short interval of evaluation, the appearance of new lesions, or progression of nontarget lesions. Computed tomography scans of the two patients with the most tumor shrinkage are illustrated in Figure 2A and B.
Figure 1.
Waterfall plot illustrating the maximum change in tumor size for each patient (N = 31). Of the 31 patients, the best overall response was PR in one patient, stable disease in 14, PD in 14 patients, and not assessable in two patients. PD, progressive disease; PR, partial response.
Figure 2.
Computed tomography scans of the two patients with the most tumor shrinkage. Scans performed (A) before treatment and (B) after two cycles of YS110. PR, partial response.
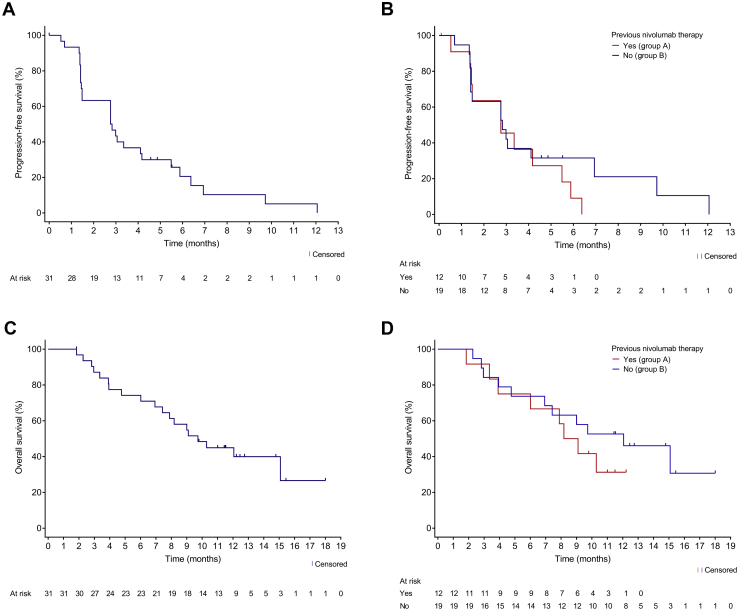
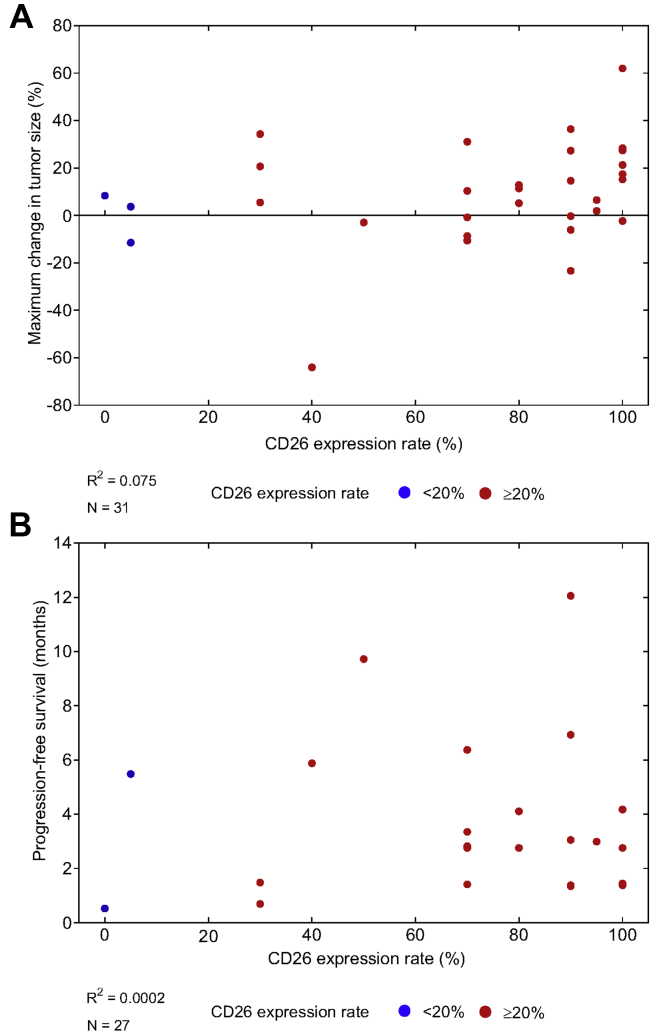
In the total cohort (N = 31), the median PFS was 2.8 months (95% CI: 1.5–4.2) and the PFS rate at 6 months was 20.6% (95% CI: 7.7–37.7) (Fig. 3A). The duration of PFS in each patient is illustrated in Supplementary Figure 1. The median OS was 9.7 months (95% CI: 6.9–not reached [NR]), and the OS rate at 6 and 12 months were 74.2% (95% CI: 55.0–86.2) and 44.9% (95% CI: 27.1–61.2), respectively (Fig. 3C). The correlation coefficients between the CD26 expression levels and tumor shrinkage was 0.075 and between the CD26 expression and PFS was 0.0002 (Fig. 4A and B).
Figure 3.
Progression-free survival (A) in the total cohort and (B) by previous nivolumab treatment. Overall survival (C) in the total cohort and (D) by previous nivolumab treatment.
Figure 4.
Relationship among CD26 expression in tumor tissue, (A) maximum change in tumor size, and (B) progression-free survival. Each dot represents an individual patient. Censored patients were excluded from this analysis.
Soluble CD26 and dipeptidyl peptidase-4 (DPP4) decreased after the first YS110 administration and thereafter remained low during the study (Supplementary Fig. 2).
Subgroup Analyses
When efficacy was evaluated in different MPM tissue types, the median PFS was 3.0, 2.8, and 1.5 months for patients with epithelioid, biphasic, and sarcomatoid MPM, respectively.
When efficacy was evaluated by previous nivolumab exposure, the best overall response in group A (nivolumab-treated group) was PR in one patient and stable disease in seven patients; the ORR and DCR were 8.3% and 58.3%, respectively in group A, and were 0% and 42.1%, respectively in group B (nivolumab-untreated group). The median PFS was 2.8 months (95% CI: 1.4–5.5) in group A and 2.8 months (95% CI: 1.4–6.9) in group B (Fig. 3B). The respective 6-month PFS rates in groups A and B were 9.1% (95% CI: 0.5–33.3) and 31.6% (95% CI: 12.9–52.2), and the respective median OS was 8.6 months (95% CI: 3.4–NR) and 12.1 months (95% CI: 4.8–NR) in groups A and B (Fig. 3D). The respective values for the 6-month OS in groups A and B were 75.0% (95% CI: 40.8–91.2) and 73.7% (95% CI: 47.9–88.1), and 31.3% (95% CI: 8.4–57.8) and 52.6% (95% CI: 28.7–71.9) for 12-month OS.
Safety
A total of 30 patients (96.8%) experienced at least one AE, and in 16 patients (51.6%), the events were related to YS110 (Table 2). The most common treatment-related AEs (≥5% of patients) were infusion-related reaction (16.1%), hiccups (9.7%), interstitial lung disease (9.7%), diarrhea (6.5%), and pruritus (6.5%, Table 2). Treatment-related grade 3 or higher AEs occurred in four patients (five grade 3 events in three patients and one grade 4 events in one patient). Six serious AEs occurred: two cases of interstitial lung disease and one case each of pneumonia bacterial, tumor lysis syndrome, hypercapnia, and hypoxia. Three patients interrupted or postponed treatment because of AEs (all infusion-related reactions, one accompanied by vomiting), and two patients discontinued treatment because of AEs (infusion-related reaction and interstitial lung disease). There were no treatment-related deaths.
Table 2.
AEs in the Safety Set (N = 31)
| Event, n (%) | AEs |
AEs Related to YS110 |
||
|---|---|---|---|---|
| Any | Grade ≥3 | Any | Grade ≥3 | |
| Any event | 30 (96.8) | 18 (58.1) | 16 (51.6) | 4 (12.9) |
| AEs occurring in ≥2 patients | ||||
| Lymphocyte count decreased | 14 (45.2) | 14 (45.2) | 0 | 0 |
| Constipation | 7 (22.6) | 0 | 0 | 0 |
| IRR | 5 (16.1) | 1 (3.2) | 5 (16.1) | 1 (3.2) |
| Anemia | 4 (12.9) | 3 (9.7) | 1 (3.2) | 0 |
| Diarrhea | 4 (12.9) | 0 | 2 (6.5) | 0 |
| Nasopharyngitis | 4 (12.9) | 0 | 0 | 0 |
| Hiccups | 4 (12.9) | 0 | 3 (9.7) | 0 |
| Pruritus | 4 (12.9) | 0 | 2 (6.5) | 0 |
| Nausea | 3 (9.7) | 0 | 1 (3.2) | 0 |
| Pyrexia | 3 (9.7) | 0 | 1 (3.2) | 0 |
| URT infection | 3 (9.7) | 0 | 0 | 0 |
| ILD | 3 (9.7) | 2 (6.5) | 3 (9.7) | 2 (6.5) |
| Hyperglycemia | 2 (6.5) | 1 (3.2) | 0 | 0 |
| Back pain | 2 (6.5) | 0 | 0 | 0 |
| Insomnia | 2 (6.5) | 0 | 0 | 0 |
| Cough | 2 (6.5) | 0 | 0 | 0 |
| Rash | 2 (6.5) | 0 | 1 (3.2) | 0 |
AE, adverse event; ILD, interstitial lung disease; IRR, infusion-related reaction; URT, upper respiratory tract.
Seven patients developed 10 events judged as infusion-related by the investigator; these include six infusion-related reactions, vomiting, seizure, dyspnea, and rash. These events were experienced by four of 12 patients (33.3%) who had previously received nivolumab and three of 19 patients (15.8%) who had not.
Discussion
This phase 2 part of a phase 1 and 2 study was conducted as an exploratory evaluation of the safety and efficacy of YS110 6 mg/kg in patients with advanced-stage MPM who are resistant to or intolerant of standard therapy, as YS110 was generally well tolerated at doses up to 6 mg/kg in Japanese patients in the phase 1 part of the study. In the phase 2 part of this study, the 6-month DCR did not exceed the predefined threshold of 23%. The best overall response with YS110 was PR, which was also observed in the phase 1 part.10 Meanwhile, DCR seemed slightly lower than that seen in the phase 1 part of this study or in the French phase 1 study (48.4% versus 55.6%10 and 50.0%,9 respectively). However, three out of 14 patients who had PD as their best overall response did experience a stable disease after the completion of the first cycle of YS110 but they did not meet the predefined time interval to be classified as stable disease, so DCR may be underestimated in the phase 2 part of this study. Further studies are warranted to fully elucidate and clarify the efficacy of YS110 for the treatment of MPM.
Although YS110 is an antibody against CD26, the association between CD26 expression and YS110 efficacy remains unknown from this study. Indeed, several of the patients who had stable disease had low CD26 expression. One explanation for this discrepancy is that current immunohistochemistry techniques may be unable to estimate CD26 expression in tumor cells correctly. To date, in vitro data indicate that a humanized monoclonal antibody against CD26 induces the inhibition of cell growth in MPM cells by cell-cycle regulation and repression of transcriptional genes.8,13, 14, 15 The inhibitory effect on CD26-positive cell proliferation in vitro was more marked in cells exhibiting stronger versus weaker CD26 positivity.16 In addition, the antitumor effects of YS110 were exhibited in a CD26-positive, tumor-bearing mouse model,13 suggesting an association between CD26 expression and anti-CD26 antibody activity in vivo and in vitro. However, contrary to expectations, no clear associations were found in the present study. This suggests that the antitumor activity of YS110 may not only be linked to the expression of CD26 in tissues but also to controlling T-cell–dependent effects by inhibiting the soluble CD26/DPP4 enzyme and then inhibiting cleavage of the chemokine CXCL10 which is truncated by DPP4 activity.17 In addition, inhibiting the CD26/DPP4 enhances CCL11-mediated eosinophil migration into solid tumors and antitumor response.18 In fact, soluble CD26 and DPP4 both decreased after YS110 administration.
It may be possible to improve the efficacy of YS110 through a more detailed evaluation of the relationship between CD26 localization and antitumor activity. Some studies have reported promising results with immune checkpoint inhibitors for patients with advanced MPM,4,19 which suggests that controlling immune function may be important in MPM. To control immune function, a combination of YS110 and immune checkpoint inhibitors may produce synergistic effects. A combination of these drugs may be safe, as the safety profile of YS110 is generally different from those of immune checkpoint inhibitors. However, interstitial lung disease, which is a known severe adverse drug reaction with immune checkpoint inhibitors, occurred in three patients. Although the effects of YS110 on the lung have not been fully elucidated, the concomitant use of YS110 and immune checkpoint inhibitors should be administered with caution. Considering the safety profile of YS110, other combination therapies, such as CD47/SIRP-α inhibitors, may be appropriate. Combination therapy with CD47/SIRP-α and CD20 inhibitors is an exciting and rapidly developing field. CD47 is highly expressed in most patients with epithelioid-type malignant mesothelioma.20 However, this combination is more often used for hematologic malignancies. Blocking CD47/SIRP-α interactions has been found to promote the destruction of tumor cells by macrophages21; however, it is not clear whether MPM cells are destroyed by phagocytosis with CD47/SIRP-α inhibition. Further studies are needed to clarify the appropriate combination therapies and the mechanisms of the antitumor effect of YS110 for MPM.
In this study, 12 patients had previously received treatment with nivolumab, including the patient who achieved a PR and four patients who had tumor shrinkage but did not meet the PR criteria; the resulting DCR was 58.3% (7 of 12 patients) including six stable disease patients. YS110 may be more effective in shrinking the tumor after immune checkpoint inhibitor use, and similar trends in PFS or OS were observed between nivolumab-treated and -naive patients in this study. Some studies have reported improved response rates to salvage chemotherapy after treatment with immune checkpoint inhibitors in patients with NSCLC.22,23 However, no PFS or OS benefit was observed in these studies, implying that the impact of immune checkpoint inhibition on T-cells was short-lived.23 In this respect, our results are consistent with the data on salvage chemotherapy after immune checkpoint inhibition in patients with NSCLC.23 It must be remembered that survival data (PFS and OS) are affected by previous lines of therapy and any poststudy treatments the patients received. Further trials are expected to confirm whether the effects of YS110 are influenced by immunotherapy.
In this study, two patients had severe interstitial lung disease, causing one patient to discontinue treatment. This was the only severe treatment-related AE that occurred in more than one patient. There were no reports of interstitial lung disease in the phase 1 part or in the French phase 1 study.9,10 Because CD26/DPP4 has widespread tissue distribution and is expressed in lung epithelial cells,24,25 the possibility that YS110 was responsible for the case of interstitial lung disease cannot be ruled out. However, YS110 has been administered in only a small number of patients to date, so the relationship between YS110 and interstitial lung disease has not yet been elucidated. Although interstitial lung disease did not cause any deaths in this study, the careful management of interstitial lung disease should be considered.
In conclusion, the 6-month DCR did not exceed the predefined threshold, but YS110 revealed modest efficacy in response rate and revealed a potential disease-stabilizing activity in PFS and OS in this difficult-to-treat patient population. YS110 was generally well tolerated. Further studies are warranted, including a more detailed evaluation of the relationship between CD26 localization and efficacy and an investigation of combination therapy with other antineoplastic agents.
CRediT Authorship Contribution Statement
Kazuhiko Nakagawa, Takumi Kishimoto, Masato Hirokawa, Hironori Matsuki, Yutaro Kaneko, Taketo Yamada and Chikao Morimoto: Conception or Design of the study. Kazuhiko Nakagawa, Takashi Kijima, Morihito Okada, Masahiro Morise, Motoyasu Kato, Katsuya Hirano, Nobukazu Fujimoto, Mitsuhiro Takenoyama, Hiroshi Yokouchi, Yuichiro Ohe, Toyoaki Hida, Keisuke Aoe, and Masayuki Takeda: Enroll patients. Hironori Matsuki: Analysis the study data. Kazuhiko Nakagawa, Takashi Kijima, Morihito Okada, Masahiro Morise, Motoyasu Kato, MD, Katsuya Hirano, Nobukazu Fujimoto, Mitsuhiro Takenoyama, Hiroshi Yokouchi, Yuichiro Ohe, Toyoaki Hida, Keisuke Aoe, Takumi Kishimoto, Masato Hirokawa, Hironori Matsuki, Yutaro Kaneko, Taketo Yamada, Chikao Morimoto, and Masayuki Takeda: Data interpretation, Writing of the manuscript, Approval the final version of the manuscript.
Acknowledgments
This study was sponsored by Kissei Pharmaceutical Co., Ltd. The sponsor was involved in designing the study, data collection, data analysis, data interpretation, and writing of the report. The authors thank Toni Dando of Springer Healthcare Communications who wrote the first draft of this manuscript. Medical writing assistance was funded by Kissei Pharmaceutical Co., Ltd.
Footnotes
Disclosure: Dr. Nakagawa reported receiving grants and personal fees from AstraZeneca K.K., Astellas Pharma Inc., Merck Sharp & Dohme K.K., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., Bristol-Myers Squibb, Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., and Merck Serono Co., Ltd./Merck Biopharma Co., Ltd.; grants, personal fees, and consulting/advisor role fees from Ono Pharmaceutical Co., Ltd., Pfizer Japan Inc., and Eli Lilly Japan K.K. during the conduct of the study outside the submitted work; personal fees from Clinical Trial Co., Ltd., Medicus Shuppan Publishers Co., Ltd., Care Net, Inc., Reno. Medical K.K., Medical Review Co., Ltd., Roche Diagnostics K.K., Bayer, Yakuhin, Ltd., Medical Mobile Communications Co., Ltd., 3H Clinical Trial Inc., Nichi-Iko Pharmaceutical Co., Ltd., Nanzando Co., Ltd., Yodosha Co., Ltd., Nikkei Business Publications, Inc., Thermo Fisher Scientific K.K., Yomiuri Telecasting Corporation, and Nippon Kayaku Co., Ltd.; personal fees and consulting/advisor role fees from Kyorin Pharmaceutical Co., Ltd. and Takeda Pharmaceutical Co., Ltd.; grants and personal fees from Taiho Pharmaceutical Co., Ltd., SymBio Pharmaceuticals Ltd., and AbbVie Inc.; and grants from inVentiv Health Japan, ICON Japan K.K., Gritsone Oncology Inc., Parexel International Corp., Kissei Pharmaceutical Co., Ltd., EPS Corporation, Syneos Health., Pfizer Research and Development Japan G.K., A2 Healthcare Corp., Quintiles Inc./IQVIA Services Japan K.K., EP-CRSU CO., Ltd., Linical Co., Ltd., Eisai Co., Ltd., CMIC Shift Zero K.K., Kyowa Hakko Kirin Co., Ltd., Bayer Yakuhin, Ltd., EPS International Co., Ltd. and Otsuka Pharmaceutical Co., Ltd. Dr. Morise reports receiving grants and nonfinancial support from F. Hoffmann-La Roche during the conduct of the study outside the submitted work; has received personal fees from Eli Lilly, Chugai Pharmaceutical Co., Ltd., AstraZeneca, Ono Pharmaceutical Co., Ltd., Pfizer, and Merck Sharp & Dohme; has worked as a principal investigator in a clinical trial for Chugai Pharmaceutical Co., Ltd., AstraZeneca, Pfizer, Merck Serono, Kissei Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., and Novartis; and received a research grant from Boehringer Ingelheim. Dr. Hirano reported receiving grants from Kissei Pharmaceutical Co., Ltd. during the conduct of the study outside the submitted work, and grants and personal fees from Ono Pharmaceutical Co., Ltd., and Eli Lilly. Dr. Fujimoto reports receiving grants from Kissei Pharmaceutical Co., Ltd. during the conduct of the study outside the submitted work; grants and personal fees from Ono Pharmaceutical Co., Ltd., Bristol-Myers Squibb, and Kyorin Pharmaceutical Co., Ltd.; personal fees from Chugai Pharmaceutical Co., Ltd. and Daiichi Sankyo; and grants from Merck Sharp & Dohme. Dr. Takenoyama reports receiving grants from Kissei Pharmaceutical Co., Ltd. during the conduct of the study outside the submitted work; grants and personal fees from AstraZeneca, Bristol-Myers Squibb, Chugai Pharmaceutical Co., Ltd., Covidien Japan, Eli Lilly Japan, Johnson & Johnson, Kyowa Hakko Kirin Co., Ltd., Merck Sharp & Dohme, Nippon Boehringer Ingelheim, Novartis Pharma, Ono Pharmaceutical Co., Ltd., Pfizer Japan, and Taiho Pharmaceutical Co., Ltd.; personal fees from Nippon Kayaku Co., Ltd.; and grants from KM Biologics. Dr. Yokouchi reports receiving grants from Kissei Pharmaceutical Co., Ltd. during the conduct of the study outside the submitted work; grants and personal fees from AstraZeneca; and grants from AbbVie, Bristol-Myers Squibb, Taiho Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Merck Biopharma, and Takeda Pharmaceutical Co., Ltd. Dr. Ohe reports receiving grants from Kissei Pharmaceutical Co., Ltd. during the conduct of the study outside the submitted work; grants and personal fees from AstraZeneca, Bristol-Myers Squibb, Chugai Pharmaceutical Co., Ltd., Eli Lilly, Kyorin Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Ono Pharmaceutical Co., Ltd., Pfizer, Taiho Pharmaceutical Co., Ltd., and Takeda; personal fees from Boehringer Ingelheim, Celltrion, Amgen, and Novartis; and grants from Janssen and Ignyta. Dr. Hida reports receiving grants from Kissei Pharmaceutical Co., Ltd. during the conduct of the study outside the submitted work; grants and personal fees from Ono Pharmaceutical, Co., Ltd., Chugai Pharmaceutical Co., Ltd., AstraZeneca, Novartis, Bristol-Myers Squibb, Merck Sharp & Dohme, Nippon Boehringer Ingelheim, Taiho Pharmaceutical Co., Ltd., Pfizer, and Takeda Pharmaceutical Co., Ltd.; and grants from Ignyta, Merck Serono, Eisai, AbbVie, Daiichi Sankyo, Astellas, and Janssen Pharmaceutical. Dr. Aoe reports receiving grants from Kissei Pharmaceutical Co., Ltd. during the conduct of the study outside the submitted work; grants and personal fees from Ono Pharmaceutical, Co., Ltd., Bristol-Myers Squibb, AstraZeneca, and Eli Lilly; and grants from Merck Sharp & Dohme (Merck), Novartis, and Kyorin Pharmaceutical, Co., Ltd. Dr. Yamada reports receiving grants from Kissei Pharmaceutical Co., Ltd., Nichirei Bioscience Co. Ltd., and the Health Sciences of the National Institute of Biomedical Innovation of Japan during the conduct of the study. Dr. Takeda reports receiving personal fees from AstraZeneca K.K., Ono Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Bristol-Myers Squibb, Chugai Pharmaceutical Co., Ltd., Novartis Pharma K.K., and Kissei Pharmaceutical Co., Ltd. during the conduct of the study. Drs. Hirokawa and Matsuki are employees of Kissei Pharmaceutical Co. Ltd., Japan. Dr. Kaneko has received personal fees and is a member of the board (Chief Executive Officer) of Y’s AC Co., Ltd. Dr. Morimoto is a company founder of Y’s AC Co., Ltd. (YS110 was patented by Y's AC Co., Ltd. and Y's AC Co., Ltd. and possesses an exclusive right of the use of patent worldwide). The remaining authors declare no conflict of interest.
Cite this article as: Nakagawa K, et al. Phase 2 Study of YS110, a Recombinant Humanized Anti-CD26 Monoclonal Antibody, in Japanese Patients With Advanced Malignant Pleural Mesothelioma. JTO Clin Res Rep. 2021;2:100178
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2021.100178.
Supplementary Data
References
- 1.Cao C., Croce B., Harris R. MPM: malignant pleural mesothelioma. Ann Cardiothorac Surg. 2012;1:544. doi: 10.3978/j.issn.2225-319X.2012.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Gerwen M., Alpert N., Wolf A. Prognostic factors of survival in patients with malignant pleural mesothelioma: an analysis of the National Cancer Database. Carcinogenesis. 2019;40:529–536. doi: 10.1093/carcin/bgz004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: malignant pleural mesothelioma. version 1.2019. http://www.nccn.org/professionals/physician_gls/pdf/mpm.pdf. Accessed April 4, 2020.
- 4.Okada M., Kijima T., Aoe K. Clinical efficacy and safety of nivolumab: results of a multicenter, open-label, single-arm, Japanese phase II study in malignant pleural mesothelioma (MERIT) Clin Cancer Res. 2019;25:5485–5492. doi: 10.1158/1078-0432.CCR-19-0103. [DOI] [PubMed] [Google Scholar]
- 5.Popat S., Curioni-Fontecedro A., Dafni U. A multicentre randomised phase III trial comparing pembrolizumab vs single-agent chemotherapy for advanced pre-treated malignant pleural mesothelioma: the European Thoracic Oncology Platform (ETOP 9–15) PROMISE-meso trial. Ann Oncol. 2020;31:1734–1745. doi: 10.1016/j.annonc.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Amatya V.J., Takeshima Y., Kushitani K., Yamada T., Morimoto C., Inai K. Overexpression of CD26/DPPIV in mesothelioma tissue and mesothelioma cell lines. Oncol Rep. 2011;26:1369–1375. doi: 10.3892/or.2011.1449. [DOI] [PubMed] [Google Scholar]
- 7.Aoe K., Amatya V.J., Fujimoto N. CD26 overexpression is associated with prolonged survival and enhanced chemosensitivity in malignant pleural mesothelioma. Clin Cancer Res. 2012;18:1447–1456. doi: 10.1158/1078-0432.CCR-11-1990. [DOI] [PubMed] [Google Scholar]
- 8.Okamoto T., Yamazaki H., Hatano R. Targeting CD26 suppresses proliferation of malignant mesothelioma cell via downmodulation of ubiquitin-specific protease 22. Biochem Biophys Res Commun. 2018;504:491–498. doi: 10.1016/j.bbrc.2018.08.193. [DOI] [PubMed] [Google Scholar]
- 9.Angevin E., Isambert N., Trillet-Lenoir V. First-in-human phase 1 of YS110, a monoclonal antibody directed against CD26 in advanced CD26-expressing cancers. Br J Cancer. 2017;116:1126–1134. doi: 10.1038/bjc.2017.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takeda M., Ohe Y., Horinouchi H. Phase I study of YS110, a recombinant humanized monoclonal antibody to CD26, in Japanese patients with advanced malignant pleural mesothelioma. Lung Cancer. 2019;137:64–70. doi: 10.1016/j.lungcan.2019.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Zucali P.A., Simonelli M., Michetti G. Second-line chemotherapy in malignant pleural mesothelioma: results of a retrospective multicenter survey. Lung Cancer. 2012;75:360–367. doi: 10.1016/j.lungcan.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Byrne M.J., Nowak A.K. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol. 2004;15:257–260. doi: 10.1093/annonc/mdh059. [DOI] [PubMed] [Google Scholar]
- 13.Inamoto T., Yamada T., Ohnuma K. Humanized anti-CD26 monoclonal antibody as a treatment for malignant mesothelioma tumors. Clin Cancer Res. 2007;13:4191–4200. doi: 10.1158/1078-0432.CCR-07-0110. [DOI] [PubMed] [Google Scholar]
- 14.Yamada K., Hayashi M., Madokoro H. Nuclear localization of CD26 induced by a humanized monoclonal antibody inhibits tumor cell growth by modulating of POLR2A transcription. PLoS One. 2013;8 doi: 10.1371/journal.pone.0062304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayashi M., Madokoro H., Yamada K. Novel antibody-drug conjugate with anti-CD26 humanized monoclonal antibody and transcription factor IIH (TFIIH) inhibitor, triptolide, inhibits tumor growth via impairing mRNA synthesis. Cancers (Basel) 2019;11:1138. doi: 10.3390/cancers11081138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inamoto T., Yamochi T., Ohnuma K. Anti-CD26 monoclonal antibody-mediated G1-S arrest of human renal clear cell carcinoma Caki-2 is associated with retinoblastoma substrate dephosphorylation, cyclin-dependent kinase 2 reduction, p27(kip1) enhancement, and disruption of binding to the extracellular matrix. Clin Cancer Res. 2006;12:3470–3477. doi: 10.1158/1078-0432.CCR-06-0361. [DOI] [PubMed] [Google Scholar]
- 17.Ohnuma K., Hatano R., Morimoto C. DPP4 in anti-tumor immunity: going beyond the enzyme. Nat Immunol. 2015;16:791–792. doi: 10.1038/ni.3210. [DOI] [PubMed] [Google Scholar]
- 18.Hollande C., Boussier J., Ziai J. Inhibition of the dipeptidyl peptidase DPP4 (CD26) reveals IL-33-dependent eosinophil-mediated control of tumor growth. Nat Immunol. 2019;20:257–264. doi: 10.1038/s41590-019-0321-5. [DOI] [PubMed] [Google Scholar]
- 19.Alley E.W., Lopez J., Santoro A. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol. 2017;18:623–630. doi: 10.1016/S1470-2045(17)30169-9. [DOI] [PubMed] [Google Scholar]
- 20.Schurch C.M., Forster S., Bruhl F., Yang S.H., Felley-Bosco E., Hewer E. The “don’t eat me” signal CD47 is a novel diagnostic biomarker and potential therapeutic target for diffuse malignant mesothelioma. Oncoimmunology. 2017;7 doi: 10.1080/2162402X.2017.1373235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuo T.C., Chen A., Harrabi O. Targeting the myeloid checkpoint receptor SIRPα potentiates innate and adaptive immune responses to promote anti-tumor activity. J Hematol Oncol. 2020;13:160. doi: 10.1186/s13045-020-00989-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park S.E., Lee S.H., Ahn J.S., Ahn M.J., Park K., Sun J.M. Increased response rates to salvage chemotherapy administered after PD-1/PD-L1 inhibitors in patients with non-small cell lung cancer. J Thorac Oncol. 2018;13:106–111. doi: 10.1016/j.jtho.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 23.Kato R., Hayashi H., Chiba Y. Propensity score-weighted analysis of chemotherapy after PD-1 inhibitors versus chemotherapy alone in patients with non-small cell lung cancer (WJOG10217L) J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2019-000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dimitrova M., Ivanov I., Todorova R. Comparison of the activity levels and localization of dipeptidyl peptidase IV in normal and tumor human lung cells. Tissue Cell. 2012;44:74–79. doi: 10.1016/j.tice.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Vliegen G., Raju T.K., Adriaensen D., Lambeir A.M., De Meester I. The expression of proline-specific enzymes in the human lung. Ann Transl Med. 2017;5:130. doi: 10.21037/atm.2017.03.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.