Abstract
Lorlatinib is a third-generation ALK inhibitor that can overcome the largest number of acquired ALK resistance mutations, including the solvent-front mutation G1202R. Here, we report, for the first time, a novel, sequentially-evolved EML4-ALK variant 3 G1202R/S1206Y double mutation in cis detected in a patient with ALK-positive NSCLC after disease progression on sequential crizotinib, alectinib, and then lorlatinib. Three-dimensional computer modeling of this double mutation and other G1202R-based double mutations with lorlatinib (ALK G1202R/L1196M, ALK G1202R/F1174C, ALK G1202R/l1198F, ALK G1202R/G1269A) were provided to reveal how these double mutations may confer resistance to lorlatinib through diverse steric hindrances in the ALK kinase domain. In addition, we performed a comprehensive literature review on published acquired double or triple ALK mutations that are resistant to lorlatinib from both patient samples and in vitro mutagenesis experiments.
Keywords: G1202R, S1206Y, Double mutation, Lorlatinib resistance, ALK+ NSCLC
Introduction
Lorlatinibis a third-generation ALK tyrosine kinase inhibitor (TKI) that can overcome the largest number of acquired ALK resistance mutations1,2 regardless of whether theyemerge in the background of the two major EML4-ALK fusion variants (variants 1 and 3).2 On the basis of a phase 2 study,3 lorlatinib is approved both in the United States (November 2, 2018) and by the European Medical Agency (February 28, 2019) for the treatment of patients with ALK-positive NSCLC who have progressed on either first-line (1L) ceritinib or 1L alectinib or crizotinib and at least another ALK TKI. Sequential use of ALK TKIs from the first generation to second generation (2G) and then to lorlatinib has led to acquired double or triple resistance mutations.4, 5, 6, 7 Here, we report a novel acquired ALK double mutation in cis that is resistant to lorlatinib after several years of lorlatinib use following sequential treatment with crizotinib and alectinib.
Methods and Results
Case Description
The patient is a never-smoking Asian woman who was diagnosed with stage IV NSCLC with malignant pleural effusion in June 2010 at age 57 years (Fig. 1A). She received carboplatin/nab-paclitaxel/bevacizumab for four cycles, followed by bevacizumab/pemetrexed maintenance for three cycles. Her tumor was profiled at the University of Colorado as part of the Lung Cancer Mutation Consortium and found to be positive for ALK by fluorescence in-situ hybridization (FISH). The patient was referred to the crizotinib phase 2 trial (PROFILE1005, NCT00932451), but tested negative for ALK by FISH by the trial-designated central laboratory, so she was enrolled into the “ALK-negative” cohort of the crizotinib phase 1 trial (PROFILE1001, NCT00585195). The false-negative result by the central laboratory in the early days of crizotinib clinical trial was likely owing to the steep learning curve of analyzing ALK FISH results because of the complexity of interpreting break-apart FISH (break-apart probes have to be separated by at least two probe sizes to be considered positive; interpreting 3' isolated ALK signals; counting 15% of tumor cells with break-apart signals based on at least 50 unique tumors cells). She received crizotinib at 250 mg twice daily from February 2011 to May 2013 with confirmed partial response as the best response. On disease progression, she was enrolled in the alectinib phase 1/2 study (NCT01871805) from May 2013 to October 2013 with progressive disease as the best response. Next-generation sequencing of a metastatic right axillary lymph node revealed the presence of EML4-ALK variant 3 fusion and ALK G1202R mutation conferring resistance to alectinib.
Figure 1.
(A) Schematic summary of the treatment course. (B) IGV of the G1202R/S1206Y in cis double mutation over the duration of treatment. Bev, bevacizumab; Carbo, carboplatin; CNS, central nervous system; IGV, integrated genome view; Pem, pemetrexed; SRS, stereotactic radiosurgery; MAF, mutant allele frequency.
She was then retreated with crizotinib with the addition of carboplatin/pemetrexed plus bevacizumab followed by maintenance pemetrexed plus bevacizumab from October 2013 to September 2015 before enrollment into the three previous ALK TKIs cohort of phase 2 lorlatinib study (NCT01970865). She achieved confirmed partial response as the best response with lorlatinib at 100 mg daily until December 2019, when she developed headache and jaw pain with brain and cervical spine magnetic resonance imaging revealing four subcentimeter brain lesions and a soft tissue mass around the odontoid. This was the first evidence of brain metastasis during her almost 10-year course of ALK+ NSCLC. Circulating tumor DNA obtained as previously described5 at that time revealed EML4-ALK variant 3 plus the emergence of an on-target ALK S1206Y resistance mutation (mutant allele frequency [MAF]: 0.49%) in addition to original ALK G1202R resistance mutation (MAF: 0.39%) in cis (Fig. 1B). This double ALK G1202R/S1206Y mutation in cis was identified about 40 months of lorlatinib treatment. After stereotactic radiation to the brain metastases and the odontoid mass, she received a commercial supply of lorlatinib at 100 mg daily in combination with chemotherapy carboplatin/pemetrexed/bevacizumab for three cycles and then pemetrexed/bevacizumab maintenance with stable disease. The patient subsequently underwent occiput to C3 fusion in June 2020 while on lorlatinib. Pemetrexed/bevacizumab chemotherapy was resumed postoperatively with a steady decrease of carcinoembryonic antigen levels from 357.7 ng/mL immediately after the procedure to 127.2 ng/mL at the last record. Repeat circulating tumor DNA testing revealed decreased but continual presence of ALK G1202R (MAF: 0.19%) and ALK S1206Y (MAF: 0.34%) (Fig. 1B). Currently, the patient remains on the combination of lorlatinib and chemotherapy. The patient provided written consent to have her treatment history published.
Review of Literature
We identified multiple double and triple mutations from both patient samples and in vitro mutagenesis experiments that confer resistance to lorlatinib after a review of the literature (Table 1). Both G1202R-paired or non–G1202R-paired mutations are identified, indicating multiple pathways leading to a common on-target phenotypic resistance to lorlatinib.6, 7, 8, 9, 10, 11, 12 Indeed, even without a preexisting solvent-front mutation but with a common preexisting acquired resistance mutation to alectinib, such as I1171X,13 subsequent treatment with lorlatinib despite its efficacy may lead to an increased risk of double mutations. Other less common acquired resistance mutations to crizotinib can also lead to double mutations when subsequently treated with lorlatinib.
Table 1.
List of Compound Mutations Resistant to Lorlatinib From Both Clinical Samples and In Vitro Mutagenesis Experiments
| Existing Pre-Lorlatinib Mutation(s) | Post-Lorlatinib Mutations | Clinical Response to ALK TKI | Reference |
|---|---|---|---|
| Clinical samples | |||
| G1202R | G1202R/L1196M | NA | Yoda, 20186 |
| G1202R/L1196M (EML4-ALK v3, tumor) | NA | Dagogo-Jack, 20197 | |
| G1202R/L1196M (plasma) | Intrinsic resistance | NA | Sharma, 201910 |
| G1202R | G1202R/F1174L (tumor) G1202R/F1174C (CTC-1) G1202R/T1151M (CTC-10) |
NA | Pailler, 20199 |
| NA | G1202R/G1269A (EML4-ALK v3, tumor) | NA | Dagogo-Jack, 20197 |
| S1206F/G1202Ra | S1206F/G1202R/G1269Aa (EML4-ALK v3, tumor) | NA | Dagogo-Jack, 20197 |
| G1202R/G1269A/L1204V (EML4-ALK v3, tumor) | NA | Dagogo-Jack, 20197 | |
| G1202R | G1202R/L1204V/G1269A | NA | Yoda, 20186 |
| I1171N/T + D1203N (EML4-ALK v3, tumor) | NA | Dagogo-Jack, 20197 | |
| I1171N/T + L1198F (EML4-ALK v2, tumor) | NA | Dagogo-Jack, 20197 | |
| G1202R/I1171N | I1171N/D1203N (plasma) | NA | Dagogo-Jack, 20197 |
| G1202R/E1145K | G1202R/F1174L | NA | Recondo, 202011 |
| D1203N | D1203N/G1123D (plasma) | NA | Dagogo-Jack, 20197 |
| L1196M | L1196M/G1202R (plasma) | NA | Dagogo-Jack, 20197 |
| L1196M | L1196M/D1203N | NA | Dagogo-Jack, 20197 |
| I1171N/L1196M (EML4-ALK v3) | L1196M/G1202R (cis) + I1171N (plasma) | NA | Hu, 202012 |
| I1171S/F1174L | I1171S/G1269A (plasma) | NA | Dagogo-Jack, 20197 |
| E1210K/D1203N | E1210K/D1203N/G1269A (plasma) | NA | Dagogo-Jack, 20197 |
| G1202R/L1196M/V1801Lb | G1202R/L1196M/C1156Yb (plasma) | NA | Dagogo-Jack, 20197 |
| C1156Y | C1156Y/L1198F | Crizotinib | Shaw, 20164 |
| ENU mutagenesis screen | |||
| Parent mutation | Acquired second mutation | In vitro sensitivityc | |
| C1156Y (EML4-ALK v1) | I1171T | NA | Yoda, 20186 |
| F1174C/I/V | NA | Yoda, 20186 | |
| L1196M | NA | Yoda, 20186 | |
| L1198F | NA | Yoda, 20186 | |
| D1203N | NA | Yoda, 20186 | |
| S1256F | NA | Yoda, 20186 | |
| G1269A | NA | Yoda, 20186 | |
| I1171N (EML4-ALK v3) | F1174L | None | Okada, 20198 |
| F1174I | None | Okada, 20198 | |
| L1196M | Ceritinib [(11 ± 0.8) nM] Brigatinib [(32 ± 6.1) nM] |
Okada, 20198 | |
| L1198H | None | Okada 20198 | |
| L1198F | Brigatinib [(49 ± 3.9) nM] | Okada, 20198 | |
| L1256F | Brigatinib [(42 ± 6.3) nM] | Okada 20198 | |
| G1269A | Ceritinib [(14 ± 2.9) nM] Brigatinib [(7.1 ± 0.4) nM] |
Okada, 20198 | |
| F1174C (EML4-ALK v1) | L1196M | NA | Yoda, 20186 |
| G1269A | NA | Yoda, 20186 | |
| G1269A (EML4-ALK v1) | I1171N/T | NA | Yoda, 20186 |
| L1196M | NA | Yoda, 20186 | |
| L1196M (EML4-ALK v1) | I1171S | NA | Yoda, 20186 |
| F1174C/L/V | NA | Yoda, 20186 | |
| L1179V | NA | Yoda, 20186 | |
| L1198F/H | NA | Yoda, 20186 | |
| L1256F | NA | Yoda, 20186 | |
| G1202R (EML4-ALK v1) | L1196M | NA | Yoda, 20186 |
| L1198F | NA | Yoda, 20186 | |
| G1202R (EML4-ALK v3) | F1174C | None | Okada, 20198 |
| F1174L | None | Okada, 20198 | |
| L1196M | None | Okada, 20198 | |
| L1198F | None | Okada, 20198 | |
| G1269Ad | None | Okada, 20198 | |
CTC, circulating tumor cell; ENU, N-ethyl-N-nitrosourea; IC50, concentration that inhibits 50%; NA, not available; TKI, tyrosine kinase inhibitor; v1, variant 1; v2, variant 2; v3, variant 3.
Case presented at WCLC 2019 by Professor Alice T. Shaw.14
G1202R was the dominant resistance mutation after brigatinib, and G1202R/C1156Y was the dominant resistance mutation after lorlatinib.
Sensitivity is considered as IC50 less than or equal to 50 nM (based on ENU clonal cells).
Patient derived cell lines.
Three-Dimensional Modeling of Lorlatinib Resistance
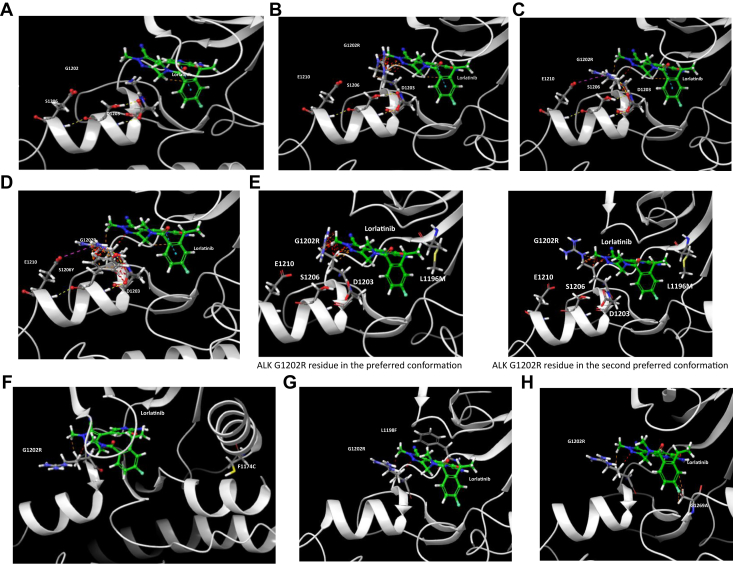
Given that ALK G1202R is one of the most frequently acquired resistance mutation to 2G ALK TKI and often sets the stage for subsequently acquired second-site resistance mutations to emerge after treatment with lorlatinib on the basis of its currently approved indications, we have modeled all the reported ALK G1202R-based double mutations in the presence of lorlatinib (Fig. 2A–H). Although the flexible ALK G1202R residue provides steric hindrance to lorlatinib in certain rotamers (Fig. 2B), the basic ALK G1202R residue could adopt a “pulled forward” rotamer to avoid steric hindrance to lorlatinib. This G1202R rotamer can be stabilized by interaction with the acidic ALK E1210 residue and also ALK D1203 and ALK S1206 residues (Fig. 2C). Therefore, although the ALK G1202R mutation reduced lorlatinib binding affinity to ALK considerably, the activity of lorlatinib would still be potent enough to achieve clinical benefit.1 However, cis mutations on top of ALK G1202R will further reduce the binding affinity of lorlatinib with ALK leading to clinical resistance. The S1206Y mutation destabilizes the ALK D1203-S1206-E1210-G1202R quaternary complex and limits G1202R movement space to create a more steric hindrance to lorlatinib (Fig. 2D). Potential resistance mechanisms owing to ALK G1202R/L1196M (Fig. 2E), ALK G1202R/F1174C (Fig. 2F), ALK G1202R/L1198F (Fig. 2G), and ALK G1202R/G1269A (Fig. 2H) are illustrated.
Figure 2.
(A) Lorlatinib in complex with ALK kinase. ALK G1202, ALK D1203, ALK S1206, and ALK E1210 are solvent-front residues (PDB ID 4CLI). (B) ALK G1202R residue in the preferred conformation. It will provide a steric hindrance to lorlatinib. (C) ALK G1202R residue in the less preferred conformation with minimized steric hindrance to lorlatinib. In this “pulled forward” conformation, the basic ALK G1202R will interact with the acidic ALK E1210 residue and form a ALK D1203-S1206-E1210-G1202R quaternary complex with ALK D1203 and ALK S1206. Therefore, lorlatinib remains active against ALK G1202R, although with an approximately 50-fold reduction in activity against nonmutated ALK.1(D) ALK S1206 is located in the center of the quaternary complex D1203-S1206-E1210-G1202R that is involved in stabilizing the “forward movement” of G1202R. The ALK S1206Y mutation will destabilize this complex and move ALK G1202R back to its normal position to confer steric hindrance to lorlatinib. (E) ALK L1196M mutation makes G1202R residue to clash with lorlatinib even at the “pulled-forward” conformation. Therefore, lorlatinib loses its potency against ALK G1202R/L1196M compound mutation (PDB ID 4CLJ). On the left, ALK G1202R residue in the preferred conformation. On the right, ALK G1202R residue in the second preferred conformation. (F) ALK F1174C mutation does not have direct interaction with Lorlatinib. It will shift ALK kinase to favor its active conformation to have a tighter binding with ATP. Therefore, ALK G1202R/F1174C further reduces the binding affinity of lorlatinib with ALK in comparison with ALK G1202R mutation (PDB ID 4CLI). (G) ALK L1198F mutation does not favor the interaction with the polar cyano group on lorlatinib, which will further reduce the already unfavored interaction with lorlatinib after ALK G1202R mutation (PDB ID 5AA9). (H) ALK G1269 residue is adjacent to the F-element on the phenyl ring of lorlatinib. ALK G1269A mutation will induce a steric hindrance to lorlatinib to further reduce the binding affinity of lorlatinib with ALK G1202R, which is already 50-fold less potent in comparison to the wild-type ALK at the kinase domain. ATP, adenosine triphosphate.
Discussion
Here we report for the first time (to our knowledge), a novel, sequentially acquired, dual ALK mutation (G1202R/S1206Y) in cis that confers resistance to lorlatinib. Considering EML4-ALK variant 3 G1202R is already a recalcitrant ALK mutation, we hypothesize that this novel G1202R/S1206Y double mutation could be highly resistant to lorlatinib. This ALK G1202R/S1206Y double mutation not only has not been reported from patient samples from the literature review but also has not been generated even under artificial N-ethyl-N-nitrosourea-accelerated mutagenesis and high-dose lorlatinib selection pressure at 1000 nM in the presence of either EML4-ALK variant 1 G1202R5 or EML4-ALK variant 3 G1202R.7 In vitro inhibition assays will be necessary to compare the resistance of this novel double mutation to other ALK G1202R-containing double mutations for this hypothesis-generating observation. One patient case containing a triple mutation S1206F/G1202R/G1269A has previously been reported.14 The clinical history was that the patient sequentially developed ALK S1206F mutation after crizotinib treatment, ALK G1202R mutation in the absence of ALK S1206F mutation after ceritinib, and then a triple mutation ALK S1206F/G1202R/G1269A after lorlatinib treatment.14
ALK G1202R is one of the most frequently acquired resistance mutations to 2G ALK TKIs.15,16 In fact, the more potent an ALK TKI against the wild-type ALK is, the higher the incidence of ALK G1202R as the acquired on-target resistance mechanism.15 Owing to the pattern of use of lorlatinib on the basis of its approved indications (post-1L alectinib, post-1L ceritinib, and post-crizotinib and at least one other ALK TKI), most of the compound mutations that are resistant to lorlatinib have evolved from an existing solvent-front mutation ALK G1202R, with ALK G1202R plus ALK L1196M being the most frequent, followed by ALK G1202R plus ALK F1174L/C, ALK G1202R plus ALK L1198F, and ALK G1202R plus ALK G1269A.6, 7, 8, 9, 10, 11, 12 A real-world study of lorlatinib revealed a decreasing median progression-free survival (PFS) with an increasing line of previous ALK TKI usage.17 From three-dimensional modeling, we postulate two resistance mechanisms of ALK G1202R-based double mutations to lorlatinib. The first mechanism involves destabilization of the quaternary complex of D1203-S1206-E1210-G1202R that moves ALK G1202R forward to allow lorlatinib to fit better with an existing G1202R mutation. The second mechanism involves a second-site steric hindrance that interferes with the binding of lorlatinib to ALK G1202R despite the presence of the intact ALK D1203-S1206-E1210-G1202R quaternary complex.
We kept this patient on lorlatinib to provide continuous central nervous system coverage. In addition, we continued lorlatinib given that EML4-ALK variant 3 was still detectable from the liquid biopsy, and the ALK G1202R/S1206Y is likely a resistance subclone. Lin et al.18 have previously reported that continuation of AKI TKIs with the addition of chemotherapy can result in a longer median PFS than just switching to chemotherapy alone, although the magnitude of increase was only 3.6 months. Notably, there was a mild decrease in the double mutation alleles ALK G1202R (from MAF of 0.39% to 0.19%) and ALK S1206Y (from MAF of 0.49% to 0.34%) after the administration of chemotherapy. Currently, a fourth-generation ALK TKI, TPX-0131, designed to overcome many of the aforementioned ALK double mutations, could potentially be the next treatment strategy for this patient when it is available for clinical trial.19
Recently, in a randomized phase 3 trial (CROWN), lorlatinib compared with crizotinib achieved an impressive blinded independent review committee (BIRC)-assessed hazard ratio (HR) of 0.28 (95% confidence interval [CI]: 0.19–0.41) for PFS in all patients, HR of 0.20 (95% CI: 0.10–0.43) for patients baseline brain metastasis, and HR of 0.32 (95% CI: 0.20–0.49) for patients without baseline brain metastasis, with all subgroup analyses favoring lorlaitnib over crizotnib.20 Given the propensity for the accumulation of sequential mutations leading to resistance to all ALK TKIs and based on the CROWN results, the frontline use of lorlatinib will be an important strategy to circumvent these recalcitrant compound mutations.
Footnotes
Disclosure: Dr. Zhu has received honoraria from AstraZeneca, Blueprint, Roche-Foundation Medicine, Roche/Genentech, Takeda, and Xcovery, and previously held stocks in Turning Point Therapeutics (until May 2020). Dr. Nagasaka has received honorarium from AstraZeneca, Caris Life Sciences, Daiichi Sankyo, Takeda, Novartis, EMD Serono, and Tempus. Drs. Madison and Schrock are employees of Foundation Medicine Inc., a wholly owned subsidiary of Roche, and have Roche stock ownership. Dr. Cui is cofounder and has stock ownership of Turning Point Therapeutics Inc. and cofounder of BrightHill Therapeutics. Dr. Ou has stock ownership of Turning Point Therapeutics and was on the scientific advisory board of Turning Point Therapeutics Inc. (until February 28, 2019); is a member of the scientific advisory board of Elevation Oncology and has stock ownership of Elevation Oncology; has received speaker honorarium from Merck, Roche/Genentech, AstraZeneca, Takeda/ARIAD, and Pfizer; has received advisory fees from Roche/Genentech, AstraZeneca, Takeda/ARIAD, Pfizer, Foundation Medicine Inc., Spectrum, Daiichi Sankyo, and Janssen/Johnson and Johnson.
References
- 1.Zou H.Y., Friboulet L., Kodack D.P. PF-06463922, an ALK/ROS1 inhibitor, overcomes resistance to first and second generation ALK inhibitors in preclinical models. Cancer Cell. 2015;28:70–81. doi: 10.1016/j.ccell.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horn L., Whisenant J.G., Wakelee H. Monitoring therapeutic response and resistance: analysis of circulating tumor DNA in patients with ALK+ lung cancer. J Thorac Oncol. 2019;14:1901–1911. doi: 10.1016/j.jtho.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solomon B.J., Besse B., Bauer T.M. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. 2018;19:1654–1667. doi: 10.1016/S1470-2045(18)30649-1. [DOI] [PubMed] [Google Scholar]
- 4.Shaw A.T., Friboulet L., Leshchiner I. Resensitization to crizotinib by the lorlatinib ALK resistance mutation L1198F. N Engl J Med. 2016;374:54–61. doi: 10.1056/NEJMoa1508887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ou S.I., Young L., Schrock A.B. Emergence of preexisting MET Y1230C mutation as a resistance mechanism to crizotinib in NSCLC with MET exon 14 skipping. J Thorac Oncol. 2017;12:137–140. doi: 10.1016/j.jtho.2016.09.119. [DOI] [PubMed] [Google Scholar]
- 6.Yoda S., Lin J.J., Lawrence M.S. Sequential ALK inhibitors can select for lorlatinib-resistant compound ALK mutations in ALK-positive lung cancer. Cancer Discov. 2018;8:714–729. doi: 10.1158/2159-8290.CD-17-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dagogo-Jack I., Rooney M., Lin J.J. Treatment with next-generation ALK inhibitors fuels plasma ALK mutation diversity. Clin Cancer Res. 2019;25:6662–6670. doi: 10.1158/1078-0432.CCR-19-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okada K., Araki M., Sakashita T. Prediction of ALK mutations mediating ALK-TKIs resistance and drug re-purposing to overcome the resistance. EBioMedicine. 2019;41:105–119. doi: 10.1016/j.ebiom.2019.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pailler E., Faugeroux V., Oulhen M. Acquired resistance mutations to ALK inhibitors identified by single circulating tumor cell sequencing in ALK-rearranged non-small-cell lung cancer. Clin Cancer Res. 2019;25:6671–6682. doi: 10.1158/1078-0432.CCR-19-1176. [DOI] [PubMed] [Google Scholar]
- 10.Sharma G.G., Cortinovis D., Agustoni F. A compound L1196M/G1202R ALK mutation in a patient with ALK-positive lung cancer with acquired resistance to brigatinib also confers primary resistance to lorlatinib. J Thorac Oncol. 2019;14:e257–e259. doi: 10.1016/j.jtho.2019.06.028. [DOI] [PubMed] [Google Scholar]
- 11.Recondo G., Mezquita L., Facchinetti F. Diverse resistance mechanisms to the third-generation ALK inhibitor lorlatinib in ALK-rearranged lung cancer. Clin Cancer Res. 2020;26:242–255. doi: 10.1158/1078-0432.CCR-19-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu J., Zhang B., Yao F. Acquired multiple mutations ALK I1171N, L1196M and G1202R mediate lorlatinib resistance in EML4-ALK-rearranged malignant pleural mesothelioma: a case report. Ther Adv Respir Dis. 2020;14:1–4. doi: 10.1177/1753466620935770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ou S.H., Milliken J.C., Azada M.C., Miller V.A., Ali S.M., Klempner S.J. ALK F1174V mutation confers sensitivity while ALK I1171 mutation confers resistance to alectinib. The importance of serial biopsy post progression. Lung Cancer. 2016;91:70–72. doi: 10.1016/j.lungcan.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Shaw A. ES14.02 first line in ALK translocated patients. J Thorac Oncol. 2019;14(suppl):S50. [Google Scholar]
- 15.Gainor J.F., Dardaei L., Yoda S. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016;6:1118–1133. doi: 10.1158/2159-8290.CD-16-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw A.T., Solomon B.J., Besse B. ALK resistance mutations and efficacy of lorlatinib in advanced anaplastic lymphoma kinase-positive non-small-cell lung cancer. J Clin Oncol. 2019;37:1370–1379. doi: 10.1200/JCO.18.02236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu V.W., Lin Y.T., Kim D.W. An international real-world analysis of the efficacy and safety of lorlatinib through early or expanded access programs in patients with tyrosine kinase inhibitor-refractory ALK-positive or ROS1-positive NSCLC. J Thorac Oncol. 2020;15:1484–1496. doi: 10.1016/j.jtho.2020.04.019. [DOI] [PubMed] [Google Scholar]
- 18.Lin J.J., Schoenfeld A.J., Zhu V.W. Efficacy of platinum/pemetrexed combination chemotherapy in ALK-positive NSCLC refractory to second-generation ALK inhibitors. J Thorac Oncol. 2020;15:258–265. doi: 10.1016/j.jtho.2019.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui J.J., Rogers E., Zhai D. TPX-0131: a next generation macrocyclic ALK Inhibitor that overcomes ALK resistant mutations refractory to currently approved ALK inhibitors. Cancer Res. 2020;80(suppl 16) Abstract nr 5226. [Google Scholar]
- 20.Shaw A.T., Bauer T.M., de Marins F. First line lorlatinib or crizotinib in advanced ALK-psoitive lung cancer. N Engl J Med. 2020;383:2018–2029. doi: 10.1056/NEJMoa2027187. [DOI] [PubMed] [Google Scholar]