Abstract
Introduction
EGFR tyrosine kinase inhibitors are one of the key drugs for treatment of NSCLC with EGFR mutations. In recent times, immune check-point inhibitors (ICIs) have also been widely used for patients with NSCLC. Although a subset of patients obtain benefit from ICIs, adverse events (AEs) that are different from those of cytotoxic chemotherapies may occur. Moreover, some patients develop AEs, which seem to be caused by the previously discontinued nivolumab.
Methods
We identified patients with NSCLC who developed AEs, which started shortly after discontinuation of nivolumab and during treatment with osimertinib. We conducted liquid chromatography-mass spectrometry analyses to estimate the concentration of serum nivolumab.
Results
Three patients with AEs were identified. Two patients developed interstitial lung disease (cases 1 and 2) and one developed hepatotoxicity (case 3) during osimertinib therapy initiated after nivolumab administration. They received several treatments, including cytotoxic chemotherapies or EGFR tyrosine kinase inhibitors other than osimertinib, followed by nivolumab for three to five cycles; nevertheless, the disease progressed. After discontinuation of nivolumab, osimertinib was administered from day 22 to 46; but treatment-related toxicities developed 56 to 96 days later. Liquid chromatography-mass spectrometry analyses revealed that the remaining levels of nivolumab in the blood (2.1 μg/mL, 12.8 μg/mL, and 31.1 μg/mL, respectively, for cases 1, 2, and 3) were enough to induce an immune response.
Conclusion
The presence of the ICI antibody that persists even after drug discontinuation may account not only for the prolonged efficacy of these agents but also for the late onset of AEs, especially when the antibodies may have interacted during subsequent treatments.
Keywords: Non−small cell lung cancer, EGFR, programmed cell death protein 1, Osimertinib, Immune-related adverse events
Introduction
EGFR tyrosine kinase inhibitors (TKIs) are key drugs for patients with sensitizing EGFR mutations involved in NSCLC. Osimertinib is a third-generation EGFR TKI that is effective against tumors with sensitizing mutations and the T790M-resistant mutation. Osimertinib was approved in March 2016 for use in Japan, and many patients are now receiving this new EGFR TKI after having been previously treated with other anticancer therapies. Interstitial lung disease (ILD) is a major adverse effect of EGFR TKIs. Its incidence is higher in Japan; however, the reasons for this are not yet known. In a global phase 3 trial comparing osimertinib and platinum plus pemetrexed in EGFR T790M–positive patients with NSCLC, the incidence of ILD was 4% among all patients and 7% among Japanese patients.1,2 Nivolumab is a human immunoglobulin G4 programmed cell death protein 1 (PD-1) antibody that is effective in the treatment of NSCLC. Among previously treated patients with NSCLC, overall survival was better with nivolumab than with docetaxel.3 Moreover, several studies revealed a durable response of nivolumab even after therapy was discontinued.4,5 Owing to the biological nature of antibodies, nivolumab may remain in the blood for extended periods of time.
However, the long-lasting effects of nivolumab may be partly responsible for the late onset of adverse events (AEs). A previous study revealed that sequential PD-1 blockade and osimertinib frequently induce immune-related AEs (irAEs).6 However, little is known about the extent of nivolumab that may contribute to this phenomenon.
Materials and Methods
We identified patients who developed AEs during treatment with osimertinib shortly after discontinuation of nivolumab (PD-1 antibody) after March 2016 when osimertinib was approved in Japan. We conducted liquid chromatography-mass spectrometry (LC-MS) analyses to estimate the concentration of serum nivolumab in these patients.7 We repeated the analyses with the remaining serum samples. AEs were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) guidelines version 4.0. We obtained ethical approval from the National Cancer Center Hospital, and confidentiality of the patient data was maintained. Written informed consent was obtained from the patients for the analysis of blood samples and for publication.
Results
Three patients with related AEs were identified. Two patients suffered from ILDs (cases 1 and 2) and one developed hepatotoxicity (case 3) during osimertinib therapy initiated after nivolumab administration. Here, we describe the detailed clinical courses of these patients.
Case 1 (Fig. 1) was a man in his late 50s who had recurrent lung adenocarcinoma with an EGFR exon 19 deletion. He was an ex-smoker and had no comorbidities. He received gefitinib, erlotinib, carboplatin, pemetrexed, and bevacizumab for 3 years. He was then treated with nivolumab at a dosage of 3 mg/kg every 2 weeks. He received three cycles of nivolumab with no obvious AEs. However, the treatment was discontinued because his disease progressed rapidly. He underwent another biopsy of the lung tumor through bronchoscopy, and a new EGFR T790M mutation was detected. Osimertinib (80 mg/d) was started 46 days after the final nivolumab administration. The efficacy of osimertinib was confirmed by computed tomography (CT) 50 days after administration, which was 96 days after the final dose of nivolumab. However, because nonsegmental, diffuse, ground-glass opacities were found in the CT scan, drug-induced ILD was suspected. Seven days later, his symptoms of dyspnea worsened and he underwent bronchoscopy for a differential diagnosis of abnormal findings in the lung. An analysis of his bronchoalveolar lavage fluid revealed that the fraction of lymphocytes had increased to 61% and the cluster of differentiation 4/cluster of differentiation 8 ratio was 1.93. There was no evidence of infection. At that time, his blood nivolumab concentration, as determined by LC-MS, was 2.1 μg/mL. Thus, he was diagnosed with grade 3 drug-induced ILD according to CTCAE guidelines. He was treated with prednisolone (0.5 mg/kg), and his symptoms improved dramatically within 1 week.
Figure 1.
Clinical course of case 1. A computed tomography scan taken 15 days after three cycles of nivolumab revealed disease progression (A). After 50 days of administration, the efficacy of osimertinib was confirmed, although abnormal findings indicating interstitial lung disease were found (B). Seven days later, the symptoms and computed tomography findings of drug–induced interstitial lung disease worsened and prednisolone was started (C). The graph exhibits the treatment timeline and longitudinal changes in the nivolumab concentrations in the blood (D).
Case 2 (Fig. 2) was a woman in her early 70s who was diagnosed with stage IV lung adenocarcinoma characterized by an EGFR exon 19 deletion. She received erlotinib, carboplatin, and pemetrexed for 2 years. When the disease progressed, she underwent another biopsy of the primary lung tumor, and a T790M resistance mutation was identified. She received an investigational drug for 9 months followed by nivolumab (3 mg/kg) every 2 weeks for three cycles. She had no AEs during nivolumab therapy, but it was stopped because of disease progression. She was started on osimertinib 22 days after the final administration of nivolumab. Thirty-four days later, at 56 days after the final administration of nivolumab, CT scan results confirmed the efficacy of osimertinib but also revealed patchy, diffuse, ground-glass opacities. There were no other findings that implied an infection, and so drug-induced ILD was suspected. The nivolumab concentration in her blood, taken on the same day on which the CT scan revealed the abnormal findings, was calculated at 12.8 μg/mL. Because the patient had no symptoms related to abnormal findings, the AE was evaluated as grade 1 according to CTCAE guidelines. The patient continued to receive osimertinib with close follow-up. Sixty-three days after the CT scan, or 128 days after the final administration of nivolumab, she underwent another CT scan, which revealed disappearance of the abnormal shadow.
Figure 2.
Clinical course of case 2. A computed tomography scan taken 15 days after three cycles of nivolumab revealed disease progression (A). After 34 days of administration, the efficacy of osimertinib was confirmed, although ground-glass opacities indicating interstitial lung disease were found (B). Although the patient continued osimertinib treatment, the abnormal shadow in computed tomography disappeared spontaneously (C). The graph exhibits the treatment timeline and longitudinal changes in the nivolumab concentrations in the blood (D).
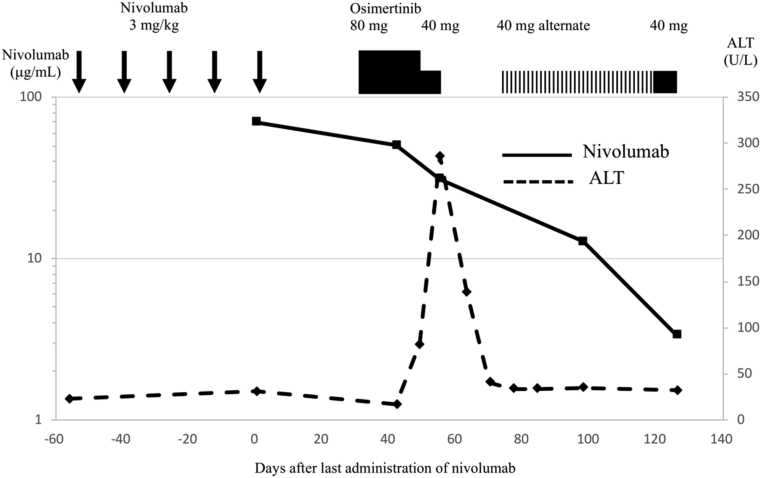
Case 3 (Fig. 3) was a woman in her early 50s who was diagnosed with stage IV lung adenocarcinoma characterized by an EGFR L858R mutation. She received gefitinib, erlotinib, afatinib, pemetrexed, and radiation therapy for the brain, followed by nivolumab at 3 mg/kg for five cycles. However, the disease progressed. Her trough serum concentration of nivolumab before the final administration was 69.7 μg/mL. Biopsy from a liver metastasis revealed a T790M mutation, and she was started on osimertinib at 80 mg/d 35 days after the final administration of nivolumab. On day 15, grade 2 elevation of transaminase levels was found, and the osimertinib dosage was reduced to 40 mg. Nonetheless, her symptoms exacerbated, accompanied by grade 1 fever and grade 2 malaise, 5 days later, and a grade 3 increase of alanine aminotransferase levels was also observed. The treatment with osimertinib was stopped 63 days after the final nivolumab administration. Her blood concentration of nivolumab was 31.1 μg/mL. After osimertinib cessation, her liver function recovered within 2 weeks without any additional treatment. She was therefore restarted on osimertinib at a dosage of 40 mg on alternate days. The dosage was subsequently increased to 80 mg/d, but there were no signs of hepatotoxicity. Her blood nivolumab concentration decreased steadily (12.7 μg/mL on day 98 and 3.4 μg/mL on day 126 after the final nivolumab administration).
Figure 3.
Clinical course of case 3. The graph exhibits the treatment timeline and longitudinal changes in the nivolumab concentrations in the blood and serum ALT activities. ALT, alanine transaminase.
Discussion
Because ICIs targeting PD-1 or its ligand, programmed death ligand 1 (PD-L1), have revealed favorable results in patients with NSCLC, anti–PD-1 or anti–PD-L1 antibodies are considered standard treatment. These antibodies disrupt suppressive signaling between cytotoxic T cells and tumors and thereby exhibit clinical benefits.8 In some cases, a durable response to these antibodies may occur even after discontinuation.4 This phenomenon can be explained by ICIs, which may affect the immune response and occur even at low concentrations. In a phase I study of nivolumab, PD-1 occupancy was 60% to 80% even after nivolumab was no longer detected in the blood (< 1.2 μg/mL).9 Furthermore, according to an in vivo study, a concentration of 1 μg/mL of nivolumab is enough to activate a T-cell response.10 Durable efficacy may provoke delayed irAEs. On August 5, 2016, postmarketing surveillance revealed 68 severe AEs in 50 patients, which occurred more than 31 days after discontinuation of nivolumab (median, 49 days; range, 31–247 days). In the present study, the patients developed ILD or hepatotoxicity after discontinuation of nivolumab. The previous study revealed that PD-(L)1 blockade followed by osimertinib induces severe irAEs more frequently than does osimertinib followed by PD-(L)1 blockade.6 There are also several case reports of ILD caused by EGFR TKI administration after ICIs11,12 and possible interaction between the AEs and previously administered ICIs. However, this is the first case series that revealed the possible relation between late-onset AEs and the concentrations of nivolumab in the blood analyzed using LC-MS. The analyses revealed that nivolumab remained in the blood for months at a level sufficient to induce an immune response. Despite the lack of evidence, nivolumab could have affected the clinical course of our patients even after discontinuation. There has also been a report of a patient with melanoma who developed AEs related to vemurafenib, which occurred even after discontinuation of anti–PD-1 antibodies.13 Although it is known that a single dose of 3 mg/kg nivolumab has a half-life of 13 days (± 7 days),14 it remains unclear as to how long it takes to sufficiently diminish the effect of nivolumab on the immune system so as not to affect subsequent treatment.
ILD is one of the most severe AEs related to both ICIs and EGFR TKIs. Previous meta-analyses found that pneumonitis occurred in about 3% and 1.12% of patients with NSCLC who received anti–PD-(L)1 antibodies or EGFR TKI, respectively.15,16 In a clinical trial that evaluated the combination of osimertinib and durvalumab, an antibody targeting PD-L1, an extremely high incidence (38%) of interstitial pneumonia was seen.17 Since the approval of nivolumab in Japan, eight cases of ILD potentially attributed to EGFR TKI use after nivolumab therapy have been reported as of July 13, 2016. An ongoing postmarketing surveillance of osimertinib has, thus far, identified 39 cases of ILD, including at least six cases in which the patients previously received nivolumab. Although these epidemiologic reports are more sporadic rather than comprehensive, and the incidence rate is yet to be determined more rigorously, many physicians have observed related abnormalities. The drug distributors and the Ministry of Health and Welfare of Japan have even released a statement to warn physicians. Details regarding the incidence of ILD after nivolumab treatment are expected to be updated.
Finally, we treated a patient who developed severe hepatotoxicity during osimertinib treatment after the failure of nivolumab. When osimertinib is administered as a single agent, hepatotoxicity is a relatively rare AE.1 However, hepatotoxicity is one of the major AEs associated with gefitinib, and the patients who were administered a combination of gefitinib and durvalumab had alanine aminotransferase levels that increased to 40%.18 ICIs may augment susceptibility to EGFR TKI–related AEs because of their contribution to immune response.
In summary, we have reported three cases of osimertinib-induced interstitial pneumonia or hepatotoxicity after the discontinuation of nivolumab. LC-MS revealed that their nivolumab concentrations were much lower than trough concentrations during treatment yet high enough to induce immune responses. Long-lasting antibodies may account for the prolonged effect of ICIs even after drug discontinuation, and they can also participate in unanticipated cross-interactions with subsequent treatments. Therefore, to avoid provoking an immune reaction that negatively alters the clinical course of the disease, careful consideration and closer follow-up are imperative during treatment after ICI therapy.
Acknowledgments
This study was supported by grants from the Practical Research for Innovative Cancer Control from the Japan Agency for Medical Research and Development under grant number JP16ck0106191.
Footnotes
Disclosure: Dr. Goto has a consulting or advisory role with Eli Lilly, Chugai, Taiho, Boehringer Ingelheim, Pfizer, Novartis, AstraZeneca, and GlaxoSmithKline; has served on the speaker’s bureau for AstraZeneca, Eli Lilly, Chugai, Taiho, Boehringer Ingelheim, Ono Pharmaceutical, Bristol Myers Squibb, Pfizer, MSD, Shionogi Pharma, and Novartis; and has received research funding from Abbvie, Eli Lilly, Taiho, Bristol Myers Squibb, Ono Pharmaceutical, Daiichi Sankyo, Pfizer, Novartis, and Kyorin. Dr. Nokihara has received research funding from Ono Pharmaceutical, Pfizer, AstraZeneca, Chugai, and Regeneron; he has also served on the speakers’ bureau for AstraZeneca, Chugai, Eisai, Eli Lilly, MSD, and Boehringer Ingelheim. Dr. Fujiwara has received grants from Japan Agency for Medical Research and Development and the Ministry of Health Labor and Welfare, Japan, during the conduct of the study; he has also served on the speakers’ bureau for AstraZeneca, Daiichi Sankyo, Taiho, Chugai, Novartis, SRL Pharma, and Bristol Myers Squibb. Dr. Ohe has had consulting or advisory roles for AstraZeneca, Chugai, Ono Pharmaceutical, Bristol Myers Squibb, Kyorin, Celltrion, and Amgen; has received research funding from AstraZeneca, Chugai, Eli Lilly, Ono Pharmaceutical, BMS, Kyorin, Dainippon-Sumitomo, Pfizer, Taiho, Novartis, Kissei, Ignyta, Takeda, Kissei, Daiichi Sankyo, and Janssen; and has served on the speakers’ bureau for AstraZeneca, Chugai, Eli Lilly, Ono Pharmaceutical, Bristol Myers Squibb, Boehringer Ingelheim, Bayer, Pfizer, MSD, and Taiho. The remaining authors declare no conflict of interest.
References
- 1.Mok T.S., Wu Y.L., Ahn M.J. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akamatsu H., Katakami N., Okamoto I. Osimertinib in Japanese patients with EGFR T790M mutation-positive advanced non-small-cell lung cancer: AURA3 trial. Cancer Sci. 2018;109:1930–1938. doi: 10.1111/cas.13623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brahmer J., Reckamp K.L., Baas P. Nivolumab versus docetaxel in advanced squamous-cell non-small-Cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takagi T., Yoshida K., Kobayashi H. Durable response after discontinuation of nivolumab therapy in patients with metastatic renal cell carcinoma. Jpn J Clin Oncol. 2018;48:860–863. doi: 10.1093/jjco/hyy106. [DOI] [PubMed] [Google Scholar]
- 5.Gettinger S.N., Horn L., Gandhi L. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33:2004–2012. doi: 10.1200/JCO.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoenfeld A.J., Arbour K.C., Rizvi H. Severe immune related adverse events are common with sequential PD- (L) 1 blockade and osimertinib. Ann Oncol. 2019;30:839–844. doi: 10.1093/annonc/mdz077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwamoto N., Shimada T., Terakado H., Hamada A. Validated LC-MS/MS analysis of immune checkpoint inhibitor nivolumab in human plasma using a fab peptide-selective quantitation method: nano-surface and molecular-orientation limited (nSMOL) proteolysis. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1023:9–16. doi: 10.1016/j.jchromb.2016.04.038. [DOI] [PubMed] [Google Scholar]
- 8.Dine J., Gordon R., Shames Y., Kasler M.K., Barton-Burke M. Immune checkpoint inhibitors: an innovation in immunotherapy for the treatment and management of patients with cancer. Asia Pac J Oncol Nurs. 2017;4:127–135. doi: 10.4103/apjon.apjon_4_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brahmer J.R., Drake C.G., Wollner I. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang C., Thudium K.B., Han M. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human Primates. Cancer Immunology Research. 2014;2:846–856. doi: 10.1158/2326-6066.CIR-14-0040. [DOI] [PubMed] [Google Scholar]
- 11.Mamesaya N., Kenmotsu H., Katsumata M., Nakajima T., Endo M., Takahashi T. Osimertinib-induced interstitial lung disease after treatment with anti-PD1 antibody. Investig New Drugs. 2016;35:105–107. doi: 10.1007/s10637-016-0389-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takakuwa O., Oguri T., Uemura T. Osimertinib-induced interstitial lung disease in a patient with non-small cell lung cancer pretreated with nivolumab: A case report. Mol Clin Oncol. 2017;7:383–385. doi: 10.3892/mco.2017.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson D.B., Wallender E.K., Cohen D.N. Severe cutaneous and neurologic toxicity in melanoma patients during vemurafenib administration following anti-PD-1 therapy. Cancer Immunol Res. 2013;1:373–377. doi: 10.1158/2326-6066.CIR-13-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto N., Nokihara H., Yamada Y. Phase I study of nivolumab, an anti-PD-1 antibody, in patients with malignant solid tumors. Investig New Drugs. 2017;35:207–216. doi: 10.1007/s10637-016-0411-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khunger M., Rakshit S., Pasupuleti V. Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer: a systematic review and meta-analysis of trials. Chest. 2017;152:271–281. doi: 10.1016/j.chest.2017.04.177. [DOI] [PubMed] [Google Scholar]
- 16.Suh C.H., Park H.S., Kim K.W., Pyo J., Hatabu H., Nishino M. Pneumonitis in advanced non-small-cell lung cancer patients treated with EGFR tyrosine kinase inhibitor: meta-analysis of 153 cohorts with 15,713 patients. Lung Cancer. 2018;123:60–69. doi: 10.1016/j.lungcan.2018.06.032. [DOI] [PubMed] [Google Scholar]
- 17.Ahn M.J., Yang J., Yu H. 136O: osimertinib combined with durvalumab in EGFR-mutant non-small cell lung cancer: results from the TATTON phase Ib trial. J Thorac Oncol. 2016;11:933–939. [Google Scholar]
- 18.Gibbons D.L., Chow L.Q., Kim D.W. 57O Efficacy, safety and tolerability of MEDI4736 (durvalumab [D]), a human IgG1 anti-programmed cell death-ligand-1 (PD-L1) antibody, combined with gefitinib (G): a phase I expansion in TKI-naïve patients (pts) with EGFR mutant NSCLC. J Thorac Oncol. 2016;11:S79. [Google Scholar]