Abstract
Introduction
The aim of this study is to evaluate the clinical backgrounds, including driver mutations, of those patients with early stage NSCLC who experienced recurrence beyond 5 years after complete resection.
Methods
We used a cohort of 512 consecutive cases of surgically resected NSCLC without other malignances from 2006 to 2011 in Aichi Cancer Center Hospital. The inclusion criteria for this cohort were patients with primary NSCLC who underwent a surgically curable operation.
Results
A total of 172 patients (32.8%) had recurrence after the surgery. Among the recurrent cases, 17 patients (3.3%) had a relapse more than 5 years after the surgery, and all except one (16 of 17, 94.1%) had driver mutations, including gene rearrangements.
Conclusions
Even in early stage NSCLC after complete resection, it was found that some cases had a relapse more than 5 years after the surgery. Most of these cases had some kind of driver mutations; so more than 5 years of postoperative surveillance may be beneficial, especially in those with driver gene mutants.
Keywords: Driver mutation, Long-term recurrence, Non–small-cell lung cancer, Postoperative surveillance
Introduction
Surgery is one of the standard treatments for patients with clinical early stage NSCLC, and there is no evidence that the mediastinum is involved before the surgical resection. The favorable results reported in the surgery series and the long-term survival data of these patients have established surgery as the treatment of choice.1
The rationale for surveillance after initial treatment of NSCLC is to detect recurrence early so that survival and quality of life can be improved. However, there are no randomized trials comparing different postoperative surveillance strategies, including the appropriate length of the observation period.2
Guidelines from the International Association for the Study of Lung Cancer and the College of American Pathologists encourage testing for EGFR mutations and ALK rearrangement in patients with newly diagnosed localized NSCLC.1 However, there is no evidence to support molecular marker testing at the time of the original diagnosis as a predictive marker of recurrence and a prognostic marker.
The aim of this study is to confirm the usefulness of the initial driver mutation status as a guide for postoperative surveillance period in patients with early stage NSCLC and to reveal the clinical backgrounds of those who had recurrence more than 5 years after the surgery.
Materials and Methods
Patients
We used a cohort of consecutive series of surgically resected patients with NSCLC from 2006 to 2011 in Aichi Cancer Center Hospital, Nagoya, Japan. The inclusion criterion of this cohort was patients with primary NSCLC who underwent a surgically curable operation. This retrospective study was approved by the Institutional Review Board of Aichi Cancer Center (no: 2019 1 449), and the need for informed consent was waived given the retrospective nature of the study design. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Genomic Test
Exons 18 to 21 of the EGFR and KRAS genes were analyzed as previously described.3 Immunohistochemical analysis of ALK, with a mouse monoclonal antibody for ALK (5A4, Dako),4,5 was done as previously described. Next-generation sequencing with tumor RNA was performed with Ion 540 chips on Ion Torrent S5 Sequencer using barcoded libraries prepared with AmpliSeq Library Preparation Kits (Thermo Fisher Scientific, Wilmington, DE) according to the manufacturer’s protocols in patients with recurrence more than 5 years after surgery. In the 10 cases, in which the tumor tissue could be collected at the time of recurrence, the presence or absence of the gene mutations was determined in pairs (Table 1).
Table 1.
The Driver Mutation Statuses of the 17 Patients of Primary and Recurrent Lesions
| Driver Gene Alteration of Primary Lung Lesion | Recurrent Lesion | Driver Gene Alteration of Reccurnt Lesion |
|---|---|---|
| CD74-ROS1 | Bone | Rebiopsy was not done |
| L858R | Brain | Rebiopsy was not done |
| 19 deletion | Lung | Rebiopsy was not done |
| CD74-ROS1 | Lung | CD74-ROS1 |
| G12C | Pleural effusion | G12C |
| Wild type | Pleural effusion | Rebiopsy was not done |
| 19deletion | Brain | Rebiopsy was not done |
| L858R | Lung | L858R |
| L858R | Lung | L858R |
| 19 deletion | Lung | 19 deletion |
| 19 deletion | Lung | 19 deletion |
| 19 deletion | Lung | 19 deletion |
| KIF5-RET | Lung | KIF5-RET |
| G12C | Lung | Rebiopsy was not done |
| L858R | Mediastinal lymph node | L858R |
| 19 deletion | Lung | Rebiopsy was not done |
| S768I | Lung | S768I |
Statistical Analysis
The univariate relationship between each independent variable was evaluated using the chi-square test. All statistical analyses were performed using JMP 12 software (SAS Institute, Cary, NC).
Results
The median time of follow-up was 61.6 months. The patients’ backgrounds are listed in Table 2. Briefly, a total of 172 patients (32.8%) had recurrence after the surgery. Among the recurrent cases, 17 patients (3.3%) had a relapse more than 5 years after the surgery and all except one of them(16 of 17, 94.1%) had driver mutations, including gene rearrangements (Tables 1 and 3), which were statistically significant when compared with the cases recurrent within 5 years.
Table 2.
Patient Background Characteristics
| Characteristic | All, n (%) | Recurrence Within 5 Years | Recurrence Beyond 5 Years | p Value |
|---|---|---|---|---|
| Sex | ||||
| Male/female | 313 (61.1)/199(38.9) | 104/51 | 10/7 | 0.678 |
| Age median [range] | 66 [22–86] | 66 [39–85] | 65 [38–80] | |
| Histologic type | ||||
| Adeno/nonadeno | 370 (72.0)/142 (38.0) | 98/57 | 15/2 | 0.073 |
| With/without STAS | 149 (29.0)/363 (71.0) | 64/91 | 5/12 | 0.491 |
| Pathologic stage | ||||
| IA/IB | 235 (45.9)/63 (12.3) | 24/22 | 9/1 | 0.030a |
| IIA/IIB | 25 (4.9)/82 (16.0) | 9/35 | 0/5 | |
| IIIA | 107 (20.9) | 65 | 2 | |
| Operative procedure | ||||
| Lobectomy/others | 474 (92.5)/38 (7.5) | 139/16 | 17/0 | 0.341 |
| Adjuvant chemotherapy | ||||
| UFT | 37 (7.2) | 11 | 1 | |
| Platinum doublets | 88 (17.1) | 58 | 1 | |
| Cytotoxic monotherapy | 7 (1.4) | 1 | 0 | |
| Driver mutation | ||||
| EGFR mutant | 201 (39.2) | 56 | 11 | 0.0004b |
| KRAS mutant | 42 (8.2) | 11 | 2 | |
| Other mutantsc | 21 (4.1) | 4 | 3 | |
| Wild type | 248 (48.5) | 84 | 1 | |
| Total | 512 | 155 | 17 | — |
Adeno, adenocarcinoma; STAS, spread through air spaces; UFT, an oral 5-fluorouracil derivative agent.
Chi-square test was performed according to stage I, II, and III between the two groups.
Chi-square test was performed with and without driver mutations between the two groups.
ALK, ROS1, and RET rearrangement.
Table 3.
The Driver Mutation Statuses of Patients With NSCLC Who Had a Relapse More Than 5 Years After Surgery
| Long Interval Recurrence (N = 17) | |
|---|---|
| Driver mutation | |
| EGFR: p.(Leu858Arg) | 4 |
| EGFR: g.(exon19del) | 6 |
| EGFR: p.(Ser768Ile) | 1 |
| KRAS: p.(Gly12Cys) | 2 |
| c. CD74:ROS1 (C6;R34) | 2 |
| c. KIF5:RET (K:R12) | 1 |
| Wild type | 1 |
The distribution of EGFR and KRAS mutations according to histologic patterns and pathologic stages is reported in Table 3. Papillary predominant adenocarcinoma (59.2%) was the most common histologic subtype for EGFR mutants. Most cases harboring EGFR mutations were of pathologic stage IA and IB (74.2%).
The precise data for somatic gene alterations are reported in Table 4. A total of 11 patients (64.7%) had EGFR-activating mutations (four with L858R, six with exon 19 deletions, and one with S768I), followed by two KRAS G12C, two CD74-ROS1, and one KIF5-RET. Only one patient had no driver mutations – none of EGFR, KRAS, BRAF, and HER2, and ALK or ROS1 gene rearrangement.
Table 4.
Distribution of EGFR and KRAS Mutations According to Histologic Subtypes and Pathologic Stage
| EGFR and KRAS Mutations | Total Number = 512, n (%) |
|---|---|
| EGFR mutations, n | 201 |
| Histologic subtypes | |
| Adenocarcinoma | 196 (97.5) |
| Papillary predominant | 119 (59.2) |
| Acinar predominant | 35 (17.5) |
| Lepidic predominant | 24 (11.9) |
| Solid predominant | 18 (8.9) |
| Pleomorphic | 1 (0.5) |
| Adenosquamous | 2 (1) |
| Squamous | 1 (0.5) |
| Clear cell | 1 (0.5) |
| Pathologic stage | |
| IA | 132 (65.7) |
| IB | 17 (8.5) |
| IIA | 4 (1.9) |
| IIB | 21 (10.4) |
| IIIA | 27 (13.5) |
| KRAS mutations, n | 42 |
| Histologic subtypes | |
| Adenocarcinoma | 35 (83.4) |
| Papillary predominant | 12 (28.5) |
| Invasive mucinous | 11 (26.1) |
| Lepidic predominant | 3 (7.1) |
| Solid predominant | 3 (7.1) |
| Acinar predominant | 1 (2.3) |
| Large | 7 (16.6) |
| Pathologic stage | |
| IA | 17 (40.4) |
| IB | 7 (16.7) |
| IIA | 3 (7.2) |
| IIB | 8 (19.0) |
| IIIA | 7 (16.7) |
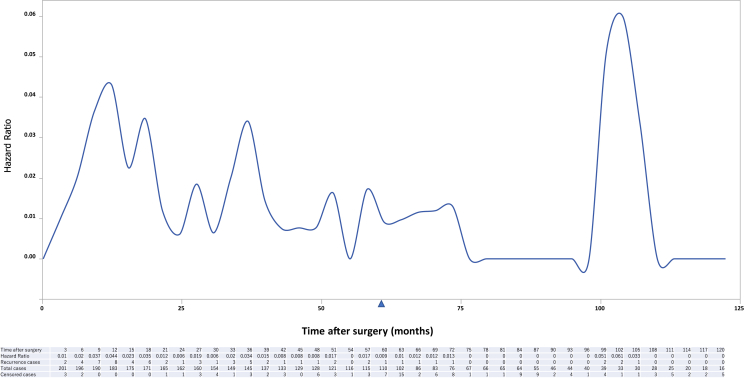
The cumulative hazard ratio (HR) of recurrence is illustrated in Figure 1 and that of EGFR mutants and cases without driver gene alterations in Figures 2 and 3, respectively. The increase in HR after 5 years is caused by the EGFR mutant, with peaks appearing at 6 and 9 years (Fig. 2). It is apparent that the risk of recurrence is highest 9 months after surgery and decreases over time in cases without driver mutations (Fig. 3).
Figure 1.
Cumulative HR of recurrence after surgery of all patients. HR, hazard ratio.
Figure 2.
Cumulative HR of recurrence after surgery of patients harboring EGFR–activating mutations. HR, hazard ratio.
Figure 3.
Cumulative HR of recurrence after surgery of patients without driver gene alterations. HR, hazard ratio.
Discussion
In this study, we analyzed the genomic background of patients who had experienced recurrence more than 5 years after complete resection for early stage NSCLC and revealed that most of them harbored driver mutations, including oncogenic fusion genes. In particular, in the EGFR mutation cases, the HR of recurrence did not reach zero even after 5 years of follow-up and recurrence cases were also recognized after 9 years.
It is well known that the treatment for breast cancer is longer than for other solid cancers. In 2012, a randomized controlled trial, Adjuvant Tamoxifen: Longer Against, revealed that extending adjuvant tamoxifen treatment from 5 years to 10 years could markedly reduce the risk of death from breast cancer.6 A hypothesis has been proposed for the mechanism by which the risk of recurrence in breast cancer continues over a long period of time, in which breast cancer cells enter the bone marrow and maintain a long-term dormancy. However, few reports have examined the risk of recurrence after more than 10 years in solid tumors other than breast cancer. For prostate cancer, recurrence more than 5 years after surgery was reported to be found in several percent of completely resected cases7; therefore, monitoring of prostate-specific antigen for recurrence for more than 5 years after surgery is recommended.8
In NSCLC, follow-up for 5 years after surgery is recommended1 and postoperative follow-up ends in 5 years in effect. In our cohort, a lot of censored cases were found in term between 60 months and 63 months after surgery, which may well reflect this guideline recommendation (Fig. 1). In a report comparing institutional surveillance patterns after NSCLC treatment with stereotactic body radiation therapy and lobectomy, a certain risk of recurrence beyond 5 years was found in the lobectomy group.9 In a study evaluating the patterns and risks of postoperative recurrence in completely resected EGFR-mutant NSCLC, thoracic recurrence, in particular, also seems to have certain risks beyond 5 years.10 Tamiya et al.11 reported the follow-up data of a prospective, multicenter, genomic examination of 876 surgically resected NSCLC cases and revealed that EGFR mutations were significantly associated with improved overall survival, given the potential value of the results in predicting outcome in patients with NSCLC and the possibility of intervention after surgery.
Several mechanisms that explain these findings may be speculated upon. First, taking EGFR L858R as an example, the transiently transfected EGFR-L858R mutant in CL1-0 lung cancer cells can promote lung cancer invasion.12 In other words, EGFR-mutant cells easily form micrometastases from the early stage of the disease and a niche. However, driver mutation–related tumor cells have less tumor mutational burden13 and are more likely to escape the host immune surveillance. In addition, it is speculated that the driver mutant tumor cells rely on the activated cascade for the survival of the tumor cells, and thus are unlikely to cause spread from the niche. Recently, Chalela et al.14 explored the prognosis impact of EGFR and KRAS mutations in the nontumoral lung of patients with adenocarcinoma and found that 21.3% of the patients had EGFR or KRAS mutations in their adenocarcinoma and also in their histologically normal lung tissue. In addition to the possible relationship of this finding with both the carcinogenesis process and the extension of a primary tumor to other organs, mutations in the normal lung tissue seem to be associated with a worse short-term prognosis.14 The long-term recurrence cases found in our study may also be derived from normal cells harboring EGFR mutations.
In a post hoc analysis of a randomized phase III trial (ADJUVANT and CTONG1104) of adjuvant gefitinib therapy in the treatment of Chinese patients who had undergone complete resection for EGFR-mutant stage II to IIIA NSCLC, temporal distribution analysis revealed that the rate of recurrence was lower in the gefitinib group in the early period after surgery, which is also consistent with our results.15 Considering these results, it may be useful to evaluate the molecular profile as much as possible during surgery in terms of postoperative surveillance.
The limitations of this study include its small sample size and retrospective nature. In the future, a prospective study, preferably multicenter, may be needed to confirm our results.
In conclusion, even in early stage NSCLC after complete resection, it was found that some cases had a relapse more than 5 years after surgery, as in breast cancer. Most of these cases had some kind of driver mutations; so it is proposed that more than 5 years of postoperative surveillance is needed, especially for these cases, and also suggesting that postoperative therapy with molecular targeted drugs might be necessary.
Footnotes
Disclosure: The authors declare no conflict of interest.
References
- 1.Solomon B.J., Mok T., Kim D.W. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 2.Calman L., Beaver K., Hind D., Lorigan P., Roberts C., Lloyd-Jones M. Survival benefits from follow-up of patients with lung cancer: a systematic review and meta-analysis. J Thorac Oncol. 2011;6:1993–2004. doi: 10.1097/JTO.0b013e31822b01a1. [DOI] [PubMed] [Google Scholar]
- 3.Kosaka T., Yatabe Y., Onozato R., Kuwano H., Mitsudomi T. Prognostic implication of EGFR, KRAS, and TP53 gene mutations in a large cohort of Japanese patients with surgically treated lung adenocarcinoma. J Thorac Oncol. 2009;4:22–29. doi: 10.1097/JTO.0b013e3181914111. [DOI] [PubMed] [Google Scholar]
- 4.Choi Y.L., Takeuchi K., Soda M. Identification of novel isoforms of the EML4-ALK transforming gene in non-small cell lung cancer. Cancer Res. 2008;68:4971–4976. doi: 10.1158/0008-5472.CAN-07-6158. [DOI] [PubMed] [Google Scholar]
- 5.Fukui T., Yatabe Y., Kobayashi Y. Clinicoradiologic characteristics of patients with lung adenocarcinoma harboring EML4-ALK fusion oncogene. Lung Cancer. 2012;77:319–325. doi: 10.1016/j.lungcan.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Davies C., Pan H., Godwin J. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–816. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venclovas Z., Jievaltas M., Milonas D. Significance of time until PSA recurrence after radical prostatectomy without neo- or adjuvant treatment to clinical progression and cancer-related death in high-risk prostate cancer patients. Front Oncol. 2019;9:1286. doi: 10.3389/fonc.2019.01286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokomizo A., Murai M., Baba S. Percentage of positive biopsy cores, preoperative prostate-specific antigen (PSA) level, pT and Gleason score as predictors of PSA recurrence after radical prostatectomy: a multi-institutional outcome study in Japan. BJU Int. 2006;98:549–553. doi: 10.1111/j.1464-410X.2006.06379.x. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell K.G., Nelson D.B., Corsini E.M. Surveillance after treatment of non-small-cell lung cancer: a call for multidisciplinary standardization. Innovations (Phila) 2019;15:57–65. doi: 10.1177/1556984519886281. [DOI] [PubMed] [Google Scholar]
- 10.Ni J., Guo T., Li Y. Patterns and risks of postoperative recurrence in completely resected EGFR-mutant non-small cell lung cancer: prognostic significance of routine immunohistochemical markers. Transl Lung Cancer Res. 2019;8:967–978. doi: 10.21037/tlcr.2019.12.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamiya A., Koh Y., Isa S.I. Impact of somatic mutations on prognosis in resected non-small-cell lung cancer: the Japan Molecular Epidemiology for lung cancer study. Cancer Med. 2020;9:2343–2351. doi: 10.1002/cam4.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai M.F., Chang T.H., Wu S.G. EGFR-L858R mutant enhances lung adenocarcinoma cell invasive ability and promotes malignant pleural effusion formation through activation of the CXCL12-CXCR4 pathway. Sci Rep. 2015;5:13574. doi: 10.1038/srep13574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vogelstein B., Papadopoulos N., Velculescu V.E., Zhou S., Diaz L.A., Kinzler K.W. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chalela R., Bellosillo B., Curull V. EGFR and KRAS mutations in the non-tumoral lung. Prognosis in patients with adenocarcinoma. J Clin Med. 2019;8:529. doi: 10.3390/jcm8040529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu S.T., Xi J.J., Zhong W.Z. The unique spatial-temporal treatment failure patterns of adjuvant gefitinib therapy: a post hoc analysis of the ADJUVANT trial (CTONG 1104) J Thorac Oncol. 2019;14:503–512. doi: 10.1016/j.jtho.2018.11.020. [DOI] [PubMed] [Google Scholar]