Abstract
Introduction
Since the July 2017 National Comprehensive Cancer Network (NCCN) malignant pleural mesothelioma (MPM) guideline revision recommended second-line immune checkpoint inhibitors (ICIs), studies have suggested a greater response to ICI among patients with nonepithelioid MPM. Nevertheless, little is known regarding adoption of ICI in routine practice and if uptake differs by histologic subtype. Our objectives were to evaluate the real-world uptake of second-line ICI among patients with MPM and to reveal its association with histologic subtype.
Methods
This was a multicenter, retrospective cohort study of real-world patients with MPM receiving at least two lines of systemic therapy between 2011 and 2019. We found the uptake of second-line ICI over time and evaluated the association between histologic subtype and ICI use, adjusting for relevant patient demographic and clinical factors.
Results
Among the 426 patients with MPM in our cohort, 310 had epithelioid and 116 nonepithelioid histologic subtype. The median age was 73 years (interquartile range: 67–78). Overall, 144 patients (33.8%) received second-line ICI and 282 (66.2%) traditional chemotherapy. ICI uptake began in early 2015 before the NCCN guideline revision and increased rapidly to 2019. After the 2017 NCCN guideline revision, patients with nonepithelioid MPM histologic subtypes had more than 3 times the odds of receiving second-line ICI (OR = 3.26; 95% confidence interval: 1.41–7.54).
Conclusions
Among real-world patients with MPM, second-line ICI uptake began over two years before the 2017 NCCN guideline recommendations and was associated with nonepithelioid histologic subtype after contemporary studies suggested increased clinical benefit in this population, reflecting prompt integration of scientific discovery into clinical practice.
Keywords: Mesothelioma, Immunotherapy, Histology, Real-world evidence, Uptake
Introduction
Malignant pleural mesothelioma (MPM) is a rare, lethal cancer with survival differences by histologic subtype.1 Epithelioid MPM comprises 60% to 70% of all diseases and is associated with a median overall survival of 12 to 27 months, whereas nonepithelioid (sarcomatoid and biphasic) histologic subtypes are less common and associated with a worse prognosis.2 The goal of systemic treatment is palliative rather than curative. Pemetrexed and cisplatin, chemotherapeutic agents, have formed the backbone of first-line systemic therapy since 2003,3 and there is no consensus agreement on subsequent lines of systemic therapy.4,5
In the past decade, the use of immune checkpoint inhibitors (ICIs) of programmed cell death-protein 1 (PD-1) and CTLA-4 (anti–PD-1 and anti–CTLA-4 agents), which enhance T-cell–mediated antitumor activity, has led to marked clinical responses in several malignancies,6,7 resulting in a paradigm shift in cancer treatment. In MPM, the immune system plays a major role in disease pathogenesis, fostering optimism that ICI therapy might benefit patients with MPM as well.1,8 Several nonrandomized phase 1 and 2 clinical trials have revealed the safety of ICI for patients with MPM and suggested potential efficacy, with 12-week disease control rates ranging from 47% to 72%.10, 11, 12, 9 As a result, the National Comprehensive Cancer Network (NCCN) MPM treatment guideline was revised on July 7, 2017, to include pembrolizumab and nivolumab (anti–PD-1 agents) with or without ipilimumab (an anti–CTLA-4 agent) as options for subsequent lines of systemic therapy.13
Interestingly, observational studies published shortly after the NCCN guideline revision have suggested that nonepithelioid MPM may be more responsive to ICI than epithelioid MPM.14,15 Moreover, the results of a phase 3 trial comparing first-line nivolumab and ipilimumab with chemotherapy revealed evidence of a greater ICI treatment effect among patients with nonepithelioid histologic subtypes compared to those with the epithelioid subtype.16 The results of these studies suggest that among patients with nonepithelioid MPM, ICI may be preferable to traditional chemotherapy.
Although the U.S. Food and Drug Administration (FDA) approved ICI for first-line MPM treatment on October 2, 2020, it is unknown when off-label ICI uptake for second-line MPM treatment began in routine clinical practice. Previous studies have reported that approximately 30% of prescribed cancer therapies in the U.S. represent off-label use often endorsed by NCCN guidelines,17,18 and given the rarity of MPM and lack of effective therapies, there has been considerable optimism for off-label ICI use.8,19 In addition, it is unclear whether the recent studies revealing differential responses to ICI by MPM histologic subtype have influenced real-world treatment selection. To further understand ICI adoption in this rare malignancy with limited other therapeutic options, we calculated the real-world uptake of second-line ICI among patients with MPM who received traditional first-line chemotherapy and explored the influence of MPM histologic subtype on ICI uptake before and after the 2017 NCCN MPM guideline revision.
Materials and Methods
Data Source
We conducted a retrospective, multicenter cohort study of patients with MPM using deidentified electronic health record (EHR) data from the Flatiron Health database,20 which contains patient-level, longitudinal data, including demographics, treatments, and disease-specific details, and is a representative sample of the U.S. oncology population with respect to age, sex, and geography.21 The Flatiron Health database comprises structured data (e.g., demographics and prescribed drugs) and data from unstructured sources (e.g., physician notes and histopathology reports) curated by means of technology-based abstraction techniques.21,22 The data set delivered for this study comprised 2170 patients diagnosed with MPM who received care at 133 distinct American cancer clinics between January 1, 2011, and December 31, 2019, and were similar in age, sex, and race or ethnicity to the U.S. population of patients with MPM according to estimates of disease incidence in the Surveillance, Epidemiology, and End Results data from 2017.23 The University of Pennsylvania Institutional Review Board approved this study and waived the requirement for written informed consent because all data were deidentified and collected as part of a routine clinical practice.
Study Population
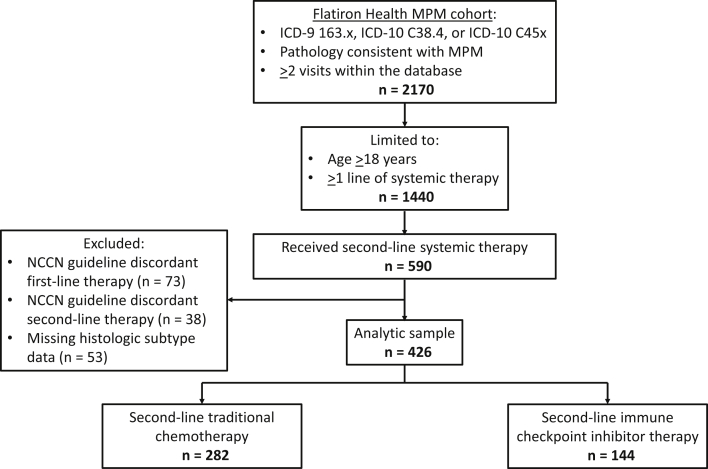
Our study cohort was selected from a broader Flatiron Health database cohort, including patients who had a diagnosis of MPM (International Classification of Diseases [ICD]-9 163.x, ICD-10 C38.4, or ICD-10 C45x), from January 1, 2011, to December 31, 2019, abstraction-confirmed histopathology reports consistent with MPM, and at least two visits within the database. We included patients aged 18 years or older who received at least two lines of systemic treatment for MPM. We limited our study to patients who received first-line chemotherapy concordant with the NCCN MPM guidelines.13 Because our goal was to evaluate the role of MPM histologic subtype in the adoption of second-line ICI, we excluded patients with missing histologic subtype data and those receiving second-line therapies not recommended by the NCCN MPM guidelines (e.g., clinical trial treatments). In this way, patients included in the final analytical sample each received first-line chemotherapy concordant with the 2017 NCCN MPM guidelines and one of the following two distinct categories of second-line systemic therapy: traditional chemotherapy (i.e., pemetrexed, carboplatin/cisplatin, bevacizumab, gemcitabine, vinorelbine, or combinations of these medications) or ICI (i.e., pembrolizumab or nivolumab ± ipilimumab). Assembly of the analytical sample is summarized in Figure 1.
Figure 1.
Assembly of the analytical sample. From the Flatiron Health MPM cohort, the analytical sample was created by selecting adult patients who received at least two lines of systemic therapy and excluding those who received NCCN guideline-discordant first- or second-line therapies and those who had missing MPM histologic subtype data. The analytical sample included 426 patients with MPM who received NCCN guideline-concordant second-line systemic therapy. NCCN guideline-concordant first-line therapies included pemetrexed, carboplatin/cisplatin, bevacizumab, gemcitabine, vinorelbine, and combinations of these medications. NCCN guideline-concordant second-line therapies included traditional chemotherapy (pemetrexed, carboplatin/cisplatin, bevacizumab, gemcitabine, vinorelbine, and combinations of these medications) and immune checkpoint inhibitor therapy (pembrolizumab and nivolumab ± ipilimumab). ICD, International Classification of Diseases; MPM, malignant pleural mesothelioma; NCCN, National Comprehensive Cancer Network.
Data Collection
Demographic and clinical data available for analysis included age, sex, race, start of second-line therapy, MPM histologic subtype (epithelioid and nonepithelioid [i.e., sarcomatoid or biphasic]), time since start of first-line therapy, baseline Eastern Cooperative Oncology Group (ECOG) performance status, center volume (low and high), type of insurance (Medicare/Medicaid, commercial health plan, other), programmed death-ligand 1 (PD-L1) status (immunohistochemical staining percentage), MPM clinical stage, and previous surgical resection for MPM. ECOG performance status was dichotomized into low (ECOG 0–1) and high (ECOG 2–4) categories, with higher scores associated with increased levels of functional disability.24 High-volume centers were defined as those contributing greater than or equal to 20 patients each to the study cohort, whereas low-volume centers each contributed to less than 20 patients.
Data Analysis
We used descriptive statistics to evaluate the demographics and clinical characteristics of patients with MPM receiving second-line systemic therapy. Continuous variables were described with medians and interquartile ranges, and categorical variables were described with the numbers and percentages of observations. Differences in the demographics and clinical characteristics between patients with epithelioid and nonepithelioid MPM were summarized using the Wilcoxon ranked sum test for continuous variables and the Pearson’s chi-square test for categorical variables. Beginning with January 2015 (i.e., the first month that both recommended ICI therapies were available for off-label use) and ending with December 2019, we used a cumulative uptake measure to determine the total number and proportion of patients receiving ICI each month. Patients receiving second-line ICI each month were maintained in the respective numerators, whereas patients becoming eligible to receive second-line ICI each month (i.e., patients who received second-line systemic therapy) were maintained in the respective denominators for the remainder of the study to calculate cumulative assessments. The cumulative uptake of second-line ICI was determined for both the overall cohorts and stratified by the specific ICI regimen and by MPM histologic subtype.
We evaluated the association between MPM histologic subtype and receipt of second-line ICI using univariable and multivariable logistic regression models. We decided a priori to evaluate the timing of second-line systemic therapy before and after the NCCN MPM guideline revision on July 7, 2017, as an effect modifier for this association using the Mantel-Haenszel test of homogeneity as part of a stratified analysis (statistical significance defined with a two-sided p < 0.10). Our stratified analysis revealed that MPM histologic subtype was associated with receipt of ICI only after the NCCN MPM guideline revision (Mantel-Haenszel test of homogeneity, p = 0.079); thus, all logistic regression models included an interaction term for the association between MPM histologic subtype and whether start of second-line therapy was before or after the NCCN guideline revision. The final multivariable model was adjusted for age, race, sex, ECOG performance status, clinical center volume, and type of insurance—chosen a priori for clinical relevance.25, 26, 27 We did not include PD-L1 status or MPM clinical stage as covariates in the final model owing to the high degrees of missingness in the data set and did not include time since start of first-line therapy as this was highly correlated with MPM histologic subtype (Pearson’s chi-square test, p < 0.001). We used multiple imputation by chained equations to impute missing values for race, ECOG performance status, and insurance.28 On the basis of our original data set, we generated 20 multiply imputed data sets, and estimates from these data sets were combined using standard methods provided in Stata/IC, version 16.1.29 All logistic regression analyses were performed on the imputed data set. We performed sensitivity analyses for the association between MPM histologic subtype and second-line ICI excluding patients with missing data for race, ECOG performance status, or insurance, patients who had greater than 12-month delays in initiation of first-line systemic therapy, and patients receiving second-line systemic therapy before the year 2015. Statistical significance was defined with a two-sided p value less than 0.05 unless otherwise specified, and all analyses were conducted using Stata/IC, version 16.1.
Results
Patient Demographics and Characteristics
Of the 426 patients with biopsy-proven MPM diagnosed between January 2011 and December 2019 who received first- and second-line systemic therapies recommended by the 2017 NCCN MPM guidelines, 310 (72.8%) had epithelioid and 116 (27.2%) nonepithelioid histologic subtypes (48 sarcomatoid, 68 biphasic). Patient demographics and clinical characteristics are presented by histologic subtype in Table 1. Overall, the median age was 73 years (interquartile range [IQR]: 67–78). Most patients were white, and 89% did not undergo a surgical resection procedure for MPM. The median time between the start of first- and second-line therapies was 8.58 months (IQR: 4.67–13.07 mo). Compared with patients with the epithelioid histologic subtype, those with nonepithelioid MPM were more likely to be male (82.8% versus 70.0%, p = 0.008), had reduced time between the initiation of first- and second-line therapies (median = 4.67 [IQR: 2.77–8.90] versus 9.80 [IQR: 6.43–14.63], p < 0.001), and had more advanced disease (stage IV disease: 44.0% versus 28.7%, p = 0.003).
Table 1.
Demographic and Clinical Characteristics of Patients With MPM Receiving Second-Line Systemic Therapy
| Variables | Total (N = 426) | Epithelioid (n = 310) | Nonepithelioida (n = 116) |
|---|---|---|---|
| Age quartile, y | |||
| 46–67 | 115 (27.0) | 81 (26.1) | 34 (29.3) |
| 68–73 | 106 (24.9) | 76 (24.5) | 30 (25.9) |
| 74–78 | 118 (27.7) | 83 (26.8) | 35 (30.2) |
| 79–84 | 87 (20.4) | 70 (22.6) | 17 (14.7) |
| Sex | |||
| Female | 113 (26.5) | 93 (30.0) | 20 (17.2) |
| Male | 313 (73.5) | 217 (70.0) | 96 (82.8) |
| Race or ethnicityb | |||
| White | 321 (75.4) | 233 (75.2) | 88 (75.9) |
| Black or African American | 19 (4.5) | 16 (5.2) | 3 (2.6) |
| Asian | 4 (0.9) | 4 (1.3) | 0 (0) |
| Hispanic or Latinx | 13 (3.1) | 11 (3.5) | 2 (1.7) |
| Other | 45 (10.6) | 30 (9.7) | 15 (12.9) |
| Missing | 24 (5.6) | 16 (5.2) | 8 (6.9) |
| Start of second-line therapyc | |||
| Before NCCN guideline revision | 270 (63.4) | 202 (65.2) | 68 (58.6) |
| After NCCN guideline revision | 156 (36.6) | 108 (34.8) | 48 (41.4) |
| Time since start of first-line therapy, mo | 8.58 (4.67–13.07) | 9.80 (6.43–14.63) | 4.67 (2.77–8.90) |
| ECOG performance statusd | |||
| 0–1 | 292 (68.5) | 222 (71.6) | 70 (60.3) |
| 2–4 | 45 (10.6) | 27 (8.7) | 18 (15.5) |
| Missing | 89 (20.9) | 61 (19.7) | 28 (24.1) |
| Treatment center volumee | |||
| Low | 229 (53.8) | 166 (53.5) | 63 (54.3) |
| High | 197 (46.2) | 144 (46.5) | 53 (45.7) |
| Type of insurance | |||
| Medicare/Medicaid | 154 (36.2) | 115 (37.1) | 39 (33.6) |
| Commercial health plan | 126 (29.6) | 83 (26.8) | 43 (37.1) |
| Other | 134 (31.5) | 105 (33.9) | 29 (25.0) |
| Missing | 12 (2.8) | 7 (2.3) | 5 (4.3) |
| MPM clinical stage | |||
| I–II | 45 (10.6) | 37 (11.9) | 8 (6.9) |
| III | 84 (19.7) | 69 (22.3) | 15 (12.9) |
| IV | 140 (32.9) | 89 (28.7) | 51 (44.0) |
| Missing | 157 (36.9) | 115 (37.1) | 42 (36.2) |
| PD-L1 statusf | |||
| <5% | 37 (8.7) | 32 (10.3) | 5 (4.3) |
| 5%–49% | 27 (6.3) | 18 (5.8) | 9 (7.8) |
| >50% | 17 (4.0) | 9 (2.9) | 8 (6.9) |
| Missing | 345 (81.0) | 251 (81.0) | 94 (81.0) |
| Prior surgical resection for MPMg | |||
| No | 378 (88.7) | 268 (86.5) | 110 (94.8) |
| Yes | 48 (11.3) | 42 (13.5) | 6 (5.2) |
| Asbestos exposure | |||
| No | 75 (17.6) | 58 (18.7) | 17 (14.7) |
| Yes | 285 (66.9) | 202 (65.2) | 83 (71.6) |
| Missing | 66 (15.5) | 50 (16.1) | 16 (13.8) |
Note: Values are given in n (%) or median (IQR).
ECOG, Eastern Cooperative Oncology Group; IQR, interquartile range; MPM, malignant pleural mesothelioma; NCCN, National Comprehensive Cancer Network; PD-L1, programmed death-ligand 1.
Sarcomatoid (n = 48) and biphasic (n = 68) histologic subtypes.
Self-reported race or ethnicity abstracted from electronic health records.
The NCCN guideline for MPM treatment was revised to include immune checkpoint inhibitor therapy as an option for second-line therapy on July 7, 2017.
Baseline ECOG performance status; grades range from 0 to 4, with higher scores associated with increasing levels of functional disability.
High-volume centers were defined as those contributing greater than 20 patients each to the study cohort, whereas low-volume centers each contributed less than 20 patients.
Highest MPM tumor PD-L1 immunohistochemical staining percentage determined before the start of second-line systemic therapy.
Surgical resections for MPM included extrapleural pneumonectomy and pleurectomy/decortication procedures.
Uptake of ICI Therapy
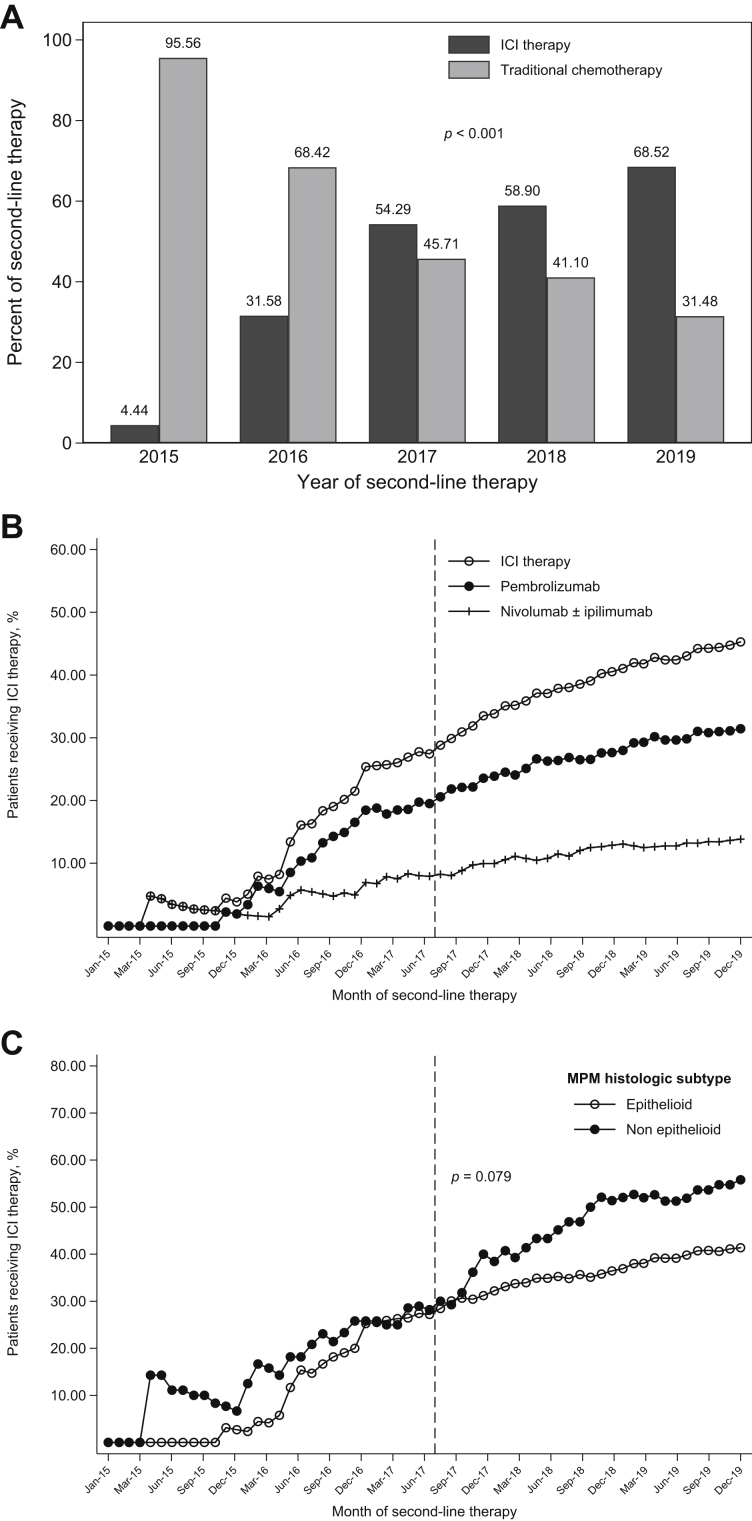
Overall, 144 patients (33.8%) received second-line ICI and 282 (66.2%) traditional chemotherapy (Fig. 1). Uptake of second-line ICI in our real-world cohort began in 2015 (Fig. 2A). In each subsequent year, a higher proportion of patients received second-line ICI (Pearson’s chi-square test, p < 0.001). Beginning the first quarter of 2016, rapid and sustained adoption of second-line ICI occurred, largely driven by pembrolizumab uptake (Fig. 2B). Before the July 2017 NCCN MPM guideline revision, the cumulative proportion of eligible patients who received second-line ICI had reached 27.4% (45 of 164 patients). By the end of the study period (December 2019), this had increased to 45.3% (144 of 318 patients). Before the July 2017 NCCN MPM guideline revision, there was similar cumulative ICI uptake by MPM histologic subtype (Fig. 2C; July 2017: 28.2% versus 27.2%). After the guideline change, there was greater uptake among patients with nonepithelioid histologic subtypes (December 2019: 55.8% versus 41.4%).
Figure 2.
Uptake of second-line ICI therapy. (A) Receipt of second-line ICI therapy was significantly associated with the year of second-line therapy (Pearson’s chi-square test, p < 0.001). ICI therapy included pembrolizumab and nivolumab ± ipilimumab. Traditional chemotherapy included pemetrexed, carboplatin/cisplatin, bevacizumab, gemcitabine, vinorelbine, and combinations of these medications. (B) Cumulative uptake of second-line ICI therapy among patients with MPM who had received traditional first-line chemotherapy concordant with the NCCN MPM guidelines. The vertical dashed lines in Figure 2B and C identify July 2017, when the NCCN MPM guideline was revised to include ICI therapy as an option for second-line systemic therapy. (C) Cumulative uptake of second-line ICI therapy by MPM histologic subtype. The start of second-line therapy before or after the NCCN guideline revision was a significant effect modifier for the association between MPM histologic subtype and receipt of ICI therapy (Mantel-Haenszel test of homogeneity, p = 0.079). Dec, December; ICI, immune checkpoint inhibitor; Jan, January; Jun, June; Mar, March; MPM, malignant pleural mesothelioma; NCCN, National Comprehensive Cancer Network; Sep, September.
Association of MPM Histologic Subtype With Second-Line ICI Therapy
The unadjusted and adjusted (for age, sex, race, ECOG performance status, center volume, and type of insurance) ORs for receipt of second-line ICI are presented in Table 2. After the 2017 NCCN MPM guideline revision, patients with nonepithelioid MPM were more likely to receive second-line ICI (unadjusted OR = 2.50; 95% confidence interval [CI]: 1.15–5.41). The effect persisted after adjustment for demographic and clinical factors in the multivariable model (adjusted OR = 3.26; 95% CI: 1.41–7.54). In the prespecified sensitivity analyses, these findings were not substantially altered when excluding patients with missing race, ECOG performance status, or insurance data, patients who had a greater than 12-month delay in initiation of first-line therapy, or patients receiving second-line therapy before the year 2015.
Table 2.
Association of MPM Histologic Subtype With Second-Line Immune Checkpoint Inhibitor Therapy
| Unadjusted OR (95% CI) | p Value | Adjusted OR (95% CI)a,b | p Value | |
|---|---|---|---|---|
| Before NCCN guideline revisionc | ||||
| Epithelioid | 1 (reference) | 0.900 | 1 (reference) | 0.767 |
| Nonepithelioidd | 0.95 (0.45–2.01) | 1.12 (0.52–2.44) | ||
| After NCCN guideline revision | ||||
| Epithelioid | 1 (reference) | 0.021 | 1 (reference) | 0.006 |
| Nonepithelioid | 2.50 (1.15–5.41) | 3.26 (1.41–7.54) |
CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; MPM, malignant pleural mesothelioma; NCCN, National Comprehensive Cancer Network.
Adjusted for age quartile (46–67, 68–73, 74–78, 79–84 y), sex (female, male), race (nonwhite, white), ECOG performance status (0–1, 2–4), center volume (low, high), and type of insurance (Medicare/Medicaid, commercial health plan, other).
Multiple imputation was used for missing ECOG performance status, race, and insurance data.
The NCCN guideline for MPM treatment was revised to include immune checkpoint inhibitor therapy as an option for second-line therapy on July 7, 2017.
Nonepithelioid histologic subtypes include sarcomatoid and biphasic MPM.
Discussion
In this retrospective cohort study of real-world patients with MPM receiving second-line systemic therapy, rapid off-label uptake of second-line ICI began over 2 years before the inclusion of ICI in the NCCN MPM guidelines. Although ICI use was first observed in 2015, there was a sustained increase in second-line ICI use between early 2016 and the end of 2019. After the 2017 NCCN MPM guidelines recommended ICI as subsequent-line therapy, patients with nonepithelioid histologic subtypes had more than three times the odds of receiving second-line ICI compared with those with the epithelioid subtype. These results reveal that oncologists treating MPM have been early adopters of ICI and may have informed their choice of therapy on the basis of contemporary data that suggested greater benefit in nonepithelioid tumors.
Historically, the adoption of medical innovations into clinical practice has been described to lag an estimated 17 years behind initial research publications30, 31, 32, 33; however, several recent studies have suggested a movement toward earlier uptake of novel therapies, especially in the context of life-threatening malignancies.34, 35, 36, 37 Although rapid adoption of novel therapies on the basis of efficacious early clinical trial results offers the potential of expedited benefit to patients, serious concerns regarding the safety, effectiveness, and applicability of these agents in a real-world population have been raised.30,34,35,38, 39, 40 In this context, our findings revealing real-world early adoption of ICI in MPM—a disease for which clinical treatment guidelines advocate for the use of off-label therapies4,5,13—represents a microcosm of the current state of diffusion, dissemination, and implementation of innovation in modern medicine. By late 2014, when early observational studies first revealed the presence of PD-L1 expression in MPM,41,42 pembrolizumab, nivolumab, and ipilimumab had each already been FDA approved for advanced melanoma treatment and, thus, were available for off-label MPM treatment. Given the tragically poor prognoses of patients with MPM experiencing disease progression after standard-of-care first-line therapy and minimal effectiveness of available second-line therapies, oncologists integrated these preliminary data into their practice rapidly without waiting for clinical trial evidence.41,42 Moreover, given the overall success of ICI in treating other malignancies and the perceived favorable safety profile compared with traditional chemotherapy, early enthusiasm for off-label ICI use in MPM treatment has been robust.8,19 Although rapid adoption of ICI may reflect rational decision making among clinicians to provide an opportunity for improved survival to patients with very poor prognoses, adoption that is too rapid can also result in harm if there is insufficient information on clinical outcomes, including potential toxicities.
Compared with patients with MPM included in ICI clinical trials, the real-world patients included in our study had notable sociodemographic and disease-specific differences.9, 11,16,43,44 For example, our cohort was older (median age of 73 y), more diverse (19% were nonwhite), and had poorer performance status (11% ECOG 2–4) compared with patients included in previous phase 1 to 3 trials of ICI in MPM. Thus, despite important differences in characteristics and prognoses among real-world patients compared with those within clinical trials, we found that off-label, second-line ICI uptake has been rapid and sustained since 2015.
Our finding of increased second-line ICI use among patients with nonepithelioid MPM after the 2017 NCCN guideline revision suggests that oncologists may have been influenced by contemporaneous early evidence revealing enhanced ICI efficacy in nonepithelioid compared with epithelioid MPM.14,15 For example, a 2018 observational study revealed that treatment with pembrolizumab was associated with an improved disease control rate and progression-free survival among patients with nonepithelioid MPM.14 It is possible that oncologists treating patients in our study cohort had similar clinical experiences that influenced their decisions to increasingly use second-line ICI among patients with nonepithelioid histologic subtypes. Nevertheless, more recent clinical trial data have failed to reveal meaningful differences in patient outcomes by MPM histologic subtype for second-line ICI compared with chemotherapy.44
Aside from the possibility of increased efficacy of ICI in nonepithelioid MPM, clinicians may have been motivated to use ICI by the inherently worse prognosis associated with nonepithelioid MPM tumors.45 In fact, a recent study evaluating clinician decision-making patterns for second-line treatment of SCLC—a disease similarly associated with a dismal prognosis—determined that real-world practice may vary by disease aggressiveness, regardless of guideline recommendations.46 In SCLC, nivolumab received accelerated FDA approval as third-line therapy in 2018 and pembrolizumab as second-line therapy in 2019 on the basis of single-arm, phase 1/2 clinical trial evidence. Similarly to MPM, adoption of ICI in SCLC was relatively rapid in the absence of robust trial evidence. This has raised concerns regarding the uncertainty regarding survival and toxicity outcomes, particularly among older patients and those with poorer performance status (i.e., ECOG > 2).47
Most previous studies using real-world data to evaluate ICI use in other malignancies, such as NSCLC, have determined ICI uptake and outcomes after FDA approval for that indication.34,48 In contrast, our study evaluated off-label ICI use before FDA approval and to our knowledge is one of few such studies across any type of malignancy. Despite being first recommended for use as second-line therapy by the NCCN in 2017, ICI (nivolumab and ipilimumab) remains only FDA approved for first-line therapy on the basis of improved survival observed in CheckMate 743, which was initially reported in 2020.16 In the second-line setting, the clinical benefit of ICI remains uncertain. Although the preliminary results of the CONFIRM trial recently reported a survival benefit for nivolumab compared with placebo,43 the PROMISE-meso trial reported no difference in outcomes between second-line pembrolizumab and chemotherapy.44 That we have reported off-label uptake of second-line ICI for MPM between 2015 and 2019 highlights the willingness of oncologists to trial novel therapies before the availability of high-quality trial evidence and despite lack of FDA approval for this indication.
Major strengths of our study are its generalizability, given its use of a contemporary and nationally representative cohort with nearly a decade of real-world EHR data, which allowed us to evaluate treatment patterns over time. The primary limitation of our study, given its retrospective and nonrandomized nature, is the possibility of unmeasured confounding. Although we adjusted for age, sex, race, ECOG performance status, center volume, and type of insurance and accounted for time in our analyses, it is possible that other important unmeasured clinical factors might be distorting our findings. For example, a key variable with a considerable amount of missingness in our data set was PD-L1 expression,41,42 which limited our ability to evaluate the impact of this factor on treatment selection. Nevertheless, an unmeasured confounder would need to be associated with both second-line ICI receipt and histologic subtype by a risk ratio of 3.01-fold each—above and beyond the measured confounders—to explain away the observed adjusted OR of 3.26.49,50 Second, as our data were EHR derived, we had missing values for race, ECOG performance status, and type of insurance. To address this, we used multiple imputation to account for missing data, and our sensitivity analysis excluding missing data was consistent with our main results. In addition, ECOG performance status at the time of second-line therapy was unavailable for most patients, so we used baseline ECOG performance status as a measure of fitness for treatment. Lastly, as the primary focus of this study was to better understand real-world treatment patterns over time, we did not evaluate patient-centered clinical outcomes, such as overall survival. Nevertheless, we plan to perform future studies to determine if second-line ICI is associated with improved outcomes as suggested by previous studies. Despite these limitations, our study is the first to characterize second-line ICI uptake in a multicenter cohort of patients with MPM treated as part of routine clinical practice.
In summary, our study found that real-world uptake of second-line ICI among patients with MPM began over two years before NCCN guideline recommendations and increased in time. After the NCCN guideline revision, clinicians preferentially prescribed second-line ICI for patients with nonepithelioid MPM, potentially influenced by contemporary observational and clinical trial evidence supporting this decision. In striking contrast to previously cited historical delays in the adoption of medical innovations,31,33 our findings suggest that oncologists treating MPM promptly integrate the results of important scientific discoveries into clinical practice, especially among patients with poor prognoses. Similar to the reported speed of ICI uptake for the treatment of other malignancies,34 the adoption of ICI for MPM treatment occurred rapidly in our study. As ICI therapies become more commonplace for MPM treatment and future studies elucidate their clinical effectiveness, it will be crucial to interpret results in the context of MPM histologic subtype and any association with biomarkers of response (e.g., PD-L1 expression).
CRediT Authorship Contribution Statement
Roger Y. Kim: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing—original draft, Writing—review and editing.
Anil Vachani: Conceptualization, Formal analysis, Methodology, Writing—review and editing.
Nandita Mitra, Stephen J. Bagley, Melina E. Marmarelis, Andrew R. Haas, Katharine A. Rendle: Formal analysis, Writing—review and editing.
Acknowledgments
This work was supported by the National Institute of Environmental Health Sciences (P30-ES013508) and the National Institutes of Health (T32 HL-007891).
Footnotes
Cite this article as: Kim RY, Mitra N, Bagley SJ, et al. Immune checkpoint inhibitor uptake in real-world patients with malignant pleural mesothelioma. JTO Clin Res Rep. 2021;2:100188.
Disclosure: Dr. Bagley reports receiving research funding to his institution from Eli Lilly and Company, Incyte Corp, GlaxoSmithKline, and Novocure and having a consulting role with Bayer and Novocure outside of the submitted work. Dr. Marmarelis reports receiving research funding to her institution from Eli Lilly and Company, Trizell, and AstraZeneca; having a consulting role with AstraZeneca, Novocure, and Boehringer Ingelheim; having stock in Gilead Sciences, Portola Pharmaceuticals, Merck, Bluebird Bio, Johnson & Johnson, and Pfizer; and receiving previous medical writing support from Novartis outside of the submitted work. Dr. Haas reports having a scientific advisory board role with Bronx Medical outside of the submitted work. Dr. Vachani reports receiving research funding to his institution from Allegro Diagnostics, Integrated Diagnostics, Janssen Research & Development, MagArray, and Bronchus Medical; having a consulting role with Allegro Diagnostics, Johnson & Johnson, Novocure, Ethicon, and AbbVie; and receiving travel support from Intuitive Surgical outside of the submitted work. The remaining authors declare no conflict of interest.
References
- 1.Yap T.A., Aerts J.G., Popat S., Fennell D.A. Novel insights into mesothelioma biology and implications for therapy. Nat Rev Cancer. 2017;17:475–488. doi: 10.1038/nrc.2017.42. [DOI] [PubMed] [Google Scholar]
- 2.Kim R.Y., Sterman D.H., Haas A.R. Malignant mesothelioma: has anything changed? Semin Respir Crit Care Med. 2019;40:347–360. doi: 10.1055/s-0039-1693406. [DOI] [PubMed] [Google Scholar]
- 3.Vogelzang N.J., Rusthoven J.J., Symanowski J. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 4.Woolhouse I., Bishop L., Darlison L. British Thoracic Society Guideline for the investigation and management of malignant pleural mesothelioma. Thorax. 2018;73(suppl 1):i1–i30. doi: 10.1136/thoraxjnl-2017-211321. [DOI] [PubMed] [Google Scholar]
- 5.Kindler H.L., Ismaila N., Armato S.G., 3rd Treatment of malignant pleural mesothelioma: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36:1343–1373. doi: 10.1200/JCO.2017.76.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber J.S., D’Angelo S.P., Minor D. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 7.Brahmer J., Reckamp K.L., Baas P. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scherpereel A., Wallyn F., Albelda S.M., Munck C. Novel therapies for malignant pleural mesothelioma. Lancet Oncol. 2018;19:e161–e172. doi: 10.1016/S1470-2045(18)30100-1. [DOI] [PubMed] [Google Scholar]
- 9.Alley E.W., Lopez J., Santoro A. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol. 2017;18:623–630. doi: 10.1016/S1470-2045(17)30169-9. [DOI] [PubMed] [Google Scholar]
- 10.Disselhorst M.J., Quispel-Janssen J., Lalezari F. Ipilimumab and nivolumab in the treatment of recurrent malignant pleural mesothelioma (INITIATE): results of a prospective, single-arm, phase 2 trial. Lancet Respir Med. 2019;7:260–270. doi: 10.1016/S2213-2600(18)30420-X. [DOI] [PubMed] [Google Scholar]
- 11.Quispel-Janssen J., van der Noort V., de Vries J.F. Programmed death 1 blockade with nivolumab in patients with recurrent malignant pleural mesothelioma. J Thorac Oncol. 2018;13:1569–1576. doi: 10.1016/j.jtho.2018.05.038. [DOI] [PubMed] [Google Scholar]
- 12.Scherpereel A., Mazieres J., Greillier L. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): a multicentre, open-label, randomised, non-comparative, phase 2 trial. Lancet Oncol. 2019;20:239–253. doi: 10.1016/S1470-2045(18)30765-4. [DOI] [PubMed] [Google Scholar]
- 13.National Comprehensive Cancer Network NCCN clinical practice guidelines in oncology: malignant pleural mesothelioma version 2. 2017. https://www.nccn.org/professionals/physician_gls/pdf/mpm.pdf Accessed.
- 14.Metaxas Y., Rivalland G., Mauti L.A. Pembrolizumab as palliative immunotherapy in malignant pleural mesothelioma. J Thorac Oncol. 2018;13:1784–1791. doi: 10.1016/j.jtho.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Pasello G., Zago G., Lunardi F. Malignant pleural mesothelioma immune microenvironment and checkpoint expression: correlation with clinical–pathological features and intratumor heterogeneity over time. Ann Oncol. 2018;29:1258–1265. doi: 10.1093/annonc/mdy086. [DOI] [PubMed] [Google Scholar]
- 16.Baas P., Scherpereel A., Nowak A.K. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet. 2021;397:375–386. doi: 10.1016/S0140-6736(20)32714-8. [DOI] [PubMed] [Google Scholar]
- 17.Kalis J.A., Pence S.J., Mancini R.S., Zuckerman D.S., Ineck J.R. Prevalence of off-label use of oral oncolytics at a community cancer center. J Oncol Pract. 2015;11:e139–e143. doi: 10.1200/JOP.2014.001354. [DOI] [PubMed] [Google Scholar]
- 18.Conti R.M., Bernstein A.C., Villaflor V.M., Schilsky R.L., Rosenthal M.B., Bach P.B. Prevalence of off-label use and spending in 2010 among patent-protected chemotherapies in a population-based cohort of medical oncologists. J Clin Oncol. 2013;31:1134–1139. doi: 10.1200/JCO.2012.42.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forde P.M., Scherpereel A., Tsao A.S. Use of immune checkpoint inhibitors in mesothelioma. Curr Treat Options Oncol. 2019;20:18. doi: 10.1007/s11864-019-0613-x. [DOI] [PubMed] [Google Scholar]
- 20.Flatiron. For life sciences. https://flatiron.com/real-world-evidence/ Available at:
- 21.Berger M.L., Curtis M.D., Smith G., Harnett J., Abernethy A.P. Opportunities and challenges in leveraging electronic health record data in oncology. Future Oncol. 2016;12:1261–1274. doi: 10.2217/fon-2015-0043. [DOI] [PubMed] [Google Scholar]
- 22.Abernethy A.P., Gippetti J., Parulkar R., Revol C. Use of electronic health record data for quality reporting. J Oncol Pract. 2017;13:530–534. doi: 10.1200/JOP.2017.024224. [DOI] [PubMed] [Google Scholar]
- 23.NIH, National Cancer Institute, Surveillance, Epidemiology, and End Results Program. SEER∗Explorer. https://www.seer.cancer.gov/explorer Available at:
- 24.Oken M.M., Creech R.H., Tormey D.C. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 25.O’Connor J.M., Seidl-Rathkopf K., Torres A.Z. Disparities in the use of programmed death 1 immune checkpoint inhibitors. Oncologist. 2018;23:1388–1390. doi: 10.1634/theoncologist.2017-0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gross C.P., Smith B.D., Wolf E., Andersen M. Racial disparities in cancer therapy: did the gap narrow between 1992 and 2002? Cancer. 2008;112:900–908. doi: 10.1002/cncr.23228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barsky A.R., Ahern C.A., Venigalla S. Gender-based disparities in receipt of care and survival in malignant pleural mesothelioma. Clin Lung Cancer. 2020;21:e583–e591. doi: 10.1016/j.cllc.2020.05.021. [DOI] [PubMed] [Google Scholar]
- 28.White I.R., Royston P., Wood A.M. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 29.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–242. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 30.Westfall J.M., Mold J., Fagnan L. Practice-based research--”Blue Highways” on the NIH roadmap. JAMA. 2007;297:403–406. doi: 10.1001/jama.297.4.403. [DOI] [PubMed] [Google Scholar]
- 31.Morris Z.S., Wooding S., Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med. 2011;104:510–520. doi: 10.1258/jrsm.2011.110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berwick D.M. Disseminating innovations in health care. JAMA. 2003;289:1969–1975. doi: 10.1001/jama.289.15.1969. [DOI] [PubMed] [Google Scholar]
- 33.Green L.W., Ottoson J.M., García C., Hiatt R.A. Diffusion theory and knowledge dissemination, utilization, and integration in public health. Annu Rev Public Health. 2009;30:151–174. doi: 10.1146/annurev.publhealth.031308.100049. [DOI] [PubMed] [Google Scholar]
- 34.O’Connor J.M., Fessele K.L., Steiner J. Speed of adoption of immune checkpoint inhibitors of programmed cell death 1 protein and comparison of patient ages in clinical practice vs pivotal clinical trials. JAMA Oncol. 2018;4 doi: 10.1001/jamaoncol.2018.0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woloshin S., Schwartz L.M. What’s the rush? The dissemination and adoption of preliminary research results. J Natl Cancer Inst. 2006;98:372–373. doi: 10.1093/jnci/djj115. [DOI] [PubMed] [Google Scholar]
- 36.Simunovic M., Coates A., Smith A., Thabane L., Goldsmith C.H., Levine M.N. Uptake of an innovation in surgery: observations from the cluster-randomized quality initiative in rectal cancer trial. Can J Surg. 2013;56:415–421. doi: 10.1503/cjs.019112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parikh R.B., Adamson B.J.S., Khozin S. Association between FDA label restriction and immunotherapy and chemotherapy use in bladder cancer. JAMA. 2019;322:1209–1211. doi: 10.1001/jama.2019.10650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Downing N.S., Aminawung J.A., Shah N.D., Krumholz H.M., Ross J.S. Clinical trial evidence supporting FDA approval of novel therapeutic agents, 2005–2012. JAMA. 2014;311:368–377. doi: 10.1001/jama.2013.282034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kesselheim A.S., Myers J.A., Avorn J. Characteristics of clinical trials to support approval of orphan vs nonorphan drugs for cancer. JAMA. 2011;305:2320–2326. doi: 10.1001/jama.2011.769. [DOI] [PubMed] [Google Scholar]
- 40.Jena A.B., Zhang J., Lakdawalla D.N. The trade-off between speed and safety in drug approvals. JAMA Oncol. 2017;3:1465–1466. doi: 10.1001/jamaoncol.2016.3337. [DOI] [PubMed] [Google Scholar]
- 41.Cedrés S., Ponce-Aix S., Zugazagoitia J. Analysis of expression of programmed cell death 1 ligand 1 (PD-L1) in malignant pleural mesothelioma (MPM) PLoS One. 2015;10 doi: 10.1371/journal.pone.0121071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mansfield A.S., Roden A.C., Peikert T. B7-H1 expression in malignant pleural mesothelioma is associated with sarcomatoid histology and poor prognosis. J Thorac Oncol. 2014;9:1036–1040. doi: 10.1097/JTO.0000000000000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fennell D., Ottensmeier C., Califano R. PS01.11 nivolumab versus placebo in relapsed malignant mesothelioma: the CONFIRM phase 3 trial. J Thorac Oncol. 2021;16(suppl):S62. doi: 10.1016/S1470-2045(21)00471-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Popat S., Curioni-Fontecedro A., Dafni U. A multicentre randomised phase III trial comparing pembrolizumab versus single-agent chemotherapy for advanced pre-treated malignant pleural mesothelioma: the European Thoracic Oncology Platform (ETOP 9–15) PROMISE-meso trial. Ann Oncol. 2020;31:1734–1745. doi: 10.1016/j.annonc.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 45.Mansfield A.S., Symanowski J.T., Peikert T. Systematic review of response rates of sarcomatoid malignant pleural mesotheliomas in clinical trials. Lung Cancer. 2014;86:133–136. doi: 10.1016/j.lungcan.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Früh M., Panje C.M., Reck M. Choice of second-line systemic therapy in stage IV small cell lung cancer (SCLC) - a decision-making analysis amongst European lung cancer experts. Lung Cancer. 2020;146:6–11. doi: 10.1016/j.lungcan.2020.03.024. [DOI] [PubMed] [Google Scholar]
- 47.Friedlaender A., Liu S.V., Passaro A., Metro G., Banna G., Addeo A. The role of performance status in small-cell lung cancer in the era of immune checkpoint inhibitors. Clin Lung Cancer. 2020;21:e539–e543. doi: 10.1016/j.cllc.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 48.Pasello G., Pavan A., Attili I. Real world data in the era of Immune Checkpoint Inhibitors (ICIs): increasing evidence and future applications in lung cancer. Cancer Treat Rev. 2020;87:102031. doi: 10.1016/j.ctrv.2020.102031. [DOI] [PubMed] [Google Scholar]
- 49.VanderWeele T.J., Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167:268–274. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 50.Mathur M.B., Ding P., Riddell C.A., VanderWeele T.J. Web site and R package for computing E-values. Epidemiology. 2018;29:e45–e47. doi: 10.1097/EDE.0000000000000864. [DOI] [PMC free article] [PubMed] [Google Scholar]