Abstract
HIV-associated neurocognitive disorders (HAND) are a leading cause of morbidity in up to 50% of individuals living with HIV, despite effective treatment with antiretroviral therapy (ART). Current evidence suggests that chronic inflammation associated with HIV is especially attributed to the dysregulated production of reactive oxygen species (ROS) that contribute to neurodegeneration and poor clinical outcomes. While ROS have beneficial effects in eliciting immune responses to infection, chronic ROS production causes damage to macromolecules such as DNA and lipids that has been linked to altered redox homeostasis associated with antioxidant dysregulation. As a result, this disruption in the balance between antioxidant-dependent mechanisms of ROS inactivation and ROS production by enzymes such as the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase family, as well as from the electron transport chain of the mitochondria can result in oxidative stress. This is particularly relevant to the brain, which is exquisitely susceptible to oxidative stress due to its inherently high lipid concentration and ROS levels that have been linked to many neurodegenerative diseases that have similar stages of pathogenesis to HAND. In this review, we discuss the possible role and mechanisms of ROS production leading to oxidative stress that underpin HAND pathogenesis even when HIV is suppressed by current gold-standard antiretroviral therapies. Furthermore, we highlight that pathological ROS can serve as biomarkers for HIV-dependent HAND, and how manipulation of oxidative stress and antioxidant-dependent pathways may facilitate novel strategies for HIV cure.
Keywords: HIV, HAND, Oxidative stress, ROS, ART, Neurodegeneration
Highlights
-
•
Production of reactive oxygen species has been linked to neurodegenerative diseases.
-
•
ROS production contributes to HIV-associated neurocognitive disorders.
-
•
ROS may be used as a biomarker for HIV-associated neurocognitive disorders.
-
•
Manipulation of antioxidant pathways may present novel HIV cure strategies.
Abbreviations
- 4-HNE
4 - hydroxynonenal
- 5HN
5-hydroxynaphthalene-1,4-dione
- 8-oxoG
8 - hydroxyguanine
- ALS
Amyotrophic lateral sclerosis
- ANI
Asymptomatic neurocognitive impairment
- ANT
Adenine nucleotide translocator
- ART
Antiretroviral therapy
- AZT
Zudovudine
- BBB
Blood brain barrier
- CNS
Central nervous system
- CSF
Cerebro-spinal fluid
- CVD
Cardiovascular disease
- ER
Endoplasmic reticulum
- ETC
Electron transport chain
- EVs
Extracellular vesicles
- FFPE
Formalin fixed paraffin embedded
- FISH
Fluorescent in situ hybridisation
- GC/MS
Gas chromatography mass spectrometry
- GFAP
Glial fibrillary acidic protein
- GIT
Gastrointestinal tract
- GSH
Glutathione
- GSR
Glutathione reductase
- Gpx1
Glutathione peroxidase
- HAD
HIV associated dementia
- HAND
HIV associated neurocognitive disorders
- HIF-1
Hypoxia inducible factor
- HIV
Human immunodeficiency virus
- HIVE
HIV-encephalitis
- HO-1
Heme-oxygenase
- HSV-1
Herpes simplex virus 1
- LCM
Laser capture microdissection
- LTR
Long terminal repeat
- MAO
Monoamine oxidase
- MAP2
Microtubule-associated protein 2
- MDA
Malondialdehyde
- MND
Mild neurocognitive disorder
- MS
Multiple sclerosis
- NAC
N-acetylcysteine
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NFL
Neurofilament light protein
- NHP
Non human primate
- NOX
NAPDH oxidase
- PBMC
Peripheral blood mononuclear cells
- PCR
Polymerase chain reaction
- PLWH
People living with HIV
- ROS
Reactive oxygen species
- RT
Reverse transcriptase
- SHIV
Simian-human immunodeficiency virus
- SIV
Simian immunodeficiency virus
- SOD
Superoxide dismutase
- Vpr
Viral protein R
- dROMs
Diacron reactive oxygen metabolites
- mtDNA
Mitochondrial DNA
- qPCR
Quantitative polymerase chain reaction
1. Background
To date, human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS) has affected more than 70 million people worldwide (World Health Organisation. Global Health Observatory (GHO), 2019). It is estimated that 38 million people are currently living with HIV/AIDS, with 690,000 people having died of HIV-related illnesses in 2019 alone (World Health Organisation. Global Health Observatory (GHO), 2019). The arrival of antiretroviral therapy (ART) regimens that suppress viral replication has brought about the transformation of HIV/AIDS from a progressive and fatal disease to one that is chronic but manageable. However, no scalable cure for HIV exists, therefore, requiring people living with HIV (PLWH) to maintain long-term treatment on suppressive ART. Although effective viral suppression strategies with ART have dramatically reduced the risk of PLWH developing AIDS-defining conditions; even a short, two-week interruption to therapy can result in a significant plasma viral load rebound. Therefore, life-long ART therapy is an absolute requirement (Finzi et al., 1999). Additionally, despite a reduced risk of developing AIDS-related opportunistic infections and cancers, PLWH exhibit heightened risk of co-morbid conditions such as cardiovascular disease (CVD), malignancies, end stage liver disease, kidney disease and neurocognitive decline (as reviewed in (Rodriguez-Penney et al., 2013)), which account for between 50 - 66% of deaths in PLWH. These non-AIDS defining conditions are typically observed in older PLWH. It appears that PLWH develop age-related conditions at an earlier stage than their HIV-seronegative counterparts, despite effective suppression of viral load with ART (Wing, 2016). Of even greater concern is the increasing prevalence of neurocognitive decline in PLWH.
Despite viral suppression with ART, between 18-69% of all PLWH exhibit some form of HIV-associated neurocognitive disorder (HAND) (Heaton et al., 2010), leading to impaired cognitive, and in some cases, motor function. Specifically, the umbrella term HAND represents 3 disorders of increasing morbidity and mortality: asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND), and HIV-associated dementia (HAD) (Heaton et al., 2010). HAD, the most severe form of HAND, was first characterized as AIDS dementia complex, a subcortical dementia that manifested as increased loss in concentration, marked motor difficulties and variable behavioral changes (Antinori et al., 2007). During the early 1990s HAD affected 16% of those with AIDS leading to marked impairment in cognitive function and poor prognosis, with a life expectancy of 6 months post-diagnosis (Antinori et al., 2007). Fortunately, effective viral suppression with ART has greatly improved the prognosis of HAND from severe dementia (i.e. HAD), to more mild neurocognitive impairment including ANI and MND (Heaton et al., 2010); albeit, HAD still occurs in 2% of individuals in Western countries and as high as 20% in the developing world. In the large multi-centre CHARTER study, MND was found to have a prevalence of 12% out of the 52% of PLWH with HAND (Heaton et al., 2010). MND produces at least a mild interference in activities of daily living and can be measured through self-report of reduced mental acuity or through observations by those close to the patient (Antinori et al., 2007). A diagnosis of MND requires patients have an acquired impairment in cognitive function in two of the listed domains with scores greater than one standard deviation below demographically corrected means (Antinori et al., 2007). ANI is the most common form of HAND, accounting for approximately 70% of HAND cases (Heaton et al., 2010), and diagnosis requires patients have an acquired neurocognitive impairment that does not impair the performance of daily activities and functions (Antinori et al., 2007). Following the inception of ANI as a diagnosis, its classification was called into question, with some claiming that the diagnoses criteria would result in an unacceptable false-positive rate, and may not be clinically relevant (Torti et al., 2011). However, despite being asymptomatic, ANI is clinically important as affected individuals are (i) 2–6 fold higher risk of developing symptomatic forms of HAND (i.e. MND or HAD), and (ii) 3 fold more likely to develop daily life problems, relative to PLWH without ANI (Grant et al., 2014). The incidence of milder forms of HAND (i.e. MND and ANI), however, remains similar to that observed prior to the introduction of ART (Heaton et al., 2010). This remaining prevalence may be due to the inability of modern ART to effectively penetrate the blood brain barrier and target latently infected cells in the central nervous system (CNS).
While the pathogenesis of HAND in viremic (i.e. high viral load) individuals, is predominantly related to ongoing productive viral infection within the CNS, recent evidence places a key role of chronic inflammation, and more specifically oxidative stress, in disease pathogenesis. Oxidative stress is driven by the unrestrained generation of reactive oxygen species (ROS) which are byproducts of normal cellular metabolism from molecular oxygen that consist of unpaired valence electrons and/or unstable bonds, that at high concentrations react readily with proteins, lipids, carbohydrates, and nucleic acids, inducing irreversible functional alterations or complete destruction (Birben et al., 2012). As such, ROS have been associated with many chronic inflammatory diseases including HAND and share similarities in the pathogenesis with other chronic neurodegenerative diseases including Alzheimer’s disease (Huang et al., 2016), Parkinson’s disease (Subramaniam and Chesselet, 2013), Amyotrophic Lateral Sclerosis (ALS) (D’Amico et al., 2013) and Multiple Sclerosis (MS) (Fischer et al., 2013), as well as infectious diseases such as bacterial and aseptic meningitis (de Menezes et al., 2009), and encephalitis associated with Influenza A (Kawashima et al., 2002) and Herpes Simplex Virus (Milatovic et al., 2002).
In this review we assess how oxidative stress and more specifically ROS may contribute to HAND, the mechanisms driving the production of ROS in HIV infection, and how animal models that recapitulate human HAND can improve our understanding of ROS as both a biomarker of disease and a targetable mechanism of disease to facilitate HIV cure.
2. What is oxidative stress and what effect does it have in neuropathological diseases?
Oxygen is a critical component of human metabolism and is required for cell functioning and energy production via oxidative phosphorylation pathways. However, during the metabolism of oxygen ROS are generated as by-products which can have detrimental effects on the body if allowed to accumulate at high levels. Specifically, ROS including superoxide anion (O2−), hydroxyl radical (OH−), hydrogen peroxide (H2O2), and hypochlorous acid (Fig. 1) are produced by the mitochondrial electron transport chain, and during intracellular metabolism of foreign compounds, toxins, and drugs (Birben et al., 2012). Whilst at low levels ROS are not particularly harmful and are also specifically generated and released by cells such as macrophage/monocytes in order to kill invading pathogens, uncontrolled ROS production is detrimental to the host. Specifically, unrestrained ROS can lead to oxidative stress, whereby an excess of ROS can activate and damage surrounding cells leading to pathology such as neurocognitive and cardiovascular diseases (as reviewed in (Liguori et al., 2018)). Furthermore, exogenous sources of ROS such as cigarette smoking, pollution, exposure to ozone, and drug use (Borgmann and Ghorpade, 2018), can also overwhelm host control mechanisms which generally have deleterious effects on the body (Birben et al., 2012). As such, strict evolutionary controls in the form of antioxidant enzymes such as superoxide dismutase (SOD), or soluble antioxidants such as reduced glutathione (GSH), regulate ROS generation to prevent damage to cellular macromolecules (Fig. 1). Failure in the balance of ROS production and metabolism, due to either the heightened activity of ROS generating enzymes or to the depletion of antioxidants, leads to oxidative stress, which can result in damage to macromolecules, lipid peroxidation, the induction of aberrant signal transduction, and activation of transcription factors that are involved in the inflammatory response (Birben et al., 2012; Ayala et al., 2014). As such, oxidative stress has been implicated in the pathogenesis of many diseases including diabetes mellitus, cancer, cardiovascular disease, and neurocognitive disorders (as reviewed in (García-Sánchez et al., 2020)). Importantly, as the brain has a high polyunsaturated fatty acid content and consumes 20–30% of inspired oxygen, it is an ideal target for oxidative stress and lipid peroxidation (Sultana et al., 2013). The neurons of the brain have a high metabolic activity, producing an estimated 1011 ROS/cell per day (Huang et al., 2016). Oxidative stress can cause cells to accumulate oxidized products such as aldehydes, isoprostanes, and base adducts from DNA oxidation. The accumulation of isoprostanes in astrocytes inhibits glutamate reuptake (Sorg et al., 1997), resulting in neurodegeneration due to the excitotoxic activity of glutamate (Schousboe and Waagepetersen, 2005) (Fig. 2). This accumulation can alter the brain and lead to neurocognitive disorders such as Alzheimer’s disease, Parkinson’s disease, ALS and MS. Thus, oxidative stress and mitochondrial dysfunction is a key contributor to the pathogenesis of many neurocognitive disorders (Guo et al., 2013).
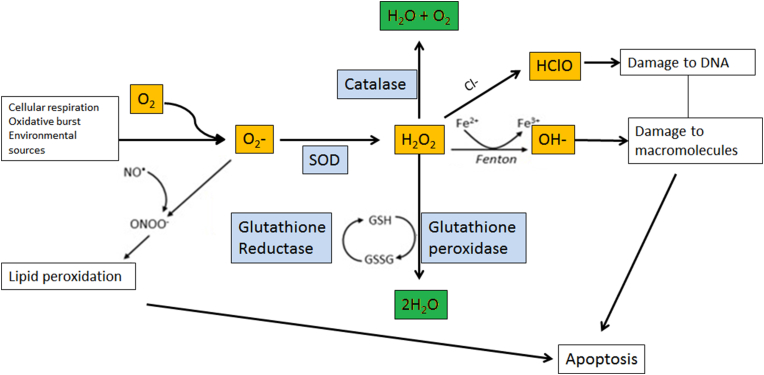
Fig. 1.
ROS pathways: Cellular respiration, oxidative burst and environmental sources produce reactive oxygen species (ROS) such as superoxide (O2−; yellow) and hydrogen peroxide (H2O2; yellow). Catalase, superoxide dismutase (SOD), glutathione reductase and glutathione peroxidase (blue) are enzymes that help to balance the production of ROS by reducing them to harmless oxygen (O2) and water (H2O; green). Reduced glutathione (GSH) also acts as a reducing agent for ROS. The addition of chloride ions (Cl−) to H2O2 results in the production of hypochlorous acid (HClO; yellow), which can damage DNA. The Fenton-Weiss-Haber reaction involves H2O2 and iron (Fe2+), and produces a reactive hydroxyl radical (OH-; yellow), which can cause major damage to macromolecules. Superoxide reacts with nitric oxide (NO•) to produce peroxynitrite (ONOO−), which causes lipid peroxidation. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
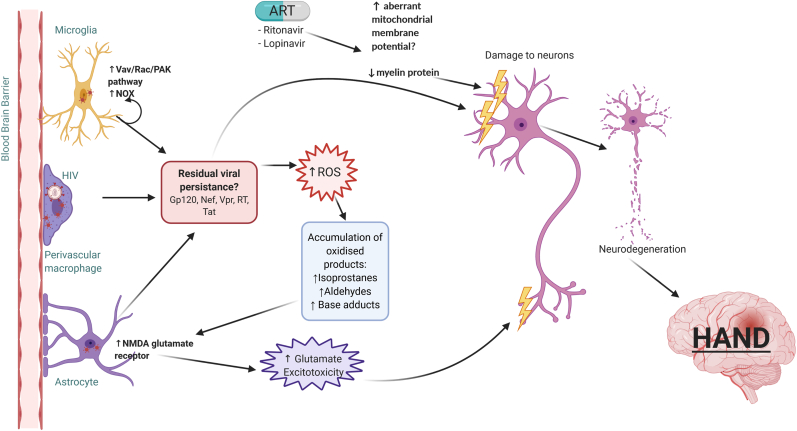
Fig. 2.
ROS generation and neurodegradation in PLWH on ART. HIV infects microglia, perivascular macrophages and astrocytes, leading to the release of HIV proteins including envelope protein Gp120, and non-structural proteins Tat, Nef, Vpr and RT. While these proteins can directly damage neurons, they also result in the production of ROS and pro-inflammatory cytokines. In microglia, viral protein Nef activates the Vav/Rac/PAK pathway, leading to NOX4 activation and ROS production. The production of ROS results in the accumulation of oxidized products including isoprostanes, aldehydes and base adducts. This leads to impaired glutamate reuptake in astrocytes due to prolonged activation of the NMDA glutamate receptor, causing indirect damage to neurons. ART medications, specifically ritonavir and lopinavir, have been found to cause aberrant mitochondrial membrane potential in neural cultures, resulting in the production of ROS. Ritonavir and lopinavir also result in the loss of myelin protein. The resulting neuronal degeneration from myelin protein loss and oxidative stress could lead to HAND.
For instance, a defining feature in the pathogenesis of Alzheimer’s disease is the deposition of amyloid β peptide in the CNS which forms insoluble plaques, and neurofibrillary tangles that accumulate in the intracellular spaces, contributing to cellular dysfunction, neurodegeneration and ultimately cognitive deficits. In Alzheimer’s disease patients, oxidative stress has been shown to initiate and enhances these processes (Huang et al., 2016). Oxidative stress markers appear decades prior to the deposition of amyloid β peptide in patients diagnosed at the prodromal stage; the symptomatic pre-dementia stage of Alzheimer’s disease (Huang et al., 2016; Praticò et al., 2002). In Parkinson’s disease, increased lipid peroxidation and oxidative DNA damage in the substantia nigra indicate the importance of oxidative stress as a causative factor (Subramaniam and Chesselet, 2013). Post mortem tissue from individuals who died with ALS consistently show oxidative damage to proteins, lipids, and DNA (Bogdanov et al., 2000), with increased concentrations of oxidative stress biomarkers such as 4-hydroxynonenal (4-HNE) found in serum and cerebrospinal fluid (CSF) (Simpson et al., 2004). Fischer and colleagues performed genome wide microarray analysis on formalin-fixed paraffin embedded (FFPE) autopsy material from 21 cases of MS; where gene ontology enrichment analysis revealed differentially expressed genes involved in hypoxia (e.g. HSD11B2, OS9), oxidative stress (e.g. SMOX, TXNIP, GSTT1) and mitochondrial dysfunction (e.g. TSFM, PYCR1, ND6) (Fischer et al., 2013).
Oxidative stress has also been implicated in the pathogenesis of various infectious neuroinflammatory diseases. In children with bacterial meningitis, an accumulation of lipid hydroperoxides has been reported in the CSF and serum where similar changes were also observed in patients with aseptic meningitis (de Menezes et al., 2009). Influenza A virus, the most common pathogenic course of acute encephalopathy, is associated with increased levels of nitrite/nitrate in both serum and CSF (Kawashima et al., 2002), as well as increased levels of free radicals as determined by the Diacron reactive oxygen metabolites (dROMs) test (Yamanaka et al., 2006). Furthermore, murine models of herpes simplex encephalitis show increased oxidative damage to neurons and other tissue in contrast to vehicle treated mice (Milatovic et al., 2002). Interestingly, Herpes Simplex Virus Type I (HSV-1) is thought to contribute to the development of Alzheimer’s disease, as HSV-1 virus can directly induce the accumulation of amyloid β peptide (Santana et al., 2013), the hallmark of Alzheimer’s disease. As mentioned previously, oxidative stress markers appear decades before the accumulation of amyloid β peptide, and it has been shown that oxidative stress enhances the effects of HSV-1 on amyloid β peptide accumulation (Santana et al., 2013). HSV-1 and the production of oxidative stress may promote the neurodegeneration events seen in Alzheimer’s disease. Therefore, oxidative stress is an important etiological factor in both infectious and idiopathic neurodegenerative disease. The likely role of oxidative stress and ROS in HAND pathogenesis is discussed in further detail below.
3. Neuropathogenesis of HAND
HIV is thought to enter the brain in part, by the continual entry of monocytes and possibly T cells into the brain parenchyma (Fischer-Smith et al., 2001). Within two weeks of infection, HIV can be detected in the CSF indicative of early penetration into the brain (Fischer-Smith et al., 2001). As a viral reservoir, the CNS provides a sanctuary space, due to the limited drug penetration across the blood brain barrier (BBB) (Barat et al., 2018). It also provides long-living cells such as macrophages, microglia and astrocytes with the potential to harbor latent infection. HIV infection has been found in perivascular macrophages, microglia (Cosenza et al., 2002) and astrocytes (Churchill et al., 2006) with integrated HIV provirus found in these cells via fluorescence in situ hybridization (FISH) or laser capture microdissection (LCM) coupled with polymerase chain reaction (PCR). The presence of replicating HIV in perivascular macrophages (Churchill et al., 2006) and microglia (Cosenza et al., 2002) has been well established. The role of astrocytes in HAND has been disputed; however, these cells are now believed to play a significant role in the development of HAND (Churchill et al., 2006). The non-productive infection of astrocytes by HIV results in significant astrocyte apoptosis, where an increased rate of loss is seen in those individuals with rapidly progressing HAD (Thompson et al., 2001). Without the presence of astrocytes, CNS immune function and redox homeostasis are not supported, and the environment becomes one of both increased neurotoxins, and oxidative stress (Schreiner et al., 2015). Increased apoptosis of astrocytes leads to reduced ROS scavenging capabilities, resulting in increased levels of ROS, and oxidative DNA damage (Schreiner et al., 2015). While direct viral damage to neurons may be occurring in HAND, it is likely that the indirect damage, inflammation and oxidative stress caused by the non-productive infection of astrocytes and other resident brain cells, is propagating neurological impairment (Fig. 2). The specific roles of viral proteins in producing ROS is discussed below.
4. Oxidative stress in PLWH
PLWH are known to exhibit heightened levels of biomarkers of oxidative stress which is thought to reflect ongoing immune activation, accelerate HIV disease pathogenesis and contribute to comorbidities including HAND (Masiá et al., 2016). Specifically, PLWH have lower levels of the anti-oxidant GSH in plasma, peripheral blood-mononuclear cells (PBMCs), monocytes, and lung epithelial lining fluid, relative to HIV-uninfected individuals, which corresponds with an increase in oxidized GSH in lymphocytes and redox imbalance (Aukrust et al., 1995) (Table 1). Plasma and PBMC markers of SOD activity, a key regulator in ROS generation, and the non-enzymatic antioxidants ascorbate (Vitamin C) and β-carotene are expressed at lower levels in PLWH relative to HIV negative controls (Treitinger et al., 2000), indicating dysregulation of oxidative stress control mechanisms in these individuals. Furthermore, monocytes from PLWH have been shown to produce more H2O2 than those from uninfected individuals (Elbim et al., 1999), the effects of which may influence both cellular activation, but also HIV itself (Table 1). This is important as H2O2 has been found to stimulate the HIV long terminal repeat (LTR) in transformed human lymphoid (Jurkat) and macrophage cell lines (THP-1) via activation of the transcription factor NF-κB at a post-transcriptional level (Kazazi et al., 1996). Therefore, HIV-induced ROS production and subsequent activation of the HIV LTR may be drive HIV and comorbid disease pathogenesis.
Table 1.
Evidence of oxidative stress and antioxidant response in PLWH with or without neurocognitive impairment.
| Reference | Tissue | Analysis methods | Virally suppressed? | Oxidative stress markers | Classification of PLWH |
|---|---|---|---|---|---|
| (Zhang et al., 2012) | Ex vivo frontal cortex tissue | In situ immunofluorescence | No | ↑8-oxoG | “HAND” or “non-HAND” |
| HIV- control | |||||
| (Ginsberg et al., 2018) | Ex vivo microglia and macrophage | Single population microarray analysis | On and off ART at time of death | ↓SOD1 | “HIVE” or “HIV+NoE” |
| ↓SOD2 | HIV- control | ||||
| (Kallianpur et al., 2016) | PBMCs and brain tissue | MRI, PCR | ART treated | ↑mtDNA 8-oxoG α ↓hippocampus volume | Domain specific Z scores |
| (Sitole et al., 2019) | Serum | Untargeted metabonomic analysis | < one year on ART | ↑aspartic acid | HIV+ |
| ↑phenylalanine | |||||
| ↑glutamic acid | HIV- control | ||||
| (Castagna et al., 1995) | CSF | Immunostaining | Unknown | ↓GSH | HIV+ with/without neurological impairment |
| (Velázquez et al., 2009) | CSF | ELISA | >70% participants on ART | ↓SOD2 activity in neurologically impaired | “HAND” or “non-HAND” |
| HIV- control | |||||
| (Bandaru et al., 2007) | CSF | Electrospray ionization tandem mass-spectrometry | No | ↑4-HNE | MSK scores – HIV dementia |
| ↑Vit E | HIV- control | ||||
| (Sacktor et al., 2004) | Ex vivo brain tissue | Immunohistochemistry | Unknown | ↑4-HNE | MSK scores – HIV dementia |
| ↑Vit E | |||||
| ↑Sphingomyelins | |||||
| ↑Ceramides | HIV- control | ||||
| (Guha et al., 2019a) | CSF | Electron microscopy | ART treated | ↑Extracellular vesicles | “HAND” or “non-HAND” |
| Immunoblotting | ↑NFL | ||||
| Untargeted mass spectrometry | HIV- control | ||||
| (Guha et al., 2019b) | CSF U87 astrocytes |
Proteomic analysis of extracellular vesicles from CSF and U87 astrocytes | ART treated | ↑GPx1 | “HAND (ANI, MND, HAD)” or “non-HAND” |
| ↑SOD1 | |||||
| ↑SOD2 | |||||
| ↑SOD3 | |||||
| ↑GSR | HIV- control | ||||
| (Turchan et al., 2003) | Ex vivo brain tissue CSF |
Immunostaining | Unknown | ↑Protein carbonyls | HIVE |
| HIV- control |
ART: antiretroviral therapy; CSF: cerebrospinal fluid; Gpx1: glutathione peroxidase 1; GSH: glutathione; GSR: glutathione reductase; HAND: HIV-associated neurocognitive disorders; HNE: 4-Hydroxy-2-nonenal; MRI: magnetic resonance imaging; MSK: Memorial Sloan Kettering scale; NFL: neurofilament light chain; PCR: polymerase chain reaction; SOD: superoxide dismutase.
5. Mechanisms driving ROS generation in the CNS of PLWH
5.1. Viral proteins and RNA
Numerous components of the HIV virion including viral proteins and/or RNA have been shown to induce ROS generation both in vivo and in vitro. Gp120, an HIV envelope glycoprotein, has been shown to have neurotoxic effects and has been associated with increased production of H2O2 and superoxide in rat cortical cell cultures, as well as an increase in the activity of the antioxidant enzyme GSH peroxidase (GPx1), which may occur as a defensive mechanism (Brooke et al., 2002). In high concentrations, the HIV envelope glycoprotein Gp120 can be directly neurotoxic and has been demonstrated to induce apoptosis in cortical cell cultures and in vivo via intracerebral injection (Meucci et al., 1998). In astrocytes, Gp120 exposure can impair glutamate reuptake, leading to prolonged activation of glutamate receptor NMDA and disruption to calcium homeostasis. This process involves Gp120 induced ROS production in astrocytes through various mechanisms: via cytochrome P450, NADPH oxidase, and the Fenton-Weiss-Haber reaction (Shah et al., 2013). Gp120 has also been shown to induce proline oxidase expression in neuroblastoma cells, resulting in increased conversion of proline to pyrroline-5-carboxylate, a conversion that generates ROS (Pandhare et al., 2015) (Fig. 3). Furthermore, repeated intrathecal gp120 treatment of rats induced overexpression of mitochondrial superoxide in neurons of the spinal cord dorsal horn (Godai et al., 2019).
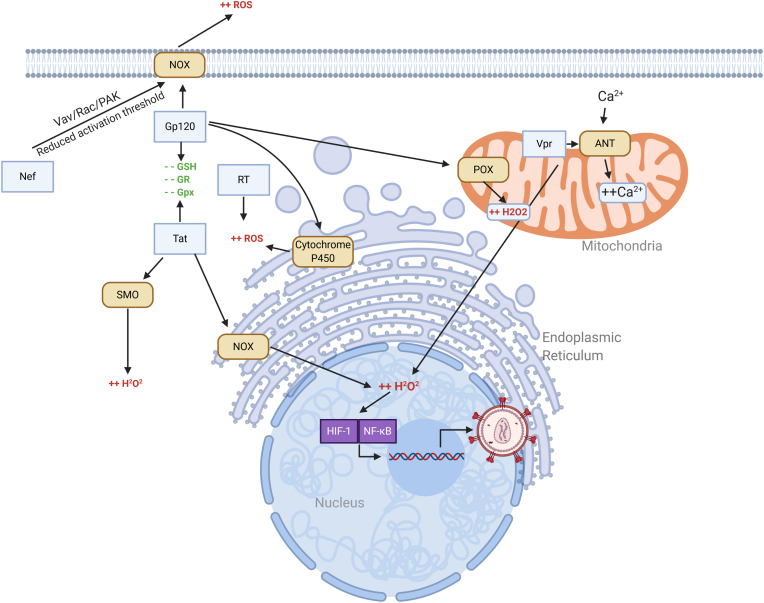
Fig. 3.
Effects of HIV proteins in the CNS on ROS production. Tat induced ROS production involves NADPH oxidase (NOX) in the ER and induction of spermine oxidase (SMO). Gp120 induces ROS production via NOX, cytochrome P450, and proline oxidase (POX). Both Tat and Gp120 suppress expression of reduced glutathione (GSH), glutathione reductase (GR) and glutathione peroxidase (GPx1). Nef activates the Vav/Rac/PAK pathway, reducing the threshold for ROS production via NOX. RT increases production of ROS via an unknown mechanism. Vpr triggers oxidative stress by causing mitochondrial dysfunction through interaction with adenine nucleotide translocator (ANT), increasing Ca2+ influx.
HIV trans-acting Tat is essential for viral replication, stimulating transcriptional elongation from the viral LTR. HIV Tat also has well-known neurotoxic properties driving cellular activation, of which ROS generation plays a key role. HIV Tat can directly depolarize neuron membranes (Wu et al., 2010), increase lipid peroxidation by generating superoxide and H2O2 (Haughey et al., 2004) and drive microglia activation and induce neuronal damage via dysregulating mitochondria removal via mitophagy (Thangaraj et al., 2018; Teodorof-Diedrich and Spector, 2018). Regulatory Tat protein induced ROS production involves NADPH oxidase 4 (NOX4), found primarily in the endoplasmic reticulum (ER), resulting in an increased concentration of H2O2 in the ER of HIV infected cells (Wu et al., 2010) (Fig. 3). Given H2O2 is known to activate the HIV LTR (Kazazi et al., 1996), production of the HIV protein Tat may be an important step in inducing and controlling LTR activation and elongation, and thus viral latency. Suppression of NOX4 in Tat-expressing cells results in the reduction of H2O2 in the ER (Wu et al., 2010). Tat has also been shown to induce ROS production by increasing the activity of spermine oxidase, resulting in activation of glutamate receptor NMDA in neuroblastoma cells (Capone et al., 2013) (Fig. 3). Tat also suppresses the expression of mitochondrial SOD in HeLa cells, resulting in decreased antioxidant defense (Flores et al., 1993). In astrocytes, Tat has been shown to increase glial fibrillary acidic protein (GFAP) expression, a cellular marker of astrocyte activation and impaired glutamate uptake (Zhou et al., 2004). Cell culture supernatants from Tat-expressing astrocytes can also induce neuron apoptosis in vitro (Zhou et al., 2004). A Tat-transgenic mouse model has shown that Tat-expressing astrocytes in the absence of HIV infection can induce neuropathologies in vivo (Kim et al., 2003). Moreover, in an immortalized endothelial cell line from rat brain capillaries, both Tat and Gp120 were shown to suppress the expression of GSH synthesizing and metabolizing enzymes (Price et al., 2005).
HIV Nef protein has shown pro-oxidant activity in microglial and neutrophil cells through activation of the Vav/Rac/PAK pathway, leading to a lower activation threshold for NADPH oxidase to a variety of stimuli, such as Ca2+ or endotoxins, resulting in superoxide synthesis (Vilhardt et al., 2002) (Fig. 3). Abundant Nef expression has been shown in the astrocytes of PLWH with neuronal damage (Ranki et al., 1995), which may lead to a reduced NADPH oxidase activation threshold in a similar fashion to microglia and neutrophils. Expression of HIV reverse transcriptase (RT) in human embryonic kidney cells has been shown to increase the production of ROS and the induction of oxidative stress responses, although the mechanisms for this remain unknown (Isaguliants et al., 2013) (Fig. 3). Myristoylated nef has also been shown to upregulate inducible nitric oxide synthase (iNOS) in mouse microglia cells, with possible downstream neurotoxic effects on other cells types such as neurons (Mangino et al., 2015).
HIV viral protein R (Vpr) can trigger oxidative stress by causing mitochondrial dysfunction through interaction with adenine nucleotide translocator (ANT), increasing Ca2+ influx (Arunagiri et al., 1997). Human CD4+ T lymphocytes demonstrate a reduction in mitochondrial membrane potential and eventual apoptosis when treated with recombinant Vpr (Arunagiri et al., 1997) (Fig. 3). Vpr can mediate the activation of hypoxia-inducible factor 1 (HIF-1), which, under hypoxic conditions, becomes stable, and translocates to the nucleus to modulate gene expression (Deshmane et al., 2009). Vpr mediates the accumulation of HIF-1 by increasing H2O2 production; which in turn, stimulates HIV gene transcription through its association with the HIV LTR (Deshmane et al., 2009). Thus, through the stimulation of HIF-1, HIV Vpr can induce HIV gene expression (Fig. 3).
HIV RNA itself can also promote ROS generation. In response to HIV single-stranded RNA (ssRNA), ROS was produced by NADPH oxidase 2 (NOX2) within activated endosomes in human and mouse immune cells following recognition via TLR-7/8 pathways (To et al., 2017). Furthermore, recent findings have shown that HIV ssRNA LTR fragments can activate microglia via the NLR family pyrin domain containing 3 (NLRP3) inflammasome leading to increased ROS generation related to impaired clearance of dysfunctional mitochondria (Rawat et al., 2019). These findings have significant implications to viral pathogenesis as ROS production in response to viral infection inhibits antiviral and humoral responses in human immune cells, enhancing viral pathogenicity (To et al., 2017). Therefore, therapeutic approaches targeting viral interaction with NOX2 pathways via TLR-7 are under investigation.
5.2. Antiretroviral therapies
Whilst essential at suppressing HIV viremia, some ART drugs have been shown to have off-target effects in the CNS, or on CNS derived cells in culture, including the generation of ROS implying a potential pathogenic role in HAND in ART-treated individuals (Louboutin and Strayer, 2014; Akay et al., 2014). PLWH on ART have higher serum oxidant levels when compared to untreated PLWH or uninfected negative controls, suggesting that the therapy itself can contribute to ROS generation (Mandas et al., 2009). Other oxidative stress markers such as plasma malondialdehyde, protein carbonyls, and F2 isoprostane have also been found at higher levels in ART-treated patients, relative to pre-ART PLWH and uninfected controls (Hulgan et al., 2003). Markers of oxidative damage to DNA such as 8-hydroxyguanine (8-oxoG) were excreted at a higher concentration in the urine of PLWH treated with zidovudine (AZT), relative to untreated PLWH and uninfected controls (de la Asuncion et al., 1998). In the same study, the authors found that skeletal muscle mitochondrial (mtDNA) DNA oxidation and lipid peroxidation was increased in mice treated with AZT, when compared to untreated controls (de la Asuncion et al., 1998).
A study of fifteen different ART medications showed varying degrees of neuronal toxicity in primary neural cultures, as demonstrated by aberrant mitochondrial membrane potential, highlighting the possibility of ART induced oxidative stress in neurons (Robertson et al., 2012) (Fig. 2). Efavirenz, in particular, has been associated with worse neurocognitive outcomes and is also linked to ROS production and impaired mitochondrial function in neurons (Stauch et al., 2017). Jensen and colleagues found that primary mouse oligodendrocyte precursor cells treated with HIV protease inhibitors Ritonavir and Lopinavir displayed a dose-dependent decrease in oligodendrocyte maturation, alongside increased ROS production (Jensen et al., 2015). Intravenous injection of protease inhibitors Ritonavir and Lopinavir into adult mice reduced frontal cortex myelin protein levels; and in human samples, prefrontal cortex tissue from individuals with HAND on ART showed a decrease in myelin protein, relative to untreated individuals with HAND and untreated HIV-negative controls (Jensen et al., 2015). As the myelin membrane generated by oligodendrocytes is essential for signal transduction, oxidative stress and the loss of myelin associated with ART may contribute to HIV associated neuropathy (Fig. 2). Furthermore, lopinavir is neurotoxic to primary rat neuroglial cultures, determined by the loss of microtubule-associated protein 2 (MAP2), evokes oxidative stress (as indicated by CellRox green, an indicator that fluoresces when oxidized by cellular ROS) and induces the endogenous antioxidant response (Stern et al., 2018). The neurotoxicity of lopinavir was blocked through pharmacological augmentation of heme-oxygenase 1 (HO-1), indicating that lopinavir induced neurotoxicity is indeed mediated by oxidative stress (Stern et al., 2018). Therefore, together these findings indicate that it is important to consider the ability of specific ART drugs to induce ROS and oxidative stress in order to minimize neurotoxicity in PLWH, whilst maintaining effective viral suppression of the individual.
6. Does oxidative stress contribute to HAND in virally suppressed individuals?
Oxidative stress and cell damage caused by ROS may be a major driver underlying the development and severity of HAND. However, due to the short half-life and propensity to be oxidized/reduced, studying the mechanisms by which ROS may drive HAND is difficult, especially in archival brain tissue. Despite these limitations, some studies using quantitative PCR (qPCR) and immunohistochemistry (IHC) techniques have assessed the genes/proteins associated with either ROS production or catabolism. Markers of oxidative stress such as protein carbonyls and 4-HNE, have been found in brain tissue and CSF of patients with HAND even when virally suppressed with ART (Turchan et al., 2003; Zhang et al., 2012; Sitole et al., 2019; Castagna et al., 1995) (Table 1). Furthermore, proteins in the CNS of patient with HAND are more nitrated, possibly as a consequence of excess iNOS in the CNS (Uzasci et al., 2014). Zhang and colleagues (Zhang et al., 2012) found higher protein levels of 8-oxoG, indicative of oxidative stress to DNA, in frontal cortex tissue from PLWH, relative to uninfected control samples (Zhang et al., 2012) (Table 1). Notably, in this study, individuals with HIV were diagnosed with “HAND” or “non-HAND” based on a dementia score; however the authors did not explore the varying severities of HAND, potentially misdiagnosing individuals with ANI or MND as “non-HAND” (Zhang et al., 2012). PBMCs from virally suppressed PLWH also showed higher levels of mitochondrial damage (as measured by mtDNA 8-oxodG levels) which was associated with lower hippocampus and subcortical volumes (Kallianpur et al., 2016), suggesting that damage to mitochondrial DNA can elicit physical damage to the CNS. Future studies should keep the updated HAND nosology (Antinori et al., 2007) in mind, correlate viral loads with the levels of 8-oxoG in tissue, and identify which cell types exhibit the most oxidative stress-related damage in the tissue (Zhang et al., 2012). Furthermore, it is important to consider opportunistic infections such as pulmonary tuberculosis, which may have their own impact on oxidative stress in the brain.
Ginsburg and colleagues also found that microglia and macrophages isolated from PLWH without encephalitis (HIV+/noE), or with encephalitis (HIVE) displayed dysregulation of several classes of gene transcripts, relative to HIV-uninfected subjects (Ginsberg et al., 2018) (Table 1). Among these alterations, down-regulation of antioxidant enzymes SOD1 and SOD2 was observed (Ginsberg et al., 2018), which could reduce the ability of microglia to kill and/or degrade ingested particles, increasing inflammation and causing the neuronal damage seen in HAND. Note, that these findings are from FFPE autopsied brain tissue; formalin fixation can reduce the quality of extracted RNA, and microarray analyses results could not be validated via qPCR due to low yield (Ginsberg et al., 2018). While studies that measure the concentration of mRNA transcripts may provide a useful picture of disease states and the mechanisms behind them, research must take into account that post transcriptional, translation, and degradation regulation may have an important impact on protein production and turnover, and that the measurement of transcripts alone cannot determine a disease associated phenotype. Thus, future research should involve measurement of both mRNA transcripts and protein itself, where possible.
7. Animal models for studying oxidative stress in HAND
Non-human primate (NHP) models have proven to be a fundamental tool in infectious disease research due to the similarities to humans in physiology, immune system biology, neuroanatomy, and gastrointestinal tract (GIT) development and anatomy. Although not identical, simian immunodeficiency virus (SIV) infection in Asian macaques mimics key pathological features of the natural progression of HIV in humans, such as CD4+ T-cell depletion, chronic systemic inflammation, lymphoid and GIT tissue pathology, neuropathology, establishment of the latent reservoir, and progression to AIDS. Accelerated CNS viral strains, such as SIV/17E-Fr + SIV/ΔB670, are valuable in assessing the direct effect of SIV infection in the CNS, due to its rapid pathogenesis which results in SIV encephalitis (SIVE) and full immunosuppressive disease within 85 days post infection (Zink et al., 1999). Non-accelerated neuropathogenic virus, including SIVmac251 and SIVsm804E-CL757, result in a more protracted progression to disease, similar to HIV infection in humans, with a 25–50% chance of encephalitis after long term infection (Lee et al., 2020). Simian-Human Immunodeficiency Virus (SHIV) is a chimeric/recombinant virus that infects NHPs to produce HIV proteins such a HIV-1 env and is used to assess HIV infection in a NHP setting. SHIV-1157ipd3N4 CNS infection has been characterized in rhesus macaques and found disease progression mimics HIV infection in humans (Hsu et al., 2018). SIV infection of rhesus macaques is thus known as a highly representative and well characterised model for HIV neuropathogenesis studies.
In a study comparing the effects of non-pathogenic SIVagm.Sab92018 infection of African green monkeys (natural reservoir hosts) versus pathogenic SIVagm.Sab92018 infection of pigtail macaques on global gene expression, the pigtail macaques with pathogenic SIV infection demonstrated higher levels of oxidative stress and DNA damage related gene induction, particularly in SOD2 gene expression (Lederer et al., 2009). Knight et al. found that colony stimulating factor 1 was overexpressed in microglia from SIV17E-Fr/ΔB670 infected rhesus macaques, when compared with uninfected macaques (Knight et al., 2018). This overexpression occurred regardless of ART treatment and was correlated with the upregulation of SOD2 and GPx1. The upregulation of SOD2 has also been observed in the macaque dorsal root ganglia during acute SIV17E-Fr/ΔB670 infection (Mangus et al., 2019). Increased activity of monoamine oxidase (MAO) has been demonstrated in SIV17E-Fr/ΔB670 infected macaques in late stage disease; MAO oxidises monoamines, producing H2O2 as a by-product (Meulendyke et al., 2012). Increased activity of MAO in rodents decreases dopamine levels, increases H2O2 levels, GSH oxidation, astrocytosis and neuronal damage (Mallajosyula et al., 2008). In a SIV17E-Fr/ΔB670 infected macaque model with or without morphine independence, Perez-Casanova et al. observed that SIV infection alone significantly increased plasma malondialdehyde (MDA) and 8–isoprostane (lipid peroxidation markers), and significantly depleted plasma GSH, catalase and GPx1 activity. These effects were further amplified through morphine dependence (Pérez-Casanova et al., 2008). In high viral load SIV17E-Fr/ΔB670 models of HIV CNS infection, the antibiotic minocycline reduces the severity of encephalitis and the expression of neuroinflammatory markers, lowers CNS viral replication, downregulates glial activation and increases neuronal counts (Ratai et al., 2010). While this study focussed on the anti-inflammatory effects of minocycline, it is important to note that minocycline also has antioxidant and ROS scavenging properties (Kraus et al., 2005), which may contribute to the neuroprotective effects it exhibits. Interestingly, Pendyala and colleagues found that while α-tocopherol (a derivative of Vitamin E) is decreased in the plasma following SIVmac251 infection, afamin (a member of the albumin protein superfamily) is reduced in the plasma of SIV infected macaques with CNS pathology, but remains unchanged in SIV infected macaques without CNS pathology (Pendyala et al., 2010). This is problematic as afamin is important for the transport of α-tocopherol across the BBB. However, when afamin is loaded with α-tocopherol in order to add BBB transport, it continues to have neuroprotective antioxidant properties in primary cell cultures that have been treated with H2O2 (Numakawa et al., 2006). Further work is necessary to determine how and why infection with SIV (and HIV) impacts the maintenance of redox homeostasis, and how oxidative stress can become a therapeutic target through the use of both novel and established drugs (such as minocycline).
8. ROS as a biomarker of HAND
To date, no effective biomarker of HAND exists, however due to the key role of oxidative stress in disease pathogenesis, ROS may offer prognostic or diagnostic potential as therapeutic biomarkers. A targeted gas chromatography/mass spectroscopy (GC/MS)-based analysis of sera from ART-treated and untreated PLWH noted significant upregulation of aspartic acid, phenylalanine and glutamic acid, an alteration of the metabolic profile which is associated with oxidative stress (Sitole et al., 2019) (Table 1). Markers of oxidative stress in viremic PLWH have been measured in plasma samples and PBMCs, which express elevated concentrations of glutamate, and decreased intracellular GSH concentrations. However, like plasma/CSF viral load and nadir CD4 T cell count, GSH levels are not predictive of HAND in ART treated patients. One study, albeit during the early ART years, suggests that the concentration of GSH in the CSF is decreased in individuals living with HAND (Castagna et al., 1995), and is associated with impaired survival (Herzenberg et al., 1997) (Table 1). Analysis of CSF in individuals with HAND determined decreased functional activity of the antioxidant enzyme SOD2 in those with cognitive impairment, compared to those who were asymptomatic (Velázquez et al., 2009) (Table 1). More recently, increased vitamin E concentrations (a marker of antioxidant defense) in the CSF and medial frontal cortex were found to predict the onset or progression of HIV associated dementia, and that the accumulation of 4-HNE (a marker of lipid peroxidation) in the CSF was associated with active dementia (Bandaru et al., 2007; Sacktor et al., 2004) (Table 1). Increased extracellular vesicles (EVs) have been observed in the CSF of ART-treated PLWH, and this correlates with the neuronal injury biomarker neurofilament light protein (NFL) in CSF (Guha et al., 2019a). PLWH with HAND (including ANI, MND and HAD) had higher CSF EV concentrations than those without HAND, and proteomic analysis of these EVs indicate an upregulation in oxidative stress response proteins including GPx1, SOD1, 2 and 3, and GSH reductase (GSR) (Guha et al., 2019b) (Table 1). Increased protein carbonyl levels in the CSF of individuals with HAND have also been observed (Turchan et al., 2003) (Table 1). Protein carbonyls or 4-HNE could prove effective biomarkers for the diagnosis and prognosis of HAND; however, to avoid contamination with artificially formed carbonylations, the CSF used to analyse these markers must be fresh, as the carbonyl group is unstable, even when stored at low temperatures. CSF is also not an easily accessible biological product, as lumbar punctures can hardly be considered a routine clinical investigation technique for many patients. Thus, indicators of oxidative stress may offer limited prognostic value in chronic HIV-infection.
9. Oxidative stress: a novel strategy for a HIV cure?
HIV cure has been shown to be possible after the success of the Berlin and London patients. The search for oxidative and antioxidant mechanisms to manipulate, as part of a cure, has resulted in the current use of N-acetylcysteine (NAC) as a useful adjunct therapy alongside ART. NAC replenishes the depleted whole blood and T cell GSH levels in individuals with HIV, and has been found to prevent HIV protein gp120-induced, oxidative stress related damage in Lipari human cultured astrocytes (Visalli et al., 2007). In primary human monocyte/macrophages, both NAC and GSH blocked or substantially reduced infection with HIV as determined by decreased reverse transcriptase activity and secreted p24 protein (Ho and Douglas, 1992). The redox potential of GSH has also been explored; Bhaskar et al. has demonstrated that an increase of ~25 mV in GSH redox potential was able to promote HIV activation from latently infected U937 monocytes (Bhaskar et al., 2015).
As mentioned previously, ROS can activate NF-κB, which in turn can reactivate latent HIV. A small molecule, 5-hydroxynaphthalene-1,4-dione (5HN) derived from leaves and bark of the black walnut tree, was shown to activate latent HIV from anti-apoptotic molecule Bcl-2-induced CD4+ T cells (Yang et al., 2009). When reduced to a semiquinone radical in T cells, 5HN generates superoxide and H2O2 to activate NF-κB in a dose-dependent manner, and thus is able to reactivate HIV, notably without causing widespread T cell activation (which would indicate that the molecule is too toxic for clinical use) (Yang et al., 2009). While the ability for ROS to mediate 5HN’s activation of NF-κB is promising, differential cellular responses to ROS give 5HN a narrow therapeutic window. 5HN has also been found to affect various cellular proteins, indicating that despite its ability to activate HIV without widespread T cell activation, it may still be too toxic for therapeutic use (Yang et al., 2009).
Oxidative stress and antioxidant mechanisms appear to play an important role in HIV latency and reactivation, particularly given the link between ROS, NF-κB, and the HIV LTR. Further research into molecules such as 5HN that can exploit this association may prove helpful in discovering new ways to reactivate HIV without the induction of global T cell activation.
10. Conclusion
HAND is the major cause of morbidity in PLWH, however, the mechanisms driving disease are unclear. Oxidative stress appears to contribute to HIV disease pathogenesis, regardless of ART, therefore, implying a key role in chronic disease pathogenesis, both in the periphery, where antioxidant enzymes and molecules are depleted, as well as in HAND. However, the relative sources, and contribution of oxidative stress to disease pathology remain ill-defined. Therefore, further research is required, using well controlled, well powered cohorts of both human participants with updated nosology, and non-human primate models, to investigate the use of ART and the presence of comorbidities or opportunistic infection may impact the production of ROS and antioxidant enzymes or molecules, regardless of disease state. Thus, understanding the presence, sources and contribution of ROS to HAND will guide the utilisation of oxidative stress markers to act as biomarkers for HAND and possibly even therapeutic mechanisms to drive reactivation of latent HIV and inform HIV cure strategies.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
This manuscript was supported by funding from the Australian National Health and Medical Research Council (NH&MRC) to M.J.C, J.D.E and T.A.A (#1157988) and RMIT University collaborative grants to M.J.C and S.S. S.B. was supported by an RMIT University Research Stipend Scholarship and T.A.A was supported by an RMIT University Vice Chancellor’s Postdoctoral Fellowship.
Authors’ contributions
S.B and T.A.A wrote the manuscript with intellectual contributions and review from C.C, M.R, J.D.E, S.S. and M.J.C.
Declaration of competing interests
The authors declare that they have no competing interests.
Acknowledgements
Figures were created using BioRender.com.
References
- Akay C., Cooper M., Odeleye A., Jensen B.K., White M.G., Vassoler F. Antiretroviral drugs induce oxidative stress and neuronal damage in the central nervous system. J. Neurovirol. 2014;20(1):39–53. doi: 10.1007/s13365-013-0227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A., Arendt G., Becker J.T., Brew B.J., Byrd D.A., Cherner M. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunagiri C., Macreadie I., Hewish D., Azad A. A C-terminal domain of HIV-1 accessory protein Vpr is involved in penetration, mitochondrial dysfunction and apoptosis of human CD4+ lymphocytes. Apoptosis. 1997;2(1):69–76. doi: 10.1023/a:1026487609215. [DOI] [PubMed] [Google Scholar]
- Aukrust P., Svardal A.M., Muller F., Lunden B., Berge R.K., Ueland P.M. Increased levels of oxidized glutathione in CD4+ lymphocytes associated with disturbed intracellular redox balance in human immunodeficiency virus type 1 infection. Blood. 1995;86(1):258–267. [PubMed] [Google Scholar]
- Ayala A., Munoz M.F., Arguelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med. Cell Longev. 2014;2014:31. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandaru V.V.R., McArthur J.C., Sacktor N., Cutler R.G., Knapp E.L., Mattson M.P. Associative and predictive biomarkers of dementia in HIV-1-infected patients. Neurology. 2007;68(18):1481–1487. doi: 10.1212/01.wnl.0000260610.79853.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barat C., Proust A., Deshiere A., Leboeuf M., Drouin J., Tremblay M.J. Astrocytes sustain long-term productive HIV-1 infection without establishment of reactivable viral latency. Glia. 2018;66(7):1363–1381. doi: 10.1002/glia.23310. [DOI] [PubMed] [Google Scholar]
- Bhaskar A., Munshi M., Khan S.Z., Fatima S., Arya R., Jameel S. Measuring glutathione redox potential of HIV-1-infected macrophages. J. Biol. Chem. 2015;290(2):1020–1038. doi: 10.1074/jbc.M114.588913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birben E., Sahiner U.M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5(1):9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanov M., Brown R.H., Matson W., Smart R., Hayden D., O’Donnell H. Increased oxidative damage to DNA in ALS patients. Free Radic. Biol. Med. 2000;29(7):652–658. doi: 10.1016/s0891-5849(00)00349-x. [DOI] [PubMed] [Google Scholar]
- Borgmann K., Ghorpade A. Methamphetamine augments concurrent astrocyte mitochondrial stress, oxidative burden, and antioxidant capacity: tipping the balance in HIV-associated neurodegeneration. Neurotox. Res. 2018;33(2):433–447. doi: 10.1007/s12640-017-9812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke S.M., McLaughlin J.R., Cortopassi K.M., Sapolsky R.M. Effect of GP120 on glutathione peroxidase activity in cortical cultures and the interaction with steroid hormones. J. Neurochem. 2002;81(2):277–284. doi: 10.1046/j.1471-4159.2002.00825.x. [DOI] [PubMed] [Google Scholar]
- Capone C., Cervelli M., Angelucci E., Colasanti M., Macone A., Mariottini P. A role for spermine oxidase as a mediator of reactive oxygen species production in HIV-Tat-induced neuronal toxicity. Free Radic. Biol. Med. 2013;63:99–107. doi: 10.1016/j.freeradbiomed.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Castagna A., Le Grazie C., Accordini A., Giulidori P., Cavalli G., Bottiglieri T. Cerebrospinal fluid S-adenosylmethionine (SAMe) and glutathione concentrations in HIV infection: effect of parenteral treatment with SAMe. Neurology. 1995;45(9):1678–1683. doi: 10.1212/wnl.45.9.1678. [DOI] [PubMed] [Google Scholar]
- Churchill M.J., Gorry P.R., Cowley D., Lal L., Sonza S., Purcell D.F.J. Use of laser capture microdissection to detect integrated HIV-1 DNA in macrophages and astrocytes from autopsy brain tissues. J. Neurovirol. 2006;12(2):146–152. doi: 10.1080/13550280600748946. [DOI] [PubMed] [Google Scholar]
- Cosenza M.A., Zhao M.L., Si Q., Lee S.C. Human brain parenchymal microglia express CD14 and CD45 and are productively infected by HIV-1 in HIV-1 encephalitis. Brain Pathol. 2002;12(4):442–455. doi: 10.1111/j.1750-3639.2002.tb00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Asuncion J.G., del Olmo M.L., Sastre J., Millan A., Pellin A., Pallardo F.V. AZT treatment induces molecular and ultrastructural oxidative damage to muscle mitochondria. Prevention by antioxidant vitamins. J. Clin. Invest. 1998;102(1):4–9. doi: 10.1172/JCI1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Menezes C.C., Dorneles A.G., Sperotto R.L., Duarte M.M.F., Schetinger M.R.C., Loro V.L. Oxidative stress in cerebrospinal fluid of patients with aseptic and bacterial meningitis. Neurochem. Res. 2009;34(7):1255–1260. doi: 10.1007/s11064-008-9903-6. [DOI] [PubMed] [Google Scholar]
- Deshmane S.L., Mukerjee R., Fan S., Del Valle L., Michiels C., Sweet T. Activation of the oxidative stress pathway by HIV-1 Vpr leads to induction of hypoxia-inducible factor 1alpha expression. J. Biol. Chem. 2009;284(17):11364–11373. doi: 10.1074/jbc.M809266200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico E., Factor-Litvak P., Santella R.M., Mitsumoto H. Clinical perspective of oxidative stress in sporadic ALS. Free Radic. Biol. Med. 2013;65 doi: 10.1016/j.freeradbiomed.2013.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbim C., Pillet S., Prevost M.H., Preira A., Girard P.M., Rogine N. Redox and activation status of monocytes from human immunodeficiency virus-infected patients: relationship with viral load. J. Virol. 1999;73(6):4561–4566. doi: 10.1128/jvi.73.6.4561-4566.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finzi D., Blankson J., Siliciano J.D., Margolick J.B., Chadwick K., Pierson T. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat. Med. 1999;5(5):512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- Fischer M.T., Wimmer I., Höftberger R., Gerlach S., Haider L., Zrzavy T. Disease-specific molecular events in cortical multiple sclerosis lesions. Brain. 2013;136(6):1799–1815. doi: 10.1093/brain/awt110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Smith T., Croul S., Sverstiuk A.E., Capini C., L’Heureux D., Regulier E.G. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J. Neurovirol. 2001;7(6):528–541. doi: 10.1080/135502801753248114. [DOI] [PubMed] [Google Scholar]
- Flores S.C., Marecki J.C., Harper K.P., Bose S.K., Nelson S.K., McCord J.M. Tat protein of human immunodeficiency virus type 1 represses expression of manganese superoxide dismutase in HeLa cells. Proc. Natl. Acad. Sci. Unit. States Am. 1993;90(16):7632–7636. doi: 10.1073/pnas.90.16.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Sánchez A., Miranda-Díaz A.G., Cardona-Muñoz E.G. The role of oxidative stress in physiopathology and pharmacological treatment with pro- and antioxidant properties in chronic diseases. Oxid Med. Cell Longev. 2020;2020:2082145. doi: 10.1155/2020/2082145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg S.D., Alldred M.J., Gunnam S.M., Schiroli C., Lee S.H., Morgello S. Expression profiling suggests microglial impairment in human immunodeficiency virus neuropathogenesis. Ann. Neurol. 2018;83(2):406–417. doi: 10.1002/ana.25160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godai K., Takahashi K., Kashiwagi Y., Liu C.H., Yi H., Liu S. Ryanodine receptor to mitochondrial reactive oxygen species pathway plays an important role in chronic human immunodeficiency virus gp120MN-induced neuropathic pain in rats. Anesth. Analg. 2019;129(1):276–286. doi: 10.1213/ANE.0000000000003916. [DOI] [PubMed] [Google Scholar]
- Grant I., Franklin D.R., Jr., Deutsch R., Woods S.P., Vaida F., Ellis R.J. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology. 2014;82(23):2055–2062. doi: 10.1212/WNL.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha D., Mukerji S.S., Chettimada S., Misra V., Lorenz D.R., Morgello S. Cerebrospinal fluid extracellular vesicles and neurofilament light protein as biomarkers of central nervous system injury in HIV-infected patients on antiretroviral therapy. AIDS. 2019;33(4):615–625. doi: 10.1097/QAD.0000000000002121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha D., Lorenz D.R., Misra V., Chettimada S., Morgello S., Gabuzda D. Proteomic analysis of cerebrospinal fluid extracellular vesicles reveals synaptic injury, inflammation, and stress response markers in HIV patients with cognitive impairment. J. Neuroinflammation. 2019;16(1):254. doi: 10.1186/s12974-019-1617-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C., Sun L., Chen X., Zhang D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res. 2013;8(21):2003–2014. doi: 10.3969/j.issn.1673-5374.2013.21.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey N.J., Cutler R.G., Tamara A., McArthur J.C., Vargas D.L., Pardo C.A. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann. Neurol. 2004;55(2):257–267. doi: 10.1002/ana.10828. [DOI] [PubMed] [Google Scholar]
- Heaton R.K., Clifford D.B., Franklin D.R., Woods S.P., Ake C., Vaida F. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzenberg L.A., De Rosa S.C., Dubs J.G., Roederer M., Anderson M.T., Ela S.W. Glutathione deficiency is associated with impaired survival in HIV disease. Proc. Natl. Acad. Sci. Unit. States Am. 1997;94(5):1967–1972. doi: 10.1073/pnas.94.5.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho W.Z., Douglas S.D. Glutathione and N-acetylcysteine suppression of human immunodeficiency virus replication in human monocyte/macrophages in vitro. AIDS Res. Hum. Retrovir. 1992;8(7):1249–1253. doi: 10.1089/aid.1992.8.1249. [DOI] [PubMed] [Google Scholar]
- Hsu D.C., Sunyakumthorn P., Wegner M., Schuetz A., Silsorn D., Estes J.D. Central nervous system inflammation and infection during early, nonaccelerated simian-human immunodeficiency virus infection in rhesus macaques. J. Virol. 2018;92(11) doi: 10.1128/JVI.00222-18. e00222-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W.J., Zhang X.I.A., Chen W.W. Role of oxidative stress in Alzheimer’s disease. Biomed Rep. 2016;4(5):519–522. doi: 10.3892/br.2016.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulgan T., Morrow J., D’Aquila R.T., Raffanti S., Morgan M., Rebeiro P. Oxidant stress is increased during treatment of human immunodeficiency virus infection. Clin. Infect. Dis. 2003;37(12):1711–1717. doi: 10.1086/379776. [DOI] [PubMed] [Google Scholar]
- Isaguliants M., Smirnova O., Ivanov A.V., Kilpelainen A., Kuzmenko Y., Petkov S. Oxidative stress induced by HIV-1 reverse transcriptase modulates the enzyme’s performance in gene immunization. Hum. Vaccines Immunother. 2013;9(10):2111–2119. doi: 10.4161/hv.25813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen B.K., Monnerie H., Mannell M.V., Gannon P.J., Espinoza C.A., Erickson M.A. Altered oligodendrocyte maturation and myelin maintenance: the role of antiretrovirals in HIV-associated neurocognitive disorders. J. Neuropathol. Exp. Neurol. 2015;74(11):1093–1118. doi: 10.1097/NEN.0000000000000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallianpur K.J., Gerschenson M., Mitchell B.I., LiButti D.E., Umaki T.M., Ndhlovu L.C. Oxidative mitochondrial DNA damage in peripheral blood mononuclear cells is associated with reduced volumes of hippocampus and subcortical gray matter in chronically HIV-infected patients. Mitochondrion. 2016;28:8–15. doi: 10.1016/j.mito.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima H., Watanabe Y., Ichiyama T., Mizuguchi M., Yamada N., Kashiwagi Y. High concentration of serum nitrite/nitrate obtained from patients with influenza-associated encephalopathy. Pediatr. Int. 2002;44(6):705–707. doi: 10.1046/j.1442-200x.2002.01650.x. [DOI] [PubMed] [Google Scholar]
- Kazazi F., Koehler J.K., Klebanoff S.J. Activation of the HIV long terminal repeat and viral production by H2O2-vanadate. Free Radic. Biol. Med. 1996;20(6):813–820. doi: 10.1016/0891-5849(95)02214-7. [DOI] [PubMed] [Google Scholar]
- Kim B.O., Liu Y., Ruan Y., Xu Z.C., Schantz L., He J.J. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am. J. Pathol. 2003;162(5):1693–1707. doi: 10.1016/S0002-9440(10)64304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight A.C., Brill S.A., Mankowski J.L., Queen S.E., Tarwater P.M. Increased microglial CSF1R expression in the SIV/macaque model of HIV CNS disease. J. Neuropathol. Exp. Neurol. 2018;77(3):199–206. doi: 10.1093/jnen/nlx115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus R.L., Pasieczny R., Lariosa-Willingham K., Turner M.S., Jiang A., Trauger J.W. Antioxidant properties of minocycline: neuroprotection in an oxidative stress assay and direct radical-scavenging activity. J. Neurochem. 2005;94(3):819–827. doi: 10.1111/j.1471-4159.2005.03219.x. [DOI] [PubMed] [Google Scholar]
- Lederer S., Favre D., Walters K.-A., Proll S., Kanwar B., Kasakow Z. Transcriptional profiling in pathogenic and non-pathogenic SIV infections reveals significant distinctions in kinetics and tissue compartmentalization. PLoS Pathog. 2009;5(2) doi: 10.1371/journal.ppat.1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.A., Beasley E., Sundar K., Smelkinson M., Vinton C., Deleage C. Simian immunodeficiency virus-infected memory CD4+ T cells infiltrate to the site of infected macrophages in the neuroparenchyma of a chronic macaque model of neurological complications of AIDS. mBio. 2020;11(2):e00602–e00620. doi: 10.1128/mBio.00602-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguori I., Russo G., Curcio F., Bulli G., Aran L., Della-Morte D. Oxidative stress, aging, and diseases. Clin. Interv. Aging. 2018;13:757–772. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louboutin J.-P., Strayer D. Role of oxidative stress in HIV-1-Associated neurocognitive disorder and protection by gene delivery of antioxidant enzymes. Antioxidants. 2014;3(4):770–797. doi: 10.3390/antiox3040770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallajosyula J.K., Kaur D., Chinta S.J., Rajagopalan S., Rane A., Nicholls D.G. MAO-B elevation in mouse brain astrocytes results in Parkinson’s pathology. PloS One. 2008;3(2) doi: 10.1371/journal.pone.0001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandas A., Iorio E.L., Congiu M.G., Balestrieri C., Mereu A., Cau D. Oxidative imbalance in HIV-1 infected patients treated with antiretroviral therapy. J. Biomed. Biotechnol. 2009;2009:749575. doi: 10.1155/2009/749575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangino G., Famiglietti M., Capone C., Veroni C., Percario Z.A., Leone S. HIV-1 myristoylated nef treatment of murine microglial cells activates inducible nitric oxide synthase, NO2 production and neurotoxic activity. PloS One. 2015;10(6) doi: 10.1371/journal.pone.0130189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangus L.M., Weinberg R.L., Knight A.C., Queen S.E., Adams R.J., Mankowski J.L. SIV-induced immune activation and metabolic alterations in the dorsal root ganglia during acute infection. J. Neuropathol. Exp. Neurol. 2019;78(1):78–87. doi: 10.1093/jnen/nly111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masiá M., Padilla S., Fernández M., Rodríguez C., Moreno A., Oteo J.A. Oxidative stress predicts all-cause mortality in HIV-infected patients. PloS One. 2016;11(4) doi: 10.1371/journal.pone.0153456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meucci O., Fatatis A., Simen A.A., Bushell T.J., Gray P.W., Miller R.J. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc. Natl. Acad. Sci. U. S. A. 1998;95(24):14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulendyke K.A., Pletnikov M.V., Engle E.L., Tarwater P.M., Graham D.R., Zink M.C. Early minocycline treatment prevents a decrease in striatal dopamine in an SIV model of HIV-associated neurological disease. J. Neuroimmune Pharmacol. 2012;7(2):454–464. doi: 10.1007/s11481-011-9332-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milatovic D., Zhang Y., Olson S.J., Montine K.S., Roberts L.J., 2nd, Morrow J.D. Herpes simplex virus type 1 encephalitis is associated with elevated levels of F2-isoprostanes and F4-neuroprostanes. J. Neurovirol. 2002;8(4):295–305. doi: 10.1080/13550280290100743. [DOI] [PubMed] [Google Scholar]
- Numakawa Y., Numakawa T., Matsumoto T., Yagasaki Y., Kumamaru E., Kunugi H. Vitamin E protected cultured cortical neurons from oxidative stress-induced cell death through the activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. J. Neurochem. 2006;97(4):1191–1202. doi: 10.1111/j.1471-4159.2006.03827.x. [DOI] [PubMed] [Google Scholar]
- Pandhare J., Dash S., Jones B., Villalta F., Dash C. A novel role of proline oxidase in HIV-1 envelope glycoprotein-induced neuronal autophagy. J. Biol. Chem. 2015;290(42):25439–25451. doi: 10.1074/jbc.M115.652776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendyala G., Trauger S.A., Siuzdak G., Fox H.S. Quantitative plasma proteomic profiling identifies the vitamin E binding protein afamin as a potential pathogenic factor in SIV induced CNS disease. J. Proteome Res. 2010;9(1):352–358. doi: 10.1021/pr900685u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Casanova A., Husain K., Noel R.J., Rivera-Amill V., Kumar A. Interaction of SIV/SHIV infection and morphine on plasma oxidant/antioxidant balance in macaque. Mol. Cell. Biochem. 2008;308(1):169–175. doi: 10.1007/s11010-007-9625-0. [DOI] [PubMed] [Google Scholar]
- Praticò D., Clark C.M., Liun F., Lee V.Y.M., Trojanowski J.Q. Increase of brain oxidative stress in mild cognitive impairment: a possible predictor of alzheimer disease. Arch. Neurol. 2002;59(6):972–976. doi: 10.1001/archneur.59.6.972. [DOI] [PubMed] [Google Scholar]
- Price T.O., Ercal N., Nakaoke R., Banks W.A. HIV-1 viral proteins gp120 and Tat induce oxidative stress in brain endothelial cells. Brain Res. 2005;1045(1-2):57–63. doi: 10.1016/j.brainres.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Ranki A., Nyberg M., Ovod V., Haltia M., Elovaara I., Raininko R. Abundant expression of HIV Nef and Rev proteins in brain astrocytes in vivo is associated with dementia. AIDS. 1995;9(9):1001–1008. doi: 10.1097/00002030-199509000-00004. [DOI] [PubMed] [Google Scholar]
- Ratai E.M., Bombardier J.P., Joo C.G., Annamalai L., Burdo T.H., Campbell J. Proton magnetic resonance spectroscopy reveals neuroprotection by oral minocycline in a nonhuman primate model of accelerated NeuroAIDS. PloS One. 2010;5(5) doi: 10.1371/journal.pone.0010523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawat P., Teodorof-Diedrich C., Spector S.A. Human immunodeficiency virus Type-1 single-stranded RNA activates the NLRP3 inflammasome and impairs autophagic clearance of damaged mitochondria in human microglia. Glia. 2019;67(5):802–824. doi: 10.1002/glia.23568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson K., Liner J., Meeker R.B. Antiretroviral neurotoxicity. J. Neurovirol. 2012;18(5):388–399. doi: 10.1007/s13365-012-0120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Penney A.T., Ludicello J.E., Riggs P.K., Doyle K., Ellis R.J., Letendre S.L. Co-morbidities in persons infected with HIV: increased burden with older age and negative effects on health-related quality of life. AIDS Patient Care STDS. 2013;27(1):5–16. doi: 10.1089/apc.2012.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor N., Haughey N., Cutler R., Tamara A., Turchan J., Pardo C. Novel markers of oxidative stress in actively progressive HIV dementia. J. Neuroimmunol. 2004;157(1-2):176–184. doi: 10.1016/j.jneuroim.2004.08.037. [DOI] [PubMed] [Google Scholar]
- Santana S., Sastre I., Recuero M., Bullido M.J., Aldudo J. Oxidative stress enhances neurodegeneration markers induced by herpes simplex virus type 1 infection in human neuroblastoma cells. PloS One. 2013;8(10) doi: 10.1371/journal.pone.0075842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schousboe A., Waagepetersen H.S. Role of astrocytes in glutamate homeostasis: implications for excitotoxicity. Neurotox. Res. 2005;8(3):221–225. doi: 10.1007/BF03033975. [DOI] [PubMed] [Google Scholar]
- Schreiner B., Romanelli E., Liberski P., Ingold-Heppner B., Sobottka-Brillout B., Hartwig T. Astrocyte depletion impairs redox homeostasis and triggers neuronal loss in the adult CNS. Cell Rep. 2015;12(9):1377–1384. doi: 10.1016/j.celrep.2015.07.051. [DOI] [PubMed] [Google Scholar]
- Shah A., Kumar S., Simon S.D., Singh D.P., Kumar A. HIV gp120- and methamphetamine-mediated oxidative stress induces astrocyte apoptosis via cytochrome P450 2E1. Cell Death Dis. 2013;4 doi: 10.1038/cddis.2013.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E.P., Henry Y.K., Henkel J.S., Smith R.G., Appel S.H. Increased lipid peroxidation in sera of ALS patients: a potential biomarker of disease burden. Neurology. 2004;62(10):1758–1765. doi: 10.1212/wnl.62.10.1758. [DOI] [PubMed] [Google Scholar]
- Sitole L.J., Tugizimana F., Meyer D. Multi-platform metabonomics unravel amino acids as markers of HIV/combination antiretroviral therapy-induced oxidative stress. J. Pharmaceut. Biomed. Anal. 2019;176:112796. doi: 10.1016/j.jpba.2019.112796. [DOI] [PubMed] [Google Scholar]
- Sorg O., Horn T.F., Yu N., Gruol D.L., Bloom F.E. Inhibition of astrocyte glutamate uptake by reactive oxygen species: role of antioxidant enzymes. Mol. Med. 1997;3(7):431–440. [PMC free article] [PubMed] [Google Scholar]
- Stauch K.L., Emanuel K., Lamberty B.G., Morsey B., Fox H.S. Central nervous system-penetrating antiretrovirals impair energetic reserve in striatal nerve terminals. J. Neurovirol. 2017;23(6):795–807. doi: 10.1007/s13365-017-0573-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern A.L., Lee R.N., Panvelker N., Li J., Harowitz J., Jordan-Sciutto K.L. Differential effects of antiretroviral drugs on neurons in vitro: roles for oxidative stress and integrated stress response. J. Neuroimmune Pharmacol. 2018;13(1):64–76. doi: 10.1007/s11481-017-9761-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S.R., Chesselet M.F. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Prog. Neurobiol. 2013:17–32. doi: 10.1016/j.pneurobio.2013.04.004. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana R., Perluigi M., Butterfield D.A. Lipid peroxidation triggers neurodegeneration: a redox proteomics view into the alzheimer disease brain. Free Radic. Biol. Med. 2013;62:157–169. doi: 10.1016/j.freeradbiomed.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teodorof-Diedrich C., Spector S.A. Human immunodeficiency virus type 1 gp120 and Tat induce mitochondrial fragmentation and incomplete mitophagy in human neurons. J. Virol. 2018;92(22) doi: 10.1128/JVI.00993-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangaraj A., Periyasamy P., Liao K., Bendi V.S., Callen S., Pendyala G. HIV-1 TAT-mediated microglial activation: role of mitochondrial dysfunction and defective mitophagy. Autophagy. 2018;14(9):1596–1619. doi: 10.1080/15548627.2018.1476810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson K.A., McArthur J.C., Wesselingh S.L. Correlation between neurological progression and astrocyte apoptosis in HIV-associated dementia. Ann. Neurol. 2001;49(6):745–752. doi: 10.1002/ana.1011. [DOI] [PubMed] [Google Scholar]
- To E.E., Vlahos R., Luong R., Halls M.L., Reading P.C., King P.T. Endosomal NOX2 oxidase exacerbates virus pathogenicity and is a target for antiviral therapy. Nat. Commun. 2017;8(1):69. doi: 10.1038/s41467-017-00057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torti C., Foca E., Cesana B.M., Lescure F.X. Asymptomatic neurocognitive disorders in patients infected by HIV: fact or fiction? BMC Med. 2011;9:138. doi: 10.1186/1741-7015-9-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treitinger A., Spada C., Verdi J.C., Miranda A.F., Oliveira O.V., Silveira M.V. Decreased antioxidant defence in individuals infected by the human immunodeficiency virus. Eur. J. Clin. Invest. 2000;30(5):454–459. doi: 10.1046/j.1365-2362.2000.00642.x. [DOI] [PubMed] [Google Scholar]
- Turchan J., Pocernich C.B., Gairola C., Chauhan A., Schifitto G., Butterfield D.A. Oxidative stress in HIV demented patients and protection ex vivo with novel antioxidants. Neurology. 2003;60(2):307–314. doi: 10.1212/01.wnl.0000042048.85204.3d. [DOI] [PubMed] [Google Scholar]
- Uzasci L., Bianchet M.A., Cotter R.J., Nath A. Identification of nitrated immunoglobulin variable regions in the HIV-infected human brain: implications in HIV infection and immune response. J. Proteome Res. 2014;13(3):1614–1623. doi: 10.1021/pr401117m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velázquez I., Plaud M., Wojna V., Skolasky R., Laspiur J.P., Meléndez L.M. Antioxidant enzyme dysfunction in monocytes and CSF of Hispanic women with HIV-associated cognitive impairment. J. Neuroimmunol. 2009;206(1-2):106–111. doi: 10.1016/j.jneuroim.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilhardt F., Plastre O., Sawada M., Suzuki K., Wiznerowicz M., Kiyokawa E. The HIV-1 Nef protein and phagocyte NADPH oxidase activation. J. Biol. Chem. 2002;277(44):42136–42143. doi: 10.1074/jbc.M200862200. [DOI] [PubMed] [Google Scholar]
- Visalli V., Muscoli C., Sacco I., Sculco F., Palma E., Costa N. N-acetylcysteine prevents HIV gp 120-related damage of human cultured astrocytes: correlation with glutamine synthase dysfunction. BMC Neurosci. 2007;8(1):106. doi: 10.1186/1471-2202-8-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing E.J. HIV and aging. Int. J. Infect. Dis. 2016;53:61–68. doi: 10.1016/j.ijid.2016.10.004. [DOI] [PubMed] [Google Scholar]
- World Health Organisation Global Health Observatory (GHO) data. 2019. http://www.who.int/gho/hiv/en/ Available from.
- Wu R.F., Ma Z., Liu Z., Terada L.S. Nox4-derived H2O2 mediates endoplasmic reticulum signaling through local Ras activation. Mol. Cell Biol. 2010;30(14):3553–3568. doi: 10.1128/MCB.01445-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka G., Kawashima H., Suganami Y., Watanabe C., Watanabe Y., Miyajima T. Diagnostic and predictive value of CSF d-ROM level in influenza virus-associated encephalopathy. J. Neurol. Sci. 2006;243(1):71–75. doi: 10.1016/j.jns.2005.11.029. [DOI] [PubMed] [Google Scholar]
- Yang H.C., Xing S., Shan L., O’Connell K., Dinoso J., Shen A. Small-molecule screening using a human primary cell model of HIV latency identifies compounds that reverse latency without cellular activation. J. Clin. Invest. 2009;119(11):3473–3486. doi: 10.1172/JCI39199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Wang M., Li H., Zhang H., Shi Y., Wei F. Accumulation of nuclear and mitochondrial DNA damage in the frontal cortex cells of patients with HIV-associated neurocognitive disorders. Brain Res. 2012;1458:1–11. doi: 10.1016/j.brainres.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Zhou B.Y., Liu Y., Kim B., Xiao Y., He J.J. Astrocyte activation and dysfunction and neuron death by HIV-1 Tat expression in astrocytes. Mol. Cell. Neurosci. 2004;27(3):296–305. doi: 10.1016/j.mcn.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Zink M.C., Suryanarayana K., Mankowski J.L., Shen A., Piatak M., Spelman J.P. High viral load in the cerebrospinal fluid and brain correlates with severity of simian immunodeficiency virus encephalitis. J. Virol. 1999;73(12):10480–10488. doi: 10.1128/jvi.73.12.10480-10488.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.