Introduction
The ALK gene rearrangement is the second most common driver genomic alteration accounting for approximately 3% to 7% of patients with approved targeted therapies in NSCLC, after EGFR gene mutations. We here describe a rare case of a patient with STRN-ALK–rearranged NSCLC.
Case Report
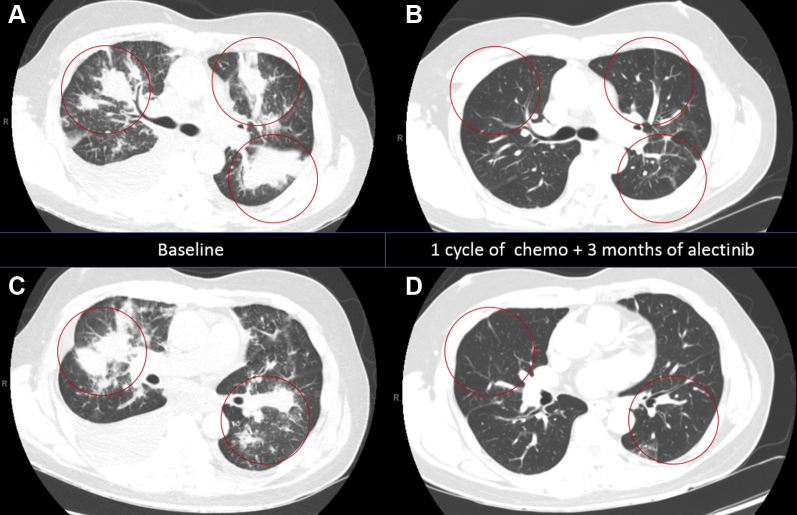
A 66-year-old nonsmoker man presented with exertional dyspnea. Computed tomography revealed a large right-sided pleural effusion (Fig. 1A and C). Cytology results from thoracentesis revealed malignant cells with immunohistochemistry (IHC) consistent with adenocarcinoma. While awaiting molecular testing, chemotherapy was initiated in an attempt to control the effusion. EGFR, ROS1, and BRAF were negative. Programmed death-ligand 1 score was 98% by 22C3. ALK testing from effusion reported “positive atypical rearrangement” on the basis of fluorescence in situ hybridization revealing 52% of nuclei were consistent with deletion of the 5′ region of ALK1, suggestive of ALK1 rearrangement. IHC for ALK was not performed. Next-generation sequencing (NGS) from liquid biopsy identified a STRN-ALK (fusion breakpoint S3, A20) with the percent cell-free DNA or amplification frequency at 1.5% and coalterations of TP53 L43fs at 2.3% and more than two (medium) MYC amplification. The patient had an excellent response 3 months after the therapy was switched to alectinib 600 mg twice daily (Fig. 1B and D) and continues to respond 6 months from the switch without any grade greater than or equal to 2 adverse events.
Figure 1.
(A, C) Initial workup with chest CT revealed a large bilateral pleural effusion and multiple bilateral lung nodules with mediastinal lymphadenopathy. Brain MRI result was negative for metastasis. (B, D) Follow-up CT scans after 1 cycle of carboplatin and pemetrexed followed by 3 months of alectinib revealed resolution of the pleural effusion and decrease in the size of multiple lung nodules and mediastinal lymphadenopathy reaching a near-complete response. Chemo, chemotherapy; CT, computed tomography; MRI, magnetic resonance imaging.
Discussion
Various ALK rearrangements have been previously described, with the EML4-ALK fusion variants being the most common.1 STRN is located in the very same short arm of chromosome 2 as ALK and EML4. Although STRN-ALK fusion has been known to occur in thyroid cancer,2 there are only a few case reports of STRN-ALK fusion reported in the literature as an oncogenic driver for NSCLC with inconsistent reports on the efficacy of ALK inhibitors.1,3,4 Yang et al.3 reported a case of a 59-year-old Chinese man with heavily pretreated metastatic lung adenocarcinoma harboring STRN-ALK fusion who had an excellent clinical, radiographic, and molecular response to crizotinib. Zhou et al.4 reported a unique case of a 43-year-old man originally presenting with metastatic adenocarcinoma with EGFR exon 19 deletion who developed STRN-ALK fusion on progression to osimertinib. The authors reported partial response after the therapy was switched to gefitinib and crizotinib.4 In contrast, Nakanishi et al.1 reported a case of a 51-year-old man with metastatic lung adenocarcinoma harboring STRN-ALK fusion who unfortunately did not respond to alectinib. As marked response was found in our case, STRN-ALK rearrangement seems to act as an oncogenic driver. Furthermore, a recent report of a 13-year-old girl with malignant peritoneal mesothelioma harboring STRN-ALK also revealed a dramatic response to ceritinib,5 supporting the use of second-generation ALK inhibitors for those with STRN-ALK–rearranged tumors.
Conclusions
The novel in-frame STRN-ALK fusion seems to be an oncogenic driver. Nevertheless, this rearrangement seems to be a rare occurrence and the efficacy of different ALK inhibitors would require further data collection and investigation. Although fluorescence in situ hybridization and IHC are sensitive and specific for ALK detection, they cannot always identify the fusion partner. It will be critical to use NGS to detect uncommon gene fusions so that patients may be offered highly effective targeted therapy. NGS through liquid biopsy may have added benefit especially when tissue samples are scarce.
Acknowledgments
The patient involved in this manuscript gave his informed consent authorizing use and disclosure of his medical information.
Footnotes
Disclosure: Dr. Nagasaka reports receiving personal fees from AstraZeneca, Caris Life Sciences, Daiichi Sankyo, Takeda, Novartis, EMD Serono, and Blue Print; has received grants from Tempus and a nonfinancial support from An Heart outside of the submitted work; and has been awarded the 2020 Karmanos Cancer Institute Cancer Immunology and Immunotherapy Pilot Award (P30 CA022453). Dr. Sukari reports receiving grants and personal fees from Eisai and personal fees from Merck outside of the submitted work. Dr. Klosowski is an employee and stockholder of Guardant Health. Dr. Yanagihara reports receiving equity interests from AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb, Celgene, Editas, and Biogen outside of the submitted work. The remaining authors declare no conflict of interest.
References
- 1.Nakanishi Y., Masuda S., Iida Y., Takahashi N., Hashimoto S. Case report of non-small cell lung cancer with STRN-ALK translocation: a nonresponder to alectinib. J Thorac Oncol. 2017;12:e202–e204. doi: 10.1016/j.jtho.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Kelly L.M., Barila G., Liu P. Identification of the transforming STRN-ALK fusion as a potential therapeutic target in the aggressive forms of thyroid cancer. Proc Natl Acad Sci U S A. 2014;111:4233–4238. doi: 10.1073/pnas.1321937111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Y., Qin S., Zhu J. A rare STRN-ALK fusion in lung adenocarcinoma identified using next-generation sequencing-based circulating tumor DNA profiling exhibits excellent response to crizotinib. Mayo Clin Proc Innov Qual Outcomes. 2017;1:111–116. doi: 10.1016/j.mayocpiqo.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou C., Zeng L., Zhang Y., Yang N. Responder of gefitinib plus crizotinib in osimertinib failure EGFR-mutant NSCLC-resistant with newly identified STRN-ALK by next-generation sequencing. J Thorac Oncol. 2019;14:e143–e144. doi: 10.1016/j.jtho.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Rüschoff J.H., Gradhand E., Kahraman A. STRN-ALK rearranged malignant peritoneal mesothelioma with dramatic response following ceritinib treatment. JCO Precis Oncol. 2019;3:1–6. doi: 10.1200/PO.19.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]