Abstract
Background
Major depressive disorder (MDD) is associated with chronic inflammation. Exercise training can treat depression in adults with MDD, potentially through reducing inflammatory activity. This improvement may occur through adaptations to repeated acute inflammatory responses. Cytokine responses to acute steady-state exercise of varying intensities were determined in women with different levels of depression.
Methods
This analysis included 19 women with MDD who each participated in four sessions consisting of 30 min of quiet rest, light, moderate, or hard intensity exercise. Blood samples were collected pre- and within 10 min post-session. Changes in the levels of IL-6, IL-8, IL-10, and TNF were evaluated in each session.
Results
Serum concentrations of IL-6, IL-8 and TNF were all significantly elevated following vigorous exercise (i.e., hard) compared to the quiet rest session. No changes in cytokine levels occurred after light and moderate exercise. Depression severity did not appear to influence the acute inflammatory response to exercise.
Limitations
The sample size was small, all female, and from a secondary data analysis, which limits the generalizability of the findings.
Conclusions
Repeat, acute increases in inflammatory activity following hard exercise sessions may prompt adaptations and lead to reductions in chronic inflammation over time. This dose-response study identified an exercise intensity threshold to induce acute inflammatory responses in women with MDD.
Keywords: Major depressive disorder, Inflammation, Exercise, Cytokines, Dose-response, Interleukin
1. Introduction
Major depressive disorder (MDD) is a leading cause of disability worldwide (World Health Organization). In the United States, MDD affects approximately 8.6% of adults annually (Kessler et al., 2012). Chronic inflammation has been implicated as a contributing factor to MDD (Miller and Raison, 2016). For example, increased levels of inflammatory cytokines occur in those with MDD compared to non-depressed populations (Young et al., 2014). Although acute inflammation during times of sickness and injury is essential to respond to infectious pathogens, chronic inflammation can lead to tissue damage and other pathophysiology (Pearson et al., 2003, Pradhan et al., 2001, Rost et al., 2001). Regular exercise training is anti-inflammatory (Fischer, 2006) and improves symptoms of depression (Schuch et al., 2016). The mechanisms through which chronic exercise training leads to symptom improvements are still not clearly understood, but decreases in chronic inflammation may mediate some of the antidepressant effects (Cotman et al., 2007).
Although regular exercise training is anti-inflammatory (Fischer, 2006), an acute bout of exercise elevates inflammatory cytokine and acute phase reactants during and post-exercise (Allen et al., 2015). Interleukin 6 (IL-6) is produced by many tissues, including contracting muscles during exercise and can downregulate the release of other cytokines, such as tumor necrosis factor (TNF) (Gleeson et al., 2011). The inflammatory response following single exercise sessions in healthy individuals varies based on exercise intensity. A recent review on cytokine levels post-exercise in healthy adults found that more intense exercise generally produces larger increases in cytokine levels, while lower intensities result in no change or only small increases (Cerqueira et al., 2020).
In contrast to the extensive data for healthy individuals, there is not systematic research on cytokine responsiveness after exercise in adults with MDD. Hallberg et al. (2010) reported increases in cytokines measured during and following a maximal exercise test, but did find cytokine increases following exercise completion in 18 adults with MDD. To our knowledge, the cytokine response to steady-state submaximal exercise of varying intensities for adults with MDD is unknown. Similarly, whether cytokine responses to exercise vary based on depression severity has not been examined. Given that there is a larger increase in IL-6 to mental stress in severe depression (Fagundes et al., 2013), inflammatory responses to the physical stress of exercise could also reflect depression severity.
A better understanding of the acute effect of differing exercise intensities can help us understand more about the inflammatory hypothesis of depression. Although increased resting levels of cytokines can be found in those with MDD, it is unknown if inflammatory response to exercise is dysregulated in MDD. Moreover, adaptations to exercise may induce a change in the regulatory set points for cytokine release (i.e., hormesis), contributing to the antidepressant effects of exercise. The primary aim of this study was to examine IL-6, IL-8, IL-10 and TNF responses to acute exercise of varying intensity (light, moderate, hard/vigorous) in women with MDD. Based on prior research in healthy controls, it was hypothesized that more intense exercise would elicit larger increases in cytokine levels. A secondary hypothesis was that depression severity would influence the inflammatory response to exercise.
2. Methods
The current study is a secondary analysis using archived samples from a larger experiment (Meyer et al., 2016). All methods were approved by the local Institutional Review Board. Written consent was obtained from all participants after the full protocol was explained.
2.1. Participants
Inclusion criteria for the larger study were: 1) between 20-60 years of age, 2) female, 3) nonsmoker, 4) current diagnosis for MDD, and 5) on a stable treatment regimen for 8 weeks prior to the first visit. Potential participants who were unable to exercise safely, used opioid or analgesic medications, suffered from a psychological disorder besides MDD or generalized anxiety disorder, were pregnant, or abused alcohol or other drugs, were ineligible. Twenty-four female participants with MDD were recruited initially from the University of Wisconsin-Madison community through a mass email and flyers posted on campus. Samples used in the present analysis come from participants who had banked samples available for all four conditions (n = 19). Participants received $100 for participation.
2.2. Procedures
Participants completed four randomly ordered experimental conditions separated by at least 1 week. Exercise was performed on a cycle ergometer (Lode Corival, Lode BV, Groningen, The Netherlands). The Borg 6–20 Rating of Perceived Exertion (RPE) scale (Borg, 1998) was used to define exercise intensity and to monitor exertion throughout each condition. The experimental conditions consisted of 30 min of either quiet rest (control condition), light-intensity exercise (RPE of 11), moderate-intensity exercise (RPE of 13), or hard- (i.e., vigorous) intensity exercise (RPE of 15). Participants were instructed to maintain pedaling speed at 60–70 revolutions per min, with resistance adjusted by the participant as necessary to achieve and maintain each exercise intensity. The 30 min of exercise included a 5 min warm-up, 20 min of steady-state exercise, and a 5 min cool down. Heart rate (HR) was measured continuously through each condition with a Polar heart rate monitor (Polar, Kempele, Finland), with HR and RPE recorded every 5 min.
2.3. Measures
2.3.1. Depression
To assess eligibility, on the first day of testing participants were screened for current MDD based on the DSM-IV with the Mini International Neuropsychiatric Interview 6.0.0 (MINI) (Sheehan et al., 1998) by a trained staff member, overseen by a clinical psychologist. The MINI has been previously demonstrated to be a valid instrument for diagnosing MDD with high sensitivity (94%) and specificity (79%) (Lecrubier et al., 1997). Depression severity was assessed with the Beck Depression Inventory (BDI-II), which assesses depression symptom severity for the preceding two weeks (Beck et al., 1996).
Current mood states were assessed before exercise and 10 min post-exercise with the Profile of Mood States (POMS) asking participants to rate each item with how they were feeling “right now.” The POMS is a 65-item measure that assesses current mood state with individual items being added together to calculate scores for 6 separate mood states: tension, depression, anger, vigor, fatigue, and confusion, along with a total score for Total Mood Disturbance (TMD). It is commonly used to assess mood states in exercise settings (Berger and Motl, 2000).
2.3.2. Inflammatory markers
Blood was collected pre- and within 10 min post-condition into serum separator vacutainers (Becton Dickinson, Franklin Lakes, NJ), allowed to clot for 15 min, and spun in a refrigerated centrifuge for 10 min at 5500 RPM. Four cytokines (IL-6, IL-8, IL-10, and TNF) were assessed at all conditions. In order to examine current resting levels of inflammation, high-sensitivity C reactive protein (CRP) was assessed for the quiet rest condition only. Serum was aliquoted into polyvials and stored in an ultracold freezer at −-80 °C until analysis. Cytokines were quantified by multi-cytokine array, and CRP was determined via a singleplex assay, using an electrochemiluminescence platform and a QuickPlex SQ 120 imager for quantification of both cytokines and CRP (Meso Scale Discovery, Rockville, MD). Each specimen was assayed in duplicate, with intra-assay coefficients of variation between 1.94 and 4.38%, and values referenced to a standard curve generated from 7 calibrators with known concentrations. The lower limit of detection was 0.1 pg/mL, with a large dynamic range up to 2,000 pg/mL. CRP is present in circulation at higher concentrations, and thus, needed to be assayed separately. After diluting sera so that CRP levels corresponded to the reference curve, values were converted to mg/L units to be consistent with the clinical literature, and calculated down to 0.1 mg/L.
2.3.3. Analyses
A natural log transformation was applied to normalize the distribution of cytokine values. Greenhouse-Geisser corrections were applied if the data distribution violated the assumption of sphericity. Heart rate and BDI-II at baseline were assessed with a repeated measures ANOVA to examine differences across the four conditions. Mood responses to exercise were examined with a 2 × 4 repeated measures ANOVA with the POMS depression subscale as the dependent variable. Inflammatory measures across time and condition were compared with a 2 level (pre, post) x 4 condition (quiet rest, light, moderate, hard) repeated measures ANOVA. In addition, for each cytokine, planned pairwise comparisons evaluated the difference from pre to post for each exercise intensity compared to quiet rest. Only when significant changes for a cytokine were found for a session compared to quiet rest, exploratory post hoc Pearson’s tests were performed to determine if the increments were associated with changes in the POMS depression score. The mean BDI-II score for each particpant across all four sessions was used to examine the effect of depression severity on cytokine responses. Four repeated measures ANCOVAs were performed with the addition of mean BDI-II as a covariate for each cytokine.
3. Results
Nineteen female participants with a mean age of 39 ± 14 years and mean body mass index of 30.2 ± 8 kg/m2 were included in this analysis. Elevated inflammation was found in our sample, indicated by mean resting levels of CRP greater than 3 mg/dL (Table 1). Repeated measures ANOVA confirmed that mean heart rate (p < 0.001; F1.78, 32.1 = 77) and RPE (p < 0.001; F1.53, 27.41 = 4522) were significantly different between conditions (Table 1). Mean BDI-II score on the day of each of the four conditions was 22.5 ± 7.3 with no significant difference in BDI-II scores across test days. POMS depression subscores were significantly different across sessions (p = 0.03; F3,54 = 3.03) and changed from pre-to-post session (p < 0.001; F3,18 = 33.7). However, the ANOVA results did not indicate a significant interaction between time and condition (p = 0.90: F3,54 = 0.194).
Table 1.
Average exercise and mood variables across each session. All values are presented as mean ± SD POMS: Profile of Mood States; BDI-II: Beck Depression Inventory II, CRP: C reactive protein.
| Quiet Rest | Light | Moderate | Hard | |||||
|---|---|---|---|---|---|---|---|---|
| Heart rate (Beats per min) | 82 ± 12 | 111 ± 18 | 126 ± 20 | 148 ± 24 | ||||
| RPE | 6.1 ± 0.08 | 10.9 ± 0.04 | 13.06 ± 0.04 | 15.04 ± 0.04 | ||||
| BDI-II Score | 23.9 ± 8.6 | 21.1 ± 7.4 | 23.8 ± 8.9 | 21.3 ± 10 | ||||
| CRP (mg/dL) | 7.1 ± 6.76 | N/A | N/A | N/A | ||||
| POMS Depression | Pre | Post | Pre | Post | Pre | Post | Pre | Post |
| 18.4 ± 11.9 | 12.6 ± 8.5 | 15.4 ± 18.6 | 8.7 ± 6.9 | 17.1 ± 11.9 | 10.0 ± 8.1 | 13.3 ± 11.1 | 7.5 ± 8.3 | |
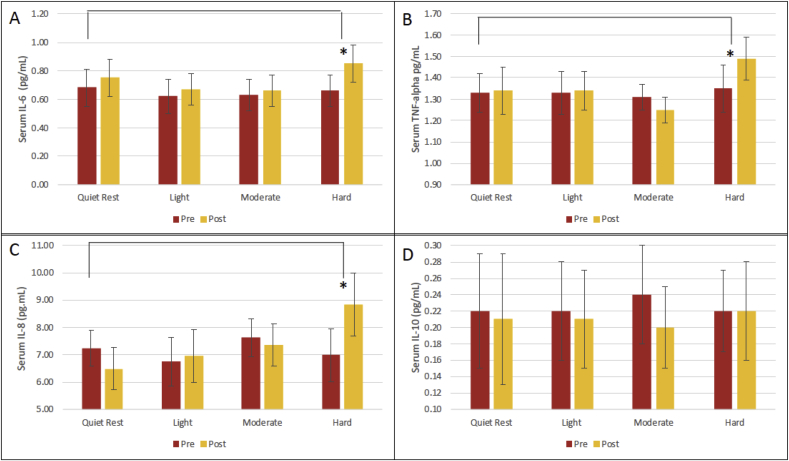
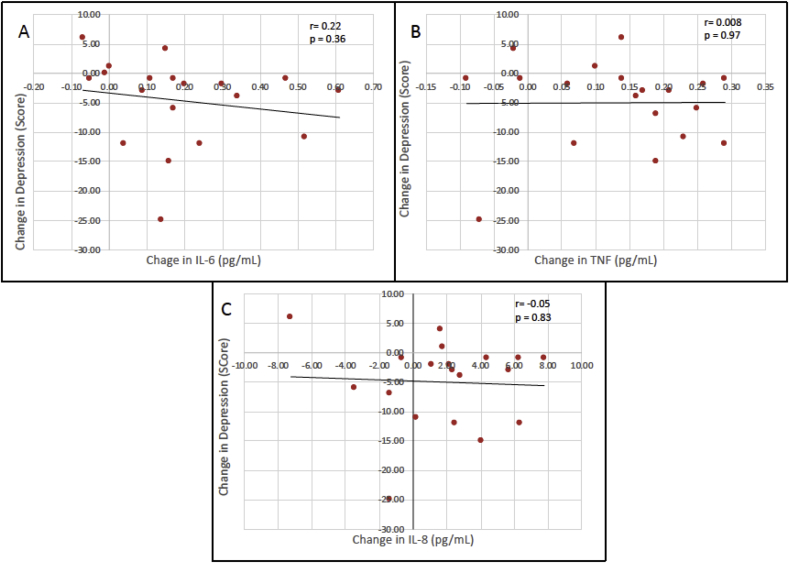
There was a significant interaction between Time and Exercise condition for IL-6 (p = 0.001; F3,54 = 6.38) (Fig. 1A) and TNF levels (p < 0.001; F2.04,36.7 = 7.66) (Fig. 1B). Planned comparisons indicated the increment from pre-to post-session was significantly different between hard exercise and quiet rest for IL-6 (p = 0.025; F1,18 = 5.95) and TNF (p = 0.021; F1,18 = 6.37). While the Time by Condition interaction was not significant for IL-8 (p > 0.05), the difference between pre- and post-session for just hard exercise did differ significantly in a pairwise comparision with quiet rest (p = 0.04; F1,18 = 4.5) (Fig. 1C). For IL-10, there was a significant effect of Time from pre-to-post session (p = 0.002; F1,18 = 12.49) but it was not different across exercise intensities (p > 0.05) (Fig. 1D). No significant associations were found for changes in depressive mood and the magnitude of the cytokine responses after hard exercise for IL-6 (r = -.22; p = 0.36), TNF (r = 0.008; p = 0.97), and IL-8 (r = −0.05; p = 0.83) (Fig. 2). The ANCOVA models did not indicate a significant effect of depression severity for any cytokine response (p > 0.05 for the effect of BDI-II in each model).
Fig. 1.
Mean (±SE) serum cytokine levels pre- and post-exercise for interleukin 6 (IL-6) (A), tumor necrosis factor (TNF) (B), interleukin 8 (IL-8) (C), and interleukin 10 (IL-10) (D). * denotes significantly different compared to quiet rest (p < 0.05).
Fig. 2.
Associations and lines of best fit for changes in POMS score and interleukin 6 (A), tumor necrosis factor (B), and interleukin 8 (C) from pre-to-post session.
4. Discussion
The main finding was that serum levels of IL-6, TNF, and IL-8 increased significantly following 30 min of steady-state hard-intensity exercise compared to quiet rest in women with MDD. Light or moderate-intensity exercise did not induce an increase in cytokine levels. Serum IL-10 differed from the other 3 cytokines and did not increase, even to the hard exercise, and when considered across all exercise conditions, tended to be lower at the end of the session. Because chronic exercise training results in a reduction in inflammatory physiology in healthy individuals (Fischer, 2006) as well as decrease in symptoms of depression (Schuch et al., 2016), it is important to further explore how different intensities of acute exercise alter inflammatory responses. Increases in specific inflammatory proteins at submaximal, yet hard, exercise may contribute to a shift in the regulatory set points for basal levels of cytokines over time, similar to the way that regular exercise training can result in a lowering of resting heart rate and blood pressure, as well as better glucoregulation. It is also known that weight loss in obese individuals will lead to a reduction in both CRP and IL-6 levels (Bastard et al., 2000), which would also be likely to occur with training and regular exercise.
The anti-inflammatory effect of exercise has been studied previously in healthy men by Starkie et al. (2003). Participants underwent three conditions: quiet rest, exercise, and an IL-6 infusion. Tumor necrosis factor and IL-6 were measured at baseline and after endotoxin (i.e., lipopolysaccharide) administration to initiate an inflammatory response (assessed by TNF levels). When comparing the three conditions, increased levels of IL-6 following both infusion and during exercise were found compared to quiet rest. The TNF response to the endotoxin was significantly decreased during both exercise and IL-6 administration when compared to levels during quiet rest. The results indicate that the IL-6 response to acute exercise-induced IL-6 may also serve to feedback and lower the extent of the TNF response to exercise. This demonstrates an anti-inflammatory effect of acute increases in IL-6 due to exercise. However, high resting levels of IL-6 are indicative of chronic inflammation (Gabay, 2006). With regard to its role in MDD, following 12 weeks of exercise training in 116 participants, Lavebratt et al. (2017) found a positive association between changes in basal levels of IL-6 and changes in depression symptom severity; those with the largest decreases in resting IL-6 had the most improvement in depression symptoms. Overall, IL-6-induced changes by exercise may be relevant in the antidepressnt effect of exercise.
Previous research on acute exercise has generally found that increases in TNF are induced by maximal and exhaustive exercise. For example, TNF increased following maximal exercise in healthy men and women (Steinberg et al., 2007), following marathon running in healthy men (Ostrowski et al., 1998), and following incremental maximal exercise in MDD (Hallberg et al., 2010). These increments are distinct from TNF responses following exercise at submaximal intensities such as 30 min of running in healthy men (75% of VO2max; Landers-Ramos et al., 2014) and 1 h of cycling in healthy men (75% of VO2max; Ullum et al., 1994), which found no increases. Our findings did not replicate this conclusion in women with MDD, as we found an increase in TNF during the submaximal condition of hard exercise. Because IL-6 increase during sustained exercise may inhibit the release of TNF (Petersen and Pedersen, 2005; Starkie et al., 2003), it is possible that the timing of the blood collection is critically important for observing a change in TNF levels. It is also possible that the reciprocal relationship between IL-6 and TNF becomes dysregulated in individuals with MDD. The differential cytokine responses to submaximal exercise and intense exercise of varying lengths provides a unique opportunity for investigating these cytokine feedback relationships in both healthy and depressed participants.
The levels of IL-8 also increased after hard exercise as compared to quiet rest. Previous research has found increased IL-8 following maximal exercise (Hallberg et al., 2010) and exhaustive exercise such as marathon-running (Ostrowski et al., 2001). IL-8 can derive from different tissue sources than the other measured cytokines, such as the epithelial cells of the skin, and it is a potent stimulator of neutrophil function. Angiogenesis is also promoted by IL-8 (Li et al., 2003) and it is believed that angiogenesis may be a contributing factor to how exercise training can improve mental health (Deslandes et al., 2009). However, in the context of increased IL-6 and TNF levels, the rise in IL-8, would more likely be a component of an integrated inflammatory response to exercise, albeit of a lower magnitude that would be observed following a bacterial infection or tissue damage.
A recent review examined cytokine changes following acute exercise in healthy men and women (Cerqueira et al., 2020). In general, larger increases were observed for higher intensity and longer duration exercise. The most consistent results were found for IL-6, where majority of the studies reported increases following moderate- and vigorous-intensity exercise. For TNF, increases were found only for exercise of more than 1 h. For IL-10, increases were found only following exercise of vigorous intensity. The conclusions about hard exercise and IL-6 are similar to our results in women with MDD. However, we did not observe an increase in IL-6 after moderate exercise, and we found an increase in TNF, even with only 30-min of more strenuous exercise. This evidence suggests possible differences in the cytokine response to exercise in women with MDD as compared to healthy population.
A secondary finding of the present study was that there wasn’t a differential effect of depression severity on the cytokine response to exercise. This result appears to differ from research showing that the inflammatory responses to cognitive demands are may be influenced by the severity of depression. For example, Fagundes et al. (2013) reported larger IL-6 responses for adults with more symptoms of depression compared to those with fewer symptoms during and following mental arithmetic and public speaking tasks. In addition, Lavebratt et al. (2017)found that participants with the most severe depressive symptoms at baseline had the largest decreases in IL-6 following 12 weeks of exercise training. Future research will have to determine if the more sustained changes over time are directly related to the acute increments that occur to single bouts of exercise when they are repeated during training.
Although significant changes in cytokines were not found following moderate- and light-intensity exercise, there were similar improvements in mood states following all conditions in this study. The improvements in depressive symptoms for this subsample of 19 participants was comparable to the results from the full sample (n = 24) (Meyer et al., 2016). This conclusion is in agreement with previous research on individuals with MDD (Bartholomew et al., 2005). However, the mood improvement was not directly associated with the extent of the cytokine responses, even after the hard exercise condition. It suggests that changes in inflammatory physiology are not the direct mediator of exercise-induced improvement in mood in the same manner as has been described for endogenous opiate hormones. It is more likely that a combination of factors, including psychological processes, such as perceived control and distraction (Simons et al., 1985; Daley, 2002), and increases in neurotransmitters (e.g., serotonin and dopamine) (Stahl, 2000) and endocannabinoids (Meyer et al., 2019) play a more important role.
Notwithstanding the important finding that more intense exercise can increase circulating levels of cytokines, several limitations should be acknowledged. The number of participants was small, which may have limited our ability to discern a significant association with the severity of depressive symptoms. In addition, the magnitude of the cytokine increments was relatively modest, but that may reflect the electrochemiluminescence assay platform used to quantify the cytokines, because it typically yields lower values than other assay systems. Here, it was also important that the experimental design was carefully controlled to always include a baseline level during the pre-condition on every exercise test day, and that all results were compared to a control condition of quiet rest. It should also be acknowledged that the effects were examined only in women. However, this decision was based on the more limited amount of research on inflammatory responses to exercise in women, which is particularly relevant to MDD, given the gender differences in the prevalence of depression (Kessler, 2003).
5. Conclusion
In conclusion, 30 min of hard exercise resulted in increases in serum IL-6, TNF, and IL-8 levels in women with MDD, while light and moderate exercise conditions did not. When considered in the context of other known changes in physiology during sustained exercise training, the acute increases in inflammatory activity may serve to prompt counter-regulatory adaptations over time that may lead to decreases in resting inflammatory activity. That interpretation would be in keeping with the extensive research on how improvements in cardiovascular function and glucoregulation occur over the course of regulatory training. However, we were not able to demonstrate that the changes in cytokine levels were directly related to depressive symptoms, either with respect to severity across individuals or with respect to the improvement in mood from pre-to-post session. Future research on exercise interventions in depressed individuals may be able to interrogate this question more specifically by taking advantage of the finding that the cytokine response to hard-intensity exercise differed from the response to light and moderate exercise.
Funding sources
This study was funded in part, by the Virginia Horne Henry Gift Fund, the University of Wisconsin-Madison Graduate School, the Wisconsin Center for Education Research, and start-up funding at Iowa State University (Meyer).
Declaration of competing interest
Dr. Raison reports the following relationships in the previous six months: consultant for Usona Institute, Sage Pharmaceuticals and Emory Healthcare.
Acknowledgements
The authors would like to thank Lauren Shlapman, Hannah Feinstein, Shawn Tipple, Matthew Patton, Caroline Wickler, and Rachel Prince for their assistance in data collection and processing.
References
- Allen J., Sun Y., Woods J.A. In: Bouchard C., editor. (Vol. 135, Academic Press; 2015. pp. 337–354. [DOI] [Google Scholar]
- Bartholomew J.B., Morrison D.M., Ciccolo J.T. Effects of acute exercise on mood and well-being in patients with major depressive disorder. Med. Sci. Sports Exerc. 2005;37(12):2032–2037. doi: 10.1249/01.mss.0000178101.78322.dd. [DOI] [PubMed] [Google Scholar]
- Bastard J.-P., Jardel C., Bruckert E., Blondy P., Capeau J., Laville M., Vidal H., Hainque B. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J. Clin. Endocrinol. Metabol. 2000;85(9):3338–3342. doi: 10.1210/jcem.85.9.6839. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Ball R., Ranieri W.F. Comparison of Beck depression inventories-IA and-II in psychiatric outpatients. J. Pers. Assess. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Berger B.G., Motl R.W. Exercise and mood: a selective review and synthesis of research employing the profile of mood states. J. Appl. Sport Psychol. 2000;12(1):69–92. doi: 10.1080/10413200008404214. [DOI] [Google Scholar]
- Borg G. Borg’s perceived exertion and pain scales. Hum. Kinet. 1998:104. [Google Scholar]
- Cerqueira É., Marinho D.A., Neiva H.P., Lourenço O. Inflammatory effects of high and moderate intensity exercise—a systematic review. Front. Physiol. 2020;10 doi: 10.3389/fphys.2019.01550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman C.W., Berchtold N.C., Christie L.-A. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Daley A.J. Exercise therapy and mental health in clinical populations: is exercise therapy a worthwhile intervention? Adv. Psychiatr. Treat. 2002;8(4):262–270. doi: 10.1192/apt.8.4.262. [DOI] [Google Scholar]
- Deslandes A., Moraes H., Ferreira C., Veiga H., Silveira H., Mouta R., Pompeu F.A.M.S., Coutinho E.S.F., Laks J. Exercise and mental health: many reasons to move. Neuropsychobiology. 2009;59(4):191–198. doi: 10.1159/000223730. [DOI] [PubMed] [Google Scholar]
- Fagundes C.P., Glaser R., Hwang B.S., Malarkey W.B., Kiecolt-Glaser J.K. Depressive symptoms enhance stress-induced inflammatory responses. Brain Behav. Immun. 2013;31:172–176. doi: 10.1016/j.bbi.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer Interleukin-6 in acute exercise and training: what is the biological relevance. 2006;12:6–33. [PubMed] [Google Scholar]
- Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res. Ther. 2006;8(2):S3. doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson M., Bishop N.C., Stensel D.J., Lindley M.R., Mastana S.S., Nimmo M.A. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011;11(9):607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- Hallberg L., Janelidze S., Engstrom G., Wisén A.G.M., Westrin Å., Brundin L. Exercise-induced release of cytokines in patients with major depressive disorder. J. Affect. Disord. 2010;126(1–2):262–267. doi: 10.1016/j.jad.2010.02.133. [DOI] [PubMed] [Google Scholar]
- Kessler R.C. Epidemiology of women and depression. J. Affect. Disord. 2003;74(1):5–13. doi: 10.1016/S0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Petukhova M., Sampson N.A., Zaslavsky A.M., Wittchen H.-U. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int. J. Methods Psychiatr. Res. 2012;21(3):169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landers-Ramos R.Q., Jenkins N.T., Spangenburg E.E., Hagberg J.M., Prior S.J. Circulating angiogenic and inflammatory cytokine responses to acute aerobic exercise in trained and sedentary young men. Eur. J. Appl. Physiol. 2014;114(7):1377–1384. doi: 10.1007/s00421-014-2861-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavebratt C., Herring M.P., Liu J.J., Wei Y.B., Bossoli D., Hallgren M., Forsell Y. Interleukin-6 and depressive symptom severity in response to physical exercise. Psychiatr. Res. 2017;252:270–276. doi: 10.1016/j.psychres.2017.03.012. [DOI] [PubMed] [Google Scholar]
- Lecrubier Y., Sheehan D., Weiller E., Amorim P., Bonora I., Harnett Sheehan K., Janavs J., Dunbar G. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. Eur. Psychiatr. 1997;12(5):224–231. doi: 10.1016/S0924-9338(97)83296-8. [DOI] [Google Scholar]
- Li A., Dubey S., Varney M.L., Dave B.J., Singh R.K. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J. Immunol. 2003;170(6):3369–3376. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- Meyer J.D., Koltyn K.F., Stegner A.J., Kim J.-S., Cook D.B. Influence of exercise intensity for improving depressed mood in depression: a dose-response study. Behav. Ther. 2016;47(4):527–537. doi: 10.1016/j.beth.2016.04.003. [DOI] [PubMed] [Google Scholar]
- Meyer J.D., Crombie K.M., Cook D.B., Hillard C.J., Koltyn K.F. Serum endocannabinoid and mood changes after exercise in major depressive disorder. Med. Sci. Sports Exerc. 2019;51(9):1909–1917. doi: 10.1249/MSS.0000000000002006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.H., Raison C.L. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016;16(1):22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski K., Rohde T., Zacho M., Asp S., Pedersen B.K. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J. Physiol. 1998;508(3):949–953. doi: 10.1111/j.1469-7793.1998.949bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski Kenneth, Rohde T., Asp S., Schjerling P., Klarlund Pedersen B. Chemokines are elevated in plasma after strenuous exercise in humans. Eur. J. Appl. Physiol. 2001;84(3):244–245. doi: 10.1007/s004210170012. [DOI] [PubMed] [Google Scholar]
- Pearson T.A., Mensah G.A., Alexander R.W., Anderson J.L., CannonCriqui M., Fadl Y.Y., Fortmann S.P., Hong Y., Myers G.L., Rifai N., Smith S.C., Taubert K., Tracy R.P., Vinicor F. Markers of Inflammation and Cardiovascular Disease. Circulation. 2003;107(3):499–511. doi: 10.1161/01.CIR.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Petersen A.M.W., Pedersen B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005;98(4):1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- Pradhan A.D., Manson J.E., Rifai N., Buring J.E., Ridker P.M. C-Reactive Protein, Interleukin 6, and Risk of Developing Type 2 Diabetes Mellitus. JAMA. 2001;286(3):327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- Rost N.S., Wolf P.A., Kase C.S., Kelly-Hayes M., Silbershatz H., Massaro J.M., D’Agostino R.B., Franzblau C., Wilson P.W.F. Plasma Concentration of C-Reactive Protein and Risk of Ischemic Stroke and Transient Ischemic Attack: The Framingham Study. Stroke. 2001;32(11):2575–2579. doi: 10.1161/hs1101.098151. [DOI] [PubMed] [Google Scholar]
- Schuch F.B., Vancampfort D., Richards J., Rosenbaum S., Ward P.B., Stubbs B. Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J. Psychiatr. Res. 2016;77:42–51. doi: 10.1016/j.jpsychires.2016.02.023. [DOI] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., Dunbar G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatr. 1998;59(Suppl. 20):22–33. [PubMed] [Google Scholar]
- Simons A.D., Epstein L.H., McGowan C.R., Kupfer D.J., Robertson R.J. Exercise as a treatment for depression: An update. Clin. Psychol. Rev. 1985;5(6):553–568. doi: 10.1016/0272-7358(85)90034-0. [DOI] [Google Scholar]
- Stahl S.M. Blue genes and the monoamine hypothesis of depression. J. Clin. Psychiatr. 2000;61(2):77–78. doi: 10.4088/JCP.v61n0201. [DOI] [PubMed] [Google Scholar]
- Starkie R., Ostrowski S.R., Jauffred S., Febbraio M., Pedersen B.K. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-α production in humans. Faseb. J. 2003;17(8):884–886. doi: 10.1096/fj.02-0670fje. [DOI] [PubMed] [Google Scholar]
- Steinberg J.G., Ba A., BReGEON F., Delliaux St, Jammes Y. Cytokine and oxidative responses to maximal cycling exercise in sedentary subjects. Med. Sci. Sports Exerc. 2007;39(6):964–968. doi: 10.1097/mss.0b013e3180398f4b. [DOI] [PubMed] [Google Scholar]
- Ullum H., Haahr P.M., Diamant M., Palmo J., Halkjaer-Kristensen J., Pedersen B.K. Bicycle exercise enhances plasma IL-6 but does not change IL-1 alpha, IL-1 beta, IL-6, or TNF-alpha pre-mRNA in BMNC. J. Appl. Physiol. 1994;77(1):93–97. doi: 10.1152/jappl.1994.77.1.93. [DOI] [PubMed] [Google Scholar]
- Young J.J., Bruno D., Pomara N. A review of the relationship between proinflammatory cytokines and major depressive disorder. J. Affect. Disord. 2014;169:15–20. doi: 10.1016/j.jad.2014.07.032. [DOI] [PubMed] [Google Scholar]