Abstract
Introduction
In the phase 3 ASCEND-4 study, ceritinib exhibited improved progression-free survival (PFS) by Blinded Independent Review Committee (BIRC) assessment versus the standard first-line chemotherapy in patients with advanced ALK-rearranged NSCLC. Here, we assessed the efficacy and safety of ceritinib in the subgroup of Asian patients from the ASCEND-4 trial.
Methods
Treatment-naive patients with stage IIIB or IV ALK-rearranged nonsquamous NSCLC were randomized in a one-to-one ratio to receive either oral ceritinib 750 mg/day (fasted) daily or intravenous chemotherapy ([cisplatin 75 mg/m2 or carboplatin area under the curve 5–6 plus pemetrexed 500 mg/m2] every three wk, followed by pemetrexed maintenance). The primary end point was PFS by BIRC assessment.
Results
Of 376 randomized patients, 158 (42.0%) were Asian (ceritinib arm: N = 76; chemotherapy arm: N = 82). The median time from randomization to the cutoff date (June 24, 2016) was 18.3 months (range = 13.5–34.2) in the Asian subgroup. The median PFS (by BIRC assessment) was 26.3 months (95% confidence interval [CI]: 8.6–not estimable) and 10.6 months (95% CI: 6.7–15.0), with an estimated 34% risk reduction in PFS (hazard ratio = 0.66, 95% CI: 0.41–1.05) in the ceritinib arm versus chemotherapy arm. The most common adverse events of any grade were diarrhea (85.5%), increased alanine aminotransferase and vomiting (73.7% each), and increased aspartate aminotransferase and nausea (69.7% each) in the ceritinib arm, and nausea (49.3%), vomiting (42.7%), and anemia (40.0%) in the chemotherapy arm.
Conclusion
Ceritinib was effective and safe in treatment-naive Asian patients with advanced ALK-rearranged NSCLC. The findings were largely consistent with that of the overall study population.
Keywords: ASCEND-4, Ceritinib, ALK, NSCLC
Introduction
ALK gene rearrangements are oncogenic driver mutations in NSCLC and occur in approximately 2% to 7% of patients with NSCLC.1,2 Most patients are young, never-smokers or with a light smoking history, and have adenocarcinoma as the histologic diagnosis.1,3,4 Data from clinical studies have revealed that targeted therapy with ALK inhibitors (ALKis) is an effective and safe treatment option in this patient population.1,5, 6, 7, 8, 9 Ceritinib is a second-generation selective oral ALKi.6,10 Ceritinib 750 mg/day administered in the fasted state received the U.S. Food and Drug Administration (FDA) accelerated approval in 2014 for the treatment of patients with advanced ALK-rearranged NSCLC who had progressed on or were intolerant to crizotinib.10, 11, 12
In the phase 1 ASCEND-1 trial, ceritinib at the recommended dose of 750 mg/day in the fasted state exhibited clinically meaningful antitumor responses and prolonged progression-free survival (PFS) in ALKi-naive patients with advanced ALK-rearranged NSCLC.13 Per investigator assessment, the overall response was 72%, and the median PFS was 18.4 months in this subset of patients.13 In the phase 2 ASCEND-3 trial, treatment with ceritinib resulted in clinically meaningful overall survival (OS), PFS, and duration of response (DOR) in chemotherapy pretreated (at least three lines), ALKi-naive patients with advanced ALK-rearranged NSCLC.14 The median PFS was 16.6 months by investigator assessment and 19.4 months by Blinded Independent Review Committee (BIRC) assessment.14 The investigator-assessed overall response rate (ORR) was 57.1% and 74.7% in patients with and without baseline brain metastases (BM), respectively.14
In the global phase 3 ASCEND-4 study (NCT01828099), ceritinib exhibited a clinically meaningful improvement in PFS versus pemetrexed-platinum chemotherapy in previously untreated patients with advanced ALK-rearranged NSCLC.15 The median PFS by BIRC assessment was 16.6 months (95% confidence interval [CI]: 12.6–27.2) in the ceritinib arm versus 8.1 months (95% CI: 5.8–11.1) in the chemotherapy arm (hazard ratio [HR] = 0.55, 95% CI: 0.42–0.73, p < 0.00001).15 Improvements in PFS were observed in patients with and without BM.15 In addition, a higher overall intracranial response was observed in ceritinib-treated patients, and the overall safety profile was consistent with that reported in previous studies.6,13, 14, 15 On the basis of these data, in May 2017, the FDA granted regular approval for ceritinib to treat patients with advanced ALK-rearranged NSCLC and extended its indication to include previously untreated patients with advanced ALK-rearranged NSCLC.11,12,15
Limited data exist on the efficacy and safety of first-line ceritinib in Asian patients with advanced ALK-rearranged NSCLC. Here, we sought to assess the efficacy and safety of ceritinib versus pemetrexed-platinum chemotherapy in the subgroup of Asian patients from the ASCEND-4 trial.
Materials and Methods
Patient Population
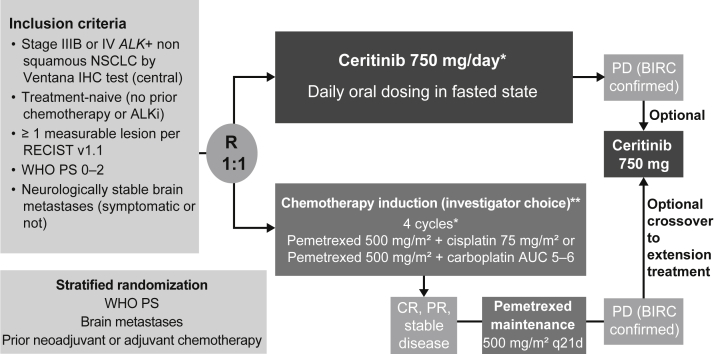
The patient population for the open-label, randomized, global, phase 3 ASCEND-4 study has been published previously (Fig. 1).15 Patients (aged ≥ 18 years) with histologically or cytologically confirmed locally advanced or metastatic ALK-rearranged nonsquamous NSCLC who were treatment-naive (no previous ALKi or no previous chemotherapy) were eligible. Previous adjuvant or neoadjuvant systemic therapy was allowed (except ALKi) if relapse had occurred more than 12 months from the end of therapy. ALK rearrangement was confirmed using the Ventana anti-ALK (D5F3) CDx assay (Ventana immunohistochemistry test, Roche, Basel, Switzerland) at a sponsor-designated central laboratory. Other key inclusion criteria included a WHO performance status (PS) of 0 to 2, at least one measurable lesion per the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1) by investigator assessment, and asymptomatic or neurologically stable BM (for ≥ 2 week).
Figure 1.
The ASCEND-4 study design. ALKi, ALK inhibitor; AUC, area under the curve; BIRC, Blinded Independent Review Committee; CR, complete response; IHC, immunohistochemistry; PD, progressive disease; PR, partial response; PS, performance status; q21d, every 21 days; R, randomized; RECIST v1.1, Response Evaluation Criteria in Solid Tumors version 1.1. Data cutoff was on June 24, 2016. ∗One cycle equal to 21 days. ∗∗At the time when ASCEND-4 was designed and initiated, pemetrexed-platinum chemotherapy followed by pemetrexed maintenance was the standard of care in patients with nonsquamous advanced NSCLC.15, 16, 17
Study Design and Treatment
The ASCEND-4 study was conducted in 134 sites across 28 countries. Eligible patients were randomized in a one-to-one ratio and received either oral ceritinib 750 mg/day in the fasted state or standard intravenous chemotherapy.15 Patients were stratified by WHO PS (0 versus 1–2), BM as per investigator assessment at screening (present versus absent), and previous neoadjuvant or adjuvant chemotherapy (yes versus no).15 Induction chemotherapy comprised cisplatin 75 mg/m2 or carboplatin area under the curve 5 to 6 plus pemetrexed 500 mg/m2 (based on investigator’s choice) every 21 days followed by pemetrexed maintenance in patients who successfully completed four cycles of chemotherapy without progressive disease (PD).15 At the start of the ASCEND-4 trial, platinum-pemetrexed doublet was the standard of care in patients with nonsquamous NSCLC.15, 16, 17 Treatment in both arms continued until patients experienced BIRC-confirmed PD per RECIST v1.1 criteria or developed unacceptable toxicity.15 An optional crossover from the chemotherapy to the ceritinib arm was allowed if patients had BIRC-confirmed RECIST-defined PD.15 A maximum of three dose reductions (150 mg per reduction to the lowest dose of 300 mg/day) was permitted for patients treated with ceritinib. Dose reductions followed package insert or local guidelines for patients in the chemotherapy arm.15 Treatment interruption or delay was allowed for onset of adverse events (AEs), physician decision, patient or guardian decision, or technical problems.
The study protocol and all amendments were reviewed by the independent ethics committee or institutional review board at each center, and written informed consent was obtained from all patients before screening. This study was conducted in accordance with the principles of the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Conference on Harmonization.
Outcomes and Assessments
The primary end point was PFS, defined as the time from randomization to the date of the first radiologically documented disease progression (determined by BIRC according to RECIST v1.1) or death owing to any cause.15 Key secondary end points included PFS by investigator assessment; ORR, disease control rate (DCR), and DOR by investigator and BIRC assessment; overall intracranial response rate (OIRR) and intracranial DCR (IDCR) by BIRC neuroradiologist assessment; and safety.15 Whole-body responses were assessed by BIRC and investigator review in accordance with RECIST v1.1.15 The intracranial response was assessed by an independent central neuroradiologist (from BIRC) using modified RECIST v1.1 to allow a more rigorous evaluation of intracranial response to the treatment.15 A maximum of five target lesions located in the brain could be selected (if the minimum size of the longest diameter was 10 mm) at baseline and evaluated at each subsequent time point.15 The efficacy end points were assessed in all patients who were assigned study treatment by randomization. The safety analyses were done in patients who received at least one dose of the study drug (ceritinib or pemetrexed or carboplatin or cisplatin). All AEs were graded according to the Common Terminology Criteria for Adverse Events version 4.03.15
Statistical Analysis
Data were analyzed using the Statistical Analysis System software version 9.4. The cutoff date for this subgroup analysis was June 24, 2016, consistent with that of the primary analysis. The distribution of DOR and PFS was estimated using the Kaplan-Meier method. Percentiles including median with 95% CIs were calculated by means of PROC LIFETEST using the method of Brookmeyer and Crowley.18 Kaplan-Meier estimates with 95% CI at specific time points were also summarized. A Cox regression model stratified by randomization stratification factors was used to estimate the HR, along with 95% CIs on the basis of the Wald test.15 The ORR, DCR, OIRR, and IDCR were presented along with 95% CI. This Asian subgroup analysis was preplanned to be conducted for the primary PFS end point. Analyses with the other end points were not preplanned and were added as supportive analyses in this report. No statistical tests were performed for this Asian subgroup analysis. The study was not powered to detect differences between the treatment arms in the Asian subgroup.
Results
Demographic and Baseline Characteristics
The median age of patients in the ceritinib arm (N = 76; women, 51.3%) and chemotherapy arm (N = 82; women, 59.8%) was 52.0 years (range = 22–79) and 51.5 years (range = 22–80), respectively. Most patients in the ceritinib and chemotherapy arms were younger than 65 years (62 patients [81.6%] and 70 patients [85.4%], respectively) and had a WHO PS score of 0 to 1. Adenocarcinoma was the primary histologic diagnosis, reported in 75 patients (98.7%) and 79 patients (96.3%) in the ceritinib and chemotherapy arms, respectively. At the time of study entry, all patients in the ceritinib and chemotherapy arms had either stage IV NSCLC (72 patients [94.7%] and 78 patients [95.1%]) or stage IIIB NSCLC (four patients [5.3%] and four patients [4.9%]), respectively. A total of 25 patients (32.9%) in the ceritinib arm and 21 patients (25.6%) in the chemotherapy arm had BM at baseline (Table 1). The patient distribution between the two treatment arms with respect to previous antineoplastic therapies was comparable.
Table 1.
Patient Demographics and Disease Characteristics at Baseline
| Characteristic | Ceritinib 750 mg N = 76 | Chemotherapy N = 82 |
|---|---|---|
| Age, median (range), y | 52.0 (22–79) | 51.5 (22–80) |
| Sex, n (%) | ||
| Female | 39 (51.3) | 49 (59.8) |
| Male | 37 (48.7) | 33 (40.2) |
| Ethnicity, n (%) | ||
| East Asian | 50 (65.8) | 52 (63.4) |
| South Asian | 3 (3.9) | 2 (2.4) |
| Southeast Asian | 21 (27.6) | 22 (26.8) |
| West Asian | 0 | 4 (4.9) |
| Other | 1 (1.3) | 2 (2.4) |
| Unknown | 1 (1.3) | 0 |
| WHO PS, n (%) | ||
| 0 | 24 (31.6) | 20 (24.4) |
| 1 | 48 (63.2) | 57 (69.5) |
| 2 | 4 (5.3) | 5 (6.1) |
| Smoking history, n (%) | ||
| Current smoker | 8 (10.5) | 7 (8.5) |
| Ex-smoker | 22 (28.9) | 18 (22.0) |
| Never-smoker | 46 (60.5) | 57 (69.5) |
| Tumor histologic or cytologic diagnosis, n (%) | ||
| Adenocarcinoma | 75 (98.7) | 79 (96.3) |
| Adenosquamous cell carcinoma | 0 | 2 (2.4) |
| Large cell carcinoma | 0 | 1 (1.2) |
| Other | 1 (1.3) | 0 |
| Metastatic site of cancer, n (%) | ||
| Lung | 71 (93.4) | 72 (87.8) |
| Lymph nodes | 61 (80.3) | 60 (73.2) |
| Bone | 34 (44.7) | 32 (39.0) |
| Pleura | 27 (35.5) | 38 (46.3) |
| Brain | 25 (32.9) | 21 (25.6) |
| Liver | 10 (13.2) | 14 (17.1) |
| Adrenal | 11 (14.5) | 8 (9.8) |
| Soft tissue | 3 (3.9) | 2 (2.4) |
| Kidney | 2 (2.6) | 2 (2.4) |
| Stage at the time of study entry, n (%) | ||
| IIIB | 4 (5.3) | 4 (4.9) |
| IV | 72 (94.7) | 78 (95.1) |
| Previous antineoplastic therapy, n (%) | ||
| Any therapy | 22 (28.9) | 27 (32.9) |
| Surgery | 16 (21.1) | 15 (18.3) |
| Radiotherapy | 13 (17.1) | 15 (18.3) |
| Medication: chemotherapy settinga | ||
| Adjuvant | 4 (5.3) | 4 (4.9) |
| Neoadjuvant | 0 | 0 |
| Prevention | 0 | 0 |
| Palliative | 0 | 0 |
| Therapeutic | 0 | 0 |
| No. of previous regimens of chemotherapy, n (%) | ||
| 0 | 72 (94.7) | 78 (95.1) |
| 1 | 4 (5.3) | 4 (4.9) |
Note: Any previous antineoplastic therapy includes patients who have had medication, radiotherapy, or surgery. Surgery excludes diagnostic biopsies.
PS, performance status.
A patient may have multiple settings.
Patient Disposition
Patient disposition is summarized in Supplementary Table 1. Of the 376 patients randomized in the ASCEND-4 study, 158 (42.0%) were Asian and were included in this subgroup analysis; of these patients, 76 were in the ceritinib arm, and 82 were in the chemotherapy arm. The efficacy end points were assessed in all 158 Asian patients (ceritinib arm, N = 76 and chemotherapy arm, N = 82). All patients randomized to the ceritinib arm (N = 76) received at least one dose of ceritinib. Of the patients randomized to the chemotherapy arm (N = 82), seven did not receive the assigned treatment owing to patient or guardian decision (four patients), physician decision (two patients), and AE (one patient), and therefore, were excluded from the safety analyses. The median duration of follow-up (from randomization to data cutoff date; N = 158) was 18.3 months (range = 13.5–34.2). At the time of data cutoff, 42 patients (55.3%) in the ceritinib arm and 18 patients (22.0%) in the chemotherapy arm were still receiving the assigned study treatment. The proportion of patients who discontinued study treatment was lower in the ceritinib arm versus the chemotherapy arm (34 patients [44.7%] versus 64 patients [78.0%]). The primary reasons for discontinuation were disease progression (18 patients [23.7%] versus 38 patients [46.3%]) and AEs (six patients [7.9%] versus four patients [4.9%]) in the ceritinib and chemotherapy arms, respectively. In the chemotherapy arm, 33 patients (40.2%) crossed over to ceritinib treatment after BIRC-confirmed PD, of whom 15 (18.3%) were ongoing, and 18 (22.0%) discontinued at the cutoff date. Discontinuation was mainly owing to PD (11 patients [13.4%]).
Efficacy
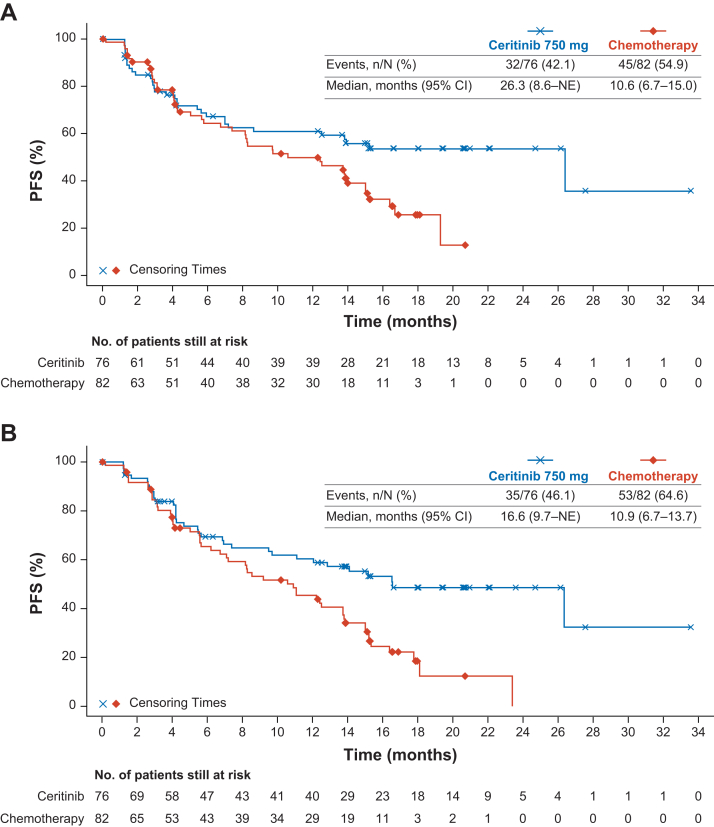
The median duration of follow-up for PFS was 12.4 months (range = 0–33.6) versus 5.7 months (range = 0–20.7) by BIRC assessment and 12.4 months (range = 0–33.6) versus 6.9 months (range = 0–23.4) by investigator assessment for the ceritinib and chemotherapy arms, respectively. The median PFS (by BIRC assessment) was 26.3 months (95% CI: 8.6–not estimable [NE]) and 10.6 months (95% CI: 6.7–15.0) in the ceritinib and chemotherapy arms, respectively (Table 2 and Fig. 2A). There were 32 events (42.1%) in the ceritinib arm and 45 events (54.9%) in the chemotherapy arm. The estimated PFS rate at 15 months was 55.9% (95% CI: 43.2–66.9) for the ceritinib arm and 39.0% (95% CI: 26.9–51.0) for the chemotherapy arm. An estimated 34% risk reduction in PFS (HR = 0.66, 95% CI: 0.41–1.05) was obtained in the ceritinib arm versus the chemotherapy arm. The median PFS (by investigator assessment) was 16.6 months (95% CI: 9.7–NE) in the ceritinib arm and 10.9 months (95% CI: 6.7–13.7) in the chemotherapy arm (Fig. 2B). There were 35 PFS events (46.1%) in the ceritinib arm versus 53 PFS events (64.6%) in the chemotherapy arm. The estimated PFS rates at 15 months were 55.3% (95% CI: 42.6–66.2) for the ceritinib arm and 34.1% (95% CI: 22.9–45.6) in the chemotherapy arm (Table 2). An estimated 45% risk reduction in PFS (HR = 0.55, 95% CI: 0.35–0.87) was obtained in the ceritinib arm versus the chemotherapy arm.
Table 2.
Summary of Whole-Body Efficacy by BIRC and Investigator Assessment
| Parameter | By BIRC Assessment |
By Investigator Assessment |
||
|---|---|---|---|---|
| Ceritinib 750 mg N = 76 | Chemotherapy N = 82 | Ceritinib 750 mg N = 76 | Chemotherapy N = 82 | |
| ORR, % [95% CI] | 65.8 [54.0–76.3] | 29.3 [19.7–40.4] | 68.4 [56.7–78.6] | 28.0 [18.7–39.1] |
| BOR, n (%) | ||||
| CR | 0 | 0 | 0 | 0 |
| PR | 50 (65.8) | 24 (29.3) | 52 (68.4) | 23 (28.0) |
| Stable disease | 11 (14.5) | 38 (46.3) | 17 (22.4) | 43 (52.4) |
| PD | 11 (14.5) | 6 (7.3) | 6 (7.9) | 5 (6.1) |
| Non-CR or non-PD | 2 (2.6) | 3 (3.7) | — | — |
| Unknown | 2 (2.6) | 11 (13.4) | 1 (1.3) | 11 (13.4) |
| DCR, % [95% CI] | 82.9 [72.5–90.6] | 79.3 [68.9–87.4] | 90.8 [81.9–96.2] | 80.5 [70.3–88.4] |
| Median PFS, mo [95% CI] | 26.3 [8.6–NE] | 10.6 [6.7–15.0] | 16.6 [9.7–NE] | 10.9 [6.7–13.7] |
| n/N (%) | 32/76 (42.1) | 45/82 (54.9) | 35/76 (46.1) | 53/82 (64.6) |
| % Event-free probability estimates [95% CI] | ||||
| 9 mo | 61.0 [48.4–71.5] | 54.7 [41.8–65.8] | 64.9 (52.5–74.8) | 53.2 (40.7–64.2) |
| 12 mo | 61.0 [48.4–71.5] | 49.8 [37.1–61.2] | 60.3 (47.9–70.7) | 45.4 (33.2–56.8) |
| 15 mo | 55.9 [43.2–66.9] | 39.0 [26.9–51.0] | 55.3 (42.6–66.2) | 34.1 (22.9–45.6) |
| Ma = 50 | Ma = 24 | Ma = 52 | Ma = 23 | |
| Median DOR,b mo [95% CI] | NE [24.7–NE] | 16.4 [7.8–NE] | NE [14.0–NE] | 11.0 [6.8–19.2] |
| n/N (%) | 14/50 (28.0) | 8/24 (33.3) | 17/52 (32.7) | 13/23 (56.5) |
| % Event-free probability estimates [95% CI] | ||||
| 9 mo | 81.2 [67.0–89.8] | 76.1 [48.0–90.4] | 81.7 (67.7–90.0) | 57.4 (32.4–76.1) |
| 12 mo | 79.0 [64.5–88.1] | 50.8 [22.5–73.5] | 75.1 (60.3–85.1) | 39.4 (17.5–60.8) |
| 15 mo | 70.4 [54.0–81.9] | 50.8 [22.5–73.5] | 64.0 (47.5–76.5) | 39.4 (17.5–60.8) |
Note: ORR is CR plus PR. DCR is CR plus PR plus stable disease plus non-CR or non-PD. Non-CR or non-PD refers to BORs that are neither CR nor PD per RECIST v1.1 criteria for patients with nonmeasurable disease only at baseline.
BIRC, Blinded Independent Review Committee; BOR, best overall response; CI, confidence interval; CR, complete response; DCR, disease control rate; DOR, duration of response; n, total number of events included in the analysis; N, total number of patients included in the analysis; NE, not estimable; ORR, overall response rate; PD, progressive disease; PFS, progression-free survival; PR, partial response; RECIST v1.1, Response Evaluation Criteria in Solid Tumors version 1.1.
Total number of patients with confirmed CR or PR.
For median DOR (by BIRC assessment), there were no responders at risk at and beyond 18 months in the chemotherapy arm, resulting in the estimated Kaplan-Meier event-free rates to be NE. For the median DOR (by investigator assessment), the estimated Kaplan-Meier event-free rates were NE, since there were no responders at risk at 21 months and beyond in the chemotherapy arm.
Figure 2.
Kaplan-Meier Plot of PFS per (A) BIRC assessment and (B) Investigator assessment. BIRC, Blinded Independent Review Committee; CI, confidence interval; n, total number of events included in the analysis; N, total number of patients included in the analysis; NE, not estimable; PFS, progression-free survival.
The ORR was higher in the ceritinib arm versus the chemotherapy arm (BIRC-assessed: 65.8% [95% CI: 54.0–76.3] versus 29.3% [95% CI: 19.7–40.4] and investigator-assessed: 68.4% [95% CI: 56.7–78.6] versus 28.0% [95% CI: 18.7–39.1]). The results of ORR by investigator assessment were consistent with those observed per BIRC assessment (Table 2). The DCR by BIRC assessment was 82.9% (95% CI: 72.5–90.6) versus 79.3% (95% CI: 68.9–87.4), and the DCR by investigator assessment was 90.8% (95% CI: 81.9–96.2) versus 80.5% (95% CI: 70.3–88.4) in the ceritinib and chemotherapy arms, respectively (Table 2). The median DOR (by BIRC assessment) was not reached in the ceritinib arm versus 16.4 months (95% CI: 7.8–NE) in the chemotherapy arm. The 12-month DOR rate was higher in the ceritinib arm versus the chemotherapy arm (79.0% [95% CI: 64.5–88.1] versus 50.8% [95% CI: 22.5–73.5]). The estimated event-free rate at 9 months was 81.2% (95% CI: 67.0–89.8) for the ceritinib arm and 76.1% (95% CI: 48.0–90.4) for the chemotherapy arm (Table 2). The median DOR (by investigator assessment) was not reached in the ceritinib arm and was 11.0 months (95% CI: 6.8–19.2) in the chemotherapy arm. The estimated event-free rate at 12 months was 75.1% (95% CI: 60.3–85.1) for the ceritinib arm and 39.4% (95% CI: 17.5–60.8) for the chemotherapy arm (Table 2). A total of 25 patients in the ceritinib arm and 22 patients in the chemotherapy arm had BM (measurable or nonmeasurable) as per BIRC neuroradiologist review. Of these, OIRR was observed in 11 patients (44.0%) in the ceritinib arm and five patients (22.7%) in the chemotherapy arm, and IDCR was observed in 20 patients (80.0%) in the ceritinib arm and in 17 patients (77.3%) in the chemotherapy arm (Table 3).
Table 3.
Best Overall Intracranial Response per BIRC Assessment
| Parameter | Ceritinib 750 mg N = 25 | Chemotherapy N = 22 |
|---|---|---|
| OIRR, n (%) [95% CI] | 11 (44.0) [24.4–65.1] | 5 (22.7) [7.8–45.4] |
| BOIR, n (%) | ||
| CR | 7 (28.0) | 2 (9.1) |
| PR | 4 (16.0) | 3 (13.6) |
| Stable disease | 0 | 2 (9.1) |
| PD | 3 (12.0) | 1 (4.5) |
| Non-CR or non-PD | 9 (36.0) | 10 (45.5) |
| Unknown | 2 (8.0) | 4 (18.2) |
| IDCR, n (%) [95% CI] | 20 (80.0) [59.3–93.2] | 17 (77.3) [54.6–92.2] |
Note: N is the total number of patients with measurable and/or nonmeasurable disease in the brain at baseline as per BIRC neuroradiology review. It is the denominator for percentage calculation. The n is the number of patients who are in the corresponding category. OIRR is CR plus PR. IDCR is CR plus PR plus stable disease plus non-CR or non-PD. Non-CR or non-PD refers to BOIRs that are neither CR nor PD per the modified RECIST v1.1 criteria for patients with nonmeasurable disease only at baseline.
BIRC, Blinded Independent Review Committee; BOIR, best overall intracranial response; CI, confidence interval; CR, complete response; IDCR, intracranial disease control rate; OIRR, overall intracranial response rate; PD, progressive disease; PR, partial response; RECIST v1.1, Response Evaluation Criteria in Solid Tumors version 1.1.
Safety
The median duration of exposure to ceritinib was longer than that of chemotherapy (64.5 weeks [range = 5.3–144.4] versus 35.0 weeks [range = 0.7–93.3]). A higher proportion of patients was exposed to ceritinib for a period of ≥ 33 weeks versus chemotherapy (55 patients [72.4%] versus 40 patients [53.3%]). The median relative dose intensity was 70.8% (range = 30.4–100.0), 96.0% (range = 67.9–103.4), 97.6% (range = 65.5–101.9), and 95.1% (range = 67.0–116.3) for patients receiving ceritinib (N = 76), pemetrexed (N = 75), cisplatin (N = 26), and carboplatin (N = 51), respectively. For ceritinib, the proportion of patients requiring ≥ 1 dose reduction and ≥ 1 dose interruption was 58 patients (76.3%) and 62 patients (81.6%), respectively.
The overall summary of AEs is reported in Supplementary Table 2. AEs of any grade (all causality) were reported in 76 patients (100%) in the ceritinib arm (N = 76) and in 73 patients (97.3%) in the chemotherapy arm (N = 75). The most frequently reported AEs of any grade (all causality) are illustrated in Table 4. Grade 3 or 4 AEs (all causality) were reported in 60 patients (78.9%) in the ceritinib arm and 50 patients (66.7%) in the chemotherapy arm. The most frequently reported grade 3 or 4 AEs (all causality) were increased hepatic function tests, namely: alanine aminotransferase (ALT) (29 patients [38.2%]); gamma-glutamyl transferase (17 patients [22.4%]); and aspartate aminotransferase (AST) (16 patients [21.1%]) in the ceritinib arm. In the chemotherapy arm, the incidence of specific individual grade 3 or 4 AEs (all causality) was lower than 15% (Table 4).
Table 4.
Most Common AEs (All Causality) in ≥ 20% of Patients, Any Grade, in Either Treatment Group
| AEs by Preferred Term | Ceritinib 750 mg N = 76 |
Chemotherapy N = 75 |
||
|---|---|---|---|---|
| Any Grade, n (%) | Grade 3 or 4, n (%) | Any Grade, n (%) | Grade 3 or 4, n (%) | |
| Total | 76 (100) | 60 (78.9) | 73 (97.3) | 50 (66.7) |
| Diarrhea | 65 (85.5) | 4 (5.3) | 12 (16.0) | 1 (1.3) |
| ALT increased | 56 (73.7) | 29 (38.2) | 17 (22.7) | 1 (1.3) |
| Vomiting | 56 (73.7) | 4 (5.3) | 32 (42.7) | 7 (9.3) |
| AST increased | 53 (69.7) | 16 (21.1) | 20 (26.7) | 1 (1.3) |
| Nausea | 53 (69.7) | 1 (1.3) | 37 (49.3) | 5 (6.7) |
| Decreased appetite | 28 (36.8) | 0 | 28 (37.3) | 1 (1.3) |
| Fatigue | 28 (36.8) | 6 (7.9) | 22 (29.3) | 4 (5.3) |
| GGT increased | 27 (35.5) | 17 (22.4) | 9 (12.0) | 1 (1.3) |
| Cough | 22 (28.9) | 0 | 13 (17.3) | 0 |
| Blood ALP increased | 21 (27.6) | 2 (2.6) | 3 (4.0) | 0 |
| Blood creatinine increased | 21 (27.6) | 2 (2.6) | 5 (6.7) | 0 |
| Weight decreased | 21 (27.6) | 3 (3.9) | 12 (16.0) | 1 (1.3) |
| Pyrexia | 20 (26.3) | 0 | 13 (17.3) | 2 (2.7) |
| Abdominal pain | 18 (23.7) | 2 (2.6) | 5 (6.7) | 0 |
| Anemia | 17 (22.4) | 4 (5.3) | 30 (40.0) | 10 (13.3) |
| Back pain | 16 (21.1) | 1 (1.3) | 12 (16.0) | 0 |
| Rash | 16 (21.1) | 1 (1.3) | 7 (9.3) | 0 |
| Constipation | 13 (17.1) | 0 | 18 (24.0) | 0 |
| Dyspnea | 12 (15.8) | 2 (2.6) | 17 (22.7) | 5 (6.7) |
| WBC count decreased | 6 (7.9) | 0 | 21 (28.0) | 6 (8.0) |
| Neutrophil count decreased | 5 (6.6) | 2 (2.6) | 19 (25.3) | 8 (10.7) |
| Neutropenia | 3 (3.9) | 0 | 15 (20.0) | 7 (9.3) |
Note: A patient with multiple occurrences of an AE under one treatment is counted only once in the AE category for that treatment. A patient with multiple AEs is counted only once in the total row. Only AEs occurring during the on-treatment period are summarized. Missing grades are included under the “any grade” column. MedDRA v19.0 was used. AEs were graded according to the CTCAE v4.03.
AE, adverse event; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTCAE v4.03, Common Terminology Criteria for Adverse Events version 4.03; GGT, gamma-glutamyl transferase; MedDRA v19.0, medical dictionary for regulatory activities version 19.0; WBC, white blood cell.
AEs of any grade that were suspected to be related to the study drug were reported in 75 patients (98.7%) and 68 patients (90.7%) in the ceritinib and chemotherapy arms, respectively. Grade 3 or 4 AEs suspected to be related to the study drug were reported in 53 patients (69.7%) and 36 patients (48.0%) in the ceritinib and chemotherapy arms, respectively. The most frequently reported AEs of any grade and grade 3 or 4 suspected to be related to study drug are illustrated in Supplementary Table 3. AEs of any grade leading to study drug discontinuation were increased blood creatinine (four patients [5.3%]), increased ALT, increased amylase, decreased renal creatinine clearance, and increased lipase (all reported in one patient [1.3% each]) in the ceritinib arm; and decreased renal creatinine clearance, alveolitis allergic, dyspnea, embolism, and pneumonia (all reported in one patient [1.3% each]) in the chemotherapy arm. The proportion of patients who had serious AEs (SAEs) of any grade (all causality) was 29 patients (38.2%) in the ceritinib arm versus 22 patients (29.3%) in the chemotherapy arm (Supplementary Table 2). One on-treatment death (1.3%) was reported in the ceritinib arm and three (4.0%) in the chemotherapy arm. All four deaths were attributed to advanced ALK-rearranged NSCLC.
In the ceritinib arm, hepatic-related AEs were reported in 60 patients (78.9%), and most (76.3%) were suspected to be ceritinib-related. Grade 3 or 4 hepatic-related AEs were reported in 42 patients (55.3%), five patients (6.6%) reported SAEs, and one patient (1.3%) reported increased ALT leading to study drug discontinuation. The most frequently reported hepatic-related AEs were increased ALT, increased AST, and increased gamma-glutamyl transferase. One patient (1.3%) had a grade 3 liver injury (SAE), suspected to be related to ceritinib, which required dose interruption. Grade 3 acute hepatitis (not an SAE), suspected to be related to ceritinib, was observed in one patient (1.3%). This required dose reduction.
Discussion
Results from this subgroup analysis of treatment-naive Asian patients with advanced ALK-rearranged NSCLC support the robustness of the primary analysis results in the overall population of the global ASCEND-4 trial. Except for the prevalence of baseline BM, the demographics and disease characteristics were well balanced between the two arms and consistent with the overall patient population in the ASCEND-4 trial. In Asian patients, ceritinib treatment resulted in an estimated risk reduction of 34% in PFS by BIRC assessment versus chemotherapy. The event-free probability estimates remained higher for the ceritinib arm versus the chemotherapy arm, indicating early and sustained advantage with ceritinib therapy. Although the median PFS by BIRC assessment in the ceritinib arm versus the chemotherapy arm points to a clinical benefit with ceritinib treatment, the upper limit of the 95% CI for the median PFS was NE, and it is likely that the median PFS of 26.3 months was overestimated. The PFS results by investigator assessment were supportive of the BIRC assessment. Furthermore, results from the other secondary end points also confirmed the favorable efficacy of ceritinib versus chemotherapy in Asian patients. Per the BIRC and investigator assessment, ORR was higher in the ceritinib arm versus the chemotherapy arm. Although BM at baseline was imbalanced between the two treatment arms per BIRC neuroradiologist review (ceritinib arm: 32.9% and chemotherapy arm: 26.8%), the higher OIRR observed in the ceritinib arm versus the chemotherapy arm in patients with measurable or nonmeasurable BM points to the efficacy of ceritinib in the central nervous system (CNS).
The efficacy results observed in Asian patients are consistent with that of the overall population randomized in the ASCEND-4 trial, thus, supporting the robustness of the results in the overall population. Overall, the proportion of PFS events and the estimated PFS rates at 12 months, 15 months, and 18 months in the Asian subgroup were consistent with that of the overall population. The proportion of Asian patients with baseline BM was similar to that of the overall population in the ceritinib arm (Asian subgroup: 32.9% versus overall population: 31.2%) but numerically lower than that of the overall population (25.6% versus 33.2%) in the chemotherapy arm. In the ceritinib arm, the OIRR for Asian patients with measurable or nonmeasurable BM was high and consistent with that of the overall population (Asian subgroup: 44.0% and overall population: 41.0%) per the BIRC neuroradiologist assessment.
Although the efficacy of ceritinib versus crizotinib has not been evaluated in a direct head-to-head trial, a recently conducted retrospective analysis revealed a significantly longer median PFS with ceritinib versus crizotinib (32.3 months versus 12.9 months, log-rank p = 0.020) in treatment-naive Asian patients with advanced ALK-rearranged NSCLC.19 The ORR observed with ceritinib in this Asian subgroup analysis (65.8% [95% CI: 54.0–76.3]) was numerically lower than that reported for crizotinib in Asian patients in the ALESIA (77%, N = 62), ALEX (76.8% [95% CI: 65.1–86.1], N = 69), PROFILE 1014 (70% [95% CI: 59.0–80.0], N = 77), and PROFILE 1029 (87.5% [95% CI: 79.6–93.2], N = 104) studies, and that reported for alectinib in Asian patients in the ALESIA (91%, N = 125) and ALEX (81.2% [95% CI: 69.9–89.6], N = 69) studies.20, 21, 22, 23 With all the limitations of indirect, cross-trial comparisons, the CNS response rate observed with ceritinib in this Asian subgroup analysis (11 of 25 patients [44%]; 95% CI: 24.4–65.1) was numerically higher than that reported for crizotinib (CNS responders: five of 23 patients [22%]; 95% CI: 8–44) but numerically lower than that reported for alectinib (CNS responders: 32 of 44 patients [73%]; 95% CI: 57–85) in Asian patients with similar conditions treated in the ALESIA study.20
The overall safety profile of ceritinib in Asian patients was largely consistent with that of the overall population treated with ceritinib 750 mg/day in the fasted state.15 No new or unexpected safety concerns emerged from this Asian subgroup analysis. AEs due to ceritinib were well managed with dose interruptions or reduction and by medication. The incidence of increased AST of any grade suspected to be related to ceritinib was numerically higher in Asian patients—that is, a difference of ≥ 15% versus the overall population (69.7% versus 50.8%)—but the incidence of grade 3 or 4 increased AST suspected to be related to ceritinib was similar between the two populations (Asian subgroup: 21.1% versus overall population: 15.9%). In the ceritinib arm, the proportion of patients with hepatic-related AEs of any grade and grade 3 or 4 in the Asian subgroup was similar to that of the overall population. Elevation in transaminases (ALT or AST laboratory values > 3× the upper limit of normal) was reported in 75% of Asian patients and 61.4% of the overall population, suggesting a numerically higher proportion of elevated transaminases in the Asian subgroup. In Asian patients, most hepatic-related AEs were managed with ceritinib dose reduction or interruption or delay and were fully reversible.
Since the publication on the primary analysis of the ASCEND-4 study, the FDA-approved dose of ceritinib was revised from 750 mg/day in the fasted state to 450 mg/day with a low-fat meal.12 This recommendation was based on the results from the phase 1 ASCEND-8 trial, which demonstrated consistent efficacy and a more favorable gastrointestinal safety profile of ceritinib in the 450-mg fed arm versus the 750-mg fasted arm. In addition, no clinically meaningful differences were detected in the pharmacokinetic profile of ceritinib between the 450-mg fed and the 750-mg fasted arms.24, 25, 26 Furthermore, results from a recent network meta-analysis conducted in the Chinese health care setting indicate that ceritinib 450 mg/day with a low-fat meal is cost-effective compared with crizotinib and alectinib in the first-line setting for advanced ALK-rearranged NSCLC.27
In conclusion, ceritinib is a highly effective and safe first-line treatment in Asian patients with advanced ALK-rearranged NSCLC. The PFS results by both BIRC and investigator assessment were largely consistent with that of the overall ASCEND-4 study population. Ceritinib also exhibited a high intracranial activity that was consistent with that of the overall population. Collectively, these results establish that ceritinib is a potent and selective first-line targeted therapy administered for patients with advanced ALK-rearranged NSCLC who are treatment-naive. The consistency of efficacy results in the Asian subgroup demonstrating robustness of the observed clinical benefit of ceritinib in the overall ASCEND-4 study population and the similar safety profile establish substantial evidence for ceritinib as a cost-effective and efficacious treatment option for Asian patients with advanced ALK-rearranged NSCLC in the first-line setting.
Acknowledgments
This study was sponsored by Novartis Pharmaceuticals Corporation. The authors thank the participating patients, their families, all study coinvestigators, and research coordinators. Medical writing support was provided by Gowri Natarajan and Shilpa Garg, Novartis Healthcare Pvt. Ltd. (Hyderabad, India). Drs. Tan and Wu contributed to study conception and design. Drs. Tan, Geater, Tsai, Lin, Yang, and Branle contributed to the collection and assembly of data. Drs. Tan, Geater, Yu, Tsai, Hsia, Chen, Lin, Lu, Sriuranpong, Yang, Sen, Branle, Shi, and Wu contributed to data analysis and interpretation. Drs. Tan, Geater, Yu, Tsai, Chen, Lin, Sriuranpong, and Yang contributed to the provision of study materials or patients. All authors contributed to manuscript writing and final approval of the manuscript.
Footnotes
Disclosure: Dr. Tan reports receiving honoraria (self) from Novartis, Roche, Pfizer, Bristol-Myers Squibb, and Takeda; advisory or consultancy from Novartis, Merck, Loxo, AstraZeneca, Roche, and Pfizer; research grant or funding (institution) from Novartis, AstraZeneca, and GlaxoSmithKline; and travel or accommodation or expenses from Roche, Pfizer, and Boehringer Ingelheim. Dr. Geater reports advisory or consultancy from Boehringer Ingelheim; honoraria (self) from AstraZeneca and Boehringer Ingelheim; research grant or funding (institution) from AstraZeneca, Roche, Novartis, and Boehringer Ingelheim; and employment at the Prince of Songkla University. Dr. Yu reports advisory or consultancy from Roche, AstraZeneca, Ono Pharmaceutical, Boehringer Ingelheim, Novartis, GlaxoSmithKline, and Merck Sharp & Dohme; and research grant or funding (institution) from Novartis. Dr. Tsai reports receiving research grant or funding (institution) from Novartis; advisory or consultancy from Novartis, Pfizer, Roche, Eli Lilly, Merck Sharp & Dohme, Bristol-Myers Squibb, and AstraZeneca; and honoraria from Novartis, Pfizer, Roche, Eli Lilly, Merck Sharp & Dohme, Bristol-Myers Squibb, and AstraZeneca. Dr. Hsia reports advisory or consultancy from Novartis, Eli Lilly, AstraZeneca, and Roche; served as local speaker for Roche; and received research grant or funding (institution) from Novartis. Dr. Chen reports receiving research grant or funding (institution) from Novartis. Dr. Lin reports receiving research grant or funding (institution) from Novartis; and advisory or consultancy from AstraZeneca, Novartis, Boehringer Ingelheim, and Roche. Dr. Sriuranpong reports receiving research grant or funding (institution) from Novartis; honoraria (self) from Novartis; and advisory or consultancy from Novartis. Dr. Yang reports receiving research grant or funding (institution) from Novartis and advisory or consultancy from Novartis, Eli Lilly, AstraZeneca, Ono Pharmaceuticals, and Boehringer Ingelheim. Drs. Sen, Branle, and Shi report receiving shareholder or stockholder or stock options from Novartis and full-time employment at Novartis. Dr. Wu reports receiving honoraria (self) from AstraZeneca, Roche, Eli Lilly, Pfizer, Merck Sharp & Dohme, Bristol-Myers Squibb, Boehringer Ingelheim, and Sanofi; advisory or consultancy from AstraZeneca, Roche, and Boehringer Ingelheim; and research grant or funding (institution) from AstraZeneca, Roche, and Novartis. Dr. Lu declares no conflict of interest. Data from this study have been previously presented at the European Society for Medical Oncology (ESMO) 2019 congress (abstract #1473P) in Barcelona, Spain, September 27-October 01, 2019
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2020.100131.
Supplementary Data
References
- 1.Kwak E.L., Bang Y.J., Camidge D.R. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rikova K., Guo A., Zeng Q. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–1203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 3.Shaw A.T., Yeap B.Y., Mino-Kenudson M. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27:4247–4253. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soda M., Choi Y.L., Enomoto M. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 5.Shaw A.T., Gandhi L., Gadgeel S. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol. 2016;17:234–242. doi: 10.1016/S1470-2045(15)00488-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw A.T., Kim D.W., Mehra R. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370:1189–1197. doi: 10.1056/NEJMoa1311107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw A.T., Kim D.W., Nakagawa K. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 8.Awad M.M., Shaw A.T. ALK inhibitors in non-small cell lung cancer: crizotinib and beyond. Clin Adv Hematol Oncol. 2014;12:429–439. [PMC free article] [PubMed] [Google Scholar]
- 9.Song Z., Wang M., Zhang A. Alectinib:. a novel second generation anaplastic lymphoma kinase (ALK) inhibitor for overcoming clinically acquired resistance. Acta PharmSin B. 2015;5:34–37. doi: 10.1016/j.apsb.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khozin S., Blumenthal G.M., Zhang L. FDA approval: ceritinib for the treatment of metastatic anaplastic lymphoma kinase-positive non-small cell lung cancer. Clin Cancer Res. 2015;21:2436–2439. doi: 10.1158/1078-0432.CCR-14-3157. [DOI] [PubMed] [Google Scholar]
- 11.US Food and Drug Administration FDA broadens ceritinib indication to previously untreated ALK-positive metastatic NSCLC. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-broadens-ceritinib-indication-previously-untreated-alk-positive-metastatic-NSCLC
- 12.US Food and Drug Administration Zykadia prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211225s000lbl.pdf
- 13.Kim D.W., Mehra R., Tan D.S.W. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol. 2016;17:452–463. doi: 10.1016/S1470-2045(15)00614-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishio M., Felip E., Orlov S. Final overall survival and other efficacy and safety results from ASCEND-3: Phase II study of ceritinib in ALKi-naive patients with ALK-rearranged NSCLC. J Thorac Oncol. 2020;15:609–617. doi: 10.1016/j.jtho.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Soria J.C., Tan D.S.W., Chiari R. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017;389:917–929. doi: 10.1016/S0140-6736(17)30123-X. [DOI] [PubMed] [Google Scholar]
- 16.Scagliotti G.V., Kortsik C., Dark G.G. Pemetrexed combined with oxaliplatin or carboplatin as first-line treatment in advanced non-small cell lung cancer: a multicenter, randomized, phase II trial. Clin Cancer Res. 2005;11:690–696. [PubMed] [Google Scholar]
- 17.Scagliotti G.V., Parikh P., von Pawel J. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 18.Brookmeyer R., Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38:29–41. [Google Scholar]
- 19.Huang S.H., Huang A.C., Wang C.C. Front-line treatment of ceritinib improves efficacy over crizotinib for Asian patients with anaplastic lymphoma kinase fusion NSCLC: the role of systemic progression control. Thorac Cancer. 2019;10:2274–2281. doi: 10.1111/1759-7714.13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou C., Kim S.W., Reungwetwattana T. Alectinib versus crizotinib in untreated Asian patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer (ALESIA): a randomised phase 3 study. Lancet Respir Med. 2019;7:437–446. doi: 10.1016/S2213-2600(19)30053-0. [DOI] [PubMed] [Google Scholar]
- 21.Mok T.S.K., Peters S., Camidge D.R. Alectinib (ALC) vs crizotinib (CRZ) in treatment-naive ALK+ non-small-cell lung cancer (NSCLC): Asian vs non-Asian subgroup analysis of the ALEX study. Ann Oncol. 2017;28(suppl 10):x191. [Google Scholar]
- 22.Wu Y.L., Lu S., Lu Y. Results of PROFILE 1029, a phase III comparison of first-line crizotinib versus chemotherapy in East Asian patients with ALK-positive advanced non-small cell lung cancer. J Thorac Oncol. 2018;13:1539–1548. doi: 10.1016/j.jtho.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Nishio M., Kim D.W., Wu Y.L. Crizotinib versus chemotherapy in Asian patients with ALK-positive advanced non-small cell lung cancer. Cancer Res Treat. 2018;50:691–700. doi: 10.4143/crt.2017.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho B.C., Kim D.W., Bearz A. ASCEND-8: a randomized phase 1 study of ceritinib, 450 mg or 600 mg, taken with a low-fat meal versus 750 mg in fasted state in patients with anaplastic lymphoma kinase (ALK)-rearranged metastatic non-small cell lung cancer (NSCLC) J Thorac Oncol. 2017;12:1357–1367. doi: 10.1016/j.jtho.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Cho B.C., Obermannova R., Bearz A. Efficacy and safety of ceritinib (450 mg/d or 600 mg/d) with food versus 750-mg/d fasted in patients with ALK receptor tyrosine kinase (ALK)-positive NSCLC: primary efficacy results from the ASCEND-8 study. J Thorac Oncol. 2019;14:1255–1265. doi: 10.1016/j.jtho.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Otoukesh S., Sanchez T., Mirshahidi S., Wallace D., Mirshahidi H. ASCEND-8 pharmacokinetic, safety, and efficacy data for ceritinib 450 mg with food in patients with anaplastic lymphoma kinase-positive non-small cell lung Cancer: a clinical perspective. Cancer Treat Res Commun. 2019;20:100149. doi: 10.1016/j.ctarc.2019.100149. [DOI] [PubMed] [Google Scholar]
- 27.Li H., Lai L., Wu B. Cost effectiveness of ceritinib and alectinib versus crizotinib in first-line anaplastic lymphoma kinase-positive advanced non-small-cell lung cancer. Clin Drug Investig. 2020;40:183–189. doi: 10.1007/s40261-019-00880-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.