Abstract
Background
Chronic sickness behavior is implicated in ME/CFS (Myalgic Encephalomyelitis/Chronic Fatigue Syndrome) and chronic pain but the level of subjective sickness behavior in these conditions has not been investigated or compared to other clinical and non-clinical samples, or to the level in experimental inflammation. Furthermore, the relationship between sickness behavior and self-rated health and functioning is not known in patients with ME/CFS and chronic pain. The aim of the present study was to investigate how sickness behavior in patients with chronic conditions differs from that in individuals with experimental acute sickness, primary care patients, the general population and healthy subjects. In addition, we wanted to explore how sickness behavior is related to self-rated health and health-related functioning.
Methods
Sickness behavior was quantified using the sickness questionnaire (SicknessQ). Self-ratings were collected at one time-point in 6 different samples. Levels of sickness behavior in patients with ME/CFS (n = 38) and patients with chronic pain (n = 190) were compared to healthy subjects with lipopolysaccharide(LPS)-induced inflammation (n = 29), primary care patients (n = 163), individuals from the general population (n = 155) and healthy subjects (n = 48), using linear regression. Correlations and moderated regression analyses were used to investigate associations between sickness behavior and self-rated health and health-related functioning in ME/CFS, chronic pain and the general population.
Results
LPS-injected individuals (M = 16.3), patients with ME/CFS (M = 16.1), chronic pain (M = 16.1) and primary care patients (M = 10.7) reported significantly higher SicknessQ scores than individuals from the general population (M = 5.4) and healthy subjects (M = 3.6) all p’s < 0.001). In turn, LPS-injected individuals, patients with ME/CFS and chronic pain reported significantly higher SicknessQ scores than primary care patients (p’s < 0.01). Higher levels of sickness behavior were associated with poorer self-rated health and health-related functioning (p’s < 0.01), but less so in patients with ME/CFS and chronic pain than in individuals from the general population.
Conclusions
Patients with ME/CFS and chronic pain report similar high levels of sickness behavior; higher than primary care patients, and comparable to levels in experimental inflammation. Further study of sickness behavior in ME/CFS and chronic pain populations is warranted as immune-to-brain interactions and sickness behavior may be of importance for functioning as well as in core pathophysiological processes in subsets of patients.
Keywords: Sickness behavior, ME/CFS, Chronic Fatigue Syndrome, Chronic pain, Endotoxin, Self-rated health, Functioning
Highlights
-
•
Investigation of the level of subjective sickness behavior, assessed with a validated questionnaire, in patients with ME/CFS (Myalgic Encephalomyelitis/Chronic Fatigue Syndrome) and in patients with chronic pain compared to clinical, non-clinical and experimental groups.
-
•
The level of sickness behavior is similarly high in ME/CFS and chronic pain, and equal to the level in experimentally induced inflammation via injection of bacterial endotoxin.
-
•
Higher levels of sickness behavior showed significant associations with lower levels of self-rated health and functioning.
1. Introduction
In recent years, the study of sickness behavior, or the sickness response, and its implications for health and functioning has received substantial attention and research support (Capuron and Miller, 2011; Dantzer and Kelley, 2007; Dantzer et al., 2008; Lacourt et al., 2018; Dantzer, 2018). In the prototypical situation, the detection of pathogens by the immune system induces the acute release of inflammatory cytokines which then alters brain function (Dantzer et al., 2008). In brief, four main pathways have been suggested for these immune-to-brain-interactions: (a) through signaling via vagal nerve afferents; (b) via cerebral endothelial cells (CEC) expressing cytokine receptors; (c) via monocyte/CEC-interactions activating microglia, and; (d) via passive transport at the circumventricular organs where the blood-brain-barrier is weaker (D’Mello and Swain, 2017). The subsequent effects on brain functions include, for example, alterations of the synthesis, release and reuptake of serotonin and dopamine. Cytokines also affect the function of the hypothalamic-pituitary-adrenal (HPA) axis (Capuron and Miller, 2011; D’Mello and Swain, 2017). On the experiential and behavioral level, following the acute release of cytokines as part of the inflammatory response the affected individual soon presents a series of typical behaviors during sickness such as general malaise, fatigue, anhedonia, loss of appetite, hyperalgesia and anxiety (Dantzer, 2004; Hart, 1988, 1990). This array of responses is assumed to represent behavioral adaptations to protect the organism from pathogens and aid recovery (Dantzer and Kelley, 2007; Hart, 1988). As such, it is believed to be an evolutionary and functional response in the short-term when the organism is fighting an infection. If these changes in the immune-to-brain-interactions persists in the long-term however, as during chronic inflammation, they may become maladaptive as they no longer contribute to recovery but to ill-health (Dantzer et al., 2008; Lacourt et al., 2018; D’Mello and Swain, 2017; Karshikoff et al., 2017; Moilanen, 2014). Importantly, from a behavioral perspective, prolonged sickness behavior may have a negative impact on health and functioning as acute sickness behavior is characterized by increases in fatigue, pain sensitivity, social avoidance and depressive symptoms (Lacourt et al., 2018; Savitz and Harrison, 2018; Harrison et al., 2009; Lasselin et al., 2016a; Karshikoff et al., 2015).
To experimentally investigate the mechanisms and consequences of sickness behavior, subjects are injected with a compound stimulating the innate immune system, such as bacterial endotoxin (lipopolysaccharide, LPS) or Typhoid vaccine (Harrison et al., 2009; Lasselin et al., 2016a). As inflammatory markers increase, a corresponding increase in sickness behavior is seen in the affected individual (Lasselin et al., 2016a; Karshikoff et al., 2015; Andreasson et al., 2018, 2019; Harrison et al., 2016). However, there is a lack of studies investigating sickness behavior in longstanding conditions with unclear pathophysiological mechanisms but where the symptomatology indicates the potential importance of immune-to-brain-interactions for symptom development. It has been argued that persistent pain, depression and fatigue could partly be a consequence of an unabated sickness response (Dantzer et al., 2008; Lacourt et al., 2018; Karshikoff et al., 2017).
Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) and chronic pain are two longstanding debilitating conditions with unclear etiology or pathophysiological mechanisms (Breivik et al., 2006; Carruthers et al., 2011). In addition to longstanding fatigue, patients with ME/CFS present with a range of symptoms pertaining to immune activation and sickness including general malaise, widespread pain, cognitive difficulties, headache and tender lymph nodes (Carruthers et al., 2011; Jonsjö et al., 2017; Davenport et al., 2011). Similarly, chronic pain is associated with depression and anxiety as well as fatigue (Breivik et al., 2006). Elevated levels of inflammatory markers, for example interleukin(IL)-6, IL-8 and tumor necrosis factor alpha (TNF-α), have been reported in patients with longstanding pain (Koch et al., 2007). In ME/CFS, preliminary results from recent studies indicate that inflammatory markers in combination with behavioral factors could be of importance for symptom burden and functioning (Karshikoff et al., 2017; Milrad et al., 2017, 2018; Lattie et al., 2012). However, the level of subjective sickness behavior has not been assessed or compared between the two patient groups nor compared to the level in other populations with validated instruments. In addition, it is not known how subjective sickness behavior in these chronic conditions relates to physical and mental health-related functioning and self-rated health.
The aims of this study were threefold. First, we wanted to investigate the level of sickness behavior as assessed with a validated sickness questionnaire (SicknessQ (Andreasson et al., 2018)) in patients with ME/CFS and in patients with chronic pain as compared to four reference groups. We hypothesized that ME/CFS and chronic pain patients would report higher levels of sickness behavior compared to healthy subjects, primary care patients and individuals from the general population but lower than healthy subjects injected with LPS. The second aim was to determine whether or not there were differences in the sickness behavior profile between ME/CFS, chronic pain and healthy subjects injected with LPS. We hypothesized that patients with ME/CFS and chronic pain would report similar levels of sickness behavior, including items on fatigue and pain given the high comorbidity between the symptom complexes in these populations. We hypothesized that patients with ME/CFS and chronic pain would report lower levels on items not relating to core symptoms in their respective conditions (i.e. non-pain or non-fatigue-related items) than LPS-injected healthy subjects. Our third aim was to investigate the associations between sickness behavior and health-related functioning and self-rated health, including if the strength of the associations differed between patients with ME/CFS, chronic pain and individuals from the general population. We hypothesized that the strength of the associations between sickness behavior and self-rated health, physical and mental health-related functioning would be similar in patients with ME/CFS, in patients with chronic pain and in individuals from the general population.
2. Methods
2.1. Participants and procedure
2.1.1. Healthy subjects
Baseline data from healthy subjects participating in an LPS experiment was used as the healthy control reference in the present study. For a detailed account of methods and materials, see (Karshikoff et al., 2015; Andreasson et al., 2019). Participants were mainly university students recruited through advertising. For inclusion, participants had to: be 18–50 years old; right-handed; medication free; non-smokers; be without history of drug abuse; be without history of or ongoing inflammatory, psychiatric and sleep disorders or chronic pain, as well as have a body mass index within the normal range. Out of the 52 healthy subjects that participated in the experiment, 48 had complete SicknessQ data at baseline and were included in the analyses for the present study (56.3% women, MAge = 28.3 years, SD = 6.8 years). The study was approved by the Regional Ethical Review Board in Stockholm, Sweden (Dnr: 2008/955-31) and all participants gave written informed consent.
2.1.2. General population
Participants in the LongGERD study were drawn from a random sample of the general population in Östhammar community, previously described in detail (Agreus et al., 2016). Adult inhabitants born on day 3, 12 and 24 of each month were sent a validated questionnaire for abdominal symptoms in 1988, 1989, 1995 and 2011. In 2011, participants who had participated in the previous surveys but who had moved out of Östhammar community received the questionnaire in addition to the current Östhammar inhabitants (total n = 1863). Responders to the 2011 survey who were available for an upper endoscopy (age below 80 years and living within 20 km of the study center, n = 947) were contacted and asked to have the investigation (Agreus et al., 2016). Responders who agreed to the investigation were slightly older than those who declined but did otherwise not differ regarding gender distribution, education and self-rated health. Responders who agreed to the investigation (n = 402) were asked to fill out an online form including the SicknessQ. A total of 155 of the participants (50% women; MAge = 53.2 years; SD = 15.9) completed the questionnaires. Missing data, due to technical problems, was assumed to be missing at random and there were no significant differences in proportion of men and women or in age between those with complete data and those without. The study was approved by the Regional Ethics Board in Uppsala (Dnr 2010/443) and all participants provided informed consent.
2.1.3. Primary care patients
Participants were consecutively recruited during the spring of 2012 from patients visiting the light emergency drop-in clinic at a primary healthcare clinic in Stockholm, previously described in detail (Andreasson et al., 2018; Lodin et al., 2019). Pregnant women, patients under 18 years of age, and patients not able to speak and read Swedish were excluded. All non-urgent visits including medical consultations regarding annual health examinations, prescriptions for addictive drugs, chronic pain conditions, and sick leave renewal or other certificates were referred to booked appointments instead of the drop-in clinic and were automatically ineligible. The three most common reasons for seeking consultation were acute infection, muscle and joint pain, and symptoms from airways (asthma and allergy). Out of 215 persons approached, 163 patients completed the SicknessQ, resulting in a response rate of 76 per cent (70.1% women; MAge = 47.9 years; SD = 16.8). The study was approved by the Regional Ethical Review Board in Stockholm (Dnr 2011/1851-31/1) and all participants gave written informed consent.
2.1.4. Chronic pain patients
Patients were consecutively recruited at a tertiary specialist clinic between 2009 and 2013 after referral from primary and tertiary care units. Patients were eligible for inclusion if they had longstanding pain (≥6 months) and were above 18 years of age. Patients were excluded if: they were under 18 years of age; they currently participated in a CBT-based treatment; they presented with severe psychiatric co-morbidity that required immediate assessment and/or treatment (e.g., high risk of suicide, psychotic symptoms, and severe depressive episode); or, they were unable to fill out the questionnaires in Swedish. Data were collected using self-report questionnaires as part of the first visit to the clinic. A total of 190 individuals completed SicknessQ and were included in this study (78.4% women; MAge = 41.0 years; SD = 13.5). The study was approved by the Regional Ethical Review Board in Stockholm, Sweden (Dnr 2010/662-31/3) and all participants gave written informed consent.
2.1.5. ME/CFS patients
Patients with ME/CFS were consecutively recruited at a tertiary specialist clinic between 2013 and 2016 as described previously in detail (Jonsjö et al., 2019). Participants were assessed by a physician and a psychologist in order to exclude other potential causes for the symptomatology. All ME/CFS participants fulfilled the 1994 Centers for Disease Control and Prevention (CDC) and the 2011 International Consensus Criteria (ICC) for ME/CFS (Carruthers et al., 2011; Fukuda et al., 1994). Patients were excluded from the study if they were non-adherent during the assessment phase, if they lacked sufficient Swedish language skills to independently fill out the questionnaires and/or if they had psychiatric comorbidity meeting criteria for schizophrenia, bipolar disorder or major depressive disorder. Questionnaire data was collected at the visit to the clinic. A total of 38 individuals had complete SicknessQ data and were included in this study (81.6% women; MAge = 50.3 years, SD = 9.3). The study was approved by the Regional Ethical Review Board in Stockholm, Sweden (Dnr 2015/370-31/4) and all participants gave written informed consent.
2.1.6. LPS-injected subjects
Out of the 48 subjects used as healthy controls as described above, 29 had been randomized to receive an injection with 0.6 ng/kg LPS (E. Coli, Lot nr:G3E0609, United States Pharmacopeia Rockville, MD) intravenously. Injected healthy subjects consists of the 29 (58.6% women, MAge = 27.4 years, SD = 6.7). Sickness behavior was assessed with the SicknessQ at 1.5 h after injection. The placebo group 21 (52.4% women) subjects were injected with saline and their ratings at 1.5 h were not used in the present study.
2.2. Measures
2.2.1. Sickness behavior
Sickness behavior was assessed with the SicknessQ, a 10-item scale developed to assess subjective sickness behavior in humans and is rated on a 4-point scale with a maximum score of 30 (Andreasson et al., 2018). Confirmatory factor analyses using Stata 15.1 (StataCorp. College Station, Texas USA) were utilized to investigate the fit of the single factor structure of the Sickness Questionnaire within each sample. The fit for each model was evaluated using published criteria including the residual Chi-Square test (ideally p > .05), the ratio Chi-Square/levels of freedom (df) (ideally <5.0), the Comparative Fit Index (CFI; ideally >0.95), the Tucker-Lewis index (TLI; ideally >0.95) and Root Means Square Error Approximation (RMSEA; ideally <0.05) (Schermelleh-Engel et al., 2003). In the healthy subjects the single-factor structure showed an ideal fit with a residual Chi-Square test p = .66, Chi-Square/df = 0.88, CFI = 1.00, TLI = 1.09 and RMSEA<0.001. In the general population sample the single-factor SicknessQ structure showed a close to ideal fit with a residual Chi-Square test p = .28, Chi-Square/df = 0.98, CFI = 0.99, TLI = 0.98 and RMSEA = 0.028. In the ME/CFS sample the single-factor structure showed a reasonable fit with a residual Chi-Square test p = .08, Chi-Square/df = 1.36, CFI = 0.89, TLI = 0.84 and RMSEA = 0.097. In the LPS-injected subjects the single-factor structure showed an ideal fit with a residual Chi-Square test p = .40, Chi-Square/df = 1.04, CFI = 0.99, TLI = 0.99 and RMSEA = 0.039. The fit of the single-factor structure for the primary care population and the chronic pain sample have been evaluated previously and showed a close to ideal fit in the primary care sample (Andreasson et al., 2018) and a perfect fit in the chronic pain sample (Åström et al., manuscript in preparation).
2.2.2. Functioning and self-rated health
Physical and mental health-related functioning and self-rated health were assessed using the RAND SF-36/SF-12 Health Survey (Sullivan and Karlsson, 1998; Gandek et al., 1998; Ware et al., 1996; McHorney et al., 1993). Self-rated health was assessed through item 1 which asks the participants to rate their health from “Excellent” to “Poor” on a five-point scale where higher scores indicate worse health (Sullivan and Karlsson, 1998; McHorney et al., 1993). SF-36 data was available for patients with ME/CFS and the general population sample while the short version SF-12 was available for patients with chronic pain (Ware et al., 1996). A close correlation between the composite (Physical; PCS, and Mental; MCS) scores between the SF-36 and SF-12 has been subsequently shown in different populations, and the Swedish versions of the instruments demonstrate good overall psychometric properties (Sullivan and Karlsson, 1998; Gandek et al., 1998; Ware et al., 1996; McHorney et al., 1993).
2.3. Statistical analyses
Patient characteristics and questionnaire data were analyzed using mean and standard deviation (SD) for quantitative measures and count/percent for qualitative measures. Linear regression was utilized to investigate differences in SicknessQ scores as well as ratings on each item between groups, adjusted for age and sex. Bivariate correlations were utilized to investigate the association between sickness behavior and self-rated health and physical and mental health-related functioning, respectively, in each sample separately. Linear regression was used to investigate differences in strength of these associations (moderation) between samples through the statistical interaction between sample group (general population; chronic pain; ME/CFS) SicknessQ (predictor) with self-rated health, physical and mental health-related functioning as the outcomes, adjusted for age and sex. Due to non-Normality, statistical inference employed the nonparametric bootstrap using 2000 repetitions. Linear regressions were performed using Stata 15.1 (StataCorp. College Station, Texas USA). All other analyses were performed using SPSS version 25 (IBM Corp. IBM SPSS statistics for Macintosh, version 25. Armonk, NY: IBM Corp, 2017). An alpha level of 0.05 (two-tailed) was used in all statistical tests.
3. Results
Descriptive statistics (range, mean values and SDs) for age, sickness behavior, self-rated health, physical and mental health-related functioning are presented in Table 1. In the primary care, chronic pain and ME/CFS samples the majority of the participants were women; 70.1%, 78.4%, and 81.6% respectively. In the general population sample, sex was equally distributed between genders (50% women), and in the healthy subjects with/without LPS-injection sex was close to equally distributed (56.3%; 58,6% women, respectively). The primary care, chronic pain and ME/CFS and general population study samples were similar in terms of age while the healthy subjects and LPS-injected subjects were significantly younger (all p’s < 0.001, Table 1).
Table 1.
Age, sickness behavior, self-rated health, physical and mental health-related functioning (range, Means and SDs).
| Age |
SicknessQ |
Self-rated health |
SF-36/12 Physical |
SF-36/12 Mental |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Range | Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | |
| Healthy subjects | 20–47 | 28.3 (6.8) | 0–10 | 3.6 (2.7) | N/A | N/A | N/A | N/A | N/A | N/A |
| General population | 21–79 | 53.2 (15.9) | 0–20 | 5.4 (4.9) | 1–5 | 2.6 (1.0) | 21.3–69.5 | 49.8 (8.8) | 11.2–65.7 | 50.6 (10.1) |
| Primary care | 18–83 | 47.9 (16.8) | 0–30 | 10.7 (6.9) | N/A | N/A | N/A | N/A | N/A | N/A |
| Chronic pain | 18–86 | 41.0 (13.5) | 0–29 | 16.1 (6.5) | 1–5 | 4.0 (1.0) | 15.6–53.9 | 30.2 (8.2) | 16.9–64.4 | 37.9 (11.6) |
| ME/CFS | 28–72 | 50.3 (9.3) | 5–28 | 16.1 (5.4) | 2–5 | 4.1 (0.8) | 15.5–53.7 | 31.1 (9.9) | 15.0–56.1 | 38.6 (11.7) |
| LPS-injected | 20–47 | 27.4 (6.7) | 2–26 | 16.3 (7.1) | N/A | N/A | N/A | N/A | N/A | N/A |
N/A: RAND SF-36/12 questionnaire not available. Mean values of SicknessQ score in the primary care sample, healthy subjects and LPS-injected subjects have been reported previously.
(Andreasson et al., 2018, 2019). SD indicates standard deviation.
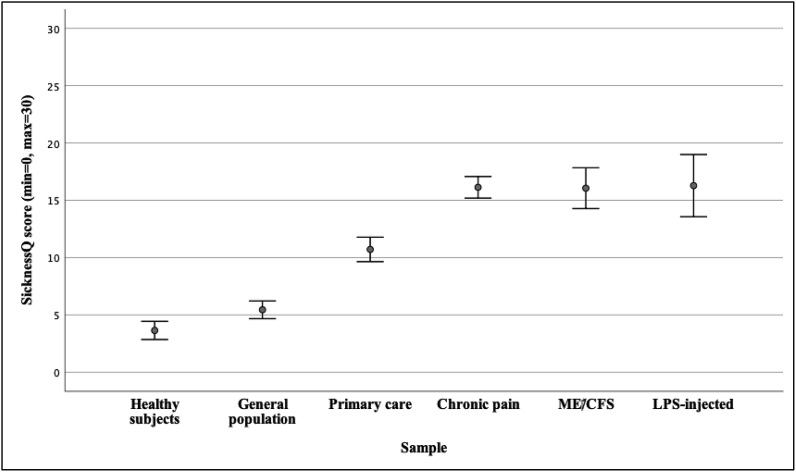
SicknessQ scores in all six samples are visually presented in Fig. 1. Mean values and standard deviations for each SicknessQ item for all groups are provided in as a supplementary table (Supplementary Table 1). Healthy subjects injected with LPS, patients with ME/CFS, patients with chronic pain, primary care patients and individuals from the general population reported significantly higher SicknessQ scores than healthy subjects (all p’s < 0.001, Table 1/Fig. 1). Healthy subjects injected with LPS, patients with ME/CFS, patients with chronic pain and primary care patients also reported significantly higher SicknessQ scores than individuals from the general population (all p’s < 0.001). In addition, healthy subjects injected with LPS, patients with ME/CFS and patients with chronic pain reported significantly higher SicknessQ scores than primary care patients (all p’s < 0.01). Healthy subjects injected with LPS, patients with ME/CFS and chronic pain reported similar levels of sickness behavior on the SicknessQ (all p’s > 0.05). In summary, patients with ME/CFS, patients with chronic pain and healthy LPS-injected subjects showed similarly high levels of sickness behavior, significantly higher than the other samples.
Fig. 1.
Means and confidence intervals (CI’s) for the SicknessQ score in each sample.
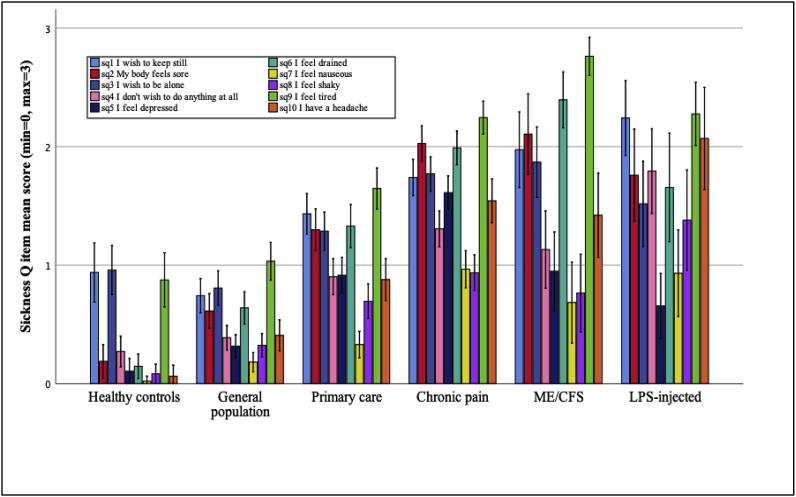
Analyses of individual items (see Fig. 2/Supplementary Table 1) showed the same pattern as for the total score where healthy subjects injected with LPS, chronic pain and ME/CFS patients showed significantly higher levels on all items compared to the general population sample and healthy subjects (all p’s < 0.05), except for item 5 (“I feel depressed”) where the LPS-injected sample did not differ significantly from the general population (p > .05). Patients with chronic pain reported significantly higher scores on item 5 (“I feel depressed”) than LPS-injected subjects (p < .001). Patients with ME/CFS reported significantly higher scores than LPS-injected subjects on item 6 (“I feel drained”) and item 9 (“I feel tired”; p’s < 0.01) (Table 2). ME/CFS patients reported significantly higher levels on item 1 (“I want to keep still”), item 6 (“I feel drained”) and item 9 (“I feel tired”) (p’s < 0.05) than patients with chronic pain, while patients with chronic pain reported a significantly higher mean score on item 5 (“I feel depressed”; p < .001) than patients with ME/CFS. In summary, patients with ME/CFS, patients with chronic pain and LPS-injected subjects showed similar sickness behavior profiles, with the exception of patients with ME/CFS showing significantly higher levels on items related to fatigue, and patients with chronic pain showing significantly higher levels on the item related to depression.
Fig. 2.
SicknessQ profile in each sample (item-by-item Means and CI’s).
Table 2.
Pearson correlations for SicknessQ and self-rated health, physical and mental health-related functioning health in the general population sample and patients with chronic pain and ME/CFS.
| Sample | Self-rated health | SF-36 Physical | SF-36 Mental |
|---|---|---|---|
| General population | .58** | -.40** | -.64** |
| Chronic pain | .39** | -.22** | -.63** |
| ME/CFS | -.08 | -.22 | -.27 |
**p < .01 (2-tailed).
*p < .05 (2-tailed).
Pearson correlations for the associations between sickness behavior and self-rated health as well as physical and mental health-related functioning are presented in Table 2. Higher levels of sickness behavior were significantly associated with worse self-rated health in the general population and chronic pain sample, while the association was not statistically significant in the ME/CFS sample. In the general population, higher levels of sickness behavior were statistically significantly associated with lower levels of both physical and mental health-related functioning. This was also seen in the chronic pain sample. In the ME/CFS sample, associations between sickness behavior and health-related functioning were not statistically significant.
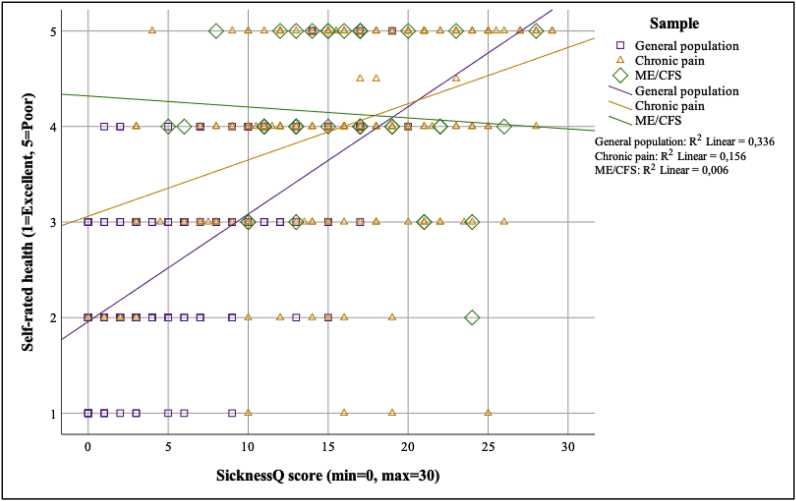
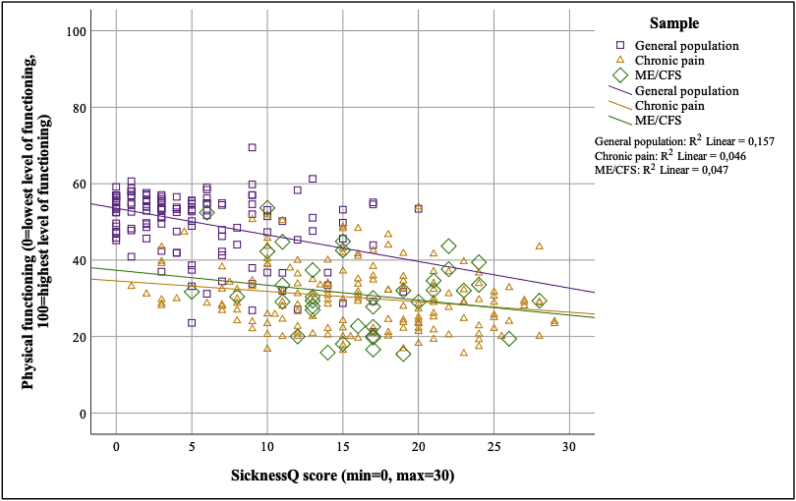
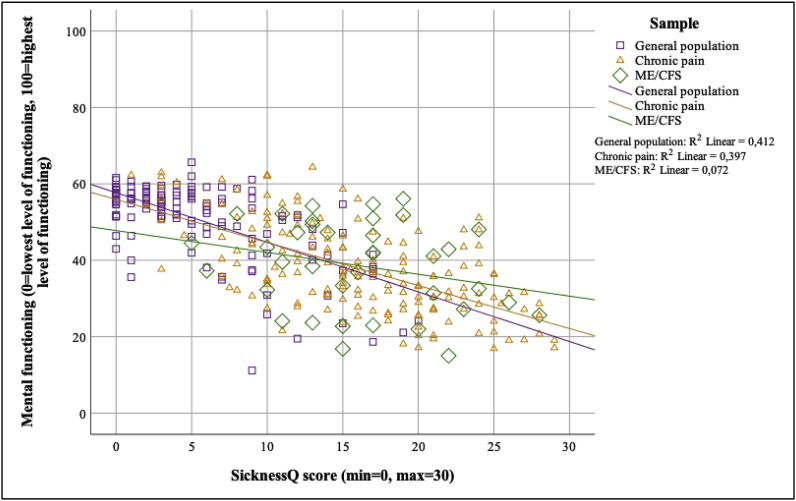
Moderated regression analyses for these associations are presented in Fig. 3, Fig. 4, Fig. 5. The strength of the associations between the SicknessQ and self-rated health was significantly weaker in the chronic pain and ME/CFS samples compared to the general population (p < .01; see Fig. 3, Fig. 4, Fig. 5 for regression slopes). The strength of the associations between the SicknessQ and the health-related physical functioning composite score were significantly weaker in chronic pain and ME/CFS compared to the general population (all p’s < 0.05). The strength of the association between the SicknessQ and the health-related mental functioning composite score were significantly weaker in ME/CFS compared to the general population (p’s < 0.05).
Fig. 3.
Associations between SicknessQ and self-rated health in the general population sample and patients with chronic pain and ME/CFS.
Fig. 4.
Associations between SicknessQ and physical health-related functioning in each sample.
Fig. 5.
Associations between SicknessQ and mental health-related functioning in each sample.
4. Discussion
The aim of the present study was to investigate the level of sickness behavior in patients with ME/CFS and chronic pain. Patients with ME/CFS and patients with chronic pain reported similar levels of sickness behavior which were significantly higher than the level in clinical and non-clinical reference groups, and equal to the level reported in experimental inflammation. In addition, we wanted to explore the associations between sickness behavior and self-rated health as well as physical and mental health-related functioning in these groups. These associations were stronger in the general population than in the chronic pain and ME/CFS patient groups.
The high levels of sickness behavior in ME/CFS and chronic pain compared to the reference groups are noteworthy. The individuals in the primary care patient sample, where the most frequent causes for seeking care were acute infection, muscle pain and airway symptoms, presumably experienced themselves as sick enough for motivating a healthcare visit. In addition, the levels of sickness behavior reported in the patients with ME/CFS and chronic pain were similar to the level of sickness behavior reported by healthy individuals at peak immune response after receiving an injection of LPS, a substance effective at activating the immune system and commonly used to investigate sickness behavior experimentally (Andreasson et al., 2018, 2019). With regards to the second aim, the levels of specific sickness behavior responses analyzed item-by-item on the SicknessQ showed similar patterns for LPS-injected individuals, chronic pain and ME/CFS samples. As such, the pattern of the differentiating levels of sickness behavior follows a gradient in the included samples, from very low levels in healthy subjects to the high and similar level in patients with ME/CFS, patients with chronic pain and LPS-injected healthy subjects. This was not expected as LPS-induced inflammation results in much higher levels of inflammatory markers compared to the levels reported in ME/CFS and chronic pain (Koch et al., 2007; Blundell et al., 2015; Schedlowski et al., 2014). One possible explanation for this similarity in subjective sickness behavior between chronic and acute conditions could be that in ME/CFS and chronic pain, vulnerability factors in combination with repeated hits on the homeostatic systems by immunological and/or psychological stressors have resulted in a dysregulated sickness behavior circuitry with a persistent low-grade pro-inflammatory state. However, there is a need for research investigating the longitudinal relationship between inflammatory markers implicated in sickness behavior and subjective sickness behavior in ME/CFS and chronic pain, and understanding the importance of such factors for symptom development and functioning.
Regarding the associations between sickness behavior and measures of health and functioning, sickness behavior as assessed with the SicknessQ was consistently related to self-rated health and health-related physical and mental functioning in individuals from the general population and patients with chronic pain, but not in the ME/CFS patients. This might be due to the plausibly persistent high level of sickness behavior in this group which could affect variability and associations. Furthermore, although data in the present study was cross-sectional, given the plausible persistent high level of sickness behavior in ME/CFS and chronic pain, these patients may have developed coping strategies over time in order to reduce the influence of sickness behavior on perceived health and functioning. Importantly, the core symptom burden alone would account for the low levels of functioning and quality of life in these groups (Breivik et al., 2006; Falk Hvidberg et al., 2015). As such, the investigation of immune-neuro-behavior interactions may add to the current knowledgebase regarding etiology and pathophysiological mechanisms in these conditions. Based on the high level of sickness behavior reported in these groups, we suggest the further study of sickness behavior and its proposed mechanisms in the chronic pain and ME/CFS populations, where longstanding alterations in the sickness behavior circuitry may be of importance for symptom development, functioning and quality of life in subsets of patients. Recent preliminary research results indicate that the immune-to-brain communication is of importance for chronic pain and ME/CFS, where sickness behavior processes on both behavioral and biological levels may affect symptoms and treatment response (Karshikoff et al., 2017; Milrad et al., 2017, 2018; Lattie et al., 2012; Lasselin et al., 2016b). More so, until pathophysiological mechanisms are accessible for targeted treatment, the development of treatments that include behavioral strategies for managing longstanding sickness behavior responses in more effective ways seem to be of importance. For chronic pain, Acceptance and Commitment Therapy (ACT) shows strong research support (Hughes et al., 2017; Society of Clinical Psychology APADivision 12). ACT-treatment also shows preliminary but promising results in improving functioning and quality of life in ME/CFS (Jonsjö et al., 2019; Jacobsen et al., 2017; Densham et al., 2016). Potentially, these treatments may be targeted at more general sickness symptoms, in addition to the main symptom pertaining to the two respective diagnoses.
Some limitations in this study should be considered when interpreting the findings. First, the cross-sectional design excludes causal interpretations of the effects of sickness behavior on health-related functioning and self-rated health. Second, the ME/CFS sample size was small, potentially affecting the non-significant association between sickness behavior and self-rated health as well as functioning in this group, as for example the association for physical functioning was similar in strength to the chronic pain sample. The sample size may also explain the poorer fit of the CFA for the ME/CFS sample, hence further study of the SicknessQ in larger samples from the ME/CFS population is needed.
In conclusion, patients with ME/CFS and chronic pain report high levels of sickness behavior symptoms compared to healthy subjects, individuals from the general population and primary care patients. Furthermore, the level of sickness behavior reported in ME/CFS and chronic pain compares to the level of sickness behavior reported during an acute and strong immune activation using bacterial endotoxin. Interventions targeted at reducing the impact of sickness behavior in addition to the core symptoms should be explored in ME/CFS and chronic pain, although the association between sickness behavior and health-related functioning was weaker in these groups compared to the general population. Thus, the present findings warrant further investigation of the sickness behavior circuitry in chronic pain and ME/CFS, in order to develop our knowledge of pathophysiological mechanisms and more effective treatments.
Funding
The LPS-study was supported by grants from Osher Center for Integrative Medicine and Center for Allergy Research at Karolinska Institutet, the Swedish Society of Medicine, Hedlund Foundation, the Swedish Heart Lung Foundation, Swedish Asthma and Allergy Association, the Swedish Research Council, Stockholm Stress Center and the Swedish Council for Working Life and Social Research. The ME/CFS study was supported by the research fund (Risk Hälsa) at Skandia Insurance Company, Ltd, Sweden. BK is supported by the Swedish Research Council.
Author contributions statement
MJ and AA designed the study. MJ, AA and MPJ carried out the statistical analyses, and JÅ contributed to data analyses. MJ and AA wrote the first draft of the manuscript. JÅ contributed to the first draft. MJ, AA, MK, BK, KL, LA, GLO collected the data. JÅ, MK, MPJ, BK, KL, LH, LA, RW, JA, ML and GLO commented on the manuscript. All the authors contributed to and have approved the final manuscript.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
We thank the following colleagues for help with data collection: Healthy subjects/LPS sample: T. Bachrach, J. Rehman, H. Vergoossen, T. Sundelin, M. Kramar, K. Sahlander, Y. Österman, G. de Forest, A. Ingvar, M. Dahl, W. Osika and W. Johnen; ME/CFS sample: S. Höglund, J. Larsson; Primary care sample: S. Kennerhed, M. Bergström; General population sample: A. Forsberg, B. Wallner, L. Kjellström, PM. Hellström.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2019.100028.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Agreus L., Hellstrom P.M., Talley N.J., Wallner B., Forsberg A., Vieth M. Towards a healthy stomach? Helicobacter pylori prevalence has dramatically decreased over 23 years in adults in a Swedish community. United Eur. Gastroenterol. J. 2016;4(5):686–696. doi: 10.1177/2050640615623369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasson A., Wicksell R.K., Lodin K., Karshikoff B., Axelsson J., Lekander M. A global measure of sickness behaviour: development of the Sickness Questionnaire. J. Health Psychol. 2018;23(11):1452–1463. doi: 10.1177/1359105316659917. [DOI] [PubMed] [Google Scholar]
- Andreasson A., Karshikoff B., Lidberg L., Akerstedt T., Ingvar M., Olgart Hoglund C. The effect of a transient immune activation on subjective health perception in two placebo controlled randomised experiments. PLoS One. 2019;14(3):e0212313. doi: 10.1371/journal.pone.0212313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell S., Ray K.K., Buckland M., White P.D. Chronic fatigue syndrome and circulating cytokines: a systematic review. Brain Behav. Immun. 2015;50:186–195. doi: 10.1016/j.bbi.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Breivik H., Collett B., Ventafridda V., Cohen R., Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur. J. Pain. 2006;10(4):287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Capuron L., Miller A.H. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol. Ther. 2011;130(2):226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers B.M., van de Sande M.I., De Meirleir K.L., Klimas N.G., Broderick G., Mitchell T. Myalgic encephalomyelitis: international Consensus criteria. J. Intern. Med. 2011;270(4):327–338. doi: 10.1111/j.1365-2796.2011.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Mello C., Swain M.G. Immune-to-Brain communication pathways in inflammation-associated sickness and depression. Curr. Top Behav. Neurosci. 2017;31:73–94. doi: 10.1007/7854_2016_37. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur. J. Pharmacol. 2004;500(1–3):399–411. doi: 10.1016/j.ejphar.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Neuroimmune interactions: from the brain to the immune system and vice versa. Physiol. Rev. 2018;98(1):477–504. doi: 10.1152/physrev.00039.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R., Kelley K.W. Twenty years of research on cytokine-induced sickness behavior. Brain Behav. Immun. 2007;21(2):153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R., O’Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport T.E., Stevens S.R., Baroni K., Van Ness M., Snell C.R. Diagnostic accuracy of symptoms characterising chronic fatigue syndrome. Disabil. Rehabil. 2011;33(19–20):1768–1775. doi: 10.3109/09638288.2010.546936. [DOI] [PubMed] [Google Scholar]
- Densham S., Williams D., Johnson A., Turner-Cobb J.M. Enhanced psychological flexibility and improved quality of life in chronic fatigue syndrome/myalgic encephalomyelitis. J. Psychosom. Res. 2016;88:42–47. doi: 10.1016/j.jpsychores.2016.07.009. [DOI] [PubMed] [Google Scholar]
- Falk Hvidberg M., Brinth L.S., Olesen A.V., Petersen K.D., Ehlers L. The health-related quality of life for patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) PLoS One. 2015;10(7):e0132421. doi: 10.1371/journal.pone.0132421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K., Straus S.E., Hickie I., Sharpe M.C., Dobbins J.G., Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann. Intern. Med. 1994;121(12):953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- Gandek B., Ware J.E., Aaronson N.K., Apolone G., Bjorner J.B., Brazier J.E. Cross-validation of item selection and scoring for the SF-12 health survey in nine countries: results from the IQOLA project. International quality of life assessment. J. Clin. Epidemiol. 1998;51(11):1171–1178. doi: 10.1016/s0895-4356(98)00109-7. [DOI] [PubMed] [Google Scholar]
- Harrison N.A., Brydon L., Walker C., Gray M.A., Steptoe A., Critchley H.D. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol. Psychiatry. 2009;66(5):407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison N.A., Voon V., Cercignani M., Cooper E.A., Pessiglione M., Critchley H.D. A neurocomputational account of how inflammation enhances sensitivity to punishments versus rewards. Biol. Psychiatry. 2016;80(1):73–81. doi: 10.1016/j.biopsych.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart B.L. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 1988;12(2):123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Hart B.L. Behavioral adaptations to pathogens and parasites: five strategies. Neurosci. Biobehav. Rev. 1990;14(3):273–294. doi: 10.1016/s0149-7634(05)80038-7. [DOI] [PubMed] [Google Scholar]
- Hughes L.S., Clark J., Colclough J.A., Dale E., McMillan D. Acceptance and commitment therapy (ACT) for chronic pain: a systematic review and meta-analyses. Clin. J. Pain. 2017;33(6):552–568. doi: 10.1097/AJP.0000000000000425. [DOI] [PubMed] [Google Scholar]
- Jacobsen H.B., Kallestad H., Landrø N.I., Borchgrevink P.C., Stiles T.C. Processes in acceptance and commitment therapy and the rehabilitation of chronic fatigue. Scand. J. Psychol. 2017;58(3):211–220. doi: 10.1111/sjop.12363. [DOI] [PubMed] [Google Scholar]
- Jonsjö M.A., Wicksell R.K., Holmström L., Andreasson A., Bileviciute-Ljungar I., Olsson G.L. Identifying symptom subgroups in patients with ME/CFS – relationships to functioning and quality of life. Fatigue: Biomed., Health Behav. 2017;5(1):33–42. [Google Scholar]
- Jonsjö M.A., Wicksell R.K., Holmström L., Andreasson A., Olsson G.L. Acceptance & commitment therapy for ME/CFS (chronic fatigue syndrome) – a feasibility study. J. Contextual Behav. Sci. 2019;12:89–97. [Google Scholar]
- Karshikoff B., Lekander M., Soop A., Lindstedt F., Ingvar M., Kosek E. Modality and sex differences in pain sensitivity during human endotoxemia. Brain Behav. Immun. 2015;46:35–43. doi: 10.1016/j.bbi.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Karshikoff B., Sundelin T., Lasselin J. Role of inflammation in human fatigue: relevance of multidimensional assessments and potential neuronal mechanisms. Front. Immunol. 2017;8:21. doi: 10.3389/fimmu.2017.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A., Zacharowski K., Boehm O., Stevens M., Lipfert P., von Giesen H.J. Nitric oxide and pro-inflammatory cytokines correlate with pain intensity in chronic pain patients. Inflamm. Res. 2007;56(1):32–37. doi: 10.1007/s00011-007-6088-4. [DOI] [PubMed] [Google Scholar]
- Lacourt T.E., Vichaya E.G., Chiu G.S., Dantzer R., Heijnen C.J. The high costs of low-grade inflammation: persistent fatigue as a consequence of reduced cellular-energy availability and non-adaptive energy expenditure. Front. Behav. Neurosci. 2018;12:78. doi: 10.3389/fnbeh.2018.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasselin J., Elsenbruch S., Lekander M., Axelsson J., Karshikoff B., Grigoleit J.S. Mood disturbance during experimental endotoxemia: predictors of state anxiety as a psychological component of sickness behavior. Brain Behav. Immun. 2016;57:30–37. doi: 10.1016/j.bbi.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Lasselin J., Kemani M.K., Kanstrup M., Olsson G.L., Axelsson J., Andreasson A. Low-grade inflammation may moderate the effect of behavioral treatment for chronic pain in adults. J. Behav. Med. 2016;39(5):916–924. doi: 10.1007/s10865-016-9769-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattie E.G., Antoni M.H., Fletcher M.A., Penedo F., Czaja S., Lopez C. Stress management skills, neuroimmune processes and fatigue levels in persons with chronic fatigue syndrome. Brain Behav. Immun. 2012;26(6):849–858. doi: 10.1016/j.bbi.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodin K., Lekander M., Petrovic P., Nilsonne G., Hedman-Lagerlöf E., Andreasson A. Cross-sectional associations between inflammation, sickness behaviour, health anxiety and self-rated health in a Swedish primary care population. Eur. J. Inflamm. 2019;17 [Google Scholar]
- McHorney C.A., Ware J.E., Jr., Raczek A.E. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med. Care. 1993;31(3):247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- Milrad S.F., Hall D.L., Jutagir D.R., Lattie E.G., Czaja S.J., Perdomo D.M. Depression, evening salivary cortisol and inflammation in chronic fatigue syndrome: a psychoneuroendocrinological structural regression model. Int. J. Psychophysiol. 2018;131:124–130. doi: 10.1016/j.ijpsycho.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milrad S.F., Hall D.L., Jutagir D.R., Lattie E.G., Ironson G.H., Wohlgemuth W. Poor sleep quality is associated with greater circulating pro-inflammatory cytokines and severity and frequency of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) symptoms in women. J. Neuroimmunol. 2017;303:43–50. doi: 10.1016/j.jneuroim.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moilanen E. Two faces of inflammation: an immunopharmacological view. Basic Clin. Pharmacol. Toxicol. 2014;114(1):2–6. doi: 10.1111/bcpt.12180. [DOI] [PubMed] [Google Scholar]
- Savitz J., Harrison N.A. Interoception and inflammation in psychiatric disorders. Biol. Psychiatr. Cogn. Neurosci. Neuroimag. 2018;3(6):514–524. doi: 10.1016/j.bpsc.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedlowski M., Engler H., Grigoleit J.S. Endotoxin-induced experimental systemic inflammation in humans: a model to disentangle immune-to-brain communication. Brain Behav. Immun. 2014;35:1–8. doi: 10.1016/j.bbi.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Schermelleh-Engel K., Moosbrugger H., Müller H. Evaluating the fit of structural equation models: tests of significance and descriptive goodness-of-fit measures. Methods Psychol. Res. 2003;8(2):23–74. [Google Scholar]
- Society of Clinical Psychology APA, Division 12 Acceptance and commitment therapy for chronic pain. http://www.div12.org/PsychologicalTreatments/treatments/chronicpain_act.html Retrieved from.
- Sullivan M., Karlsson J. The Swedish SF-36 Health Survey III. Evaluation of criterion-based validity: results from normative population. J. Clin. Epidemiol. 1998;51(11):1105–1113. doi: 10.1016/s0895-4356(98)00102-4. [DOI] [PubMed] [Google Scholar]
- Ware J., Jr., Kosinski M., Keller S.D. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med. Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.