Abstract
Patients with NSCLC in East Asia, including Japan, frequently contain EGFR mutations. In 2018, we published the latest full clinical practice guidelines on the basis of those provided by the Japanese Lung Cancer Society Guidelines Committee. The purpose of this study was to update those recommendations, especially for the treatment of metastatic or recurrent EGFR-mutated NSCLC. We conducted a literature search of systematic reviews of randomized controlled and nonrandomized trials published between 2018 and 2019 that multiple physicians had reviewed independently. On the basis of those studies and the advice from the Japanese Society of Lung Cancer Expert Panel, we developed updated guidelines according to the Grading of Recommendations, Assessment, Development, and Evaluation system. We also evaluated the benefits of overall and progression-free survival, end points, toxicities, and patients’ reported outcomes. For patients with NSCLC harboring EGFR-activating mutations, the use of EGFR tyrosine kinase inhibitors (EGFR TKIs), especially osimertinib, had the best recommendation as to first-line treatment. We also recommended the combination of EGFR TKI with other agents (platinum-based chemotherapy or antiangiogenic agents); however, it can lead to toxicity. In the presence of EGFR uncommon mutations, except for an exon 20 insertion, we also recommended the EGFR TKI treatment. However, we could not provide recommendations for the treatment of EGFR mutations with immune checkpoint inhibitors, including monotherapy, and its combination with cytotoxic chemotherapy, because of the limited evidence present in the literature. The 2020 Japanese Lung Cancer Society Guidelines can help community-based physicians to determine the most appropriate treatments and adequately provide medical care to their patients.
Keywords: Non–small cell lung cancer, Epidermal growth factor receptor, Systematic review, Guidelines
Introduction
Therapies for stage IV NSCLC are evolving day by day, and the progress achieved over the past decade has been impressive. Since 2010, the Japanese Society of Lung Cancer (JLCS) Guidelines Committee has provided treatment guidelines for community-based physicians to adequately treat their patients. Based on this, we published the latest full clinical practice guidelines in 2018.1 In this study, we conducted a literature search and systematic review from 2018 to 2019 to update these guidelines, specifically focusing on studies that involved mutations in the sequence encoding for EGFR, which is one of the most altered regions.
Materials and Methods
Process of Systematic Review
We conducted a systematic review on the basis of the Grading of Recommendations, Assessment, Development, and Evaluation system,2 to develop lung cancer medical treatment guidelines in Japan. The detailed collection of these reviews is described in the Supplementary Data. The recommendation (R) level was determined on the basis of the Grading of Recommendations, Assessment, Development, and Evaluation grid method3 and the votes from the JLCS Expert Panel, composed of individuals with various occupations, and whose conflicts of interest were strictly controlled according to the regulations of the JLCS.
Key Outcomes of Interest
We focused on the efficacy of overall survival (OS) and progression-free survival (PFS) as the key outcomes when assessing clinical questions (CQs). In addition, toxicity and patient-reported outcomes were evaluated according to the recommended level determined by the Expert Panel. Because of the difficulty in analyzing the cost-effectiveness in the Japanese universal health insurance system, the impact of treatment costs was not reflected in this set of guidelines. The voting results for each CQ provided by the Expert Panel are summarized in Supplementary Tables 4 to 11. The objectivity of the guidelines was also enhanced through public comments to the JLCS members in July 2020.
Results
Characteristics of the Identified Studies
Six nonrandomized and 18 randomized controlled trials (RCTs) summarized in Tables 1 and 2 met our criteria for study selection as they assessed CQs in patients with NSCLC harboring EGFR mutations. Besides the RCTs analyzed when we created the guidelines published in 2018,1 we included six new RCTs and updated the results of two RCTs. In addition, we recently analyzed two exploratory RCTs: (1) the combination therapy with an immune checkpoint inhibitor (ICI); and (2) cytotoxic chemotherapy targeting EGFR mutations (one including ALK fusions) (summarized in Table 3). In this review, three meta-analysis studies were performed on each CQ using multiple RCTs with the same design, and the resulting funnel plots are illustrated in Supplementary Figures 1 to 3. In contrast, for the analysis of the efficacy of ICI monotherapy, we adopted a high-quality meta-analysis already published.4 The quality assessments of all the included interventional studies are provided in Supplementary Tables 2 (for RCTs) and 3 (for nonrandomized trials). RCTs that were not approved by the Expert Panel were not considered in the development of the current recommendations.5, 6, 7, 8 One notable RCT was not approved because it was not possible to extract sufficient meta-analytical data from published literature.9
Table 1.
Characteristics of RCTs
| Study | Design | No. | Patient | Intervention | Comparison | Median Follow-Up (Updated) |
|---|---|---|---|---|---|---|
| Mitsudomi et al., 201010 (WJTOG3405) |
Phase 3 | 172 | NSCLC harboring EGFR mutation (19del or L858R), 75 y or younger, stage IIIB/IV or rec, ECOG PS 0–1, treatment-naive |
Gefitinib | Cisplatin plus docetaxel | 81 d (59.1 mo)11 |
| Maemondo et al., 201012 (NEJ002) |
Phase 3 | 228 | NSCLC harboring EGFR mutation, 75 y or younger, stage IIIB/IV or rec, ECOG PS 0–1, treatment-naive |
Gefitinib | Carboplatin plus paclitaxel | 527 d (704 d)13 |
| Zhou et al., 201114 (OPTIMAL) |
Phase 3 | 154 | NSCLC harboring EGFR mutation (19del or L858R), 18 y or older, stage IIIB/IV or rec, ECOG PS 0–2, treatment-naive |
Erlotinib | Carboplatin plus gemcitabine | 15.6 mo (25.9 mo)15 |
| Rosell et al., 201216 (EURTAC) |
Phase 3 | 174 | NSCLC harboring EGFR mutation (19del or L858R), 18 y or older, stage IIIB/IV, ECOG PS 0–2, treatment-naive |
Erlotinib | Platinum-doubleta | 18.9 mo (Not described)17 |
| Wu et al., 201518 (ENSURE) |
Phase 3 | 217 | NSCLC harboring EGFR mutation (19del or L858R), 18 y or older, stage IIIB/IV, ECOG PS 0–2, treatment-naive |
Erlotinib | Cisplatin plus gemcitabine | 27.1–28.9 mo |
| Sequist et al., 201319 (LUX-Lung 3) |
Phase 3 | 345 | Lung adenocarcinoma harboring EGFR mutation, 18 y or older, stage IIIB/IV, ECOG PS 0–1, treatment-naive |
Afatinib | Cisplatin plus pemetrexed | 16.4 mo (41 mo)21 |
| Wu et al., 201420 (LUX-Lung 6) |
Phase 3 | 364 | Lung adenocarcinoma harboring EGFR mutation, 18 y or older, stage IIIB/IV, ECOG PS 0–1, treatment-naive |
Afatinib | Cisplatin plus gemcitabine | 16.6 mo (33 mo)21 |
| Soria et al., 201825 (FLAURA) |
Phase 3 | 556 | NSCLC harboring EGFR mutation (19del or L858R), 18 y or older, locally advanced, or metastatic, ECOG PS 0–1, treatment-naive |
Osimertinib | Gefitinib or erlotinib | 15.0 mo (35.8 mo)26 |
| Hosomi et al., 202027 (NEJ009) |
Phase 3 | 345 | Non-SCC NSCLC harboring EGFR mutation (exons19, 21, or 18), 20 y or older, stage IIIB/IV or rec, ECOG PS 0–1, treatment-naive |
Gefitinib plus CBDCA/PEM | Gefitinib | 45 mo |
| Noronha et al., 202028 | Phase 3 | 350 | NSCLC harboring EGFR mutation (exons19, 21, or 18), 18 y or older, stage IIIB/IV, ECOG PS 0–2, treatment-naive | Gefitinib plus CBDCA/PEM | Gefitinib | 17 mo |
| Seto et al., 201429 (JO25567) |
Phase 2 | 152 | Non-SCC NSCLC harboring EGFR mutation (19del or L858R), 20 y or older, stage IIIB/IV or rec, ECOG PS 0–1, treatment-naive |
Erlotinib plus bevacizumab | Erlotinib | 20.4 mo (34.7 mo)30 |
| Saito et al., 201931 (NEJ026) |
Phase 3 | 224 | non-SCC NSCLC harboring EGFR mutation (19del or L858R), 20 y or older, stage IIIB/IV or rec, ECOG PS 0–2, treatment-naive |
Erlotinib plus bevacizumab | Erlotinib | 12.4 mo (39.2 mo)32 |
| Stinchcombe et al., 201933 | Phase 2 | 88 | NSCLC harboring EGFR mutation (19del or L858R), stage IV, ECOG PS 0–1, treatment-naive | Erlotinib plus bevacizumab | Erlotinib | 33 mo |
| Nakagawa et al., 201934 (RELAY) |
Phase 3 | 449 | NSCLC harboring EGFR mutation (19del or L858R), 18 y or older, stage IV, or rec, ECOG PS 0–1, treatment-naive |
Erlotinib plus ramucirumab | Erlotinib plus placebo | 20.7 mo |
| Wu et al., 201735 (ARCHER1050) |
Phase 3 | 452 | NSCLC harboring EGFR mutation (19del or L858R), 18 y or older, stage IIIB/IV, ECOG PS 0–1, treatment-naive, no brain metastases |
Dacomitinib | Gefitinib | 22.1 mo (31.3 mo)36 |
| Yang et al., 201737 (CTONG0901) |
Phase 3 | 256 | NSCLC harboring EGFR mutation (19del or L858R), 18 y or older, advanced, or metastatic, ECOG PS 0–2, EGFR TKI naive |
Erlotinib | Gefitinib | 22.1 mo |
| Park et al., 201638 (LUX-Lung 7) |
Phase 2 | 319 | Lung adenocarcinoma harboring EGFR mutation, 18 y or older, stage IIIB/IV, ECOG PS 0–1, treatment-naive |
Afatinib | Gefitinib | 27.3 mo |
| Mok et al., 201750 (AURA3) |
Phase 3 | 419 | NSCLC harboring EGFR T790M resistant mutation, 18 y or older, locally advanced, or metastatic, ECOG PS 0–2, after first-line EGFR TKI therapy |
Osimertinib | Platinum-doubletb | 8.3 mo |
19del, exon 19 deletion; CBDCA, carboplatin; ECOG, Eastern Cooperative Oncology Group; PEM, pemetrexed; PS, performance status; RCT, randomized controlled trial; rec, recurrent disease; SCC, squamous cell carcinoma; TKI, tyrosine kinase inhibitor.
Cisplatin or carboplatin plus docetaxel or gemcitabine.
Cisplatin or carboplatin plus pemetrexed.
Table 2.
Study Characteristics of Nonrandomized Trials
| Study | Design | No. | Patient | Intervention | Median Follow-Up, mo |
|---|---|---|---|---|---|
| Inoue et al., 200939 (NEJ001) |
Phase 2 | 30 | NSCLC harboring EGFR mutation (19del, L858R, L861Q, or G719X), stage IIIB/IV or rec, poor ECOG PS (20–74 y with PS 3–4, 75–79 y with PS 2–4, and 80 y or older with PS 1–4), treatment-naive |
Gefitinib | 17.8 |
| Maemondo et al., 201240 (NEJ003) |
Phase 2 | 31 | NSCLC harboring EGFR mutation (19del, L858R, L861Q, or G719X), stage IIIB/IV or rec, 75 y or older, ECOG PS 0–2, treatment-naive | Gefitinib | 27.5 |
| Yang et al., 201546 (LUX-lung 2,3,6) |
Pooled | 75 | Lung adenocarcinoma harboring EGFR uncommon mutation (group 1; point mutation or duplications in exon18–21, group 2; de novo T790M, group 3; exon 20 insertion), 18 y or older, stage IIIB/IV, ECOG PS 0–1, treatment-naive |
Afatinib | 34.7 |
| Cho et al., 202047 (KCSG-LU15-09) |
Phase 2 | 37 | NSCLC harboring EGFR mutation (other than 19del, L858R, T790M, or exon 20 insertions), metastatic or rec, 19 y or older, ECOG PS 0–2, EGFR TKI naive |
Osimertinib | 20.6 |
| Ramalingam et al., 201849 (AURA) |
Phase 1 | 60 | NSCLC harboring EGFR mutation (including T790M), locally advanced or metastatic, 18 y or older, WHO PS 0–1, treatment-naive | Osimertinib | 19.1 |
19del, exon 19 deletion; ECOG, Eastern Cooperative Oncology Group; PS, performance status; rec, recurrent disease; TKI, tyrosine kinase inhibitor.
Table 3.
Characteristics of RCTs in an Exploratory Analysis
| Study | Design | No. | Patient | Intervention | Comparison | Median Follow-Up, mo |
|---|---|---|---|---|---|---|
| Reck et al., 201953 (IMpower150) |
Phase 3 (exploratory) | 124 | Non-SCC NSCLC harboring EGFR mutation (19del or L858R), stage IV, 18 y or older, ECOG PS 0–1, disease progression or intolerance to treatment with at least one TKI | ABCPa | BCPb | 19.6 |
| West et al., 201954 (IMpower130) |
Phase 3 (exploratory) | 44 | Non-SCC NSCLC harboring EGFR mutation or ALK translocation, stage IV, 18 y or older, ECOG PS 0–1, disease progression, or intolerance to treatment with at least one TKI | Atezolizumab plus CBDCA/nabPTX | CBDCA/nabPTX | 18.5 |
19del, exon 19 deletion; CBDCA, carboplatin; ECOG, Eastern Cooperative Oncology Group; nabPTX, nab-paclitaxel; PS, performance status; PTX, paclitaxel; RCT, randomized controlled trial; TKI, tyrosine kinase inhibitor.
ABCP, Atezolizumab plus CBDCA/PTX/bevacizumab.
BCP, CBDCA/PTX/bevacizumab.
General Remarks
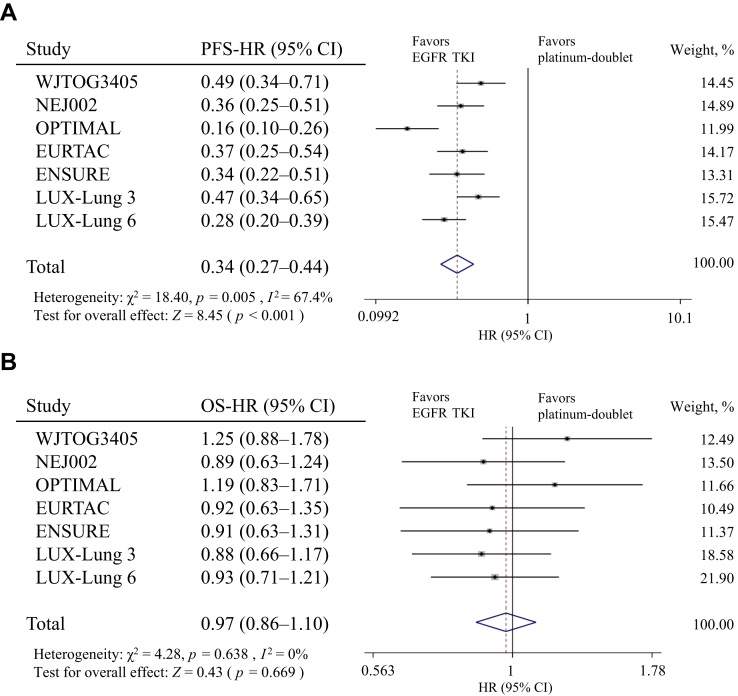
Seven phase 3 trials comparing EGFR tyrosine kinase inhibitors (TKIs) and platinum-doublet chemotherapy in patients with NSCLC harboring EGFR mutations were analyzed.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 Among them, EGFR TKI administration consistently prolonged PFS (as the primary end point) compared with that of platinum-doublet (pooled hazard ratio [HR]: 0.34; 95% confidence interval [CI]: 0.27–0.44; p < 0.001); however, a strong heterogeneity in the overall PFS effect across the seven trials was noted (χ2: 18.40; p = 0.005; I2: 67.4%) (Fig. 1A). On the contrary, the risk of death was not significantly heterogeneous (χ2: 4.28; p = 0.638; I2: 0%) (Fig. 1B) or different between EGFR TKI and platinum-doublet chemotherapy treatments (pooled HR: 0.97; 95% CI: 0.86–1.10; p = 0.669). In addition, in a large-scale observational study, no difference in the PFS when providing erlotinib as first- or third-line pharmacotherapy to patients with EGFR mutations were observed.22 On the basis of these results, the best sequence of EGFR TKI and cytotoxic chemotherapy treatments is still unclear. EGFR TKIs administration resulted in improved quality of life and less toxicity than those obtained with cytotoxic chemotherapy; however, the profiles and degrees of toxicities were different depending on each EGFR TKI.23,24
Figure 1.
The Forest plot of HR for (A) PFS and (B) OS of patients with NSCLC receiving EGFR TKI versus platinum-based chemotherapy treatments. CI, confidence interval; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; TKI, tyrosine kinase inhibitor.
On the basis of a comprehensive evaluation of these results, our guideline recommends the use of EGFR TKIs as the first-line pharmacotherapy in patients with NSCLC harboring EGFR mutations (particularly exon 19 deletion and L858R mutation).
Recommendations
EGFR-Activating Mutations (Exon 19 Deletion and L858R Mutation)
Good General Condition with Eastern Cooperative Oncology Group Performance Status 0 to 1
Regarding the question of what is the optimal first-line treatment for patients with Eastern Cooperative Oncology Group performance status (ECOG PS) 0 to 1 (CQ1), the following are the recommendations of the Expert Panel: (1) osimertinib is strongly recommended (R1, evidence level [EL] B, agreement rate [AR] 93%); (2) gefitinib plus carboplatin/pemetrexed is weakly recommended (R2, EL-A); (3) erlotinib plus bevacizumab or ramucirumab is weakly recommended (R2, EL-A); (4) dacomitinib is weakly recommended (R2, EL-B); and (5) gefitinib, erlotinib, or afatinib is weakly recommended (R2, EL-A). Because the comparison in the clinical trial included in (5) was platinum-doublet chemotherapy, the evaluation criteria for evidence differed from other items.
FLAURA was a phase 3 trial that compared osimertinib with first-generation EGFR TKIs (gefitinib or erlotinib) in patients with locally advanced or metastatic NSCLC harboring EGFR mutations (exon 19 deletion or L858R mutation). In this trial, the PFS (primary endpoint) was significantly prolonged in the osimertinib arm (HR: 0.46; 95% CI: 0.37–0.57; p < 0.001).25 The update, reported in 2019, reported that OS was also significantly improved (HR: 0.80; 95% CI: 0.64–1.00; p = 0.046). The subgroup analysis of OS indicated a slight interaction between race and type of EGFR mutations. The OS HRs for race were 1.00 (95% CI: 0.75–1.32) and 0.54 (95% CI: 0.38–0.77) in patients with Asian and non-Asian, respectively. In addition, the OS HRs for EGFR mutations were 0.68 (95% CI: 0.51–0.90) and 1.00 (95% CI: 0.71–1.40) in exon 19 deletion and L858R mutation, respectively.26 Regarding toxicity, osimertinib tended to have a milder skin rash and liver dysfunction than the first-generation EGFR TKIs.
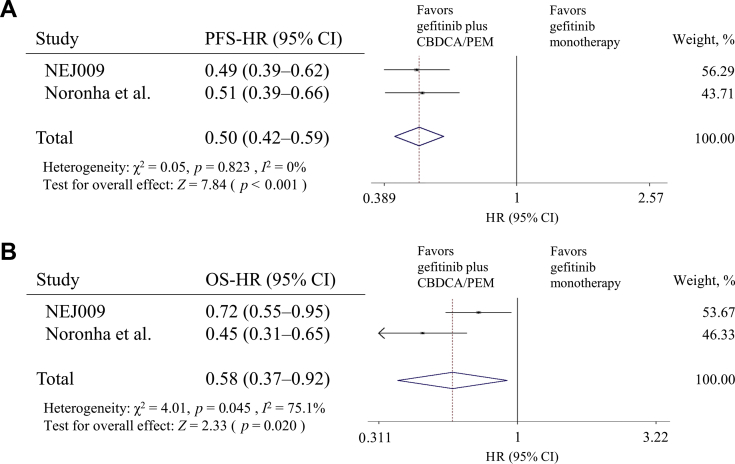
In 2020, two phase 3 trials (NEJ009 and Noronha et al.28) yielded improved PFS and OS when comparing the results of gefitinib and a combination of gefitinib and platinum-based chemotherapy (carboplatin plus pemetrexed) in patients with NSCLC harboring EGFR mutations.27,28 Both trials consistently yielded improved PFS (pooled HR: 0.50; 95% CI: 0.42–0.59; p < 0.001) (Fig. 2A) and OS (pooled HR: 0.58; 95% CI: 0.37–0.92; p = 0.020) (Fig. 2B). Adverse events (AEs) of grade 3 or worse were observed more frequently when combining gefitinib with cytotoxic chemotherapy, and their hematologic toxicities were particularly enhanced.
Figure 2.
Forest plot of HR for (A) PFS and (B) OS of patients with NSCLC receiving the combination of gefitinib and platinum-based chemotherapy (carboplatin plus pemetrexed) versus gefitinib monotherapy. CBDCA, carboplatin; CI, confidence interval; HR, hazard ratio; OS, overall survival; PEM, pemetrexed; PFS, progression-free survival.
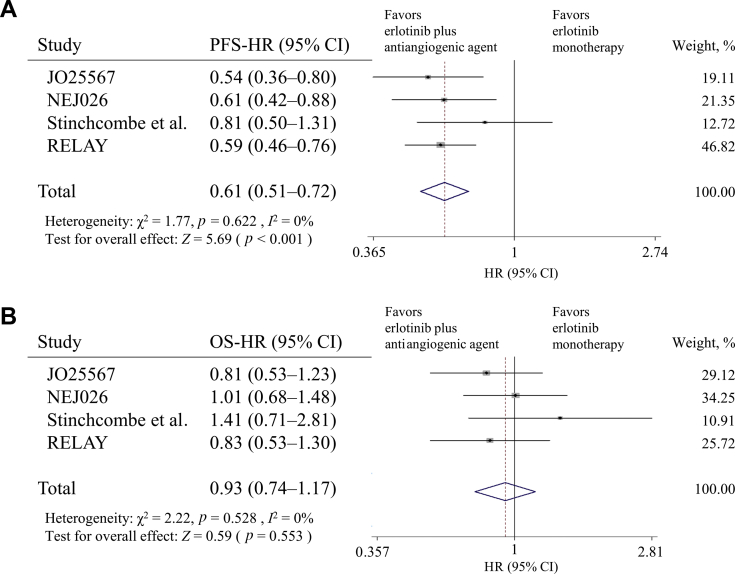
Four RCTs compared erlotinib plus antiangiogenic agents (bevacizumab or ramucirumab) and erlotinib monotherapy in patients with NSCLC harboring EGFR-activating mutations (exon 19 deletion and L858R mutation).29, 30, 31, 32, 33, 34 Most trials reported the superiority of the PFS with nonheterogeneity (χ2: 1.77; p = 0.622; I2: 0%), and the pooled efficacy was significant (pooled HR: 0.61; 95% CI: 0.51–0.72; p < 0.001) (Fig. 3A). However, the OS did not exhibit any obvious improvement (pooled HR: 0.93; 95% CI: 0.74–1.17; p = 0.528) (Fig. 3B). In the combination arm, the antiangiogenic drug-related toxicities, such as hypertension, proteinuria, and bleeding, were frequently observed.
Figure 3.
The forest plot of HR for (A) PFS and (B) OS of patients with NSCLC receiving the combination of erlotinib and antiangiogenic agent versus erlotinib monotherapy. CI, confidence interval; HR, hazard ratio; OS, overall survival; PFS, progression-free survival.
ARCHER1050 was a phase 3 trial that compared dacomitinib with gefitinib in patients with stage IIIB/IV NSCLC harboring EGFR mutations (exon 19 deletion or L858R mutation). In this trial, patients with brain metastases were excluded. In the dacomitinib arm, the PFS, as a primary end point, was significantly prolonged (HR: 0.59; 95% CI: 0.47–0.74; p < 0.0001).35 The OS also generally exhibited improved trends (HR: 0.760; 95% CI: 0.582–0.993); however, the original hypothesis was rejected by the gatekeeping procedure; therefore, the improvement was not significant.36 Regarding toxicity, dacomitinib was worse than gefitinib in inducing diarrhea, paronychia, and acne-like rash.
None of the other approved EGFR TKIs (gefitinib, erlotinib, and afatinib) exhibited a clear survival advantage. The study CTONG0901, which compared gefitinib and erlotinib, revealed similar survival effects.37 In a randomized phase 2 LUX-Lung 7 trial, which compared afatinib and gefitinib, the PFS was significantly prolonged in the afatinib arm. However, the improvement in OS in the afatinib arm was not significant, and the drug was more toxic than gefitinib.38
The votes of the Expert Panel in this CQ1 with multiple treatment strategies are presented in Supplementary Table 5. Because of insufficient evidence to directly compare the recommended regimens mentioned above, the Expert Panel evaluated them on the basis of balancing the benefits and harms of each regimen. Finally, our guideline concludes that osimertinib monotherapy is the most recommended among patients in good general condition. A combination of EGFR TKI and other agents is also recommended; however, its toxicity should be noted.
Moderate General Condition (ECOG PS 2)
Regarding the question of what is the optimal first-line treatment for patients with ECOG PS 2 (CQ2), the use of EGFR TKI (gefitinib or erlotinib) is strongly recommended (R1, EL-C, AR 86%). The use of gefitinib plus carboplatin/pemetrexed cannot be determined because of uncertain outcomes.
In two phase 3 trials comparing erlotinib with platinum-doublet chemotherapy, patients with EGFR-mutant NSCLC with PS 2 were enrolled at around 6% to 14% of the total population14,16,18 Both trials tended to prolong PFS in the erlotinib arm compared with the platinum-doublet arm, albeit in a small number of cases. Gefitinib was safe and effective against patients with poor PS with EGFR mutations in two nonrandomized phase 2 trials,39,40 whereas afatinib and dacomitinib have not been adequately and prospectively considered. The efficacy of osimertinib in patients with PS 2 has not been confirmed in a reliable study; however, its toxicity other than interstitial lung disease may be milder than that of gefitinib or erlotinib.25
In a phase 3 trial comparing gefitinib plus platinum-based chemotherapy (carboplatin/pemetrexed) and gefitinib monotherapy, patients with NSCLC with PS 2 were included in 21% to 22%.28 A subgroup analysis revealed that the combination arm improved the OS in patients with PS 2 compared with gefitinib monotherapy (HR: 0.57; 95% CI: 0.33–0.98). The evaluation of patients with PS 2 noted AEs greater than grade 3 to be more significantly frequent in the combination treatment than EGFR TKI monotherapy (58% versus 28%).41
The votes of the Expert Panel in this CQ2, including the two treatment strategies mentioned above, are presented in Supplementary Table 6. In the Expert Panel, the subgroup analysis against PS 2 by Noronha et al.28 was evaluated to have a high risk of bias because of the unclear concealment and incomplete outcome data. Therefore, our guideline recommends EGFR TKI (especially gefitinib, or erlotinib) monotherapy. In contrast, any recommendation for the combination of gefitinib and chemotherapy could not be determined by our consensus.
Poor General Condition (ECOG PS ≥3)
Regarding the question of what is the optimal first-line treatment for patients with poor general condition (CQ3), gefitinib is strongly recommended (R1, EL-C, AR 75%).
NEJ001 was a nonrandomized phase 2 trial that evaluated gefitinib monotherapy in patients harboring EGFR mutations with poor PS, which included 73% of patients with PS 3 to 4.39 In the PS 3 to 4 group, gefitinib exhibited favorable effects (overall response rate [ORR]: 66%; median PFS: 6.5 mo; median OS: 17.8 mo), improving PS in approximately 80% of this population. In contrast, two retrospective studies reported that poor PS might be a risk factor for the development of interstitial pneumonia because of gefitinib.42,43
The Expert Panel discussed the pros and cons of the treatment, especially for PS 4. Therefore, gefitinib was recommended in such a population as well. However, it is necessary to thoroughly evaluate whether EGFR TKIs could be expected to improve PS or symptoms.
EGFR Uncommon Mutations
Regarding the question on what is the optimal first-line treatment for patients harboring uncommon EGFR mutations with a good general condition (CQ4), the following are the recommendations: (1) EGFR TKIs are weakly recommended in patients harboring uncommon EGFR mutations, except for exon 20 insertion and de novo T790M mutations (R2, EL-C, AR 87%); (2) EGFR TKIs are strongly not recommended in patients harboring EGFR exon 20 insertion mutations (NR1, EL-C, AR 70%); and (3) osimertinib is weakly recommended in patients harboring de novo T790M mutations (R2, EL-D, AR 67%).
Except for EGFR-activating mutations (exon 19 deletion and L858R mutation), uncommon mutations in exons 18 to 21 are generally identified in about 10% of the cases.44 The efficacy of EGFR TKIs was slightly inferior in the presence of uncommon rather than activating mutations.45 Some of the above phase 3 trials excluded patients with these mutations.10,14,16,18 The uncommon mutation is not clearly defined. Therefore, in the present guideline, all mutations in the exons 18 to 21 regions, excluding exon 19 deletion and L858R mutation, are classified as uncommon mutations. The coexistence of tumors with common and uncommon mutations was classified as an uncommon mutation.
The efficacy of EGFR TKIs varied and depended on the type of these uncommon mutations; meanwhile, the ORR was reported as 48.4% in a retrospective analysis.45 In the pooled analysis of three prospective trials for uncommon mutations, excluding the T790M mutation and the exon 20 insertion, the use of afatinib resulted in an ORR of 71.1% and a median PFS of 10.7 months.46 In a phase 2 trial of osimertinib for the same patients, the ORR was 50%, and the median PFS was 8.2 months.47 Because these results had different frequencies and treatment efficacies of uncommon mutations, the Expert Panel concluded that the superiority of each EGFR TKI when treating patients with uncommon mutations, except for T790M and exon 20 insertion, should not be determined.
Exon 20 insertion is rare, and few retrospective studies have reported that the ORRs of EGFR TKIs were less than 10%.46,48 Thus, treatment with EGFR TKIs is not recommended as first-line pharmacotherapy.
The detection of de novo T790M mutations in treatment-naive patients who participated in clinical trials that used EGFR TKIs was extremely rare. In a phase 1 trial that tested osimertinib for treatment of patients with EGFR mutations, a partial response was observed in six of seven (86%) treatment-naive patients with NSCLC harboring de novo T790M mutations.49 Efficacy data were limited.
Regarding whether osimertinib is recommended as second-line treatment for patients harboring EGFR T790M–resistant mutation after the progression of EGFR TKIs (CQ5), the Expert Panel strongly recommended osimertinib (R1, EL-B, AR 100%).
AURA3 was a phase 3 trial that compared osimertinib and platinum plus pemetrexed treatments in patients with NSCLC who progressed after receiving a first- or second-generation EGFR TKIs and acquired T790M resistance mutations. The PFS medians, as the primary endpoint, was 10.1 months in the osimertinib arm and 4.4 months in the platinum plus pemetrexed arm, which were significant (HR: 0.30; 95% CI: 0.23–0.41; p < 0.001). In addition, the frequency of grade 3 AEs or greater was lower in the osimertinib rather than the platinum plus pemetrexed treatment group (6% versus 34%).50
Cytotoxic Chemotherapy
Regarding the question as to whether cytotoxic chemotherapy is recommended for patients harboring oncogenic driver alterations (CQ6), the Expert Panel concluded that this is strongly recommended (e.g. platinum-doublet) (R1, ELA, AR 100%).
The administration of kinase inhibitor was the best treatment for patients harboring oncogenic driver alterations; however, in the analyzed RCTs, most patients received cytotoxic chemotherapy. According to the post hoc analyses of RCTs, the prognosis of patients receiving cytotoxic chemotherapy was slightly better.11,13 The same tendency was observed in a Japanese real-world observational study.51 If the patient is resistant to osimertinib, or resistant to other EGFR TKIs without T790M-acquired mutation, cytotoxic chemotherapy, such as platinum-doublet, is recommended.
ICI Monotherapy
Regarding the question as to whether ICI monotherapy is recommended for patients harboring oncogenic driver alterations (CQ7), the Expert Panel states that recommendations cannot be determined because of uncertain outcomes; as such, no specific recommendations can be made (EL-B, AR 73%).
Most of the phase 3 trials that evaluated the efficacy of ICI monotherapy as a first-line treatment excluded patients harboring EGFR mutations and ALK fusions. A meta-analysis of RCTs that compared programmed cell death protein 1/programmed death-ligand 1 (PD-L1) inhibitors with docetaxel as a second-line treatment did not illustrate OS superiority in the EGFR-mutant subgroup (pooled HR: 1.11; 95% CI: 0.80–1.53; p = 0.54).4 A retrospective study reported that the ORR of programmed cell death protein 1/PD-L1 inhibitors in EGFR-mutant or ALK-positive patients was 3.6%.52 The Expert Panel determined that the efficacy of ICI monotherapy on oncogenic driver alterations might be inferior to that of each TKI or platinum-based chemotherapy. The administration of ICI as the first- or second-line treatment is not recommended, although administration as a later-line treatment could be considered. However, evidence supporting the above perspective remains insufficient.
Combination of ICIs and Cytotoxic Chemotherapy
Regarding the question as to whether the combination of ICI and cytotoxic chemotherapy recommended for patients harboring oncogenic driver alterations (CQ8), the Expert Panel states that recommendations cannot be determined because of uncertain outcomes; as such, no specific recommendations can be made (EL-C, AR 64%).
The exploratory analysis of a phase 3 trial (IMpower150) that evaluated the treatment of patients with NSCLC harboring EGFR mutations with PD-L1 inhibitor atezolizumab plus carboplatin/paclitaxel plus bevacizumab indicated that the addition of atezolizumab slightly improved the PFS (HR: 0.61; 95% CI: 0.36–1.03) and OS (HR: 0.61; 95% CI: 0.29–1.28).53 However, it should be noted that the number of cases evaluated was limited to 79 (out of which, exon 19 deletion and L858R mutation were 58). A phase 3 trial (IMpower130) that evaluated the efficacy of atezolizumab plus carboplatin/nab-paclitaxel also conducted an exploratory analysis of EGFR mutations and ALK fusions. According to the Kaplan-Meier curve of that trial, the addition of atezolizumab had a few advantages in PFS and OS.54 The Expert Panel pointed this out as a bias because of the nonstratification in subgroup analyses. As with CQ7, the evidence for CQ8 is still inadequate.
Discussion
This set of guidelines has unique features that are unlike that of the European Society for Medical Oncology and National Comprehensive Cancer Network guidelines.55, 56, 57 First, because of Japan’s universal health insurance system, the selection of treatment options was not affected by costs. Second, CQs were specially developed according to the patient’s condition, such as the older adults and those with poor PS. However, we will continue to make yearly revisions and provide recommendations that meet the needs in the actual clinical practice in Japan.
Limitations
The evidence reviewed in the guidelines included some limitations. First, few studies have directly compared the response to EGFR TKIs from different generations. Second, the information about factors that were harmful to patients between the studies, such as patient’s reported outcomes and treatment costs, was insufficient. In addition, the results obtained from a unique population, such as the one containing the uncommon EGFR mutation or patients with poor PS, relied on nonrandomized or observational studies. Therefore, the Expert Panel made consensus recommendations on the basis of limited evidence from the literature.
Acknowledgments
The authors thank Mr. Shinichi Abe, Ms. Mutsumi Yamazaki, Ms. Misako Kaji, and Ms. Mariko Henmi, for their significant contributions to the literature search. The authors also thank Ms. Mayumi Ito, Ms. Etsuko Yaguchi, Ms. Sachiko Arai, and Ms. Yoshiko Kudoh for their support as the secretariat of the Japanese Society of Lung Cancer.
Footnotes
Disclosure: Dr. Ninomiya reports receiving honoraria outside of the current work from AstraZeneca, Merck Sharp & Dohme, Bristol-Myers Squibb, Boehringer Ingelheim, Chugai Pharmaceutical, Eli Lilly, Taiho Pharmaceutical, Ono Pharmaceutical, Nippon Kayaku, and Kyowa Kirin. Dr. Teraoka reports receiving honoraria outside of the current work from Chugai Pharmaceutical, Novartis, AstraZeneca, Taiho Pharmaceutical, Ono Pharmaceutical, and Boehringer Ingelheim. Dr. Zenke reports receiving honoraria outside of the current work from AstraZeneca, Boehringer Ingelheim, Chugai Pharmaceutical, Eli Lilly, Merck Sharp & Dohme, Ono Pharmaceutical, Bristol-Myers Squibb, and Taiho Pharmaceutical; and grants outside of the current work from AstraZeneca, Merck Sharp & Dohme, and Merck Biopharma. Dr. Kenmotsu reports receiving honoraria outside of the current work from Chugai Pharmaceutical, Ono Pharmaceutical, Boehringer Ingelheim, Eli Lilly, Kyowa Kirin, Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Daiichi Sankyo, AstraZeneca, Pfizer, and Taiho Pharmaceutical; and grants outside of the current work from Chugai Pharmaceutical, Boehringer Ingelheim, Novartis, and AstraZeneca. Dr. Nakamura reports receiving grants outside of the current work from Bristol-Myers Squibb, Astellas, Taiho Pharmaceutical, Chugai Pharmaceutical, Eli Lilly, Merck Biopharma, and AstraZeneca. Dr. Okuma reports receiving honoraria outside of the current work from Chugai Pharmaceutical, Boehringer Ingelheim, AstraZeneca, Merck Sharp & Dohme, Ono Pharmaceutical, Bristol-Myers Squibb, Taiho Pharmaceutical, and Novartis; and grants outside of the current work from AbbVie and Chugai Pharmaceutical. Dr. Tamiya reports receiving honoraria outside of the current work from Chugai Pharmaceutical, AstraZeneca, Eli Lilly, Boehringer Ingelheim, Ono Pharmaceutical, Bristol-Myers Squibb, Merck Sharp & Dohme, Taiho Pharmaceutical, Pfizer, and Kissei; and grants outside of the current work from AstraZeneca, Ono Pharmaceutical, and Bristol-Myers Squibb. Dr. Nosaki reports receiving honoraria outside of the current work from AstraZeneca, Bristol-Myers Squibb, Chugai Pharmaceutical, Eli Lilly, Merck Sharp & Dohme, Nippon Kayaku, Novartis, Pfizer, and Taiho Pharmaceutical; and grants outside of the current work from Takeda Pharmaceutical. Dr. Morise reports receiving honoraria outside of the current work from Eli Lilly, Chugai Pharmaceutical, AstraZeneca, Ono Pharmaceutical, Pfizer, and Merck Sharp & Dohme; and grants outside of the current work from Boehringer Ingelheim and Eli Lilly. Dr. Aokage reports receiving honoraria outside of the current work from Mochida Pharmaceutical, Merck Sharp & Dohme, AstraZeneca, Johnson and Johnson, Taiho Pharmaceutical, Ono Pharmaceutical, Eli Lilly, Covidien, Bristol-Myers Squibb, Care-net, and Chugai Pharmaceutical; and grants outside of the current work from AstraZeneca and Merck Sharp & Dohme. Dr. Kozuki reports receiving honoraria outside of the current work from Chugai Pharmaceutical, AstraZeneca, Eli Lilly, Taiho Pharmaceutical, Bristol-Myers Squibb, Ono Pharmaceutical, Merck Sharp & Dohme, Pfizer, Kyowa Kirin, Boehringer Ingelheim, Nippon Kayaku, and Novartis; and grants outside of the current work from Chugai Pharmaceutical, AstraZeneca, Eli Lilly, Taiho Pharmaceutical, Bristol-Myers Squibb, Merck Sharp & Dohme, Kyowa Kirin, and Merck Biopharma. Dr. Sakamoto reports receiving honoraria outside of the current work from Chugai Pharmaceutical, Kyowa Kirin, AstraZeneca, Merck Sharp & Dohme, Merck Biopharma, Eli Lilly, and Ono Pharmaceutical. Dr. K. Tanaka reports receiving honoraria outside of the current work from AstraZeneca, Chugai Pharmaceutical, Eli Lilly, Ono Pharmaceutical, Taiho Pharmaceutical, Novartis, and AbbVie; and grants outside of the current work from Chugai Pharmaceutical, Boehringer Ingelheim, and Ono Pharmaceutical. Dr. H. Tanaka reports receiving honoraria outside of the current work from Boehringer Ingelheim, Chugai Pharmaceutical, and AstraZeneca. Dr. Tanizaki reports receiving honoraria outside of the current work from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai Pharmaceutical, Eli Lilly, and Taiho Pharmaceutical. Dr. Miura reports receiving honoraria outside of the current work from Chugai Pharmaceutical, Eli Lilly, Boehringer Ingelheim, Ono Pharmaceutical, AstraZeneca, and Merck Sharp & Dohme. Dr. Miyauchi reports receiving honoraria outside of the current work from AstraZeneca, Chugai Pharmaceutical, Ono Pharmaceutical, Boehringer Ingelheim, Taiho Pharmaceutical, Eli Lilly, Kyowa Kirin, Daiichi Sankyo, Merck Sharp & Dohme, Bristol-Myers Squibb, Novartis, and Merck Biopharma; and grants outside of the current work from Chugai Pharmaceutical, Ono Pharmaceutical, Boehringer Ingelheim, and Eli Lilly. Dr. Yamaguchi reports receiving honoraria outside of the current work from Ono Pharmaceutical, Bristol-Myers Squibb, Chugai Pharmaceutical, AstraZeneca, Merck Sharp & Dohme, Eli Lilly, and Taiho Pharmaceutical. Dr. Goto reports receiving honoraria and grant outside of the current work from Taiho Pharmaceutical. Dr. Sasaki reports receiving honoraria outside of the current work from Pfizer, Boehringer Ingelheim, Chugai Pharmaceutical, Merck Sharp & Dohme, Ono Pharmaceutical, AstraZeneca, Eli Lilly, and Novartis; and grants outside of the current work from Pfizer and Boehringer Ingelheim. Dr. Daga reports receiving honoraria outside of the current work from Chugai Pharmaceutical, Merck Sharp & Dohme, Taiho Pharmaceutical, and Ono Pharmaceutical; and grants outside of the current work from Chugai Pharmaceutical, AstraZeneca, and Pfizer. Dr. Morita reports receiving honoraria outside of the current work from AstraZeneca, Bristol-Myers Squibb, Chugai Pharmaceutical, Eli Lilly, Merck Sharp & Dohme, Pfizer, Taiho Pharmaceutical, Boehringer Ingelheim, and Ono Pharmaceutical; and grants outside of the current work from Boehringer Ingelheim and Ono Pharmaceutical. Dr. Imamura reports receiving honoraria outside of the current work from Chugai Pharmaceutical and Ono Pharmaceutical, and grants outside of the current work from Otsuka Pharmaceutical. Dr. Suzuki reports receiving honoraria outside of the current work from Taiho Pharmaceutical and Takeda Pharmaceutical. Dr. Oizumi reports receiving honoraria outside of the current work from AstraZeneca, Bristol-Myers Squibb, Chugai Pharmaceutical, Eli Lilly, Pfizer, Ono Pharmaceutical, Merck Biopharma, Merck Sharp & Dohme, Takeda Pharmaceutical, and Taiho Pharmaceutical; and grants outside of the current work from AstraZeneca AbbVie, Bristol-Myers Squibb, Chugai Pharmaceutical, Pfizer, Kissei, Kyowa Kirin, Ono Pharmaceutical, Merck Biopharma, Takeda Pharmaceutical, and Taiho Pharmaceutical. Dr. Hida reports receiving honoraria outside of the current work from Chugai Pharmaceutical, Ono Pharmaceutical, AstraZeneca, Novartis, Bristol-Myers Squibb, Merck Sharp & Dohme, Boehringer Ingelheim, Kissei, Taiho Pharmaceutical, Pfizer, Takeda Pharmaceutical, and Merck Biopharma; and grants outside of the current work from Chugai Pharmaceutical, Ono Pharmaceutical, AstraZeneca, Novartis, Bristol-Myers Squibb, Merck Sharp & Dohme, Boehringer Ingelheim, Kissei, Taiho Pharmaceutical, Pfizer, Takeda Pharmaceutical, Merck Biopharma, AbbVie, Daiichi Sankyo, Astellas, and Janssen Pharmaceutical. Dr. Hotta reports honoraria outside of the current work from Pfizer, Eli Lilly, AstraZeneca, Bristol-Myers Squibb, Ono Pharmaceutical, Merck Sharp & Dohme, Chugai Pharmaceutical, Nippon Kayaku, Taiho Pharmaceutical, Boehringer Ingelheim, Novartis, Daiichi Sankyo, and Kyorin Pharmaceutical; and grants outside of the current work from Eli Lilly, AstraZeneca, Bristol-Myers Squibb, Merck Sharp & Dohme, Chugai Pharmaceutical, and Astellas. Dr. Takiguchi reports receiving honoraria outside of the current work from Eli Lilly, Chugai Pharmaceutical, Merck Sharp & Dohme, Taiho Pharmaceutical, Novartis, Boehringer Ingelheim, AstraZeneca, Ono Pharmaceutical, and Chugai Pharmaceutical; and grants outside of the current work from Eli Lilly, Chugai Pharmaceutical, Merck Sharp & Dohme, Takeda Pharmaceutical, Taiho Pharmaceutical, Daiichi Sankyo, Novartis, Kyowa Kirin, Boehringer Ingelheim, Ono Pharmaceutical, and Chugai Pharmaceutical. The remaining authors declare no conflict of interest.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2020.100107.
Supplementary Data
References
- 1.Akamatsu H., Ninomiya K., Kenmotsu H. Japanese Lung Cancer Society Guideline for non-small cell lung cancer, stage IV. Int J Clin Oncol. 2019;24:731–770. doi: 10.1007/s10147-019-01431-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GRADE working group. http://www.gradeworkinggroup.org/ Accessed October 8, 2020.
- 3.Jaeschke R., Guyatt G.H., Dellinger P. Use of the GRADE grid to reach decisions on clinical practice guidelines when consensus is elusive. BMJ. 2008;337:a744. doi: 10.1136/bmj.a744. [DOI] [PubMed] [Google Scholar]
- 4.Lee C.K., Man J., Lord S. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non-small cell lung carcinoma: a systematic review and meta-analysis. JAMA Oncol. 2018;4:210–216. doi: 10.1001/jamaoncol.2017.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nie K., Zhang Z., Zhang C. Osimertinib compared docetaxel-bevacizumab as a third-line treatment in EGFR T790M mutated non-small-cell lung cancer. Lung Cancer. 2018;121:5–11. doi: 10.1016/j.lungcan.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Xu L., Qi Q., Zhang Y., Cui J., Liu R., Li Y. Combination of icotinib and chemotherapy as first-line treatment for advanced lung adenocarcinoma in patients with sensitive EGFR mutations: a randomized controlled study. Lung Cancer. 2019;133:23–31. doi: 10.1016/j.lungcan.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Arrieta O., Barrón F., Padilla M.S. Effect of metformin plus tyrosine kinase inhibitors compared with tyrosine kinase inhibitors alone in patients with epidermal growth factor receptor-mutated lung adenocarcinoma: a phase 2 randomized clinical trial. JAMA Oncol. 2019;5 doi: 10.1001/jamaoncol.2019.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Q., Wu Y.L., Cheng Y. CTONG 1509: phase 3 study of bevacizumab with or without erlotinib in untreated Chinese patients with advanced EGFR-mutated NSCLC. Ann Oncol. 2019;30(suppl 5):v602–v660. [Google Scholar]
- 9.Shi Y.K., Wang L., Han B.H. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28:2443–2450. doi: 10.1093/annonc/mdx359. [DOI] [PubMed] [Google Scholar]
- 10.Mitsudomi T., Morita S., Yatabe Y. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harboring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomized phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 11.Yoshioka H., Shimokawa M., Seto T. Final overall survival results of WJTOG3405, a randomized phase III trial comparing gefitinib versus cisplatin with docetaxel as the first-line treatment for patients with stage IIIB/IV or postoperative recurrent EGFR mutation-positive non-small-cell lung cancer. Ann Oncol. 2019;30:1978–1984. doi: 10.1093/annonc/mdz399. [DOI] [PubMed] [Google Scholar]
- 12.Maemondo M., Inoue A., Kobayashi K. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 13.Inoue A., Kobayashi K., Maemondo M. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naïve non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002) Ann Oncol. 2013;24:54–59. doi: 10.1093/annonc/mds214. [DOI] [PubMed] [Google Scholar]
- 14.Zhou C., Wu Y.L., Chen G. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicenter, open-label, randomized, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 15.Zhou C., Wu Y.L., Chen G. Final overall survival results from a randomized, phase III study of erlotinib versus chemotherapy as first-line treatment for EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802) Ann Oncol. 2015;26:1877–1883. doi: 10.1093/annonc/mdv276. [DOI] [PubMed] [Google Scholar]
- 16.Rosell R., Carcereny E., Gervais R. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicenter, open-label, randomized phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 17.Leon L.F., Golsorkhi A., Liu S. Overall survival analyses of first–line erlotinib versus chemotherapy in the EURTAC study population controlling for the use of post-study therapy. Ann Oncol. 2014;25:iv447. [Google Scholar]
- 18.Wu Y.L., Zhou C., Liam C.K. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol. 2015;26:1883–1889. doi: 10.1093/annonc/mdv270. [DOI] [PubMed] [Google Scholar]
- 19.Sequist L.V., Yang J.C., Yamamoto N. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y.L., Zhou C., Hu C.P. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harboring EGFR mutations (LUX-Lung 6): an open-label, randomized phase 3 trial. Lancet Oncol. 2014;15:213–222. doi: 10.1016/S1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]
- 21.Yang J.C., Wu Y.L., Schuler M. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomized, phase 3 trials. Lancet Oncol. 2015;16:141–151. doi: 10.1016/S1470-2045(14)71173-8. [DOI] [PubMed] [Google Scholar]
- 22.Rosell R., Moran T., Queralt C. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 23.Oizumi S., Kobayashi K., Inoue A. Quality of life with gefitinib in patients with EGFR-mutated non-small cell lung cancer: quality of life analysis of the North East Japan Study Group 002 Trial. Oncologist. 2012;17:863–870. doi: 10.1634/theoncologist.2011-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Y.L., Hirsh V., Sequist L.V. Does EGFR mutation type influence patient-reported outcomes in patients with advanced EGFR mutation-positive non-small-cell lung cancer? Analysis of two large, phase III studies comparing afatinib with chemotherapy (LUX-Lung 3 and LUX-Lung 6) Patient. 2018;11:131–141. doi: 10.1007/s40271-017-0287-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soria J.C., Ohe Y., Vansteenkiste J. Osimertinib in untreated EGFR-mutated advanced non-small cell lung cancer. N Engl J Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 26.Ramalingam S.S., Vansteenkiste J., Planchard D. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382:41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 27.Noronha V., Patil V.M., Joshi A. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. 2020;38:124–136. doi: 10.1200/JCO.19.01154. [DOI] [PubMed] [Google Scholar]
- 28.Hosomi Y., Morita S., Sugawara S. Gefitinib alone versus gefitinib plus chemotherapy for non-small-cell lung cancer with mutated epidermal growth factor receptor: NEJ009 study. J Clin Oncol. 2020;38:115–123. doi: 10.1200/JCO.19.01488. [DOI] [PubMed] [Google Scholar]
- 29.Seto T., Kato T., Nishio M. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harboring EGFR mutations (JO25567): an open-label, randomized, multicenter, phase 2 study. Lancet Oncol. 2014;15:1236–1244. doi: 10.1016/S1470-2045(14)70381-X. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto N., Seto T., Nishio M. Erlotinib plus bevacizumab (EB) versus erlotinib alone (E) as first-line treatment for advanced EGFR mutation-positive non-squamous non–small-cell lung cancer (NSCLC): survival follow-up results of JO25567. J Clin Oncol. 2018;36(suppl 15) doi: 10.1016/j.lungcan.2020.11.020. 9007–9007. [DOI] [PubMed] [Google Scholar]
- 31.Saito H., Fukuhara T., Furuya N. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomized, multicenter, phase 3 trial. Lancet Oncol. 2019;20:625–635. doi: 10.1016/S1470-2045(19)30035-X. [DOI] [PubMed] [Google Scholar]
- 32.Maemondo M., Fukuhara T., Saito H. NEJ026: final overall survival analysis of bevacizumab plus erlotinib treatment for NSCLC patients harboring activating EGFR-mutations. J Clin Oncol. 2020;38(suppl 15) 9506–9506. [Google Scholar]
- 33.Stinchcombe T.E., Jänne P.A., Wang X. Effect of erlotinib plus bevacizumab vs erlotinib alone on progression-free survival in patients with advanced EGFR-mutant non-small cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol. 2019;5:1448–1455. doi: 10.1001/jamaoncol.2019.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakagawa K., Garon E.B., Seto T. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer: a randomized, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:1655–1669. doi: 10.1016/S1470-2045(19)30634-5. [DOI] [PubMed] [Google Scholar]
- 35.Wu Y.L., Cheng Y., Zhou X. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomized, open-label, phase 3 trial. Lancet Oncol. 2017;18:1454–1466. doi: 10.1016/S1470-2045(17)30608-3. [DOI] [PubMed] [Google Scholar]
- 36.Mok T.S., Cheng Y., Zhou X. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non-small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018;36:2244–2250. doi: 10.1200/JCO.2018.78.7994. [DOI] [PubMed] [Google Scholar]
- 37.Yang J.J., Zhou Q., Yan H.H. A phase III randomized controlled trial of erlotinib vs gefitinib in advanced non-small cell lung cancer with EGFR mutations. Br J Cancer. 2017;116:568–574. doi: 10.1038/bjc.2016.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park K., Tan E.H., O’Byrne K. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomized controlled trial. Lancet Oncol. 2016;17:577–589. doi: 10.1016/S1470-2045(16)30033-X. [DOI] [PubMed] [Google Scholar]
- 39.Inoue A., Kobayashi K., Usui K. First-line gefitinib for patients with advanced non-small cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J Clin Oncol. 2009;27:1394–1400. doi: 10.1200/JCO.2008.18.7658. [DOI] [PubMed] [Google Scholar]
- 40.Maemondo M., Minegishi Y., Inoue A. First-line gefitinib in patients aged 75 years or older with advanced non-small cell lung cancer harboring epidermal growth factor receptor mutations: NEJ 003 study. J Thorac Oncol. 2012;7:1417–1422. doi: 10.1097/JTO.0b013e318260de8b. [DOI] [PubMed] [Google Scholar]
- 41.Prabhash K., Noronha V., Patil V., Joshi A., Chougule A. P1.01-88 PS 2 patients with advanced EGFR mutant NSCLC: subgroup analysis of a phase III randomized trial comparing gefitinib to gefitinib with chemotherapy. J Thorac Oncol. 2019;14(suppl):S395. [Google Scholar]
- 42.Kudoh S., Kato H., Nishiwaki Y. Interstitial lung disease in Japanese patients with lung cancer: a cohort and nested case-control study. Am J Respir Crit Care Med. 2008;177:1348–1357. doi: 10.1164/rccm.200710-1501OC. [DOI] [PubMed] [Google Scholar]
- 43.Ando M., Okamoto I., Yamamoto N. Predictive factors for ILD, antitumor response, and survival in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2006;24:2549–2556. doi: 10.1200/JCO.2005.04.9866. [DOI] [PubMed] [Google Scholar]
- 44.Beau-Faller M., Prim N., Ruppert A.M. Rare EGFR exon 18 and exon 20 mutations in non-small-cell lung cancer in 10,117 patients: a multicenter observational study by the French ERMETIC-IFCT network. Ann Oncol. 2014;25:126–131. doi: 10.1093/annonc/mdt418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu J.Y., Yu C.J., Chang Y.C., Yang C.H., Shih J.Y., Yang P.C. Effectiveness of tyrosine kinase inhibitors on “uncommon” epidermal growth factor receptor mutations of unknown clinical significance in non-small cell lung cancer. Clin Cancer Res. 2011;17:3812–3821. doi: 10.1158/1078-0432.CCR-10-3408. [DOI] [PubMed] [Google Scholar]
- 46.Yang J.C., Sequist L.V., Geater S.L. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harboring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16:830–838. doi: 10.1016/S1470-2045(15)00026-1. [DOI] [PubMed] [Google Scholar]
- 47.Cho J.H., Lim S.H., An H.J. Osimertinib for patients with non-small cell lung cancer harboring uncommon EGFR mutations: a multicenter, open-label, phase II trial (KCSG-LU15-09) J Clin Oncol. 2020;38:488–495. doi: 10.1200/JCO.19.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yasuda H., Kobayashi S., Costa D.B. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. Lancet Oncol. 2012;13:e23–e31. doi: 10.1016/S1470-2045(11)70129-2. [DOI] [PubMed] [Google Scholar]
- 49.Ramalingam S.S., Yang J.C., Lee C.K. Osimertinib as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer. J Clin Oncol. 2018;36:841–849. doi: 10.1200/JCO.2017.74.7576. [DOI] [PubMed] [Google Scholar]
- 50.Mok T.S., Wu Y.L., Ahn M.J. Osimertinib or platinum-pemetrexed in EGFR T790M-Positive Lung cancer. N Engl J Med. 2017;376:629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inoue A., Yoshida K., Morita S. Characteristics and overall survival of EGFR mutation-positive non-small cell lung cancer treated with EGFR tyrosine kinase inhibitors: a retrospective analysis of 1660 Japanese patients. Jpn J Clin Oncol. 2016;46:462–467. doi: 10.1093/jjco/hyw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gainor J.F., Shaw A.T., Sequist L.V. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res. 2016;22:4585–4593. doi: 10.1158/1078-0432.CCR-15-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reck M., Mok T.S.K., Nishio M. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomized, open-label phase 3 trial. Lancet Respir Med. 2019;7:387–401. doi: 10.1016/S2213-2600(19)30084-0. [DOI] [PubMed] [Google Scholar]
- 54.West H., McCleod M., Hussein M. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicenter, randomized, open-label, phase 3 trial. Lancet Oncol. 2019;20:924–937. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 55.Besse B., Adjei A., Baas P. 2nd ESMO Consensus Conference on Lung Cancer: non-small-cell lung cancer first line/second and further lines of treatment in advanced disease. Ann Oncol. 2014;25:1475–1484. doi: 10.1093/annonc/mdu123. [DOI] [PubMed] [Google Scholar]
- 56.Wu Y.L., Planchard D., Lu S. Pan-Asian adapted clinical practice guidelines for the management of patients with metastatic non-small-cell lung cancer: a CSCO-ESMO initiative endorsed by JSMO, KSMO, MOS, SSO, and TOS. Ann Oncol. 2019;30:171–210. doi: 10.1093/annonc/mdy554. [DOI] [PubMed] [Google Scholar]
- 57.Ettinger D.S., Wood D.E., Aggarwal C. NCCN guidelines insights: non-small cell lung cancer version 1.2020. J Natl Compr Canc Netw. 2019;17:1464–1472. doi: 10.6004/jnccn.2019.0059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.