Abstract
Objective
This study investigated the stress-buffering effect of social support on immune function and infectious risk in women with breast cancer, during and after chemotherapy.
Method
Data were collected from 50 women with breast cancer before and after their chemotherapy, as well as three months later. Stress was measured by daily hassles related to cancer and social support by marital status (MS) and perceived support from friends (Ps-fr). Blood was collected to measure innate immune markers (i.e., T cells, NK cells and neutrophils). Infections were evaluated using a semi-structured interview. Moderation, mediation and moderated mediation models were computed to test the hypotheses.
Results
Higher stress at baseline was found to significantly predict a higher occurrence of infections during chemotherapy, but not three months later. The relationship between stress and infections was not significantly explained by any of the immune markers. The interaction between stress and social support was tested using MS alone and combined with Ps-fr. A protective effect of social support on the deleterious effect of stress on infectious risk was found. Single patients reporting lower Ps-fr showed the strongest association between stress and infections, while the weakest association was found in patients in a committed relationship with a higher level of Ps-fr.
Conclusions
Women experiencing more stress before the beginning of chemotherapy would appear to be at a higher risk of developing infections during their treatment. Results of this study also suggest that this effect could be buffered by the presence of a romantic partner and by higher Ps-fr.
Keywords: Cancer, Infectious diseases, Psychoneuroimmunology, Social factors influencing health, Social support, Stress and health
Highlights
-
•
The buffering effect of social support in the relationship between stress, immune function and infectious risk has yet to be explored in cancer.
-
•
Results of this study showed that a higher stress level prior to chemotherapy was associated with an increased risk for infections during chemotherapy.
-
•
The relationship between stress and infections was not significantly mediated by immune alterations.
-
•
The buffering effect of social support was supported: Single patients reporting lower support from their friends showed the strongest association between stress and infections.
-
•
The weakest association was found in patients in a committed relationship with a higher perceived support from friends.
1. Introduction
Studies in recent decades suggest that social support has a significant impact on health. Indeed, in a number of prospective epidemiological studies (reviewed in Holt-Lunstad et al., 2010, and House et al., 1988), social support was linked to a marked decrease in early mortality risk. More specifically, lack of social support may influence mortality risk on a scale comparable to, or even exceeding, certain well-recognized health determinants such as cigarette smoking, obesity, and excessive alcohol use (Holt-Lunstad et al., 2010; House et al., 1988). In the context of cancer, a meta-analysis found that, among the studies that controlled for the effect of certain confounding variables, married patients (k = 40), those who had a higher perception of social support (k = 21) and those with a larger network (k = 23), had a lower relative risk of cancer-related mortality of 12%, 20% and 25%, respectively, compared to those with lower social support indicators (Pinquart and Duberstein, 2010). The stress-buffering hypothesis postulates that social support could have a beneficial effect on health by protecting the person from the potentially harmful effects of stressful events on health, especially when experiencing multiple stressors (Cohen and Wills, 1985).
Social support is a multidimensional construct often conceptualized in terms of functional and structural support (Cohen and Wills, 1985; Uchino, 2006). Structural support is the level of integration of individuals into their social network and is typically measured by marital status or the number of relationships or cohabiting individuals (Taylor, 2011). Functional support refers to the different functions fulfilled by available help, such as emotional, instrumental and informational support (Berkman et al., 2000; Cohen and Wills, 1985). Based on their review of the literature, Cohen and Wills (1985) postulated that structural support would impact health more directly and that functional support would act more as a stress buffer. According to this hypothesis, functional support would impact health indirectly, by attenuating the deleterious effects of stressors through the reduction of negative affects and physiological stress response and through improvement of health-related behaviors (Cohen and Wills, 1985).
One possible biological pathway through which social support could influence health is immunity. Most studies have examined the main effect of social support on immune function and have found associations, both cross-sectionally and longitudinally, with various immune alterations (Kiecolt-Glaser et al., 2010; Uchino et al., 1996; Uchino et al., 2012). Previous reviews of this literature have revealed that a higher social support was related to an increased proliferation of lymphocytes to mitogens and increased NK cell activity (Uchino et al., 1996) as well as chronic low grade inflammation (e.g. interleukin-6 [IL-6]; Kiecolt-Glaser et al., 2010; Uchino et al., 2018; Uchino et al., 2012). With regard to stress, a meta-analysis (Segerstrom and Miller, 2004) including more than 300 studies highlighted relationships between different types of stress measures and a variety of immune alterations (e.g., reduced NK cell activity). The authors found that, although globally scores on stressful life event inventories and immune parameters were not significantly related, these associations were significant in immunosuppressed individuals, namely older adults and people with HIV/AIDS.
Given that stress has been associated with immune dysregulation (Segerstrom and Miller, 2004), a number of studies have tested the stress-buffering hypothesis (Bosch et al., 2009; Kang et al., 1998; Kiecolt-Glaser et al., 1991; Marsland et al., 2007; Turner-Cobb et al., 2000). While some studies provided support for one or more aspects of this model in relation to immune functioning, two obtained results supporting the complete model (Kang et al., 1998; Kiecolt-Glaser et al., 1991). Both studies observed that, among people facing a stressful situation (academic exam and dementia caregiving), those reporting a higher social support showed lower immune function changes than those with poorer support. The study by Kiecolt-Glaser et al. (1991) conducted in caregivers of patients with dementia is of particular interest because it measured both immunity and infections. As compared to spouses of healthy individuals, spouse caregivers showed significantly more alterations of functional immune markers (i.e., lower proliferation of lymphocytes to mitogens) and reported more days spent with an infectious disease (4.3 vs. 2.3), but not more infectious episodes at follow-up. Moreover, in line with the stress-buffering effect hypothesis, caregivers with greater support showed a lower immunosuppression.
In the context of cancer, links between social support and immune function have also been observed. The cross-sectional study by Lekander et al. (1996) showed that, three months after chemotherapy for breast cancer, greater perceived social support was associated with a higher number of leukocytes, a higher number and percentage of granulocytes, as well as with a reduced percentage of lymphocytes. However, no association was observed during chemotherapy. Similarly, Hughes et al. (2014) found that breast cancer patients who reported lower social support before the beginning of their treatments had higher levels of IL-6 six months later. In ovarian cancer patients, Lutgendorf et al. (2005) observed that, on the day of surgery, a higher functional social support was significantly related to higher levels of NK cell activity, both in the peripheral blood and at the site of the tumor.
The stress-buffering effect of social support has also been investigated in cancer patients, with mixed results (Turner-Cobb et al., 2004; Von Ah et al. (2007). Von Ah et al.’s (2007) study showed that a higher perceived stress was associated with lower NK cell activity and interferon gamma (IFN-γ) levels. However, satisfaction with social support did not significantly moderate the relationship between stress and immunity. Conversely, Turner-Cobb et al. (2004) observed, among women with metastatic breast cancer, that those reporting more stressful life events had a better delayed type hypersensitivity response when they had a larger social network, a structural measure of social support. However, the perceived quality of the support, as well as the satisfaction towards the support received did not moderate this association.
Although prior evidence shows some support for the stress-buffering effect of social support in cancer, no study has yet evaluated the possible clinical impact of this effect, through immune function, in this population. One health indicator that appears particularly relevant in oncology is the occurrence of infections, especially during chemotherapy. Indeed, chemotherapy weakens the immune system, putting patients at a higher risk of developing infections (Rizzo et al., 2011). Infections can have serious repercussions on the course and efficacy of chemotherapy treatments, through a reduction of doses or increased delays in the administration of cycles (Abbas, 2009; Crawford et al., 2004). Given that stress may alter immune function and that social support may mitigate its impact, and even enhance immune function, it is important to study associations between stress, social support, immune function, and infectious risk in patients receiving chemotherapy. Longitudinal studies are especially warranted to better understand possible causal links between these factors.
The objective of this prospective, naturalistic study was to investigate the stress-buffering effect of social support in the relationship between stress, immune function, and infections during and after chemotherapy treatments in women with breast cancer. It was hypothesized that higher stress at baseline would predict more infections during chemotherapy and within the three months following the end of chemotherapy. It was also hypothesized that immune alterations would mediate the relationships between high stress and infections. It was finally postulated, in accordance with Will and Cohen’s theory (1985) and despite the inconsistent results in cancer patients, that functional social support at baseline would moderate these immune mediated associations.
2. Method
2.1. Participants
This is a secondary analysis of a study on the relationships between insomnia, immunity and infections in cancer patients (Ruel et al., 2019). Eligible patients had received a first diagnosis of stage I-III breast cancer (the main study had two more patients with gynecological cancer) and were about to start one of the following chemotherapy protocols: carboplatin/paclitaxel (taxol-carbo; 6 cycles), docetaxel/cyclophosphamide (TC; 4 cycles), doxorubicin/cyclophosphamide (AC; 4 cycles), doxorubicin/cyclophosphamide + paclitaxel (AC-Taxol®; 4 + 12 cycles), or 5-fluorouracil/epirubicin/cyclophosphamide + docetaxel (FEC-D; 3 + 3 cycles). Only women receiving these chemotherapy regimens were included to maximize homogeneity in their frequency of administration and their aplastic potential. In addition, participants had to: 1) be aged between 18 and 80; 2) live within 50 km of the research center; and 3) be able to read and understand French. A patient was not eligible if she: 1) had a severe psychiatric disorder (e.g., schizophrenia, bipolar disorder) or severe cognitive impairments (e.g., dementia, Parkinson’s disease or Mini-Mental State Examination [MMSE] score ≤ 23, Folstein et al., 1975); 2) had a substance use disorder; 3) had a sleep disorder other than insomnia (e.g., sleep apnea); 4) had a medical condition that could significantly alter immune function (immunosuppressive therapy, bone marrow transplant or chemotherapy in the last 18 months).
2.2. Recruitment
Recruitment was carried out in two hospitals of the CHU de Québec-Université Laval (L’Hôtel-Dieu de Québec and the Hôpital du St-Sacrement) as well as the Hôtel-Dieu de Lévis hospital. The study was approved by the ethics committee of both centers. During a consultation with their oncologist, patients were asked to provide written consent to be contacted by phone to assess their eligibility and to present the study in detail. Those interested and eligible were invited to the first evaluation. From May 2012 to August 2015, 129 patients consented to be contacted, 89 (69%) were eligible and 53 (60%) agreed to participate (see Ruel et al., 2019, for more details).
2.3. Procedure
The initial study used a prospective longitudinal design with eight time points. Three of them were used in the current study: baseline (T1), which took place at least one week before the beginning of chemotherapy, post-treatment (T2), which took place 10–20 days following the last chemotherapy treatment, and 3-month follow-up (T3). T1 took place at home or at the hospital. The Structured Clinical interview for DSM-IV (SCID; First et al., 1996) and the MMSE (Folstein et al., 1975) were administered to screen for psychiatric disorders and cognitive impairments. After the patients’ eligibility was confirmed, they were asked to provide their written consent. A blood sample was drawn by a nurse and a battery of questionnaires was given to be completed at home. It included a demographic and medical questionnaire, the Inventory of Recent Life Experiences for Cancer Patients (IRLE-C; Fillion et al., 2001), the Perceived Social Support From Friends and Family (PSS; Procidano and Heller, 1983), and a health behaviours questionnaire. At T2 and T3, the same battery of questionnaires was completed, blood samples were collected and an adapted version of the Structured Interview for the Assessment of Infectious Illness Symptoms (SIAIIS; Orts et al., 1995) was administered to measure infections that occurred since the last interview. In addition to being administered at T2 and T3, this interview was conducted on four occasions during chemotherapy, all between T1 and T2: 10–14 days after the first two chemotherapy cycles (immunosuppression phase) and just before the subsequent cycle (recovery phase).
2.4. Measures
2.4.1. Stress
Stress was assessed using the French-Canadian version of the IRLE-C (Fillion et al., 2001). This self-report scale measures the extent to which a person experienced cancer-related daily hassles in the past month. This scale was designed to minimize contamination with subjective and physical distress, thus reducing the risk of overlap between this measure and the infectious symptoms. It includes 30 items (e.g., “Having to wait to find out the results of tests”, “Not being able to care for yourself”), that are scored on a Likert scale ranging from 1 (not at all) to 4 (very much). The total score ranges from 30 to 120, a higher score indicates a greater level of stress. It has shown satisfactory validity, a good internal consistency (0.94) and a very good test-retest reliability over two weeks (r = .70; Fillion et al., 2001).
2.4.2. Social support
Two social support indices were used. Marital status, a structural indicator, was divided into two categories: 1) “in a committed relationship” (i.e., married people and cohabiting partners); and “single” (i.e., single, divorced, separated, or widowed). The second index was the Friends subscale (PSS-fr) score of the French-Canadian version of the PSS (Vézina, 1988). The PSS is a 40-item questionnaire (20 for family, 20 for friends) measuring the extent to which individuals perceive that their needs for social support are met (Procidano and Heller, 1983). Each subscale score varies from 0 to 20. For the purpose of moderation analyses and since no empirical cut-off is available, a median split was used to categorize participants into two groups (score ≤ 18 = low level of support). The Family subscale (PSS-fa) was not used because scores exhibited a ceiling effect (the mean was near the maximal value). The English version has shown satisfactory internal consistency (Cronbach α of 0.88 for the PSS-fr and 0.90 for the PSS-fa). The total PSS score showed an adequate construct validity with the Perceived Support Network Inventory (r = 0.57; Orts et al., 1995).
2.4.3. Innate immune functioning
Immune markers were selected based on theoretical and clinical grounds. Neutrophils (absolute count and percentage) were selected because of their crucial role in the prevention of infections (Bodey et al., 1966). Count and ratio of CD16+, CD56+ and CD16+/56+ cells were selected given that quantitative and functional measurements of NK cells have been related to both stress and social support (Miller et al., 2002). Finally, because better social support was found to predict a lower percentage of lymphocytes in a sample of women with breast cancer three months after receiving chemotherapy (Lekander et al., 1996), count and ratio of CD3 + lymphocytes T were also retained, for a total of 10 immune markers.
Twenty ml of blood were taken at each time point, in the morning between 8:00 and 12:00, to control for diurnal variations. Enumeration of T and NK cells was obtained by five color flow cytometry using the BD FACSCanto II cytometer (BD Biosciences; San Jose, California). Within 24 h following its collection, 50 μl of blood samples were stained adding 5 μl of each antibody (CD45-V500-C, CD4-APC-H7, CD25-APC, CD56-PE-Cy7 and CD16-V450) and incubated for 15 min. Then, 900 μl of lysis reagent was used to disintegrate erythrocytes. Absolute values of lymphocyte markers were calculated by multiplying results of the flow cytometry with the absolute lymphocyte count obtained from the complete blood count, which was performed using the Coulter LH Series Hematology Analyzer (Beckman Coulter; Mississauga, Ontario).
2.4.4. Infections
Infections were assessed using a translated and slightly adapted version of the SIAIIS (Orts et al., 1995). The original English version showed an excellent convergence with a medical diagnosis of infection. The SIAIIS contains 24 main questions assessing, for a defined period of time, the occurrence of infectious symptoms (e.g., fever, sore throat, earache, cough), number and length of infectious episodes (e.g., cold, oral herpes, gastroenteritis), as well as medical consultations, medication use and their consequences on the person’s functioning. This tool allows to evaluate infections that are the most likely to occur during chemotherapy (i.e., respiratory, gastrointestinal, urinary tract infections, and herpes-related). To better meet the needs of this study, a few symptoms were added (e.g., ulcers, vaginitis) and the reference period was changed to include infections since the last time point.
To be considered as an infectious episode, each symptom of a set of symptoms reported by the patient had to be judged as being the result of an infectious process using this scale: score of 0 = no symptom, or the symptom is clearly explainable by a cause other than infection (e.g., chemotherapy side effect); score varying from 1 to 3 = symptom clearly caused by an infection and weighted according to its relative contribution to the episode (e.g., fever = 3, cough = 2, nausea = 1). Thus, an infectious episode was considered to have occurred if there was a sufficient number of symptoms concomitantly present to obtain a total score of 3 and over (e.g., the combination of fever, diarrhea and vomiting suggesting the presence of a gastroenteritis). In the main study, ninety-three interviews were listened to in order to calculate the inter-judge agreement, which turned out to be high: 94.5% for the presence of an infectious episode, 94.5% for the number of episodes and 83.5% for the number of infectious symptoms (Ruel et al., 2019).
2.5. Statistical analyses
Raw data was entered by two independent assistants to minimize errors. Descriptive and inferential analyses were performed using SAS 9.4 software (SAS Institute, 2014).
2.5.1. Composite score of infections
A global infection score was created using sums of all infections to have occurred between two assessment times. Since the length of these intervals varied among participants, the sums of episodes and symptoms were adjusted by the average number of days of each interval, so that the data reflect infections occurring in an equivalent period for all participants. The mean duration of chemotherapy was 108 days (SD = 39, range: 49 to 223) and that of the period between T2 and T3 was 96 days (SD = 6, range: 79 to 113). The composite infection score was calculated by averaging three Z-standardized indicators, derived from the interview: (1) number of episodes; (2) number of different symptoms; and (3) proportion of days spent with an episode.
2.5.2. Selection of covariates
The covariates were first selected based on their theoretical relevance, based on the existing PNI literature, then a statistical selection was made. To be included in the models as covariates, potential confounding variables had to show a moderate to large correlation (≥±0.30, Frigon and Laurencelle, 1993) between the independent (stress) and the dependent variable (infections). Possible confounding variables investigated were: demographic characteristics (age, occupation, education, income), health behaviors (physical activity, caffeine and alcohol consumption, diet, tobacco use), cancer stage, chemotherapy regimen, and medications used (anxiolytics, antidepressants and hematopoietic growth factors). Only one variable (i.e., tobacco use) was found to meet this criterion, and was thus included in all subsequent inferential analyses.
2.5.3. Main analyses
A linear regression analysis was performed to verify the presence of a total predictive effect between the independent variable (stress) at T1 and the dependent variable (infections) at T2 and at T3. When this relationship was significant, a moderation analysis was conducted to verify if the strength of this total effect varied with the moderator level (social support), by adding a moderator × stress interaction to the regression model. Two social support indicators were tested, measured at T1: (a) marital status alone; and (b) combination of marital status and perceived social support from friends (PSS-fr); “in a relationship with a higher level of support from friends”, “in a relationship with a lower level of support from friends”, “single with a higher level of support from friends” and “single with a lower level of support from friends”).
Mediation and moderated mediation models were computed using Hayes’s (2013) guidelines and the PROCESS macro (version 2.16 for SAS). These analyses were favored over traditional mediation testing that use hierarchical regressions since non-parametric bootstrap confidence intervals are estimated to determine the significance of the indirect effect. The bootstrapping approach (k = 5000 samples) estimates the standard error and 95% bias-corrected confidence intervals of the product coefficient for the indirect (mediation) relationship (in mediation models) and on the differences of indirect effect between moderator levels (in moderated mediation models). When there were missing data on any of the variables included in a model, the participant was removed from this analysis (6–11 withdrawals, see notes to Table 2, Table 3).
Table 2.
Standardized total and indirect effect of stress at T1 and infections during chemotherapy (T2), standard error (SE), 95% bias corrected confidence intervals (CI), ratio of indirect to total effect.
| Path |
Effect (β) |
SE |
95% BC CI |
Ratio of indirect to total effect |
|---|---|---|---|---|
| Total effect | 0.43∗ | 0.13 | [0.15 to 0.70] | |
| Indirect effect | ||||
| CD3+ number | 0.00 | 0.03 | [-0.03 to 0.10] | 1.7% |
| CD3+ ratio | 0.00 | 0.02 | [-0.06 to 0.05] | 0.1% |
| CD16+ number | 0.03 | 0.03 | [-0.03 to 0.15] | 7.1% |
| CD16+ ratio | 0.04 | 0.04 | [-0.02 to 0.15] | 10.4% |
| CD56+ number | −0.01 | 0.04 | [-0.15 to 0.04] | 1.8% |
| CD56+ ratio | −0.02 | 0.04 | [-0.16 to 0.02] | 5.6% |
| CD16+/56+ number | 0.02 | 0.04 | [-0.04 to 0.13] | 4.8% |
| CD16+/56+ ratio | 0.03 | 0.04 | [-0.04 to 0.11] | 6.4% |
| Neutrophils, absolute count | −0.01 | 0.03 | [-0.10 to 0.03] | 2.2% |
| Neutrophils, percentage | −0.01 | 0.03 | [-0.11 to 0.02] | 1.4% |
Note. The standardized beta (β) quantifies the variation of the dependent variable, in standard deviation, associated with each increase of one standard deviation on the independent variable (for total effect) or via a mediator (for indirect effect). N = 44 for all analyses except the percentage of neutrophils for which N = 39.
∗ Effects are significant at alpha = 5% when confidence intervals (CI) exclude zero.
Table 3.
Standardized conditional indirect effects (IE) of stress at T1 on infections during chemotherapy (T2) through immune markers at different levels of social support (moderator), indices of moderated mediation, 95% bias corrected confidence intervals.
| Immune markers | IE single people β | IE in a relationship β | Index of moderated mediation β (Boot SE) | [95% BC CI] |
|---|---|---|---|---|
| CD3+ number | 0.07 | −0.01 | −0.08 (0.26) | [-0.40 to 0.56] |
| CD3+ ratio | 0.01 | −0.05 | −0.06 (0.20) | [-0.41 to 0.43] |
| CD16+ number | 0.21 | 0.00 | −0.21 (0.36) | [-0.80 to 0.43] |
| CD16+ ratio | 0.07 | 0.00 | −0.07 (0.25) | [-0.43 to 0.41] |
| CD56+ number | 0.05 | −0.01 | −0.06 (0.28) | [-0.48 to 0.58] |
| CD56+ ratio | 0.00 | −0.04 | −0.04 (0.16) | [-0.31 to 0.24] |
| CD16/56+ number | 0.20 | −0.01 | −0.20 (0.45) | [-1.06 to 0.39] |
| CD16/56+ ratio | 0.06 | −0.02 | −0.08 (0.37) | [-0.45 to 0.36] |
| Neutrophils, absolute count | 0.02 | −0.04 | −0.06 (0.14) | [-0.30 to 0.24] |
| Neutrophils, percentage | 0.00 | −0.02 | −0.0 (0.19) | [-0.40 to 0.69] |
Note. Standardized beta (β) quantifies the variation of the dependent variable, in standard deviation, associated with each increase of one standard deviation on the independent variable, through the mediator. N = 44 for all analyses except the percentage of neutrophils for which N = 39.
∗ Effects are significant when confidence intervals (CI) exclude zero.
First, to explore the unique mediation hypothesis, a series of single mediation models were computed for each of the immune markers separately. Second, a moderated mediation model was tested for each of the mediators to investigate the moderating effect of social support, on the immune mediated relationships between stress and infections. This model compared the size of the mediated association at different levels of the moderator. For this analysis, only marital status was used as a moderator to ensure that each cell was large enough to perform the analysis. Direct and indirect regression coefficients were fully standardized to allow comparisons between models.
3. Results
3.1. Descriptive statistics
3.1.1. Demographic and medical characteristics
Participants were 50 women aged from 35 to 73 years old (see Table 1). Most of them had a stage I or II cancer (74.5%).
Table 1.
Participants’ demographic and medical characteristics (N = 50).
| M (SD) | N (%) | |
|---|---|---|
| Age; M (range: 35–73) | 55.1 (10.4) | |
| Education completed; n (%) | ||
| Secondary/High school or less | 16 (31.4) | |
| College | 15 (29.4) | |
| University | 19 (37.3) | |
| Family income (CAN $); n (%) | ||
| $20 000 or less | 4 (7.8) | |
| $20 001 – $40 000 | 4 (7.8) | |
| $40 001 – $60 000 | 16 (31.4) | |
| $60 001 – $80 000 | 9 (17.6) | |
| $80 001 – $100 000 | 4 (7.8) | |
| $100 000 – $120 000 | 5 (9.8) | |
| $120 000 or more | 3 (5.9) | |
| Missing | 5 (9.8) | |
| Marital status; n (%) | ||
| In a committed relationship (married/common-law partner) | 32 (64.0) | |
| Single | 18 (36.0) | |
| Separated/divorced | 9 (18.0) | |
| Single | 6 (12.0) | |
| Widowed | 3 (6.0) | |
| Occupation; n (%) | ||
| Full-time employed | 1 (2.0) | |
| Unpaid family work | 17 (33.3) | |
| Medical leave | 13 (25.5) | |
| Retired | 16 (31.4) | |
| Other | 3 (5.9) | |
| Cancer stage; n (%) | ||
| I | 17 (33.3) | |
| II | 21 (41.2) | |
| III | 12 (23.5) | |
| Type of surgery; n (%) | ||
| Total mastectomy | 14 (28) | |
| Partial mastectomy | 36 (72) | |
| Number of chemotherapy cycles; n (%) | ||
| 4 or less | 26 (52.0) | |
| 5-8 | 19 (38.0) | |
| 9-16 | 5 (10.0) | |
3.1.2. Stress and social support
The average IRLE-C score at T1 was 47.40 (SD = 11.20; range = 31–86). For marital status, 64.0% were in a relationship and 36.0% were single. The mean PSS total score was considerably high in our sample (M = 36.02, SD = 5.30) and the mean score on the PSS-fa and PSS-fr subscales were 18.40 (SD = 2.99) and 17.62 (SD = 3.07), respectively.
3.1.3. Infections
During chemotherapy (between T1 and T2), patients (n = 49) reported a total of 63 infectious episodes, for an average of 1.29 (SD = 1.84, range: 0 to 8) per patient. The most frequent were respiratory tract infections (RTI; 44.9%), other infections (20.4%; e.g., oral, skin) and oral herpes (12.2%). During the same period, patients reported a total of 103 distinct infectious symptoms, for an average of 2.10 (SD = 2.13, range: 0 to 8) per patient. The most common were sore throat (34.7%), stuffy/runny nose (28.6%) and fever (24.5%). Between T2 and T3, patients (n = 43) reported a total of 32 infectious episodes, for an average of 0.74 (SD = 1.03, range: 0 to 4) per patient. The most frequent were RTI (32.6%), urinary (9.3%) and other infections (9.3%; i.e., skin, pericarditis). During the same interval, patients reported a total of 83 distinct infectious symptoms, for an average of 1.93 (SD = 2.62, range: 0 to 11) per patient. The most frequent were stuffy/runny nose (34.9%), sore throat (30.2%), and productive cough (23.3%).
3.2. Main analyses
3.2.1. Stress predicting infections
Consistent with our hypothesis, stress at baseline (T1) significantly and positively predicted infections during chemotherapy (T2), β = 0.47, p = .001. To our knowledge, no standard exists for the interpretation of standardized regression coefficients. However, according to Nieminen et al. (2013), in an unadjusted analysis (with only one predictor), the β value is equivalent to a Pearson’s r. Hence, based on Cohen’s criteria (1988), a β value of 0.47 would be considered a strong relationship. The relationship between stress at T1 and infections at T3 was not significant, β = 0.07, p = .63. To verify if stress measured at a more proximal point in time would predict infections at T3, the relationship with stress at T2 was assessed. As this association was also not significant, β = 0.01, p = .95, no further analyses were carried out on infections at T3.
3.2.2. Moderating effect of social support
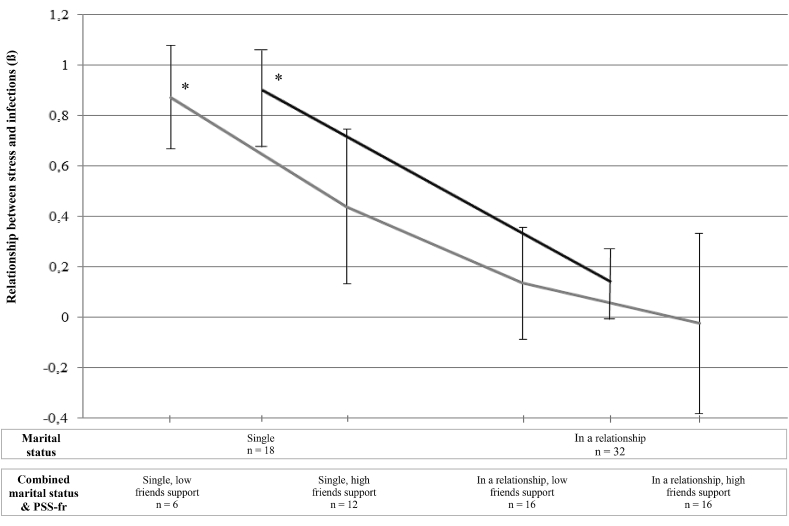
The moderating effect of social support, assessed by marital status and the PSS-fr score, was tested for the time interval during chemotherapy (from T1 to T2). After controlling for tobacco use, the interaction between stress and social support was significant when entering marital status alone, R2 change = 0.12, F (1,44) = 9.65, p = .003, as well as the combination of marital status and the PSS-fr score, R2 change = 0.14, F (3,40) = 3.47, p = .025 (see Fig. 1). However, the interaction between stress and social support was not significant when entering the PSS-fr score alone, R2 change = 0.05, F (1,43) = 3.12, p = .085.
Fig. 1.
Moderating effect of social support on the relationship between stress at baseline and infections during chemotherapy. PSS-Fr = Perceived Social Support From Friends and Family- Friends subscale. ∗p < .05.
More specifically, when entering marital status alone, stress at T1 had a strong and significant association with infections during chemotherapy in single patients, β = 0.87; SE = 0.18; t = 4.75, p = .0001, while stress did not significantly explain infections in patients in a relationship, β = .01, SE = 0.15; t = 0.9, p = .37 (see Fig. 1). When entering both measures of social support, stress at T1 was only significantly associated with infections during chemotherapy in the subsample of single patients with lower perceived support from friends, β = 0.87; SE = 0.21; t = 4.25, p = .0001. For the other subgroups, relationships between stress and infections were not significant, but their strength decreased proportionally with increased social support (single with higher support from friends, β = 0.44; SE = 0.31; t = 1.43. p = .160; in a relationship with lower support from friends: β = 0.13; SE = 0.22; t = 0.61. p = .544, and in a relationship with higher support from friends, β = −0.02; SE = 0.36; t = - 0.07. p = .948; see Fig. 1).
3.2.3. Mediating effect of immune function
A mediation analysis was conducted to test the hypothesis that greater immune alterations during chemotherapy would mediate the relationship between high stress at T1 and infections at T2. The total effect of stress on infections was positive and significant, β = 0.43, SE = 0.13; 95% CI [0.16, 0.70]. However, contrary to the hypothesis, none of the tested immune markers significantly mediated the association between stress and infections. The markers explained from 0.12% to 10.41% of the total effect (see Table 2).
3.2.4. Moderating effect of social support on the immune mediated relationship between stress and infections
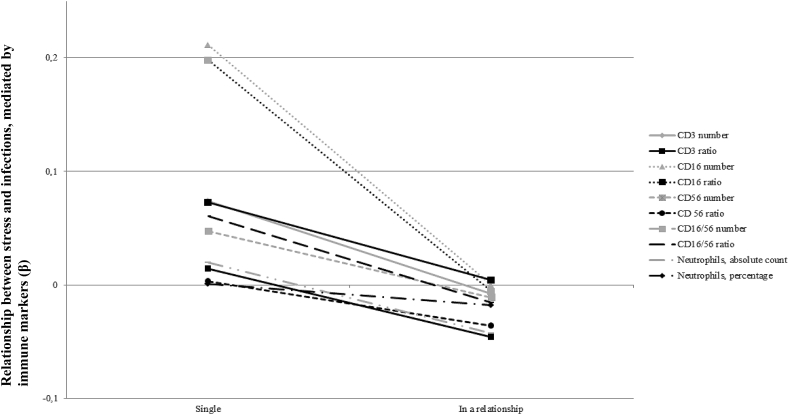
Ten models of moderated mediation were analysed, one for each immune marker measured during chemotherapy, using stress at T1 as the independent variable, infections at T2 as the dependent variable and marital status as the moderator. Tobacco use was included as a covariate. Although none of the mediational relationships was significant, all associations between stress and infections were weaker in the subgroup of patients in a relationship than in the single subgroup (see Fig. 2 and Table 3).
Fig. 2.
Immune mediated effect of stress at baseline on infections during chemotherapy, as a function of marital status.
4. Discussion
The purpose of this naturalistic and prospective study was to investigate the stress-buffering effect of social support in the relationship between stress, immune function, and infections in women with breast cancer receiving chemotherapy. The study first examined the relationship between stress and infections during and after chemotherapy. It then explored the moderating role of social support on the relation between stress and infections during chemotherapy. Subsequently, it investigated the mediating effect of immune function in this relationship, and finally, the moderating role of social support in the mediated relationships. To our knowledge, this is the first study testing the stress-buffering effect of social support in an integrated analytical model, including immune function alterations as a possible mediating physiological mechanism, as well as a real clinical outcome, that is infections. Overall, it was found that higher stress levels at baseline, measured by self-reported daily hassles related to cancer, was a significant predictor of increased infections during chemotherapy, but not within the three months following this treatment. However, the relationship between stress at baseline and infections during chemotherapy was not significantly explained by any of the immune markers. Consistent with the stress-buffering theory, a protective effect of social support from the adverse effect of pre-treatment stress on infectious risk during chemotherapy was found, particularly when measured by marital status alone, but also when combining marital status and the functional perceived support from friends. However, functional social support alone did not significantly moderate this relationship.
Results supporting a stress-buffering effect of social support on infectious risk in women treated for breast cancer, that predominantly involved its structural aspect, namely marital status, warrant some discussion. More precisely, when the sample was divided by marital status, stress was a significant predictor of infections in single patients but not in those in a committed relationship. When using the combined measure of social support, the association between stress and infections decreased proportionally with increasing level of support: single patients with a lower perceived support from friends showed the strongest association, followed in order by singles with higher perceived support from friends, patients in a relationship with lower perceived support from friends and patients in a relationship with higher support from friends.
These findings are inconsistent with Cohen and Wills’ (1985) theory, who postulated that structural support has a direct effect on health rather than an indirect one. However, our results are in line with those of other studies conducted in the context of cancer (Turner-Cobb et al., 2004; Von Ah et al., 2007). Both studies failed to find a stress-buffering effect of functional social support on immunity, measured by NK cell activity, interferon-gamma cytokine level and delayed-type hypersensitivity. Moreover, the cross-sectional study of Turner-Cobb et al. (2004) found a stress-buffering effect of structural social support (number of contacts) on immune functioning (delayed-type hypersensitivity).
A first contribution of this study was to clarify the moderating effect of two different aspects of social support, on the relationship between stress and health. A preliminary analysis using functional support alone did not support a stress-buffering effect but, did so when combined with marital status. Hence, it would appear that the weaker effect of functional support becomes detectable only when also taking into account marital status. This is in line with recent studies which highlighted the complexity of the social support construct and how its various components are distinctly associated with health outcomes (Holt-Lunstad et al., 2010; Kroenke et al., 2017).
Although it was not hypothesized that marital status would have a stronger moderating effect than functional support, this result is not surprising given that it is well established that being married has a protective effect on health and survival, including cancer survival (Rendall et al., 2011; Tatangelo et al., 2017). For instance, the meta-analysis by Pinquart and Duberstein (2010) revealed a significantly lower relative cancer mortality risk of 12%–16% in married compared to unmarried individuals. In addition, two epidemiological studies found a significant reduction of cancer death in married patients, ranging from 12 to 33% in the study investigating various cancers (Aizer et al., 2013), and from 15 to 26% in the one conducted in women with invasive breast cancer (Hinyard et al., 2017).
Another significant contribution of this study is the attempt to test a possible physiological mechanism through which social support may have an impact on health, which is a gap in research on social support that has repeatedly been highlighted in the literature (Thoits, 2011). Contrary to what was expected, innate immune markers did not significantly explain the relationship between stress and infections. It is possible that the selected markers were not the ones most involved in this relationship. For instance, markers of adaptive immunity have more frequently been linked to clinical outcomes, notably in vaccination studies (reviewed in Hayward et al., 2019). Moreover, previous studies showing that increased levels of inflammation are linked to higher stress and lower social integration and social support suggest that inflammation markers could also be involved (Jaremka et al., 2013; Kiecolt-Glaser et al., 2010; Uchino et al., 2018). Low statistical power, due to the small sample size could also explain this absence of a significant mediated relationship.
Notwithstanding, it is interesting to note that, although not significant, all the immune mediated relationships were stronger in single patients than those in a relationship, which suggests a stress-buffering effect of being in relationship (see Fig. 2). Other possible mechanisms could be involved. Laboratory studies, conducted in the general population, showed that the support of a life partner could reduce stress reactivity, measured by cortisol (Ditzen et al., 2007; Meuwly et al., 2012). For instance, it has been proposed that physical touch could be a couple-specific behavior that may reduce stress reactivity (Ditzen et al., 2007).
Results of this study should be interpreted taking into account the following limitations. First, the fact that it was exclusively conducted in women with breast cancer limits the generalization of the results to other populations. This is important given the differential impact of social support in men and women. For instance, laboratory studies suggest that women only benefit from the support of their spouse when it is physically manifested while men benefit from it when both manifested physically or verbally (Ditzen et al., 2007; Kirschbaum et al., 1995). In addition, epidemiological studies in the general population and in cancer patients have found a greater benefit of being married on survival in men than in women (Aizer et al., 2013; Rendall et al., 2011; Tatangelo et al., 2017). Together these results suggest that the protective effect of being in a relationship may be greater in men and emphasize the need to also study the role of social support in this population. The fact that only marital status, and not marital quality, was measured is another limitation of this study. Indeed, dyadic coping has been found to be associated with inflammation in women (Gouin et al., 2016). Furthermore, as mentioned previously, our participants reported high levels of functional social support in general, thus limiting the ability to find significant associations with the other study variables because of a ceiling effect. Given that this phenomenon has also been observed in other studies on social support in the context of cancer, researchers should therefore invest more efforts in the future in recruiting participants who are likely to have lower functional social support (e.g., less educated patients, minority people; Costanzo et al., 2005; Von Ah et al., 2007). Finally, the study is limited by the small sample, which reduced the statistical power to detect some associations. This was particularly likely for the moderation analysis using the combined measure of social support which involved subgroups with a small number of cases, making the regression analysis more unstable. This emphasizes the importance of replicating these results in a larger sample.
The strengths of this study include its longitudinal design that allowed us to investigate the predictive relationships between baseline stress and its possible impact over time, during and after chemotherapy. In addition, many variables were investigated as possible confounders, which increase the internal validity of the study. Another important innovative aspect was the measurement of a real possible clinical impact (i.e., infections) of stress and social support that is relevant for cancer patients undergoing chemotherapy. Finally, the study was conducted in a natural context, which is particularly relevant since previous work investigating the relationships between stress and infections has often been done in the laboratory with healthy individuals (e.g., Cohen and Wills, 1985; Cohen et al., 1998).
In sum, results of this study suggest that women experiencing more pre-treatment stress related to their breast cancer may be at higher risk of developing infections during their treatment, but not within the three months following them. Women who are single, especially those with a weaker perception of support from friends, appear to be at a greater risk, while women in a relationship may be protected from this deleterious effect of stress. Although replication is warranted, these results have some clinical implications. In particular, they suggest that it is crucial to take more into account social factors when trying to identify women who are at a higher risk of complications during chemotherapy. Because they are more vulnerable to infections, women with weaker support may be less likely to benefit from chemotherapy, by having their doses reduced and more likely to have complications leading to hospitalizations and even death (Crawford et al., 2004). While our results may not be very encouraging for single patients who are about to receive chemotherapy, it is important to emphasize that they also suggest that support from friends, may compensate, at least to a certain extent, for the lack of a partner. Interestingly, results of a recent meta-analysis studying the effect of psychosocial interventions on cancer survival suggest that unmarried patients may be more likely to benefit from these interventions (Mirosevic et al., 2019). Although further studies are needed to draw more definitive conclusions, this indicates that clinicians should more particularly offer such resources to single patients with higher levels of cancer-related stress. Efforts should also be invested in developing interventions to increase social support because, despite repeated calls to do so, such interventions are still lacking (Hogan et al., 2002; Holt-Lunstad et al., 2017).
Funding
The original research was supported by a grant from the Canadian Institutes of Health Research (MOP-69073) awarded to Josée Savard, Ph.D.
Declaration of competing interest
None.
References
- Abbas A.K. Elsevier Masson; 2009. Les bases de l’immunologie fondamentale et clinique. Issy-Les-Moulineaux (Hauts-de-Seine): Issy-Les-Moulineaux Hauts-de-Seine. c2009. [Google Scholar]
- Aizer A.A., Chen M.H., McCarthy E.P., Mendu M.L., Koo S., Wilhite T.J. Marital status and survival in patients with cancer. J. Clin. Oncol. 2013;31(31):3869–3876. doi: 10.1200/jco.2013.49.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman L.F., Glass T., Brissette I., Seeman T.E. From social integration to health: durkheim in the new millennium. Soc. Sci. Med. 2000;51(6):843–857. doi: 10.1016/S0277-9536(00)00065-4. [DOI] [PubMed] [Google Scholar]
- Bodey G.P., Buckley M., Sathe Y.S., Freireich E.J. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann. Intern. Med. 1966;64(2):328–340. doi: 10.7326/0003-4819-64-2-328. [DOI] [PubMed] [Google Scholar]
- Bosch J.A., Fischer J.E., Fischer J.C. Psychologically adverse work conditions are associated with CD8+ T cell differentiation indicative of immunesenescence. Brain Behav. Immun. 2009;23(4):527–534. doi: 10.1016/j.bbi.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Cohen Sheldon, Frank Ellen, Doyle William, J., Skoner David, P., Rabin Bruce, S., Gwaltney Jack., M. Jr. Types of stressors that increase susceptibility to the common cold in healthy adults. Health psychology. 1998;17(3):214–223. doi: 10.1037//0278-6133.17.3.214. [DOI] [PubMed] [Google Scholar]
- Cohen S., Wills T.A. Stress, social support, and the buffering hypothesis. Psychol. Bull. 1985;98(2):310–357. doi: 10.1037/0033-2909.98.2.310. [DOI] [PubMed] [Google Scholar]
- Costanzo E.S., Lutgendorf S.K., Sood A.K., Anderson B., Sorosky J., Lubaroff D.M. Psychosocial factors and interleukin-6 among women with advanced ovarian cancer. Cancer. 2005;104(2):305–313. doi: 10.1002/cncr.21147. [DOI] [PubMed] [Google Scholar]
- Crawford J., Dale D.C., Lyman G.H. Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer. 2004;100(2):228–237. doi: 10.1002/cncr.11882. [DOI] [PubMed] [Google Scholar]
- Ditzen B., Neumann I.D., Bodenmann G., von Dawans B., Turner R.A., Ehlert U., Heinrichs M. Effects of different kinds of couple interaction on cortisol and heart rate responses to stress in women. Psychoneuroendocrinology. 2007;32(5):565–574. doi: 10.1016/j.psyneuen.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Fillion L., Kohn P., Gagnon P., Van Wijk M., Cunningham A. The inventory of recent life Experiences for cancer patients (irle-C): a decontaminated measure of cancer-based hassles. Psychol. Health. 2001;16(4):443–459. doi: 10.1080/08870440108405518. [DOI] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. Biometrics Research Department, New York State Psychiatric Institute; New York: 1996. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P, Version 2.0) [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frigon J.-Y., Laurencelle L. Analysis of covariance: a proposed algorithm. Educ. Psychol. Meas. 1993;53(1):1–18. doi: 10.1177/0013164493053001001. [DOI] [Google Scholar]
- Gouin J.-P., Scarcello S., da Estrela C., Paquin C., Barker E. Dyadic coping and inflammation in the context of chronic stress. Health Psychol. 2016;35 doi: 10.1037/hea0000395. [DOI] [PubMed] [Google Scholar]
- Hayes A.F. The Guilford Press; New York: 2013. Introduction to Mediation, Moderation, and Conditional Process Analysis : A Regression-Based Approach. [Google Scholar]
- Hayward S.E., Dowd J.B., Fletcher H., Nellums L.B., Wurie F., Boccia D. A systematic review of the impact of psychosocial factors on immunity: implications for enhancing BCG response against tuberculosis. SSM - population health. 2019;10 doi: 10.1016/j.ssmph.2019.100522. 100522-100522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinyard L., Wirth L.S., Clancy J.M., Schwartz T. The effect of marital status on breast cancer-related outcomes in women under 65: a SEER database analysis. Breast. 2017;32:13–17. doi: 10.1016/j.breast.2016.12.008. [DOI] [PubMed] [Google Scholar]
- Hogan B.E., Linden W., Najarian B. Social support interventions: do they work? Clin. Psychol. Rev. 2002;22(3):381–440. doi: 10.1016/S0272-7358(01)00102-7. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J., Robles T.F., Sbarra D.A. Advancing social connection as a public health priority in the United States. Am. Psychol. 2017;72(6):517. doi: 10.1037/amp0000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt-Lunstad J., Smith T.B., Layton J.B. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 2010;7(7) doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House J.S., Landis K.R., Umberson D. Social relationships and health. Science. 1988;241(4865):540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- Hughes S., Jaremka L.M., Alfano C.M., Glaser R., Povoski S.P., Lipari A.M. Social support predicts inflammation, pain, and depressive symptoms: longitudinal relationships among breast cancer survivors. Psychoneuroendocrinology. 2014;42:38–44. doi: 10.1016/j.psyneuen.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaremka L.M., Lindgren M.E., Kiecolt-Glaser J.K. Synergistic relationships among stress, depression, and troubled relationships: insights from psychoneuroimmunology. Depress. Anxiety. 2013;30(4):288–296. doi: 10.1002/da.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D.H., Coe C.L., Karaszewski J., McCarthy D.O. Relationship of social support to stress responses and immune function in healthy and asthmatic adolescents. Res. Nurs. Health. 1998;21(2):117–128. doi: 10.1002/(sici)1098-240x(199804)21:2<117::aid-nur3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser J.K., Dura J.R., Speicher C.E., Trask O.J., Glaser R. Spousal caregivers of dementia victims: longitudinal changes in immunity and health. Psychosom. Med. 1991;53(4):345–362. doi: 10.1097/00006842-199107000-00001. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser J.K., Gouin J.-P., Hantsoo L. Close relationships, inflammation, and health. Neurosci. Biobehav. Rev. 2010;35(1):33–38. doi: 10.1016/j.neubiorev.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C., Klauer T., Filipp S.-H., Hellhammer D.H. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosom. Med. 1995;57(1):23–31. doi: 10.1097/00006842-199501000-00004. [DOI] [PubMed] [Google Scholar]
- Kroenke C.H., Michael Y.L., Poole E.M., Kwan M.L., Nechuta S., Leas E. Postdiagnosis social networks and breast cancer mortality in the after Breast Cancer Pooling Project. Cancer. 2017;123(7):1228–1237. doi: 10.1002/cncr.30440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekander M., Furst C.J., Rotstein S., Blomgren H., Fredrikson M. Social support and immune status during and after chemotherapy for breast cancer. Acta Oncol. 1996;35(1):31–37. doi: 10.3109/02841869609098476. [DOI] [PubMed] [Google Scholar]
- Lutgendorf S.K., Sood A.K., Anderson B., McGinn S., Maiseri H., Dao M. Social support, psychological distress, and natural killer cell activity in ovarian cancer. J. Clin. Oncol. 2005;23(28):7105–7113. doi: 10.1200/jco.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Marsland A.L., Sathanoori R., Muldoon M.F., Manuck S.B. Stimulated production of interleukin-8 covaries with psychosocial risk factors for inflammatory disease among middle-aged community volunteers. Brain Behav. Immun. 2007;21(2):218–228. doi: 10.1016/j.bbi.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Meuwly N., Bodenmann G., Germann J., Bradbury T.N., Ditzen B., Heinrichs M. Dyadic coping, insecure attachment, and cortisol stress recovery following experimentally induced stress. J. Fam. Psychol. 2012;26(6):937–947. doi: 10.1037/a0030356. [DOI] [PubMed] [Google Scholar]
- Miller G.E., Cohen S., Ritchey A.K. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychol. 2002;21(6):531–541. doi: 10.1037/0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Mirosevic S., Jo B., Kraemer H.C., Ershadi M., Neri E., Spiegel D. Not just another meta-analysis": sources of heterogeneity in psychosocial treatment effect on cancer survival. Cancer Med. 2019;8(1):363–373. doi: 10.1002/cam4.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieminen P., Lehtiniemi H., Vähäkangas K., Huusko A., Rautio A. Standardised regression coefficient as an effect size index in summarising findings in epidemiological studies. Epidemiology, Biostatistics and Public Health. 2013;10(4) doi: 10.2427/8854. [DOI] [Google Scholar]
- Orts K., Sheridan J.F., Robinson-Whelen S., Glaser R., Malarkey W.B., Kiecolt-Glaser J.K. The reliability and validity of a structured interview for the assessment of infectious illness symptoms. J. Behav. Med. 1995;18(6):517–529. doi: 10.1007/BF01857893. [DOI] [PubMed] [Google Scholar]
- Pinquart M., Duberstein P.R. Associations of social networks with cancer mortality: a meta-analysis. Crit. Rev. Oncol. Hematol. 2010;75(2):122–137. doi: 10.1016/j.critrevonc.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procidano M.E., Heller K. Measures of perceived social support from friends and from family: three validation studies. Am. J. Community Psychol. 1983;11(1):1–24. doi: 10.1007/BF00898416. [DOI] [PubMed] [Google Scholar]
- Rendall M.S., Weden M.M., Favreault M.M., Waldron H. The protective effect of marriage for survival: a review and update. Demography. 2011;48(2):481–506. doi: 10.1007/s13524-011-0032-5. [DOI] [PubMed] [Google Scholar]
- Rizzo T., Odle T.G., Oberleitner M.G. In: fourth ed. Fundukian L.J., editor. vol. 2. Gale; Detroit: 2011. Chemotherapy; pp. 945–948. (The Gale Encyclopedia of Medicine). [Google Scholar]
- Ruel S., Ivers H., Savard M.-H., Gouin J.-P., Lemieux J., Provencher L. Insomnia, immunity, and infections in cancer patients: results from a longitudinal study. Health Psychol. 2019 doi: 10.1037/hea0000811. 10.1037/hea0000811. [DOI] [PubMed] [Google Scholar]
- Segerstrom S.C., Miller G.E. Psychological stress and the human immune system: a meta-analytic study of 30 Years of inquiry. Psychol. Bull. 2004;130(4):601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatangelo G., McCabe M., Campbell S., Szoeke C. Gender, marital status and longevity. Maturitas. 2017;100:64–69. doi: 10.1016/j.maturitas.2017.03.002. [DOI] [PubMed] [Google Scholar]
- Taylor S.E. Oxford University Press; New York, NY, US: 2011. Social Support: A Review the Oxford Handbook of Health Psychology; pp. 189–214. [Google Scholar]
- Thoits P.A. Mechanisms linking social ties and support to physical and mental health. J. Health Soc. Behav. 2011;52(2):145–161. doi: 10.1177/0022146510395592. [DOI] [PubMed] [Google Scholar]
- Turner-Cobb J.M., Koopman C., Rabinowitz J.D., Terr A.I., Sephton S.E., Spiegel D. The interaction of social network size and stressful life events predict delayed-type hypersensitivity among women with metastatic breast cancer. Int. J. Psychophysiol. 2004;54(3):241–249. doi: 10.1016/j.ijpsycho.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Turner-Cobb J.M., Sephton S.E., Koopman C., Blake-Mortimer J., Spiegel D. Social support and salivary cortisol in women with metastatic breast cancer. Psychosom. Med. 2000;62(3):337–345. doi: 10.1097/00006842-200005000-00007. [DOI] [PubMed] [Google Scholar]
- Uchino B.N. Social support and health: a review of physiological processes potentially underlying links to disease outcomes. J. Behav. Med. 2006;29(4):377–387. doi: 10.1007/s10865-006-9056-5. [DOI] [PubMed] [Google Scholar]
- Uchino B.N., Cacioppo J.T., Kiecolt-Glaser J.K. The relationship between social support and physiological processes: a review with emphasis on underlying mechanisms and implications for health. Psychol. Bull. 1996;119(3):488–531. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- Uchino B.N., Trettevik R., Kent de Grey R.G., Cronan S., Hogan J., Baucom B.R.W. Social support, social integration, and inflammatory cytokines: a meta-analysis. Health Psychol. 2018;37(5):462–471. doi: 10.1037/hea0000594. [DOI] [PubMed] [Google Scholar]
- Uchino B.N., Vaughn A.A., Carlisle M., Birmingham W. Oxford University Press; New York, NY, US: 2012. Social Support and Immunity the Oxford Handbook of Psychoneuroimmunology; pp. 214–233. [Google Scholar]
- Vézina A. 1988. Le travail et le réseau de support comme facteurs d’adaptation chez les veuves d’âge moyen. (8462 CaQQLA Thèse (Ph.D.)), Université Laval, Québec, Canada. Available from: Bibliothèque de l’Université Laval Ariane database. [Google Scholar]
- Von Ah D., Kang D.H., Carpenter J.S. Stress, optimism, and social support: impact on immune responses in breast cancer. Res. Nurs. Health. 2007;30(1):72–83. doi: 10.1002/nur.20164. [DOI] [PubMed] [Google Scholar]