Abstract
Long-term cognitive and affective impairments are common problems in the survivors of sepsis, which weakens their vocational and daily life ability. Neuroinflammation has been reported to exert a key role in the development of cognitive deficit in different disorders including epilepsy, Alzheimer’s disease (AD) and stroke. Mice treated with lipopolysaccharide (LPS), an endotoxin produced by gram-negative bacteria, show a robust but short-lived neuroinflammation and develop long-term memory and affective problems. In this study, we test the hypothesis that pharmacological blockade of the EP2 receptor for prostaglandin E2 reduces neuroinflammation and prevents long-term affective and memory deficits in a mouse model of LPS-induced, sepsis-associated encephalopathy (SAE). Our results show that an EP2 antagonist, TG6-10-1, promotes the recovery of body weight, mitigates neuroinflammation as judged by inflammatory cytokines and microgliosis, prevents the loss of synaptic proteins, and ameliorates depression-like behavior in the sucrose preference test as well as memory loss in the novel object recognition test. Our results point to a new avenue to ameliorate neuroinflammation and long-term affective and cognition problems of sepsis survivors.
Keywords: Sepsis-associated encephalopathy, Lipopolysaccharide, EP2 receptor, Novel object recognition test, Sucrose preference test, Neuroinflammation
Highlights
-
•
In the LPS mouse model of sepsis-associated encephalopathy (SAE), inhibition of EP2 receptors reduced neuroinflammation.
-
•
Inhibition of EP2 receptors restored depressed expression of synaptic proteins induced by SAE.
-
•
Neuroinflammation and cognitive deficits can occur without obvious neurodegeneration.
-
•
Inhibition of EP2 receptors reduced depressive behavior and eliminated a memory deficit in the novel object recognition test.
1. Introduction
Sepsis, defined as a life-threatening syndrome with organ dysfunction caused by dysregulated immune response to an infection, often leads to diffuse brain injury (Singer et al., 2016). Up to 70% of sepsis patients develop sepsis associated encephalopathy (SAE), which often manifests as delirium and coma (Gofton and Young, 2012; Chung et al., 2020). In addition, sepsis survivors have a higher risk of long-term cognitive deficits and seizure susceptibility (Iwashyna et al., 2010; Reznik et al., 2017; Sewal et al., 2017). The endotoxin lipopolysaccharide (LPS), a cell wall component of gram-negative bacteria, has been used to model the inflammatory driver of sepsis in rodents. LPS injection causes up-regulated expression of pro-inflammatory genes (Jeong et al., 2010), microglia activation (Huang et al., 2020), breakdown of blood brain barrier (BBB) (Danielski et al., 2018), as well as long-term cognitive deficits (Andonegui et al., 2018). Cyclooxygenase-2 (COX-2), a rate-limiting enzyme in prostaglandin (PG) synthesis, contributes to the development of neuroinflammation in disorders including status epilepticus (Serrano et al., 2011) and Alzheimer’s disease (Guan and Wang, 2019). The COX-2 inhibitor, meloxicam, has also been reported to attenuate BBB disruption in a LPS induced sepsis model (Kikuchi et al., 2019). Additionally, COX-2 knockout mice have an enhanced bacterial clearance ability in the Pseudomonas aeruginosa pneumonia mouse sepsis model (Sadikot et al., 2007).
Although the detailed mechanism of how COX-2 regulates inflammatory and immune responses in the brain is not fully elucidated, prostaglandin E2 (PGE2), a pivotal product of COX-2, and its downstream EP2 receptor, appear to promote the development of inflammatory responses. Our previous studies have reported that EP2 receptor signaling pathways regulate classical activation of microglia (Quan et al., 2013), which plays a key role in neuroinflammation. In EP2 conditional knockout mice, LPS causes less robust inflammation in both plasma and hippocampus (Johansson et al., 2013) than in wild-type mice. EP2 global knockout mice also have higher survival rate in a sepsis model induced by intrauterine Streptococcus Pyogenes(SP) infection (Mason et al., 2013). Additionally, we have demonstrated that inhibition of EP2 receptors blunts expression of inflammatory cytokines, reduces microgliosis, and prevents disruption of BBB in different status epilepticus models (Rojas et al., 2020; Jiang et al., 2019).
Although the immune response to LPS is briefer than that of sepsis patients, it does induce systemic inflammation and long-term cognitive impairments, which are common in sepsis patients (Buras et al., 2005). Moreover, 62% of the positive microbiological culture isolates in intensive care units were gram-negative organisms (Vincent et al., 2009). As the most potent microbial mediator in the pathogenesis of sepsis, LPS has been widely used to model sepsis (Opal, 2010). In this study, we test the hypothesis that pharmacological blockade of EP2 receptors reduces neuroinflammation and prevents long-term depression-like behavior and memory deficit in a mouse model of LPS induced SAE. Our results indicate that inhibition of EP2 receptors reduces expression of COX-2 in brain, reverses reduction of synaptic protein levels in the brain, and provides protection against long-lasting depression like behavior and memory deficit in LPS treated mice. Therefore, our study provides new insights into the role of EP2 receptors in sepsis and a novel therapeutic strategy for preventing depression like behavior and cognition deficit of sepsis survivors.
2. Material and Methods
2.1. Animals
Six- to ten-week-old adult C57BL/6 mice (Charles River, United States) were used in the EP2 antagonist treatment experiments, and six-month-old adult mice were used to study the time-course of inflammatory response. Mice were housed under a 12 h light-dark cycle with food and water ad libitum. All animal experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Emory University and conducted in accordance with its guidelines.
2.2. Experiment design
2.2.1. Molecular study
To induce systemic inflammation, mice were injected with LPS (Escherichia coli O111:B4, Sigma, USA; 3 mg/kg in saline, i.p). Mice were then randomized to treatment with TG6-10-1 (10 mg/kg in vehicle, i.p) or vehicle (10% DMSO, 50% PEG 400, 40% ddH2O) 0.5 h and 3.5 h after LPS injection. Mice were euthanized between 0.5 and 8 h after LPS injection.
2.2.2. Behavior study
Mice were injected with LPS (5 mg/kg in saline, i.p) to induce systemic inflammation. Mice were randomized 0.5 h later to treatment with TG6-10-1 (10 mg/kg in vehicle, sc) or vehicle (2% dimethylacetamide and 98% corn oil). The mice were monitored at least twice a day in the first week, and then once a day after the first week.
2.3. Nesting behavior
Two days after LPS injection, mice were single housed and a new nesting pad of pressed cotton was placed in mouse cages. The next morning, a nesting score was given to the mice according to a 5-point nest-rating scale (Deacon, 2006).
2.4. Sucrose preference test
Three weeks after LPS injection, the sucrose preference test (SPT) was conducted to test depression like behavior. Mice were single housed with an extra nesting pad for this test. Two identical bottles (with scale on the surface) filled with 1% sucrose (weight/volume) or mouse standard drinking water were put in the mouse cages. 24 h later, both the position and sipper tube were changed to avoid location and odor effects. During the experiment, mice had free access to both bottles continuously for 48 h. Estimation of sucrose preference was done by the following equation: ratio of sucrose consumption = ml sucrose / (ml sucrose water + ml standard water).
2.5. Novel object recognition and open field test
We chose the novel object recognition test because it causes less stress on the animals tested compared with other memory tests and requires low demands of specific equipment (Lueptow, 2017; Bellantuono et al., 2020). The results of novel object recognition test can be influenced by both hippocampal and cortical lesions (Antunes and Biala, 2012). There were three stages, including habituation (5 min), training (10 min) and testing (5 min). The 5 min habituation stage was also performed as an open-field test for motor ability assessment. To reduce stress and anxiety, mice were handled by the investigator a few minutes each day for seven days before the experiment. The test room was the same room used for mice housing but in an adjacent cubicle in order to isolate noise and control odor. The NOR apparatus was 40 cm × 40 cm x 30 cm. ANY-maze, a behavior tracking software, was used to track mice, compute time spent and distance travelled. On day 27 after LPS, mice were allowed 5 min to explore the open-field arena freely, in the absence of objects. At this stage, the center part of mouse was tracked to compute travelled distance by Any-maze. On the next day, mice were allowed to explore two identical objects in the arena for 10 min, then removed to their home cage. After 2 h, one object was replaced by a similar sized novel object and mice were given 5 min to explore freely. Between each test, 70% ethanol solution was used to clean the arena and objects. Before experiments, predefined areas which consist of small areas around both novel and known objects were located in the arena. Mice heads instead of the body center were tracked. When their heads were within a predefined area with a distance of ≤ 2 cm from the object, with behaviors including sniffing, touching and gnawing, we considered they were exploring objects. Excluded from the total exploration time was any time spent with the object where a mouse simply propped the forepaws onto the object with the head pointing away from the object (Rojas et al., 2016). A trained researcher reviewed the output videos to detect misbehavior of the animals that cannot be distinguished by the tracking software. Mice with total exploring time ≤ 15 s, spending more than 75% time in one object during training or only exploring one object in the testing stage were excluded from the data analysis (Bellantuono et al., 2020). Based on these criteria 13 out of 56 mice were excluded from analysis.
2.6. RT-qPCR
Mice were perfused with cold PBS and decapitated to obtain the brain. Total RNA of mouse cortex or hippocampus was isolated using TRIzol (Invitrogen) with the PureLink RNA Mini Kit (Invitrogen). RNA concentration and purity were measured by A260 value and A260/A280 ratio, respectively. For cDNA synthesis we used 1 μg of total RNA, 4 μl qScript cDNA Supermix (Bio-Rad Laboratories) in a reaction volume of 20 μl at 25 °C for 5 min, 42 °C for 30 min, and 85 °C for 5 min. Quantitative real-time PCR (qRT-PCR) was performed on mixtures of 2 μl 5 × diluted cDNA, 1 μl 10 μM forward and reverse primers, and 10 μl iQ SYBR Green Supermix (Bio-Rad Laboratories) with a final volume of 20 μl in the iQ5 Multicolor Real-Time PCR Detection System (Bio-Rad Laboratories). PCR cycling conditions were as follows: 95 °C for 2 min followed by 40 cycles of 95 °C for 15 s and then 60 °C for 1 min. Melting-curve analysis was used to verify single-species PCR product. Fluorescent data were acquired at the 60 °C step. The geometric mean of cycle thresholds for GAPDH, β-actin and HPRT1 was used as an internal control for each sample. Primers used for qRT-PCR were as Table 1.
Table 1.
Sequence of employed primers.
| Genes | Forward Primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|
| GAPDH | TGTCCGTCGTGGATCTGAC | CCTGCTTCACCACCTTCTTG |
| β-actin | AAGGCCAACCGTGAAAAGAT | GTGGTACGACCAGAGGCATAC |
| HPRT1 | GGAGCGGTAGCACCTCCT | CTGGTTCATCATCGCTAATCAC |
| COX-2 | CTCCACCGCCACCACTAC | TGGATTGGAACAGCAAGGAT |
| IL-1β | TGAGCACCTTCTTTTCCTTCA | TTGTCTAATGGGAACGTCACAC |
| IL-6 | TCTAATTCATATCTTCAACCAAGAGG | TGGTCCTTAGCCACTCCTTC |
| TNF-α | TCTTCTGTCTACTGAACTTCGG | AAGATGATCTGAGTGTGAGGG |
| CCL2 | CATCCACGTGTTGGCTCA | GCTGCTGGTGATCCTCTTGTA |
| iNOS | CCTGGAGACCCACACACTG | CCATGATGGTCACATTCTGC |
2.7. Western blot
Cortex was homogenized on ice in 0.5 ml RIPA buffer (ThermoFisher Scientific) containing a mixture of protease and phosphatase inhibitors (ThermoFisher Scientific). Protein concentration was measured in the collected supernatant by BCA protein assay kit (Thermo Fisher Scientific) after centrifugation (12,000×g, 15 min, 4 °C). 30 μg of total proteins was separated by 4–15% SDS-PAGE, and electroblotted onto PVDF membranes (Millipore). The membrane was blocked with 5% non-fat milk at room temperature for 2 h, then incubated overnight at 4 °C with primary antibodies: rabbit anti-COX-2 (1:1000, Abcam, ab15191), rabbit anti-PSD-95 (1:1000, Cell Signaling Technology, 3450), rabbit anti-Synaptophysin (1:1000, Cell Signaling Technology, 5461) and mouse anti-β-actin (1:5000, Abcam, ab6276). After incubating with anti-mouse IRDye 680RD secondary antibody (1:10000) or anti-rabbit IRDye 800CW secondary antibody (1:10000) at room temperature for 1 h, the blots were washed with 1X Tris-Buffered Saline, 0.1% Tween 20 and scanned. The band intensities were quantified by Image lab (Bio-Rad).
2.8. Immunohistochemistry
Half brains from perfused mice were placed in 4% paraformaldehyde solution for 24 h and then in 30% sucrose solution in PBS until the brains sunk. Coronal sections (30 μm) through the hippocampus were cut with a cryostat CM 1850 (Leica, Wetzlar, Germany) and placed in PBS. The free-floating sections were washed three times with PBS for 5 min each and then blocked at room temperature for 2 h with PBS containing 20% normal goat serum (NGS), 1% IgG free bovine serum albumin (BSA), and 0.3% Triton X-100. Then, sections were incubated overnight with gentle shaking at 4 °C with primary antibody rabbit anti-Iba-1 (1:1000, Wako) or rabbit anti-GFAP (1:1000, abcam). After incubating with Alexa Fluor goat anti-rabbit 488-conjugated secondary antibody (1:500, Thermo Fisher Scientific) at room temperature for 2 h, the sections were mounted with anti-fade mountant with DAPI (Thermo Fisher Scientific) and placed in a dark environment to dry. Images were taken using an Axio Observer A1 fluorescence microscope and the AxioVision AC 4.1 software.
2.9. Nissl staining
Sections from mouse brain (30 μM) were used for Nissl staining to observe neuron damage. Sections were immersed in 0.5% cresyl violet for 10 min followed by distilled water. Then, the sections were transferred to 50% alcohol, 75% alcohol, 95% alcohol, 2 × 100% alcohol for 3 min respectively. Following dehydration, the sections were immersed in xylenes for 5 min and covered with permount media. Images were taken using the software AxioVision AC 4.7 (Zeiss).
2.10. FluoroJade B (FJB) staining
Sections from mouse brain (30 μM) were used for FJB staining to label degenerating neurons. Sections were immersed in 80% alcohol for 2 min followed by 2 min in 70% alcohol and 2 min in distilled water. Then, the sections were transferred to 0.06% potassium permanganate solution for 10 min. The sections were rinsed for 2 min in distilled water and then transferred to 0.0004% FluoroJade B staining solution for 30 min in the dark. Following staining, the sections were rinsed with three 1 min changes of water. The sections were dried, immersed in xylene and then mounted under D.P.X. mounting media. Images were taken using the software AxioVision AC 4.7 (Zeiss).
2.11. Pharmacokinetics of TG6-10-1
Male C57Bl/6 mice were injected with TG6-10-1 by the ip route (5 mg/kg, in 10% DMSO, 50% PEG 400, 40% ddH2O), or sc route (50 mg/kg, 2% DMA in olive oil). At various times after injection, 3 mice per timepoint were euthanized and plasma and brain isolated. Samples were extracted and analyzed by LC/MS/MS at SAI Ltd (India).
2.12. Statistical analyses
All data are presented as mean ± SD or SEM. Statistical analyses were performed with GraphPad Prism software (8.0). One- or two-way ANOVA with post-hoc Bonferroni or Dunnett’s test, paired t-test, or Fisher’s exact test were performed as appropriate to reveal significant differences. Estimation statistics (Ho et al., 2019) was used for the sucrose preference test. Outliers were searched for by the Grubbs’ test, and two outliers were found and removed before analyzing in Fig. 3A p < 0.05 was considered statistically significant.
Fig. 3.
Inhibition of EP2 receptor reduces COX-2 expression after LPS injection. (A–B) The mRNA levels of COX-2 were measured by qRT-PCR in mouse hippocampus and kidney at 6 h after LPS (3 mg/kg, i.p) injection. (A) Administration of EP2 antagonist TG6-10-1 (10 mg/kg, ip, 0.5 h and 3.5 h after LPS injection) reduced mRNA expression of COX-2 (n = 4–7 mice per group, ∗∗∗∗p < 0.0001 for LPS + vehicle vs saline + vehicle, #p = 0.0171 for LPS + tg6-10-1 vs LPS + vehicle, one-way ANOVA and Bonferroni of the ΔΔCT values). Data are shown as mean ± SEM. (B) Administration of EP2 antagonist TG6-10-1 reduced mRNA expression of COX-2 in the kidney of the same mice (n = 5–7 mice per group, ∗∗∗p = 0.0002 for LPS + vehicle vs saline + vehicle, #p = 0.0142 for LPS + tg6-10-1 vs LPS + vehicle, one-way ANOVA and Bonferroni of the ΔΔCT values). Data are shown as mean ± SEM. (C) Administration of EP2 antagonist TG6-10-1 moderately reduced expression of inflammatory genes in the same mice (n = 6–7 mice per group, p = 0.0138, two-tailed paired t-test of the ΔΔCT values; mean reduction = 19.5% of the six mediators). Data are shown as mean + SEM. (D–E) In the same mice, TG6-10-1 significantly reduced COX-2 protein expression induced by LPS in mouse cortex, measured 6 h after LPS injection (n = 5–7 mice per group, ∗p = 0.0314 for LPS + tg6-10-1 vs LPS + vehicle, one-way ANOVA and Bonferroni). Data are shown as mean ± SEM.
3. Results
3.1. COX-2 induction is an early event in LPS induced neuroinflammation
Mice were injected with LPS (3 mg/kg, i.p) to study the expression of COX-2 and other pro-inflammatory genes at denoted time points in the brain as shown in Fig. 1. COX-2 mRNA displayed a rapid rise to 4.12 ± 0.172-fold from control at 2 h. IL-1β, IL-6, TNF-α, and CCL2 expression had a similar time course after LPS, whereas iNOS expression lagged (Fig. 1).
Fig. 1.
Time course of inflammatory genes expression in the cortex of LPS injected mice. mRNA levels of inflammatory mediators in mouse cortex were measured by quantitative real-time PCR (qRT-PCR) at the denoted time points after injection of LPS (3 mg/kg, i.p). The mRNA level of most inflammatory molecules, including COX-2, IL-1β, IL-6, TNF-α, CCL2, peaked at 2 h whereas iNOS showed a delayed peak. (n = 3–6 mice per time point, ∗p < 0.05; ∗∗ p < 0.01; ∗∗∗ p < 0.001; ∗∗∗∗ p < 0.0001 compared with control mice, one-way ANOVA and Dunnett’s test of the ΔΔCT values from the qRT-PCR). Data are shown as mean ± SEM.
3.2. EP2 antagonist facilitates the recovery of body weight
Based on the time course of COX-2 gene expression in the brain, and the observation that LPS elevates brain level of PGE2 (Golia et al., 2019; Zhao et al., 2019), the EP2 antagonist, TG6-10-1, was administered to mice at 0.5 h and 3.5 h (10 mg/kg, i.p) after LPS injection. In the long-term study, mice were injected with 5 mg/kg LPS to induce depressive-like behavior as well as memory deficit, and TG6-10-1 (10 mg/kg, s.c) was injected at 0.5 h after LPS injection (Fig. 2A). The doses and dose scheduling of TG6-10-1 were chosen to maintain adequate brain exposure (concentrations above the Schild KB) for 6 h (molecular study) or >24 h (behavioral study) (Fig. 2B). There was a similar survival rate in mice treated with vehicle (78.9%) and TG6-10-1 (81.8%) (Fig. 2C). At day 3, mice treated with the EP2 antagonist had a somewhat improved ability to build a good nest (nest score ≥ 4) than vehicle treated mice (Fig. 2D). Mice in the LPS and vehicle treated group lost 16.3% body weight at day 2 and began to regain body weight at day 4. However, LPS and TG6-10-1 treated mice began to regain body weight at day 3, which showed a small but significant difference (n = 15–17 in each group, p = 0.0001) (Fig. 2E). These data suggest that EP2 antagonism throughout the period of COX-2 elevation is beneficial in the mouse model of LPS induced systemic inflammation.
Fig. 2.
EP2 antagonist, TG6-10–1, treatment promotes the recovery of LPS injected mice. (A) Experiment design for both molecular study and behavior study in the model of systemic inflammation. For molecular study, 3 mg/kg LPS was used to establish the systemic inflammation model; for behavior study, 5 mg/kg LPS was injected to the mice. (B) Plasma and brain concentrations of TG6-10-1 after ip or sc injection. The terminal plasma half-life by the ip route is 1.8 h, and by the sc route 9.3 h. For comparison the Schild KB for EP2 is shown (18 nM). (C) Survival curve of vehicle or TG6-10-1 (10 mg/kg, s.c) treated mice injected with LPS (5 mg/kg, i.p),(n = 19–22, p = 0.921, log-rank (Mantel–Cox test)). (D) Animal nesting behavior at day 3 after 5 mg/kg LPS injection. Mice that achieved a nesting score ≥ 4 were considered as nest + (n = 14–17, p = 0.0669, Fisher’s exact test). (E) Mouse body weight changes after 5 mg/kg LPS injection (p = 0.0001, two-way ANOVA). Data are shown as mean ± SEM.
3.3. EP2 antagonist reduces brain COX-2 expression in the LPS model of SAE
Since there was a rapid induction of COX-2 mRNA, mice were sacrificed at 6 h after LPS injection to explore the effect of EP2 antagonist on COX-2 expression. Hippocampus was used to study inflammatory gene expression by qRT-PCR and cortex was used to explore protein expression by western-blot. COX-2 mRNA level in hippocampus was upregulated 6 h after LPS in vehicle treated mice by 2.20 ± 0.15-fold; this effect was mildly reduced in TG6-10-1 treated mice (to 1.63 ± 0.09-fold of saline + vehicle, n = 4–7, p = 0.0171, Fig. 3A). Moreover, to evaluate whether there was a peripheral effect of EP2 antagonist, COX-2 mRNA level in the kidney was evaluated by qRT-PCR. The EP2 antagonist produced a similar reduction of the COX-2 mRNA level in kidney (n = 5–7, p = 0.0142, Fig. 3B). The induction of six pro-inflammatory genes including COX-2, IL-6, IL-1β, CCL2, TNF-α, iNOS in the hippocampus was also decreased by an average of 24.9% (n = 6–7, p = 0.0138; Fig. 3C). Additionally, COX-2 protein was induced in cortex of LPS injected mice (1.54 ± 0.10-fold) (Fig. 3D and E), and TG6-10-1 inhibited COX-2 protein expression in mouse cortex by 54.1% (n = 5–7 mice per group, p = 0.0314).
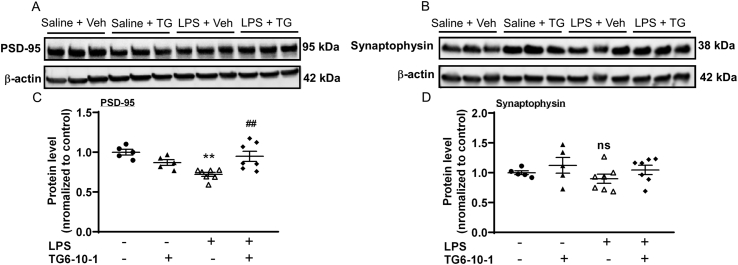
3.4. EP2 antagonist reverses the reduction of postsynaptic protein levels caused by LPS
Synaptic alterations that contribute to cognitive deficits can happen following neuroinflammation. We measured the level of two synaptic proteins in cortex, postsynaptic density protein 95 (PSD-95) and synaptophysin, a presynaptic protein, after LPS injection. As shown in Fig. 4, LPS reduced the level of PSD-95 protein to 72.1 ± 2.57% of saline + vehicle, whereas TG6-10-1 restored it to 94.8 ± 6.39% of saline + vehicle (n = 5–7 mice per group, p = 0.0033). For synaptophysin, LPS decreased the protein level to 89.8 ± 7.72% without reaching significance, and TG6-10-1 restored the level to that of saline + vehicle.
Fig. 4.
Restoration by EP2 antagonist of synaptic protein levels in cortex reduced by systemic inflammation. (A–B) Synaptic protein levels in cortex were measured by western-blot. (C) LPS (3 mg/kg, i.p) injection resulted in reduction of PSD-95 (∗∗p = 0.0011 for LPS + vehicle vs saline + vehicle), whereas TG6-10-1 treatment opposed this effect (##p = 0.0033 for LPS + TG6-10-1 vs LPS + vehicle, n = 5–7 mice per group, one-way ANOVA and Bonferroni). Data are shown as mean ± SEM. (D) LPS decreased synaptophysin protein levels without reaching significance in the same mice. (ns, p ≥ 0.05, n = 5–7 mice per group, one-way ANOVA and Bonferroni).
3.5. EP2 antagonist attenuates the activation of microglia after LPS injection
Experiments were then performed to examine the effect of EP2 antagonist on gliosis. Three days after LPS injection, mice were sacrificed to investigate the activation of glia cells. Compared with saline group, many Iba1-stained microglia in LPS group that were treated with vehicle showed an amoeboid morphology with enlarged cell body (Fig. 5A and B). However, there was no obvious change in glial fibrillary acidic protein (GFAP) staining between saline treatment and LPS treatment (Fig. 5C and D). EP2 antagonist significantly reduced the Iba-1 area (%) in both CA1 (n = 7–9 in LPS vehicle/TG6-10-1 group, p < 0.001) and CA3 regions (n = 7–9 in LPS vehicle/TG6-10-1 group, p < 0.05) compared with vehicle treatment in LPS injected mice.
Fig. 5.
EP2 receptor antagonist treatment ameliorates microgliosis induced by systemic inflammation. (A–B) Representative fluorescence images (200 × total magnification) show positive Iba1 immunostaining (green) as a microglia mark in the hippocampal CA1 and CA3 regions. (A3, B3) Three days after LPS (5 mg/kg, i.p) injection, microgliosis is obvious in both CA1 and CA3 regions compared with saline group (A1, B1). (A4, B4) Administration of TG6-10-1 (10 mg/kg, s.c, 0.5 h after LPS injection) reduced microgliosis in both CA1 and CA3 region compared with LPS-vehicle group. (C–D) Quantification of microgliosis by measuring the area of Iba-1. TG6-10-1 treatment reduced the Iba-1 area significantly (n = 5 in saline-vehicle/TG6-10-1 group, n = 7–9 in LPS vehicle/TG6-10-1 group, ∗p = 0.013; ∗∗∗ p = 0.0003, one-way ANOVA and Bonferroni test). (C–D) Representative fluorescence images (200 × total magnification) show positive GFAP immunostaining (green) as a astrocyte mark in the hippocampus CA1 and CA3 regions. No significant difference was found betweeen LPS-vehicle group and LPS-TG6-10-1 group, (n = 3–5 in each group; ns, p ≥ 0.05 by one-way ANOVA and Bonferroni test, Data are shown as mean + SEM). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
To further explore whether neuroinflammation induced by LPS results in neuron loss or degenerating neurons, Nissl staining and FJB staining were performed three days after LPS injection. Nissl staining showed that there was no obvious neuron loss in LPS treated mice (n = 5, Fig. 6A and B), and that hippocampal cellular architecture was preserved. Sections from a status epilepticus mouse model induced by pilocarpine were used as a positive control for FJB staining. Interestingly, unlike the case after status epilepticus, degenerating neurons were not found in hippocampus CA1 and CA3 regions (n = 7, Fig. 6C and D). Neurodegeneration was similarly absent in cerebral cortex, amygdala, piriform cortex and thalamus (data not shown).
Fig. 6.
Systemic inflammation does not cause obvious neuron loss. (A–B) Representative images (50 × total magnification) of nissl staining showed no obvious difference between saline treated mice and LPS treated mice (n = 4–5). (C) Representative image (50 × total magnification) shows no obvious cellular FJB staining in the hippocampus three days after LPS (5 mg/kg, i.p) injection (n = 7). (D) The representative images of pilocarpine treated mouse hippocampus at day four were used as a positive control. Image B was taken at 50 × total magnification.
3.6. EP2 antagonist improves depressive behavior and prevents memory deficits following SAE
Depression-like behaviors following systemic inflammation have been reported (Anderson et al., 2015). Here, depressive behavior was evaluated by the sucrose preference test (SPT) 21 days after LPS injection. There were three cohorts in the test: saline group treated with vehicle or TG6-10-1; LPS group treated with vehicle; LPS group treated with TG6-10-1. As Fig. 7A–B shows, the saline group showed a preference to 1% sucrose solution (0.740 ± 0.117). LPS and vehicle treated mice displayed lower preference (0.616 ± 0.0962) than saline group, but LPS and TG6-10-1 treated mice showed no difference compared with saline group (n = 17–22, ∗∗p < 0.01 for saline-vehicle/TG6-10-1 vs LPS-vehicle). Data were also interpreted by estimation statistics (Ho et al., 2019) with a 95% confidence interval (95% CI), and the result was consistent with the ANOVA analysis (Fig. 7B).
Fig. 7.
EP2 receptor antagonist, TG6-10–1, improves depression-related behavior and prevents the development of long-term memory deficit. (A–B) Depression-like behavior was tested by sucrose preference test. (A) The mean difference for 2 comparisons (LPS-vehicle group and LPS-TG6-10-1 group) against the shared control (saline group) are shown in the above Cumming estimation plot. The vertical dashed lines to the right of each distribution indicate the SD of the distribution with the mean of each group shown by an open space. The raw data are plotted on the upper axes (∗∗p < 0.01, one-way ANOVA and Dunnett’s test). (B) Mean differences are plotted as bootstrapped sampling distributions. Each mean difference is depicted as a dot. Each 95% confidence interval is indicated by the ends of the vertical error bars. (C) Scheme of open field and novel object recognition test. (D) LPS (5 mg/kg, i.p) has no effect on mouse motor activity on day 27 (n = 15–22 in each group, ns = p > 0.05, one-way ANOVA and Dunnett’s test). (E) 28 days after LPS injection, vehicle-treated mice were unable to distinguish between the novel and familiar objects, while the group treated with TG6-10-1 maintained the ability to recognize the novel object (n = 17 in saline-vehicle/TG6-10-1 group, n = 13 in LPS-vehicle/TG6-10-1 groups, ∗∗∗p = 0.0001, ∗∗∗∗p < 0.0001, paired t-test).
To assess if the EP2 antagonist was able to prevent the development of long-term cognitive deficit, the novel object recognition (NOR) test was adopted to test memory. First, motor activity was tested by open field test, with the scheme shown in Fig. 7C. As Fig. 7D shows, during a 5 min exploration period, all three groups of mice travelled a similar distance on day 27 after saline or LPS (n = 15–22 in each group, ns, p ≥ 0.05, one-way ANOVA and Dunnett’s test). LPS-treated mice that had been administered vehicle 28 days previously failed to distinguish between the novel and familiar objects; they spent 14.8 ± 1.29 s exploring the novel object and 12.4 ± 1.70 s exploring the familiar object (n = 13, ns, p ≥ 0.05), indicating that memory acquisition is impaired following systemic inflammation. By contrast, mice treated with the EP2 antagonist were able to differentiate between novel and familiar objects (20.8 ± 2.27 s on the novel object compared to 10.8 ± 0.953 s on the familiar object; n = 13, ∗∗∗p < 0.001) (Fig. 7E). The impaired ability to recognize novel objects was not due to diminished motor activity since all cohorts travelled a similar distance in the open field test.
4. Discussion
Sepsis survivors have a higher risk of developing long-term depression and cognitive impairments (Prescott and Angus, 2018), which limits their return to work and school and degrades quality of life. Neuroinflammation has been considered to facilitate cognitive decline in sepsis survivors (Annane and Sharshar, 2015) and contributes to the development of depression (Brites and Fernandes, 2015). The EP2 receptor for PGE2 appears to be responsible for most of the neuroinflammation produced by COX-2 in seizure models (Rojas et al., 2020; Jiang et al., 2013, 2019). The major findings of this study are that in the LPS mouse model of SAE, brief exposure to a selective, brain-permeant EP2 receptor antagonist reduced neuroinflammation as manifested by microgliosis and elevated levels of cytokines and COX-2, restored the depressed levels of critical pre- and postsynaptic proteins, and concomitantly reduced depressive behavior and eliminated a long-term memory deficit in the novel object recognition test.
Activated microglia cells were found in patients who died from sepsis (Lemstra et al., 2007; Zrzavy et al., 2019) and are a main feature of neuroinflammation in preclinical models. Inhibition of microglial activation reduced long-term cognitive impairment in a rat model of sepsis (Michels et al., 2015). Microglia activation has also been proposed to contribute to the development of depression (Brites and Fernandes, 2015). In our study, microglia but not astrocytes in the hippocampus underwent structural changes consistant with activation after LPS. Our finding that an EP2 antagonist mitigated microglia activation is consistent with what was found in EP2 conditional knockout mice (Johansson et al., 2013). Our previous studies reported that EP2 mediates both activation (Quan et al., 2013) and death (Fu et al., 2015) of primary microglia. A report (Golia et al., 2019) that mRNA encoding EP2 and COX-2 are upregulated in microglia isolated from LPS treated mouse brain reinforces the idea that the EP2 receptor could be a valuable target for regulating the activity of microglia.
Fluorojade B staining is a reliable histological staining of neurons undergoing degeneration (Schmued and Hopkins, 2000). Exposure to both pilocarpine and kainic acid can induce status epilepticus and cause FJB positive staining (Curia et al., 2008). Here, brain sections from LPS treated mice showed no FJB staining. One possibility is that LPS causes brain dysfunction via dysregulated inflamammatory responses instead of destroying neurons directly. One study reported that neurodegeneration was observed in substantia nigra ten months after LPS injection (Zhao et al., 2020). However, aging itself can promote neurodegeneration in substantial nigra. It is possible that neurodegeneration observed in the previous study was the result of systemic inflammation and aging. Therefore, a second hit may be required for neuroinflammation induced neurodegeneration.
The sucrose preference test has been considered as an optimal way to assess mouse anhedonia, which is the core symptom of depression (Cryan et al., 2002; Liu et al., 2018a). Previous study has shown that a single dose of LPS (5 mg/kg, ip) could decrease sucrose preference (Anderson et al., 2015), as confirmed by our study. In preclinical studies COX-2 has been demonstrated to play a pivotal role in depression (Song et al., 2018) and inhibition of COX-2 rescued depressive behavior in both chronic unpredictable mild stress and LPS induced depression models (Song et al., 2019). However, the detailed mechanism by which the COX-2 - PGE2 signaling pathway regulates depression is still unknown. Our work indicates that EP2 receptors may play a contributing role in the mechanism by which COX-2 mediates depression. Because EP2 inhibition was beneficial for both preventing depression and cognitive impairment, it is possible that COX-2 - PGE2 - EP2 is a common pathway for depression and memory deficits caused by LPS. In addition, TG6-10-1 also reduced the level of COX-2 mRNA in kidney after LPS, indicating EP2 may also have a role on inflammation in the peripheral system (Golden et al., 2019).
Mice that underwent a systemic inflammatory response developed a memory deficit after 28 days, which was prevented by fleeting exposure to an EP2 antagonist. Memory problems can be caused by loss of neurons in memory circuits, or disruption of synaptic transmission in these circuits. It has been proposed that neuroinflammation contributes to neurodegeneration (Ransohoff, 2016), but in our studies LPS caused no neurodegeneration although it did reduce the levels of pre- and postsynaptic proteins in cortex. The protective effect of EP2 inhibition on cognitive deficits was associated with restored levels of synaptic proteins that had been downregulated during the acute phase of sepsis.
An hypothesis presented in Fig. 8 outlines how neuroinflammation in the absence of neuronal loss might impair cognitive processes. In this scheme, LPS initiates microglial activation. Even though the brain is rarely found as a primary infection source, there are several possible ways in which sepsis can cause neuroinflammation: peripheral cytokines crossing leaky BBB (Danielski et al., 2018), infiltration of monocytes and neutrophils (Andonegui et al., 2018; Trzeciak et al., 2019), or direct activation of brain cells by LPS through toll like receptor 4 (TLR4) (Chakravarty and Herkenham, 2005). Activation of pro-inflammatory cytokines has been reported to alter cognitive function by reducing the level of synaptic proteins (Liu et al., 2018b). PGE2 generated by the induced microglial COX-2 is well known from in vitro studies (Shie et al., 2005) to participate in an autocrine pathway that exacerbates microglial activation. The activated microglia can damage synapses, either by phagocytosis (Wu et al., 2015; York et al., 2018; Savage et al., 2019) or by emitting cytokines such as IL-1β or IL-6 that cause synapse loss (Mishra et al., 2012; Pozzi et al., 2018).
Fig. 8.
Hypothesis for septic-associated encephalopathy and cognitive deficits in the absence of frank neurodegeneration. PGE2 derived from microglial COX-2 activates EP2 receptors in an autocrine fashion, which exacerbates microglial activation. Activated microglia cause loss of synapses, either by phagocytosis akin to synaptic pruning, or by the action of released cytokines. The resulting synaptic damage impairs higher-order cognitive processes including memory and affective behaviors.
The COX-2 - PGE2 - EP2 signaling pathway regulates long-term potentiation (LTP) under physiological conditions (Li et al., 2018; Akaneya and Tsumoto, 2006). However,in pathological states, LPS impairs LTP and causes cognitive problems via excessive upregulation of COX-2 and EP2 receptor (Yang and Chen, 2008). The chief features of the model in Fig. 8 are the key role that EP2 receptor activation plays, the appearance of cognitive deficits in the absence of neurodegeneration, and the proposal that functional deficits in synaptic transmission underlie cognitive deficits. In the future it will be important to understand precisely how peripheral inflammation results in neuroinflammation, and how activated microglia impair synaptic transmission.
5. Conclusion
Taken together, these results suggest that EP2 receptor inhibition prevents cognitive deficit in SAE, and are consistent with the notion that resolved neuroinflammation itself can help improve cognitive deficits. Additionally, it is clear that neuroinflammation and cognitive deficits can occur without obvious neurodegeneration. Microgliosis and associated altered synaptic transmission may be one possible driver for the initiation of long-term affective and cognitive deficits in sepsis.
Funding
This work was supported by the National Institutes of Health, Grant Number R01 NS097776 (RD), U01 AG052460 (TG), and R01 NS112308 (RD).
Declaration of competing interest
The authors declare that they have no conflicts of interests.
Acknowledgements
C.J. is a member of the joint training program between Emory University School of Medicine and Xiangya School of Medicine, Central South University, and is supported by the China Scholarship Council. We appreciate the help of Dr. Asheebo Rojas and Dr. Varvel Nicholas.
Contributor Information
Chunxiang Jiang, Email: jchunxiang@126.com.
Ray Dingledine, Email: rdingle@emory.edu.
References
- Akaneya Y., Tsumoto T. Bidirectional trafficking of prostaglandin E2 receptors involved in long-term potentiation in visual cortex. J. Neurosci. : Off. J. Soc. Neurosci. 2006;26(40):10209–10221. doi: 10.1523/jneurosci.3028-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S.T., Commins S., Moynagh P.N., Coogan A.N. Lipopolysaccharide-induced sepsis induces long-lasting affective changes in the mouse. Brain Behav. Immun. 2015;43:98–109. doi: 10.1016/j.bbi.2014.07.007. [DOI] [PubMed] [Google Scholar]
- Andonegui G., Zelinski E.L., Schubert C.L., Knight D., Craig L.A., Winston B.W., Spanswick S.C., Petri B., Jenne C.N., Sutherland J.C., Nguyen R., Jayawardena N., Kelly M.M., Doig C.J., Sutherland R.J., Kubes P. Targeting inflammatory monocytes in sepsis-associated encephalopathy and long-term cognitive impairment. JCI insight. 2018;3(9) doi: 10.1172/jci.insight.99364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annane D., Sharshar T. Cognitive decline after sepsis. Lancet Respir. Med. 2015;3(1):61–69. doi: 10.1016/s2213-2600(14)70246-2. [DOI] [PubMed] [Google Scholar]
- Antunes M., Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cognit. Process. 2012;13(2):93–110. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellantuono I., de Cabo R., Ehninger D., Di Germanio C., Lawrie A., Miller J., Mitchell S.J., Navas-Enamorado I., Potter P.K., Tchkonia T., Trejo J.L., Lamming D.W. A toolbox for the longitudinal assessment of healthspan in aging mice. Nat. Protoc. 2020;15(2):540–574. doi: 10.1038/s41596-019-0256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brites D., Fernandes A. Neuroinflammation and depression: microglia activation, extracellular microvesicles and microRNA dysregulation. Front. Cell. Neurosci. 2015;9:476. doi: 10.3389/fncel.2015.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buras J.A., Holzmann B., Sitkovsky M. Animal models of sepsis: setting the stage. Nat. Rev. Drug Discov. 2005;4(10):854–865. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- Chakravarty S., Herkenham M. Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J. Neurosci. : Off. J. Soc. Neurosci. 2005;25(7):1788–1796. doi: 10.1523/jneurosci.4268-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H.Y., Wickel J., Brunkhorst F.M., Geis C. Sepsis-associated encephalopathy: from delirium to dementia? J. Clin. Med. 2020;9(3) doi: 10.3390/jcm9030703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan J.F., Markou A., Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol. Sci. 2002;23(5):238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Curia G., Longo D., Biagini G., Jones R.S., Avoli M. The pilocarpine model of temporal lobe epilepsy. J. Neurosci. Methods. 2008;172(2):143–157. doi: 10.1016/j.jneumeth.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielski L.G., Giustina A.D., Badawy M., Barichello T., Quevedo J., Dal-Pizzol F., Petronilho F. Brain barrier breakdown as a cause and consequence of neuroinflammation in sepsis. Mol. Neurobiol. 2018;55(2):1045–1053. doi: 10.1007/s12035-016-0356-7. [DOI] [PubMed] [Google Scholar]
- Deacon R.M. Assessing nest building in mice. Nat. Protoc. 2006;1(3):1117–1119. doi: 10.1038/nprot.2006.170. [DOI] [PubMed] [Google Scholar]
- Fu Y., Yang M.S., Jiang J., Ganesh T., Joe E., Dingledine R. EP2 receptor signaling regulates microglia death. Mol. Pharmacol. 2015;88(1):161–170. doi: 10.1124/mol.115.098202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gofton T.E., Young G.B. Sepsis-associated encephalopathy. Nat. Rev. Neurol. 2012;8(10):557–566. doi: 10.1038/nrneurol.2012.183. [DOI] [PubMed] [Google Scholar]
- Golden J., Illingworth L., Kavarian P., Escobar O., Delaplain P., Isani M., Wang J., Lim J., Bowling J., Bell B., Gayer C.P., Grishin A., Ford H.R. Augusta, Ga.; 2019. EP2 Receptor Blockade Attenuates COX-2 Upregulation during Intestinal Inflammation. Shock. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golia M.T., Poggini S., Alboni S., Garofalo S., Ciano Albanese N., Viglione A., Ajmone-Cat M.A., St-Pierre A., Brunello N., Limatola C., Branchi I., Maggi L. Interplay between inflammation and neural plasticity: both immune activation and suppression impair LTP and BDNF expression. Brain Behav. Immun. 2019;81:484–494. doi: 10.1016/j.bbi.2019.07.003. [DOI] [PubMed] [Google Scholar]
- Guan P.P., Wang P. Integrated communications between cyclooxygenase-2 and Alzheimer’s disease. Faseb. J. : Off. Publ. Federation Am. Soc. Exp. Biol. 2019;33(1):13–33. doi: 10.1096/fj.201800355RRRR. [DOI] [PubMed] [Google Scholar]
- Ho J., Tumkaya T., Aryal S., Choi H., Claridge-Chang A. Moving beyond P values: data analysis with estimation graphics. Nat. Methods. 2019;16(7):565–566. doi: 10.1038/s41592-019-0470-3. [DOI] [PubMed] [Google Scholar]
- Huang W.Y., Liu K.H., Lin S., Chen T.Y., Tseng C.Y., Chen H.Y., Wu H.M., Hsu K.S. NADPH oxidase 2 as a potential therapeutic target for protection against cognitive deficits following systemic inflammation in mice. Brain Behav. Immun. 2020;84:242–252. doi: 10.1016/j.bbi.2019.12.006. [DOI] [PubMed] [Google Scholar]
- Iwashyna T.J., Ely E.W., Smith D.M., Langa K.M. Long-term cognitive impairment and functional disability among survivors of severe sepsis. Jama. 2010;304(16):1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong H.K., Jou I., Joe E.H. Systemic LPS administration induces brain inflammation but not dopaminergic neuronal death in the substantia nigra. Exp. Mol. Med. 2010;42(12):823–832. doi: 10.3858/emm.2010.42.12.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Quan Y., Ganesh T., Pouliot W.A., Dudek F.E., Dingledine R. Inhibition of the prostaglandin receptor EP2 following status epilepticus reduces delayed mortality and brain inflammation. Proc. Natl. Acad. Sci. U.S.A. 2013;110(9):3591–3596. doi: 10.1073/pnas.1218498110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Yu Y., Kinjo E.R., Du Y., Nguyen H.P., Dingledine R. Suppressing pro-inflammatory prostaglandin signaling attenuates excitotoxicity-associated neuronal inflammation and injury. Neuropharmacology. 2019;149:149–160. doi: 10.1016/j.neuropharm.2019.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson J.U., Pradhan S., Lokteva L.A., Woodling N.S., Ko N., Brown H.D., Wang Q., Loh C., Cekanaviciute E., Buckwalter M., Manning-Bog A.B., Andreasson K.I. Suppression of inflammation with conditional deletion of the prostaglandin E2 EP2 receptor in macrophages and brain microglia. J. Neurosci. : Off. J. Soc. Neurosci. 2013;33(40):16016–16032. doi: 10.1523/jneurosci.2203-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi D.S., Campos A.C.P., Qu H., Forrester S.J., Pagano R.L., Lassegue B., Sadikot R.T., Griendling K.K., Hernandes M.S. Poldip2 mediates blood-brain barrier disruption in a model of sepsis-associated encephalopathy. J. Neuroinflammation. 2019;16(1):241. doi: 10.1186/s12974-019-1575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemstra A.W., Groen in’t Woud J.C., Hoozemans J.J., van Haastert E.S., Rozemuller A.J., Eikelenboom P., van Gool W.A. Microglia activation in sepsis: a case-control study. J. Neuroinflammation. 2007;4:4. doi: 10.1186/1742-2094-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Serafin E., Baccei M.L. Prostaglandin signaling governs spike timing-dependent plasticity at sensory synapses onto mouse spinal projection neurons. J. Neurosci. : Off. J. Soc. Neurosci. 2018;38(30):6628–6639. doi: 10.1523/jneurosci.2152-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.Y., Yin C.Y., Zhu L.J., Zhu X.H., Xu C., Luo C.X., Chen H., Zhu D.Y., Zhou Q.G. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat. Protoc. 2018;13(7):1686–1698. doi: 10.1038/s41596-018-0011-z. [DOI] [PubMed] [Google Scholar]
- Liu Y., Zhang Y., Zheng X., Fang T., Yang X., Luo X., Guo A., Newell K.A., Huang X.F., Yu Y. Galantamine improves cognition, hippocampal inflammation, and synaptic plasticity impairments induced by lipopolysaccharide in mice. J. Neuroinflammation. 2018;15(1):112. doi: 10.1186/s12974-018-1141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueptow L.M. Novel object recognition test for the investigation of learning and memory in mice. JoVE : JoVE. 2017:126. doi: 10.3791/55718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason K.L., Rogers L.M., Soares E.M., Bani-Hashemi T., Erb Downward J., Agnew D., Peters-Golden M., Weinberg J.B., Crofford L.J., Aronoff D.M. Intrauterine group A streptococcal infections are exacerbated by prostaglandin E2. J. Immunol. 2013;191(5):2457–2465. doi: 10.4049/jimmunol.1300786. (Baltimore, Md. : 1950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels M., Vieira A.S., Vuolo F., Zapelini H.G., Mendonca B., Mina F., Dominguini D., Steckert A., Schuck P.F., Quevedo J., Petronilho F., Dal-Pizzol F. The role of microglia activation in the development of sepsis-induced long-term cognitive impairment. Brain Behav. Immun. 2015;43:54–59. doi: 10.1016/j.bbi.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Mishra A., Kim H.J., Shin A.H., Thayer S.A. Synapse loss induced by interleukin-1β requires pre- and post-synaptic mechanisms. J. Neuroimmune Pharmacol. : Off. J. Soc. NeuroImmune Pharmacol. 2012;7(3):571–578. doi: 10.1007/s11481-012-9342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opal S.M. Endotoxins and other sepsis triggers. Contrib. Nephrol. 2010;167:14–24. doi: 10.1159/000315915. [DOI] [PubMed] [Google Scholar]
- Pozzi D., Menna E., Canzi A., Desiato G., Mantovani C., Matteoli M. The communication between the immune and nervous systems: the role of IL-1β in synaptopathies. Front. Mol. Neurosci. 2018;11:111. doi: 10.3389/fnmol.2018.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott H.C., Angus D.C. Enhancing recovery from sepsis: a review. Jama. 2018;319(1):62–75. doi: 10.1001/jama.2017.17687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan Y., Jiang J., Dingledine R. EP2 receptor signaling pathways regulate classical activation of microglia. J. Biol. Chem. 2013;288(13):9293–9302. doi: 10.1074/jbc.M113.455816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff R.M. How neuroinflammation contributes to neurodegeneration. Science (New York, N.Y.) 2016;353(6301):777–783. doi: 10.1126/science.aag2590. [DOI] [PubMed] [Google Scholar]
- Reznik M.E., Merkler A.E., Mahta A., Murthy S.B., Claassen J., Kamel H. Long-term risk of seizures in adult survivors of sepsis. Neurology. 2017;89(14):1476–1482. doi: 10.1212/wnl.0000000000004538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas A., Ganesh T., Manji Z., O’Neill T., Dingledine R. Inhibition of the prostaglandin E2 receptor EP2 prevents status epilepticus-induced deficits in the novel object recognition task in rats. Neuropharmacology. 2016;110(Pt A):419–430. doi: 10.1016/j.neuropharm.2016.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas A., Ganesh T., Wang W., Wang J., Dingledine R. A rat model of organophosphate-induced status epilepticus and the beneficial effects of EP2 receptor inhibition. Neurobiol. Dis. 2020;133:104399. doi: 10.1016/j.nbd.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadikot R.T., Zeng H., Azim A.C., Joo M., Dey S.K., Breyer R.M., Peebles R.S., Blackwell T.S., Christman J.W. Bacterial clearance of Pseudomonas aeruginosa is enhanced by the inhibition of COX-2. Eur. J. Immunol. 2007;37(4):1001–1009. doi: 10.1002/eji.200636636. [DOI] [PubMed] [Google Scholar]
- Savage J.C., St-Pierre M.K., Hui C.W., Tremblay M.E. Microglial ultrastructure in the Hippocampus of a lipopolysaccharide-induced sickness mouse model. Front. Neurosci. 2019;13:1340. doi: 10.3389/fnins.2019.01340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmued L.C., Hopkins K.J. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874(2):123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Serrano G.E., Lelutiu N., Rojas A., Cochi S., Shaw R., Makinson C.D., Wang D., FitzGerald G.A., Dingledine R. Ablation of cyclooxygenase-2 in forebrain neurons is neuroprotective and dampens brain inflammation after status epilepticus. J. Neurosci. : Off. J. Soc. Neurosci. 2011;31(42):14850–14860. doi: 10.1523/jneurosci.3922-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewal R.K., Modi M., Saikia U.N., Chakrabarti A., Medhi B. Increase in seizure susceptibility in sepsis like condition explained by spiking cytokines and altered adhesion molecules level with impaired blood brain barrier integrity in experimental model of rats treated with lipopolysaccharides. Epilepsy Res. 2017;135:176–186. doi: 10.1016/j.eplepsyres.2017.05.012. [DOI] [PubMed] [Google Scholar]
- Shie F.S., Montine K.S., Breyer R.M., Montine T.J. Microglial EP2 is critical to neurotoxicity from activated cerebral innate immunity. Glia. 2005;52(1):70–77. doi: 10.1002/glia.20220. [DOI] [PubMed] [Google Scholar]
- Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.D., Coopersmith C.M., Hotchkiss R.S., Levy M.M., Marshall J.C., Martin G.S., Opal S.M., Rubenfeld G.D., van der Poll T., Vincent J.L., Angus D.C. The third international consensus definitions for sepsis and septic shock (Sepsis-3) Jama. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Q., Fan C., Wang P., Li Y., Yang M., Yu S.Y. Hippocampal CA1 betaCaMKII mediates neuroinflammatory responses via COX-2/PGE2 signaling pathways in depression. J. Neuroinflammation. 2018;15(1):338. doi: 10.1186/s12974-018-1377-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Q., Feng Y.B., Wang L., Shen J., Li Y., Fan C., Wang P., Yu S.Y. COX-2 inhibition rescues depression-like behaviors via suppressing glial activation, oxidative stress and neuronal apoptosis in rats. Neuropharmacology. 2019;160:107779. doi: 10.1016/j.neuropharm.2019.107779. [DOI] [PubMed] [Google Scholar]
- Trzeciak A., Lerman Y.V., Kim T.H., Kim M.R., Mai N., Halterman M.W., Kim M. Long-term microgliosis driven by acute systemic inflammation. J. Immunol. 2019;203(11):2979–2989. doi: 10.4049/jimmunol.1900317. (Baltimore, Md. : 1950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J.L., Rello J., Marshall J., Silva E., Anzueto A., Martin C.D., Moreno R., Lipman J., Gomersall C., Sakr Y., Reinhart K. International study of the prevalence and outcomes of infection in intensive care units. Jama. 2009;302(21):2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- Wu Y., Dissing-Olesen L., MacVicar B.A., Stevens B. Microglia: dynamic mediators of synapse development and plasticity. Trends Immunol. 2015;36(10):605–613. doi: 10.1016/j.it.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Chen C. Cyclooxygenase-2 in synaptic signaling. Curr. Pharmaceut. Des. 2008;14(14):1443–1451. doi: 10.2174/138161208784480144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York E.M., Bernier L.P., MacVicar B.A. Microglial modulation of neuronal activity in the healthy brain. Dev. Neurobiol. 2018;78(6):593–603. doi: 10.1002/dneu.22571. [DOI] [PubMed] [Google Scholar]
- Zhao J., Bi W., Xiao S., Lan X., Cheng X., Zhang J., Lu D., Wei W., Wang Y., Li H., Fu Y., Zhu L. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci. Rep. 2019;9(1):5790. doi: 10.1038/s41598-019-42286-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Wang Y., Zhou R., Li Y., Gao Y., Tu D., Wilson B., Song S., Feng J., Hong J.S., Yakel J.L. A novel role of NLRP3-generated IL-1beta in the acute-chronic transition of peripheral lipopolysaccharide-elicited neuroinflammation: implications for sepsis-associated neurodegeneration. J. Neuroinflammation. 2020;17(1):64. doi: 10.1186/s12974-020-1728-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrzavy T., Hoftberger R., Berger T., Rauschka H., Butovsky O., Weiner H., Lassmann H. Pro-inflammatory activation of microglia in the brain of patients with sepsis. Neuropathol. Appl. Neurobiol. 2019;45(3):278–290. doi: 10.1111/nan.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]