Abstract
Introduction
Geriatric depression is frequently accompanied by cognitive complaints and inflammation that increase risk for treatment-resistant depression and dementia. Memantine, a neuroprotective drug, can improve depression, inflammation, and help prevent cognitive decline. In our six-month clinical trial, escitalopram/memantine (ESC/MEM) improved mood and cognition compared to escitalopram/placebo treatment (ESC/PBO; NCT01902004). In this report, we examined the impact of baseline inflammation on mood and cognitive outcomes.
Materials and methods
We measured a panel of inflammatory cytokine markers using Human 38-plex magnetic cytokine/chemokine kits (EMD Millipore, HCYTMAG-60K-PX38) in 90 older adults 60 years and older with major depression enrolled in a 6-month double-blind placebo-controlled trial of escitalopram + memantine (ESC/MEM) in depressed older adults with subjective memory complaints. Four cytokine factors were derived and linear models were estimated to examine the predictive ability of cytokine levels on treatment induced change in depression and cognition.
Results
Of the 90 randomized participants, 62 completed the 6-month follow up assessment. Both groups improved significantly on depression severity (HAM-D score), but not on cognitive outcomes at six months. Cytokine factor scores were not significantly different between ESC/MEM (n = 45) and ESC/PBO (n = 45) at baseline. Pro-inflammatory biomarkers at baseline predicted a decline in executive functioning in the ESC/PBO group but not in the ESC/MEM group, interaction F(1,52) = 4.63, p = .04.
Discussion
In this exploratory analysis, the addition of memantine to escitalopram provided a protective effect on executive functioning in older depressed adults. Future studies are needed to replicate the association of cytokine markers to antidepressant and neuroprotective treatment-related change in cognition in geriatric depression.
Keywords: Inflammation, Geriatric depression, Cognitive decline, Escitalopram, Memantine
1. Introduction
Geriatric depression and cognitive dysfunction are often comorbid (Charlton et al., 2014; Lee et al., 2007). Evidence of cognitive impairment has been found in up to two thirds of non-demented older adults with depression (Lanza et al., 2020). Underlying inflammation is linked to increased risk for Alzheimer’s disease and treatment-resistant depression (Zwicker et al., 2018). Existing antidepressants are able to reduce peripheral inflammation in humans and in animal models, while anti-inflammatory agents have been tried as add-on antidepressant treatment strategies with some promise (Eyre et al., 2016, 2017; Kohler et al., 2016; Lindqvist et al., 2017; Lu et al., 2019; Sun et al., 2020). Specifically, escitalopram can influence the metabolic pathways responsible for oxidative stress and inflammatory mechanisms of depression (Bhattacharyya et al., 2019). Additionally, neuroprotective agents like memantine have been reported to produce antidepressant and neuroprotective effects in animal models via neuroplastic effects on hippocampal cell proliferation and decreased neuroinflammation (Takahashi et al., 2018; Wei et al., 2016).
Neuroinflammation and excitotoxicity contribute to the pathophysiology of both depression and neurodegeneration (Bauer and Teixeira, 2019; Bhalla et al., 2009; Conwell et al., 1998; Lavretsky et al., 1998, 2020; Pelton et al., 2016; Reynolds et al., 2006; Rush et al., 2006; Steffens, 2008; Vega et al., 2016). Antidepressants coupled with drugs that target glutamate transmission and excitotoxicity, therefore, offer a promising novel “mood plus cognitive enhancer” neuroprotective approach to treatment. Memantine, an NMDA antagonist, inhibits calcium influx and excitotoxicity while preserving the physiological activation of the receptor.
We recently conducted a randomized, double-blind, placebo-controlled trial of escitalopram combined with placebo (ESC/PBO) or memantine (ESC/MEM) in depressed elderly with subjective memory complaints (NCT01902004). No differences were observed in the depression remission rate at 6- or 12-months following initiation of treatment (Lavretsky et al., 2020). However, compared to ESC/PBO, ESC/MEM treatment produced improvements in delayed recall and executive functioning at 12-month follow-up. The current report examines the effects of inflammation at baseline on clinical and cognitive outcomes at 6-month follow up.
Several studies have highlighted an association between depression symptoms and increased markers of peripheral inflammation (Dantzer et al., 2008; Miller and Raison, 2016), which theoretically spurs neuro-inflammation downstream the manifests as depression or cognitive decline (Franceschi and Campisi, 2014). The evidence linking inflammation to increased cognitive dysfunction in aging is more mixed, and may vary as a function of the presence and/or stage of neurodegenerative disease progression (Lai et al., 2017; Lassale et al., 2019; Ng et al., 2018; Yang et al., 2015). Nonetheless, inflammation remains a commonly suspected mechanism of cognitive impairment in aging (Franceschi and Campisi, 2014).
Despite the fact that inflammation is a targeted mechanism in both depression and cognitive impairment among older adults, there are surprisingly few studies that approach these symptoms as concurrent outcomes of a similar mechanistic process. The parent clinical trial of the current exploratory study offered a unique opportunity to investigate inflammation as a predictor of treatment response for both depression and cognitive outcomes. We examined baseline markers of peripheral inflammation and change scores following study treatment, testing differential treatment response between groups for either depression or cognitive function. The rationale was that our findings could uniquely contribute to mechanistic understanding of these aging-related symptoms through manipulating neurotransmitter systems intricately connected to neuro-inflammation (Haroon et al., 2017).
2. Methods
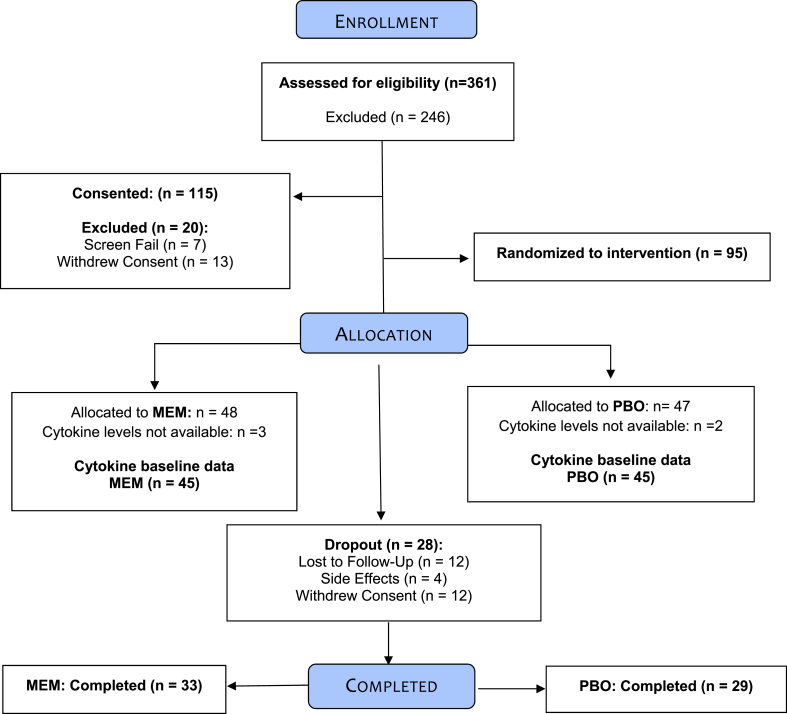
Study methods have been previously described and will be briefly summarized. This study was approved by the University of California, Los Angeles (UCLA) Institutional Review Board and all participants signed informed consent. Between October 2013 and May 2018, we recruited individuals from the UCLA Neuropsychiatric Hospital inpatient and outpatient service and from community advertising. Three hundred and sixty-one individuals were assessed via phone screening, yielding 115 participants for in-person diagnostic interview. Of these, 95 met inclusion criteria and underwent randomization to one of the treatment arms. The sample used in the present study included n = 45 randomized to receive either escitalopram with placebo and n = 45 randomized to receive escitalopram with memantine, who also had baseline inflammation data available. (please see CONSORT diagram; Fig. 1).
Fig. 1.
CONSORT diagram.
The Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (SCID DSM–5) was administered by a study psychiatrist or a trained, masters-level research associate to diagnose Major Depressive Disorder (MDD) and rule out other diagnoses (e.g., psychosis). Inclusion criteria were: 1) presence of MDD according to DSM-5 criteria; 2) score of ≥16 on the 24-item Hamilton Rating Scale for Depression (HAM-D) (Hamilton, 1960); 3) subjective memory complaints (affirmative response to the question, “Have you experienced memory problems over the past six months?” during phone screening); 4) did not have dementia (described below); and 4) age ≥ 60 years. Exclusion criteria were: 1) lifetime history of any psychiatric disorder (except MDD, co-morbid anxiety, or insomnia); 2) recent and/or current unstable medical or neurological disorders; 3) diagnosis of dementia; or 4) known allergic reaction to escitalopram or memantine. Participants were free of psychotropic medications for at least two weeks before starting the trial (four weeks in the case of fluoxetine). No participants were currently taking a cognitive enhancer at study entry.
2.1. Screening for dementia
Participants were screened for dementia using the following procedures: 1) administration of the Clinical Dementia Rating Scale (Berg, 1988), scores of >0.5 were excluded; 2) review of a standard battery of hematologic studies, blood chemistries, liver and thyroid function tests, B12 and folate levels, and RPR test; 3) a neurological and psychiatric examination; 4) review of neuropsychological scores on the study test battery; and a score of ≤24 on the Mini-Mental State Examination (Folstein et al., 1975, 1985). Those who met diagnosis of dementia were excluded.
2.2. Diagnosis of mild cognitive impairment (MCI)
Whether or not eligible participants met criteria for MCI was determined using established guidelines (Langa and Levine, 2014; Petersen, 2004). MCI was defined as: 1) a stage between normal cognition and dementia (Clinical Dementia Rating Scale (CDR) score of 0.5 (Hughes et al., 1982)); 2) patient-reported decline in cognition; 3) objective impairment on neurocognitive testing; 4) no significant functional impairment. Objective impairment on neurocognitive testing was defined as scoring one standard deviation (SD) below age- and education-specific norms on at least two screening memory tests (Hopkins Verbal Learning Test, Revised, [either Total or Delayed scores] and Wechsler Memory Scale Third Edition, WMS-III, verbal paired associates, [either Total or Delayed scores]). Participants who met this criterion and had a CDR score of 0.5 were classified as having amnestic MCI (either single or multiple domains) (Winblad et al., 2004).
2.2.1. Randomization
Eligible participants were randomized in a 1:1 ratio to escitalopram/placebo or escitalopram/memantine using a computer-generated random assignment scheme. A block randomization strategy (with randomly selected blocks of length 4 and 6) was used to maintain balance throughout the trial.
2.2.2. Intervention procedures
All study participants received a 14-day supply of the study medications including 10–20 mg of escitalopram daily open-label throughout the trial. Matching capsules containing memantine (MEM) or placebo were given and titrated from 5 mg/day up to 10 mg twice daily (i.e., 20 mg per day) during the first four weeks. Depending on Clinical Global Impressions (CGI) scale ratings at the end of week 4, participants were either titrated up to 20 mg escitalopram/day (CGI ≥3) or were continued on the same dose (CGI rating of 1 or 2). If participants reported side effects attributed to the study medications they were instructed to decrease their dosage. The minimum allowed dosages were 5 mg once daily for MEM and 10 mg once daily for escitalopram.
2.3. Study measures
2.3.1. Markers of inflammation
ACD-anticoagulated blood was transported at room temperature and processed within 18 h of blood draw. Whole blood was centrifuged at 2000 rpm for 10 min and plasma immediately stored at −80 °C. Human 38-plex magnetic cytokine/chemokine kits (EMD Millipore, HCYTMAG-60K-PX38) were used per manufacturer’s instructions. The panel includes IL-1RA, IL-10, IL-1α, IL-1β, IL-6, IFN-α2, TNF/TNF-α, TNF-β/LT-α, sCD40L, IL-12p40, IFN-γ, IL-12/IL-12p70, IL-4, IL-5, IL-13, IL-9, IL-17A, GRO/CXCL1, IL-8/CXCL8, eotaxin/CCL11, MDC/CCL22, fractalkine/CX3CL1, IP-10/CXCL10, MCP-1/CCL2, MCP-3/CCL7, MIP-1α/CCL3, MIP-1β/CCL4, IL-2, IL-7, IL-15, GM-CSF, Flt-3L/CD135, G-CSF, IL-3, EGF, FGF-2, TGF-α, and VEGF. Fluorescence was quantified using a Luminex 200™ instrument. Cytokine/chemokine concentrations were calculated using Milliplex Analyst software version 4.2 (EMD Millipore). Luminex assay and analysis were performed by the UCLA Immune Assessment Core. Only those cytokines with no more than 20% of samples were undetectable were included in analyses. Nineteen cytokines (IL-17A, IL-2, IFN-γ, VEGF, IL-12p70, IL-8, MIP-1β, TNF-α, IL-6, IL-1RA, IL-10, Eotaxin, MCP-1, IP-10, MDC, sCD40L, GRO, Fractalkine, and IFN-α2) were identified in this manner. The specimens were processed in three different batches (28.9%, 56.7% and 14.4% were processed separately). There were no differences in the number of ESC/PBO and ESC/MEM samples processed in the different batches.
2.3.2. Neuropsychological battery
The following test battery was administered at baseline and 6 months. We transformed raw scores to z-scores using the sample mean and standard deviation, reversing z-scores when necessary so that higher z-scores represent better performance for all measures. These z-scores were aggregated into domains, chosen o priori, based on the general processes involvedaccording to standard neuropsychological practice (REF) and consistent with our prior report from this sample (Harvey, 2019; Lavretsky et al., 2019): Learning (California Verbal Learning Test-II [Trial 1 through 5 Total] (Delis Kaplan et al., 2000), Rey–Osterrieth Complex Figure Test [3-min recall] (Meyers and Meyers, 1995), Verbal Pairs Associate [immediate recall]) (Wechsler, 2009); Delayed Recall (California Verbal Learning Test-II [long delayed free recall], Rey–Osterrieth Complex Figure Test [30-min delayed recall], Verbal Paired Associates (delayed recall), and Executive Functioning (Trail Making Test B (Heaton et al., 2004; Reitan, 1958), Stroop interference (CJ and SM, 1978), Controlled Oral Word Association test [FAS] (Heaton et al., 2004; Strauss et al., 2006).
2.4. Statistical approach
Data were inspected for outliers, homogeneity of variance and other assumptions to ensure their appropriateness for parametric statistical tests. The cytokine concentration levels were log-transformed and in order to reduce the number of cytokine markers in analyses, we used the iterated principal factor method with varimax rotation to obtain factor scores from the log-transformed cytokine concentrations at baseline. The number of factors was determined by using two criteria: (1) use of a scree plot (plot of eigenvalues on the y-axis and the number of factors on the x-axis) to determine the point where the slope of the curve leveled off to indicate the number of factors that should be kept, and (2) the total amount of variability of the original items explained by each factor solution. Following Hair et al. (2010), factor loading of 0.5 and above was chosen as the cut-off (Hair et al., 2010).
We first used general linear models to examine the association of the cytokine factor scores with cognitive domain scores at baseline, controlling for age, sex, BMI and batch. We also examined models including depression (HAM-D) scores to evaluate whether any shared variance with depression washed out emergent relationships between inflammation and cognition, or if these relationships were distinct from depression. We then estimated similar general linear models to examine whether the factor scores at baseline were associated with 6-month change in cognitive domain scores. As above, age, sex, BMI and batch were used as covariates. Given that this is the first study to examine the association of cytokine markers to anti-depressant treatment induced change in cognition in older depressed patients, we set the significance level at p ≤ .05 for all analyses.
3. Results
3.1. Sample
Baseline demographic and clinical variables are presented in Table 1. Treatment groups did not differ in any of these measures. Sixty-two subjects completed study: 33 ESC/MEM (out of 45 at baseline = 73% completers) and 29 ESC/PBO (out of 45 at baseline = 64% completers), see Fig. 1. Completers and drop-outs did not differ in any of the baseline measures (see Supplementary Table 1). Mean daily escitalopram dose was 9.9 mg (SD = 1.5; range: 5–20 mg). Mean daily memantine dose was 19.3 mg (SD = 2.6; range 10–20 mg). Measures of tolerability and dropouts due to side-effects did not differ between the groups. Remission rate within ESC/MEM was 47.9%, compared to 31.9% in ESC/PBO at 6 months (χ2(1) = 2.0, p = .15). Changes in HAM-D, learning, delayed recall and executive functioning scores were not significantly different between groups. Both groups improved significantly in HAM-D and neither group improved in cognitive outcomes at the end of the 6-month intervention. Please refer to our earlier paper describing the results of the parent clinical trial for more detailed results (Lavretsky et al., 2020).
Table 1.
Demographic and clinical characteristics.
| ESC/PBO (n = 45) | ESC/MEM (n = 45) | P value | ||
|---|---|---|---|---|
| Sex, n(%) | Male | 21 (46.7%) | 21 (46.7%) | 1.0 |
| Female | 24 (53.3%) | 24 (53.3%) | ||
| Race, n(%) | White | 31 (69%) | 35 (77%) | 0.34 |
| Black | 2 (4%) | 4 (9%) | ||
| Hispanic | 8 (18%) | 3 (7%) | ||
| Other | 4 (9%) | 3 (7%) | ||
| Age, mean (SD) | 72.62 (6.8) | 70.95 (7.0) | 0.26 | |
| Education years, mean (SD) | 16.24 (2.7) | 15.64 (2.4) | 0.26 | |
| BMI | 26.53 (5.6) | 26.57 (6.2) | 0.97 | |
| MMSE | 27.58 (1.7) | 28.20 (1.7) | 0.09 | |
| MCI, n(%) | 6 (13.3%) | 7 (15.6%) | 0.9 | |
| Age onset | 46.07 (22.5) | 43.85 (23.3) | 0.66 | |
| Number of depressive episodes | 4.67 (4.0) | 5.44 (5.2) | 0.46 | |
| Chronic Depression | 32 (73%) | 35 (78%) | 0.58 | |
| HAM-D, mean (SD) | 17.80 (2.38) | 17.80 (2.30) | 1.0 | |
Notes: MCI = Mild Cognitive Impairment; BMI = Body Mass Index; MMSE = Mini-Mental Status Examination; HAM-D = Hamilton Depression Scale.
3.2. Cytokine factor analysis
Four factors were chosen as the optimal number of factors to be retained, accounting for 74% of the variance. The factor loadings are presented in Table 2. Interestingly, three of the four factors identified mirrored clear biological functions. All the cytokines included in Factor 1 are typically associated to T cell responses, particularly type 1 and type 17 helper T cells (Th1 and Th17) (Damsker et al., 2010). Factor 2 includes proto-typical pro-inflammatory cytokines and chemokines (IL-8, MIP-1 β, IL-6 and TNF-α) together with their prototypical regulators IL-10 and IL-1RA, thus bearing an innate inflammatory signature (Turner et al., 2014; Zhang and An, 2007). Factor 3 only includes chemokines primarily driving the recruitment of eosinophils/basophils, T cells and monocytes (Turner et al., 2014; Zhang and An, 2007). Factor 4 was the only one without a clear biological function. Factor 4 included sCD40L and Fractalkine, both involved in vascular inflammation; while all four analytes are associated with neuroinflammation.
Table 2.
Cytokine factor loadings.
| Factor1 | Factor2 | Factor3 | Factor4 | |
|---|---|---|---|---|
| IL-17A | 0.88 | 0.17 | 0.08 | −0.03 |
| IL-2 | 0.82 | 0.24 | 0.01 | 0.31 |
| IFN- γ | 0.82 | 0.40 | 0.14 | −0.08 |
| VEGF | 0.77 | 0.31 | −0.05 | 0.17 |
| IL-12p70 | 0.76 | 0.31 | 0.17 | 0.32 |
| IL-8 | 0.27 | 0.89 | −0.24 | 0.04 |
| MIP-1β | 0.31 | 0.84 | 0.17 | 0.06 |
| TNF-α | 0.31 | 0.81 | 0.23 | 0.08 |
| IL-6 | 0.36 | 0.75 | 0.29 | 0.18 |
| IL-1RA | 0.15 | 0.64 | −0.30 | 0.32 |
| IL-10 | 0.47 | 0.50 | 0.31 | 0.31 |
| Eotaxin | 0.07 | 0.02 | 0.91 | 0.05 |
| MCP-1 | −0.04 | 0.13 | 0.87 | −0.03 |
| IP-10 | 0.11 | −0.02 | 0.81 | 0.10 |
| MDC | 0.14 | 0.04 | 0.81 | 0.14 |
| sCD40L | 0.04 | 0.18 | 0.29 | 0.77 |
| GRO | 0.10 | −0.01 | −0.24 | 0.73 |
| Fractalkine | 0.20 | 0.22 | 0.35 | 0.69 |
| IFN-α2 | 0.47 | 0.15 | 0.12 | 0.60 |
3.3. Baseline analyses
Cytokine factor scores were not significantly different between treatment groups at baseline. Factors 1, 2 and 3 were not significantly associated with any cognitive domain. However, increased scores on Factor 4 were associated with worse Learning and Delayed Recall scores: F(1,81) = 3.92, p = .05; F(1,81) = 4.31, p = .04, respectively (see Supplementary Table 2 for beta coefficients, standard errors and 95% confidence interavals for all associations). When depression (HAM-D) scores were added to the models, the results were sustained for delayed recall: Delayed Recall - F(1, 80) = 3.89, p = .05, and to a lesser extent for Learning - F(1, 80) = 3.46, p = .07.
3.4. Longitudinal analyses
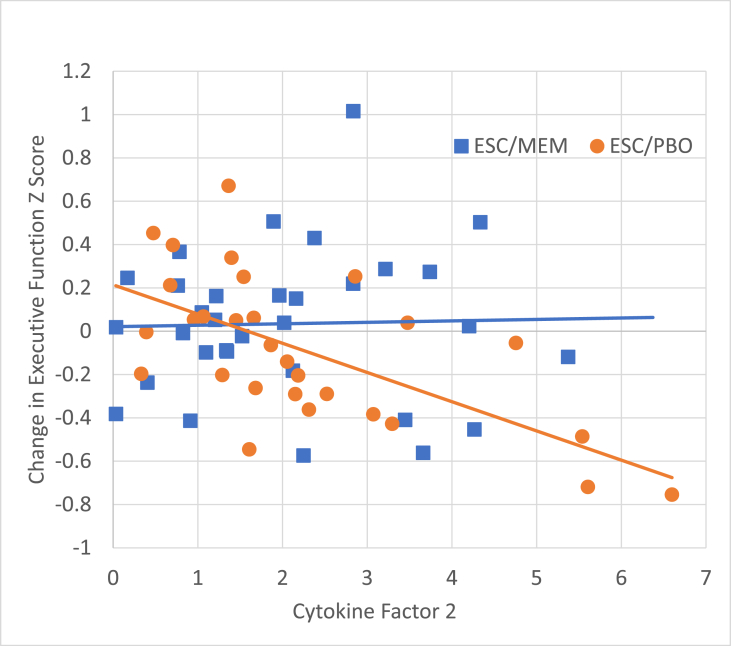
Cytokine factor scores at baseline were not associated with change in depression (HAM-D). We found that Factor 2 was differentially associated with change in Executive Function scores as a function of treatment group: interaction term F(1,52) = 4.63, p = .04. As seen in Fig. 2, baseline Factor 2 scores predicted decline in Executive Function only in the ESC/PBO group (slope = −0.13, p = .003) while the ESC/MEM exhibited no relationship between baseline inflammation scores and Executive Function decline (slope = 0.0, p = .9), despite similar distribution of Factor 2 baseline scores (see Supplementary Table 3 for beta coefficients, standard errors and 95% confidence interavals for all associations). No other association between baseline factor scores and change in cognitive domain scores were significant.
Fig. 2.
Cytokine Factor 2 predicted a decline in executive functioning in the ESC/PBO group (slope = −0.13, p = .003) but not in the ESC/MEM group (slope = 0.0, p = .9).
4. Discussion
In this study, we examined the relationship between inflammatory markers and neuropsychological functioning in older adults with depression treated with a combination of escitalopram combined with memantine or placebo. First, we found a cross-sectional relationship linking a set of inflammatory markers with learning and memory function that persisted after controlling for depression. Second, we found that increased pro-inflammatory factors (Factor 2) at baseline predicted decline in executive function, but only in the ESC/PBO group, with no such relationship observed in the ESC/MEM group. Notably, we did not find relationships among the same factors in both sets of analyses and in each set of analyses only one of the four factors was found to be related to cognition.
Elevated inflammation has been implicated in both depression and cognitive decline in aging (Ownby, 2010; Rosenblat et al., 2014), and this report extends this literature in a few key ways. Most previous studies tended to focus on the relationship of inflammation to either depression or cognitive impairment as an outcome (Elderkin-Thompson et al., 2012) not taking into account frequent comorbidity and shared underlying mechanisms (Kanchanatawan et al., 2018; Morimoto and Alexopoulos, 2013). In addition, most studies examined the role of isolated inflammatory markers, most commonly C-reactive Protein (CRP), Il-6, TNF-α (Lindqvist et al., 2017; Strawbridge et al., 2015; Yang et al., 2019), while we aggregated factors from a panel of cytokines that appear to represent concurrent function.
Most but not all cytokines included in the panel have been studied with respect to mood or cognition. Factor 2 contains a set of well-known pro-inflammatory markers often described in cognition and mood studies (da Fonseca et al., 2014; Elderkin-Thompson et al., 2012; Lai et al., 2017; Ng et al., 2018). While the cytokines that comprise Factor 4 are less well-studied than other markers in terms of cognitive outcomes, there is a growing literature uncovering their role in cognition and neurodegeneration. In mice models, IFN-a in CSF is associated with cognitive impairment (Sas et al., 2009). Higher levels of soluble CD40L has been associated with HIV-associated neuroinflammation (Ramirez et al., 2010). GRO is also implicated in inflammatory response to reactive oxygen species in mice models (Shen et al., 2010). Fractalkine moderates microglia activity in the CNS, its receptor CX3CL1 protects neurons from microglial neurotoxicity (Limatola and Ransohoff, 2014), and is linked to cognition and neurodegenerative diseases; however, the nature of these relationships requires further study (Finneran and Nash, 2019).
Our finding of the lack of influence of Factor 2 on cognitive outcomes in the ESC/MEM is intriguing and suggests that memantine may protect against pro-inflammatory cognitive decline. This observation further elaborates on the role of peripheral inflammation in geriatric depression and cognitive decline, and adds to our recent report from the same study using the functional enrichment transcriptome analysis that demonstrated that escitalopram-based remission was associated with functions related to cellular proliferation, apoptosis, and inflammatory response (Grzenda et al., 2020). Remission in the ESC/MEM group, however, was characterized by processes related to cellular clearance, metabolism, and cytoskeletal dynamics. Both treatment arms modulated inflammatory responses, albeit via different effector pathways. Memantine is an NMDA receptor agonist used to treat moderate to severe Alzheimer’s disease by reducing glutamatergic excitotoxicity (Cacabelos et al., 1999; Rogawski and Wenk, 2003). Dysfunctional glucose metabolism is emerging as a key player in the development of Alzheimer’s disease (Kuehn, 2020) (given the moniker “Type III Diabetes” (de la Monte and Wands, 2008)), and can increase oxidative stress and subsequently drive up neuroinflammation (Rosales-Corral et al., 2015). A similar mechanism has been proposed in the development of depression (Dantzer and Walker, 2014). Inflammation can lead to glutamatergic-related excitotoxicity and some have posited that this is the pathway that links inflammation to susceptibility for depression (Haroon et al., 2017). In line with our findings, studies of ketamine, another NMDA antagonist, have also suggested that it not only prevents glutamatergic excitotoxicity but also may have anti-inflammatory properties (Hudetz et al., 2009; Proescholdt et al., 2001). Taken together, our findings point to a possible role of memantine in protecting inflammation-driven cognitive decline in depressed older adults.
This study has limitations. The study was not specifically powered or designed for the presented analyses and will require replication and further study. The sample is relatively homogeneous with respect to demographic variables that might relate to the relatively preserved cognition or the level of inflammation in this cohort. In addition, cognitive function varied somewhat, although none of the participants had dementia, some met criteria for the mild cognitive impairment. Therefore, it will be important to understand these findings in studies with longitudinal follow-up to identify those who may be developing Alzheimer’s type or other neurodegenerative disorders. Participants were not required to fast before blood collection took place, introducing the possibility of uncontrolled biologic variability. While blood-based markers of inflammation are widely used as a proxy to study neuroinflammation given their easier accessibility, their signal gets diluted in the circulation compared to the peripheral site of inflammation; thus, it would be helpful to study the response of memantine treatment in measures more proximal to the central nervous system (e.g., cerebrospinal fluid) (Bettcher et al., 2018). Alternatively, ultra-sensitive methods for cytokine detection, like Simoa, may be used to improve detection of diluted signals of tissue inflammatory responses in the circulation. Several publications have confirmed the ultra-sensitivity of this technology and support its utility for the development of immune signatures and the identification of disease biomarkers (Rissin et al., 2010).
Importantly, we did not find a consistent pattern with respect to inflammatory factors and cognition. The most well-studied inflammatory markers related to cognition (i.e., Factor 2) did not correlate with cognitive functioning at baseline, only in analysis of change scores. Furthermore, associations varied among cognitive domains, with relationships found for learning and memory at baseline, and change in executive function over time. Given the exploratory nature of this study and the higher likelihood of spurious findings compared to hypothesis-driven analyses, it is critical to interpret our findings with caution. Furthermore, we acknowledge the incertitude of drawing links between highly complex, microscopic immune response and more abstract downstream performance on cognitive testing. While numerous studies take a similar theory-driven approach, our goal for the present study was to begin exploring these relationships to generate hypotheses for future research.
In summary, the present study highlights intriguing links between inflammation and cognitive outcomes in depressed older adults who were treated with escitalopram combined with memantine or placebo. The adverse effects of increased inflammation is well-studied in both depression and neurodegenerative disease, and it is important to consider overlapping neurobiological pathways for these burdensome disorders of aging. Our findings also implicate a role of modulating glutamate activity in protecting older adults with depression from inflammation related cognitive decline, a novel finding that requires further study.
Declaration of competing interest
None reported.
Acknowledgements
This work was funded by the NIH grants R01MH097892; AT009198 to HL and K08CA241337 to KVD. This work was further supported by the National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2020.100167.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Bauer M.E., Teixeira A.L. Inflammation in psychiatric disorders: what comes first? Ann. N. Y. Acad. Sci. 2019;1437:57–67. doi: 10.1111/nyas.13712. [DOI] [PubMed] [Google Scholar]
- Berg L. Clinical dementia rating (CDR) Psychopharmacol. Bull. 1988;24:637–639. [PubMed] [Google Scholar]
- Bettcher B.M., Johnson S.C., Fitch R., Casaletto K.B., Heffernan K.S., Asthana S., Zetterberg H., Blennow K., Carlsson C.M., Neuhaus J., Bendlin B.B., Kramer J.H. Cerebrospinal fluid and plasma levels of inflammation differentially relate to CNS markers of alzheimer’s disease pathology and neuronal damage. J Alzheimers Dis. 2018;62:385–397. doi: 10.3233/JAD-170602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla R.K., Butters M.A., Becker J.T., Houck P.R., Snitz B.E., Lopez O.L., Aizenstein H.J., Raina K.D., DeKosky S.T., Reynolds C.F., 3rd Patterns of mild cognitive impairment after treatment of depression in the elderly. Am. J. Geriatr. Psychiatr. 2009;17:308–316. doi: 10.1097/JGP.0b013e318190b8d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S., Ahmed A.T., Arnold M., Liu D., Luo C., Zhu H., Mahmoudiandehkordi S., Neavin D., Louie G., Dunlop B.W., Frye M.A., Wang L., Weinshilboum R.M., Krishnan R.R., Rush A.J., Kaddurah-Daouk R. Metabolomic signature of exposure and response to citalopram/escitalopram in depressed outpatients. Transl. Psychiatry. 2019;9:173. doi: 10.1038/s41398-019-0507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacabelos R., Takeda M., Winblad B. The glutamatergic system and neurodegeneration in dementia: preventive strategies in Alzheimer’s disease. Int. J. Geriatr. Psychiatr. 1999;14:3–47. doi: 10.1002/(sici)1099-1166(199901)14:1<3::aid-gps897>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Charlton R.A., Lamar M., Zhang A., Yang S., Ajilore O., Kumar A. White-matter tract integrity in late-life depression: associations with severity and cognition. Psychol. Med. 2014;44:1427–1437. doi: 10.1017/S0033291713001980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CJ G., SM F. 1978. Stroop Color and Word Test. [Google Scholar]
- Conwell Y., Duberstein P.R., Cox C., Herrmann J., Forbes N., Caine E.D. Age differences in behaviors leading to completed suicide. Am. J. Geriatr. Psychiatr. 1998;6:122–126. [PubMed] [Google Scholar]
- da Fonseca A.C., Matias D., Garcia C., Amaral R., Geraldo L.H., Freitas C., Lima F.R. The impact of microglial activation on blood-brain barrier in brain diseases. Front. Cell. Neurosci. 2014;8:362. doi: 10.3389/fncel.2014.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsker J.M., Hansen A.M., Caspi R.R. Th1 and Th17 cells: adversaries and collaborators. Ann. N. Y. Acad. Sci. 2010;1183:211–221. doi: 10.1111/j.1749-6632.2009.05133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R., O’Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R., Walker A.K. Is there a role for glutamate-mediated excitotoxicity in inflammation-induced depression? J. Neural. Transm. 2014;121:925–932. doi: 10.1007/s00702-014-1187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte S.M., Wands J.R. Alzheimer’s disease is type 3 diabetes-evidence reviewed. J Diabetes Sci Technol. 2008;2:1101–1113. doi: 10.1177/193229680800200619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis Kaplan E., Kramer J.H., DC D.D. 2000. California verbal learning test-II. [DOI] [PubMed] [Google Scholar]
- Elderkin-Thompson V., Irwin M.R., Hellemann G., Kumar A. Interleukin-6 and memory functions of encoding and recall in healthy and depressed elderly adults. Am. J. Geriatr. Psychiatr. 2012;20:753–763. doi: 10.1097/JGP.0b013e31825d08d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre H., Siddarth P., Cyr N., Yang H., Cole S., Forbes M., Lavretsky H. Comparing the immune-genomic effects of vilazodone and paroxetine in late-life depression: a pilot study. Pharmacopsychiatry. 2017;50:256–263. doi: 10.1055/s-0043-107033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre H.A., Air T., Pradhan A., Johnston J., Lavretsky H., Stuart M.J., Baune B.T. A meta-analysis of chemokines in major depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2016;68:1–8. doi: 10.1016/j.pnpbp.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finneran D.J., Nash K.R. Neuroinflammation and fractalkine signaling in Alzheimer’s disease. J. Neuroinflammation. 2019;16:30. doi: 10.1186/s12974-019-1412-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M., Anthony J.C., Parhad I., Duffy B., Gruenberg E.M. The meaning of cognitive impairment in the elderly. J. Am. Geriatr. Soc. 1985;33:228–235. doi: 10.1111/j.1532-5415.1985.tb07109.x. [DOI] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Franceschi C., Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl. 1):S4–S9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- Grzenda A., Siddarth P., Laird K.T., Yeargin J., Lavretsky H. Transcriptomic signatures of treatment response to the combination of escitalopram and memantine or placebo in late-life depression. Mol. Psychiatr. 2020 doi: 10.1038/s41380-020-0752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hair J.F., Black W.C., Babin B.J., Anderson R.E., Tatham R.L. Pearson Prentice Hall; Upper Saddle River, NJ: 2010. Multivariate Data Analysis. [Google Scholar]
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E., Miller A.H., Sanacora G. Inflammation, glutamate, and glia: a trio of trouble in mood disorders. Neuropsychopharmacology. 2017;42:193–215. doi: 10.1038/npp.2016.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey P.D. Domains of cognition and their assessment. Dialogues Clin. Neurosci. 2019 Sep;21(3):227–237. doi: 10.31887/DCNS.2019.21.3/pharvey. PMID: 31749647; PMCID: PMC6829170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton R., Miller S., Taylor M., Grant I. FL Psychol Assess Resour; Lutz: 2004. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults. [Google Scholar]
- Hudetz J.A., Iqbal Z., Gandhi S.D., Patterson K.M., Byrne A.J., Hudetz A.G., Pagel P.S., Warltier D.C. Ketamine attenuates post-operative cognitive dysfunction after cardiac surgery. Acta Anaesthesiol. Scand. 2009;53:864–872. doi: 10.1111/j.1399-6576.2009.01978.x. [DOI] [PubMed] [Google Scholar]
- Hughes C.P., Berg L., Danziger W., Coben L.A., Martin R.L. A new clinical scale for the staging of dementia. Br. J. Psychiatr. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Kanchanatawan B., Thika S., Anderson G., Galecki P., Maes M. Affective symptoms in schizophrenia are strongly associated with neurocognitive deficits indicating disorders in executive functions, visual memory, attention and social cognition. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2018;80:168–176. doi: 10.1016/j.pnpbp.2017.06.031. [DOI] [PubMed] [Google Scholar]
- Kohler O., Krogh J., Mors O., Benros M.E. Inflammation in depression and the potential for anti-inflammatory treatment. Curr. Neuropharmacol. 2016;14:732–742. doi: 10.2174/1570159X14666151208113700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn B.M. Jama; 2020. In Alzheimer Research, Glucose Metabolism Moves to Center Stage. [DOI] [PubMed] [Google Scholar]
- Lai K.S.P., Liu C.S., Rau A., Lanctot K.L., Kohler C.A., Pakosh M., Carvalho A.F., Herrmann N. Peripheral inflammatory markers in Alzheimer’s disease: a systematic review and meta-analysis of 175 studies. J. Neurol. Neurosurg. Psychiatry. 2017;88:876–882. doi: 10.1136/jnnp-2017-316201. [DOI] [PubMed] [Google Scholar]
- Langa K.M., Levine D.A. The diagnosis and management of mild cognitive impairment: a clinical review. Jama. 2014;312:2551–2561. doi: 10.1001/jama.2014.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza C.E., Sejunaite K., Steindel C., Scholz I., Riepe M.W. On the conundrum of cognitive impairment due to depressive disorder in older patients. Ginsberg S.D., editor. PloS One. 2020;15(4) doi: 10.1371/journal.pone.0231111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassale C., Batty G.D., Steptoe A., Cadar D., Akbaraly T.N., Kivimaki M., Zaninotto P. Association of 10-year C-reactive protein trajectories with markers of healthy aging: findings from the English longitudinal study of aging. J Gerontol A Biol Sci Med Sci. 2019;74:195–203. doi: 10.1093/gerona/gly028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavretsky H., Laird K.T., Krause-Sorio B., Heimberg B.F., Yeargin J., Grzenda A., Wu P., Thana-Udom K., Ercoli L.M., Siddarth P. A randomized double-blind placebo-controlled trial of combined escitalopram and memantine for older adults with major depression and subjective memory complaints. Am. J. Geriatr. Psychiatr. 2020;28:178–190. doi: 10.1016/j.jagp.2019.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavretsky H., Lesser I.M., Wohl M., Miller B.L. Relationship of age, age at onset, and sex to depression in older adults. Am. J. Geriatr. Psychiatr. 1998;6:248–256. [PubMed] [Google Scholar]
- Lee J.S., Potter G.G., Wagner H.R., Welsh-Bohmer K.A., Steffens D.C. Persistent mild cognitive impairment in geriatric depression. Int. Psychogeriatr. 2007;19:125–135. doi: 10.1017/S1041610206003607. [DOI] [PubMed] [Google Scholar]
- Limatola C., Ransohoff R.M. Modulating neurotoxicity through CX3CL1/CX3CR1 signaling. Front. Cell. Neurosci. 2014;8:229. doi: 10.3389/fncel.2014.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D., Dhabhar F.S., James S.J., Hough C.M., Jain F.A., Bersani F.S., Reus V.I., Verhoeven J.E., Epel E.S., Mahan L., Rosser R., Wolkowitz O.M., Mellon S.H. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology. 2017;76:197–205. doi: 10.1016/j.psyneuen.2016.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Xu X., Jiang T., Jin L., Zhao X.D., Cheng J.H., Jin X.J., Ma J., Piao H.N., Piao L.X. Sertraline ameliorates inflammation in CUMS mice and inhibits TNF-alpha-induced inflammation in microglia cells. Int. Immunopharm. 2019;67:119–128. doi: 10.1016/j.intimp.2018.12.011. [DOI] [PubMed] [Google Scholar]
- Meyers J., Meyers K. 1995. Rey Complex Figure Test and Recognition Trial. [Google Scholar]
- Miller A.H., Raison C.L. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto S.S., Alexopoulos G.S. Cognitive deficits in geriatric depression: clinical correlates and implications for current and future treatment. Psychiatr. Clin. 2013;36:517–531. doi: 10.1016/j.psc.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng A., Tam W.W., Zhang M.W., Ho C.S., Husain S.F., McIntyre R.S., Ho R.C. IL-1beta, IL-6, TNF- alpha and CRP in elderly patients with depression or alzheimer’s disease: systematic review and meta-analysis. Sci. Rep. 2018;8:12050. doi: 10.1038/s41598-018-30487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ownby R.L. Neuroinflammation and cognitive aging. Curr. Psychiatr. Rep. 2010;12:39–45. doi: 10.1007/s11920-009-0082-1. [DOI] [PubMed] [Google Scholar]
- Pelton G.H., Harper O.L., Roose S.P., Marder K., D’Antonio K., Devanand D.P. Combined treatment with memantine/es-citalopram for older depressed patients with cognitive impairment: a pilot study. Int. J. Geriatr. Psychiatr. 2016;31:648–655. doi: 10.1002/gps.4375. [DOI] [PubMed] [Google Scholar]
- Petersen R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Proescholdt M., Heimann A., Kempski O. Neuroprotection of S(+) ketamine isomer in global forebrain ischemia. Brain Res. 2001;904:245–251. doi: 10.1016/s0006-8993(01)02465-9. [DOI] [PubMed] [Google Scholar]
- Ramirez S.H., Fan S., Dykstra H., Reichenbach N., Del Valle L., Potula R., Phipps R.P., Maggirwar S.B., Persidsky Y. Dyad of CD40/CD40 ligand fosters neuroinflammation at the blood-brain barrier and is regulated via JNK signaling: implications for HIV-1 encephalitis. J. Neurosci. 2010;30:9454–9464. doi: 10.1523/JNEUROSCI.5796-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R. Validity of the Trail Making Test as an indicator of organic brain damage. Percept. Mot. Skills. 1958;8:271–276. [Google Scholar]
- Reynolds C.F., 3rd, Dew M.A., Pollock B.G., Mulsant B.H., Frank E., Miller M.D., Houck P.R., Mazumdar S., Butters M.A., Stack J.A., Schlernitzauer M.A., Whyte E.M., Gildengers A., Karp J., Lenze E., Szanto K., Bensasi S., Kupfer D.J. Maintenance treatment of major depression in old age. N. Engl. J. Med. 2006;354:1130–1138. doi: 10.1056/NEJMoa052619. [DOI] [PubMed] [Google Scholar]
- Rissin D.M., Kan C.W., Campbell T.G., Howes S.C., Fournier D.R., Song L., Piech T., Patel P.P., Chang L., Rivnak A.J., Ferrell E.P., Randall J.D., Provuncher G.K., Walt D.R., Duffy D.C. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat. Biotechnol. 2010;28:595–599. doi: 10.1038/nbt.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski M.A., Wenk G.L. The neuropharmacological basis for the use of memantine in the treatment of Alzheimer’s disease. CNS Drug Rev. 2003;9:275–308. doi: 10.1111/j.1527-3458.2003.tb00254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosales-Corral S., Tan D.X., Manchester L., Reiter R.J. Diabetes and Alzheimer disease, two overlapping pathologies with the same background: oxidative stress. Oxid Med Cell Longev. 2015;2015 doi: 10.1155/2015/985845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblat J.D., Cha D.S., Mansur R.B., McIntyre R.S. Inflamed moods: a review of the interactions between inflammation and mood disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2014;53:23–34. doi: 10.1016/j.pnpbp.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Rush A.J., Trivedi M.H., Wisniewski S.R., Nierenberg A.A., Stewart J.W., Warden D., Niederehe G., Thase M.E., Lavori P.W., Lebowitz B.D., McGrath P.J., Rosenbaum J.F., Sackeim H.A., Kupfer D.J., Luther J., Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR∗D report. Am. J. Psychiatr. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Sas A.R., Bimonte-Nelson H., Smothers C.T., Woodward J., Tyor W.R. Interferon-alpha causes neuronal dysfunction in encephalitis. J. Neurosci. 2009;29:3948–3955. doi: 10.1523/JNEUROSCI.5595-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S., Callaghan D., Juzwik C., Xiong H., Huang P., Zhang W. ABCG2 reduces ROS-mediated toxicity and inflammation: a potential role in Alzheimer’s disease. J. Neurochem. 2010;114:1590–1604. doi: 10.1111/j.1471-4159.2010.06887.x. [DOI] [PubMed] [Google Scholar]
- Steffens D.C. Separating mood disturbance from mild cognitive impairment in geriatric depression. Int. Rev. Psychiatr. 2008;20:374–381. doi: 10.1080/09540260802094589. [DOI] [PubMed] [Google Scholar]
- Strauss E., Sherman E., Spreen O. Oxford University Press; New York: 2006. A Compendium of Neuropsychological Tests. [Google Scholar]
- Strawbridge R., Arnone D., Danese A., Papadopoulos A., Herane Vives A., Cleare A.J. Inflammation and clinical response to treatment in depression: a meta-analysis. Eur. Neuropsychopharmacol. 2015;25:1532–1543. doi: 10.1016/j.euroneuro.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Sun Y., Drevets W., Turecki G., Li Q.S. The relationship between plasma serotonin and kynurenine pathway metabolite levels and the treatment response to escitalopram and desvenlafaxine. Brain Behav. Immun. 2020;87:404–412. doi: 10.1016/j.bbi.2020.01.011. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Nakagawasai O., Nemoto W., Kadota S., Isono J., Odaira T., Sakuma W., Arai Y., Tadano T., Tan-No K. Memantine ameliorates depressive-like behaviors by regulating hippocampal cell proliferation and neuroprotection in olfactory bulbectomized mice. Neuropharmacology. 2018;137:141–155. doi: 10.1016/j.neuropharm.2018.04.013. [DOI] [PubMed] [Google Scholar]
- Turner M.D., Nedjai B., Hurst T., Pennington D.J. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta. 2014;1843:2563–2582. doi: 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Vega J.N., Zurkovsky L., Albert K., Melo A., Boyd B., Dumas J., Woodward N., McDonald B.C., Saykin A.J., Park J.H., Naylor M., Newhouse P.A. Altered brain connectivity in early postmenopausal women with subjective cognitive impairment. Front. Neurosci. 2016;10:433. doi: 10.3389/fnins.2016.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale. fourth ed. 2009. [Google Scholar]
- Wei X., Gao H., Zou J., Liu X., Chen D., Liao J., Xu Y., Ma L., Tang B., Zhang Z., Cai X., Jin K., Xia Y., Wang Q. Contra-directional coupling of Nur77 and Nurr1 in neurodegeneration: a novel mechanism for memantine-induced anti-inflammation and anti-mitochondrial impairment. Mol. Neurobiol. 2016;53:5876–5892. doi: 10.1007/s12035-015-9477-7. [DOI] [PubMed] [Google Scholar]
- Winblad B., Palmer K., Kivipelto M., Jelic V., Fratiglioni L., Wahlund L.O., Nordberg A., Bäckman L., Albert M., Almkvist O. Mild cognitive impairment–beyond controversies, towards a consensus: report of the international working group on mild cognitive impairment. J. Intern. Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- Yang C., Wardenaar K.J., Bosker F.J., Li J., Schoevers R.A. Inflammatory markers and treatment outcome in treatment resistant depression: a systematic review. J. Affect. Disord. 2019;257:640–649. doi: 10.1016/j.jad.2019.07.045. [DOI] [PubMed] [Google Scholar]
- Yang J., Fan C., Pan L., Xie M., He Q., Li D., Wang S. C-reactive protein plays a marginal role in cognitive decline: a systematic review and meta-analysis. Int. J. Geriatr. Psychiatr. 2015;30:156–165. doi: 10.1002/gps.4236. [DOI] [PubMed] [Google Scholar]
- Zhang J.M., An J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007;45:27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwicker A., Fabbri C., Rietschel M., Hauser J., Mors O., Maier W., Zobel A., Farmer A., Aitchison K.J., McGuffin P., Lewis C.M., Uher R. Genetic disposition to inflammation and response to antidepressants in major depressive disorder. J. Psychiatr. Res. 2018;105:17–22. doi: 10.1016/j.jpsychires.2018.08.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.