Abstract
Exposure to violence (ETV) has been linked to epigenomics mechanisms such as DNA methylation (DNAm). We used epigenetic profiling of blood collected from 32 African American young adult males who lived in Washington DC to determine if changes in DNAm at CpG sites affiliated with nervous and immune system were associated with exposure to violence. Pathway analysis of differentially methylated regions comparing high and low ETV groups revealed an enrichment of gene sets annotated to nervous system and immune ontologies. Many of these genes are known to interact with each other which suggests DNAm alters gene function in the nervous and immune system in response to ETV. Using data from a unique age group, young African American adult males, we provide evidence that lifetime ETV could impact DNA methylation in genes impacted at Central Nervous System and Immune Function sites.
Method
Methylation analysis was performed on DNA collected from the blood of participants classified with either high or low lifetime ETV. Illumina®MethylationEPIC Beadchips (~850k CpG sites) were processed on the iScan System to examine whole-genome methylation differences. Differentially methylated CpG-sites between high (n = 19) and low (n = 13) groups were identified using linear regression with violence and substance abuse as model covariates. Gene ontology analysis was used to identify enrichment categories from probes annotated to the nearest gene.
Results
A total of 595 probes (279 hypermethylated; 316 hypomethylated) annotated to 383 genes were considered differentially methylated in association with ETV. Males with high ETV showed elevated methylation in several signaling pathways but were most impacted at Central Nervous System and Immune Function affiliated sites. Eight candidate genes were identified that play important biological roles in stress response to violence with HDAC4 (10%), NR4A3 (11%), NR4A2 (12%), DSCAML1(12%), and ELAVL3 (13%) exhibiting higher levels in the low ETV group and DLGAP1 (10%), SHANK2 (10%), and NRG1(11%) having increased methylation in the high ETV group. These findings suggest that individuals subjected to high ETV may be at risk for poor health outcomes that have not been reported previously.
Keywords: DNA methylation, Epigenetic, Exposure to violence, African americans, Young adults
Highlights
-
•
Exposure to violence has been linked to DNA methylation.
-
•
Total of 595 probes annotated to 383 genes were differentially methylated.
-
•
Differential methylation in central nervous system and immune signaling pathway.
-
•
Methylation at NRG1 and NR4A2 possibly associated with stress response to violence.
1. Introduction
For many African Americans (AA), experiencing violence, discrimination, and environmentally induced stressors, such as childhood neglect or abuse, are well documented (Jacobs et al., 2014; Pew Research Center, 2016). AAs carry a disproportionate burden of incidence, morbidity, and mortality from chronic diseases such as hypertension and obesity (Allport et al., 2019; Barengolts et al., 2019; Yang et al., 2019; Li et al., 2019; Faucher et al., 2019; Assari et al., 2019; Nagy et al., 2020; Goode et al., 2017; Go et al., 2014) in addition to exposure to violence (ETV) which has also been shown to have a negative impact on health (Griggs et al., 2019; Goldmann et al., 2011; Woodson et al., 2010; Paranjape and Kaslow, 2010; Mitchell et al., 2010; Paranjape et al., 2009; McGee et al., 2001; Moffitt and Klaus-Grawe Think, 2013; Olofsson et al., 2012). Therefore, it is worthwhile to examine if lifetime exposure to violence is a contributing factor in AA health disparity. Lifetime ETV is defined in this study as the cumulative effects of ETV during childhood, before age 18, and exposure to community violence after age 18. The key questions we would like to understand in this study is how lifetime exposure to violence, especially during the most vulnerable periods of childhood and adolescence, translates into changes at a molecular level. One possible mechanism is through methylation changes since ETV has been shown to affect the epigenome (Olofsson et al., 2012). Cytosine DNA methylation (DNAm) in humans occurs primarily at CG dinucleotides, which are also called CpG sites (Alberts, 2008). Over half of the promoters in human genes contain CpG islands which are CpG-rich regions that are binding sites for regulatory factors that modulate gene transcription. The most studied type of epigenetic regulation is by DNAm of the promoter regions (Mansell et al., 2019). DNAm generally acts as an On-Off switch that turns genes On when the DNA in not methylated and Off when the DNA is methylated (Alberts, 2008). Cytosine methylation occurs at the 5th position of the base which faces into the major groove of the DNA helix. Since transcription factors bind to the major groove of DNA, DNAm at CpG islands often blocks transcriptional activators from binding to the promoter regions (Alberts, 2008). That is one of the reasons why DNAm usually turns genes off. Another reason why DNAm turns genes off is that methyl-DNA-binding proteins, such as MeCP2, which is mutated in Rett Syndrome patients, binds to methylated cytosines and acts as a transcriptional repressor (Lyst et al., 2013). Blood is usually the surrogate tissue used in humans to DNAm changes caused by environmental or social stressors because other tissues are not generally available in humans (Ebrahimi et al., 2020). In mouse studies, epigenetic changes in the blood often correspond to epigenetic changes in the brain, thus justifying a surrogate tissue approach in humans (McKay et al., 2011). In recent years, contrasting results have been reported about the use of blood to study brain alterations. However, blood can be used to study peripheral rather than central biomarkers.
One remarkable finding over the past couple of decades is that recent epigenetic studies, including those focused on DNAm, have found an association between adverse life experiences (such as exposure to community and family violence, discrimination, and trauma) and modulation of gene regulatory regions that can change behaviors, influence personality, and increase the risk for mental health disorders and psychosocial stressors (Vick and Burris, 2017; Barker et al., 2018; Jovanovic et al., 2017). Other studies have demonstrated the association between DNAm and perceived discrimination among African American women (Barcelona de Mendoza et al., 2018), with the highest rates of perceived discrimination (35%) by African American in comparison to other women (Jacobs et al., 2014). Results from another recent DNAm study in Brazil showed altered gene expression across the lifespan of those who experienced repeated community and domestic violence (Serpeloni et al., 2020). A meta-analysis conducted across five studies exploring the association between DNAm, disadvantaged neighborhoods, and cardiovascular disease risk indicated an association between DNAm changes in expression to the stress- and inflammation-related genes and disadvantaged neighborhoods, and risk of cardiovascular disease (Giurgescu et al., 2019). In a longitudinal study, childhood victimization predicted elevated levels of C-Reactive Protein (CRP) at age 18 with an association that was specific to women (Baldwin et al., 2018). Increased epigenetic aging and heart rate in children ages 6–13 who experienced direct, but not witnessed, violence has also been shown (Jovanovic et al., 2017). Additionally, increased levels of DNAm at CpG sites across the genome have also been associated with socioeconomic status (SES) in a cohort of young adults (McDade et al., 2019). While several studies have investigated the relationship of epigenomic changes and stressful life events, such as exposure to violence, in disadvantage neighborhoods, there is no study that investigate the genomewide changes among African American young adults. To address this issue, we explored the relationship between DNAm and exposure to different levels of violence, using genome wide DNAm data extracted from whole blood. To our knowledge, there are no published studies that have examined how lifetime exposure to violence is associated with DNAm across the epigenome among AA young adult males. We hypothesized that higher levels of lifetime exposure to violence would result in DNAm changes involved in the immune response in African American young adult males.
2. Methods
2.1. Participants and procedure
This study selected 32 males who scored in the highest (n = 19) and lowest (n = 13) 30% of 638 African American males and females (aged 18–25) from economically and socially disadvantaged neighborhoods of Washington DC on a self-reported scale measuring lifetime exposure to violence (Lifetime ETV) for epigenetic profiling. The Lifetime ETV scale combined 34 items measuring ETV during childhood (before age 18) and 35 items on exposure to community violence since adulthood. This study compares the high and low ETV male groups to determine if ETV resulted in changes to DNAm sites affiliated with immune function. This study controlled for drug use in the past 30 days. The IRB was approved by Howard University Office of Regulatory Research Compliance (IRB-13-PED-06).
To qualify for inclusion in the study, respondents had to be between the ages of 18 and 25 as of their most recent birthday, self-identify as African American or Black, screen as HIV negative (to exclude those with HIV compromised immune systems from the larger study on immune function), and currently live in one of the predominantly disadvantaged wards in Washington, DC. The full study entailed a comprehensive survey about participants’ ETV before and after the age of 18, adverse life experiences, discrimination, current and childhood socioeconomic characteristics, current health problems and symptoms, current drug use, sleep quality measures, depressive symptom measures, and current HIV risk behaviors.
While mouse epigenetic studies of behavior can involve brain tissues, such tissues are impossible to collect in humans except from human brain banks. Instead, people who study epigenetic regulation of behavior in humans utilize a surrogate tissue such as blood or saliva which can be collected in non-invasive manners (Solomon et al., 2018; Murata et al., 2019). Other tissues have been collected in humans for epigenetic studies, such as fat or muscle biopsies (Taylor et al., 2019), but such collections are much more invasive than collecting blood or saliva and consequently more difficult to collect. Despite the limitation of surrogate tissues in humans, several studies have identified epigenetic biomarkers in genes that correlate with stressful conditions in humans (Sen et al., 2015a, 2015b; Intarasunanont et al., 2012).
2.2. Survey of exposure to childhood and community violence
To measure exposure to childhood violence, exposure to community violence as adults, the survey included questions from previously developed and tested instruments. The childhood exposure to violence scale contained 34 questions that asked participants to respond to circumstances that might have happened during their childhood from birth through age 18. The response options were “1 time,” “2 times,” “3 times,” “4 times,” “5 times or more,” “no times,” and “prefer not to answer.” This scale has a test-retest reliability coefficient of 0.90 and Cronbach’s α= 0.85 (Finkelhor et al., 2010, 2015; Stith and Hamby, 2002; Little and Hamby, 2001). Community exposure to violence as adults was measured by 35 items. Participants were asked to describe the violence that they experienced, saw, or heard about since they turned 18. The responses were “never,” “once or twice,” “a few times,” “many times,” or “prefer not to answer.” This scale has an internal consistency of 0.85, test-retest reliability of 0.90, and Cronbach’s α= 0.61, 0.79, and 0.86, respectively for violence experienced, seen, and heard (Richters and Saltzman, 1990).
2.3. Genomic DNA extraction method
High molecular weight genomic DNA was extracted from 300 μL of whole blood from participants using the Qiagen DNAeasy DNA extraction kit for blood and tissue (Qiagen Sciences Inc.) according to the manufacturer’s protocol. The concentration and integrity of the DNAs were measured using a NanoDrop 2000c Microvolume Spectrophotometer. The DNA samples (200 ng aliquots) were used in genome-wide DNAm analysis by the Genome Sciences Core at Wayne State University.
2.4. Global methylation analysis
Methylation analysis was performed using Illumina®MethylationEPIC Beadchips prepared as described in the Illumina® Infinium® HD Assay Methylation Protocol Guide (15019519 v01) before processing on the Illumina iScan System. Input DNA (250 ng) was bisulfite treated using the Zymo EZ DNA Methylation Kit. Zymo’s Human Methylated and Non-methylated DNA controls are treated with samples. Controls are PCR amplified and run on a gel to confirm both methylated and unmethylated bands are present. After bisulfite conversion was confirmed, the bisulfite treated DNA was manually prepared for sequence-specific array-based hybridization using whole-genome amplification (WGA), enzymatic endpoint fragmentation and chemical precipitation. The WGA product was re-suspended and captured by array hybridization. Arrays were then mounted in the Tecan GenePaint automated slide processor on the Tecan Freedom Evo® robotic liquid handling system for primer extension and staining. The amount of fluorescence was measured and used to determine the methylation level of the CpG sites.
2.5. Differential methylation analysis
Raw data from the Infinium assays underwent quality control including staining, extension, hybridization, and bisulfite conversion checks (Aryee et al., 2014). After probe correction to remove probes with low intensity and normalization (Triche et al., 2013), differentially methylated CpG-sites (expressed as M values) between high (n = 19) and low (n = 13) groups were identified using linear regression with violence and substance abuse as model covariates (Ritchie et al., 2015). Substance abuse was calculated by averaging self-reported alcohol, marijuana, cocaine, glue, and heroin use during the thirty-day window prior to the survey. Average Delta β values indicating the differential methylation were calculated by subtracting the average β value of high violence from that of low violence groups. The differentially methylated probes with gene annotation (|Delta β| ≥ 0.1; p-value ≤ 0.05) were further analyzed for significant biological pathways using gene ontology analysis (Huang et al., 2009).
3. Results
3.1. Descriptive characteristic of participants
Descriptive characteristics and childhood SES variables of participants are summarized in Table 1. The mean age of the participants was 20.7 ± 2.4 and they were all unmarried (1 was engaged). 21.9 % did not finish high school and 62.5% reported completing high school or GED. 81.3 % made less than $15,000 a year and 68.8% grew up in families with incomes <$40,000 a year and 28.1% were unemployed. 72% were exposed to violence during childhood, two-thirds were exposed to community violence during their young adulthood and 78.1% were exposed to both childhood and community violence. 62.5% of participants had at least one drink during their lifetime, 53.1% had ever smoked a cigarette and 68.7% had ever used marijuana. About 47% had restless sleep, and 34.4% felt lonely and fearful in the past week.
Table 1.
Descriptive characteristic and lifetime exposure to violence of participants (N = 32).
| Variables | Mean (SD) or percent |
|---|---|
| Age 18-25 | 20.7 (2.4) |
| Income | |
| < $14,999 | 81.3% |
| $15,000-$29,999 | 12.5% |
| $30,000+ | 6.2% |
| Education | |
| Did not finish HS | 21.9% |
| High school or GED | 62.5% |
| Finished vocational or trade school | 3.1% |
| Attend or graduated college | 12.5% |
| Unemployment | 28.1% |
| Source of childhood family income | |
| People who worked | 71.9 |
| Welfare or public assistance | 12.5 |
| Worked and welfare | 15.5 |
| Childhood Household Income | |
| < $14,999 | 40.6 |
| $15,000-$29,999 | 18.8 |
| $30,000-$39,999 | 9.4 |
| $40,000-$49,999 | 12.5 |
| $50,000 and more | 18.8% |
| Rented Residence (% yes) | 59.4% |
| Lifetime childhood ETV | 72.0% |
| Lifetime community ETV as adults | 75.0% |
| Lifetime childhood and community ETV | 78.1% |
| Ever drank alcohol (yes) | 62.5% |
| Ever smoked cigarette (yes) | 53.1% |
| Ever used marijuana (yes) | 68.7% |
| Felt lonely past week | 34.4% |
| Restless sleep past week | 46.9% |
| Felt fearful past week | 34.4% |
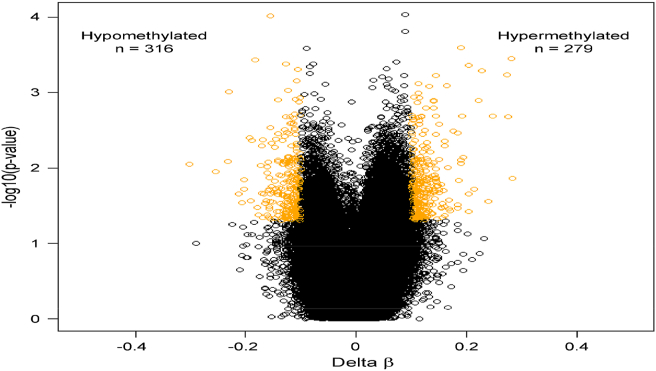
The results of genome-wide DNAm analysis of 19 participants with high ETV showed significantly higher DNAm at multiple loci/genes in comparison with the 13 participants with low ETV. Of the 866091 probes on the EPIC Beadchips analyzed, 476292 passed QC across all 32 samples. Probes that had a greater than 10% change in β (p-value ≤ 0.05) between the groups of high and low Lifetime exposure to violence were classified as differentially methylated sites (DMS) (Fig. 1). In total, 595 probes annotated to 383 genes were considered DMS. (See Appendix A. supplementary table showing all 383 genes).
Fig. 1.
Volcano plot of differentially methylated CpG sites. CpG sites with an absolute Delta β of 10% (p-value ≤ 0.05) are depicted in yellow. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Gene Ontology analysis of genes annotated to DMS (top 30 in Table 2), revealed 85 significant categories (FDR ≤ 0.05) with several related to the nervous system: nervous system development (GO:0007399), anterograde trans-synaptic signaling (GO:0098916), chemical synaptic transmission (GO:0007268), synaptic signaling (GO:0099536), trans-synaptic signaling (GO:0099537), neurogenesis (GO:0022008) and central nervous system development (GO:0007417). Only one category, inflammatory response to antigenic stimulus (GO:0002437), was related to immune function.
Table 2.
Top 30 Gene Ontology categories from genes annotated to DMS.
| Term | Description | Fold Enrichment | FDR |
|---|---|---|---|
| GO:0022610 | biological adhesion | 2.219861 | 3.03E-09 |
| GO:0007155 | cell adhesion | 2.196325 | 6.84E-09 |
| GO:0098742 | cell-cell adhesion via plasma-membrane adhesion molecules | 4.655715 | 1.21E-06 |
| GO:0007275 | multicellular organism development | 1.486545 | 1.44E-06 |
| GO:0044707 | single-multicellular organism process | 1.40827 | 2.12E-06 |
| GO:0032501 | multicellular organismal process | 1.350336 | 2.27E-06 |
| GO:0048731 | system development | 1.51563 | 4.27E-06 |
| GO:0007156 | homophilic cell adhesion via plasma membrane adhesion molecules | 5.364321 | 5.8E-06 |
| GO:0044700 | single organism signaling | 1.356961 | 2.52E-05 |
| GO:0048856 | anatomical structure development | 1.392844 | 3.19E-05 |
| GO:0023052 | Signaling | 1.345472 | 4.49E-05 |
| GO:0007399 | nervous system development | 1.744177 | 4.96E-05 |
| GO:0044767 | single-organism developmental process | 1.382599 | 5.69E-05 |
| GO:0032502 | developmental process | 1.372452 | 6.16E-05 |
| GO:0009887 | organ morphogenesis | 2.203251 | 7E-05 |
| GO:0098609 | cell-cell adhesion | 2.060874 | 0.000116 |
| GO:0007154 | cell communication | 1.325265 | 0.000152 |
| GO:0007267 | cell-cell signaling | 1.827299 | 0.00051 |
| GO:0030198 | extracellular matrix organization | 3.032487 | 0.001149 |
| GO:0043062 | extracellular structure organization | 3.023435 | 0.001197 |
| GO:0040011 | Locomotion | 1.776931 | 0.001277 |
| GO:0051239 | regulation of multicellular organismal process | 1.543705 | 0.001295 |
| GO:0048513 | animal organ development | 1.482196 | 0.001522 |
| GO:0001501 | skeletal system development | 2.502209 | 0.002694 |
| GO:0048870 | cell motility | 1.803652 | 0.002756 |
| GO:0051674 | localization of cell | 1.803652 | 0.002756 |
| GO:0007165 | signal transduction | 1.292314 | 0.003003 |
| GO:0098916 | anterograde trans-synaptic signaling | 2.294712 | 0.003452 |
| GO:0007268 | chemical synaptic transmission | 2.294712 | 0.003452 |
| GO:0099536 | synaptic signaling | 2.294712 | 0.003452 |
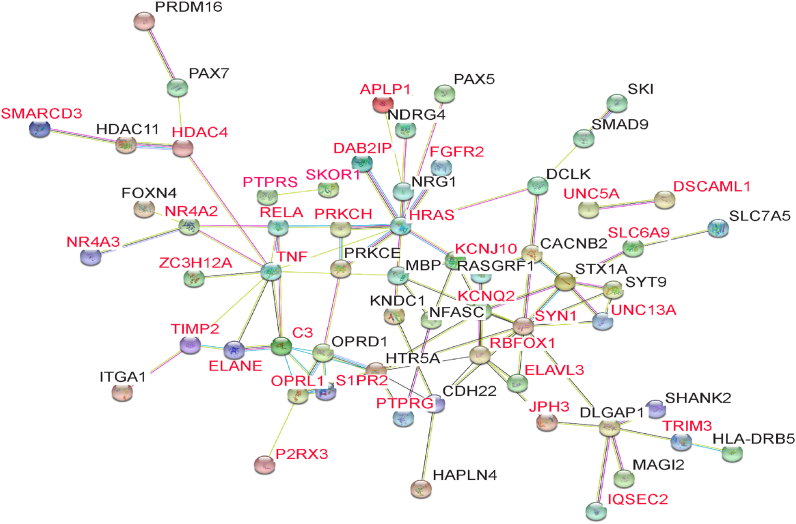
Interaction analysis of the 90 genes categorized as related to the nervous system or immune response revealed interactions between 53 genes in 3 clusters∗ (Fig. 2). Our interaction network analysis identified several gene network pathways some that clustered in the central nervous system or immune response pathways. Neuregulin 1 (NRG1) which promotes excitatory neurons has been implicated in schizophrenia (Mei and Xiong, 2008) but also is involved in pro-regenerative immune response (Alizadeh et al., 2018). NR4A2 is expressed in both T cells (Raveney et al., 2013) and dopamageneric neurons (Luo et al., 2008). The large cluster contains several genes involved in innate immunity including Doublecortin Like Kinase 1 (DCLK1) a regulator of IL17, tripartite motif-containing protein 3 (TRIM3), myelin basic protein (MBP), complement C3 (C3), NR4A3 (Nagaoka et al., 2017; Odagiu et al., 2016; Boulet et al., 2019) and TNF (Zhang et al., 2018; Ozato et al., 2008; Nakagawa et al., 2003; Kerepesi et al., 2006; Francisco et al., 2015). Additional immune related genes include RELA Proto-Oncogene (RELA) a NF-KB subunit expressed in the macrophage (Pittet et al., 2011), HDAC11 which regulates interferon signaling (Cao et al., 2019) and HRAS a critical component of protective immunity (Iborra et al., 2011). Genes with neurological functions include controllers of neuronal apoptosis (APLP1 (Tang et al., 2007), NDRG4 (Wen et al., 2019)), neuronal differentiation (TMP-2 (Perez-Martinez and Jaworski, 2005)), excitatory neurons (FGFR2 (Stevens et al., 2010)), neuronal migration (DAB2IP (Lee et al., 2012)), hippocampal neurons (PRKCH (Buchser et al., 2010)), V2b neurons (FoxN4 (Li et al., 2005)) and axonal growth (RasGRF1). Several genes have ties to neurological disorders including autism (SMAD9 bmp regulator SKI/SMAD4 (Zhang et al., 2017), CACNB2 (Breitenkamp et al., 2014), STX1A (Durdiakova et al., 2014) (Nakamura et al., 2008), UNC13A (Lipstein et al., 2017) RBFOX1 (Lee et al., 2016))), Alzheimer’s disease (Elavl3 (Ogawa et al., 2018) (Scheckel et al., 2016), DLGAP1 (Hadar et al., 2016), SHANK2 (Eltokhi et al., 2018), HLA-DRB5 MS (Caillier et al., 2008)), bipolar disorder (PAX-5 B-cell differentiation downregulated in bipolar disorder (Ohtsuka et al., 2013)) and Parkinson’s disease (HDAC4 (Wu et al., 2017)). The genes that did not cluster included a site annotated to the protocadherin family (PCDH) which is highly expressed in the nervous system and has epigenetic alterations influencing PCDH regulation which has been implicated in neurological disorders (El Hajj et al., 2017). Although only one immune category was enriched in the GO analysis, several of the genes in the nervous system categories also have immune functions. Within the two smaller clusters protein tyrosine phosphatase sigma (PTPRS) is expressed in dendritic cells and has been shown to regulate interferon (Bunin et al., 2015) while both UNC5A and DSCAML1 are receptors that belong to the immunoglobin family (Lai Wing Sun et al., 2011; Ly et al., 2008). SLC6A9 is a glycine transporter and glycine is important in immune modulation (Zhong et al., 2003) as well as an inhibitory neurotransmitter in the central nervous system (Legendre, 2001; Umeda et al., 2019).
Fig. 2.
Interaction network for genes annotated to differentially methylated sites related to nervous system and immune response. Genes with hypermethylated sites are depicted in red.
∗Not all of the genes from the nervous system or immune response category have known interactions. For example, UNC5A and DSCAML1 are connected to each other but no other gene. It’s not currently known if these genes function in a coordinated way. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
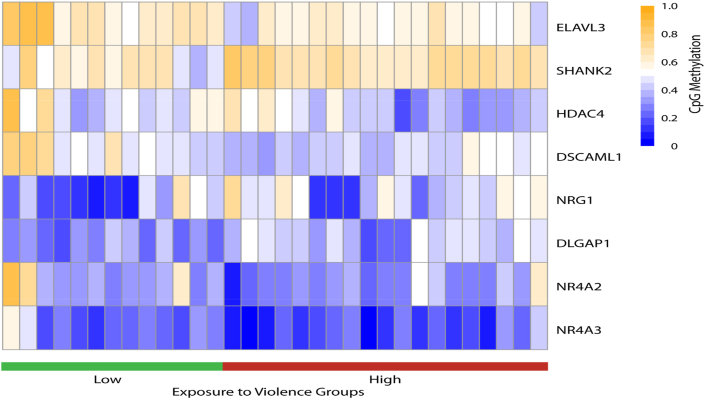
We also carried out heatmap analysis of the methylation findings for 8 candidate genes identified from our genome-wide methylation analysis to play important biological roles in stress response to violence (Fig. 3). We observed variable methylation levels within and across the low and high violence groups. Overall, we observed that for NR4A3, NR4A2, DSCAML1, HDAC4, and ELAV3 there was relatively higher methylation in the low violence group compared to high violence group. In contrast, for DLGAP1, NRG1, and SHANK2, there was relatively lower methylation in the low violence when compared to the higher violence exposure.
Fig. 3.
Heatmap for selected genes showing methylation levels (hypomethylation - blue; hypermethylation - orange) across individual subjects classified into the low (green) and high (red) ETV categories. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
There are several environmental determinants associated with health disparities in the USA including exposure to violence. Exposure to violence can directly influence health through biological mechanisms and can be measured as indicator of stressors. The well characterized biological pathway whereby environmental exposure to violence are transmitted through the body to elicit physiological response is via the so-called hypothalamic-pituitary-adrenal axis (HPA axis) system. Signals from the central nervous system in the form of chemical signals or electrical potential are sent to the HPA axis signifying the release of corticotrophin releasing hormone (CRH). The CRH in turn stimulates biosynthesis and release of adrenocorticotrophic hormone that triggers the production of glucocorticoids (cortisol) which are the stress response markers (Welberg et al., 2001).
However, the underlying biological mechanism whereby exposure to violence may alter stressor in the body is unknown. In this study we carried out genome-wide DNAm analysis and found that individuals with high levels of self-reported violence victimization had significant hypermethylation of key CpG sites located in the genes influencing nervous system development, cell adhesion, and cellular signaling as compared to those similarly situated individuals without high violence exposure. Previous studies have shown that experiencing community and domestic violence was associated with gene methylation involved in the neural development in adolescents (Serpeloni et al., 2019), and epigenomic mechanisms possibly associated with risk for health problems later in life in maltreated children. Additionally, increased levels of DNAm at CpG sites across the genome were found to be associated with low socioeconomic status (SES) in a cohort of young adults (McDade et al., 2019).
In our current studies we have observed that high exposure to violence is significantly associated with increased DNAm of genetic networks involved in the central nervous system and the immune system. Several studies suggest that epigenetic mechanisms such as DNAm changes plays dynamic roles in gene expression throughout the life of neurons and in neurodegenerative diseases including Rett syndrome, fragile X syndrome and Alzheimer’s disease (Christopher et al., 2017). Similarly, there are emerging evidence to suggest that aberrant epigenetic DNAm may participate in defects in immune-mediated pathologies (Calle-Fabregat et al., 2020).
In our previous studies we observed that young men and women exposed to violence experienced adverse physical and mental health outcomes including depression, and sleep disturbances (Saadatmand et al., 2017). In women, exposure to violence significantly affected sleep as a result of direct personal violence. In contrast, men exposed to direct personal violence such as gun violence or witnessing violent death had considerably greater impact upon depression and depressive mood. In our more recent study (Saadatmand et al., 2019) we reported that exposure to violence was significantly associated with marijuana use in both men and women. In an exploratory study, Uddin, et al. (Uddin et al., 2013), also suggest that sex differences in DNAm may contribute to sex differences in the prevalence of PTSD and depression.
Thus, our current observation of differential methylation of several genes in the central nervous system and the immune signaling pathway suggests that this is a potential biological mechanism for disrupting normal biological processing including sleeping patterns, depressive moods and drug use with regards to the exposure to violence. In a review of epigenetic findings in both animal and human studies, Lockwood et al. (2015), concluded that epigenetics could play an important role in depression and suicide in humans. A review of previous research concludes that DNAm plays a central role in learning and memory processes and in drug addiction (Bali et al., 2011). Results from a study showed a distinct DNAm pattern in insufficient sleep with differences related to compromised neuroplasticity and neurodegeneration (involving genes, such as ERC2, MAGI2, CAST, and CDK5R1) (Lahtinen et al., 2019).
Naumova et al. (2012), found that children raised in an institution since birth showed greater epigenome-wide DNAm compared with high-poverty children living with their families, particularly in genes related to immune regulation and cellular signaling. More recently, based on a cross-sectional sample of high-risk youth, Cecil, et al. (Cecil et al., 2016), sought to characterize the DNAm ‘signatures’ of different forms of maltreatment, using an epigenome-wide approach. They found that physical maltreatment showed the strongest associations with DNAm, implicating multiple genes previously associated with psychiatric and physical disorders (e.g., GABBR1, GRIN2D, CACNA2D4, PSEN2).
Of particular interest is the identification of genes including Neuroregulin 1 (NRG1) and nuclear receptor subfamily 4 Group A member 2 (NR4A2) from our GO analysis to be involved in both the nervous system and immune signal pathways. In support of our observation, a recent study by Uddin et al. (2018), identified methylation at 2 CpG sites in an epigenome-wide association studies to be associated with PSTD. One study also found methylation of NRG1 to be associated with inflammation (Song et al., 2016). Taken together, these observations suggests that aberrant methylation of key regulatory genes in the central nervous system and immune signal pathways maybe a potential mechanism for inducing chronic inflammatory changes in various psychiatry disorders including depression, anxiety, and PTSD. Based on their study, Safe et al. (2016), suggest that NR4A2 is important for regulating both inflammation and resolution of inflammatory signaling in activated immune cells and glial cells.
Furthermore, our results showed a gene interaction analysis of the 90 significant genes categorized as related to the nervous system or immune response revealed interactions in 3 clusters. The findings of this study suggest that young African American men who are exposed to high levels of lifetime violence maybe at risk for many health risks and diseases that have not been reported in literature related to this age group. However, we note that no clinical data are available about the participants’ psychiatric symptoms. This will be pursued in a future study.
4.1. Study limitations and strengths
The results of this study should be considered in light of several limitations. First, the sample was only from the baseline phase of a longitudinal study and we cannot infer casual associations between exposure to violence and DNAm. Second, this study was based on 32 DNA samples of young African American, living in Washington DC, who were exposed to low and high exposure to violence and might not have enough power analysis. However, given the lack of study on DNAm among young African American, and the high cost associated with the DNAm testing, this study is novel in its approach and the importance of studying the impact of life adversities, such as exposure to violence on DNAm. This study might be a blueprint for a larger study of examining how environmental influences such as exposure to violence become biologically embedded and find causal pathways between DNAm and exposure to violence among young African American males and females.
5. Conclusion
While replication is required, this study suggests that overall genes that are differentially methylated are involved in pathways including neurological apoptosis, differentiation and migration, axonal growth, and neurological disorders including autism, Alzheimer’s, bipolar disorder, and Parkinson’s disease. Future studies will investigate how methylation of these gene network drives health disparities. These analyses lay groundwork for building a portrait of the potential contribution of violence exposure on methylation processes in young African American men.
Declaration of competing interest
The authors have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Acknowledgements
Funding: This project has been funded in whole or in part with U.S. Government funds from the following National Institutes of Health (NIH): National Institute of Minority Health & Health Disparities (NIMHD) (Grant # 4RO1MD005851), National Center for Advancing Translational Sciences (NCATS) “Re-Engineering the Clinical Research Enterprise,” (Grant # UL1TR000101), Office of the Director (Grant #UH3 OD023285) and the District of Columbia Department of Health (DCDOH), USA. (The HIV Prevention Grant #16Z202).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the DCDOH.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2021.100247.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Alberts B. fifth ed. Garland Science; New York: 2008. Molecular Biology of the Cell. [Google Scholar]
- Alizadeh A., Santhosh K.T., Kataria H., Gounni A.S., Karimi-Abdolrezaee S. Neuregulin-1 elicits a regulatory immune response following traumatic spinal cord injury. J. Neuroinflammation. 2018;15(1):53. doi: 10.1186/s12974-018-1093-910.1186/s12974-018-1093-9. Epub 2018/02/23. [pii]. PubMed PMID: 29467001; PubMed Central PMCID: PMC5822667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allport L., Song M., Leung C.W., McGlumphy K.C., Hasson R.E. Influence of parent stressors on adolescent obesity in African American youth. J Obes. 2019;2019:1316765. doi: 10.1155/2019/1316765. Epub 2019/12/25. PubMed PMID: 31871784; PubMed Central PMCID: PMCPMC6913272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryee M.J., Jaffe A.E., Corrada-Bravo H., Ladd-Acosta C., Feinberg A.P., Hansen K.D. Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics. 2014;30(10):1363–1369. doi: 10.1093/bioinformatics/btu049btu049. Epub 2014/01/31. [pii]. PubMed PMID: 24478339; PubMed Central PMCID: PMC4016708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assari S., Wisseh C., Bazargan M. Obesity and polypharmacy among African American older adults: gender as the moderator and multimorbidity as the mediator. Int. J. Environ. Res. Publ. Health. 2019;16(12) doi: 10.3390/ijerph16122181. Epub 2019/06/23. PubMed PMID: 31226752; PubMed Central PMCID: PMCPMC6617277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin J.R., Arseneault L., Caspi A., Fisher H.L., Moffitt T.E., Odgers C.L. Childhood victimization and inflammation in young adulthood: a genetically sensitive cohort study. Brain Behav. Immun. 2018;67:211–217. doi: 10.1016/j.bbi.2017.08.025. Epub 2017/09/05. PubMed PMID: 28867281; PubMed Central PMCID: PMCPMC5710993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bali P., Im H.I., Kenny P.J. Methylation, memory and addiction. Epigenetics. 2011;6(6):671–674. doi: 10.4161/epi.6.6.15905. Epub 2011/05/19. PubMed PMID: 21586900; PubMed Central PMCID: PMCPMC3142366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcelona de Mendoza V., Huang Y., Crusto C.A., Sun Y.V., Taylor J.Y. Perceived racial discrimination and DNA methylation among African American women in the InterGEN study. Biol. Res. Nurs. 2018;20(2):145–152. doi: 10.1177/1099800417748759. Epub 2017/12/21. PubMed PMID: 29258399; PubMed Central PMCID: PMCPMC5741522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barengolts E., Green S.J., Chlipala G.E., Layden B.T., Eisenberg Y., Priyadarshini M. Predictors of obesity among gut microbiota biomarkers in African American men with and without diabetes. Microorganisms. 2019;7(9) doi: 10.3390/microorganisms7090320. Epub 2019/09/08. PubMed PMID: 31491976; PubMed Central PMCID: PMCPMC6780321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker E.D., Walton E., Cecil C.A.M. Annual Research Review: DNA methylation as a mediator in the association between risk exposure and child and adolescent psychopathology. JCPP (J. Child Psychol. Psychiatry) 2018;59(4):303–322. doi: 10.1111/jcpp.12782. Epub 2017/07/25. PubMed PMID: 28736860. [DOI] [PubMed] [Google Scholar]
- Boulet S., Daudelin J.F., Odagiu L., Pelletier A.N., Yun T.J., Lesage S. The orphan nuclear receptor NR4A3 controls the differentiation of monocyte-derived dendritic cells following microbial stimulation. Proc. Natl. Acad. Sci. U. S. A. 2019;116(30):15150–15159. doi: 10.1073/pnas.18212961161821296116. Epub 2019/07/10. [pii]. PubMed PMID: 31285338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitenkamp A.F., Matthes J., Nass R.D., Sinzig J., Lehmkuhl G., Nurnberg P. Rare mutations of CACNB2 found in autism spectrum disease-affected families alter calcium channel function. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0095579PONE-D-13-44841. Epub 2014/04/23. [pii]. PubMed PMID: 24752249; PubMed Central PMCID: PMC3994086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchser W.J., Slepak T.I., Gutierrez-Arenas O., Bixby J.L., Lemmon V.P. Kinase/phosphatase overexpression reveals pathways regulating hippocampal neuron morphology. Mol. Syst. Biol. 2010;6:391. doi: 10.1038/msb.2010. Epub 2010/07/29. 52msb201052 [pii]. PubMed PMID: 20664637; PubMed Central PMCID: PMC2925531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunin A., Sisirak V., Ghosh H.S., Grajkowska L.T., Hou Z.E., Miron M. Protein tyrosine phosphatase PTPRS is an inhibitory receptor on human and murine plasmacytoid dendritic cells. Immunity. 2015;43(2):277–288. doi: 10.1016/j.immuni.2015.07.009S1074-7613(15)00276-9. Epub 2015/08/02. [pii]. PubMed PMID: 26231120; PubMed Central PMCID: PMC4547994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillier S.J., Briggs F., Cree B.A.C., Baranzini S.E., Fernandez-Vina M., Ramsay P.P. Uncoupling the roles of HLA-DRB1 and HLA-DRB5 genes in multiple sclerosis. J. Immunol. 2008;181(8):5473–5480. doi: 10.4049/jimmunol.181.8.5473. PubMed PMID: ISI:000260025300039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calle-Fabregat C., Morante-Palacios O., Ballestar E. Understanding the relevance of DNA methylation changes in immune differentiation and disease. Genes. 2020;11(1) doi: 10.3390/genes11010110. Epub 2020/01/23. PubMed PMID: 31963661; PubMed Central PMCID: PMCPMC7017047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Sun L., Aramsangtienchai P., Spiegelman N.A., Zhang X.Y., Huang W.S. HDAC11 regulates type I interferon signaling through defatty-acylation of SHMT2. P Natl Acad Sci USA. 2019;116(12):5487–5492. doi: 10.1073/pnas.1815365116. PubMed PMID: ISI:000461679000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecil C.A., Smith R.G., Walton E., Mill J., McCrory E.J., Viding E. Epigenetic signatures of childhood abuse and neglect: implications for psychiatric vulnerability. J. Psychiatr. Res. 2016;83:184–194. doi: 10.1016/j.jpsychires.2016.09.010. Epub 2016/09/20. PubMed PMID: 27643477. [DOI] [PubMed] [Google Scholar]
- Christopher M.A., Kyle S.M., Katz D.J. Neuroepigenetic mechanisms in disease. Epigenet. Chromatin. 2017;10(1):47. doi: 10.1186/s13072-017-0150-4. Epub 2017/10/19. PubMed PMID: 29037228; PubMed Central PMCID: PMCPMC5644115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durdiakova J., Warrier V., Banerjee-Basu S., Baron-Cohen S., Chakrabarti B. STX1A and Asperger syndrome: a replication study. Mol. Autism. 2014;5(1):14. doi: 10.1186/2040-2392-5-142040-2392-5-14. Epub 2014/02/20. [pii]. PubMed PMID: 24548729; PubMed Central PMCID: PMC3932791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi P., Luthman H., McGuigan F.E., Akesson K.E. Epigenome-wide cross-tissue correlation of human bone and blood DNA methylation - can blood be used as a surrogate for bone? Epigenetics. 2020:1–14. doi: 10.1080/15592294.2020.1788325. Epub 2020/07/22. PubMed PMID: 32692944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hajj N., Dittrich M., Haaf T. Epigenetic dysregulation of protocadherins in human disease. Semin. Cell Dev. Biol. 2017;69:172–182. doi: 10.1016/j.semcdb.2017.07.007. Epub 2017/07/12. S1084-9521(17)30396-8 [pii]10.1016/j.semcdb.2017.07.007. PubMed PMID: 28694114. [DOI] [PubMed] [Google Scholar]
- Eltokhi A., Rappold G., Sprengel R. Distinct phenotypes of Shank2 mouse models reflect neuropsychiatric spectrum disorders of human patients with SHANK2 variants. Front. Mol. Neurosci. 2018;11:240. doi: 10.3389/fnmol.2018.00240. Epub 2018/08/04. PubMed PMID: 30072871; PubMed Central PMCID: PMC6060255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faucher M.A., Greathouse K.L., Hastings-Tolsma M., Padgett R.N., Sakovich K., Choudhury A. Exploration of the vaginal and gut microbiome in African American women by body mass index, class of obesity, and gestational weight gain: a pilot study. Am. J. Perinatol. 2019 doi: 10.1055/s-0039-1692715. Epub 2019/06/27. PubMed PMID: 31242511. [DOI] [PubMed] [Google Scholar]
- Finkelhor D., Turner H., Ormrod R., Hamby S.L. Trends in childhood violence and abuse exposure: evidence from 2 national surveys. Arch. Pediatr. Adolesc. Med. 2010;164(3):238–242. doi: 10.1001/archpediatrics.2009.283. Epub 2010/03/03. PubMed PMID: 20194256. [DOI] [PubMed] [Google Scholar]
- Finkelhor D., Turner H.A., Shattuck A., Hamby S.L. Prevalence of childhood exposure to violence, crime, and abuse: results from the national survey of children’s exposure to violence. JAMA Pediatr. 2015;169(8):746–754. doi: 10.1001/jamapediatrics.2015.0676. Epub 2015/06/30. PubMed PMID: 26121291. [DOI] [PubMed] [Google Scholar]
- Francisco N.M., Hsu N.J., Keeton R., Randall P., Sebesho B., Allie N. TNF-dependent regulation and activation of innate immune cells are essential for host protection against cerebral tuberculosis. J. Neuroinflammation. 2015;12:125. doi: 10.1186/s12974-015-0345-110.1186/s12974-015-0345-1. Epub 2015/06/27. [pii]. PubMed PMID: 26112704; PubMed Central PMCID: PMC4488051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giurgescu C., Nowak A.L., Gillespie S., Nolan T.S., Anderson C.M., Ford J.L. Neighborhood environment and DNA methylation: implications for cardiovascular disease risk. J. Urban Health. 2019;96(Suppl. 1):23–34. doi: 10.1007/s11524-018-00341-1. Epub 2019/01/13. PubMed PMID: 30635842; PubMed Central PMCID: PMCPMC6430282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go A.S., Mozaffarian D., Roger V.L., Benjamin E.J., Berry J.D., Blaha M.J. Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129(3):399–410. doi: 10.1161/01.cir.0000442015.53336.12. Epub 2014/01/22. PubMed PMID: 24446411. [DOI] [PubMed] [Google Scholar]
- Goldmann E., Aiello A., Uddin M., Delva J., Koenen K., Gant L.M. Pervasive exposure to violence and posttraumatic stress disorder in a predominantly African American urban community: the detroit neighborhood health study. J. Trauma Stress. 2011;24(6):747–751. doi: 10.1002/jts.20705. Epub 2011/12/07. PubMed PMID: 22144187; PubMed Central PMCID: PMCPMC4433006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode R.W., Styn M.A., Mendez D.D., Gary-Webb T.L. African Americans in standard behavioral treatment for obesity, 2001-2015: what have we learned? West. J. Nurs. Res. 2017;39(8):1045–1069. doi: 10.1177/0193945917692115. Epub 2017/03/23. PubMed PMID: 28322668; PubMed Central PMCID: PMCPMC5519343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggs S., Ratner H.H., Hannigan J.H., Delaney-Black V., Chiodo L.M. Violence exposure, conflict, and health outcomes in inner-city African American adolescents. Nurs. Forum. 2019;54(4):513–525. doi: 10.1111/nuf.12365. Epub 2019/07/17. PubMed PMID: 31309581; PubMed Central PMCID: PMCPMC6856364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadar A., Milanesi E., Squassina A., Niola P., Chillotti C., Pasmanik-Chor M. RGS2 expression predicts amyloid-beta sensitivity, MCI and Alzheimer’s disease: genome-wide transcriptomic profiling and bioinformatics data mining. Transl. Psychiatry. 2016;6(10):e909. doi: 10.1038/tp.2016.179tp2016179. Epub 2016/10/05. [pii]. PubMed PMID: 27701409; PubMed Central PMCID: PMC5315547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Sherman B.T., Lempicki R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Iborra S., Soto M., Stark-Aroeira L., Castellano E., Alarcon B., Alonso C. H-ras and N-ras are dispensable for T-cell development and activation but critical for protective Th1 immunity. Blood. 2011;117(19):5102–5111. doi: 10.1182/blood-2010-10-315770blood-2010-10-315770. Epub 2011/03/30. [pii]. PubMed PMID: 21444916. [DOI] [PubMed] [Google Scholar]
- Intarasunanont P., Navasumrit P., Waraprasit S., Chaisatra K., Suk W.A., Mahidol C. Effects of arsenic exposure on DNA methylation in cord blood samples from newborn babies and in a human lymphoblast cell line. Environ. Health. 2012;11:31. doi: 10.1186/1476-069X-11-31. PubMed PMID: 22551203; PubMed Central PMCID: PMCPMC3506565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs E.A., Rathouz P.J., Karavolos K., Everson-Rose S.A., Janssen I., Kravitz H.M. Perceived discrimination is associated with reduced breast and cervical cancer screening: the Study of Women’s Health across the Nation (SWAN) J Womens Health (Larchmt) 2014;23(2):138–145. doi: 10.1089/jwh.2013.4328. PubMed PMID: 24261647; PubMed Central PMCID: PMCPMC3922246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T., Vance L.A., Cross D., Knight A.K., Kilaru V., Michopoulos V. Exposure to violence accelerates epigenetic aging in children. Sci. Rep. 2017;7(1):8962. doi: 10.1038/s41598-017-09235-9. Epub 2017/08/23. PubMed PMID: 28827677; PubMed Central PMCID: PMCPMC5566406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerepesi L.A., Hess J.A., Nolan T.J., Schad G.A., Abraham D. Complement component C3 is required for protective innate and adaptive immunity to larval strongyloides stercoralis in mice. J. Immunol. 2006;176(7):4315–4322. doi: 10.4049/jimmunol.176.7.4315. Epub 2006/03/21. 176/7/4315 [pii]10.4049/jimmunol.176.7.4315. PubMed PMID: 16547268. [DOI] [PubMed] [Google Scholar]
- Lahtinen A., Puttonen S., Vanttola P., Viitasalo K., Sulkava S., Pervjakova N. A distinctive DNA methylation pattern in insufficient sleep. Sci. Rep. 2019;9(1):1193. doi: 10.1038/s41598-018-38009-0. Epub 2019/02/06. PubMed PMID: 30718923; PubMed Central PMCID: PMCPMC6362278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Wing Sun K., Correia J.P., Kennedy T.E. Netrins: versatile extracellular cues with diverse functions. Development. 2011;138(11):2153–2169. doi: 10.1242/dev.044529138/11/2153. Epub 2011/05/12. [pii]. PubMed PMID: 21558366. [DOI] [PubMed] [Google Scholar]
- Lee G.H., Kim S.H., Homayouni R., D’Arcangelo G. Dab2ip regulates neuronal migration and neurite outgrowth in the developing neocortex. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0046592PONE-D-12-21254. Epub 2012/10/12. [pii]. PubMed PMID: 23056358; PubMed Central PMCID: PMC3464295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.A., Damianov A., Lin C.H., Fontes M., Parikshak N.N., Anderson E.S. Cytoplasmic Rbfox1 regulates the expression of synaptic and autism-related genes. Neuron. 2016;89(1):113–128. doi: 10.1016/j.neuron.2015.11.025S0896-6273(15)01031-4. Epub 2015/12/22. [pii]. PubMed PMID: 26687839; PubMed Central PMCID: PMC4858412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P. The glycinergic inhibitory synapse. Cell. Mol. Life Sci. 2001;58(5–6):760–793. doi: 10.1007/PL00000899. Epub 2001/07/05. PubMed PMID: 11437237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.G., Misra K., Matise M.P., Xiang M.Q. Foxn4 acts synergistically with Mash1 to specify subtype identity of V2 interneurons in the spinal cord. P Natl Acad Sci USA. 2005;102(30):10688–10693. doi: 10.1073/pnas.0504799102. PubMed PMID: ISI:000230853300051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Wang Z., Hardy T., Huang Y., Hui Q., Crusto C.A. Association of obesity with DNA methylation age acceleration in African American mothers from the InterGEN study. Int. J. Mol. Sci. 2019;20(17) doi: 10.3390/ijms20174273. Epub 2019/09/05. PubMed PMID: 31480455; PubMed Central PMCID: PMCPMC6747309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipstein N., Verhoeven-Duif N.M., Michelassi F.E., Calloway N., van Hasselt P.M., Pienkowska K. Synaptic UNC13A protein variant causes increased neurotransmission and dyskinetic movement disorder. J. Clin. Invest. 2017;127(3):1005–1018. doi: 10.1172/JCI90259. Epub 2017/02/14. doi: 90259 [pii]10.1172/JCI90259. PubMed PMID: 28192369; PubMed Central PMCID: PMC5330740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little L., Hamby S.L. Memory of childhood sexual abuse among clinicians: characteristics, outcomes, and current therapy attitudes. Sex Abuse. 2001;13(4):233–248. doi: 10.1177/107906320101300402. Epub 2001/10/27. PubMed PMID: 11677925. [DOI] [PubMed] [Google Scholar]
- Lockwood L.E., Su S., Youssef N.A. The role of epigenetics in depression and suicide: a platform for gene-environment interactions. Psychiatr. Res. 2015;228(3):235–242. doi: 10.1016/j.psychres.2015.05.071. Epub 2015/07/15. PubMed PMID: 26163724. [DOI] [PubMed] [Google Scholar]
- Luo G.R., Chen Y., Li X.P., Liu T.X., Le W.D. Nr4a2 is essential for the differentiation of dopaminergic neurons during zebrafish embryogenesis. Mol. Cell. Neurosci. 2008;39(2):202–210. doi: 10.1016/j.mcn.2008.06.010S1044-7431(08)00163-2. Epub 2008/07/22. [pii]. PubMed PMID: 18638558. [DOI] [PubMed] [Google Scholar]
- Ly A., Nikolaev A., Suresh G., Zheng Y., Tessier-Lavigne M., Stein E. DSCAM is a netrin receptor that collaborates with DCC in mediating turning responses to netrin-1. Cell. 2008;133(7):1241–1254. doi: 10.1016/j.cell.2008.05.030S0092-8674(08)00690-9. Epub 2008/07/01. [pii]. PubMed PMID: 18585357; PubMed Central PMCID: PMC2491333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyst M.J., Ekiert R., Ebert D.H., Merusi C., Nowak J., Selfridge J. Rett syndrome mutations abolish the interaction of MeCP2 with the NCoR/SMRT co-repressor. Nat. Neurosci. 2013;16(7):898–902. doi: 10.1038/nn.3434. PubMed PMID: 23770565; PubMed Central PMCID: PMCPMC3786392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansell G., Gorrie-Stone T.J., Bao Y., Kumari M., Schalkwyk L.S., Mill J. Guidance for DNA methylation studies: statistical insights from the Illumina EPIC array. BMC Genom. 2019;20(1):366. doi: 10.1186/s12864-019-5761-7. PubMed PMID: 31088362; PubMed Central PMCID: PMCPMC6518823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade T.W., Ryan C.P., Jones M.J., Hoke M.K., Borja J., Miller G.E. Genome-wide analysis of DNA methylation in relation to socioeconomic status during development and early adulthood. Am. J. Phys. Anthropol. 2019;169(1):3–11. doi: 10.1002/ajpa.23800. Epub 2019/02/17. PubMed PMID: 30771258. [DOI] [PubMed] [Google Scholar]
- McGee Z.T., Davis B.L., Brisbane T., Collins N., Nuriddin T., Irving S. Urban stress and mental health among African-American youth: assessing the link between exposure to violence, problem behavior, and coping strategies. J. Cult. Divers. 2001;8(3):94–104. Epub 2002/02/22. PubMed PMID: 11855219. [PubMed] [Google Scholar]
- McKay J.A., Xie L., Harris S., Wong Y.K., Ford D., Mathers J.C. Blood as a surrogate marker for tissue-specific DNA methylation and changes due to folate depletion in post-partum female mice. Mol. Nutr. Food Res. 2011;55(7):1026–1035. doi: 10.1002/mnfr.201100008. Epub 2011/04/27. PubMed PMID: 21520493. [DOI] [PubMed] [Google Scholar]
- Mei L., Xiong W.C. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat. Rev. Neurosci. 2008;9(6):437–452. doi: 10.1038/nrn2392nrn2392. Epub 2008/05/15. [pii]. PubMed PMID: 18478032; PubMed Central PMCID: PMC2682371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell S.J., Lewin A., Horn I.B., Valentine D., Sanders-Phillips K., Joseph J.G. How does violence exposure affect the psychological health and parenting of young African-American mothers? Soc. Sci. Med. 2010;70(4):526–533. doi: 10.1016/j.socscimed.2009.10.048. Epub 2009/11/26. PubMed PMID: 19932932; PubMed Central PMCID: PMCPMC2853478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt T.E., Klaus-Grawe Think T. Childhood exposure to violence and lifelong health: clinical intervention science and stress-biology research join forces. Dev. Psychopathol. 2013;25(4 Pt 2):1619–1634. doi: 10.1017/S0954579413000801. Epub 2013/12/18. PubMed PMID: 24342859; PubMed Central PMCID: PMCPMC3869039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y., Fujii A., Kanata S., Fujikawa S., Ikegame T., Nakachi Y. Evaluation of the usefulness of saliva for DNA methylation analysis in cohort studies. Neuropsychopharmacol. Rep. 2019;39(4):301–305. doi: 10.1002/npr2.12075. PubMed PMID: 31393092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka M., Yashiro T., Uchida Y., Ando T., Hara M., Arai H. The orphan nuclear receptor NR4A3 is involved in the function of dendritic cells. J. Immunol. 2017;199(8):2958–2967. doi: 10.4049/jimmunol.1601911jimmunol.1601911. Epub 2017/09/13. [pii]. PubMed PMID: 28893954. [DOI] [PubMed] [Google Scholar]
- Nagy M.R., McGlumphy K.C., Dopp R., Lewis T.C., Hasson R.E. Association between asthma, obesity, and health behaviors in African American youth. J. Asthma. 2020;57(4):410–420. doi: 10.1080/02770903.2019.1571083. Epub 2019/02/01. PubMed PMID: 30702005. [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Ma B.Y., Uemura K., Oka S., Kawasaki N., Kawasaki T. Role of mannan-binding protein, MBP, in innate immunity. Anticancer Res. 2003;23(6a):4467–4471. Epub 2003/12/12. PubMed PMID: 14666735. [PubMed] [Google Scholar]
- Nakamura K., Anitha A., Yamada K., Tsujii M., Iwayama Y., Hattori E. Genetic and expression analyses reveal elevated expression of syntaxin 1A ( STX1A) in high functioning autism. Int. J. Neuropsychopharmacol. 2008;11(8):1073–1084. doi: 10.1017/S1461145708009036S1461145708009036. Epub 2008/07/03. [pii]. PubMed PMID: 18593506. [DOI] [PubMed] [Google Scholar]
- Naumova O.Y., Lee M., Koposov R., Szyf M., Dozier M., Grigorenko E.L. Differential patterns of whole-genome DNA methylation in institutionalized children and children raised by their biological parents. Dev. Psychopathol. 2012;24(1):143–155. doi: 10.1017/S0954579411000605. Epub 2011/11/30. PubMed PMID: 22123582; PubMed Central PMCID: PMCPMC3470853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odagiu L., Boulet S., Daudelin J.F., Labrecque N. NR4A3 controls CD8+T cell metabolism and differentiation. J. Immunol. 2016;196 PubMed PMID: ISI:000380288302146. [Google Scholar]
- Ogawa Y., Kakumoto K., Yoshida T., Kuwako K.I., Miyazaki T., Yamaguchi J. Elavl3 is essential for the maintenance of Purkinje neuron axons. Sci. Rep. 2018;8(1):2722. doi: 10.1038/s41598-018-21130-510.1038/s41598-018-21130-5. Epub 2018/02/11. [pii]. PubMed PMID: 29426875; PubMed Central PMCID: PMC5807307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka N., Badurek S., Busslinger M., Benes F.M., Minichiello L., Rudolph U. GABAergic neurons regulate lateral ventricular development via transcription factor Pax5. Genesis. 2013;51(4):234–245. doi: 10.1002/dvg.22370. Epub 2013/01/26. PubMed PMID: 23349049; PubMed Central PMCID: PMC3740952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson N., Lindqvist K., Shaw B.A., Danielsson I. Long-term health consequences of violence exposure in adolescence: a 26-year prospective study. BMC Publ. Health. 2012;12:411. doi: 10.1186/1471-2458-12-411. Epub 2012/06/22. PubMed PMID: 22716027; PubMed Central PMCID: PMCPMC3419075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozato K., Shin D.M., Chang T.H., Morse H.C., 3rd TRIM family proteins and their emerging roles in innate immunity. Nat. Rev. Immunol. 2008;8(11):849–860. doi: 10.1038/nri2413nri2413. Epub 2008/10/07. [pii]. PubMed PMID: 18836477; PubMed Central PMCID: PMC3433745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranjape A., Kaslow N. Family violence exposure and health outcomes among older African American women: do spirituality and social support play protective roles? J Womens Health (Larchmt) 2010;19(10):1899–1904. doi: 10.1089/jwh.2009.1845. Epub 2010/09/14. PubMed PMID: 20831432; PubMed Central PMCID: PMCPMC2956386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranjape A., Sprauve-Holmes N.E., Gaughan J., Kaslow N.J. Lifetime exposure to family violence: implications for the health status of older African American women. J Womens Health (Larchmt) 2009;18(2):171–175. doi: 10.1089/jwh.2008.0850. Epub 2009/02/03. PubMed PMID: 19183088; PubMed Central PMCID: PMCPMC2945718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Martinez L., Jaworski D.M. Tissue inhibitor of metalloproteinase-2 promotes neuronal differentiation by acting as an anti-mitogenic signal. J. Neurosci. 2005;25(20):4917–4929. doi: 10.1523/JNEUROSCI.5066-04.2005. Epub 2005/05/20. 25/20/4917 [pii]10.1523/JNEUROSCI.5066-04.2005. PubMed PMID: 15901773; PubMed Central PMCID: PMC1282460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pew Research Center . 2016. On Views of Race and Inequality, Blacks and Whites Are Worlds Apart.https://www.pewsocialtrends.org/2016/06/27/on-views-of-race-and-inequality-blacks-and-whites-are-worlds-apart/ Available from: [Google Scholar]
- Pittet L.A., Quinton L.J., Yamamoto K., Robson B.E., Ferrari J.D., Algul H. Earliest innate immune responses require macrophage RelA during pneumococcal pneumonia. Am. J. Respir. Cell Mol. Biol. 2011;45(3):573–581. doi: 10.1165/rcmb.2010-0210OC2010-0210OC. Epub 2011/01/11. [pii]. PubMed PMID: 21216972; PubMed Central PMCID: PMC3175578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raveney B.J.E., Oki S., Yamamura T. Nuclear receptor NR4A2 orchestrates Th17 cell-mediated autoimmune inflammation via IL-21 signalling. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0056595. ARTN e5659510.1371/journal.pone.0056595. PubMed PMID: ISI:000315186000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richters, JE., & Saltzman, W. reportSurvey of Exposure to Community Violence: Self-Report Version. 1990. Rockville, MD: National Institute of Mental Health.
- Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. doi: 10.1093/nar/gkv007gkv007. Epub 2015/01/22. [pii]. PubMed PMID: 25605792; PubMed Central PMCID: PMC4402510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadatmand F., Harrison R., Bronson J., Crouse D., Jackson M. Gender differences and the impact of exposure to violence on depressive symptoms and sleep habits among young African American adults. J. Family Strengths. 2017;17(1) Epub 2017/08/01. PubMed PMID: 30288367; PubMed Central PMCID: PMCPMC6168193. [PMC free article] [PubMed] [Google Scholar]
- Saadatmand F., Harrison R., Bronson J., Dearfield C., Crouse D., Douglas M. Violence exposure, drug use and HIV/AIDS risk taking behaviors: the role of gender. J. Natl. Med. Assoc. 2019 doi: 10.1016/j.jnma.2019.05.003. Epub 2019/06/17. PubMed PMID: 31202486; PubMed Central PMCID: PMCPMC6908765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe S., Jin U.H., Morpurgo B., Abudayyeh A., Singh M., Tjalkens R.B. Nuclear receptor 4A (NR4A) family - orphans no more. J. Steroid Biochem. Mol. Biol. 2016;157:48–60. doi: 10.1016/j.jsbmb.2015.04.016. Epub 2015/04/29. PubMed PMID: 25917081; PubMed Central PMCID: PMCPMC4618773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheckel C., Drapeau E., Frias M.A., Park C.Y., Fak J., Zucker-Scharff I. Regulatory consequences of neuronal ELAV-like protein binding to coding and non-coding RNAs in human brain. Elife. 2016;5 doi: 10.7554/eLife.10421. ARTN e1042110.7554/eLife.10421. PubMed PMID: ISI:000371880400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A., Heredia N., Senut M.C., Hess M., Land S., Qu W. Early life lead exposure causes gender-specific changes in the DNA methylation profile of DNA extracted from dried blood spots. Epigenomics. 2015;7(3):379–393. doi: 10.2217/epi.15.2. PubMed PMID: 26077427; PubMed Central PMCID: PMCPMC4501025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A., Heredia N., Senut M.C., Land S., Hollocher K., Lu X. Multigenerational epigenetic inheritance in humans: DNA methylation changes associated with maternal exposure to lead can be transmitted to the grandchildren. Sci. Rep. 2015;5:14466. doi: 10.1038/srep14466. PubMed PMID: 26417717; PubMed Central PMCID: PMCPMC4586440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpeloni F., Radtke K.M., Hecker T., Sill J., Vukojevic V., de Assis S.G. Does prenatal stress shape postnatal resilience? - an epigenome-wide study on violence and mental health in humans. Front. Genet. 2019;10:269. doi: 10.3389/fgene.2019.00269. Epub 2019/05/02. PubMed PMID: 31040859; PubMed Central PMCID: PMCPMC6477038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpeloni F., Natt D., Assis S.G., Wieling E., Elbert T. Experiencing community and domestic violence is associated with epigenetic changes in DNA methylation of BDNF and CLPX in adolescents. Psychophysiology. 2020;57(1) doi: 10.1111/psyp.13382. Epub 2019/05/07. PubMed PMID: 31059136; PubMed Central PMCID: PMCPMC7003421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon O., MacIsaac J., Quach H., Tindula G., Kobor M.S., Huen K. Comparison of DNA methylation measured by Illumina 450K and EPIC BeadChips in blood of newborns and 14-year-old children. Epigenetics. 2018;13(6):655–664. doi: 10.1080/15592294.2018.1497386. PubMed PMID: 30044683; PubMed Central PMCID: PMCPMC6140901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M., He Y., Zhou H., Zhang Y., Li X., Yu Y. Combined analysis of DNA methylome and transcriptome reveal novel candidate genes with susceptibility to bovine Staphylococcus aureus subclinical mastitis. Sci. Rep. 2016;6:29390. doi: 10.1038/srep29390. Epub 2016/07/15. PubMed PMID: 27411928; PubMed Central PMCID: PMCPMC4944166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens H.E., Smith K.M., Maragnoli M.E., Fagel D., Borok E., Shanabrough M. Fgfr2 is required for the development of the medial prefrontal cortex and its connections with limbic circuits. J. Neurosci. 2010;30(16):5590–5602. doi: 10.1523/JNEUROSCI.5837-09.201030/16/5590. Epub 2010/04/23. [pii]. PubMed PMID: 20410112; PubMed Central PMCID: PMC2868832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stith S.M., Hamby S.L. The anger management scale: development and preliminary psychometric properties. Violence Vict. 2002;17(4):383–402. doi: 10.1891/vivi.17.4.383.33683. Epub 2002/10/02. PubMed PMID: 12353588. [DOI] [PubMed] [Google Scholar]
- Tang X., Milyavsky M., Goldfinger N., Rotter V. Amyloid-beta precursor-like protein APLP1 is a novel p53 transcriptional target gene that augments neuroblastoma cell death upon genotoxic stress. Oncogene. 2007;26(52):7302–7312. doi: 10.1038/sj.onc.1210542. Epub 2007/05/30. doi: 1210542 [pii]10.1038/sj.onc.1210542. PubMed PMID: 17533371. [DOI] [PubMed] [Google Scholar]
- Taylor D.L., Jackson A.U., Narisu N., Hemani G., Erdos M.R., Chines P.S. Integrative analysis of gene expression, DNA methylation, physiological traits, and genetic variation in human skeletal muscle. Proc. Natl. Acad. Sci. U. S. A. 2019;116(22):10883–10888. doi: 10.1073/pnas.1814263116. PubMed PMID: 31076557; PubMed Central PMCID: PMCPMC6561151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triche T.J., Jr., Weisenberger D.J., Van Den Berg D., Laird P.W., Siegmund K.D. Low-level processing of Illumina Infinium DNA methylation BeadArrays. Nucleic Acids Res. 2013;41(7):e90. doi: 10.1093/nar/gkt090gkt090. Epub 2013/03/12. [pii]. PubMed PMID: 23476028; PubMed Central PMCID: PMC3627582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M., Sipahi L., Li J., Koenen K.C. Sex differences in DNA methylation may contribute to risk of PTSD and depression: a review of existing evidence. Depress. Anxiety. 2013;30(12):1151–1160. doi: 10.1002/da.22167. Epub 2013/08/21. PubMed PMID: 23959810; PubMed Central PMCID: PMCPMC4530966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M., Ratanatharathorn A., Armstrong D., Kuan P.F., Aiello A.E., Bromet E.J. Epigenetic meta-analysis across three civilian cohorts identifies NRG1 and HGS as blood-based biomarkers for post-traumatic stress disorder. Epigenomics. 2018;10(12):1585–1601. doi: 10.2217/epi-2018-0049. Epub 2018/11/21. PubMed PMID: 30456986; PubMed Central PMCID: PMCPMC6331697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda K., Negishi M., Katoh H. RasGRF1 mediates brain-derived neurotrophic factor-induced axonal growth in primary cultured cortical neurons. Biochem. Biophys. Rep. 2019;17:56–64. doi: 10.1016/j.bbrep.2018.11.011S2405-5808(18)30275-9. Epub 2018/12/26. [pii]. PubMed PMID: 30582008; PubMed Central PMCID: PMC6295856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick A.D., Burris H.H. Epigenetics and health disparities. Curr. Epidemiol. Rep. 2017;4(1):31–37. doi: 10.1007/s40471-017-0096-x. Epub 2017/03/04. PubMed PMID: 28255530; PubMed Central PMCID: PMCPMC5327425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welberg L.A., Seckl J.R., Holmes M.C. Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin-releasing hormone: possible implications for behaviour. Neuroscience. 2001;104(1):71–79. doi: 10.1016/s0306-4522(01)00065-3. Epub 2001/04/20. PubMed PMID: 11311532. [DOI] [PubMed] [Google Scholar]
- Wen L., Liu L., Li J., Tong L., Zhang K., Zhang Q. NDRG4 protects against cerebral ischemia injury by inhibiting p53-mediated apoptosis. Brain Res. Bull. 2019;146:104–111. doi: 10.1016/j.brainresbull.2018.12.010. Epub 2018/12/30. S0361-9230(18)30292-2 [pii]10.1016/j.brainresbull.2018.12.010. PubMed PMID: 30593880. [DOI] [PubMed] [Google Scholar]
- Woodson K.M., Hives C., Sanders-Phillips K. Violence exposure and health related risk among African American adolescent female detainees: a strategy for reducing recidivism. J. Offender Rehabil. 2010;49(8):571–584. doi: 10.1080/10509674.2010.519669. Epub 2011/03/05. PubMed PMID: 21373205; PubMed Central PMCID: PMCPMC3045759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., Yang X., Zhang L., Zhang Y., Feng L. Nuclear accumulation of histone deacetylase 4 (HDAC4) exerts neurotoxicity in models of Parkinson’s disease. Mol. Neurobiol. 2017;54(9):6970–6983. doi: 10.1007/s12035-016-0199-210.1007/s12035-016-0199-2. Epub 2016/10/28. [pii]. PubMed PMID: 27785754. [DOI] [PubMed] [Google Scholar]
- Yang Y., Cai Q., Zheng W., Steinwandel M., Blot W.J., Shu X.O. Oral microbiome and obesity in a large study of low-income and African-American populations. J. Oral Microbiol. 2019;11(1):1650597. doi: 10.1080/20002297.2019.1650597. Epub 2019/09/07. PubMed PMID: 31489128; PubMed Central PMCID: PMCPMC6713186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Takaku M., Zou L.Y., Gu A.D., Chou W.C., Zhang G. Reversing SKI-SMAD4-mediated suppression is essential for T(H)17 cell differentiation. Nature. 2017;551(7678):105. doi: 10.1038/nature24283. PubMed PMID: ISI:000414222900054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zoltan M., Riquelme E., Xu H., Sahin I., Castro-Pando S. Immune cell production of interleukin 17 induces stem cell features of pancreatic intraepithelial neoplasia cells. Gastroenterology. 2018;155(1):210–223. doi: 10.1053/j.gastro.2018.03.041. Epub 2018/04/01. S0016-5085(18)30348-2 [pii]10.1053/j.gastro.2018.03.041. PubMed PMID: 29604293; PubMed Central PMCID: PMC6035075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z., Wheeler M.D., Li X., Froh M., Schemmer P., Yin M. L-Glycine: A novel antiinflammatory, immunomodulatory, and cytoprotective agent. Curr. Opin. Clin. Nutr. Metab. Care. 2003;6(2):229–240. doi: 10.1097/01.mco.0000058609.19236.a4. Epub 2003/02/18. PubMed PMID: 12589194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.