Abstract
Offspring adrenal function may be negatively affected in utero by maternal stressors such as microbial infection. Maternal supplementation with immunomodulatory compounds such as omega-3 polyunsaturated fatty acids (n-3 PUFA) may help minimize the adverse effects of maternal stress on fetal hypothalamic-pituitary-adrenal development and improve offspring health. Presently, n-3 PUFA sources are primarily fish-based, but n-3 PUFA microalgae (AL) may be an alternative. Previously, it was determined that maternal AL or fish oil (FO) supplementation to sows, in addition to maternal stress induced by Escherichia coli lipopolysaccharide (LPS) challenge appeared to have a greater influence on the stress response of male offspring compared to females. To further elaborate on these findings, this study assessed the effects of maternal AL or FO supplementation combined with a maternal LPS challenge on adrenal gene expression in male offspring fed a nursery diet containing low-quality protein sources. Forty-eight sows were fed gestation diets starting on gestation day (gd) 75 containing either 3.12% AL, 3.1% FO, or a control diet containing 1.89% corn oil. On gd 112, half the sows in each treatment were administered 10 μg/kg LPS i.m. Piglets were weaned at 21 days of age onto a common low-quality plant-based protein diet, and one week after weaning, four piglets per sow were administered 40 μg/kg LPS i.m. Two hours later, the piglets were euthanized to obtain adrenal tissue, and total RNA was extracted to carry out transcriptome analysis using the Affymetrix GeneChip WT Plus assay and subsequent validation by real-time PCR. Analysis revealed that adrenal steroidogenesis, fatty acid metabolism and immune function were significantly influenced by maternal diet and stress. Increased expression of immune-related genes including lymphocyte antigen 96, TLR-2 and NF-κB suggests that maternal AL supplementation may increase offspring sensitivity to inflammation after weaning. Decreased expression of lymphocyte antigen 96 in male offspring from sows receiving maternal LPS challenge also suggests a possible role of maternal stress in diminishing the offspring immune response to immune stress challenge. Increased expression of the genes encoding the 11BHSD2 enzyme in offspring from sows fed FO may also reduce the magnitude of the stress response. These data provide insight to the immune and metabolic mechanisms that may be influenced by maternal diet and stress.
Keywords: Microalgae, Fish oil, Maternal stress, Microarray, Adrenal, Immune function
Highlights
-
•
Expression of adrenal steroidogenesis genes were influenced by maternal treatment.
-
•
Expression of lipid metabolism genes and immune function genes were enriched.
-
•
Maternal algae supplementation may increase offspring sensitivity to inflammation.
-
•
Maternal stress may reduce the offspring immune response to immune challenges.
-
•
Maternal fish oil supplementation may reduce the offspring stress response.
1. Introduction
The adrenal gland is important for both hormone and neurotransmitter signalling. Its main function includes the synthesis of steroid hormones within the adrenal cortex including androgens, mineralocorticoids and glucocorticoids. In addition to its role in the hypothalamic-pituitary-adrenal (HPA) axis, or stress axis, it is also involved in regulating the immune response during tissue injury or pathogen invasion, in part through the production of inflammatory mediators (Huang et al., 2010; Mohn et al., 2011).
During fetal development, the adrenal gland may be vulnerable to adverse programming resulting from exposure to maternal stressors such as microbial infection during pregnancy (Fisher et al., 2014; Harris and Seckl, 2011). Maternal stress including simulated infection has previously been shown to alter developmental programming of both the HPA axis and the immune system in sheep and swine (Fisher-Heffernan et al., 2015; Fisher et al., 2014; Kranendonk et al., 2006; Lay et al., 2011). This may leave offspring vulnerable to inflammatory, metabolic and neurodegenerative diseases in adulthood (Bronson and Bale, 2014; Gur et al., 2017; Mdaki et al., 2016). Developmental programming is believed to occur through epigenetic mechanisms whereby DNA modifications, or changes in chromatin organization, result in differential expression of genes throughout development (Weaver et al., 2017). Disrupting this normal developmental process in various tissues can result in changes in immune or neuroendocrine phenotypes that may predispose the offspring to disease in neonatal and/or adult life (Harris and Seckl, 2011).
The microbe-associated molecular pattern (MAMP) lipopolysaccharide (LPS) from Gram-negative bacteria is a commonly used microbial stressor that, at sufficient concentrations, activates the HPA axis. Recognition of Escherichia coli LPS by sentinel immune cells occurs mainly through activation of toll-like receptor 4 (TLR-4; Gnauck et al., 2016), that in turn initiates signalling cascades leading to the production of several mediators that contribute to inflammation (Huang et al., 2010). While inflammation is an important innate effector response against pathogens, it is a non-specific response that can result in damage to the surrounding tissues (Karrow, 2006).
To help control inflammation associated with maternal stressors, the maternal diets of humans may be supplemented with immune-modulating compounds such as omega-3 polyunsaturated fatty acids (n-3 PUFA); these may provide health benefits to the offspring and may help to control inappropriate tissue programming resulting from maternal infection (Bronson and Bale, 2014; Saccone et al., 2015). Omega-3 PUFA are known for their anti-inflammatory effects, and the long-chain PUFA docosahexanoic acid (DHA) and eicosapentanoic acid (EPA) are precursors to several anti-inflammatory metabolites including resolvins and maresins (Fritsche, 2015). Maternal dietary supplementation with DHA and EPA may result in healthier offspring that are less vulnerable to disease. While most sources of DHA and EPA are derived from fish products such as fish oil (FO), alternative sources including microalgae (AL) are also being investigated; AL may prove to be a more sustainable source of n-3 PUFA (Robertson et al., 2015).
Animal models have been extensively used to investigate causes and mechanisms of fetal programming that may not be easily investigated in humans (Harris and Seckl, 2011). The swine model may provide valuable insight compared to other animal models such as rodents or sheep, because pigs are more physiologically and genetically similar to humans (Dawson et al., 2013; Roura et al., 2016). Conducting such research with pigs may also directly benefit producers, as peri-weaning morbidity and mortality are concerns for the swine industry (Campbell et al., 2013), and it may be possible to enrich maternal diets with n-3 PUFA to produce healthier piglets that are less susceptible to peri-weaning disease. This may in turn allow for the use of less-expensive ingredients, such as low-quality plant-based protein sources in nursery diets without compromising piglet growth or health (Huber et al., 2018; Skinner et al., 2014).
Previously, ovine research has shown that the combination of maternal dietary n-3 PUFA supplementation and an LPS challenge can differentially modulate the offspring stress response, as characterized by fever and cortisol responses, when offspring are later exposed to an LPS challenge (Fisher-Heffernan et al., 2015; Fisher et al., 2014; You et al., 2019). In pigs, there appear to be differences in the stress response between male and female offspring, with male offspring being more sensitive than female offspring to maternal AL or FO dietary supplementation and/or maternal LPS exposure (You et al., 2019). In addition, more pronounced differences were observed among these male offspring from differing maternal treatments. To further elaborate on these findings, the aim of this study was to investigate the effects of maternal AL and FO supplementation in addition to maternal LPS challenge on the differential gene expression of the adrenal gland in male offspring that were fed a low-quality protein diet after weaning.
2. Methods
The animal trials were conducted at the Arkell Swine Research Station at the University of Guelph (Guelph, ON, Canada). The vitamin E and the Menhaden FO for this experiment were provided by Grand Valley Fortifiers (Cambridge, ON), and the AL (Aurantiochytrium limacinum biomass [AURA; CCAP 4087/2] containing 70% crude fat and 17% DHA) was provided by Alltech Inc. (Nicholasville, KY, USA). The experimental protocol (AUP # 3124) was approved by the University of Guelph Animal Care Committee and followed Canadian Council of Animal Care guidelines (CCAC, 2009).
2.1. Sow experimental methods
Experimental methods for the sow trial were conducted as per You et al. (2019). Briefly, forty-eight Landrace x Yorkshire cross-bred sows (12 sows per block x 4 blocks) were selected based on breeding date and parity (≥2) and were randomly assigned to one of three dietary treatments: diets supplemented with 3.1% FO, 3.12% AL, or a 1.89% corn oil (CO) control diet. Sows were fed 2.5 kg per day of the assigned diets from gestation day (gd) 75, as per Lee et al. (2019b) until farrowing (approx. gd 114), and were given unlimited access to fresh water. The experimental diets were formulated to meet the estimated nutrient requirements for sows in late gestation (National Research Council, 2012). AL and FO diets were formulated based on a preliminary trial to match total DHA content, maximizing fetal PUFA enrichment, and were formulated to have similar n-6 PUFA concentrations as the CO diet (Lee et al., 2019b). Ingredient and calculated nutrient composition of maternal gestation diets are shown in Table 1.
Table 1.
Formulations of sow experimental diets.
| Days on feed | 3.12% ALa |
3.1% FOb |
COc |
|---|---|---|---|
| 39 | 39 | 39 | |
| Ingredient Composition (%) | |||
| Corn (NRCd; 8.3% CPe) | 74.28 | 74.06 | 77.06 |
| Corn Oil | 1.55 | 1.79 | 1.89 |
| Fish Oilf | – | 3.10 | – |
| Microalgaeg | 3.12 | – | – |
| Soybean Meal | 17.38 | 17.38 | 17.38 |
| L-Lysine | 0.07 | 0.07 | 0.07 |
| Salt | 0.40 | 0.40 | 0.40 |
| Limestone | 1.52 | 1.52 | 1.52 |
| Mono-Cal Phosphate | 1.17 | 1.17 | 1.17 |
| Vitamin/Mineral Premixh | 0.50 | 0.50 | 0.50 |
| Vitamin Ef | 0.02 | 0.02 | 0.02 |
| Calculated Nutrient contenti | |||
| ME, kcal/kg | 3097 | 3153 | 3090 |
| CP (%) | 14.48 | 14.61 | 14.46 |
| SIDj Lysine (%) | 0.64 | 0.65 | 0.64 |
| SID Methionine + Cysteine (%) | 0.43 | 0.44 | 0.43 |
| SID Threonine (%) | 0.45 | 0.46 | 0.45 |
| SID Tryptophan (%) | 0.13 | 0.13 | 0.13 |
| Analyzed nutrient content | |||
| Dry matter (%) | 86.98 | 88.17 | 87.53 |
| CP (%) | 14.34 | 15.03 | 14.44 |
| Phosphorus (%) | 0.57 | 0.58 | 0.54 |
| Sodium (%) | 0.18 | 0.17 | 0.17 |
| Calcium (%) | 0.81 | 0.78 | 0.88 |
| Potassium (%) | 0.65 | 0.67 | 0.65 |
| Magnesium (%) | 0.14 | 0.14 | 0.13 |
| Eicosapentanoic acid (EPA; mg/g) | 0.19 | 3.49 | 0.08 |
| Docosahexanoic acid (DHA; mg/g) | 3.76 | 3.16 | 0.03 |
| Total omega-3 PUFAk (mg/g) | 5.43 | 10.21 | 1.70 |
| Ratio n-3:n-6l | 0.21 | 0.37 | 0.07 |
AL, microalgae.
FO, fish oil.
CO, 1.89% corn oil control.
NRC, National Research Council.
CP, crude protein.
Fish oil and Vitamin E provided by Grand Valley Fortifiers (Cambridge, ON, CA).
Algae provided by Alltech Inc. (Nicholasville, KY, USA) and supplied as dried biomass containing 15.8% CP, 70% CF and 17% DHA.
Supplied per kg of diet: vitamin A, 12,000 IU as retinyl acetate; vitamin D3, 1200 IU as cholecalciferol; vitamin E, 48 IU as DL-α-tocopherol acetate; vitamin K, 3 mg as menadione; vitamin B12, 0.03 mg; pantothenic acid, 18 mg; riboflavin, 6 mg; choline, 600 mg; folic acid, 2.4 mg; niacin, 30 mg; thiamine, 18 mg; pyridoxine, 1.8 mg; biotin, 200 μg; Cu, 18 mg as CuSO4·5H2O; Fe, 120 mg as FeSO4; Mn, 24 mg as MnSO4; Zn, 126 mg as ZnO; Se, 0.36 mg as FeSeO3; I, 0.6 mg as KI; DSM.
Calculated on the basis of the NRC (2012) ingredient values.
SID, standardized ileal digestible.
PUFA, polyunsaturated fatty acids.
Ratio n-3:n-6, ratio of omega-3 PUFA to omega-6 PUFA.
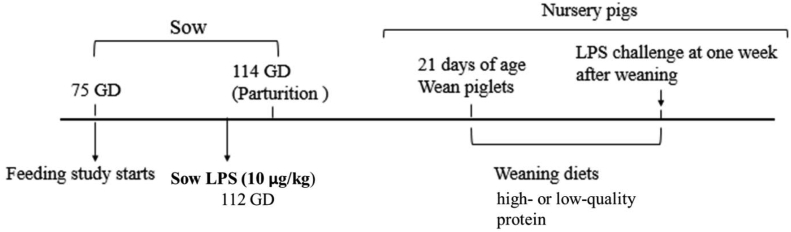
On gd 112, eight sows per dietary treatment were challenged i.m. with 10 μg/kg of LPS from E. coli O55:B5 (Sigma-Aldrich, Oakville, ON) dissolved in 2 mL of saline, as per Lee et al. (2019b). The remaining eight sows per dietary treatment received a 2 mL injection of saline as a control. Rectal temperature was recorded hourly up to 6 hr post LPS injection to monitor fever response, and general temperament was also observed. Sow experimental timeline is shown in Fig. 1.
Fig. 1.
Schematic timeline of events for animal trials. GD, gestation day; LPS, Lipopolysaccharide. Adapted from You et al. (2019).
2.2. Piglet experimental methods
Piglet experimental procedures were conducted as per You et al. (2019). Within 24 hr of birth, litters were standardized to a maximum of 12 piglets by cross-fostering within treatments and piglets were weaned at 21 days of age. At parturition, each litter was assigned an identification (ID) code, and each piglet was given a unique ID number corresponding to the litter ID code. At weaning, all of the piglets (n = 475) were randomly mixed and sorted into 8 pens (n = 6/pen), and were placed on a low-quality protein diet. Piglets were given unlimited access to feed and fresh water for the duration of the trial. Nursery diets were formulated to meet the estimated nutrient requirements for nursery phase 1 (National Research Council, 2012, Table 2). One week after weaning, 1 male offspring per litter (n = 48, n = 8/maternal treatment) was randomly selected and received a 40 μg/kg i.m. injection of LPS from E. coli O55:B5. After the LPS challenge (2 hr), the pigs were euthanized by administration of 0.3 mL/kg pentobarbital by cardiac puncture. Immediately following euthanasia, adrenal tissue was collected from the piglets and snap frozen in liquid nitrogen. Samples were then stored at −80 °C until RNA isolation and microarray analysis could be conducted. The piglet experimental timeline is shown in Fig. 1.
Table 2.
Formulation of offspring experimental diets.
| Diet | Low Quality |
|---|---|
| Days on feed | 7 |
| Ingredient Composition (%) | |
| Corn (NRCa; 8.3% CPb) | 45.28 |
| Wheat | 15.00 |
| Fat, Animal/vegetable blend | 5.00 |
| Soybean meal | 30.00 |
| L- Lysine | 0.37 |
| L- Methionine | 0.15 |
| L- Tryptophan | 0.02 |
| L- Threonine | 0.24 |
| Salt | 0.50 |
| Limestone | 1.30 |
| Monocalcium Phosphate | 1.52 |
| Mineral/Vitamin premixc | 0.60 |
| Vitamin Ed | 0.02 |
| Calculated nutrient contente | |
| ME, kcal/kg | 3471 |
| CP (%) | 20.85 |
| SIDf Lysine (%) | 1.21 |
| SID Methionine + Cysteine (%) | 0.71 |
| SID Threonine (%) | 0.86 |
| SID Tryptophan (%) | 0.24 |
| Analyzed nutrient content (%) | |
| Dry matter | 87.08 |
| CP | 21.18 |
| Phosphorus | 0.63 |
| Calcium | 0.87 |
| Sodium | 0.18 |
| Potassium | 0.89 |
| Magnesium | 0.17 |
NRC, National Research Council.
CP, crude protein.
Supplied per kg of diet: vitamin A, 12,000 IU as retinyl acetate; vitamin D3, 1200 IU as cholecalciferol; vitamin E, 48 IU as DL-α-tocopherol acetate; vitamin K, 3 mg as menadione; vitamin B12, 0.03 mg; pantothenic acid, 18 mg; riboflavin, 6 mg; choline, 600 mg; folic acid, 2.4 mg; niacin, 30 mg; thiamine, 18 mg; pyridoxine, 1.8 mg; biotin, 200 μg; Cu, 18 mg as CuSO4·5H2O; Fe, 120 mg as FeSO4; Mn, 24 mg as MnSO4; Zn, 126 mg as ZnO; Se, 0.36 mg as FeSeO3; I, 0.6 mg as KI; DSM.
Vitamin E provided by Grand Valley Fortifiers (Cambridge, ON, CA).
Calculated on the basis of the NRC (2012) ingredient values.
SID, standardized ileal digestible.
2.3. Microarray analysis
Following the LPS challenge, adrenal tissue was selected from 24 of the male offspring (n = 4/maternal gestation dietary treatment and maternal LPS status). The adrenal gland was selected for microarray analysis due to previously observed differences in the offspring cortisol response following LPS challenge (You et al., 2019). Total RNA was extracted from 20-30 mg of adrenal tissue using the Qiagen RNeasy extraction kit (Qiagen, Valencia, CA, USA) as per the manufacturer’s specifications. Following RNA extraction, the RNA pellet was re-suspended in 40 μL of nuclease-free water.
RNA quality and quantity were then assessed using a Nanodrop 1000 spectrophotometer (Thermo Scientific, NanoDrop Products, Wilmington, DE, USA). RNA integrity was assessed using an Agilent 2100 bioanalyzer (Agilent Technologies, Inc., Santa Clara, CA, USA), as per manufacturer’s protocol. Samples were prepared for microarray analysis using Applied Biosystems’ GeneChip WT plus reagent kit (Applied Biosystems, Foster City, CA, USA) as per manufacturer’s instructions.
Following sample preparation, samples were loaded onto Affymetrix Porcine Gene 1.0 ST GeneChips ™ for hybridization following the manufacturer’s protocol. GeneChips were then incubated in a hybridization oven for 16 hr at 65 °C and were spun at a speed of 60 rpm.
After 16 hr of incubation, hybridization cocktail was removed from the GeneChips, 160 μL of holding buffer was added, and the GeneChips were loaded into a fluidics station (Affymetrix GeneChip fluidics station 450) for washing and staining using the provided wash and staining solutions. GeneChips were then analyzed using the Affymetrix GeneChip Scanner 3000.
2.4. Real-time polymerase chain reaction (RT-PCR) analysis
Total RNA (1 μg) from the adrenal gland was reverse transcribed into cDNA using the High Capacity cDNA Reverse Transcription (RT) Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. Real-time PCR was performed in triplicate for the genes 11BHSD1, 11BHSD2, TLR-2, LY96 and PPARG using commercially available porcine TaqMan Assays (Applied Biosystems) and the 7500 fast RT-PCR System (Applied Biosystems). In a 96-well plate, 5 μL of 1:10 cDNA samples were loaded in triplicate for each gene, and were combined with 1 μL of primers, 10 μL of TaqMan™ Fast advanced master mix and 4 μL of nuclease-free water for a total reaction volume of 20 μL. Samples were held for 2 min at 50 °C and for 10 min at 95 °C followed by 40 amplification cycles at 95 °C for 15 s, and at 60 °C for 60 s. β-actin was selected as a house keeping gene to account for variation in the PCR. The relative quantification was expressed as a ratio of the target gene to the control gene using the delta-delta cycle threshold method as described in Zhang et al. (2018).
2.5. Data analysis
Raw data were obtained from the GeneChip scanner in the form of a.CEL file. These data were initially analyzed using transcriptome analysis console 4.0 (TAC 4.0; Applied Biosystems). Data were normalized to a control treatment and were set to detect fold-differences of <-1.2 or >1.2; a P-value of <0.05 was considered significant. The fold-difference cut-offs of <-1.2 and >1.2 were selected to capture genes where a small increase or decrease in expression may have a pronounced physiologic effect. The list of Gene IDs, gene symbols and fold-differences were obtained for differentially expressed genes with a significant P-value, and data underwent secondary analysis using Qiagen Ingenuity Pathway Analysis software (IPA; Ingenuity Systems Inc., Redwood City, CA, USA) to determine the canonical pathways, biological functions and proteins involved. Project datasets were created using the gene IDs, fold-change and P-values obtained from TAC 4.0 analysis, and were then analyzed using the IPA core expression analysis. Pathways, functions and proteins with a P-value of <0.05 and an activation z-score of <-2.0 or >2.0 were considered significant.
PCR results were analyzed using PROC GLIMMIX of SAS version 9.4. A multiple means comparison with a Tukey adjustment was conducted, similar to Karrow et al. (2017), and the statistical model included the fixed effects of maternal gestation dietary treatment, maternal LPS status, and their interaction, and included the random effect of block. Significant differences are reported where P < 0.05 and statistical trends are reported where 0.05≤ P ≤ 0.1.
3. Results
Due to RNA quality following isolation and quantification, only 19 out of 24 samples could be used for the transcriptome analysis (n = 4 for FO + CON, n = 3 for FO + LPS, AL + CON, AL + LPS, CO + CON and CO + LPS). The raw data from the microarray analysis are available in the NCBI Gene Expression Omnibus (accession number GSE135782).
3.1. Microarray analysis of differentially expressed adrenal genes
Of the 25,470 genes on the GeneChip Porcine Gene 1.0 ST array, a range of 449 to 2335 genes were differentially expressed, depending on the treatment selected for data normalization (Table 3). The factorial design of the maternal treatments allowed for the investigation of the effects of maternal LPS challenge within a maternal dietary treatment, as well as the effects of a maternal dietary treatment within an LPS treatment on the male offspring.
Table 3.
Overview of differentially expressed male offspring adrenal genes in pigs that were fed a low-quality protein diet and were from sows supplemented with AL, FO or CO then challenged with LPS or saline (n = 4 for FO + CON, n = 3 for all other treatments).
| Treatment Comparison | Number of Genes |
% of total genes | ||
|---|---|---|---|---|
| Up-regulated | Down-regulated | Total | ||
| ALa+LPSb vs AL + CONc | 1211 | 1114 | 2325 | 9.13 |
| FOd+LPS vs FO + CON | 352 | 521 | 873 | 3.43 |
| COe+LPS vs CO + CON | 283 | 183 | 466 | 1.83 |
| AL + LPS vs CO + LPS | 492 | 383 | 875 | 3.44 |
| AL + CON vs CO + CON | 266 | 319 | 585 | 2.30 |
| FO + LPS vs CO + LPS | 393 | 376 | 769 | 3.02 |
| FO + CON vs CO + CON | 241 | 208 | 449 | 1.76 |
| AL + LPS vs FO + LPS | 729 | 737 | 1466 | 5.76 |
| AL + CON vs FO + CON | 207 | 315 | 522 | 2.05 |
AL, microalgae.
LPS, lipopolysaccharide.
CON, saline control.
FO, fish oil.
CO, corn oil.
3.2. Lipid metabolism
When investigating the effect of maternal diet and LPS treatments on the male offspring, lipid metabolism was one of the top enriched pathways across treatments (Table 4). A total of 13 genes (P < 0.05) were enriched in pigs from the maternal AL + LPS treatment compared to CO + LPS, and 58 genes (P < 0.05) were enriched in pigs from the maternal AL + CON treatment compared to FO + CON. Genes that were found to be differentially expressed included fatty acid elongase (ELOVL7), PPAR-γ, acyl-CoA dehydrogenases, acyl-CoA synthetases, phospholipase A2, arachidonate lipoxygenase, prostaglandin synthases and prostaglandin reductase, as outlined in Table 4.
Table 4.
Differentially expressed male offspring adrenal genes pertaining to lipid metabolism in pigs that were fed a low-quality protein diet and were from sows supplemented with AL, FO or CO then challenged with LPS or saline (n = 4 for FO + CON, n = 3 for all other treatments).
| Treatment Comparison | Gene | Description | Fold change | P-value |
|---|---|---|---|---|
| ALa+LPSb vs AL + CONc | ACADL | Acyl-CoA Dehydrogenase Long-chain | −1.74 | 0.005 |
| ECHDC1 | Ethylmalonyl-CoA decarboxylase 1 | −1.63 | 0.022 | |
| ACSL3 | Acyl-CoA synthetase long-chain family 3 | −1.62 | 0.015 | |
| ACADM | Acyl-CoA Dehydrogenase medium-chain | −1.51 | 0.047 | |
| PLA2G16 | Phospholipase A2 group 16 | −1.47 | 0.004 | |
| ECHS1 | Enoyl CoA hydratase short chain 1 | 1.48 | 0.046 | |
| FOd+LPS vs. FO + CON | ACSL5 | Acyl-CoA synthetase long-chain family 5 | −1.22 | 0.020 |
| PTGIS | Prostaglandin I synthase | 1.30 | 0.036 | |
| COe+LPS vs. CO + CON | LIPE | Lipase | 1.22 | 0.031 |
| ELOVL7 | ELOVL fatty acid elongase 7 | 1.28 | 0.040 | |
| FFAR2 | Free-fatty acid receptor 2 | 1.34 | 0.040 | |
| AL + LPS vs CO + LPS | PPARG | Peroxisome proliferator-activated receptor gamma | −1.54 | 0.015 |
| ACACA | Acetyl-CoA carboxylase alpha | 1.30 | 0.039 | |
| AL + CON vs. CO + CON | ALOXE3 | Arachidonate Lipoxygenase 3 | −1.42 | 0.007 |
| FO + LPS vs. CO + LPS | PLA2G16 | Phospholipase A2 group 16 | 1.22 | 0.024 |
| PLA2G12A | Phospholipase A2 group 12A | 1.51 | 0.006 | |
| PTGR2 | Prostaglandin reductase 2 | 1.53 | 0.046 | |
| PTGES2 | Prostaglandin E synthase 2 | 1.76 | 0.004 | |
| FO + CON vs. CO + CON | ALOXE3 | Arachidonate Lipoxygenase 3 | −1.27 | 0.042 |
| LPCAT2 | Lysophosphatidylcholine acyltransferase 2 | 1.38 | 0.016 | |
| AL + LPS vs FO + LPS | ACADL | Acyl-CoA Dehydrogenase Long-chain | −1.53 | 0.012 |
| ACADSB | Acyl-CoA Dehydrogenase Short/Branched chain | −1.40 | 0.049 | |
| PLA2G16 | Phospholipase A2 group 16 | −1.39 | 0.013 | |
| PPARG | Peroxisome proliferator-activated receptor gamma | −1.39 | 0.046 | |
| AL + CON vs. FO + CON | ECHCD3 | Enoyl CoA hydratase domain containing 3 | −1.64 | 0.014 |
| PLA2G7 | Phospholipase A2 group 7 | 1.59 | 0.033 |
AL, microalgae.
LPS, lipopolysaccharide.
CON, saline control.
FO, fish oil.
CO, corn oil.
3.3. Steroidogenesis
The expression of adrenal genes involved in steroidogenesis were also enriched when investigating the effect of maternal diet and LPS treatments (Table 5). These genes included the cytochrome P450 enzymes CYP1A1, CYP1A2, CYP4F2 and CYP39A1, and hydroxysteroid dehydrogenases HSD17B8, HSD11B1, HSD11B2, HSDL1 and HSDL2 (Table 5).
Table 5.
Differentially expressed male offspring adrenal genes pertaining to steroidogenesis in pigs that were fed a low-quality protein diet and were from sows supplemented with AL, FO or CO then challenged with LPS or saline (n = 4 for FO + CON, n = 3 for all other treatments).
| Treatment Comparison | Gene | Description | Fold change | P-value |
|---|---|---|---|---|
| ALa+LPSb vs AL + CONc | AIG1 | Androgen-induced 1 | −1.82 | 0.019 |
| HSDL2 | Hydroxysteroid Dehydrogenase like 2 | −1.77 | 0.011 | |
| HSD11B1 | 11β Hydroxysteroid dehydrogenase type 1 | −1.67 | 0.030 | |
| AVPR1 | Arginine vasopressin receptor 1 | −1.62 | 0.015 | |
| HSDL1 | Hydroxysteroid Dehydrogenase like 1 | 1.24 | 0.039 | |
| FOd+LPS vs FO + CON | MRAP2 | Melanocortin 2 receptor accessory protein 2 | −1.58 | 0.025 |
| CYP39A1 | Cytochrome P450 39A1 | −1.44 | 0.017 | |
| CYP1a2 | Cytochrome P450 1A2 | 1.21 | 0.039 | |
| CYP4F2 | Cytochrome P450 4F2 | 1.42 | 0.040 | |
| CRHBP | Corticotropic releasing hormone binding protein | 1.44 | 0.047 | |
| AL + LPS vs COe+LPS | CYP2J2L | Cytochrome P450 2J2 like | 1.52 | 0.035 |
| AL + CON vs CO + CON | ADRA2A | Adrenoreceptor alpha 2A | −1.27 | 0.025 |
| ADRBK2 | β-Adrenergic receptor kinase 2 | 1.40 | 0.008 | |
| GLCCI1 | Glucocorticoid induced 1 | 1.45 | 0.003 | |
| FO + LPS vs CO + LPS | CYP2J2L | Cytochrome P450 2J2 like | 1.42 | 0.022 |
| FO + CON vs CO + CON | ADRBK2 | β-Adrenergic receptor kinase 2 | 1.21 | 0.023 |
| HSD11B2 | 11β Hydroxysteroid dehydrogenase type 2 | 1.71 | 0.023 | |
| AL + LPS vs FO + LPS | HSD11B2 | 11β Hydroxysteroid dehydrogenase type 2 | −1.57 | 0.019 |
| HSDL2 | Hydroxysteroid dehydrogenase like 2 | −1.49 | 0.038 | |
| HSDL1 | Hydroxysteroid dehydrogenase like 1 | 1.22 | 0.041 | |
| CYP1A1 | Cytochrome P450 1A1 | 1.31 | 0.020 | |
| AL + CON vs FO + CON | HSD17B8 | 17β Hydroxysteroid Dehydrogenase type 8 | −1.31 | 0.033 |
AL, microalgae.
LPS, lipopolysaccharide.
CON, saline control.
FO, fish oil.
CO, corn oil.
3.4. Immune function
When investigating the effect of maternal diet and LPS treatments on the male offspring, immune response was also one of the top enriched pathways across treatments (Table 6). The expression of several immune-related genes was higher in pigs from dams fed diets supplemented with AL and FO compared to those fed CO, and in pigs from sows that were challenged with LPS compared to those that received saline as a control. Commonly enriched pathways included cell-mediated immune response, antibody-mediated immune response, hypersensitivity response, free-radical scavenging, inflammatory response and immune cell trafficking. Commonly enriched molecules included LY96 (MD-2), TLR-2, MYD88, NF-κB, IL-1R and IL-6R (Table 6).
Table 6.
Differentially expressed male offspring adrenal genes pertaining to immune function in pigs that were fed a low-quality protein diet and were from sows supplemented with AL, FO or CO then challenged with LPS or saline (n = 4 for FO + CON, n = 3 for all other treatments).
| Treatment Comparison | Gene | Description | Fold change | P-value |
|---|---|---|---|---|
| ALa+LPSb vs AL + CONc | CD180 | CD180 | −2.55 | 0.029 |
| IFNGR1 | Interferon gamma receptor 1 | −2.26 | 0.023 | |
| SLA-DRA | MHC II SLA-DRA | −1.78 | 0.010 | |
| LY96 | Lymphocyte antigen 96 | −1.62 | 0.035 | |
| CD83 | CD83 | −1.61 | 0.034 | |
| MYD88 | MyD-88 | −1.57 | 0.010 | |
| SLA-DQA1 | MHC II SLA-DQA1 | −1.55 | 0.034 | |
| IL10 | Interleukin-10 | −1.46 | 0.018 | |
| IL1RL1 | Interleukin-1 receptor like 1 | 1.28 | 0.022 | |
| NFKB2 | Nuclear factor kappa B2 | 1.47 | 0.001 | |
| IL17B | Interleukin 17b | 1.76 | 0.007 | |
| SLA-DRB1 | MHC II SLA-DRB1 | 1.85 | 0.021 | |
| FOd+LPS vs FO + CON | LY96 | Lymphocyte antigen 96 | −1.40 | 0.045 |
| HP | Haptoglobin | 1.81 | 0.012 | |
| COe+LPS vs CO + CON | SLA-DRB1 | MHC II SLA-DRB1 | −1.66 | 0.010 |
| LY96 | Lymphocyte antigen 96 | 1.55 | 0.036 | |
| AL + LPS vs CO + LPS | LY96 | Lymphocyte antigen 96 | −1.66 | 0.029 |
| SLA-DQA1 | MHC II SLA-DQA1 | −1.50 | 0.044 | |
| CD34 | CD34 | −1.42 | 0.004 | |
| NFKB2 | Nuclear factor kappa B2 | 1.26 | 0.036 | |
| SLA-DRB1 | MHC II SLA-DRB1 | 1.77 | 0.022 | |
| HP | Haptoglobin | 2.02 | 0.040 | |
| SLA-DOB | MHC II SLA-DOB | 2.60 | 0.010 | |
| AL + CON vs CO + CON | SLA-DRB1 | MHC II SLA-DRB1 | −1.74 | 0.009 |
| SLA-DOA | MHC II SLA-DOA | 1.34 | 0.035 | |
| IL2RG | Interleukin-2 receptor gamma | 1.43 | 0.017 | |
| TLR2 | Toll-like receptor 2 | 1.50 | 0.004 | |
| LY96 | Lymphocyte antigen 96 | 1.51 | 0.044 | |
| FO + LPS vs CO + LPS | TLR2 | Toll-like receptor 2 | −1.33 | 0.045 |
| IL1RL1 | Interleukin-1 receptor like 1 | −1.29 | 0.004 | |
| SLA-DRB1 | MHC II SLA-DRB1 | 1.47 | 0.040 | |
| FO + CON vs CO + CON | SLA-DRB1 | MHC II SLA-DRB1 | −2.07 | 0.006 |
| NOD1 | Nucleotide-binding oligomerization domain-containing protein 1 | −1.36 | 0.037 | |
| TLR9 | Toll-like receptor 9 | 1.25 | 0.037 | |
| LY96 | Lymphocyte antigen 96 | 1.47 | 0.029 | |
| SLA-DQA1 | MHC II SLA-DQA1 | −1.84 | 0.005 | |
| CD83 | CD83 | −1.75 | 0.009 | |
| SLA-DRA | MHC II SLA-DRA | −1.58 | 0.024 | |
| CD34 | CD34 | −1.42 | 0.001 | |
| IL12B | Interleukin 12b | 1.29 | 0.036 | |
| IL1RL1 | Interleukin 1 receptor like1 | 1.34 | 0.004 | |
| IL23R | Interleukin 23 receptor | 1.49 | 0.014 | |
| IL17B | Interleukin 17b | 1.56 | 0.017 | |
| AL + LPS vs FO + LPS | TLR2 | Toll-like receptor 2 | 1.39 | 0.015 |
AL, microalgae.
LPS, lipopolysaccharide.
CON, saline control.
FO, fish oil.
CO, corn oil.
3.5. Real-time PCR validation of several differentially expressed adrenal genes
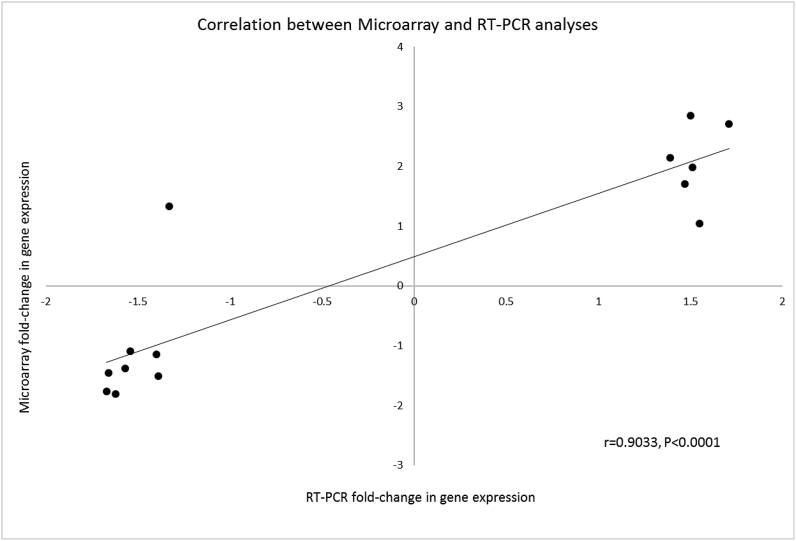
The target genes HSD11B1, HSD11B2, LY96, TLR2 and PPARG showing significant fold-change in expression among treatments following microarray analysis were further validated using RT-PCR. The fold-differences in expression were similar for each of these genes between treatments with the exception of TLR-2 for the comparison of FO + LPS vs. CO + LPS; the correlation between microarray and RT-PCR analyses was 0.90 (Fig. 2).
Fig. 2.
Correlation between fold-change in gene expression from microarray and real-time polymerase chain reaction (RT-PCR) in the adrenal gland of male offspring fed a low-quality protein diet from dams fed diets supplemented with microalgae, fish oil or a control diet and were challenged with lipopolysaccharide from Escherichia coli, or saline as a control in late gestation.
4. Discussion
This study is the first to investigate the effects of maternal AL and FO supplementation in combination with maternal LPS challenge in late gestation on adrenal mRNA expression in swine. The animals in this study were previously used to the assess acute-phase response to an LPS challenge (You et al., 2019). The results from this study showed differential effects of maternal gestation dietary treatment and maternal LPS status, leading to the hypothesis that the maternal treatments would also influence the adrenal transcriptome of the offspring. Previous differences in the acute-phase response were also noted between male and female offspring, with maternal treatment more profoundly influencing male offspring compared to female offspring (You et al., 2019). Therefore, it was expected that differences in gene expression in adrenal tissue would be more pronounced within male offspring from differing maternal treatments, and hence, males were selected for the present study.
4.1. Lipid metabolism
Several genes that are involved in lipid metabolism were found to be differentially expressed. This includes the expression of phospholipase A2 (PLA2) group 16. The expression of PLA2 was higher in offspring from FO + LPS sows compared to offspring from AL + LPS and CO + LPS sows. PLA2 is responsible for hydrolysis of fatty acids from membrane phospholipids, indicating increased lipid metabolism and lipolysis (Liu et al., 2018). Due to the increased concentrations of EPA and DHA in the offspring from FO sows at the time of sampling (You et al., 2019), there would likely be increased EPA and DHA available for metabolism by lipoxygenase and cyclooxygenase enzymes, resulting in increased production of anti-inflammatory metabolites (Duncan et al., 2008; Fritsche, 2015). It is interesting to note that the increased expression of PLA2 group 16 occurred in offspring from sows challenged with LPS; this may suggest a role of maternal stress in preferentially using lipid stores as a source of energy.
The expression of PPAR-γ was found to be lower in offspring from the AL + LPS sows compared to those from both the FO + LPS and CO + LPS sows. PPAR-γ is involved in lipid metabolism in both adipose tissue and in immune cells such as dendritic cells and macrophages (Ahmadian et al., 2013). PPAR-γ is also involved in the immune response; inhibiting the production of pro-inflammatory cytokines, by limiting the transcription of inflammatory genes, and promoting the differentiation of anti-inflammatory immune cell phenotypes (Ahmadian et al., 2013; Martin, 2010). The decreased expression of PPAR-γ in offspring from maternal AL + LPS sows may therefore indicate reduced lipid metabolism in offspring, and therefore they may be more susceptible to inflammation, as potentially indicated by the increased cortisol response in these pigs following LPS challenge (You et al., 2019).
4.2. Steroidogenesis
LPS administration can directly stimulate the production of the steroid hormones cortisol and aldosterone in the adrenal glands (Huang et al., 2010; Vakharia and Hinson, 2005). Based on findings from previous studies and the differences observed in the cortisol and fever responses in these same offspring following LPS challenge (You et al., 2019), it was anticipated that genes involved in steroidogenesis would be differentially expressed. The enzymes 11-β-hydroxysteroid dehydrogenase type 1 and 2 (11BHSD1/2) are responsible for activating and inactivating cortisol, respectively (Alikhani-Koopaei et al., 2004). The expression of the 11BHSD enzymes can be affected by maternal stress; stress has been associated with reduced placental expression of 11BHSD2 (Marques et al., 2015), allowing more cortisol to cross the placenta and enter fetal circulation. Expression of the 11BHSD enzymes within the adrenal cortex has also been characterized in rats and in humans (Mazzocchi et al., 1998; Shimojo et al., 1996); however, the effects of stress on adrenal 11BHSD expression are not well characterized (Zallocchi et al., 2004). In the present study, expression of 11BHSD2 was higher in offspring from FO sows compared to offspring from AL and CO sows, suggesting that maternal FO supplementation may reduce the magnitude of cortisol response from the adrenal gland in offspring following a stress challenge. However, results from the cortisol analysis do not agree with this observation (You et al., 2019), which may be due to the time of tissue sampling; expression of 11BHSD2 may be upregulated in offspring from sows fed the FO diet in attempt to attenuate the cortisol response following LPS challenge, possibly resulting in a quicker resolution of the stress response.
The primary cytochrome P450 enzymes involved in steroidogenesis in the adrenal gland were not found to be differentially expressed amongst treatments. Similarly, a previous study by Hazard et al. (2008) found that the expression of genes involved in adrenal steroidogenesis were not differentially expressed following acute ACTH challenge, but may be differentially expressed when pigs are subjected to a chronic ACTH challenge. Therefore, the lack of significant findings in this area may be attributed to the acute nature of the LPS challenge in this study.
4.3. Immune function
The enrichment of several immune-related pathways was observed within the adrenal gland. While the major function of the adrenal gland is the production of hormones and the release of catecholamines, there is also evidence that the adrenal gland is involved in the immune response. The adrenal gland can be directly activated by LPS through TLR-4 signalling (Mohn et al., 2011; Vakharia and Hinson, 2005). This results in the production of cortisol, aldosterone and other mediators including cytokines such as IL-1, nitric oxide and prostaglandins (Martinez Calejman et al., 2011; Mohn et al., 2011; Vakharia and Hinson, 2005). Collectively, these findings indicate that the adrenal gland has a role in the immune and stress responses to LPS, and these can occur in an ACTH-independent manner.
In the present study, the expression of lymphocyte antigen 96 (LY96), also known as MD-2, was differentially expressed in most treatments; MD-2 is a co-receptor required for recognition of LPS by TLR-4 on endothelial and immune cells (Gnauck et al., 2016). The expression of MD-2 was higher in pigs from sows supplemented with AL and FO receiving saline compared to pigs from sows fed the CO control diets and was higher in pigs from AL + CON and FO + CON sows compared to those from the same diets that received LPS. This suggests that the combination of maternal AL or FO supplementation and LPS challenge may decrease offspring sensitivity to future stressors, including LPS, although this appears to contradict the fever and cortisol responses observed in these offspring following LPS challenge (You et al., 2019). While MD-2 expression is essential for the LPS signalling cascade, there are many other molecules involved; the increased expression of MD-2 alone may not be sufficient to alter the offspring sensitivity to an LPS challenge, as may be reflected in the fever and cortisol responses to LPS. The differential expression of MD-2 also suggests that the male offspring from the maternal FO and AL dietary treatments may be more sensitive to future microbial stress compared to offspring from the maternal CO dietary treatment, and this finding is reflected in the fever response of offspring following LPS challenge (You et al., 2019) as well as cytokine concentrations in pigs fed AL following LPS challenge (Lee et al., 2019a).
TLR-2 expression was also found to be differentially expressed within offspring from differing maternal treatments, with increased expression in pigs from sows fed AL compared to pigs from sows fed FO and CO, all of which received saline injections. TLR-2 is typically involved in the recognition of MAMPs from Gram-positive bacteria, heterodimerizing with either TLR-1 or TLR-6 (Conti et al., 2014; Peddireddy et al., 2017), whereas MAMPs from Gram-negative bacteria are typically recognized by TLR-4. Certain studies have shown a role of TLR-2 in the immune response to LPS, indicating that TLR-2 activation may be required for TLR-4 signalling induced by LPS in certain tissues (Conti et al., 2014; Good et al., 2012; Huang et al., 2010). Studies have also reported that TLR-2 knockout mice have a decreased magnitude of immune response following LPS challenge (Good et al., 2012). Therefore, the increased expression of TLR-2 in offspring from AL + CON sows may further contribute to increased sensitivity of these offspring to future microbial stressors resulting in the activation of both the TLR-2 and TLR-4 pathways. TLR-2 and TLR-4 signalling pathways can also be activated by alarmins; endogenous molecules that indicate tissue or cellular injury (Yang et al., 2017). Differential programming of the TLR-2 and TLR-4 pathways may therefore differentially affect alarmin signalling; resulting in offspring being increasingly susceptible to inflammation and potentially increasing the risk of inflammatory disease (Roh and Sohn, 2018).
The expression of the transcription factor NF-κB was also differentially modulated by maternal treatments; NF-κB expression was increased in pigs from the AL sows that were challenged with LPS compared to their saline controls and compared to sows from the CO control diet that were challenged with LPS. NF-κB is released at the end of the LPS signalling cascade (Martinez Calejman et al., 2011), whereby phosphorylation of IκB results in its’ dissociation from NF-κB, allowing NF-κB to translocate into the nucleus, bind to response elements on DNA and induce the transcription of several pro-inflammatory cytokines (Gnauck et al., 2016). Studies have shown that NF-κB expression increases within the adrenal gland in rats following LPS stimulation (Medicherla et al., 2002), and that n-3 PUFA can reduce NF-κB expression in macrophages with or without exposure to LPS (Allam-Ndoul et al., 2016; Novak et al., 2003). Contrary to previous research, in the present study, the higher expression of NF-κB resulting from the combination of maternal AL supplementation and maternal LPS challenge may predispose offspring to inflammation in later life and increase sensitivity to future stressors. This was also reflected in the offspring fever response following LPS challenge (You et al., 2019), as well as cytokine concentrations following LPS challenge in pigs fed diets supplemented with AL (Lee et al., 2019a). This may be attributed to the nature of the algae supplement used, as algae species are known to possess bioactive compounds including carotenoids and beta-glucans, as well as antinutritive factors (Yaakob et al., 2014), that may influence the effects of the n-3 PUFA on the offspring.
The results from the microarray analysis give insight into how these molecular mechanisms may contribute to differences in phenotype and what those differences might mean to swine producers. While increasing the immune response may help protect against invading microbes, increased immune response also increases energy requirements for immune function (Rauw, 2012); in growing animals this might mean a trade-off between growth and immune function (Van Der Most et al., 2011). An increased cortisol response following exposure to stressors may provide short-term advantages, but chronic or excessive stress responses can dampen the immune response and can leave offspring vulnerable to disease (Dhabhar, 2014). Changes in the expression of genes involved in the immune and cortisol responses may indicate that offspring from sows fed AL during gestation may have an increased immune response to stressors after weaning. This was highlighted by the increased cortisol response following LPS challenge in offspring from the AL + LPS sows compared to those from the CO + LPS sows (You et al., 2019). Growth performance in these offspring was not affected in the nursery phase (Lee et al., 2019b); however, there may be negative effects of a heightened immune response on overall growth in the growing or finishing phases in these pigs. This warrants further investigation to validate the use of AL and FO in maternal gestation diets.
One potential limitation of this study is that microarray analysis may underestimate the fold-change in mRNA transcripts (Hazard et al., 2008), and therefore it is possible that important immune-related or steroidogenic genes were not found to be differentially expressed when in fact they were. Additionally, mRNA was extracted from the whole adrenal gland, and it is therefore possible that expression of certain genes were increased within the adrenal cortex and decreased within the adrenal medulla, or vice versa, resulting in no overall changes in expression as suggested by Murani et al. (2011).
It is also of note that microarray analysis was only conducted on adrenal tissue of male offspring; differences between male and female offspring stress and immune responses have been characterized in response to maternal stress (Fisher-Heffernan et al., 2015; Fisher et al., 2014). Therefore, microarray analysis of adrenal tissue from female offspring may reveal different effects of either maternal dietary supplementation, or maternal LPS status than were presently observed. Finally, it is important to consider that the tissues analyzed were from pigs fed a low-quality protein diet. While the pigs in question were only fed the low-quality diet for 1 week, it was previously observed that the offspring nursery diet quality affected the concentrations of the cytokines IL-6, IL-10 and IL-1ra following offspring LPS immune challenge (Lee et al., 2019b). Offspring fed the low-quality protein diet may therefore be more vulnerable to inflammation and disease than those fed a high-quality protein diet. It is therefore possible that the results of the microarray analysis were also influenced by the low-quality protein diet, and any anti-inflammatory effects of maternal AL or FO supplementation may be more pronounced in offspring fed a high-quality protein diet. It is also worth noting that only a portion of the swine genome is annotated (Liu et al., 2017); thus, it is possible as further elucidation of gene ontology occurs, further insight into the results obtained from microarray analysis of the adrenal gland will provide a more comprehensive view of the mechanisms at play in these offspring.
5. Conclusions
Gene expression within the adrenal gland of male offspring fed a low-quality protein diet was influenced by both maternal diet (FO and AL) and maternal LPS status. Adrenal steroidogenesis, lipid metabolism and immune function pathways were significantly influenced. Increased expression of immune-related genes including lymphocyte antigen 96, TLR-2 and NF-κB suggests that maternal AL supplementation may increase offspring sensitivity to inflammation after weaning. Decreased expression of lymphocyte antigen 96 in male offspring from sows receiving maternal endotoxin challenge also suggests a possible role of maternal stress in diminishing the offspring immune response to immune and/or stress challenges. Increased expression of the genes encoding the 11BHSD2 enzyme in offspring from sows fed FO may also reduce the magnitude of the stress response. These data provide insight into immune and metabolic mechanisms that may be influenced by maternal diet and maternal stress. Future analysis of gene expression in the adrenal gland of female offspring and offspring fed a high-quality protein diet, or gene expression of other endocrine tissues may provide more insight into the effects of maternal AL and FO supplementation and maternal LPS challenge at the genome level.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgements
The authors would like to thank the staff at the Arkell research station as well as Douglas Wey at the University of Guelph for their assistance throughout this trial. The authors would like to thank the students and volunteers who assisted throughout this trial. The authors would like to thank Alltech Inc. for providing the AL and for the use of their facilities for the microarray and PCR analyses. The authors would like to thank Grand Valley Fortifiers supplying the FO. Financial support for this project was provided by Alltech Inc. (#052363), the Natural Sciences and Engineering Research Council of Canada (#401018) and the Ontario Ministry of Agriculture, Food and Rural Affairs.
References
- Ahmadian M., Suh J.M., Hah N., Liddle C., Atkins A.R., Downes M., Evans R.M. PPARγ signaling and metabolism: the good, the bad and the future. Nat. Med. 2013;19:557–566. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alikhani-Koopaei R., Fouladkou F., Frey F.J., Frey B.M. Epigenetic regulation of 11 beta-hydroxysteroid dehydrogenase type 2 expression. J. Clin. Invest. 2004;114:1146–1157. doi: 10.1172/JCI21647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allam-Ndoul B., Guénard F., Barbier O., Vohl M.-C. Effect of n-3 fatty acids on the expression of inflammatory genes in THP-1 macrophages. Lipids Health Dis. 2016;15:69. doi: 10.1186/s12944-016-0241-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson S.L., Bale T.L. Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology. 2014;155:2635–2646. doi: 10.1210/en.2014-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J.M., Crenshaw J.D., Polo J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013;4:19. doi: 10.1186/2049-1891-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F., Boucherit N., Baldassarre V., Trouplin V., Toman R., Mottola G., Mege J.-L., Ghigo E. Coxiella burnetii lipopolysaccharide blocks p38α-MAPK activation through the disruption of TLR-2 and TLR-4 association. Front. Cell. Infect. Microbiol. 2014;4:182. doi: 10.3389/fcimb.2014.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson H.D., Loveland J.E., Pascal G., Gilbert J.G.R., Uenishi H., Mann K.M., Sang Y., Zhang J., Carvalho-Silva D., Hunt T., Hardy M., Hu Z., Zhao S.H., Anselmo A., Shinkai H., Chen C., Badaoui B., Berman D., Amid C., Kay M., Lloyd D., Snow C., Morozumi T., Cheng R.P.Y., Bystrom M., Kapetanovic R., Schwartz J.C., Kataria R., Astley M., Fritz E., Steward C., Thomas M., Wilming L., Toki D., Archibald A.L., Bed’Hom B., Beraldi D., Huang T.H., Ait-Ali T., Blecha F., Botti S., Freeman T.C., Giuffra E., Hume D.A., Lunney J.K., Murtaugh M.P., Reecy J.M., Harrow J.L., Rogel-Gaillard C., Tuggle C.K. Structural and functional annotation of the porcine immunome. BMC Genom. 2013;14:1–16. doi: 10.1186/1471-2164-14-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar F.S. Effects of stress on immune function: the good, the bad, and the beautiful. Immunol. Res. 2014;58:193–210. doi: 10.1007/s12026-014-8517-0. [DOI] [PubMed] [Google Scholar]
- Duncan R.E., Sarkadi-Nagy E., Jaworski K., Ahmadian M., Sul H.S. Identification and functional characterization of adipose-specific phospholipase A 2 (AdPLA) J. Biol. Chem. 2008;283:25428–25436. doi: 10.1074/jbc.M804146200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher-Heffernan R.E., Or’Rashid M.M., AlZahal O., Quinton M., Boermans H.J., McBride B.W., Regnault T.R.H., Karrow N.A. Fishmeal supplementation during ovine pregnancy and lactation protects against maternal stress-induced programming of the offspring immune system. BMC Vet. Res. 2015;11:266. doi: 10.1186/s12917-015-0573-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R.E., Or’Rashid M., Quinton M., AlZahal O., Boermans H.J., McBride B.W., Karrow N.A. Maternal supplementation with fishmeal protects against late gestation endotoxin-induced fetal programming of the ovine hypothalamic-pituitary-adrenal axis. J. Dev. Orig. Health Dis. 2014;5:206–213. doi: 10.1017/S2040174414000191. [DOI] [PubMed] [Google Scholar]
- Fritsche K.L. The science of fatty acids and inflammation. Adv. Nutr. 2015;6:293S–301S. doi: 10.3945/an.114.006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnauck A., Lentle R.G., Kruger M.C. The characteristics and function of bacterial lipopolysaccharides and their endotoxic potential in humans. Int. Rev. Immunol. 2016;35:189–218. doi: 10.3109/08830185.2015.1087518. [DOI] [PubMed] [Google Scholar]
- Good D.W., George T., Watts B.A., III Toll-like receptor 2 is required for LPS-induced Toll-like receptor 4 signaling and inhibition of ion transport in renal thick ascending limb. J. Biol. Chem. 2012;287:20208–20220. doi: 10.1074/jbc.M111.336255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur T.L., Shay L., Palkar A.V., Fisher S., Varaljay V.A., Dowd S., Bailey M.T. Prenatal stress affects placental cytokines and neurotrophins, commensal microbes, and anxiety-like behavior in adult female offspring. Brain Behav. Immun. 2017;64:50–58. doi: 10.1016/j.bbi.2016.12.021. [DOI] [PubMed] [Google Scholar]
- Harris A., Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm. Behav. 2011;59:279–289. doi: 10.1016/J.YHBEH.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Hazard D., Liaubet L., Sancristobal M., Mormède P. Gene array and real time PCR analysis of the adrenal sensitivity to adrenocorticotropic hormone in pig. BMC Genom. 2008;9:101. doi: 10.1186/1471-2164-9-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.-L., Chiang M.-F., Lin C.-W., Pu H.-F. Lipopolysaccharide directly stimulates aldosterone production via toll-like receptor 2 and toll-like receptor 4 related PI3K/Akt pathway in rat adrenal zona glomerulosa cells. J. Cell. Biochem. 2010;111:872–880. doi: 10.1002/jcb.22774. [DOI] [PubMed] [Google Scholar]
- Huber L.-A., Hooda S., Fisher-Heffernan R.E., Karrow N.A., De Lange C.F.M. Effect of reducing the ratio of omega-6-to-omega-3 fatty acids in diets of low protein quality on nursery pig growth performance and immune response. J. Anim. Sci. 2018;96:4348–4359. doi: 10.1093/jas/sky296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrow N.A. Activation of the hypothalamic–pituitary–adrenal axis and autonomic nervous system during inflammation and altered programming of the neuroendocrine–immune axis during fetal and neonatal development: lessons learned from the model inflammagen. lipopolysac. Brain. Behav. Immun. 2006;20:144–158. doi: 10.1016/j.bbi.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Karrow N.A., Lee A.V., Quinton M., McBride B.W., Fisher-Heffernan R.E. Ovine hippocampal mRNA expression in offspring from dams supplemented with fishmeal and stress challenged in late pregnancy with endotoxin. Anim. Nutr. 2017;3:39–45. doi: 10.1016/j.aninu.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranendonk G., Hopster H., Fillerup M., Ekkel E.D., Mulder E.J.H., Wiegant V.M., Taverne M.A.M. Lower birth weight and attenuated adrenocortical response to ACTH in offspring from sows that orally received cortisol during gestation. Domest. Anim. Endocrinol. 2006;30:218–238. doi: 10.1016/J.DOMANIEND.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Lay D.C., Kattesh H.G., Cunnick J.E., Daniels M.J., Kranendonk G., McMunn K.A., Toscano M.J., Roberts M.P. Effect of prenatal stress on subsequent response to mixing stress and a lipopolysaccharide challenge in pigs1. J. Anim. Sci. 2011;89:1787–1794. doi: 10.2527/jas.2010-3612. [DOI] [PubMed] [Google Scholar]
- Lee A., You L., Oh S.-Y., Li Z., Code A., Zhu C., Fisher-Heffernan R., Regnault T., De Lange C., Huber L.-A., Karrow N. Health benefits of supplementing nursery pig diets with microalgae or fish oil. Animals. 2019;9:80. doi: 10.3390/ani9030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A.V., You L., Oh S.Y., Li Z., Fisher-Heffernan R.E., Regnault T.R.H., de Lange C.F.M., Huber L., Karrow N.A. Microalgae supplementation to late gestation sows and its effects on the health status of weaned piglets fed diets containing high- or low-quality protein sources. Vet. Immunol. Immunopathol. 2019;218:109937. doi: 10.1016/j.vetimm.2019.109937. [DOI] [PubMed] [Google Scholar]
- Liu H., Smith T.P.L., Nonneman D.J., Dekkers J.C.M., Tuggle C.K. A high-quality annotated transcriptome of swine peripheral blood. BMC Genom. 2017;18:479. doi: 10.1186/s12864-017-3863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Jin L., Zhao L., Long K., Song Y., Tang Q., Ma J., Wang X., Tang G., Jiang Y., Zhu L., Li X., Li M. Identification of a novel antisense long non-coding RNA PLA2G16-AS that regulates the expression of PLA2G16 in pigs. Gene. 2018;671:78–84. doi: 10.1016/J.GENE.2018.05.114. [DOI] [PubMed] [Google Scholar]
- Marques A.H., Bjørke-monsen A., Teixeria A., Silverman M. Maternal stress , nutrition and physical activity : impact on immune function , CNS development and psychopathology. Brain Res. 2015;1617:28–46. doi: 10.1016/j.brainres.2014.10.051. [DOI] [PubMed] [Google Scholar]
- Martin H. Role of PPAR-gamma in inflammation. Prospects for therapeutic intervention by food components. Mutat. Res. Mol. Mech. Mutagen. 2010;690:57–63. doi: 10.1016/J.MRFMMM.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Martinez Calejman C., Astort F., Di Gruccio J.M., Repetto E.M., Mercau M., Giordanino E., Sanchez R., Pignataro O., Arias P., Cymeryng C.B. Lipopolysaccharide stimulates adrenal steroidogenesis in rodent cells by a NFκB-dependent mechanism involving COX-2 activation. Mol. Cell. Endocrinol. 2011;337:1–6. doi: 10.1016/J.MCE.2010.12.036. [DOI] [PubMed] [Google Scholar]
- Mazzocchi G., Rossi G.P., Neri G., Malendowicz L.K., Albertin G., Nussdorfer G.G. 11β-Hydroxysteroid dehydrogenase expression and activity in the human adrenal cortex. FASEB J. 1998;12:1533–1539. doi: 10.1096/fasebj.12.14.1533. [DOI] [PubMed] [Google Scholar]
- Mdaki K.S., Larsen T.D., Wachal A.L., Schimelpfenig M.D., Weaver L.J., Dooyema S.D.R., Louwagie E.J., Baack M.L. Maternal high-fat diet impairs cardiac function in offspring of diabetic pregnancy through metabolic stress and mitochondrial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2016;310:H681–H692. doi: 10.1152/ajpheart.00795.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicherla R., Leers-Sucheta S., Luo Y., Azhar S. Age-dependent modulation of NF-κB expression in rat adrenal gland. Mech. Ageing Dev. 2002;123:1211–1227. doi: 10.1016/S0047-6374(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Mohn C.E., Fernandez-Solari J., De Laurentiis A., Bornstein S.R., Ehrhart-Bornstein M., Rettori V. Adrenal gland responses to lipopolysaccharide after stress and ethanol administration in male rats. Stress. 2011;14:216–226. doi: 10.3109/10253890.2010.532254. [DOI] [PubMed] [Google Scholar]
- Murani E., Ponsuksili S., D’Eath R.B., Turner S.P., Evans G., Tholking L., Kurt E., Klont R., Foury A., Mormede P., Wimmers K. Differential mRNA expression of genes in the porcine adrenal gland associated with psychosocial stress. J. Mol. Endocrinol. 2011;46:165–174. doi: 10.1530/JME-10-0147. [DOI] [PubMed] [Google Scholar]
- National Research Council . eleventh ed. National Academies Press; Washington, D.C: 2012. Nutrient Requirements of Swine. [DOI] [Google Scholar]
- Novak T.E., Babcock T.A., Jho D.H., Helton W.S., Espat N.J. NF-κB inhibition by ω-3 fatty acids modulates LPS-stimulated macrophage TNF-α transcription. Am. J. Physiol. Cell. Mol. Physiol. 2003;284:L84–L89. doi: 10.1152/ajplung.00077.2002. [DOI] [PubMed] [Google Scholar]
- Peddireddy V., Doddam S.N., Ahmed N. Mycobacterial dormancy systems and host responses in tuberculosis. Front. Immunol. 2017;8:1–19. doi: 10.3389/fimmu.2017.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauw W.M. Immune response from a resource allocation perspective. Front. Genet. 2012;3:267. doi: 10.3389/fgene.2012.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson R., Guihéneuf F., Bahar B., Schmid M., Stengel D., Fitzgerald G., Ross R., Stanton C. The anti-inflammatory effect of algae-derived lipid extracts on lipopolysaccharide (LPS)-Stimulated human THP-1 macrophages. Mar. Drugs. 2015;13:5402–5424. doi: 10.3390/md13085402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh J.S., Sohn D.H. Damage-associated molecular patterns in inflammatory diseases. Immune Netw. 2018;18:e27. doi: 10.4110/in.2018.18.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roura E., Koopmans S.-J., Lallès J.-P., Le Huerou-Luron I., De Jager N., Schuurman T., Val-Laillet D. Critical review evaluating the pig as a model for human nutritional physiology. 2016. [DOI] [PubMed]
- Saccone G., Berghella V., Maruotti G.M., Sarno L., Martinelli P. Omega-3 supplementation during pregnancy to prevent recurrent intrauterine growth restriction: systematic review and meta-analysis of randomized controlled trials. Ultrasound Obstet. Gynecol. 2015;46:659–664. doi: 10.1002/uog.14910. [DOI] [PubMed] [Google Scholar]
- Shimojo M., Condon J., Whorwood C.B., Stewart P.M. Adrenal 11 beta-hydroxysteroid dehydrogenase. Endocr. Res. 1996;22:771–780. doi: 10.1080/07435809609043775. [DOI] [PubMed] [Google Scholar]
- Skinner L.D., Levesque C.L., Wey D., Rudar M., Zhu J., Hooda S., de Lange C.F.M. Impact of nursery feeding program on subsequent growth performance, carcass quality, meat quality, and physical and chemical body composition of growing-finishing pigs. J. Anim. Sci. 2014;92:1044–1054. doi: 10.2527/jas2013-6743. [DOI] [PubMed] [Google Scholar]
- Vakharia K., Hinson J.P. Lipopolysaccharide directly stimulates cortisol secretion by human adrenal cells by a cyclooxygenase-dependent mechanism. Endocrinology. 2005;146:1398–1402. doi: 10.1210/en.2004-0882. [DOI] [PubMed] [Google Scholar]
- Van Der Most P.J., De Jong B., Parmentier H.K., Verhulst S. Trade-off between growth and immune function: a meta-analysis of selection experiments. Funct. Ecol. 2011;25:74–80. doi: 10.1111/j.1365-2435.2010.01800.x. [DOI] [Google Scholar]
- Weaver I.C.G., Korgan A.C., Lee K., Wheeler R.V., Hundert A.S., Goguen D. Stress and the emerging roles of chromatin remodeling in signal integration and stable transmission of reversible phenotypes. Front. Behav. Neurosci. 2017;11:1–19. doi: 10.3389/fnbeh.2017.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaakob Z., Ali E., Zainal A., Mohamad M., Takriff M.S. An overview: biomolecules from microalgae for animal feed and aquaculture. J. Biol. Res. (Thessalonike, Greece) 2014;21:6. doi: 10.1186/2241-5793-21-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Han Z., Oppenheim J.J. Alarmins and immunity. Immunol. Rev. 2017;280:41–56. doi: 10.1111/imr.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You L., Lee A.V., Oh S.-Y., Fisher-Heffernan R.E., Edwards M., de Lange K., Karrow N.A. Effect of lipopolysaccharide-induced immune stimulation and maternal fish oil and microalgae supplementation during late pregnancy on nursery pig hypothalamic–pituitary–adrenal function1. J. Anim. Sci. 2019;97:2940–2951. doi: 10.1093/jas/skz166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallocchi M., Matkovic L., Damasco M.C. Adrenal 11-beta hydroxysteroid dehydrogenase activity in response to stress. Can. J. Physiol. Pharmacol. 2004;82:422–425. doi: 10.1139/Y04-035. [DOI] [PubMed] [Google Scholar]
- Zhang J.D., Ruschhaupt M., Biczok R. 2018. ddCt Method for qRT-PCR Data Analysis. [Google Scholar]