Abstract
Social-cognitive difficulties can negatively impact interpersonal communication, shared social experience, and meaningful relationships. This pilot investigation examined the relationship between social-cognitive functioning and inflammatory markers in people with multiple sclerosis (MS) and demographically-matched healthy individuals. Additionally, we compared the immune marker profile in serum and urine-matched samples. Social cognitive functioning was objectively assessed using The Awareness of Social Inference Test – Short (TASIT-S) and subjectively assessed using self-reports of abilities in emotion recognition, emotional empathy, and cognitive theory of mind. In people with MS and healthy individuals, there were moderate-to-large negative relationships between pro-inflammatory biomarkers (serum IL-1β, IL-17, TNF-α, IP-10, MIP-1α, and urine IP-10, MIP-1β) of the innate immune system and social-cognitive functioning. In MS, a higher serum concentration of the anti-inflammatory marker IL-1ra was associated with better social-cognitive functioning (i.e., self-reported emotional empathy and TASIT-S sarcasm detection performance). However, there were mixed findings for anti-inflammatory serum markers IL-4 and IL-10. Overall, our findings indicate a relationship between pro-inflammatory cytokines and social-cognitive abilities. Future studies may provide greater insight into biologically-derived inflammatory processes, sickness behaviour, and their connection with social cognition.
Keywords: Multiple sclerosis, Social cognition, Inflammation, TASIT-S, Theory of mind, Emotion perception, Sarcasm, Emotional empathy, Sickness behaviour, Innate immunity
Highlights
-
•
Innate immunity (serum IL-1β, IL-17, TNF-α, IP-10, MIP-1α, and urine IP-10, MIP-1β) negatively affects social cognition.
-
•
Given their relationship, activation of IL-1ra may be a potential therapeutic target to improve social-cognitive abilities.
-
•
Higher concentration of pro-inflammatory markers in the periphery do not always lead to higher excretion levels in urine.
-
•
Inflammatory dysregulation, may, in part, underlie social-cognitive difficulties.
1. Introduction
Inflammation has broad-ranging effects across the body and can disrupt brain functioning. Biological markers of the innate immune system and sickness behaviour have been recently implicated in poor social cognition, including reduced mentalising of another’s affective state or psychological perspective, reduced social interaction, and increased social disconnectedness (Bollen et al., 2017; Hennessy et al., 2014; Moieni and Eisenberger, 2018). Multiple sclerosis (MS) is an immune-mediated neuroinflammatory and neurodegenerative disease (Dendrou et al., 2015) for which poor social processing and difficulties in social functioning are a common outcome (Batista et al., 2017; Chalah and Ayache, 2017). However, despite the involvement of dysregulated inflammatory processes in MS (Kothur et al., 2016; Lim et al., 2017), whether these biomarkers play a role in poor social functioning is not understood.
Social cognition concerns the ability to perceive, interpret, and process interpersonal cues and socially relevant stimuli that enable a person to understand another person’s intentions, thoughts, and feelings (McDonald et al., 2013, 2018). Difficulties in social-cognitive functioning are reported in around 30–40% of people with MS (pwMS) (Genova et al., 2016) and may include reduced facial emotion recognition (Henry et al., 2011, Lenne et al., 2014) and Theory of Mind (ToM; the ability to accurately predict and interpret another’s mental state that may differ from one’s own beliefs, emotions, and desires) (Cotter et al., 2016; Pöttgen et al., 2013) abilities. Poor social-cognitive functioning negatively impacts communication, shared experience, and the ability to form meaningful relationships, which may contribute to social isolation and higher caregiver burden (Adams et al., 2019; Caplan et al., 2015). However, the identification of possible prognostic markers of social-cognitive difficulties may assist in identifying pwMS who may benefit from more extensive neuropsychological testing.
In pwMS, dysregulated cytokine production can result in aberrant concentrations of pro-inflammatory markers interleukin 1-beta (IL-1β), IL-17, tumour necrosis factor-alpha (TNF-α), and interferon gamma (IFN-γ); as well as anti-inflammatory markers IL-1 receptor antagonist (IL-1ra), IL-4, and IL-10 (Dendrou et al., 2015; Opdenakker and Van Damme, 2011; Sorenson et al., 2017; Turner et al., 2014). Prior research examining cytokine profiles indicate IL-1ra, IL-4, IL-10, TNF-α, and IFN-γ to be pleiotropic, having dualistic properties (Turner et al., 2014). For example, while IL-1ra concentrations can increase with inflammation, it also acts as an inflammatory regulator on IL-1α and IL-1β receptors. Thus, the terms pro- and anti-inflammatory are not absolute.
Research has shown that inflammatory processes and sickness behaviour can negatively affect social experience. Three prior double-blind placebo-controlled randomised crossover design studies using the International Affective Picture System (Bradley and Lang, 2007) and the Reading the Mind in the Eyes Test (RMET) (Baron-Cohen et al., 2001) found endotoxin-induced inflammation reduced the ability to recall emotional faces and the ability to mentalise the affective and psychological states in others (Bollen et al., 2017; Grigoleit et al., 2011; Moieni et al., 2015). However, in another double-blind placebo-controlled crossover design fMRI study using the RMET, no such effects on social-cognitive performance were found (Kullmann et al., 2014). A key distinction between these studies was the differing dose to trigger the inflammatory response. Changes in behavioural and physiological functioning from endotoxic processes can be dose-dependent (Grigoleit et al., 2011). For example, Kullmann et al. (2014) used 0.4 ng/kg of body weight to induce low-grade inflammation, while Moieni et al. (2015) used 0.8 ng/kg of body weight for a more robust inflammatory response. Thus, it remains possible that inflammatory markers that typically modulate responses to illnesses selectively affect social-cognitive processes.
The innate immune mediators, particularly interferon-gamma-inducible protein-10 (IP-10), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1 beta (MIP-1β), and granulocyte colony-stimulating factor (G-CSF), have been implicated in MS (Carrieri et al., 1998; Cheng and Chen, 2014; Opdenakker and Van Damme, 2011; Rust et al., 2016). Dysregulation of innate immunity (such as that which would occur during an MS relapse) can result in increased concentrations of these circulating inflammatory mediators in the brain, cerebrospinal fluid, and blood (Broux et al., 2012; Cheng and Chen, 2014). For instance, IP-10 and G-CSF can markedly increase during disease activity in relapsing-remitting MS, which may exacerbate inflammatory processes, demyelination, and lesion development (Balashov et al., 1999; Rust et al., 2016; Scarpini et al., 2002). Dysregulated innate immune responses have been reported to affect cognitive functioning in other neurological disorders such as schizophrenia, Alzheimer’s disease, and autism (Novellino et al., 2020); however, their relationship with social-cognitive abilities in MS has not yet been examined.
The present study was a pilot investigation to delineate the possible relationship between the various serum- and urine-based inflammatory markers known to be implicated in MS and social-cognitive functioning. We first hypothesised that, in pwMS, there would be a negative association between pro-inflammatory markers (i.e., IL-1β, IL-17, IFN-γ, TNF-α, IP-10, MIP-1β and MCP-1) and social-cognitive abilities. Second, we hypothesised that, in pwMS, there would be a positive association between anti-inflammatory markers (i.e., IL-1ra, IL-4 and IL-10) and social-cognitive abilities. We compared these relationships to those found in healthy individuals without MS and examined if urine markers were associated with respective serum markers.
2. Materials and methods
2.1. Participants
Participants comprised 20 pwMS (17 relapsing-remitting, 1 secondary-progressive, 2 primary-progressive) and 20 healthy control (HC) individuals without MS, demographically-matched for sex, age, and education (Table 1). PwMS were diagnosed according to the McDonald criteria (Novellino et al., 2020; Thompson et al., 2018). MS phenotypes were not analysed separately as this study was interested in the level of inflammation found rather than understanding the disease types’ unique pathological features. Participants with MS completed the Disease Steps Scale (Hohol et al., 1999), which indicated ‘mild’ to ‘moderate’ levels of disability in the present sample (Mdn = 1.50, IQR = 1.00–2.75). Exclusion criteria included any psychotic, bipolar, or related disorder; a history of brain injury or other neurological illness such as stroke or epilepsy; a history of alcohol or drug abuse; inability to speak and read English fluently; uncorrected visual difficulties affecting task completion; and pregnancy. Smoking was not an exclusion criterion in this study; we had three participants, two MS and one healthy participant, who indicated they were smokers (between 10 and 15 per day). Additional exclusion criteria included use of steroid medications within the past two months or MS disease relapse within the past 14 days for the MS participants as assessed by the Relapse Status Checklist (Brown et al., 2006) to reduce the potential confounding effect of disease-related inflammatory dysregulation that occurs during an MS relapse. All aspects of this study were approved by the Tasmania Health and Medical Research Ethics Committee (H00156630) and adhered to the World Medical Association’s Declaration of Helsinki.
Table 1.
Descriptive and inferential statistics for demographic information, TASIT-S, SEQ subscales, IRI Perspective Taking, and HADS in MS and HC individuals.
| Variable | MS n = 20 |
HC n = 20 |
t or χ2 | df | p | 95% CI Difference |
V or d | |
|---|---|---|---|---|---|---|---|---|
| LL | UL | |||||||
| Male | 4 (40%) | 6 (60%) | ||||||
| Female | 16 (53.3%) | 14 (46.7%) | .53 | 1 | .465 | .12c | ||
| Age, years (SD) | 47.25 (11.04) | 44.35 (11.43) | .81 | 38 | .420 | −4.30 | 10.10 | .25 |
| Education, years (SD) | 12.45 (1.76) | 13.25 (1.99) | 1.34 | 38 | .187 | −2.01 | .41 | .42 |
| TASIT-S, mean (SD) | ||||||||
| Part 1 Emotion Evaluation Test | 6.70 (1.34) | 7.45 (1.05) | 1.97 | 38 | .056 | −1.52 | 0.02 | .62 |
| Part 2 Sincerity | 13.00 (2.29) | 12.85 (3.18) | .17 | 38 | .865 | −1.63 | 1.93 | .05 |
| Part 2 Sarcasmb | 16.15 (3.69) | 18.30 (1.81) | 2.34 | 27.64 | .027a | −4.03 | 0.27 | .74 |
| Part 3 Lies | 11.40 (2.06) | 11.65 (2.60) | .34 | 38 | .738 | −1.75 | 1.25 | .11 |
| Part 3 Sarcasm | 15.85 (2.25) | 17.10 (2.13) | 1.80 | 38 | .079 | −2.65 | 0.15 | .57 |
| SEQ, mean (SD) | ||||||||
| Emotion Recognition | 18.85 (3.39) | 19.85 (3.15) | .97 | 38 | .340 | −3.10 | 1.10 | .31 |
| Emotional Empathy | 19.00 (2.90) | 18.70 (2.30) | .36 | 38 | .719 | −1.38 | 1.98 | .12 |
| IRI, mean (SD) | ||||||||
| Perspective Taking | 18.35 (7.10) | 15.60 (7.42) | 1.20 | 38 | .239 | −1.90 | 7.40 | .38 |
| HADS, mean (SD) | ||||||||
| Anxiety | 7.40 (4.43) | 5.50 (3.30) | 1.54 | 38 | .420 | −0.60 | 4.40 | .49 |
| Depression | 5.50 (3.00) | 3.20 (3.44) | 2.25 | 38 | .030a | 0.23 | 4.37 | .71 |
Note: Descriptive and inferential statistics for between-group comparisons on demographics, TASIT-S, SEQ subscales Emotion Recognition and Emotional Empathy, IRI Perspective Taking subscale, and the HADS. Frequencies are reported for sex. Means and standard deviations for all other variables are reported. CI, Confidence Interval; d, Cohen's d effect size; df, degrees of freedom; HADS, Hospital Anxiety and Depression Scale; IRI, Interpersonal Reactivity Index; LL, Lower Level; SEQ, Social and Emotional Questionnaire; TASIT-S, The Awareness of Social Inference Test-Short; UL, Upper Level; and V, Cramer's V effect size.
Denotes significant between-group differences (p < .05). Cohen's d effect size > .5 are in bold.
Denotes a significant Levene's Test for Equality of Variances; thus, equal variances not assumed was interpreted.
Denotes Cramer's V effect size.
2.2. Data and sample collection
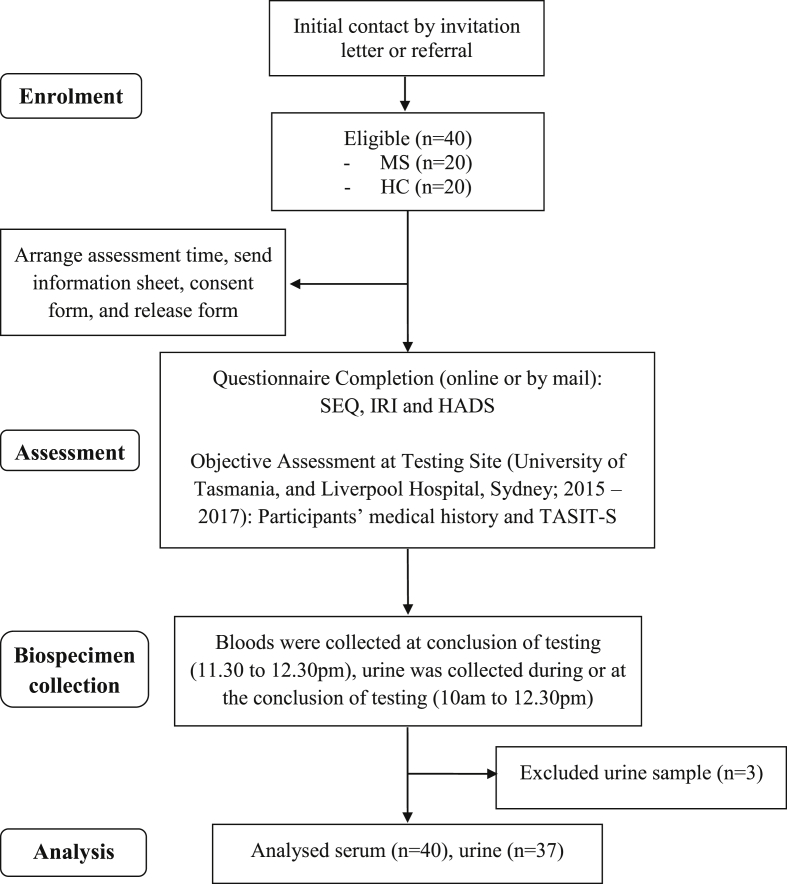
Following recruitment (by invitation letter and referral), participants completed a questionnaire containing demographic and disease-related questions, and standardised questionnaires to assess self-reported social-cognitive functioning and mood within seven days before attending a face-to-face testing session. The self-report social cognitive questionnaires included two subscales of The Social and Emotional Questionnaire (SEQ) (Bramham et al., 2009) – Emotion Recognition and Emotional Empathy, and the Perspective Taking subscale of the Interpersonal Reactivity Index (IRI) (Davis, 1980). All objective assessment occurred in the morning (start time between 9 and 10am) and in a temperature-controlled room (24°C) to control for time of day and temperature effects on performance and biological markers. At the testing session, participants were firstly interviewed to ascertain their disease history and characteristics before completing a battery of neuropsychological tests that included tests of general cognitive functioning (to be reported elsewhere) and a social-cognitive test, The Awareness of Social Inference Test - Short (TASIT-S) (Honan et al., 2016). TASIT-S was administered approximately 50 min into the testing session. Following testing, 30 mL of venous blood was extracted from the participant by a qualified phlebotomist. Mid-stream urine was also collected either during or immediately after testing. The venous blood was processed to extract the serum and then stored at approximately −80 °C until transferred for analysis. Prior to biochemical analysis, blinding measures such as unsorting and re-labelling were undertaken (Fig. 1).
Fig. 1.
Flow diagram of the study. MS, multiple sclerosis; HC, healthy control; SEQ, Social and Emotional Questionnaire; IRI, Interpersonal Reactivity Index; HADS, Hospital Anxiety and Depression Scale; TASIT-S, The Awareness of Social Inference Test-Short. Self-reported questionnaires (i.e., SEQ, IRI and HADS) were completed < 7 days before the objective assessment at testing site.
2.3. Social cognitive and neuropsychological tests
TASIT-S (Honan et al., 2016) is an objectively assessed social cognition task comprised of three parts. Part 1 is a dynamic Emotional Evaluation Test (EET) containing 10 short video vignettes of professional actors depicting five basic facial emotions: happy, sad, anger, fear, disgust, and no specific emotion – neutral. Part 2 Social Inference Minimal is a task that examines the comprehension of conversational meanings from paralinguistic cues (tone of voice, facial expression, and gesture). It comprises nine video vignettes depicting four sincere and five sarcastic (tapping into ToM) social exchanges. Each vignette requires the participant to answer questions that assess the participants’ understanding of an actor’s belief (what they are doing), meaning (what they are trying to say), intention (what they are thinking), and feeling (how they are feeling). Part 3 Social Inference Enriched is another social-cognitive task similar to Part 2, but with additional contextual information to aid interpretation. It is comprised of nine video vignettes depicting four blatant lies and five sarcastic social exchanges.
Self-reported social-cognitive abilities were assessed using two 5-item subscales of the SEQ (Bramham et al., 2009) – Emotion Recognition and Emotional Empathy. In the SEQ, statements are rated on a 5-point scale from 1 = strongly disagree to 5 = strongly agree. Self-reported cognitive ToM was assessed using the 7-item Perspective Taking subscale from the IRI (Davis, 1980). In the IRI, statements are rated on a 5-point scale from A = does not describe me well to E = describes me well. Higher scores on the SEQ and IRI are indicative of higher social-cognitive abilities.
The Hospital Anxiety and Depression Scale (HADS) (Zigmond and Snaith, 1983) was administered to profile self-reported anxiety and depression levels over the preceding week. The HADS comprises 14-items where various statements are rated on a 4-point scale where 0 = not at all to 3 = most of the time. Scores are summed for anxiety and depression subscales with higher scores indicative of higher symptomatology.
2.4. Profiling of immunological markers
Cytokines, chemokines, and growth and colony-stimulating factors were concurrently quantified according to the manufacturer’s protocols using commercial Human Cytokine 27-plex magnetic bead-based immunoassay kits (Bio-Rad, CA, USA). In accordance with current practice, median fluorescence intensities (FI) were used to analyse immune profile data. Due to its increased sensitivity, this technique has higher statistical power to detect variance compared to the use of absolute concentration values (Breen et al., 2015). The urine markers are expressed as FI value/creatinine (Allen et al., 2004). Notably, serum concentrations of IFN-γ, GM-CSF and VEGF, and urine concentrations of IL-1β, MIP-1α, and IL-4 were minimally or undetectable and thus were not analysed and reported.
2.5. Statistical analysis
Statistical analyses were conducted using IBM SPSS Statistics for Windows, Version 26 (IBM Corporation, 2019). The normality of the variables was checked using histograms, visual inspection of the residual/Q-Q plots, and Shapiro-Wilk tests. Homogeneity of variance was examined utilising Levene’s test with equal variances not assumed, interpreted as required. Before analyses, the data were inspected case-by-case for outliers. Subsequently, one MS (urine IP-10) and two HC (1 urine IL-1ra; 1 urine TNF-α, G-CSF, and GM-CSF) cases were removed. Independent samples t-tests assessed between-group differences on the social-cognitive measures. To normalise the biomarker data, logarithmic and square root transformations were applied; however, the variables could not be normalised, thus Spearman’s ρ, and Mann-Whitney U analyses (with Hodges-Lehman 95% confidence intervals of the difference) were used. Due to a priori hypotheses about the direction of between-group effects and correlations, p-values < .05 were deemed to be statistically significant. Effect sizes that were at least moderate in size per the guidelines of Cohen (2013) were reported. This included correlations >0.30 and Cohen’s d > 0.50.
Figures are provided for statistically significant biomarker comparisons and correlations, while tables containing complete descriptive data and inferential statistics for the variables are included in the supplemental information (S1 to S4).
3. Results
3.1. Demographic and between-group differences on TASIT-S, SEQ, IRI, and HADS
Descriptive and inferential statistics for the questionnaire and social-cognitive measures are shown in Table 1. There were no significant between-group differences in sex, age, and education. On TASIT-S, pwMS had poorer Part 1 EET scores and Part 2 Sarcasm scores (moderate-to-large effects). Although significance was not met for Part 3 Sarcasm, a moderate effect for poorer scores in pwMS was present. On the HADS, pwMS self-reported higher depression scores (moderate-to-large effect) and higher anxiety scores (a moderate effect).
3.2. Between-group differences on pro- and anti-inflammatory biomarkers
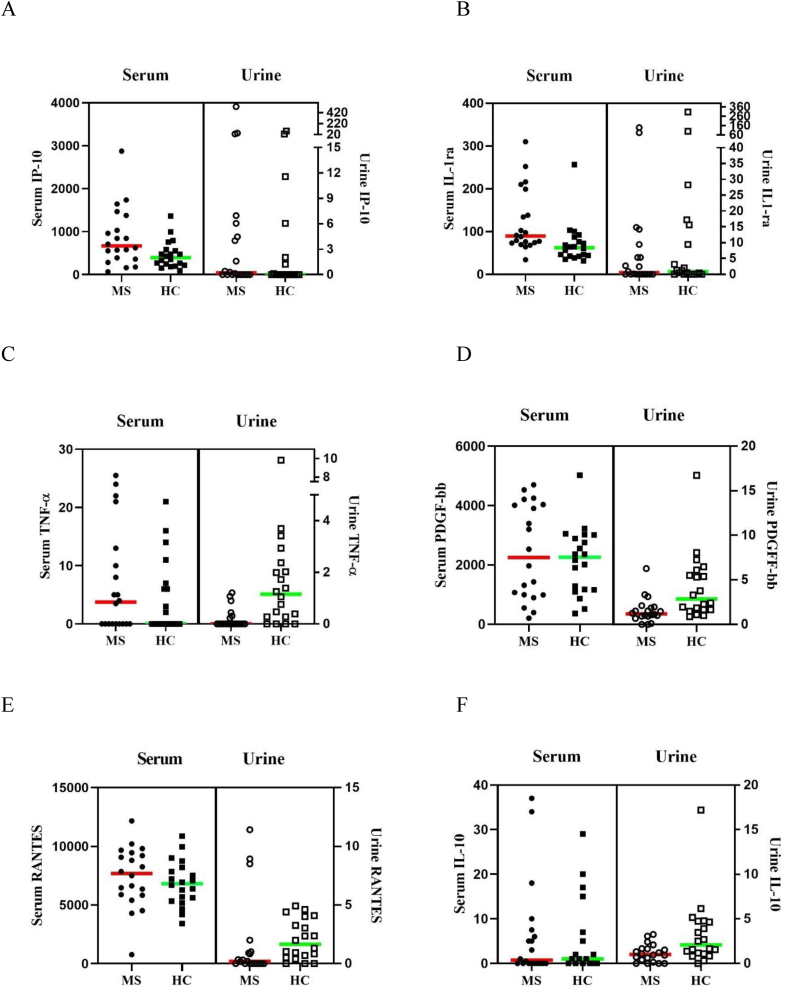
PwMS had significantly higher serum concentrations of pro-inflammatory IP-10 (U = 117.00, p = .024, d = 0.76, Fig. 2A) and anti-inflammatory IL-1ra (U = 90.00, p = .002, d = 1.07, Fig. 2B) than HC (large effects). PwMS also had significantly lower urine concentrations of pro-inflammatory IFN-γ (U = 293.00, p = .011, d = 0.78), TNF-α (U = 306.00, p = .001, d = 1.23, Fig. 2C), GM-CSF (U = 294.00, p = .003, d = 1.06), PDGF-bb (U = 317.00, p = .001, d = 1.16, Fig. 2D), RANTES (U = 282.50, p = .024, d = 0.75, Fig. 2E), and anti-inflammatory IL-10 (U = 297.00, p = .008, d = 0.91, Fig. 2F) than HC (large effects) (for all comparisons, see Supplementary Tables S1 and S2). Intra-correlational analyses between serum and urine biomarkers are shown in Table 2.
Fig. 2.
Significant between-group differences for pro-inflammatory IP-10 (A) and anti-inflammatory IL-1ra (B) markers from serum (with non-significant urine comparisons), and significant between-group differences for pro-inflammatory TNF-α (C), PDGF-bb (D), RANTES (E) and anti-inflammatory IL-10 (F) markers from urine (with non-significant serum comparisons) in people with multiple sclerosis and matched healthy control participants. Interferon-gamma and Granulocyte-macrophage colony-stimulating factor is not shown due to having minimal or undetectable levels from serum. IL-1ra, Interleukin 1 receptor antagonist; IL-10, Interleukin 10; IP-10, Interferon-gamma inducible protein 10; PDGF-bb, Platelet-derived growth factor 2 b subunits; RANTES, Regulated on activation normal T-cell expressed and secreted; and TNFα, Tumour necrosis factor-alpha. Concentrations are shown in fluorescence intensity values for serum and fluorescence intensity/creatinine values for urine.
Table 2.
Spearman’s ρ correlations between serum and urine markers stratified by MS and HC individuals.
| Biomarker dyads Serum correlated with Urine samples |
Overall ρ Correlation n = 40 |
MS ρ Correlation n = 20 |
HC ρ Correlation n = 20 |
|---|---|---|---|
| Pro-inflammatory biomarkers | |||
| IL-17 | −.24 | −.08 | −.39 |
| TNFα | .11a | .06 | .41a |
| G-CSF | −.11a | −.06 | −.10a |
| IP-10 | .01a | −.14a | .16 |
| MCP-1(MCAF) | .05 | .28 | −.12 |
| MIP-1β | .41∗ | .47∗ | .32 |
| PDGF-bb | −.11 | −.08 | −.03 |
| RANTES | .01 | .04 | .01 |
| Anti-inflammatory biomarkers | |||
| IL-1ra | .24a | −.09 | .18a |
| IL-10 | −.03 | −.01 | −.16 |
| Head-to-head serum marker comparison | |||
| IL-1ra vs IL-17 | −.16 | −.54* | .07 |
| IL-1ra vs IL-1β | .11 | −.21 | .18 |
Note. Spearman’s ρ correlation for serum versus urine derived biomarkers overall and stratified by multiple sclerosis and healthy matched control participants. Abbreviations: G-CSF, granulocyte colony-stimulating factor; IL-1β, interleukin 1 beta; IL-1ra, interleukin 1 receptor antagonist; IL-4, interleukin 4; IL-10, interleukin 10; IL-17, interleukin 17; IP-10, interferon inducible protein-10; MCP-1(MCAF), monocyte chemotactic protein-1 and activating factor; MIP-1β, macrophage inflammatory protein-1 beta; PDGF-bb, platelet-derived growth factor-two b subunits; RANTES, regulated upon activation normal T-cell expressed and secreted; TNFα, tumour necrosis factor-alpha. Intra-correlations are not shown for pro-inflammatory markers IL-1β, IFNγ, GM-CSF, MIP-1α, VEGF and anti-inflammatory IL-4 due to minimal or undetectable concentration levels.
*Denotes significant correlation p < .05.
Denotes the removal of 1 outlier case before analyses.
3.3. Pro-inflammatory biomarkers correlated with social-cognitive functioning
3.3.1. Objective social-cognitive measures
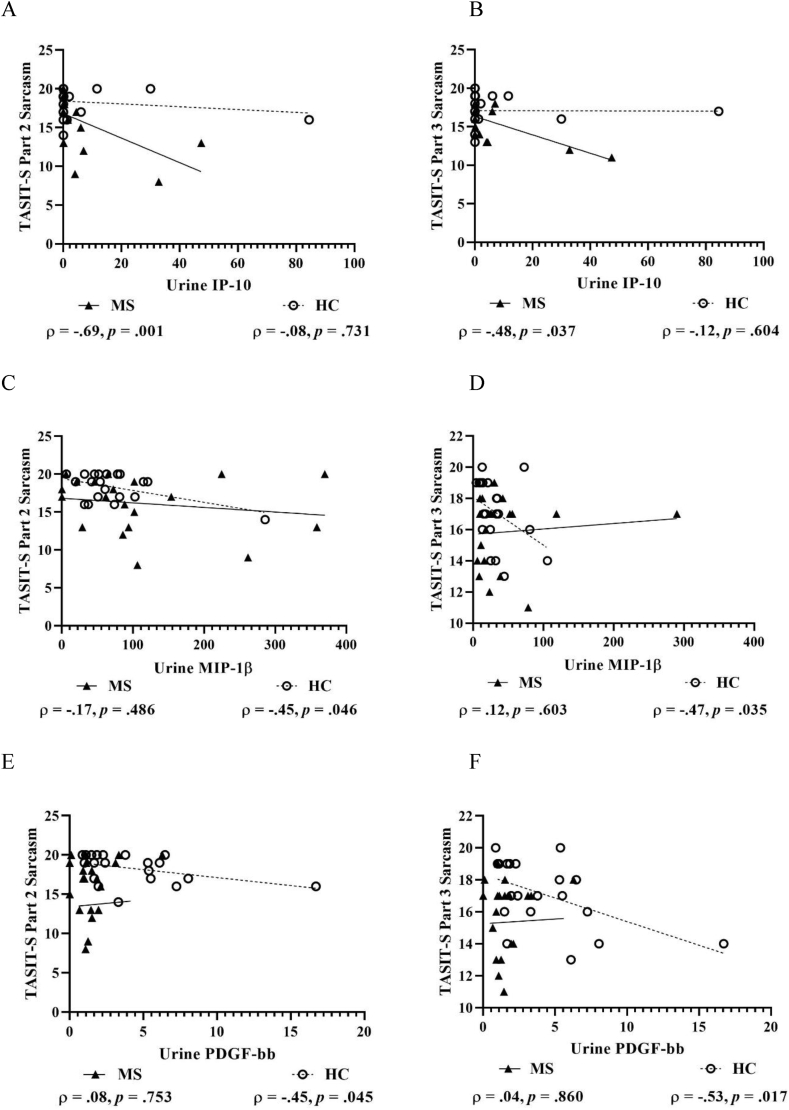
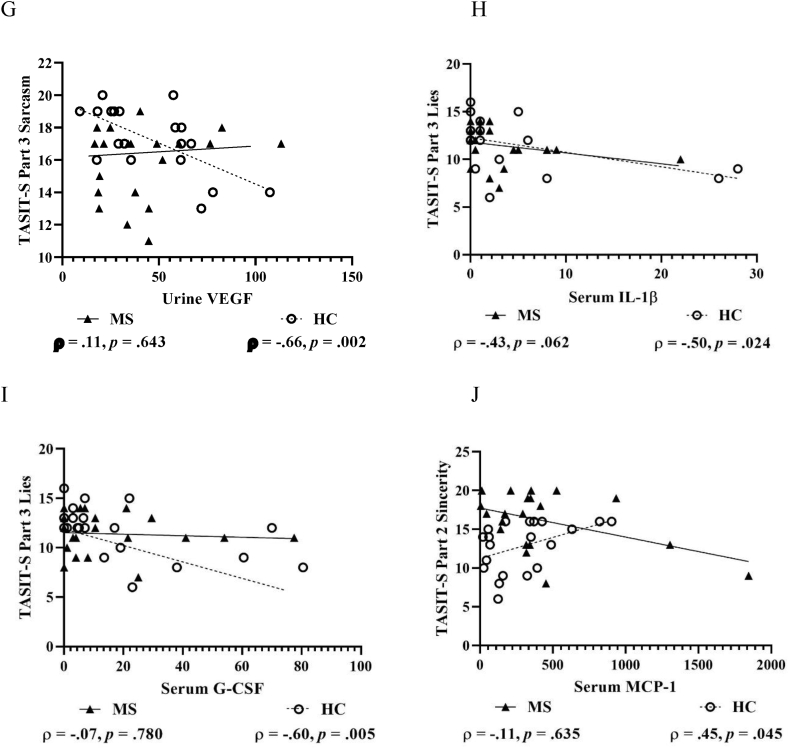
For TASIT-S Part 1 EET, no significant correlations were found with pro-inflammatory markers from serum or urine in pwMS or HCs. For TASIT-S Part 2 and 3 Sarcasm, urine IP-10 showed large negative correlations in pwMS, but not in HCs (Fig. 3A–B). Conversely, urine MIP-1β and PDGF-bb showed moderate-to-large negative correlations in HCs, but not in pwMS (Fig. 3C–F), which also applied for urine VEGF and TASIT-S Part 3 Sarcasm (Fig. 3G). Further, serum IL-1β and G-CSF showed large negative correlations with TASIT-S Part 3 Lies in HCs, but not in pwMS (Fig. 3H–I), while serum MCP-1 showed a moderate-to-large positive correlation with TASIT-S Part 2 Sincerity in HCs (Fig. 3J).
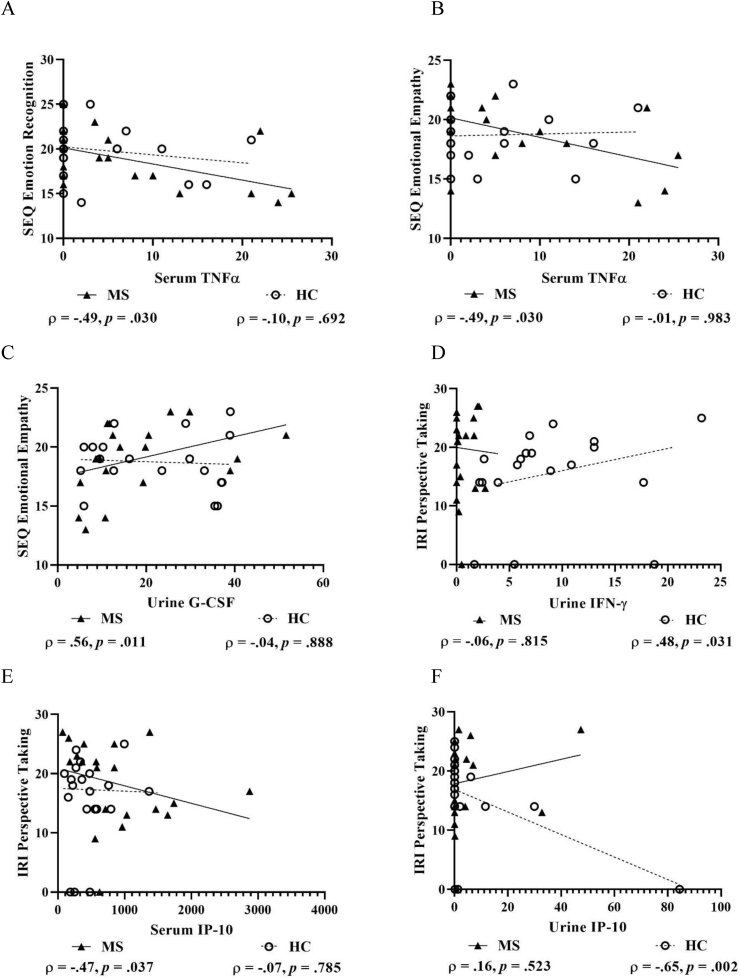
Fig. 4.
Scatterplots with selected correlations between pro-inflammatory markers and subjectively assessed social-cognitive measures in people with multiple sclerosis and matched healthy control participants. Figures show pro-inflammatory serum TNF-α and both SEQ Emotion Recognition (A) and SEQ Emotional Empathy (B), urine G-CSF and SEQ Emotional Empathy (C) urine IFNγ and IRI Perspective Taking (D), serum IP-10 and IRI Perspective Taking (E), and urine IP-10 and IRI Perspective Taking (F). G-CSF, Granulocyte colony-stimulating factor; IFN-γ, Interferon-gamma; IP-10, Interferon-gamma inducible protein 10; IRI, Interpersonal Reactivity Index; SEQ, Social and Emotional Questionnaire; and TNFα, Tumour necrosis factor-alpha. An alternative analysis was performed on Figure F (urine IP-10 & IRI Perspective Taking), removing the HC outlier, which showed minimal change in the strength, direction and significance of the correlation (ρ = −0.60, p = .007); thus, this case was retained. Concentrations are shown in fluorescence intensity values for serum and fluorescence intensity/creatinine values for urine.
Fig. 3.
Scatterplots with selected correlations between pro-inflammatory markers and objectively assessed social-cognitive measures in people with multiple sclerosis (MS) and matched healthy control (HC) participants. Figures show pro-inflammatory markers from urine IP-10 and TASIT-S Part 2 Sarcasm (A) and Part 3 Sarcasm (B), MIP-1β and TASIT-S Part 2 Sarcasm (C) and Part 3 Sarcasm (D), PDGF-bb and TASIT-S Part 2 Sarcasm (E) and Part 3 Sarcasm (F), VEGF and TASIT-S Part 3 Sarcasm (G) markers, and pro-inflammatory markers from serum IL-1β and TASIT-S Part 3 Lies (H), G-CSF and TASIT-S Part 3 Lies (I), and MCP-1 and TASIT-S Part 2 Sincerity (J). G-CSF, Granulocyte colony-stimulating factor; IFNγ, Interferon-gamma; IL-1β, Interleukin 1 beta; IP-10, Interferon-gamma inducible protein 10; IRI, Interpersonal Reactivity Index; IL-17, Interleukin 17; MCP-1, Monocyte chemoattractant protein-1; MCP-1, Macrophage inflammatory protein-1β; PDGF-bb, Platelet-derived growth factor-2 b subunits; TASIT-S, The Awareness of Social Inference Test-Short; TNFα, Tumour necrosis factor-alpha; and VEGF, Vascular endothelial growth factor. Concentrations are shown in fluorescence intensity values for serum and fluorescence intensity/creatinine values for urine.
For TASIT-S in pwMS, positive correlations that were at least moderate in size, but not statistically significant, were found between both serum IP-10 (MS ρ = 0.42, p = .066; HCs ρ = −0.30, p = .205) and urine IL-17 (MS ρ = 0.36, p = .120; HCs ρ = 0.07, p = .789) and Part 1 EET. However, negative correlations were found between serum TNF-α (MS ρ = −0.30, p = .204; HCs ρ = −0.07, p = .773) and Part 2 Sarcasm, and between urine MIP-1β (MS ρ = −0.34, p = .139; HCs ρ = −0.03, p = .887) and Part 3 Lies.
In HCs, positive correlations that were at least moderate in size, but not statistically significant, were found between TASIT-S Part 1 EET and serum RANTES (MS ρ = 0.03, p = .897; HCs ρ = 0.34, p = .148). While negative correlations were found between Part 2 Sincerity and both serum G-CSF (MS ρ = −0.08, p = .727; HCs ρ = −0.41, p = .070) and MIP-1α (MS ρ = 0.04, p = .880; HCs ρ = −0.35, p = .134); Part 2 Sarcasm and serum G-CSF (MS ρ = −0.13, p = .596; HCs ρ = −0.34, p = .145) and urine markers IFN-γ (MS ρ = 0.26, p = .231; HCs ρ = −0.38, p = .065), TNF-α (MS ρ = −0.12, p = .614; HCs ρ = −0.37, p = .121), MCP-1 (MS ρ = −0.06, p = .814; HCs ρ = −0.42, p = .069), and VEGF (MS ρ = 0.13, p = .573; HCs ρ = −0.38, p = .095); Part 3 Lies and both serum TNF-α (MS ρ = 0.10, p = .689; HCs ρ = −0.38, p = .101) and IP-10 (MS ρ = 0.17, p = .474; HCs ρ = −0.32, p = .165); and Part 3 Sarcasm and urine G-CSF (MS ρ = 0.02, p = .951; HCs ρ = −0.36, p = .133).
3.3.2. Subjective social-cognitive measures
For the SEQ subscales, only serum TNF-α showed large negative correlations with Emotion Recognition and Emotional Empathy in pwMS, but not in HCs (Fig. 4A & B). There was also a large positive correlation found between urine G-CSF and Emotional Empathy in pwMS that was not present in HCs (Fig. 4C). For the IRI subscale, only urine IFN-γ showed a moderate-to-large positive correlation with Perspective Taking in HCs, but not in pwMS (Fig. 4D). Further, serum IP-10 showed a moderate-to-large negative correlation with Perspective Taking in pwMS, which was not present in HCs (Fig. 4E). Conversely, urine IP-10 showed a large negative correlation with Perspective Taking in HCs, but not in pwMS (Fig. 4F).
For the SEQ subscales in pwMS, positive correlations that were at least moderate in size, but not statistically significant, were found between Emotion Recognition and urine G-CSF (MS ρ = 0.43, p = .056; HCs ρ = 0.13, p = .590); and between Emotional Empathy and urine VEGF (MS ρ = 0.30, p = .204; HCs ρ = −0.15, p = .521). However, negative correlations were found between Emotion Recognition and urine GM-CSF (MS ρ = −0.36, p = .115; HCs ρ = −0.17, p = .494); and between Emotional Empathy and serum IP-10 (MS ρ = −0.34, p = .138; HCs ρ = −0.15, p = .541) and MIP-1α (MS ρ = −0.37, p = .113; HCs ρ = −0.07, p = .785) and urine GM-CSF (MS ρ = −0.35, p = .131; HCs ρ = −0.14, p = .561) and IP-10 (MS ρ = −0.31, p = .199; HCs ρ = −0.04, p = .857). For the IRI subscale in pwMS, only serum IL-1β showed a moderate negative correlation with Perspective Taking (MS ρ = −0.31, p = .188; HCs ρ = 0.01, p = .966). Whereas in HCs, moderate positive correlations were found between IRI Perspective Taking and urine MIP-1β (MS ρ = 0.13, p = .572; HCs ρ = 0.30, p = .198), PDGF-bb (MS ρ = −0.12, p = .604; HCs ρ = 0.32, p = .163), and VEGF (MS ρ = 0.13, p = .599; HCs ρ = 0.33, p = .162).
3.4. Anti-inflammatory biomarkers correlated with social-cognitive functioning
3.4.1. Objective social-cognitive measures
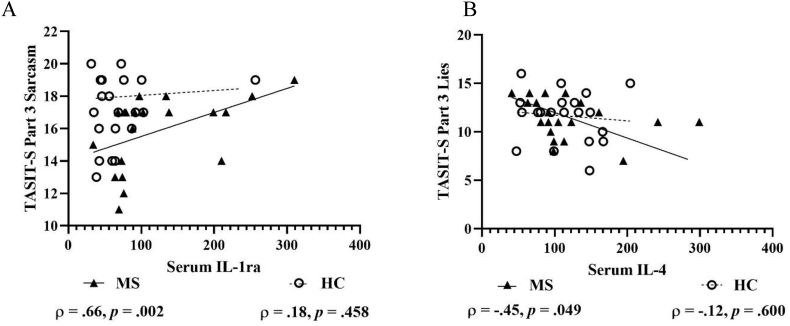
On TASIT-S in pwMS, there was a large positive correlation between Part 3 Sarcasm and serum IL-1ra (Fig. 5A) and a moderate-to-large negative correlation between Part 3 Lies and serum IL-4 (Fig. 5B). In HCs, there were no significant correlations between TASIT-S and anti-inflammatory markers from serum and urine.
Fig. 5.
Scatterplots with selected correlations between anti-inflammatory markers and objectively assessed social-cognitive measures in people with multiple sclerosis and matched healthy control participants. Figures show anti-inflammatory serum IL-1ra and TASIT-S Part 3 Sarcasm (A) and serum IL-4 and TASIT-S Part 3 Lies (B). IL-1ra, Interleukin 1 receptor antagonist; IL-4, Interleukin 4; and TASIT-S, The Awareness of Social Inference Test-Short.
Concentrations are shown in fluorescence intensity values for serum.
For TASIT-S in pwMS, a positive correlation that was moderate in size, but not statistically significant, was found between Part 2 Sarcasm and serum IL-1ra (MS ρ = 0.36, p = .116; HCs ρ = 0.07, p = .780), however a moderate negative correlation was found between Part 2 Sincerity and serum IL-4 (MS ρ = −0.36, p = .122; HCs ρ = −0.22, p = .361). In HCs, moderate negative correlations were found between TASIT-S Part 2 Sincerity and IL-1ra from serum (MS ρ = 0.04, p = .879; HCs ρ = −0.36, p = .115) and urine (MS ρ = −0.06, p = .803; HCs ρ = −0.32, p = .183), Part 2 Sarcasm and serum IL-4 (MS ρ = −0.04, p = .858; HCs ρ = −0.32, p = .165), and IL-10 (MS ρ = 0.21, p = .365; HCs ρ = −0.32, p = .175), urine IL-1ra (MS ρ = 0.15, p = .516; HCs ρ = −0.31, p = .196) and IL-10 (MS ρ = 0.16, p = .500; HCs ρ = −0.43, p = .058), and Part 3 Lies and serum IL-10 (MS ρ = −0.27 p = .256; HCs ρ = −0.40, p = .084).
3.4.2. Subjective social-cognitive measures
No significant correlations were found between the anti-inflammatory markers and subjective social-cognitive measures. For the SEQ subscales in pwMS, positive correlations that were at least moderate in size, but not statistically significant, were found between Emotion Recognition and serum IL-10 (MS ρ = 0.44, p = .054; HCs ρ = −0.25, p = .285) and between Emotional Empathy and serum IL-4 (MS ρ = 0.32, p = .175; HCs ρ = −0.23, p = .341). However, there was a moderate negative correlation between Emotion Recognition and urine IL-10 (MS ρ = −0.30, p = .196; HCs ρ = 0.21, p = .372). In HCs, a moderate positive correlation was found between Emotional Empathy and serum IL-1ra (MS ρ = 0.28, p = .233; HCs ρ = 0.38, p = .103). For the IRI subscale, there was a moderate positive correlation between Perspective Taking and urine IL-10 (MS ρ = −0.05, p = .821; HCs ρ = 0.31, p = .177), and moderate negative correlations with both serum IL-1ra (MS ρ = 0.23, p = .339; HCs ρ = −0.32, p = .174) and IL-4 (MS ρ = 0.14, p = .564; HCs ρ = −0.37, p = .114).
Further alternative exploratory analyses were conducted to examine whether sex, age, smoking, depression, and anxiety could explain any additional variance in the above relationships. The strength of all relationships remained similar with these variables added as covariates, with the maximum change in correlation size being Δr = 0.05 (or 25% of variance). A chi-square test of independence showed that there were no significant association between group and time of year tested, χ2 (3, N = 40) = 3.47, p = .325.
4. Discussion
This study investigated the relationship between various inflammatory markers and social-cognitive functioning in pwMS and demographically-matched healthy individuals. Our results highlight a novel role that the innate immune system may be linked to a disruption in social-cognitive functioning. Specifically, higher levels of serum IL-1β, IL-17, TNF-α, IP-10, MIP-1α, and urine IP-10, and MIP-1β were associated with poorer social-cognitive abilities relating to the detection of blatant lies and sarcasm in conversation, and poorer self-reported emotion recognition, emotional empathy, and perspective taking abilities. These immune markers are known as pro-inflammatory mediators in the context of immune dysregulation in MS or sickness behaviour in healthy individuals.
Surprisingly, two cytokines most associated with MS, IFN-γ and IL-17 in the serum, were not higher in pwMS than HCs, suggesting its pathological dysregulation may be limited to the brain and not the peripheral regions of the body (Stromnes et al., 2008; van Langelaar et al., 2018). However, we found a negative correlation between serum IL-1ra and IL-17, indicating inhibition of IL-17 production via the IL-1-IL-17 signalling axis by IL-1ra (Table 2) (Nakae et al., 2003). This possible explanation for the attenuated IL-17 is supported by findings of prior alternative research that serum concentration of IL-17 and IFN-γ, cytokines associated with cell-mediated innate immunity (helper T cells of the Th1 and Th17 axes), can be similar in people with relapsing-remitting MS (the type of MS characterising the majority of the current sample) and healthy individuals (Arellano et al., 2017; Ghaffari et al., 2017). Our result reflects a broader implication and link with immune regulators such as the aryl hydrocarbon receptor and interactions with the kynurenine pathway in modulating MS progression, relevant to our MS cohort (Bessede et al., 2014; Yan et al., 2010). It would be of interest to examine how the kynurenine pathway fits into our current hypothesis considering its role in both mood and immune regulation in MS (Tan et al., 2021).
A novel aspect of this study was collecting paired serum and urine samples, which allowed us to investigate unique immune fingerprints in compartmentalised biological systems. Interestingly, we found that healthy individuals excreted a greater concentration of inflammatory markers in urine than pwMS, whereas the corresponding serum levels were in opposing trend (Fig. 2A–F). Potentially, this discrepancy may either reflect differential urinary filtration processes (An and Gao, 2015), biomarker-specific fluctuation (Schenk et al., 2019), epitopic remnant accumulation that is theorised in autoimmune disease (Opdenakker et al., 2020), or is an artefact of poor urine quality from bladder dysfunction and reduced fluid intake (Katsavos and Anagnostouli, 2013). However, we controlled for creatinine levels to minimise potential confounder variables such as fluid consumption and age. Another possible explanation is based on the generalised assumption that increased serum metabolite concentration will result in increased urine metabolite excretion (Ritscher et al., 2020). However, while this may hold true for healthy individuals, in the context of MS or an alternative disease state, the possible incapacity to excrete these inflammatory mediators as efficiently as healthy individuals, may have resulted in these findings. Despite blood-based immune profiling being an established and widely used technique, the addition of a urinary immune profile may provide a feasible and non-invasive alternative technique to fully extrapolate the effects of inflammation in pwMS (Prasad et al., 2016). This approach of using urine samples may provide a new avenue for enabling longitudinal sampling in pwMS with greater disability levels.
In both pwMS and healthy individuals, we found higher concentration of numerous pro-inflammatory biomarkers to be associated with lower social-cognitive functioning (Fig. 3, Fig. 4). Social-cognitive processing recruits a unique neural network called the “social brain”, which includes the amygdala, insular cortex, superior temporal sulcus, anterior and posterior cingulate, temporoparietal junction, and ventromedial and orbitofrontal cortices (Wang et al., 2017). Our findings indicate that the social brain may be particularly vulnerable to pro-inflammatory processes related to innate immunity. Our results support current findings that inflammatory processes, or sickness behaviour, can negatively shape social perception (Grigoleit et al., 2011; Moieni et al., 2015), and negatively affect social-cognitive abilities (Bollen et al., 2017; Eisenberger et al., 2010; Hennessy et al., 2014). Thus, in pwMS, social cognition may be particularly affected, given that dysregulated inflammatory processes are a characteristic of MS.
As expected, in pwMS, we found elevated serum concentration of anti-inflammatory IL-1ra relates to better social-cognitive abilities, specifically improved sarcasm detection and better self-reported emotion recognition and emotional empathy (Fig. 5A and S4). These findings were in agreement with recent evidence in clinical and pre-clinical MS models, which suggest a multi-faceted role of the IL-1 system in MS pathophysiology, whereby IL-1ra is the only known endogenous neuroprotective antagonistic cytokine to downregulate the pro-inflammatory action of IL-1α/β (Musella et al., 2020). Furthermore, in MS, the results of prior pharmacological research reports that disease modifying therapies, such as glatiramer acetate, natalizumab, laquinimod, and interferon beta, can restore the inflammatory imbalance by increasing circulating concentration of IL-1ra; thus, reducing relapse rates and moderating the development of new brain lesions (Group, 1993; Jacobs et al., 1996; Nicoletti et al., 1996; Ruiz et al., 2019). Extending to other anti-inflammatory markers, IL-4 and IL-10, our findings were mixed (Table S3). Similar to our findings in IL-1ra, in pwMS, elevated serum concentrations of IL-4 and IL-10 were related to better self-reported social cognitive abilities. However, serum IL-4 and IL-10 were associated with poorer social-cognitive performance on objective assessment (TASIT-S; Honan et al., 2016). This contradictory finding may reflect the lack of concordance that is often seen between subjective and objective cognitive assessments (Honan et al., 2015). Nevertheless, it remains clinically valuable to evaluate one’s perception of functioning as this too can be predictive of functional outcomes (Honan et al., 2015). Together, our findings indicate that IL-1ra may be an important therapeutic target for improved social-cognitive functioning.
There are limitations associated with this pilot study. The small sample size means that we have not sufficiently captured other MS types and do not have the statistical power to examine the differences that might exist among different disease profiles. However, given our pilot results show promise, we would expect that a larger exploratory study with a broader cohort of participants will be able to more thoroughly examine the relationship between inflammatory biomarkers and social-cognitive functioning, and how this may be mediated or moderated by particular disease characteristics. Given that we examined pwMS who were not in a relapse phase, our findings are limited to inflammatory processes during remission phases. Future research may benefit from exploring pwMS during relapse phases and further extending to other MS subtypes. Comparing urinary-based immune markers to those in cerebrospinal fluid may also help identify surrogate biomarkers for interventionist strategies to regulate inflammation in individuals experiencing social-cognitive difficulties. For example, current pharmaceutical interventions may benefit from research into complementary medicines such as probiotics, vitamin D, and resveratrol that may reduce inflammation (Morshedi et al., 2019).
5. Conclusion
Overall, our findings highlight the important implications that inflammatory processes, sickness behaviour, and the innate immune system, have on everyday social-cognitive functioning. In pwMS, better social-cognitive performance was associated with higher serum concentration of IL-1ra. Thus, it may be possible to improve social-cognitive abilities by limiting inflammatory processes, and IL-1ra may be a potential therapeutic target for future clinical trials. In both pwMS and healthy individuals, poorer social-cognitive functioning was related to pro-inflammatory biomarkers with an innate immunity signature (i.e., IL-1β, IL-17, TNF-α, IP-10, and MIP-1α, and higher urine concentration of IP-10, and MIP-1β). However, the findings were mixed for IL-4 and IL-10, such that they were negatively associated with objective social-cognitive performance, and positively related to better self-reports of social-cognitive abilities. Further cross-sectional and longitudinal research examining relationships between inflammatory markers and social-cognitive functioning according to various disease-related factors (MS subtypes) and biological sample type (serum, urine, and cerebrospinal fluid) is warranted.
Declaration of competing interest
There are no known conflicts of interest reported.
Acknowledgement
The authors thank Professor Ingrid van der Mei from the Menzies Institute for Medical Research Hobart, MS Limited Tasmania, and Dr Kurien Koshy for assistance with participant recruitment. For assistance in the collection of behavioural data, we thank John Murphy (UTAS), Caitlin Turner (UTAS), Sarah Harms (UTAS), and Christina Kozlowski (MQ). We thank Dr Jeff Beckett (UTAS) and Roxanne Maher (UTAS) for the collection and processing of biological samples.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2021.100254.
Contributor Information
Jason A. Turner, Email: jturner7@utas.edu.au.
Christine Padgett, Email: Christine.Padgett@utas.edu.au.
Skye McDonald, Email: mcdonald@unsw.edu.au.
Kiran D.K. Ahuja, Email: kiran.ahuja@utas.edu.au.
Heather M. Francis, Email: heather.francis@mq.edu.au.
Chai K. Lim, Email: edwin.lim@mq.edu.au.
Cynthia A. Honan, Email: Cynthia.Honan@utas.edu.au.
Contributions
CAH, CKL, SM conceptualised and designed the study. JAT, CAH, CKL wrote the manuscript. CAH, HMF, KDKA, CKL were involved in data acquisition. JAT, CKL, CAH, and CP analysed and/or interpreted the data. All authors contributed to the review and approved the final version of the manuscript.
Study funding
The authors acknowledge the importance of funding from a Multiple Sclerosis Research Australia (MSRA) Incubator grant (2016–17) awarded to CAH, CKL, and SM.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Adams A.G., Henry J.D., Molenberghs P., Robinson G.A., Nott Z., von Hippel W. The relationship between social cognitive difficulties in the acute stages of stroke and later functional outcomes. Soc. Neurosci. 2019;1–12 doi: 10.1080/17470919.2019.1668845. [DOI] [PubMed] [Google Scholar]
- Allen R., Mage D., Gondy G., Christensen C., Barr D., Needham L. The use of a creatinine correction for reporting children’s urinary pesticide concentrations. Epidemiology. 2004;15(4):S69–S70. doi: 10.1038/sj.jea.7500343. https://journals.lww.com/epidem/Fulltext/2004/07000/the_Use_of_A_Creatinine_Correction_for_Reporting.171.aspx [DOI] [PubMed] [Google Scholar]
- An M., Gao Y. Urinary biomarkers of brain diseases. Dev. Reprod. Biol. 2015;13(6):345–354. doi: 10.1016/j.gpb.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arellano G., Acuña E., Reyes L.I., Ottum P.A., De Sarno P., Villarroel L., Ciampi E., Uribe-San Martín R., Cárcamo C., Naves R. Th1 and Th17 cells and associated cytokines discriminate among clinically isolated syndrome and multiple sclerosis phenotypes [original research] Front. Immunol. 2017;8(753) doi: 10.3389/fimmu.2017.00753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balashov K.E., Rottman J.B., Weiner H.L., Hancock W.W. CCR5(+) and CXCR3(+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc. Natl. Acad. Sci. U. S. A. 1999;96(12):6873–6878. doi: 10.1073/pnas.96.12.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S., Wheelwright S., Hill J., Raste Y., Plumb I. The “Reading the Mind in the Eyes” Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J. Child Psychol. Psychiatry. 2001;42(2):241–251. doi: 10.1111/1469-7610.00715. [DOI] [PubMed] [Google Scholar]
- Batista S., d’Almeida O.C., Afonso A., Freitas S., Macario C., Sousa L., Castelo-Branco M., Santana I., Cunha L. Impairment of social cognition in multiple sclerosis: amygdala atrophy is the main predictor. Mult. Scler. 2017;23(10):1358–1366. doi: 10.1177/1352458516680750. [DOI] [PubMed] [Google Scholar]
- Bessede A., Gargaro M., Pallotta M.T., Matino D., Servillo G., Brunacci C., Bicciato S., Mazza E.M.C., Macchiarulo A., Vacca C., Iannitti R., Tissi L., Volpi C., Belladonna M.L., Orabona C., Bianchi R., Lanz T.V., Platten M., Della Fazia M.A., Piobbico D., Zelante T., Funakoshi H., Nakamura T., Gilot D., Denison M.S., Guillemin G.J., DuHadaway J.B., Prendergast G.C., Metz R., Geffard M., Boon L., Pirro M., Iorio A., Veyret B., Romani L., Grohmann U., Fallarino F., Puccetti P. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature. 2014;511(7508):184–190. doi: 10.1038/nature13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bio-Rad Laboratories, Inc., 1000 Alfred Nobel Drive Hercules, California 94547, United States of America.
- Bollen J., Trick L., Llewellyn D., Dickens C. The effects of acute inflammation on cognitive functioning and emotional processing in humans: a systematic review of experimental studies. J. Psychosom. Res. 2017;94:47–55. doi: 10.1016/j.jpsychores.2017.01.002. [DOI] [PubMed] [Google Scholar]
- Bradley M.M., Lang P.J. Handbook of Emotion Elicitation and Assessment. Oxford University Press; 2007. The international affective picture system (IAPS) in the study of emotion and attention; pp. 29–46. [Google Scholar]
- Bramham J., Morris R.G., Hornak J., Bullock P., Polkey C.E. Social and emotional functioning following bilateral and unilateral neurosurgical prefrontal cortex lesions. J. Neuropsychol. 2009;3(Pt 1):125–143. doi: 10.1348/174866408x293994. [DOI] [PubMed] [Google Scholar]
- Breen E.J., Polaskova V., Khan A. Bead-based multiplex immuno-assays for cytokines, chemokines, growth factors and other analytes: median fluorescence intensities versus their derived absolute concentration values for statistical analysis. Cytokine. 2015;71(2):188–198. doi: 10.1016/j.cyto.2014.10.030. [DOI] [PubMed] [Google Scholar]
- Broux B., Pannemans K., Zhang X., Markovic-Plese S., Broekmans T., Eijnde B.O., Van Wijmeersch B., Somers V., Geusens P., van der Pol S., van Horssen J., Stinissen P., Hellings N. CX(3)CR1 drives cytotoxic CD4(+)CD28(-) T cells into the brain of multiple sclerosis patients. J. Autoimmun. 2012;38(1):10–19. doi: 10.1016/j.jaut.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Brown R.F., Tennant C.C., Sharrock M., Hodgkinson S., Dunn S.M., Pollard J.D. Relationship between stress and relapse in multiple sclerosis: Part I. Important features. Mult. Scler. 2006;12(4):453–464. doi: 10.1191/1352458506ms1295oa. [DOI] [PubMed] [Google Scholar]
- Caplan B., Bogner J., Brenner L., Manskow U.S., Sigurdardottir S., Røe C., Andelic N., Skandsen T., Damsgård E., Elmståhl S., Anke A. Factors affecting caregiver burden 1 Year after severe traumatic brain injury: a prospective nationwide multicenter study. J. Head Trauma Rehabil. 2015;30(6):411–423. doi: 10.1097/HTR.0000000000000085. [DOI] [PubMed] [Google Scholar]
- Carrieri P.B., Provitera V., De Rosa T., Tartaglia G., Gorga F., Perrella O. Profile of cerebrospinal fluid and serum cytokines in patients with relapsing-remitting multiple sclerosis: a correlation with clinical activity. Immunopharmacol. Immunotoxicol. 1998;20(3):373–382. doi: 10.3109/08923979809034820. [DOI] [PubMed] [Google Scholar]
- Chalah M.A., Ayache S.S. Deficits in social cognition: an unveiled signature of multiple sclerosis. J. Int. Neuropsychol. Soc. 2017;23(3):266–286. doi: 10.1017/S1355617716001156. [DOI] [PubMed] [Google Scholar]
- Cheng W., Chen G. Chemokines and chemokine receptors in multiple sclerosis. Mediat. Inflamm. 2014;2014 doi: 10.1155/2014/659206. 659206-659206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Academic press; 2013. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- Corporation I.B.M. IBM Corporation; Armonk, NY, United States of America.: 2019. IBM SPSS Statistics for Windows, Version 26.0. [Google Scholar]
- Cotter J., Firth J., Enzinger C., Kontopantelis E., Yung A., Drake R.J. Social cognition in multiple sclerosis: a systematic review and meta-analysis. Neurology. 2016;87:1727–1736. doi: 10.1212/WNL.0000000000003236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. A multidimensional approach to individual differences in empathy. JSAS Catalog Sel. Doc. Psychol. 1980;10 https://www.apa.org/pubs/journals/psp [Google Scholar]
- Dendrou C.A., Fugger L., Friese M.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015;15(9):545–558. doi: 10.1038/nri3871. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I., Inagaki T.K., Mashal N.M., Irwin M.R. Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain Behav. Immun. 2010;24(4):558–563. doi: 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genova H.M., Cagna C.J., Chiaravalloti N.D., DeLuca J., Lengenfelder J. Dynamic assessment of social cognition in individuals with multiple sclerosis: a pilot study. J. Int. Neuropsychol. Soc. 2016;22(1):83–88. doi: 10.1017/s1355617715001137. [DOI] [PubMed] [Google Scholar]
- Ghaffari S.A., Nemati M., Hajghani H., Ebrahimi H., Sheikhi A., Jafarzadeh A. Circulating concentrations of interleukin (IL)-17 in patients with multiple sclerosis: Evaluation of the effects of gender, treatment, disease patterns and IL-23 receptor gene polymorphisms. Iranian J. Neurol. 2017;16(1):15–25. https://pubmed.ncbi.nlm.nih.gov/28717429 [PMC free article] [PubMed] [Google Scholar]
- Grigoleit J.S., Kullmann J.S., Wolf O.T., Hammes F., Wegner A., Jablonowski S., Engler H., Gizewski E., Oberbeck R., Schedlowski M. Dose-dependent effects of endotoxin on neurobehavioral functions in humans. PloS One. 2011;6(12) doi: 10.1371/journal.pone.0028330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group T.I.M.S.S. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993;43(4):655–661. doi: 10.1212/wnl.43.4.655. [DOI] [PubMed] [Google Scholar]
- Hennessy M.B., Deak T., Schiml P.A. Sociality and sickness: have cytokines evolved to serve social functions beyond times of pathogen exposure? Brain Behav. Immun. 2014;37:15–20. doi: 10.1016/j.bbi.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry A., Tourbah A., Chaunu M.P., Rumbach L., Montreuil M., Bakchine S. Social cognition impairments in relapsing-remitting multiple sclerosis. J. Int. Neuropsychol. Soc. 2011;17(6):1122–1131. doi: 10.1017/S1355617711001147. [DOI] [PubMed] [Google Scholar]
- Hohol M.J., Orav E.J., Weiner H.L. Disease steps in multiple sclerosis: a longitudinal study comparing Disease Steps and EDSS to evaluate disease progression. Multiple Sclerosis J. 1999;5(5):349–354. doi: 10.1177/135245859900500508. [DOI] [PubMed] [Google Scholar]
- Honan C.A., Brown R.F., Batchelor J. Perceived cognitive difficulties and cognitive test performance as predictors of employment outcomes in people with multiple sclerosis. J. Int. Neuropsychol. Soc. 2015;21(2):156–168. doi: 10.1017/s1355617715000053. [DOI] [PubMed] [Google Scholar]
- Honan C.A., McDonald S., Sufani C., Hine D.W., Kumfor F. The awareness of social inference test: development of a shortened version for use in adults with acquired brain injury. Clin. Neuropsychol. 2016;30(2):243–264. doi: 10.1080/13854046.2015.1136691. [DOI] [PubMed] [Google Scholar]
- Jacobs L.D., Cookfair D.L., Rudick R.A., Herndon R.M., Richert J.R., Salazar A.M., Fischer J.S., Goodkin D.E., Granger C.V., Simon J.H., Alam J.J., Bartoszak D.M., Bourdette D.N., Braiman J., Brownscheidle C.M., Coats M.E., Cohan S.L., Dougherty D.S., Kinkel R.P., Mass M.K., Munschauer F.E., 3rd, Priore R.L., Pullicino P.M., Scherokman B.J., Whitham R.H. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG) Ann. Neurol. 1996;39(3):285–294. doi: 10.1002/ana.410390304. [DOI] [PubMed] [Google Scholar]
- Katsavos S., Anagnostouli M. Biomarkers in multiple sclerosis: an up-to-date overview. Multiple Sclerosis Int. 2013 doi: 10.1155/2013/340508. 340508-340508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothur K., Wienholt L., Brilot F., Dale R.C. CSF cytokines/chemokines as biomarkers in neuroinflammatory CNS disorders: a systematic review. Cytokine. 2016;77:227–237. doi: 10.1016/j.cyto.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Kullmann J.S., Grigoleit J.S., Wolf O.T., Engler H., Oberbeck R., Elsenbruch S., Forsting M., Schedlowski M., Gizewski E.R. Experimental human endotoxemia enhances brain activity during social cognition. Soc. Cognit. Affect Neurosci. 2014;9(6):786–793. doi: 10.1093/scan/nst049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenne B., Barthelemy G., Nandrino J., Sequeira H., Pinti A., Mecheri H., Kwiatkowski A., Hautecoeur P. Impaired recognition of facial emotional expression in multiple sclerosis. Neuropsychol. Trends. 2014;15:67–83. doi: 10.7358/neur-2014-015-lenn. [DOI] [Google Scholar]
- Lim C.K., Bilgin A., Lovejoy D.B., Tan V., Bustamante S., Taylor B.V., Bessede A., Brew B.J., Guillemin G.J. Kynurenine pathway metabolomics predicts and provides mechanistic insight into multiple sclerosis progression. Sci. Rep. 2017;7 doi: 10.1038/srep41473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald S., Honan C., Kelly M., Byom L., Rushby J. 2nd. Taylor & Francis Group; Oxfordshire, United Kingdom: 2013. Disorders of Social Cognition and Social Behaviour in Adults with TBI; pp. 119–159. [Google Scholar]
- McDonald S., Honan C., Allen S.K., El-Helou R., Kelly M., Kumfor F., Piguet O., Hazelton J.L., Padgett C., Keage H.A.D. Normal adult and adolescent performance on TASIT-S, a short version of the Assessment of Social Inference Test. Clin. Neuropsychol. 2018;32(4):700–719. doi: 10.1080/13854046.2017.1400106. 2018/05/19. [DOI] [PubMed] [Google Scholar]
- Moieni M., Eisenberger N.I. Effects of inflammation on social processes and implications for health. Ann. N. Y. Acad. Sci. 2018;1428(1):5–13. doi: 10.1111/nyas.13864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moieni M., Irwin M.R., Jevtic I., Breen E.C., Eisenberger N.I. Inflammation impairs social cognitive processing: a randomized controlled trial of endotoxin. Brain Behav. Immun. 2015;48:132–138. doi: 10.1016/j.bbi.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshedi M., Hashemi R., Moazzen S., Sahebkar A., Hosseinifard E.-S. Immunomodulatory and anti-inflammatory effects of probiotics in multiple sclerosis: a systematic review. J. Neuroinflammation. 2019;16(1):231. doi: 10.1186/s12974-019-1611-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musella A., Fresegna D., Rizzo F.R., Gentile A., De Vito F., Caioli S., Guadalupi L., Bruno A., Dolcetti E., Buttari F., Bullitta S., Vanni V., Centonze D., Mandolesi G. ‘Prototypical’ proinflammatory cytokine (IL-1) in multiple sclerosis: role in pathogenesis and therapeutic targeting. Expert Opin. Ther. Targets. 2020;24(1):37–46. doi: 10.1080/14728222.2020.1709823. [DOI] [PubMed] [Google Scholar]
- Nakae S., Saijo S., Horai R., Sudo K., Mori S., Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc. Natl. Acad. Sci. Unit. States Am. 2003;100(10):5986. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti F., Patti F., Marco R.D., Zaccone P., Nicoletti A., Meroni P., Reggio A. Circulating serum levels of IL-1ra in patients with relapsing remitting multiple sclerosis are normal during remission phases but significantly increased either during exacerbations or in response to IFN-β treatment. Cytokine. 1996;8(5):395–400. doi: 10.1006/cyto.1996.0054. [DOI] [PubMed] [Google Scholar]
- Novellino F., Saccà V., Donato A., Zaffino P., Spadea M.F., Vismara M., Arcidiacono B., Malara N., Presta I., Donato G. Innate immunity: a common denominator between neurodegenerative and neuropsychiatric diseases. Int. J. Mol. Sci. 2020;21(3):1115. doi: 10.3390/ijms21031115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opdenakker G., Van Damme J. Probing cytokines, chemokines and matrix metalloproteinases towards better immunotherapies of multiple sclerosis. Cytokine Growth Factor Rev. 2011;22(5):359–365. doi: 10.1016/j.cytogfr.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Opdenakker G., Abu El-Asrar A., Van Damme J. Remnant epitopes generating autoimmunity: from model to useful paradigm. Trends Immunol. 2020, May;41(5):367–378. doi: 10.1016/j.it.2020.03.004. [DOI] [PubMed] [Google Scholar]
- Pöttgen J., Dziobek I., Reh S., Heesen C., Gold S.M. Impaired social cognition in multiple sclerosis. J. Neurol. Neurosurg. Psychiatr. 2013;84(5):523. doi: 10.1136/jnnp-2012-304157. [DOI] [PubMed] [Google Scholar]
- Prasad S., Tyagi A.K., Aggarwal B.B. Detection of inflammatory biomarkers in saliva and urine: potential in diagnosis, prevention, and treatment for chronic diseases. Exp. Biol. Med. 2016;241(8):783–799. doi: 10.1177/1535370216638770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritscher S., Hoyer M., Georges C., Wunder C., Wallemacq P., Persu A., Obermüller N., Toennes S.W. Benefit of serum drug monitoring complementing urine analysis to assess adherence to antihypertensive drugs in first-line therapy. PloS One. 2020;15(8) doi: 10.1371/journal.pone.0237383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz F., Vigne S., Pot C. Resolution of inflammation during multiple sclerosis. Semin. Immunopathol. 2019;41(6):711–726. doi: 10.1007/s00281-019-00765-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust H., Kuhle J., Kappos L., Derfuss T. Severe exacerbation of relapsing-remitting multiple sclerosis after G-CSF therapy. Neurol. Neuroimmunol. Neuroinflam. 2016;3(2):e215. doi: 10.1212/nxi.0000000000000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpini E., Galimberti D., Baron P., Clerici R., Ronzoni M., Conti G., Scarlato G. IP-10 and MCP-1 levels in CSF and serum from multiple sclerosis patients with different clinical subtypes of the disease. J. Neurol. Sci. 2002;195(1):41–46. doi: 10.1016/s0022-510x(01)00680-3. [DOI] [PubMed] [Google Scholar]
- Schenk H.M., van Ockenburg S.L., Nawijn M.C., De Jonge P., Rosmalen J.G.M. Identification of inflammatory markers suitable for non-invasive, repeated measurement studies in biobehavioral research: a feasibility study. PloS One. 2019;14(9) doi: 10.1371/journal.pone.0221993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorenson M., Furst J., Mathews H., Jason L.A. Dysregulation of cytokine pathways in chronic fatigue syndrome and multiple sclerosis. Fatigue: Biomed. Health Behav. 2017;5(3):145–158. doi: 10.1080/21641846.2017.1335237. [DOI] [Google Scholar]
- Stromnes I.M., Cerretti L.M., Liggitt D., Harris R.A., Goverman J.M. Differential regulation of central nervous system autoimmunity by TH1 and TH17 cells. Nat. Med. 2008;14(3):337–342. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L.S.Y., Francis H.M., Lim C.K. Exploring the roles of tryptophan metabolism in MS beyond neuroinflammation and neurodegeneration: a paradigm shift to neuropsychiatric symptoms. Brain Behav. Immun. Health. 2021, March;12:100201. doi: 10.1016/j.bbih.2021.100201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A.J., Banwell B.L., Barkhof F., Carroll W.M., Coetzee T., Comi G., Correale J., Fazekas F., Filippi M., Freedman M.S., Fujihara K., Galetta S.L., Hartung H.P., Kappos L., Lublin F.D., Marrie R.A., Miller A.E., Miller D.H., Montalban X., Mowry E.M., Sorensen P.S., Tintoré M., Traboulsee A.L., Trojano M., Uitdehaag B.M.J., Vukusic S., Waubant E., Weinshenker B.G., Reingold S.C., Cohen J.A. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162–173. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- Turner M.D., Nedjai B., Hurst T., Pennington D.J. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta Mol. Cell Res. 2014;1843(11):2563–2582. doi: 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- van Langelaar J., van der Vuurst de Vries R.M., Janssen M., Wierenga-Wolf A.F., Spilt I.M., Siepman T.A., Dankers W., Verjans G.M.G.M., de Vries H.E., Lubberts E., Hintzen R.Q., van Luijn M.M. T helper 17.1 cells associate with multiple sclerosis disease activity: perspectives for early intervention. Brain. 2018;141(5):1334–1349. doi: 10.1093/brain/awy069. [DOI] [PubMed] [Google Scholar]
- Wang Y., Metoki A., Alm K.H., Olson I.R. bioRxiv; 2017. White Matter and Social Cognition. [DOI] [Google Scholar]
- Yan Y., Zhang G.X., Gran B., Fallarino F., Yu S., Li H., Cullimore M.L., Rostami A., Xu H. IDO upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. J. Immunol. 2010;185(10):5953–5961. doi: 10.4049/jimmunol.1001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, Jun;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.