Abstract
Following spinal cord injury, 18–26% of patients are diagnosed with depressive disorders, compared to 8–12% in the general population. As increased inflammation strongly correlates with depression in both animal and human studies, we hypothesized that the immune activation inherent to SCI could increase depression-like behavior. Thus, we proposed that reducing immune activation with minocycline, a microglial inhibitor, would decrease depression-like behavior following injury. Male Sprague-Dawley rats were given minocycline in their drinking water for 14 days following a moderate, mid-thoracic (T12) spinal contusion. An array of depression-like behaviors (social activity, sucrose preference, forced swim, open field activity) were examined prior to injury as well as on days 9–10, 19–20, and 29–30 post-injury. Peripheral cytokine levels were analyzed in serum collected prior to injury and 10 days post-injury. Hierarchical cluster analysis divided subjects into two groups based on behavior: depressed and not-depressed. Depressed subjects displayed lower levels of open field activity and social interaction relative to their not-depressed counterparts. Depressed subjects also showed significantly greater expression of pro-inflammatory cytokines both before and after injury and displayed lower levels of hippocampal neurogenesis than not-depressed subjects. Intriguingly, subjects who later showed depressive behaviors had higher baseline levels of the pro-inflammatory cytokine IL-6, which persisted throughout the duration of the experiment. Minocycline, however, did not affect serum cytokine levels and did not block the development of depression; equal numbers of minocycline versus vehicle-treated subjects appeared in both phenotypic groups. Despite this, these data overall suggest that molecular correlates of inflammation prior to injury could predict the development of depression after a physical stressor.
Keywords: Spinal cord injury, Depression, Inflammation, Minocycline, Rat model
1. Introduction
Approximately 22% of people with spinal cord injury (SCI) suffer from major depressive disorder (MDD) (Williams and Murray 2015), an incidence nearly three times greater than the 8.1% in the general US population (Brody et al., 2018). Further, 16–34% of people living with SCI report significant clinical symptoms of depression without meeting the criteria for MDD (Bombardier et al., 2012; Krause et al., 2000; Migliorini et al., 2009). These symptoms of depression significantly affect quality of life. Moreover, people with SCI and depression are more likely to develop urinary tract infections and pressure ulcers, and they tend to show lower levels of functional independence, experiencing greater unemployment and lower community involvement (Elliott and Frank 1996; Herrick et al., 1994). Depression after SCI clearly affects both physical and psychological wellbeing.
The increased incidence of depression after SCI may not be surprising considering the overwhelming life changes evoked by injury. However, depression-like behavior is also observed in animal models of SCI, in the absence of the socioeconomic challenges and changes in functional independence that may affect the clinical population. Approximately one-third of rats with SCI develop depression-like behavior, a percentage concomitant with the clinical population (Brakel et al., 2019; Luedtke et al., 2014; Maldonado-Bouchard et al., 2016; Wu et al., 2014b). Importantly, these rats do not exhibit depression-like behavior prior to injury (Brakel et al., 2019). This evidence of depression in animal models post-injury indicates that physiological or molecular changes inherent to SCI may contribute to the rise in depression. Specifically, we posit that inflammation after injury may underlie the development of this affective disorder.
There is strong evidence to support the premise of inflammation as a cause of depression. Inflammatory cytokines have not only been observed in depressed patients, they have also been shown to induce depression when administered therapeutically for other illnesses, such as cancer and hepatitis infection (Capuron et al. 2002, 2009). Diseases characterized by chronic inflammation, including multiple sclerosis, psoriasis, inflammatory bowel disease, and arthritis are also associated with increased likelihoods of developing depression (Jensen et al., 2016; Marrie 2017; Olivier et al., 2010; Siegert and Abernethy 2005). Importantly, inflammation is a hallmark of SCI, which produces a marked increase in the immune reaction in the spinal cord, periphery, and even the brain (Davies et al., 2007; Ferguson et al., 2004; Popovich et al., 1997; Wu et al. 2014a, 2014b). Further, elevated pro-inflammatory cytokines are observed in both the periphery and brain in rodent models of SCI-induced depression (do Espírito Santo et al., 2019; Maldonado-Bouchard et al., 2016; Wu et al., 2014a; Wu et al., 2014b). These data suggest that targeting inflammation may be an effective strategy in the treatment of depression.
Researchers have begun to investigate the potential of anti-inflammatory treatments as antidepressants. For example, in a preclinical rat model of depression after SCI, Farrell and Houle (2019) targeted tumor necrosis factor (TNF), a common inflammatory factor often found elevated in depression, with XPro1595, a soluble TNF inhibitor. Contrary to their hypothesis, they found that TNF inhibition increased the incidence of depression in female rats after SCI (Farrell and Houle 2019). However, they also saw an increase in NF-κB signaling with XPro1595 administration, suggesting that the synthesis of other cytokines may be increased with this treatment, which could contribute to the development of depression. Broader targeting of additional post-SCI inflammatory cytokines may be needed to block the development of depression after SCI (Farrell and Houle 2019).
In a clinical trial, Allison and Ditor (2015) also investigated the effects of an anti-inflammatory diet on depression in people with SCI. They found that patients on the anti-inflammatory diet had slightly lower depression scores and IL-1β levels (Allison and Ditor 2015). While neither of these studies provided conclusive evidence for a direct effect of anti-inflammatory treatment on depression outcomes, they suggest that multiple cytokines could act synergistically to induce depression and that a global inhibitor of inflammation may be effective in the SCI model.
Minocycline, a tetracycline antibiotic known for its anti-inflammatory properties and ability to cross the blood-brain barrier, has been the focus of a number of recent clinical trials. It has proven to be significantly better than placebo for the treatment of unipolar depression (Rosenblat and McIntyre 2018), although its efficacy has been called into question with a more recent clinical trial (Husain et al., 2020). Minocycline has also been shown to attenuate depression-like behavior in numerous animal models of depression, including the olfactory bulbectomised (OB) rat and in rats with depression induced with lipopolysaccharide (LPS) (Henry et al., 2008; O’Connor et al., 2009), restraint stress (Hinwood et al., 2013) and inescapable shock (Arakawa et al., 2012). The current experiment aimed to extend this data to a model of protracted inflammation, the rodent model of SCI. We hypothesized that oral administration of minocycline for two weeks after spinal cord injury could also reduce inflammation and block the development of depression-like symptoms.
2. Materials and methods
2.1. Subjects
Thirty-three young adult (2–3 months old), male Sprague Dawley rats served as subjects. Three subjects, one from each dose group, were subsequently removed from the study based on a priori exclusion criteria which included, 1) a baseline sucrose preference of at least 75%, 2) a BBB score <8 on day 1 post SCI, and 3) recovery of a minimum of 3 points on the BBB scale across the 28 day testing period. The 3 excluded subjects did not have baseline sucrose preferences of 75%. All subjects were single-housed on a 12-h light-dark cycle, with food and water available ad libitum. All of the experiments described here were reviewed and approved by the Institutional Animal Care and Use Committee at Texas A&M University and all NIH guidelines for the care and use of animal subjects were followed.
2.2. Surgery
Subjects were given a moderate contusion injury, as previously described (Brakel et al., 2019). Briefly, subjects were anesthetized with 5% isoflurane. When a surgical level of anesthesia was reached, the isoflurane concentration was lowered and maintained at 2–3%. A laminectomy (T12 vertebra) was performed, exposing the T13-L1 spinal cord. A moderate contusion was administered using an Infinite Horizons Impactor (Precision Systems and Instrumentation) to apply a 150 kDyne force with a 1 s dwell time to the exposed spinal cord. The surgical site was then closed with Michel clips. Immediately after injury, 3 ml of 0.9% sterile saline was administered subcutaneously to offset fluid loss from the surgery, and 100,000 units/kg of penicillin G (i.p.) were given to prevent infection. Animals recovered for 24 h in warm cages maintained at approximately 30 °C. After the injury, subjects’ bladders were manually expressed twice daily (6:00–8:30 and 16:30–18:00) until they voided on their own for three consecutive days.
2.3. Minocycline administration
Subjects were randomly assigned to three groups and administered minocycline (minocycline hydrochloride; Sigma-Aldrich, St. Louis, MO, USA) through their drinking water (0, 0.33, or 1 mg/ml; n = 10 for each) for the first 14 days post-injury. Water bottles were weighed every morning to track the liquid consumption of each animal, and the minocycline solution was replaced every 7 days. The oral route of minocycline was chosen to more closely simulate clinical administration, as well to avoid additional stress with repeated i.p. injections of the drug. High performance liquid chromatography data suggests that there is minimal degradation of minocycline in solution at room temperature over 7 days (Pearson and Trissel 1993).
2.4. Timeline overview
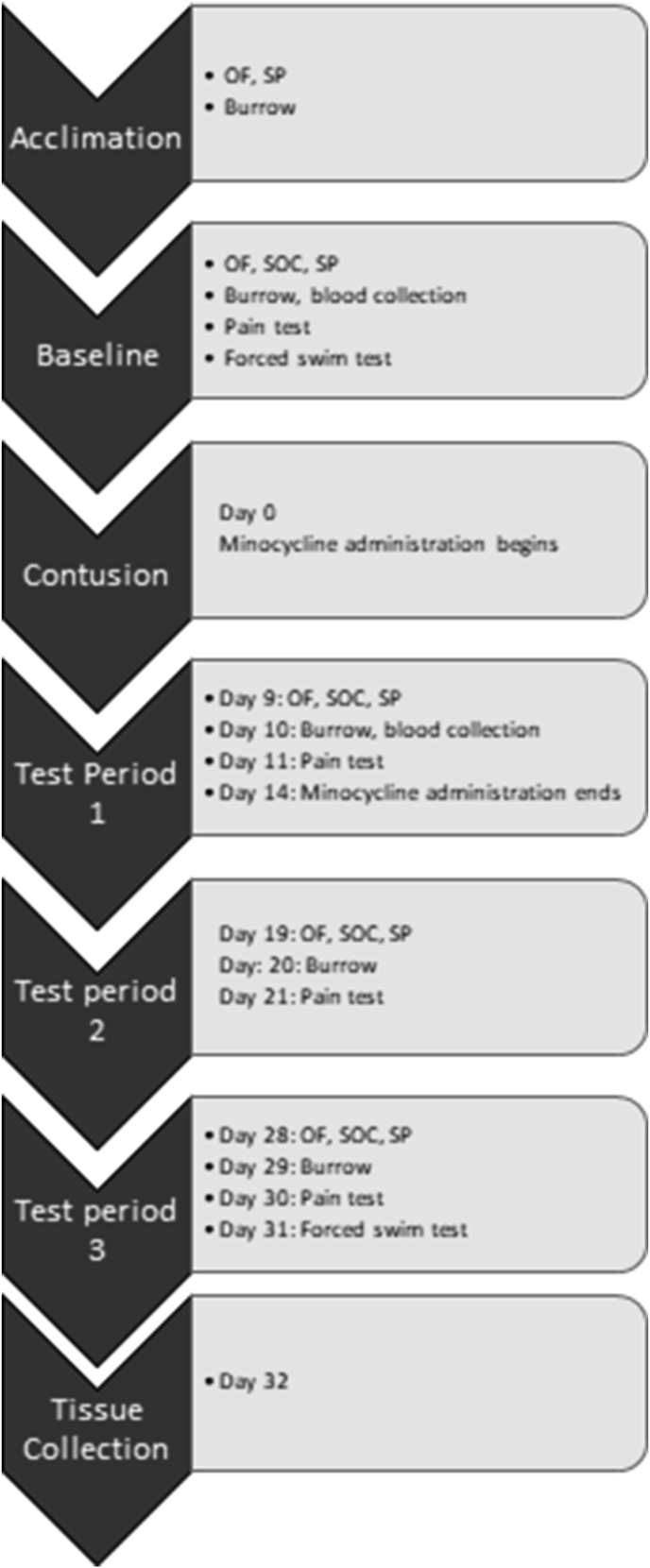
The timeline for this experiment is shown in Fig. 1. We used an array of tests commonly used to assess psychological well-being in rodents (Brakel et al., 2019; Luedtke et al., 2014; Maldonado-Bouchard et al., 2016). These tests have been associated with common clinical signs of depression such as anhedonia, fatigue, social isolation, and suicidal ideation. Subjects were acclimated to the behavioral tests of depression for one week prior to baseline testing. Baseline tests of depression were then conducted one week prior to surgery. Depression behaviors were re-assessed on days 9–11, 19–21, and 28–30 post-injury. The forced swim test was conducted only on Day 31 post-injury to prevent habituation to the task. Blood serum was also collected before and after injury, in conjunction with the baseline and testing phases. Behavioral testing was conducted during the animals’ light cycle.
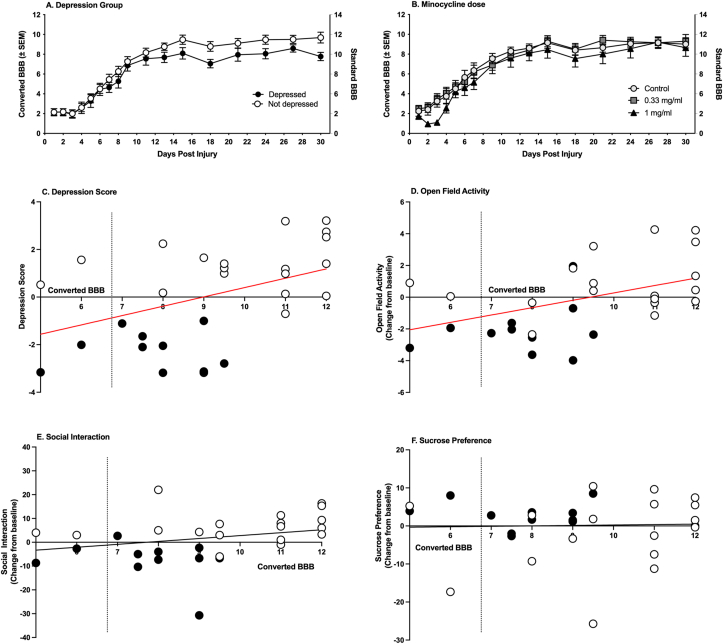
Fig. 1.
Behavioral testing schedule. Subjects were acclimated to the open field (OF)/social (SOC) arena, sucrose preference (SP) test, and burrowing tubes for 10 days. Baseline testing was conducted the week preceding the contusion injury. Minocycline was administered for 14 days immediately following SCI. Depression behavior tests, pain sensitivity assays, and serum collection were repeated in 3 testing periods post-injury. Spinal and brain tissue was collected 32 days post-injury.
2.5. Tests of depression-like behavior
2.5.1. Sucrose preference
Anhedonia was measured with the sucrose preference test. Subjects were first acclimated to a 2% sucrose solution until a minimum of a 75% sucrose preference was established. For testing, the subjects were presented with two bottles, containing 2% sucrose or water, in their home cages for 2 h. The positions of the bottles were reversed after 1 h to control for any side-preference bias. Bottles were weighed before and after the 2-h test, and sucrose preference was calculated as the percent sucrose consumed out of the total liquid (sucrose plus water) consumption. A decrease in sucrose preference is indicative of anhedonia, or a loss of interest in previously pleasurable activities (Casarotto and Andreatini 2007; Willner et al., 1987).
2.5.2. Open field activity
Subjects were placed in a 90 × 60 × 20 cm black box and allowed to explore for 5 min. Ethovision video tracking software was used to measure the total distance traveled over the time as well as the total time spent in the center of the arena (defined as ≥10 cm from a wall of the arena) by experimenters blinded to condition. Open field activity is a common measure of psychomotor retardation or fatigue (Bison et al., 2011; El Arfani et al., 2017).
2.5.3. Social interaction
In the social interaction test, subjects were placed in a 90 × 60 × 20 cm black box with a novel, uninjured rat of the same age and approximate weight. The time the SCI subject spent initiating social contact (sniffing, grooming, or pursuing the other animal) was measured and calculated as a percent of the total 5-min test session by experimenters blinded to condition. Decreased social interaction indicates anhedonia (Yirmiya 1996).
2.5.4. Burrowing
Burrowing was used as another measure of fatigue and anhedonia (Deacon 2006). A 10 (inner diameter) x 30 (height) cm capped PVC pipe filled with wood chips was placed into the subject’s home cage for 2 h. The percent of wood chips displaced by burrowing was measured by weighing the tube before and after the task.
2.5.5. Forced swim
In the forced swim tests, the rats were released into a clear plastic container (73 × 40 × 46.4 cm) filled with 28 cm of 23 °C water. The rat was filmed for 10 min and then removed and dried off. The time the rat spent immobile, operationalized as floating in the water without struggling and making only the necessary movements to keep its head above water, was determined by experimenters blinded to condition with post hoc video analyses. Greater immobility is associated with the clinical symptoms of hopelessness and suicidal ideation (Castagné et al., 2010; Hernández and Fernández-Guasti 2018).
2.6. Assessment of locomotor recovery
The recovery of hindlimb function was scored using the Basso, Beattie, and Bresnahan (BBB) scale (Basso et al., 1995). A numerical assignment on this scale corresponds to specific milestones in locomotor recovery. For example, 0 indicates no hind limb movement, 1 is slight movement of one or two joints, 10 is occasional stepping with no coordination, and 21 is perfect stepping with perfect coordination between the fore- and hindlimbs (i.e. uninjured stepping). After injury, the locomotor capacity (BBB) of subjects was observed for 5 min and scored by a trained observer on days 1–7, 9, 11, 13, 15, 18, 21, 24, and 27 post-SCI. BBB scores were transformed to help assure that the data were amendable to parametric analyses (Ferguson et al., 2004). This transformation pools BBB scores 2–4, removing a discontinuity in the scale, and scores 14–21, which are very seldom used under the present injury parameters. By pooling these scores, an ordered scale is created that is relatively continuous, with units of approximately equivalent intervals. Meeting these criteria allows for application of metric operations (computation of mean performance across legs), improves the justification for parametric statistical analyses, and increases statistical power.
2.7. Assessment of sensory function
At-level allodynia was assessed using the girdle test before injury and on days 4, 11, 22, and 28 post-injury. Animals were acclimated to the testing room for 10 min and gently handled to prevent unnecessary anxiety. During testing, the subjects were loosely held to ensure they were calm and did not vocalize because of stress. A von Frey filament (Semmes–Weinstein Anesthesiometer, Stoelting Co., Chicago, IL) with a bending force of 204.14 mN (26g force) was applied to a 4 × 11 grid across the girdle region of each subject. Because animals do not normally vocalize with this stimulus, a vocalization response indicates that a noxious stimulus was experienced. Vocalization responses were recorded and mapped onto a grid map for each animal. The number of vocalizations (Nv) were reported as a percent of the total stimulation events, given the following formula: (NV x 100)/total number of filament applications (44).
Below-level pain reactivity was assessed with von Frey filaments. Subjects were placed into restraining tubes (7.00 cm [internal diameter] × 20.00 cm [length]) to allow their legs to hang freely from the tube. After 15 min of acclimation to the tube alone, mechanical reactivity was assessed using von Frey stimulation. Nylon monofilaments (Semmes–Weinstein Anesthesiometer, Stoelting Co., Chicago, IL) of increasing strength were applied sequentially at approximately 2 s intervals to the L5 dermatome on the plantar surface of the hind paws. Stimuli were presented until subjects exhibited a motor withdrawal (spinal) and vocal (supraspinal) response. If one or both responses (motor and vocal) were not observed, testing was terminated at a force of 300 g. Each subject was tested twice on each foot in a counterbalanced ABBA order.
Thermal reactivity was assessed with the tail flick test. Subjects were restrained in plexiglass tubes (7.00 cm [internal diameter] × 20.00 cm [length]) and placed on the tail-flick apparatus (IITC Life Science Inc., Woodland Hills, CA, USA). Their tails were positioned in the 0.5 cm deep groove on the apparatus so that the area 1.5 inches from the tip of the tail was directly under a thermal light source. The light was set at an intensity that produced a 3–4 s tail-flick latency in an uninjured rat. The latency to flick the tail away from the radiant heat source was recorded. Two tests occurred at 2-min intervals.
2.8. Tissue collection
At 32 days post-injury, subjects were euthanized with pentobarbital (100 mg/kg, i.p.). All tissue collection occurred rapidly after the animal reached a deep, unresponsive level of anesthesia. Fifteen of the thirty remaining subjects were perfused with 4% paraformaldehyde (PFA) for histological reconstruction of the lesion and analysis of hippocampal neurogenesis. Whole brains and a 1.5 cm section of spinal cord, centered on the lesion site, were extracted and placed in ice-cold 4% PFA for 24 h for further fixation. After 24 h, the tissue was transferred to a 30% sucrose solution for cryoprotection and preservation before freezing and sectioning for histology.
Fresh brain and spinal tissue were collected from the remaining fifteen subjects for protein analysis. A 1.5 cm section of spinal cord, centered on the lesion site, and the brain were collected and flash-frozen in liquid nitrogen. The frontal cortex, hypothalamus, and hippocampi were dissected and flash-frozen separately. The frozen tissue was stored at −80 °C until processing.
2.9. Histological lesion assessment
To examine lesion size, frozen spinal cord segments were sectioned from the rostral to the caudal end in 20 μm thick sections, and every 10th slice was preserved for staining. All sections were stained with cresyl violet for Nissl substance and luxol fast blue for myelin.
The total cross-sectional area of the cord and spared tissue was assessed at the lesion center (averaged across three sections −600, 0, and +600 μm from the lesion center) using MicroBrightField software (MBF Bioscience, Williston, VT). Four indices of lesion magnitude were derived: lesion; residual gray matter (GM); residual white matter (WM); and width. As described by Grau et al. (2004), an experimenter blind to the experimental treatments determined the lesion extent by tracing around the boundaries of cystic formations and areas of dense gliosis. Residual GM was defined as Nissl-stained areas that contained neurons and glia of approximately normal densities. WM was defined as spared myelin-stained areas lacking dense gliosis and swollen fibers.
The total area of each cross-section was derived by summing the areas of damage, GM, and WM. The width of each section was then determined from the most lateral points along the transverse plane. These analyses yielded six parameters for each section: WM area, GM area, spared tissue (white + gray), damaged tissue area, net area (white + gray + damage), and section width. To control for variability in section area across subjects, we applied a correction factor derived from standard undamaged cord sections, taken from age-matched controls (Grau et al., 2004). This correction factor is based on section widths and is multiplied by all area measurements to standardize area across analyses. By standardizing area across sections, we were able to estimate the degree to which tissue was ‘‘missing’’ (i.e., tissue loss from atrophy, necrosis, or apoptosis). An accurate assessment of the degree to which a spinal cord has been affected includes both the remaining ‘‘damaged’’ tissue as well as resolved lesioned areas. When we sum the amount of missing tissue and the measured damaged area, we derive an index of the relative lesion (percent relative lesion) in each section that is comparable across sections. We also computed the percent of GM and WM remaining in each section, relative to intact spinal cords.
2.10. Cytokine and protein assays
Blood was collected for serum cytokine assays, via a saphenous vein draw, during the last 2 h of the light cycle. Samples were collected three days before injury and 10 days after injury. Prior to collection, the leg (alternated across collections) was shaved to expose the saphenous vein. The animal was then gently restrained in a towel while the leg was disinfected and treated with topical lidocaine. The saphenous vein was firmly held above the collection site and punctured with a 20-gauge needle. Blood was collected in 300 μl serum microvettes (Sarsvedt) coated with clotting factors. Samples were allowed to clot for 30–60 min, per manufacturer instructions, and then centrifuged at 2000×g for 15 min at 4 °C. The resulting serum was used in a multiplex assay (RECYMAG65K27PMX, Millipore) to assess 27 unique pro-inflammatory cytokines and chemokines. All samples were run in duplicate, following the manufacturer’s instructions.
The frozen frontal cortex and hippocampus samples were homogenized in 500 μl of lysis buffer (100 mM Tris, 1 M NaCl, 4 mM EDTA, 2% Triton X-100, 0.1% sodium azide, 5 μl/ml phenylmethyl-sulphonyl fluoride, and protease inhibitor cocktail (Thermo Scientific, 78442) and allowed to sit on ice for 10 min before centrifugation. The resulting supernatant was collected and stored at −20 °C for later use.
A rat BDNF ELISA (CYT306, Millipore) was used to detect BDNF levels in the hippocampus and frontal cortex of 12 subjects. Samples in this assay were diluted to 0.25 mg/ml total protein with the included sample diluent, and the assay was conducted according to the manufacturer instructions.
A serotonin (5-HT) ELISA (IBL-America, IB89540) was used to detect 5-HT levels in the hippocampus and frontal cortex of 12 subjects. Samples were diluted 1:30 (0.9 mg/ml) with the included sample diluent, and the manufacturer instructions were followed.
2.11. Immunofluorescent staining for neurogenesis
Hippocampal neurogenesis was also assessed as a marker of depression. Decreased neurogenesis has been observed in multiple models of stress and depression, but anti-depressants consistently restore normal neurogenesis, giving rise to its validity as at least a marker, and possibly a causative agent, of depression (Malberg and Duman 2003). After perfusion and whole brain collection, the brains were embedded in sectioning compound and frozen. The whole brain was sectioned into twelve serial sets of free floating, coronal sections (50 μm thick), using a freezing microtome.
Fifteen subjects were included in these analyses. Four sections of the rostral hippocampus were selected for each subject and stained with goat anti-DCX C-18 and DCX N-19 (sc-8066 and sc-8067, respectively, Santa Cruz Biotechnology). The sections were washed 3 times in 0.01 M PBS and then transferred to the primary antibody solution (0.2% DCX N, 0.2% DCX C, 0.5% Tween, 5% normal horse serum, in 0.01 M PBS). They incubated at room temperature with rotation for 23 h. The sections were washed again and incubated in secondary stain (0.5% donkey anti-goat IgG 555, 5% Tween, 5% normal horse serum, in 0.01 M PBS) for 1.2 h. Sections were then rinsed and mounted on slides. They were allowed to dry overnight and coverslipped with Vectashield anti-fade mounting medium (H-1400, Vectashield, Burlingame, CA).
DCX + cells in the suprapyramidal (upper) and infrapyramidal (lower) blades of the dentate gyrus of each section were counted using Stereo Investigator (MBF life sciences), using a 25% sampling rate, a 70 × 70 μm frame, and a dissector height of 15 μm. The cell count per area for upper and lower blades was calculated separately for each subject and the mean value for the upper blades and lower blades (across 4 sections) was found for each subject.
2.12. Statistical analyses
2.12.1. Assessment of depression-like behavior and minocycline dose
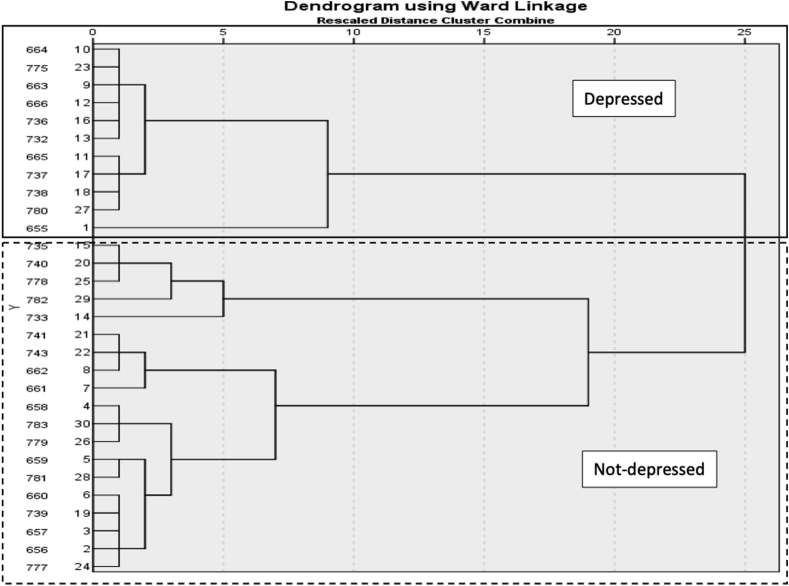
Hierarchical cluster analysis divided the sample population into phenotypic clusters that exhibited similar behaviors across tasks. Specifically, change from baseline scores collected on Day 29–30 post-injury (ensuring that minocycline had cleared the system) for sucrose preference, open field and social activity were first standardized by z scores. Then, hierarchical cluster analysis was performed using Ward’s method and applying squared Euclidean distance as the distance measure. The number of appropriate clusters was obtained by looking for a break in the agglomeration coefficient change and by observing the dendrogram (shown in Fig. 2), which visually depicts the distance between linked clusters. Two clusters were identified and based on lower open field and social activity scores, the first cluster was termed “depressed” and the second “not-depressed.” Repeated measures ANOVAs and post hoc unpaired Welch’s T-tests were subsequently used to compare the behavior of depressed and not-depressed groups at each time point, as well as to compare serum cytokine levels and brain 5-HT and BDNF levels. Repeated measures ANOVAs were used to examine recovery of locomotor and sensory function across depression and minocycline dose groups.
Fig. 2.
Hierarchical clustering dendrogram. Hierarchical clustering of subjects based off of change-from-baseline behavior (standardized by z scores) at days 29–30 resulted in two major clusters. The smaller group displayed more depression-like behaviors and was named “Depressed.” Each line on the far left of the dendrogram represents one subject. The relatedness of clusters is represented by the length of the horizontal lines extending from their link—longer lines are more distantly related.
A two-group discriminant function analysis was also performed to develop an equation that could be used to examine the relationships between depression, motor and sensory function, and cytokine expression. Discriminant analysis is a statistical test used to determine which continuous variables best distinguish two or more naturally occurring groups. It generates a function using an optimal combination of the variables and coefficients that can be used to predict group membership. Again, using Day 29–30 sucrose preference, open field, and social activity, the discriminant function analysis compared the depressed and not-depressed groups. The structure matrix produced was examined. The canonical discriminant function coefficients were retained for the depression score equation.
2.12.2. Correlations between cytokines and behavior
Acknowledging cytokine redundancy, principal component analyses were used to generate principal component scores, derived from the linear combination of cytokines that differed significantly between depressed and not-depressed groups. Separate analyses were conducted at baseline and on Day 10, respectively, to generate scores for each groups of differential cytokines. Pearson product moment correlations were used to examine the relationships between the derived principal components and behavior. Based on the a priori prediction that increased cytokines would be associated with increased depression-like behavior and decreased recovery of motor and sensory function, one-tailed tests were used to determine statistical significance.
2.12.3. Analysis of neurogenesis
As the data for neurogenesis were not normally distributed, Mann-Whitney tests and Kruskal-Wallis tests were used to compare the numbers of DCX + cells in the depressed and not-depressed groups, as well as the minocycline dose groups. Based on substantial data indicating that depression is associated with decreased neurogenesis, as well as the small sample size, a one-tailed test was used to determine significance. All statistical analyses were conducted with SPSS software, version 27.0.
3. Results
3.1. One-third of SCI rats develop depression-like behavior
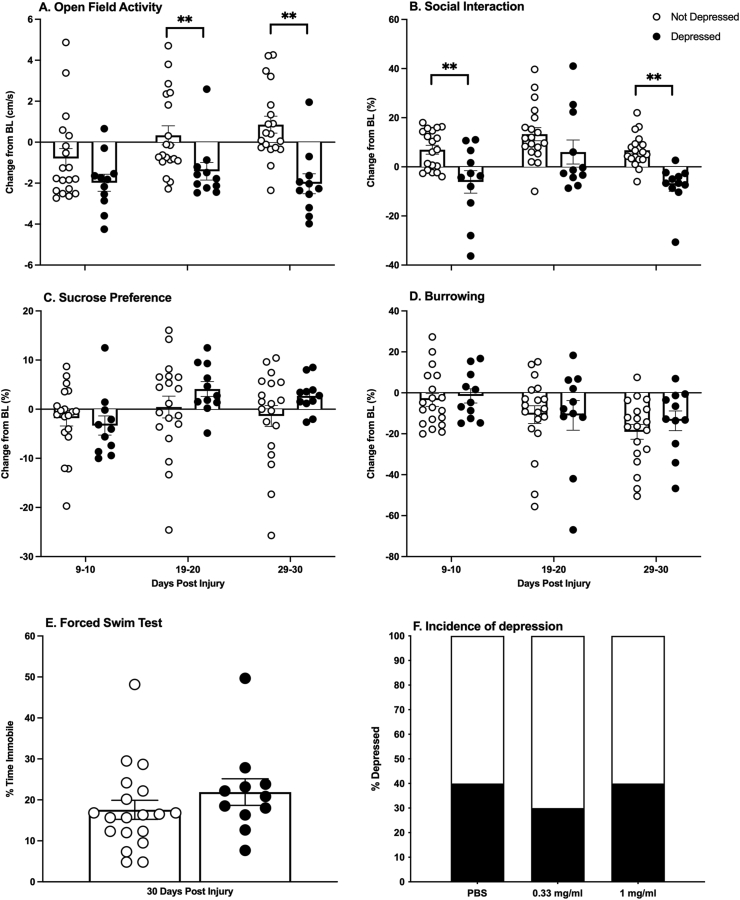
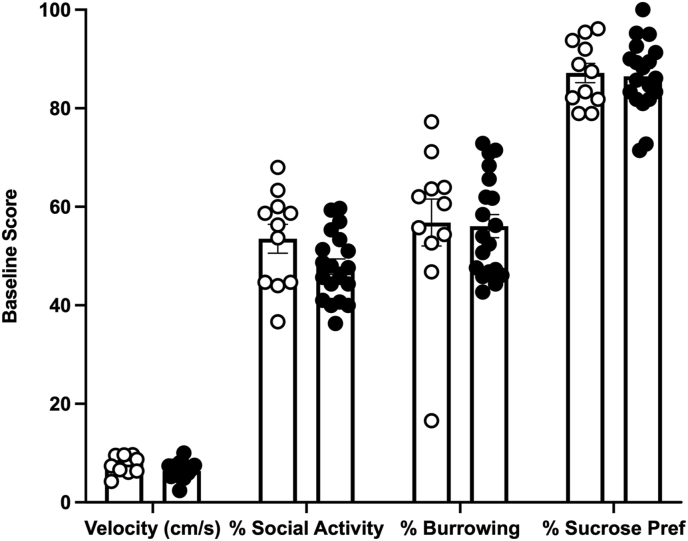
After injury, 33% of the subjects exhibited depression-like behavior. Unrestricted hierarchical cluster analyses showed that the subjects separated into two groups (Fig. 2). These groups were categorized as depressed or not-depressed with repeated measures ANOVAs. Comparing performances on each of the depression tests, there was a main effect of depression group on open field activity (F (1, 28) = 15.27, p = 0.0005), as well as an effect of time (F (1.809, 50.66) = 7.842, p = 0.002), and an interaction between depression group and time (F (2, 56) = 7.84, p = 0.003). The depressed group had lower open field activity on Days 19–20 and 29–30 post-injury (t (28) = −2.52, p = 0.018; t (28) = −4.38, p < 0.001, respectively, Fig. 3A). Whereas the not-depressed group increased activity in the open field over sessions, the depressed group remained low at all timepoints. There was also a main effect of depression group (F (1, 28) = 9.474, p = 0.005) and time on social interaction (F (1.92, 53.64) = 9.98, p = 0.0003), but no depression group by time interaction. Subjects classified as depressed displayed lower social interaction on days 9–10 and 29–30 (t (28) = −3.14, p = 0.004; t (28) = −5.17, p < 0.001, respectively, Fig. 3B). There was also a trend for higher immobility on the forced swim test in this cluster of subjects, but it was not statistically significant (Fig. 3E). Depression-like behaviors did not differ across clusters prior to injury (Fig. 4).
Fig. 3.
Depression behaviors. Animals that clustered in the depressed group explored an open field arena less than their not-depressed counterparts (A). They also initiated social interaction with a novel conspecific less than the not-depressed group (B). They did not differ significantly on any other behavioral tests (C–E). All behaviors but forced swim are presented as change-from-baseline. There were no differences in the incidence of depression between the dose groups (F). Depressed n = 11, Not depressed n = 19; Mean ± SEM, ∗∗p < 0.05.
Fig. 4.
Baseline behavioral scores. There were no baseline behavioral differences between the subjects that later clustered into the depressed and not-depressed groups. Depressed n = 11, Not depressed n = 19; Mean ± SEM.
Comparing the incidence of depression across minocycline dose groups, minocycline did not block the development of depression-like behavior. Repeated measures ANOVAs revealed no effects of dose on any of the behaviors assessed (F < 1.0 for all tests, p > 0.05). Thirty-three to forty percent of each dose group developed depression-like behaviors (Fig. 3F).
There was no significant difference in the amount of water (with or without minocycline) consumed across the 14 days of administration (F (2,27) = 1.97, p = 0.159). All minocycline dose groups consumed similar amounts of liquid, resulting in an average of 11.9 ± 0.8 mg of minocycline per day for the low dose group and 30.5 ± 2.8 mg/day for the high dose. Compared to previous published studies, this consumption should be adequate to create an anti-inflammatory effect (Burke et al., 2014; Camargos et al., 2020; Hinwood et al. 2012, 2013; Molina-Hernández et al., 2008).
Recognizing that depression is not categorical, and occurs on a continuum, a discriminant function analyses was also used to generate an equation and depression scores that could be correlated with other continuous behavioral and molecular outcomes. As for the cluster analysis, sucrose preference, open-field activity, and social exploration scores on Day 29–30 were used to define the depressed and not-depressed groups. Using the canonical discriminant function coefficients, the following depression equation was generated:
| Depression score = −0.09 - 0.071SP+ 0.5170FA+ 0.131SOC |
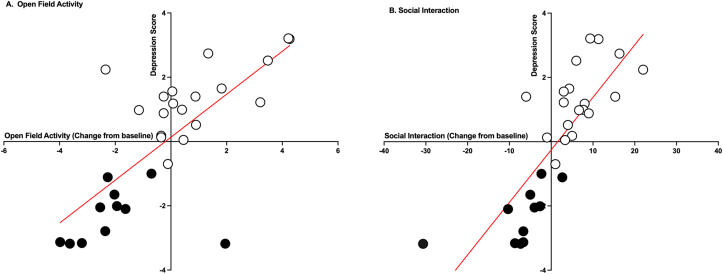
where SP is the change from baseline for sucrose preference, SOC is change from baseline for social exploration, and OFA is change from baseline for open-field activity for Day 29–30 post injury. Wilk’s lambda tests confirmed that the depressed and not-depressed clusters did not have identical means, and the equation was acceptable for classifying subjects (λ = 0.232; p < 0.001). Classification with the equation concurred with the cluster analysis, correctly classifying 96.7% of subjects as either depressed or not-depressed. The depression scores generated were highly correlated with Day 29–30 scores for open field activity and social activity (r (30) = 0.73, 0.80, respectively, p < 0.001, Fig. 5), but did not correlate with any of the other tests of depression-like behavior.
Fig. 5.
Depression scores correlations. The depression scores correlated with open field activity change-from-baseline scores at Day 29; r (30) = 0.73, p < 0.001 (A) and social activity change-from-baseline scores at Day 30; r (30) = 0.80, p < 0.001 (B). Not-depressed subjects (shown with the white circles) had higher scores than depressed subjects (represented by the black circles) for both behaviors.
3.2. Effects of depression on functional recovery
Overall, there was no significant effect of depression on locomotor recovery as assessed with the BBB scale (F (1,28) = 1.94, p = 0.175, Fig. 6A). However, BBB scores collected on Day 30 were significantly correlated with depression scores calculated with the discriminant function analyses (r (30) = 0.44, p < 0.01). Despite recovery of weight support (Converted BBB score = 7), lower BBB scores were associated with increased depression-like behavior (Fig. 6C). BBB scores on day 30 were also significantly correlated with open field activity on Day 29–30 (r (30) = 0.47, p < 0.01, Fig. 6D), but not with social behavior or sucrose preference (r (30) = 0.28, 0.03 respectively, p < 0.05, Fig. 6E and F), tests that were also used to categorize the subjects and derive depression scores. Oral minocycline treatment did not affect locomotor recovery across the 30 days post injury (F (2, 27) = 0.92, p = 0.409, Fig. 6B).
Fig. 6.
Locomotor recovery. Neither depression (A) nor minocycline (B) significantly affected locomotor recovery. All groups achieved stepping by Day 9, when post-injury behavioral depression testing began. However, depression scores (C) and open field activity scores (D) were correlated with BBB scores at Day 30 post injury (r (30) = 0.44, r (30) = 0.47, respectively, p < 0.01). The red line-of-best fit denotes a significant correlation. BBB scores were not correlated with social behavior (E) or sucrose preference (F). Depressed (black circles) n = 11, Not depressed (white circles) n = 19; Control n = 10, 0.33 mg/ml n = 10, 1 mg/ml n = 10. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Sensory scores did not differ between depressed and not-depressed animals, indicating that pain was unlikely to have played a role in the development of depression-like symptoms. There was also no effect of minocycline on any of the tests of thermal or mechanical reactivity. There was no correlation between depression scores or BBB scores, collected on Day 30, and sensory function.
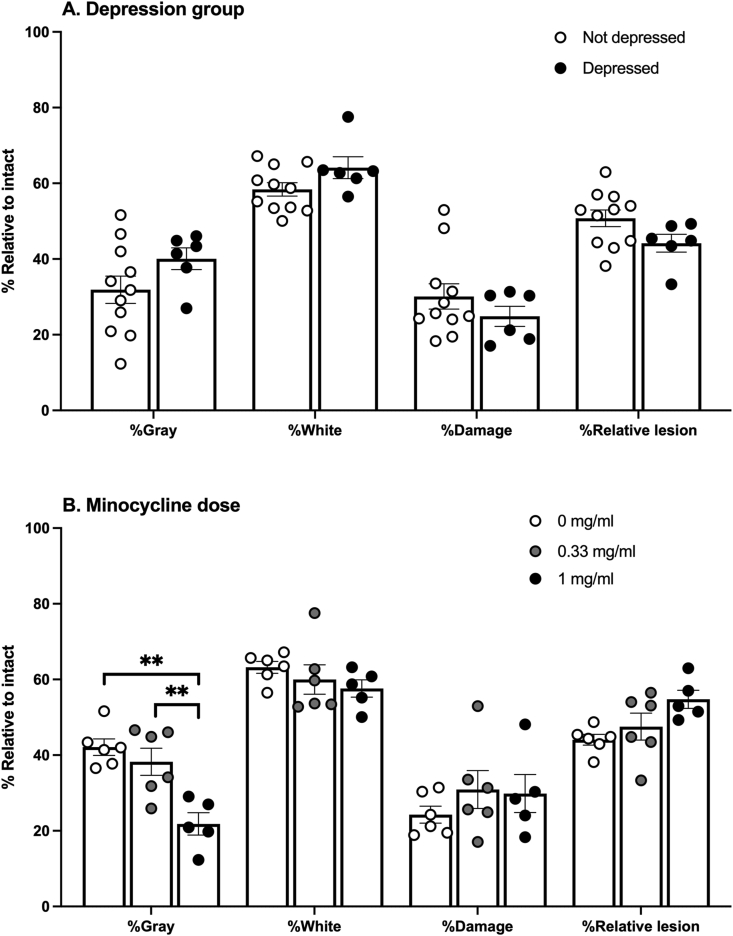
3.3. Neither depression nor minocycline affected lesion size
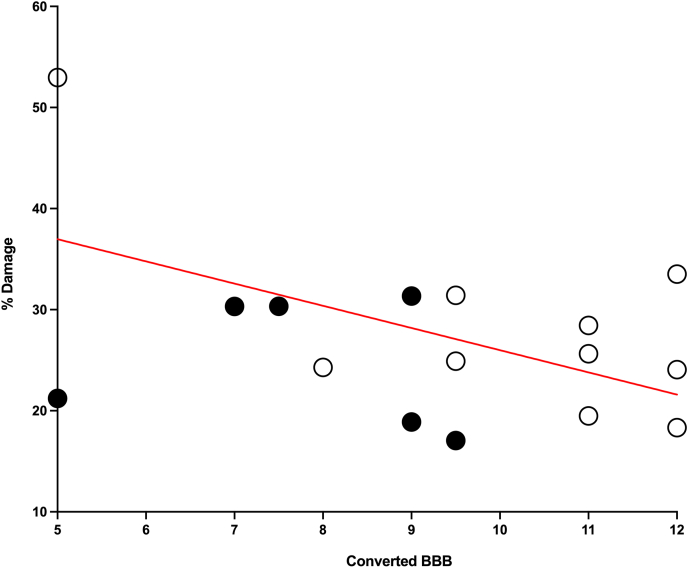
Replicating previous studies, % Damage was significantly correlated with Day 30 BBB scores (r (17) = −0.56, p < 0.05, Fig. 7). However, there were no differences in lesion size, gray matter, and white matter sparing between the depressed and not-depressed groups (Fig. 8A). Additionally, despite its reported neuroprotective properties, oral minocycline did not impact lesion size or spared white matter. Conversely, the high dose minocycline group had less spared gray matter than the vehicle controls, at the center of the lesion (F (2,14) = 6.06, p = 0.013, Fig. 8B).
Fig. 7.
Percent damage and recovery. Percent damage at the center of the spinal cord lesion was negatively correlated with BBB scores on Day 30 post injury (r (17) = −0.56, p < 0.05). Black circles represent subjects from the depressed group, and white circles represent subjects from the not-depressed group.
Fig. 8.
Spinal cord tissue damage in depression groups and minocycline groups. Percent spared gray and white matter, percent damage, and percent relative lesion size did not differ between depressed and not-depressed subjects (A). Subjects that received 1 mg/ml of minocycline had lower levels of spared gray matter compared to 0.33 mg/ml subjects and control subjects (B). Depressed n = 6, Not depressed n = 12; Control n = 6, 0.33 mg/ml n = 6, 1 mg/ml n = 5; Mean ± SEM, ∗∗p < 0.05.
3.4. Subjects that develop depression have elevated pro-inflammatory cytokine profiles
None of the cytokine levels in the minocycline groups differed from those of the vehicle controls either prior to or 10 days post-injury (data not shown), suggesting that oral minocycline did not reduce peripheral inflammation after injury.
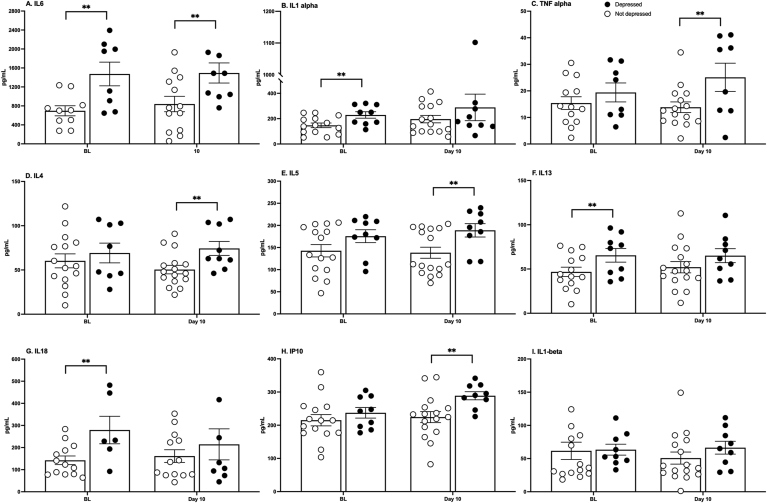
However, there were differences in cytokine levels both before and after injury between the depressed and not-depressed groups. Of the 27 cytokines and chemokines measured, 5 were upregulated at 10 days post injury in the depressed group: IL-6, TNF-α, IP-10, IL-4, and IL-5 (t (19) = 2.62, p = 0.017; t (21) = 2.39, p = 0.026; t (22) = 3.23, p = 0.004; t (22) = 2.59, p = 0.017; t (22) = 2.63, p = 0.015, respectively, Fig. 9). Intriguingly, these animals also exhibited pro-inflammatory profiles before injury, with upregulated IL-6, IL-1α, IL-13, and IL-18 (t (15) = 3.24, p = 0.006; t (20) = 2.55, p = 0.019; t (20) = 2.35, p = 0.029; t (16) = 2.61, p = 0.019, respectively, Fig. 9).
Fig. 9.
Serum cytokines. Cytokine levels were significantly higher in depressed compared with not-depressed subjects prior to injury and 10 days post injury. IL-6, IL-1ɑ, IL-13, and IL-18 were elevated before injury (A-B, F-G). IL-6, TNF-ɑ, IL-4, IL-5, and IP10 were elevated 10 days after injury (A, C-E, H). Individual differences in baseline (BL) cytokine levels (1.5–2.2X greater in depressed subjects) may predict vulnerability to the development of an affective disorder. Group size was influenced by the success of the ELISAs and varied slightly among cytokines. Mean ± SEM ∗∗p < 0.05.
Pearson product moment correlations were used to further examine the relationships between inflammation and behavior (Table 1). As cytokines do not act independently, principal component analyses (PCA) were used derive scores representing the linear combination of cytokines that differed significantly between depressed and not-depressed groups at baseline and on Day 10, respectively. At baseline, PCA1 explained more than 79.62% of the variance across depression groups. All cytokines loaded positively on baseline PCA1, with coefficients greater than 0.82. There was no correlation between the depression scores calculated with the discriminant function analysis and baseline PCA1 scores. There was, however, a strong and significant positive correlation between scores of immobility on the forced swim test and PCA1 (r (14) = 0.71, p < 0.01) derived at baseline. As cytokine levels increased, immobility increased. Scores of locomotor function (BBB) collected on Day 30 post-injury also correlated significantly with baseline PCA1 scores (r (14) = −0.55, p < 0.05). Higher baseline cytokines were associated with decreased recovery of locomotor function. No other correlations between baseline cytokines and behavior were significant.
Table 1.
Pearson correlation coefficients (r) between behavior and cytokine PCA factors.
| Baseline (PCA1) (↑cytokines) |
Day 10 (PCA1) (↑cytokines) |
|
|---|---|---|
| OF 10 | 0.15 | 0.00 |
| OF 30 | −0.31 | −0.34 |
| SOC 10 | 0.08 | 0.00 |
| SOC 30 | −0.20 | −0.25 |
| SP 10 | 0.05 | 0.00 |
| SP 30 | 0.02 | 0.27 |
| BUR 10 | 0.08 | 0.19 |
| BUR 30 | −0.23 | −0.17 |
| FST (immobility) | 0.71b | 0.37 |
| Depression score | −0.29 | −0.44a |
| BBB 30 | −0.55a | −0.63b |
| TF 10 | −0.31 | −0.24 |
| TF 30 | 0.37 | 0.21 |
OF: open field, SOC: social activity, SP: sucrose preference, BUR: burrowing, FST: forced swim test, BBB: locomotor score, TF: tail flick. a: p < 0.05, b: p < 0.01.
For Day 10 post injury, PCA again identified a principal component that explained more than 78.49% of the variance across depression groups. All cytokines loaded positively on PCA1, with coefficients greater than 0.77. Depression scores were significantly negatively correlated with Day 10 PCA1 scores (r (20) = −0.44, p < 0.05). As cytokines increased, depression scores decreased, denoting increased depression-like behavior. Day 10 PCA1 scores were also strongly correlated with BBB scores collected on post-injury day 30 (r (20) = −0.61, p < 0.01). Higher PCA1 scores were associated with decreased locomotor recovery. No other correlations, between baseline cytokines and behavior were significant.
3.5. Serotonin is decreased in brains of depressed subjects
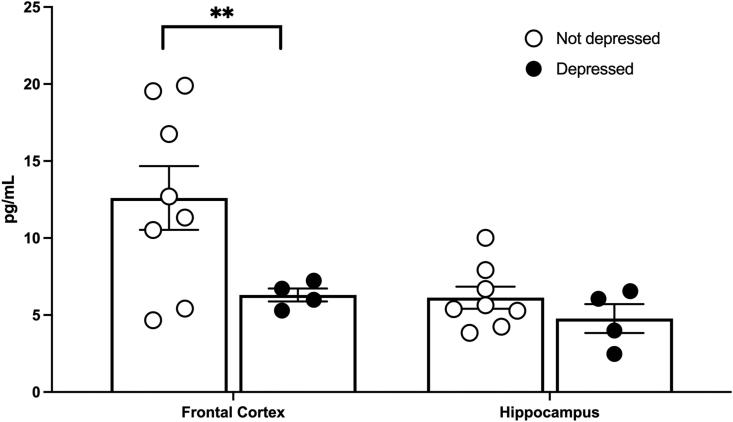
Commensurate with behavior, subjects that displayed depression-like behavior had lower serotonin expression in the frontal cortex than their not-depressed counterparts (t (7.57) = 2.99, p = 0.02, Fig. 10). Serotonin expression was also lower in the hippocampus, but it was not significant with the small sample size. BDNF levels did not differ between depressed and not-depressed groups (data not shown). Neither serotonin nor BDNF levels differed among minocycline dose groups.
Fig. 10.
Serotonin expression in the brain. Depressed subjects displayed lower serotonin in the frontal cortex and trended towards lower serotonin in the hippocampus (A). Depressed n = 4, Not depressed n = 9; Mean ± SEM ∗∗p < 0.05.
3.6. Neurogenesis
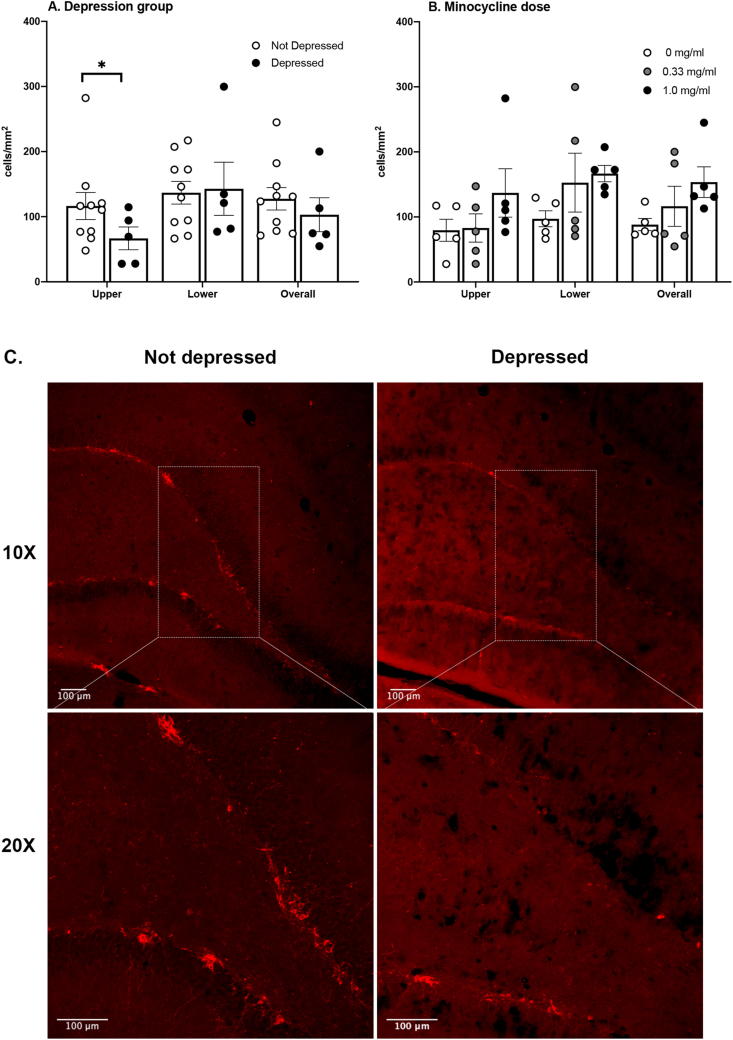
Neurogenesis was assessed in the upper (suprapyramidal) and lower (infrapyramidal) blades of the dentate gyrus by stereological counting of immature neurons stained for doublecortin (DCX). One subject was removed from the analysis because of its poor tissue quality and staining. Mann-Whitney U tests showed that the depressed subjects had fewer DCX + cells in the upper blade of the dentate gyrus than the not-depressed subjects (p = 0.05, Fig. 11A). There was no significant effect of minocycline dose on neurogenesis (Fig. 11B).
Fig. 11.
Neurogenesis is lower in depressed subjects. Depressed subjects had lower DCX expression in the upper, suprapyramidal, blade of the dentate gyrus (A). There was no significant effect of minocycline on DCX expression in the dentate gyrus (B). Representative images from the suprapyramidal blade of the dentate gyrus illustrate the differences between the depressed and not depressed groups (C). Depressed n = 5, Not depressed n = 10; Control n = 5, 0.33 mg/ml n = 6, 1 mg/ml n = 5; Mean ± SEM, ∗p < 0.05.
4. Discussion
Approximately one-third of the subjects displayed depression-like behaviors in the month following injury. This is commensurate with both human data (Williams and Murray 2015) and our previous findings in the rodent model of depression after SCI (Brakel et al., 2019; Luedtke et al., 2014; Maldonado-Bouchard et al., 2016). Also consistent with the literature, depression itself was associated with increased peripheral inflammation post-injury, decreased serotonin levels in the prefrontal cortex, and decreased neurogenesis. Oral minocycline was not sufficient to reduce peripheral inflammation after SCI, and it did not block the development of depression-like behavior. Intriguingly, however, we found that rats that developed depression-like behaviors not only displayed elevated pro-inflammatory cytokine expression post-injury, but also prior to injury. Inflammation may be a cause of, or a biomarker for, susceptibility to depression.
Replicating previous studies, there were clear post-injury differences in cytokine expression across depression groups (do Espírito Santo et al., 2019; Maldonado-Bouchard et al., 2016; Wu et al., 2014a; Wu et al., 2014b). The depressed subjects had elevated serum cytokines compared to not-depressed subjects. Surprisingly, we found that the depressed subjects also had elevated baseline pro-inflammatory cytokine expression relative to not-depressed subjects, before SCI and before the subjects exhibited depression-like symptoms. There was no significant difference between the levels of pro-inflammatory cytokines expressed prior to injury and on Day 10 post injury. Protracted elevations of cytokines in the subset of depressed subjects, therefore, may reflect pre-existing individual differences rather than a differential response to SCI. This finding seems counter to the general consensus that SCI causes persistent inflammation. Elevated levels of TNF-α, IL-6, IL-1RA, and IL-2 have been seen in serum of some people with SCI years after their injuries (Davies et al., 2007; Hayes et al., 2002). However, time-course studies of SCI-induced inflammation in rodents have shown that while TNF-α, IL-6, and IL-1β increase for the first few hours after an injury, levels return to baseline after 24 h (Yang et al., 2005). Serum IL-6, specifically, is elevated 24 h after a contusion injury in rats, but it returns to baseline levels after 7 days (Yang et al., 2018). The chronically high inflammation observed in some human patients may be due to long-term physiological modifications (i.e., secondary infections, pressure sores) brought about by living with an SCI. Alternatively, they may reflect individuals with higher basal inflammation, irrespective of injury. Although our data do not rule out a role for post-injury cytokines in the development of depression, they do suggest that pre-existing individual differences predict psychological wellbeing after SCI.
There is increasing evidence to support the premise that basal cytokine expression may be predictive of susceptibility to depression. For example, a landmark study, the Whitehall Study, followed hundreds of white-collar office workers for twelve years, collecting data on their mental health status as well as their peripheral blood cytokine profiles (Gimeno et al., 2009). The study found that baseline levels of C reactive protein and IL-6 predicted depressive symptoms that manifest up to 12 years later. Studies looking at both juvenile and aged populations have found similar results: IL-6 measured in 9 year-old children was predictive of depression and other mental disturbances 9 years later, and both IL-6 and CRP measured in adults over 60 were predictive of depression identified 5 years later (Khandaker et al., 2014; Zalli et al., 2016). These inflammatory molecules are clearly ubiquitous across populations and are important to the development of depression. Similarly, in animal models Hodes et al. (2014) found that leukocytes from mice susceptible to depression induced with mild social defeat stress produced more IL-6 when stimulated with lipopolysaccharide (LPS) than those from resilient mice. Additionally, they found that when IL-6 activity was blocked with a monoclonal antibody, or eliminated through an IL-6 knockout, mice subjected to social defeat stress were less likely to develop depression-like behaviors (Hodes et al., 2014). Clearly, the inflammatory potential before an injury or stressor plays an important role in susceptibility to depression.
Interestingly, in addition to associations with depression, we found a strong and significant correlation between baseline cytokine expression and locomotor recovery after SCI. BBB scores collected on Day 30 post injury were not only correlated with differential cytokine expression after injury but also before injury. A long-standing question in the SCI field has been why, with controlled and systematic injuries, there is differential recovery of locomotor and sensory function among rats. Individual differences prior to injury may not only impact psychological wellbeing, they may also significantly affect the prognosis for recovery of locomotor function.
It is also important to note that our data do not negate the hypothesis that elevated cytokines post injury are critical to the development of depression. Indeed, depression scores collected after SCI were significantly correlated with the principal component scores derived for cytokine expression at Day 10 post injury. The data presented here instead suggests that elevated cytokines alone may not be sufficient to drive the development of depression. In the context of pathophysiological changes induced by SCI, and other stressors, we predict that the consequences of high levels of peripheral cytokines would change. It is well-established that injury changes the permeability of the blood-spinal cord barrier, allowing white blood cells to infiltrate the injured parenchyma and contribute to secondary injury (Bilgen et al., 2002; Cohen et al., 2009; Noble and Wrathall 1989; Popovich et al., 1996; Whetstone et al., 2003). Similarly, while studies suggest that SCI may not increase the leakiness of the blood-brain barrier, increasing permeability to albumin, it does change the selective transport of cytokines from the blood into the underlying brain. Under basal conditions cytokines do cross the blood–brain barrier via saturable transport systems to act on astrocytes, neurons and microglia (Banks et al. 1994a, 1994b, 1995), but SCI further increases the transport of TNFα into the brain for up to 5 days even after a distant L1-L2 compression injury (Pan et al., 1999). Spinal cord injury also leads to dysfunction of the blood-cerebrospinal fluid barrier after a thoracic contusion injury (Shechter et al., 2013). Changes in the blood-cerebrospinal fluid barrier can result in the production of different proteins by the choroid plexus epithelial cells, contributing to changes in the cerebrospinal fluid, and decreased expression of tight junction and transporter proteins in the barrier which will alter immune cell trafficking (Shechter et al., 2013; Solar et al., 2020). While monocytes and lymphocytes normally enter the healthy brain in small numbers through the choroid plexus (Baruch and Schwartz 2013; Louveau et al., 2015; Shechter et al., 2013), SCI appears to trigger further opening of the gates separating the brain from the blood. SCI induced disruption of the central nervous system barriers would enable peripheral mediators of inflammation to infiltrate the brain, and change behavior.
Indeed, decreased neurogenesis, which is strongly associated with depression, may be a consequence of increased peripheral cytokine and immune cell infiltration across compromised CNS barriers after SCI. Studies have reported severity-dependent decreases in hippocampal neurogenesis after SCI, relative to uninjured controls (Felix et al., 2012; Jure et al., 2017; Soltani Zangbar et al., 2020; Wu et al. 2014c, 2016). Our data suggests that depression is also linked with neurogenesis after SCI; rats that displayed depression-like behavior had decreased hippocampal neurogenesis relative to their not-depressed conspecifics. This finding concurs with previous literature showing that stress and depression correlate with decreased hippocampal neurogenesis (Czéh et al., 2002; Malberg and Duman 2003; Pham et al., 2003). Malberg et al. (2003), for example, found that inescapable foot shock produced depression behaviors and decreased neurogenesis in rats. Repeated social defeat stress also decreases hippocampal granule cell proliferation and survival (Czéh et al., 2002), and physical restraint stress decreases neuronal proliferation in the dentate gyrus (Czéh et al., 2002; Malberg and Duman 2003; Pham et al., 2003). These changes in neurogenesis, with stress and SCI, may result from increased inflammation in the brain. The inflammatory cytokines IL-6, IL-1β, and TNF-α have all been shown to decrease hippocampal neuronal proliferation and differentiation (Kim et al., 2016). IL-1β activates NF-κB, which then blocks the transcription of genes necessary for neurogenesis. An NF-κB antagonist restores hippocampal cell proliferation after systemic inflammation, while blocking IL-6 in vitro promotes proliferation (Monje et al. 2003, 2011). Serotonin, which was also decreased in the frontal cortex of the depressed rats, also influences neurogenesis. Standard antidepressants, such as sertraline, venlafaxine, and escitalopram, and especially those that work on the serotonin system, like fluoxetine, consistently promote neurogenesis while reducing depressive symptoms (Anacker et al., 2011; Jiang et al., 2005; Mahar et al., 2014; Peng et al., 2008; Santarelli et al., 2003; Sen et al., 2008; Shimizu et al., 2003). From these data, it is clear that neurogenesis and inflammation interact with many of the same molecular mechanisms that we attribute to depression, and that inflammation plays an important role in recovery from and possibly resilience to this affective disorder.
While we found intriguing associations between inflammation and depression, unfortunately minocycline did not reduce peripheral cytokine levels in the rodent SCI model and it did not block SCI-induced depression. This lack of effect contradicts many published studies investigating the anti-depressant efficacy of minocycline in both animal models and clinical studies. As noted previously, minocycline has been reported as an effective antidepressant in people with unipolar depression and in animal models (Arakawa et al., 2012; Henry et al., 2008; Hinwood et al., 2012; O’Connor et al., 2009; Rosenblat and McIntyre 2018). Notably, however, others have reported null effects with minocycline treatment in both rats and mice (Deak et al., 2005; Vogt et al., 2016). Further, the largest randomized, placebo-controlled trial of anti-inflammatory treatments for depression has been recently completed and found no evidence that minocycline was superior to placebo, and no effects of minocycline treatment on serum C-reactive protein, a marker of inflammation (Husain et al., 2020). Many variables including the route of administration, length of treatment, and etiology of depression may significantly affect the efficacy of anti-inflammatory strategies.
In the current study, several experimental factors may have contributed to the lack of minocycline effects. First, we selected the oral route of minocycline to simulate the clinical administration of this treatment. However, because the subjects received the minocycline through their drinking water, we could not control the exact dose each animal received or whether they drank immediately before behavioral testing and serum collection. As the half-life of minocycline in rats is only a few hours (Elewa et al., 2006), it is possible that the subjects did not ingest a biologically relevant amount in an appropriate time frame to have anti-inflammatory effects. Also, while high performance liquid chromatography studies suggest that minocycline should remain stable in solution for up to 7 days at room temperature (Pearson and Trissel 1993), we did not verify this is in the current study. Second, although sufficient to reduce inflammation in milder inflammatory conditions, the dose of minocycline used may not have been sufficient to reduce the robust inflammation inherent to SCI. Indeed, a subset of data collected from a Phase II minocycline trial in people with SCI also failed to show a decrease in CSF levels of pro-inflammatory cytokines after 7 days of i.v. minocycline treatment (Casha et al., 2018). Notably, although minocycline has been repeatedly shown to be neuroprotective (Camargos et al., 2020; Elewa et al., 2006; Li et al., 2016; Mejia et al., 2001; Ulndreaj et al., 2017), we saw no effect of minocycline on lesion size or locomotor recovery in the current study, indicating that our dose or route of administration may not have been biologically meaningful. There was, in fact, a puzzling decrease in gray matter volume in the high dose minocycline group which should be further explored. An intraperitoneal route of administration may be more appropriate in this model, using doses that have been reported to reduce lesion size in an SCI model.
The oral administration of minocycline may also have affected the efficacy of treatment through disruption of the gut microbiome. Minocycline is an antibiotic, and even brief doses of antibiotics can significantly deplete the gut microbiota (Becattini et al., 2016; Park et al., 2019). It is conceivable that the disrupted gut flora, combined with SCI-induced gut dysbiosis (Kigerl et al., 2016; Zhang et al., 2018), might increase inflammation in the SCI model neutralizing any anti-inflammatory effects inherent to the drug (Brakel and Hook 2019). Countering this argument, however, minocycline has been shown to improve the integrity of the gut-blood barrier in animal models of chronic unpredictable stress, increasing the expression of tight junction proteins, relative to vehicle controls exposed to stress alone (Yang et al., 2020). Similarly, in several rat and mouse models of depression, it has been shown that minocycline increases the expression anti-inflammatory gut metabolites such as butyrate, suggesting that it increases the expression of beneficial bacterial species (Schmidtner et al., 2019; Wong et al., 2016; Yang et al., 2020). Notably, beneficial gut-mediated anti-inflammatory effects of minocycline, in the current study, might have been masked by the concurrent use of penicillin. A limitation of the current study is that, similar to what would occur in the clinical setting, all rats also received penicillin immediately following SCI and for at least one day following injury. It is unknown what effects the combination of SCI, minocycline, and a secondary antibiotic might have on the gut microbiome and barrier integrity. Whether the route of administration affects the outcomes of minocycline administration is also an empirical question, as all of the aforementioned studies of the microbiome used an intraperitoneal route of administration. Understanding the effects of minocycline, and the interactions with standard clinical practices, will be imperative in future experiments. It is possible that the anti-inflammatory and neuroprotective properties of minocycline may be masked by the co-administration of other microbiota-depleting antibiotics or when coupled with injury-induced dysbiosis. As inflammation has been implicated in many of the adverse consequences of SCI, we must understand the parameters that could yield beneficial, adverse, or no effects of anti-inflammatories with therapeutic potential.
Overall, the data presented here show that after SCI a subset of rats display depression-like behavior, as well as increased peripheral inflammation, decreased serotonin in the prefrontal cortex, and decreased hippocampal neurogenesis. While oral minocycline administration was not sufficient to reduce inflammation or depression after spinal injury, the current study yielded the unexpected finding that depression after SCI is associated with elevated serum cytokines prior to injury. Adding to evidence across species and stressors, these results lead to an intriguing hypothesis that pre-existing peripheral inflammation might predispose an individual to develop depression symptoms after SCI. Determining whether this pre-existing inflammation is causally related to depression, at a behavioral and molecular level, warrants detailed investigation in future studies.
Declaration of competing interest
The authors have no conflict of interest.
Acknowledgements
This work was generously supported by funding from the Gillson Longenbaugh Foundation and Mission Connect.
References
- Allison D.J., Ditor D.S. Targeting inflammation to influence mood following spinal cord injury: a randomized clinical trial. J. Neuroinflammation. 2015;12:204. doi: 10.1186/s12974-015-0425-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker C., Zunszain P.A., Cattaneo A., Carvalho L.A., Garabedian M.J. Antidepressants increase human hippocampal neurogenesis by activating the glucocorticoid receptor. Mol. Psychiatr. 2011;16:738–750. doi: 10.1038/mp.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa S., Shirayama Y., Fujita Y., Ishima T., Horio M. Minocycline produced antidepressant-like effects on the learned helplessness rats with alterations in levels of monoamine in the amygdala and no changes in BDNF levels in the hippocampus at baseline. Pharmacol. Biochem. Behav. 2012;100:601–606. doi: 10.1016/j.pbb.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Banks W.A., Kastin A.J., Ehrensing C.A. Blood-borne interleukin-1 alpha is transported across the endothelial blood-spinal cord barrier of mice. J. Physiol. 1994;479(Pt 2):257–264. doi: 10.1113/jphysiol.1994.sp020293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks W.A., Kastin A.J., Gutierrez E.G. Penetration of interleukin-6 across the murine blood-brain barrier. Neurosci. Lett. 1994;179:53–56. doi: 10.1016/0304-3940(94)90933-4. [DOI] [PubMed] [Google Scholar]
- Banks W.A., Plotkin S.R., Kastin A.J. Permeability of the blood-brain barrier to soluble cytokine receptors. Neuroimmunomodulation. 1995;2:161–165. doi: 10.1159/000096887. [DOI] [PubMed] [Google Scholar]
- Baruch K., Schwartz M. CNS-specific T cells shape brain function via the choroid plexus. Brain Behav. Immun. 2013;34:11–16. doi: 10.1016/j.bbi.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Basso D., Beattie M., Bresnahan J. A new sensitive locomotor rating scale for locomotor recovery after spinal cord contusion injuries in rats. J. Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Becattini S., Taur Y., Pamer E.G. Antibiotic-induced changes in the intestinal microbiota and disease. Trends Mol. Med. 2016;22:458–478. doi: 10.1016/j.molmed.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilgen M., Dogan B., Narayana P.A. In vivo assessment of blood-spinal cord barrier permeability: serial dynamic contrast enhanced MRI of spinal cord injury. Magn. Reson. Imaging. 2002;20:337–341. doi: 10.1016/s0730-725x(02)00504-0. [DOI] [PubMed] [Google Scholar]
- Bison S., Razzoli M., Arban R., Michielin F., Bertani S., Carboni L. Effect of the p38 MAPK inhibitor SB-239063 on Lipopolysaccharide-induced psychomotor retardation and peripheral biomarker alterations in rats. Eur. J. Pharmacol. 2011;661:49–56. doi: 10.1016/j.ejphar.2011.04.020. [DOI] [PubMed] [Google Scholar]
- Bombardier C.H., Kalpakjian C.Z., Graves D.E., Dyer J.R., Tate D.G., Fann J.R. Validity of the Patient Health Questionnaire-9 in assessing major depressive disorder during inpatient spinal cord injury rehabilitation. Arch. Phys. Med. Rehabil. 2012;93:1838–1845. doi: 10.1016/j.apmr.2012.04.019. [DOI] [PubMed] [Google Scholar]
- Brakel K., Aceves A.R., Aceves M., Hierholzer A., Nguyen Q.-N., Hook M.A. Depression-like behavior corresponds with cardiac changes in a rodent model of spinal cord injury. Exp. Neurol. 2019;320:112969. doi: 10.1016/j.expneurol.2019.112969. [DOI] [PubMed] [Google Scholar]
- Brakel K., Hook M.A. SCI and depression: does inflammation commandeer the brain? Exp. Neurol. 2019;320:112977. doi: 10.1016/j.expneurol.2019.112977. [DOI] [PubMed] [Google Scholar]
- Brody D.J., Pratt L.A., Hughes J.P. 2018. Prevalence of Depression Among Adults Aged 20 and over: United States, 2013-2016. NCHS Data Brief; pp. 1–8. [PubMed] [Google Scholar]
- Burke N.N., Kerr D.M., Moriarty O., Finn D.P., Roche M. Minocycline modulates neuropathic pain behaviour and cortical M1–M2 microglial gene expression in a rat model of depression. Brain Behav. Immun. 2014;42:147–156. doi: 10.1016/j.bbi.2014.06.015. [DOI] [PubMed] [Google Scholar]
- Camargos Q.M., Silva B.C., Silva D.G., de Brito Toscano E.C., da Silva Oliveira B. Minocycline treatment prevents depression and anxiety-like behaviors and promotes neuroprotection after experimental ischemic stroke. Brain Res. Bull. 2020;155:1–10. doi: 10.1016/j.brainresbull.2019.11.009. [DOI] [PubMed] [Google Scholar]
- Capuron L., Fornwalt F.B., Knight B.T., Harvey P.D., Ninan P.T., Miller A.H. Does cytokine-induced depression differ from idiopathic major depression in medically healthy individuals? J. Affect. Disord. 2009;119:181–185. doi: 10.1016/j.jad.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L., Gumnick J.F., Musselman D.L., Lawson D.H., Reemsnyder A. Neurobehavioral effects of interferon-α in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- Casarotto P., Andreatini R. Repeated paroxetine treatment reverses anhedonia induced in rats by chronic mild stress or dexamethasone. Eur. Neuropsychopharmacol. 2007;17:735–742. doi: 10.1016/j.euroneuro.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Casha S., Rice T., Stirling D.P., Silva C., Gnanapavan S. Cerebrospinal fluid biomarkers in human spinal cord injury from a Phase II minocycline trial. J. Neurotrauma. 2018;35:1918–1928. doi: 10.1089/neu.2018.5899. [DOI] [PubMed] [Google Scholar]
- Castagné V., Moser P., Roux S., Porsolt R.D. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Current protocols in pharmacology. 2010;49(5.8):1–5. doi: 10.1002/0471141755.ph0508s49. 8. 14. [DOI] [PubMed] [Google Scholar]
- Cohen D.M., Patel C.B., Ahobila-Vajjula P., Sundberg L.M., Chacko T. Blood-spinal cord barrier permeability in experimental spinal cord injury: dynamic contrast-enhanced MRI. NMR Biomed. 2009;22:332–341. doi: 10.1002/nbm.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czéh B., Welt T., Fischer A.K., Erhardt A., Schmitt W. Chronic psychosocial stress and concomitant repetitive transcranial magnetic stimulation: effects on stress hormone levels and adult hippocampal neurogenesis. Biol. Psychiatr. 2002;52:1057–1065. doi: 10.1016/s0006-3223(02)01457-9. [DOI] [PubMed] [Google Scholar]
- Davies A.L., Hayes K.C., Dekaban G.A. Clinical correlates of elevated serum concentrations of cytokines and autoantibodies in patients with spinal cord injury. Arch. Phys. Med. Rehabil. 2007;88:1384–1393. doi: 10.1016/j.apmr.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Deacon R.M. Burrowing in rodents: a sensitive method for detecting behavioral dysfunction. Nat. Protoc. 2006;1:118. doi: 10.1038/nprot.2006.19. [DOI] [PubMed] [Google Scholar]
- Deak T., Bellamy C., D’Agostino L.G., Rosanoff M., McElderry N.K., Bordner K.A. Behavioral responses during the forced swim test are not affected by anti-inflammatory agents or acute illness induced by lipopolysaccharide. Behav. Brain Res. 2005;160:125–134. doi: 10.1016/j.bbr.2004.11.024. [DOI] [PubMed] [Google Scholar]
- do Espírito Santo C.C., da Silva Fiorin F., Ilha J., Duarte M.M.M.F., Duarte T., Santos A.R.S. Spinal cord injury by clip-compression induces anxiety and depression-like behaviours in female rats: the role of the inflammatory response. Brain Behav. Immun. 2019;78:91–104. doi: 10.1016/j.bbi.2019.01.012. [DOI] [PubMed] [Google Scholar]
- El Arfani A., Parthoens J., Demuyser T., Servaes S., De Coninck M. Accelerated high-frequency repetitive transcranial magnetic stimulation enhances motor activity in rats. Neuroscience. 2017;347:103–110. doi: 10.1016/j.neuroscience.2017.01.045. [DOI] [PubMed] [Google Scholar]
- Elewa H.F., Hilali H., Hess D.C., Machado L.S., Fagan S.C. Minocycline for short-term neuroprotection. Pharmacotherapy. 2006;26:515–521. doi: 10.1592/phco.26.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott T.R., Frank R.G. Depression following spinal cord injury. Arch. Phys. Med. Rehabil. 1996;77:816–823. doi: 10.1016/s0003-9993(96)90263-4. [DOI] [PubMed] [Google Scholar]
- Farrell K., Houle J.D. Systemic inhibition of soluble TNF with XPro1595 exacerbates a post-spinal cord injury depressive phenotype in female rats. J. Neurotrauma. 2019;36(21):2964–2976. doi: 10.1089/neu.2019.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix M.S., Popa N., Djelloul M., Boucraut J., Gauthier P. Alteration of forebrain neurogenesis after cervical spinal cord injury in the adult rat. Front. Neurosci. 2012;6:45. doi: 10.3389/fnins.2012.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson A.R., Hook M.A., Garcia G., Bresnahan J.C., Beattie M.S., Grau J.W. A simple post hoc transformation that improves the metric properties of the BBB scale for rats with moderate to severe spinal cord injury. J. Neurotrauma. 2004;21:1601–1613. doi: 10.1089/neu.2004.21.1601. [DOI] [PubMed] [Google Scholar]
- Gimeno D., Kivimäki M., Brunner E.J., Elovainio M., De Vogli R. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol. Med. 2009;39:413–423. doi: 10.1017/S0033291708003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau J.W., Washburn S.N., Hook M.A., Ferguson A.R., Crown E.D. Uncontrollable stimulation undermines recovery after spinal cord injury. J. Neurotrauma. 2004;21:1795–1817. doi: 10.1089/neu.2004.21.1795. [DOI] [PubMed] [Google Scholar]
- Hayes K., Hull T., Delaney G., Potter P., Sequeira K. Elevated serum titers of proinflammatory cytokines and CNS autoantibodies in patients with chronic spinal cord injury. J. Neurotrauma. 2002;19:753–761. doi: 10.1089/08977150260139129. [DOI] [PubMed] [Google Scholar]
- Henry C.J., Huang Y., Wynne A., Hanke M., Himler J. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J. Neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández A., Fernández-Guasti A. Male rats with same-sex preference show higher immobility in the forced swim test, but similar effects of fluoxetine and desipramine than males that prefer females. Pharmacol. Biochem. Behav. 2018;171:39–45. doi: 10.1016/j.pbb.2018.05.017. [DOI] [PubMed] [Google Scholar]
- Herrick S.M., Elliott T.R., Crow F. Social support and the prediction of health complications among persons with spinal cord injuries. Rehabil. Psychol. 1994;39:231. doi: 10.1007/BF01989628. [DOI] [PubMed] [Google Scholar]
- Hinwood M., Morandini J., Day T.A., Walker F.R. Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex. Cerebr. Cortex. 2012;22:1442–1454. doi: 10.1093/cercor/bhr229. [DOI] [PubMed] [Google Scholar]
- Hinwood M., Tynan R.J., Charnley J.L., Beynon S.B., Day T.A., Walker F.R. Chronic stress induced remodeling of the prefrontal cortex: structural re-organization of microglia and the inhibitory effect of minocycline. Cerebr. Cortex. 2013;23:1784–1797. doi: 10.1093/cercor/bhs151. [DOI] [PubMed] [Google Scholar]
- Hodes G.E., Pfau M.L., Leboeuf M., Golden S.A., Christoffel D.J. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc. Natl. Acad. Sci. Unit. States Am. 2014;111:16136–16141. doi: 10.1073/pnas.1415191111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain M.I., Cullen C., Umer M., Carvalho A.F., Kloiber S. Minocycline as adjunctive treatment for treatment-resistant depression: study protocol for a double blind, placebo-controlled, randomized trial (MINDEP2) BMC Psychiatr. 2020;20:173. doi: 10.1186/s12888-020-02553-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P., Ahlehoff O., Egeberg A., Gislason G., Hansen P.R., Skov L. Psoriasis and new-onset depression: a Danish nationwide cohort study. Acta Derm. Venereol. 2016;96:39–42. doi: 10.2340/00015555-2183. [DOI] [PubMed] [Google Scholar]
- Jiang W., Zhang Y., Xiao L., Van Cleemput J., Ji S.-P. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic-and antidepressant-like effects. J. Clin. Invest. 2005;115:3104–3116. doi: 10.1172/JCI25509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jure I., Pietranera L., De Nicola A.F., Labombarda F. Spinal cord injury impairs neurogenesis and induces glial reactivity in the Hippocampus. Neurochem. Res. 2017;42:2178–2190. doi: 10.1007/s11064-017-2225-9. [DOI] [PubMed] [Google Scholar]
- Khandaker G.M., Pearson R.M., Zammit S., Lewis G., Jones P.B. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA psychiatry. 2014;71:1121–1128. doi: 10.1001/jamapsychiatry.2014.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigerl K.A., Hall J.C., Wang L., Mo X., Yu Z., Popovich P.G. Gut dysbiosis impairs recovery after spinal cord injury. J. Exp. Med.: jem. 2016:20151345. doi: 10.1084/jem.20151345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.-K., Na K.-S., Myint A.-M., Leonard B.E. The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2016;64:277–284. doi: 10.1016/j.pnpbp.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Krause J.S., Kemp B., Coker J. Depression after spinal cord injury: relation to gender, ethnicity, aging, and socioeconomic indicators. Arch. Phys. Med. Rehabil. 2000;81:1099–1109. doi: 10.1053/apmr.2000.7167. [DOI] [PubMed] [Google Scholar]
- Li Z., Wei H., Piirainen S., Chen Z., Kalso E. Spinal versus brain microglial and macrophage activation traits determine the differential neuroinflammatory responses and analgesic effect of minocycline in chronic neuropathic pain. Brain Behav. Immun. 2016;58:107–117. doi: 10.1016/j.bbi.2016.05.021. [DOI] [PubMed] [Google Scholar]
- Louveau A., Harris T.H., Kipnis J. Revisiting the mechanisms of CNS immune privilege. Trends Immunol. 2015;36:569–577. doi: 10.1016/j.it.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedtke K., Bouchard S.M., Woller S.A., Funk M.K., Aceves M., Hook M.A. Assessment of depression in a rodent model of spinal cord injury. J. Neurotrauma. 2014;31:1107–1121. doi: 10.1089/neu.2013.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahar I., Bambico F.R., Mechawar N., Nobrega J.N. Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci. Biobehav. Rev. 2014;38:173–192. doi: 10.1016/j.neubiorev.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Malberg J.E., Duman R.S. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562–1571. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- Maldonado-Bouchard S., Peters K., Woller S.A., Madahian B., Faghihi U. Inflammation is increased with anxiety-and depression-like signs in a rat model of spinal cord injury. Brain Behav. Immun. 2016;51:176–195. doi: 10.1016/j.bbi.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie R.A. Comorbidity in multiple sclerosis: implications for patient care. Nat. Rev. Neurol. 2017;13:375. doi: 10.1038/nrneurol.2017.33. [DOI] [PubMed] [Google Scholar]
- Mejia R.O.S., Ona V.O., Li M., Friedlander R.M. Minocycline reduces traumatic brain injury-mediated caspase-1 activation, tissue damage, and neurological dysfunction. Neurosurgery. 2001;48:1393–1401. doi: 10.1097/00006123-200106000-00051. [DOI] [PubMed] [Google Scholar]
- Migliorini C., New P., Tonge B. Comparison of depression, anxiety and stress in persons with traumatic and non-traumatic post-acute spinal cord injury. Spinal Cord. 2009;47:783. doi: 10.1038/sc.2009.43. [DOI] [PubMed] [Google Scholar]
- Molina-Hernández M., Tellez-Alcántara N.P., Pérez-García J., Olivera-Lopez J.I., Jaramillo-Jaimes M.T. Antidepressant-like actions of minocycline combined with several glutamate antagonists. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2008;32:380–386. doi: 10.1016/j.pnpbp.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Monje F.J., Cabatic M., Divisch I., Kim E.-J., Herkner K.R. Constant darkness induces IL-6-dependent depression-like behavior through the NF-κB signaling pathway. J. Neurosci. 2011;31:9075–9083. doi: 10.1523/JNEUROSCI.1537-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje M.L., Toda H., Palmer T.D. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Noble L.J., Wrathall J.R. Distribution and time course of protein extravasation in the rat spinal cord after contusive injury. Brain Res. 1989;482:57–66. doi: 10.1016/0006-8993(89)90542-8. [DOI] [PubMed] [Google Scholar]
- O’Connor J.C., Lawson M.A., Andre C., Moreau M., Lestage J. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatr. 2009;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier C., Robert P.D., Daihung D., Urbà G., Catalin M.P. The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch. Dermatol. 2010;146:891–895. doi: 10.1001/archdermatol.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W., Kastin A.J., Bell R.L., Olson R.D. Upregulation of tumor necrosis factor alpha transport across the blood-brain barrier after acute compressive spinal cord injury. J. Neurosci. : the official journal of the Society for Neuroscience. 1999;19:3649–3655. doi: 10.1523/JNEUROSCI.19-09-03649.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M.J., Pilla R., Panta A., Pandey S., Sarawichitr B. Reproductive senescence and ischemic stroke remodel the gut microbiome and modulate the effects of estrogen treatment in female rats. Translational Stroke Research. 2019:1–19. doi: 10.1007/s12975-019-00760-5. [DOI] [PubMed] [Google Scholar]
- Pearson S.D., Trissel L.A. Stability and compatibility of minocycline hydrochloride and rifampin in intravenous solutions at various temperatures. Am. J. Hosp. Pharm. 1993;50:698–702. [PubMed] [Google Scholar]
- Peng Q., Masuda N., Jiang M., Li Q., Zhao M. The antidepressant sertraline improves the phenotype, promotes neurogenesis and increases BDNF levels in the R6/2 Huntington’s disease mouse model. Exp. Neurol. 2008;210:154–163. doi: 10.1016/j.expneurol.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham K., Nacher J., Hof P.R., McEwen B.S. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur. J. Neurosci. 2003;17:879–886. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- Popovich P.G., Horner P.J., Mullin B.B., Stokes B.T. A quantitative spatial analysis of the blood-spinal cord barrier. I. Permeability changes after experimental spinal contusion injury. Exp. Neurol. 1996;142:258–275. doi: 10.1006/exnr.1996.0196. [DOI] [PubMed] [Google Scholar]
- Popovich P.G., Wei P., Stokes B.T. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J. Comp. Neurol. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Rosenblat J.D., McIntyre R.S. Efficacy and tolerability of minocycline for depression: a systematic review and meta-analysis of clinical trials. J. Affect. Disord. 2018;227:219–225. doi: 10.1016/j.jad.2017.10.042. [DOI] [PubMed] [Google Scholar]
- Santarelli L., Saxe M., Gross C., Surget A., Battaglia F. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Schmidtner A.K., Slattery D.A., Glasner J., Hiergeist A., Gryksa K. Minocycline alters behavior, microglia and the gut microbiome in a trait-anxiety-dependent manner. Transl. Psychiatry. 2019;9:223. doi: 10.1038/s41398-019-0556-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S., Duman R., Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol. Psychiatr. 2008;64:527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter R., Miller O., Yovel G., Rosenzweig N., London A. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity. 2013;38:555–569. doi: 10.1016/j.immuni.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu E., Hashimoto K., Okamura N., Koike K., Komatsu N. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol. Psychiatr. 2003;54:70–75. doi: 10.1016/s0006-3223(03)00181-1. [DOI] [PubMed] [Google Scholar]
- Siegert R., Abernethy D. Depression in multiple sclerosis: a review. J. Neurol. Neurosurg. Psychiatr. 2005;76:469–475. doi: 10.1136/jnnp.2004.054635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solar P., Zamani A., Kubickova L., Dubovy P., Joukal M. Choroid plexus and the blood-cerebrospinal fluid barrier in disease. Fluids Barriers CNS. 2020;17:35. doi: 10.1186/s12987-020-00196-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltani Zangbar H., Ghadiri T., Vafaee M.S., Ebrahimi Kalan A., Karimipour M. A potential entanglement between the spinal cord and hippocampus: theta rhythm correlates with neurogenesis deficiency following spinal cord injury in male rats. J. Neurosci. Res. 2020;98:2451–2467. doi: 10.1002/jnr.24719. [DOI] [PubMed] [Google Scholar]
- Ulndreaj A., Badner A., Fehlings M.G. Promising neuroprotective strategies for traumatic spinal cord injury with a focus on the differential effects among anatomical levels of injury. F1000Research. 2017;6 doi: 10.12688/f1000research.11633.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt M.A., Mallien A.S., Pfeiffer N., Inta I., Gass P., Inta D. Minocycline does not evoke anxiolytic and antidepressant-like effects in C57BL/6 mice. Behav. Brain Res. 2016;301:96–101. doi: 10.1016/j.bbr.2015.12.015. [DOI] [PubMed] [Google Scholar]
- Whetstone W.D., Hsu J.Y., Eisenberg M., Werb Z., Noble-Haeusslein L.J. Blood-spinal cord barrier after spinal cord injury: relation to revascularization and wound healing. J. Neurosci. Res. 2003;74:227–239. doi: 10.1002/jnr.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R., Murray A. Prevalence of depression after spinal cord injury: a meta-analysis. Arch. Phys. Med. Rehabil. 2015;96:133–140. doi: 10.1016/j.apmr.2014.08.016. [DOI] [PubMed] [Google Scholar]
- Willner P., Towell A., Sampson D., Sophokleous S., Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology. 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Wong M.L., Inserra A., Lewis M.D., Mastronardi C.A., Leong L. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol. Psychiatr. 2016;21:797–805. doi: 10.1038/mp.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Stoica B.A., Luo T., Sabirzhanov B., Zhao Z. Isolated spinal cord contusion in rats induces chronic brain neuroinflammation, neurodegeneration, and cognitive impairment: involvement of cell cycle activation. Cell Cycle. 2014;13:2446–2458. doi: 10.4161/cc.29420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Zhao Z., Kumar A., Lipinski M.M., Loane D.J. Endoplasmic reticulum stress and disrupted neurogenesis in the brain are associated with cognitive impairment and depressive-like behavior after spinal cord injury. J. Neurotrauma. 2016;33:1919–1935. doi: 10.1089/neu.2015.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Zhao Z., Sabirzhanov B., Stoica B.A., Kumar A. Spinal cord injury causes brain inflammation associated with cognitive and affective changes: role of cell cycle pathways. J. Neurosci. 2014;34:10989–11006. doi: 10.1523/JNEUROSCI.5110-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Zhao Z., Sabirzhanov B., Stoica B.A., Kumar A. Spinal cord injury causes brain inflammation associated with cognitive and affective changes: role of cell cycle pathways. J. Neurosci. : the official journal of the Society for Neuroscience. 2014;34:10989–11006. doi: 10.1523/JNEUROSCI.5110-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Jones N.R., Blumbergs P.C., Van Den Heuvel C., Moore E.J. Severity-dependent expression of pro-inflammatory cytokines in traumatic spinal cord injury in the rat. J. Clin. Neurosci. 2005;12:276–284. doi: 10.1016/j.jocn.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Yang Q., Luo L., Sun T., Yang L., Cheng L.F. Chronic minocycline treatment exerts antidepressant effect, inhibits neuroinflammation, and modulates gut microbiota in mice. Psychopharmacology. 2020;237:3201–3213. doi: 10.1007/s00213-020-05604-x. [DOI] [PubMed] [Google Scholar]
- Yang Z., Bramlett H.M., Moghieb A., Yu D., Wang P. Temporal profile and severity correlation of a panel of rat spinal cord injury protein biomarkers. Mol. Neurobiol. 2018;55:2174–2184. doi: 10.1007/s12035-017-0424-7. [DOI] [PubMed] [Google Scholar]
- Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain Res. 1996;711:163–174. doi: 10.1016/0006-8993(95)01415-2. [DOI] [PubMed] [Google Scholar]
- Zalli A., Jovanova O., Hoogendijk W., Tiemeier H., Carvalho L. Low-grade inflammation predicts persistence of depressive symptoms. Psychopharmacology. 2016;233:1669–1678. doi: 10.1007/s00213-015-3919-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Zhang W., Zhang J., Jing Y., Yang M. Gut microbiota dysbiosis in male patients with chronic traumatic complete spinal cord injury. J. Transl. Med. 2018;16:353. doi: 10.1186/s12967-018-1735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]