Abstract
Background
Cognitive dysfunction adversely effects multiple functional outcomes and social roles after TBI. We hypothesize that chronic systemic inflammation exacerbates cognitive deficits post-injury and diminishes functional cognition and quality of life (QOL). Yet few studies have examined relationships between inflammation and cognition after TBI. Associations between early chronic serum inflammatory biomarker levels, cognitive outcomes, and QOL 6-months and 12-months after moderate-to-severe TBI were identified using unweighted (uILS) and weighted (wILS) inflammatory load score (ILS) formation.
Methods
Adults with moderate-to-severe TBI (n = 157) completed neuropsychological testing, the Functional Impairment Measure Cognitive Subscale (FIM-Cog) and self-reported Percent Back to Normal scale 6 months (n = 139) and 12 months (n = 136) post-injury. Serial serum samples were collected 1–3 months post-TBI. Cognitive composite scores were created as equally weighted means of T-scores derived from a multidimensional neuropsychological test battery. Median inflammatory marker levels associated with 6-month and 12-month cognitive composite T-scores (p < 0.10) were selected for ILS formation. Markers were quartiled, and quartile ranks were summed to generate an uILS. Marker-specific β-weights were derived using penalized ridge regression, multiplied by standardized marker levels, and summed to generate a wILS. ILS associations with cognitive composite scores were assessed using multivariable linear regression. Structural equation models assessed ILS influences on functional cognition and QOL using 12-month FIM-Cog and Percent Back to Normal scales.
Results
ILS component markers included: IL-1β, TNF-α, sIL-4R, sIL-6R, RANTES, and MIP-1β. Increased sIL-4R levels were positively associated with overall cognitive composite T-scores in bivariate analyses, while remaining ILS markers were negatively associated with cognition. Multivariable receiver operator curves (ROC) showed uILS added 14.98% and 31.93% relative improvement in variance captured compared to the covariates only base model (age, sex, education, Glasgow Coma Scale score) when predicting cognitive composite scores at 6 and 12 months, respectively; wILS added 33.99% and 36.87% relative improvement in variance captured. Cognitive composite mediated wILS associations with FIM-Cog scores at 12 months, and both cognitive composite and FIM-Cog scores mediated wILS associations with QOL.
Conclusions
Early chronic inflammatory burden is associated with cognitive performance post-TBI. wILS explains greater variance in cognitive composite T-scores than uILS. Linking inflammatory burden associated with cognitive deficits to functional outcome post-TBI demonstrates the potential impact of immunotherapy interventions aimed at improving cognitive recovery post-TBI.
Keywords: Traumatic brain injury, Cognitive dysfunction, Inflammation, Inflammatory load score, Biomarker, Neuropsychological deficits, Functional cognition, Quality of life, Ridge regression
Highlights
-
•
Chronic inflammation influences cognitive dysfunction after traumatic brain injury.
-
•
Ridge regression generated stable β-values for novel weighted inflammatory scoring.
-
•
Weighted inflammatory scores explain more cognitive variance than unweighted scores.
-
•
Systemic inflammatory burden also impacts functional cognition and quality of life.
1. Introduction
Traumatic brain injury (TBI) contributes to functional limitations, disability, and reduced quality of life (QOL) (Riggio and Wong, 2009; Walker and Pickett, 2007). Cognitive symptoms are an especially common and important area of concern for TBI survivors, with a majority of individuals with moderate-to-severe TBI having persistent and marked cognitive impairment at two years post-injury (Schretlen and Shapiro, 2003). TBI survivors commonly self-report persistent cognitive issues as a major concern (Khan et al., 2016). Cognition influences functional outcomes more than other factors, including demographic and injury characteristics (Spitz et al., 2012). Cognitive dysfunction post-TBI often factors into justification and goal-planning for rehabilitation care as ongoing impairment reduces social participation (Sashika et al., 2017).
TBI affects the cognitive domains of executive functioning, memory, and attention; dysfunction in each of these domains can lead to decreased performance in other cognitive domains (Rabinowitz and Levin, 2014). Lannoo et al. found individuals with moderate-to-severe TBI [Glasgow Coma Scale scores (GCS) ranging from 3-12] perform significantly worse than non-head trauma controls on neuropsychological measures six months post-injury (Lannoo et al., 1998). Memory is an important cognitive domain affected after TBI, with verbal memory being affected more than visual memory (Vakil et al., 2019). Memory deficits affect functional outcomes after TBI like return to work (Mani et al., 2017). Studies have reported memory deficits persisting through five years post-TBI (Marsh, 2018), particularly among those with severe TBI (Tate et al., 1991; Dikmen et al., 1987). Executive functioning affects high-order cognitive abilities that require planning, problem solving, and emotional regulation. Studies demonstrate that impaired executive function, verbal fluency, and processing speed hinder ability to return to work or school (Spitz et al., 2012; Mani et al., 2017; Ownsworth and McKenna, 2004; Ruff et al., 1993).
Heterogeneity in long-term cognitive recovery is not fully understood among individuals post-TBI (Millis et al., 2001). Some contributors to cognitive outcomes include baseline characteristics. Older age worsens verbal memory and recognition test performance (Vakil et al., 2019) and lowers processing speed, executive function, and memory test performance (Green et al., 2008; Rabinowitz et al., 2018). Premorbid education predicts cognitive recovery and reflects cognitive reserve (Schneider et al., 2014). Another important contributor to cognition includes TBI severity (Dikmen et al., 1995).
Functional cognition, measured by the Functional Independence Measure (FIM) cognitive subscale (FIM-Cog), refers to how an individual uses cognitive skills required for daily activities. Among individuals with moderate-to-severe TBI, FIM-Cog is associated with lower community participation, inability to drive, and unemployment at one year post-injury (McGarity et al., 2017). Our previous work suggests that PTD heavily influences functional cognition measures compared to objective measures of cognitive performance (Failla et al., 2016). Together, impairments like cognitive dysfunction and PTD affect daily skills requiring functional cognition, which in turn impacts other function-associated metrics like participation and QOL. Importantly, biologic variability may contribute to cognitive outcome post-TBI, including inflammatory pathway effects.
Our previous work shows that sustained high levels of CSF neuroinflammatory markers can increase PTD risk (Juengst et al., 2015a) and odds of an unfavorable Glasgow Outcome Scale (GOS) score (Kumar et al., 2016). Acute neuroinflammation post-TBI may lead to a chronic, sustained inflammatory response in survivors in both the central nervous system and systemically (Johnson et al., 2013; Kumar et al., 2015). However, chronic systemic inflammation has only recently been recognized as an important consequence of TBI. Our group showed that in the first three months post-injury, serum inflammatory biomarkers are elevated relative to controls and contribute significantly to GOS outcomes at 6 and 12 months post-injury (Kumar et al., 2014). TBI is associated with increased dementia risk, cognitive decline, and chronic neurodegeneration in multiple studies (Schretlen and Shapiro, 2003; Johnson et al., 2013; Lee et al., 2013; Wang et al., 2012). However, no TBI studies to date have characterized chronic inflammatory profiles and their influence on cognitive outcome. Identifying associations between serum inflammatory marker levels and neuropsychological testing scores after TBI may facilitate long-term prognostication of cognitive outcomes.
In our previous work by Kumar et al. (2014), a novel inflammatory load score (ILS) was generated to assess overall inflammatory burden and relationships with GOS after TBI to show inflammation’s aggregate effect on outcome. Similar ILSs have been employed to study overall inflammatory load in other settings such as sepsis (Andaluz-Ojeda et al., 2012), acute coronary syndromes (Correia et al., 2010), and thyroid disease (Wakelkamp et al., 2003). However, ILS generation methods vary, and each of these studies created an ILS by grouping data into binary, tertiary, or quaternary categories. This approach reduces available information due to biomarker stratification, and does not reflect the relative strength of biomarker associations. A primary challenge in creating a weighted load score is the high degree of collinearity among inflammatory biomarkers, many of which have diverse biological roles within inflammatory signaling pathways despite their degree of statistical relatedness. Adjusted β-weights are similarly challenging to derive in this scenario as regression coefficients are unstable in the presence of multicollinearity (Farrar and Glauber, 1967).
Thus, this study’s primary goal was to examine associations between inflammatory marker levels measured in serum samples collected 1–3 months post-TBI and cognitive performance at 6 and 12 months post-injury. Our study examines how long-term neuropsychological performance is shaped by early chronic systemic inflammatory burden. We created and comparatively assessed multiple methods of ILS formation to assess this relationship and create a sensitive and informative ILS, developing both an unweighted (uILS) and weighted (wILS) score as aggregate measures of inflammation’s association with cognitive performance. We hypothesized that increased inflammatory burden in the first three months post-injury would be associated with worse cognitive performance measured with neuropsychological testing 6 and 12 months post-injury.
2. Methods
2.1. Participants
Data from prospectively recruited individuals (N = 157) with moderate-to-severe TBI were collected and analyzed in accordance with University of Pittsburgh Institutional Review Board approved protocols. Informed consent was obtained from patients or next-of-kin. Individuals were recruited from inpatient rehabilitation at a University of Pittsburgh Medical Center (UPMC) level 1 trauma center and followed for up to 15 months post-injury. Individuals included in this analysis were 16–79 years of age, had non-penetrating moderate-to-severe TBI based on admission GCS (3–12), and/or had their closed head injury verified by ICD-9 diagnosis code or medical documentation of functional or medical complications (i.e. positive anatomic neuroimaging findings or focal neurologic signs) on day of injury. Individuals with penetrating TBI, previous documented TBI, untreated endocrine or autoimmune disorders, or ongoing neurodegenerative disease were excluded. Participants completed neuropsychologic testing or were designated as cognitively unable to complete neuropsychological testing at 6 and/or 12 months post-injury.
2.2. Demographic and injury data
Demographic and injury severity variables were collected via medical record review or participant/proxy interview including sex, age at injury and at neuropsychological testing, race, baseline years of education, mechanism of injury, length of acute hospitalization in days, cumulative neuroimaging findings on computed tomography (CT) of the head gathered from review of all available CT reports for scans acquired during subjects’ acute hospital stay, Injury Severity Score (ISS) (Baker et al., 1974), and best Glasgow Coma Scale (GCS) score within 24 hours of injury (Teasdale and Jennett, 1974). Initial CT imaging reports were not available for some individuals who did not receive acute care at a UPMC facility.
2.3. Serum biomarker collection
Serum samples were collected monthly for individuals with TBI and collected once from healthy controls without TBI. Cytokine levels were measured in serum (n = 933 samples in total) using a Luminex™ bead array assay (Millipore, Billerica, Massachusetts). Multiplex assays used microsphere technology, tagging assay beads with several fluorescent-labeled markers. A fluorescence detection laser optic system analyzed the binding of each protein to the multiplex bead. The Human High Sensitivity T-cell Magnetic Bead Panel included interleukin (IL)-10, IL-12(p70), IL-13, IL-1β, IL-2, IL-21, IL-4, IL-23, IL-5, IL-6, IL-7, IL-8, Macrophage Inflammatory Protein (MIP)-1α, MIP-1β, Tumor Necrosis Factor (TNF)-α, Fractalkine, Granulocyte Macrophage Colony Stimulating Factor (GM-CSF), Interferon-inducible T-cell alpha chemoattractant (ITAC), and Interferon (IFN)-γ. The intra-assay coefficient of variability (%CV) was <5%. The inter-assay %CV was <20%. The Human Neurodegenerative Disease Magnetic Bead included soluble Intracellular Adhesion Molecule (sICAM)-1, Regulated upon Activation, Normal T-cell Expressed and Secreted (RANTES), Neural Cell Adhesion Molecule (NCAM), and soluble Vascular Adhesion Molecule (sVCAM-1). The intra-assay %CV was <6%. The inter-assay %CV was <13%. The Human Soluble Cytokine Receptor Magnetic Bead Panel included soluble (s) CD30, soluble glycoprotein (sgp)130, soluble IL-1 receptor (sIL-1R)-I, sIL-1RII, sIL-2α, sIL-4R, sIL-6R, sTNFRI, and sTNFRII. The intra-assay %CV was <10% while inter-assay %CV was <15%.
2.4. Cognitive outcome data
Individuals completed neuropsychological testing at 6 and/or 12 months post-TBI. A subgroup of TBI survivors with monthly inflammatory data was unable to complete neuropsychological testing at 6 and/or 12 months due to the severity of their cognitive deficits, and they were identified and grouped as cognitively unable to complete testing. Cognitive composite scores were formulated using results from nine neuropsychological tests, administered by trained staff. Testing components were then scored and organized into four domains.
The verbal fluency domain included the Delis-Kaplan Executive Function Systems Verbal Fluency section (Delis and Kaplan, 2001) and the Controlled Oral Word Association test (Borkowski et al., 1967). The attention and processing speed domain included Trail Making Test A (Reitan and Wolfson, 1985), the Symbol Search subtest from the Wechsler Adult Intelligence Scale-R (Wechsler, 1997), and the oral section of the Symbol Digit Modalities Test (Smith, 2002). The memory domain included the California Verbal Learning Test II-Long Delay Free Recall score (Delis, 1987) and the Rey-Osterrieth Complex Figure Test delayed recall score (Osterrieth, 1944). The executive function domain included the Trail Making Test B (Reitan and Wolfson, 1985), Wisconsin Card Sorting Test Percent Conceptual responses (Heaton, 1981), and the Delis-Kaplan Executive Function Systems Verbal Fluency Category switching accuracy score (Delis and Kaplan, 2001).
Raw test scores were converted into T-scores using publisher-recommended normative data, correcting test performance for age, race, sex, and education level when indicated. To obtain a domain score, participants completed at least one of the domain-specific tests. T-scores of individual, domain-specific tests were averaged to create the reported domain T-score. The overall composite score is an equally weighted average of the four domains scores specified above and excluded individuals missing any domain scores. Individual T-score performance in any cognitive domain was considered “impaired” if the T-score was >1 standard deviation below the normalized mean of 50 (i.e. T-score ≤40).
2.5. Functional outcome data
Participants’ functional outcomes were assessed using the Functional Independence Measure (FIM) (Dodds et al., 1993) and Percent Back to Normal patient questionnaire (Powell et al., 2001) at 6 and/or 12 months post-injury. The cognitive subscale score for the FIM (FIM-Cog) was calculated with the components of expression, comprehension, social interaction, problem-solving, and memory. Each component is rated from 1 to 7, with scores<5 indicating need for caregiver assistance. The sum of these five components yields the FIM-Cog Score, ranging from 5-35. The Percent Back to Normal measure is a single question asking individuals how close they feel to being back to normal, on a scale of 0–100 percent. In this study, we used self-reported Percent Back to Normal as a proxy for QOL and self-reported function after TBI. Trained research staff administered both measures via in-person interview.
2.6. Statistical methods
Statistical analyses were performed using Statistical Analysis Software (SAS 9.4) (SAS Institute Inc) and R version 3.6.2 (R Core Team, 2019). To determine relationships between demographic and clinical variables and cognitive composite scores, Spearman’s rank correlations was used to assess continuous demographic and clinical variable relationships with cognitive composite scores; Kruskal-Wallis tests were used for categorical variables. For demographic variable relationships with cognitive impairment status, Kruskal Wallis tests were used for continuous variables and χ2 tests, or Fisher’s exact test when appropriate, were used for categorical variables.
Median values for each individual’s respective inflammatory biomarker levels obtained one, two, and/or three months post-injury were calculated as a single-value describing early chronic inflammatory burden, while minimizing the potential effects of extreme observations. Biomarker medians were then standardized to have mean 0 and standard deviation 1. Levels were then included in separate, simple linear regression models for each cognitive domain at the 6- and 12-month timepoints.
Inflammatory biomarkers tested in these regressions were selected for ILS formulation if they met statistical significance threshold criteria of p < 0.10 at both time points when assessed for associations with overall cognitive performance at 6 and/or 12 months. P-value cut-offs were more liberal to allow for inclusion of potentially biologically significant markers into the ILS score and establish trends for markers to influence cognitive performance at both 6 and 12 months.
Using these selected markers, we then created an uILS using methods described in detail by Kumar et al. (2014). To create this uILS, selected biomarkers were quartiled, preserving the direction of their associations with overall cognitive composite and summed together. Individuals’ biomarker data was assigned a rank (1–4) for each marker depending on which quartile the selected serum marker level was located. For example, individual markers in the lowest quartile (25th percentile or lower) were assigned a score of 1, and individual markers in the highest quartile (75th percentile or higher) were assigned a score of 4. If increasing levels of an individual inflammatory marker were positively associated with cognitive outcome, the quartile assignments were reversed to account for the direction of this association.
Using markers selected above, a weighted inflammatory load score (wILS) was also created for the overall cognitive composite. Covariate and biomarker adjusted ridge regression was performed to obtain stable β-values for each inflammatory marker in the presence of high multicollinearity. We used the most regularized model such that error was within one standard error of the minimum mean squared error (MSE) using the R package ‘glmnet’ version 3.0-2 (Friedman et al., 2010). Individuals’ standardized biomarker values were then multiplied by ridge regression-derived β-coefficients, while preserving the direction of association, and summed to generate a wILS for each study participant. Multivariable linear regression was used with both uILS and wILS to delineate the relative and absolute incremental predictive capacity of uILS and wILS for cognitive domain T-scores at 6 and 12 months compared to a base model adjusted for age, sex, years of education, and GCS.
Kruskall-Wallis tests were used to assess relationships between inflammatory markers selected from bivariate regressions with cognitive impairment status by grouping subjects as follows: unimpaired, impaired, and unable to complete cognitive testing. For graphing, markers were scaled by a multiple of 10X to fit a 0 to 45 range.
We used a structural equation model (SEM) to elucidate inflammatory effects on function post-TBI. Specifically, we simultaneously evaluated how ILS generated based on cognitive performance scores also captured variance in FIM-Cog scores at 12 months, mediated through the overall cognitive composite score, and how the ILS relationship to percent back to normal at 12 months was mediated by FIM-Cog scores. We applied Rasch-adjustment (Heinemann et al., 1994) to FIM-Cog and checked the normality assumption of the residuals for both FIM-Cog (Rasch-adjusted) and percent back to normal. Covariate-adjusted regression models used in the SEM analyses were fit simultaneously using the R package ‘lavaan’ version 0.6-6 (Rosseel et al., 2017). Root mean square error of approximation (RMSEA), standardized root mean square residual (SRMR) were used to examine SEM residuals, while the comparative fit index (CFI) and Tucker-Lewis index (TLI) were used to examine the fit of the SEM. The mediation percentage was calculated as the change in the effect of the ILS due to mediation by the FIM-cog and overall cognitive composite relative to the total effect on QOL.
3. Results
3.1. Description of population
Our cohort included n = 157 subjects with moderate-to-severe TBI with inflammatory data. Of these subjects, n = 119 and n = 125 completed all domains of cognitive testing at 6 and 12 months, respectively, with n = 20 and n = 11 participants cognitively unable to complete neuropsychological testing at 6 and 12 months, respectively. Table 1a, Table 1ba and 1b present detailed demographic and injury data by cognitive impairment status, including those unable to complete cognitive testing as a group. At 6 months post-injury, years of education (p < 0.0001), GCS (p < 0.0001), acute hospitalization length of stay (LOS) (p < 0.0001), FIM cognitive subscale score (p < 0.0001), and the CT finding of cerebral contusion (p = 0.0494) all differed by cognitive impairment status. At 12 months post-injury, years of education (p < 0.0001), GCS (p < 0.0001), acute hospitalization LOS (p < 0.0001), and FIM cognitive subscale score (p < 0.0001) differed by cognitive impairment status. Average FIM cognitive subscale scores at 6 and 12 months were much lower in those unable to complete cognitive testing (10.82 at 6 mo., 11.40 at 12 mo.) than those able to complete testing (33.25 and 29.76 at 6 mo., 33.12 and 30.51 at 12 mo. for unimpaired and impaired subjects respectively). Table 2 shows demographic and injury information significantly associated with overall cognitive composite T-scores at 6 and 12 months. Overall cognitive performance significantly differed with years of education (p < 0.0001; p = 0.0008), GCS (p < 0.0001; p < 0.0001), acute hospitalization LOS in days (p < 0.0001; p < 0.0001), FIM Cognitive subscale scores (p < 0.0001; p < 0.0001) at the corresponding timepoint when neuropsychological data was collected . Age and sex were not significantly associated with overall cognitive composite T scores at 6 or 12 months in bivariate analysis.
Table 1a.
Demographic and clinical variables by cognitive impairment status at 6 Months post-injury.
| 6 Month Cohort (n = 139) | ||||
|---|---|---|---|---|
| Not Cognitively Impaired (n = 63) | Cognitively impaired (n = 56) | Cognitively Unable to Complete Testing (n = 20) | p-value | |
| Age at Injury, mean (SE) | 40.22 (2.34) | 36.39 (2.25) | 35.10 (4.02) | 0.3525 |
| Sex, Men (%) | 50 (79.37%) | 43 (76.79%) | 14 (70.00%) | 0.6862 |
| Race, n (%) | 0.1302 | |||
| Caucasian | 58 (92.06%) | 53 (94.64%) | 16 (80.00%) | |
| African American and Other | 5 (7.94%) | 3 (5.36%) | 4 (20.00%) | |
| Years of Education, Mean (SE) | 13.65 (0.26) | 12.29 (0.23) | 13.58 (0.45) | 0.0010∗ |
| Mechanism of injury, n (%) | 0.7357 | |||
| Automobile/MVA | 29 (46.03%) | 25 (44.64%) | 11 (55.00%) | |
| Fall | 19 (13.67%) | 15 (26.79%) | 3 (15.00%) | |
| Violent/Gunshot Wound | 1 (1.59%) | 0 (0.00%) | 1 (5.00%) | |
| Motorcycle | 10 (15.87%) | 10 (17.86%) | 4 (20.00%) | |
| Other | 4 (6.35%) | 6 (10.71%) | 1 (5.00%) | |
| Best in 24 GCS, Median (IQR) | 10 (7–13) | 7 (6–8) | 6 (3.5–9) | <.0001∗ |
| Non-head ISS, Mean (SE)∗∗ | 10.73 (1.54) | 11.37 (1.90) | 15.54 (2.83) | 0.2287 |
| Acute Hospital Length of stay, Mean Days (SE) | 14.21 (1.00) | 23.80 (1.64) | 32.40 (3.29) | <.0001∗ |
| CT Head Findings, % with finding∗∗∗ | ||||
| Subarachnoid Hemorrhage | 65% | 63% | 80% | 0.4311 |
| Subdural Hematoma | 62% | 68% | 80% | 0.2424 |
| Epidural Hematoma | 12.7% | 20% | 15% | 0.5002 |
| Intraventricular Hemorrhage | 27% | 30% | 40% | 0.5477 |
| Intraparenchymal Hemorrhage | 43% | 45% | 65% | 0.2263 |
| Intracerebral Hemorrhage | 2% | 4% | 0% | 0.5588 |
| Diffuse Axonal Injury | 8% | 14% | 15% | 0.4444 |
| Cerebral Contusion | 46% | 57% | 75% | 0.0494∗ |
| FIM Cognitive Subscale: 6 Months, Mean (SE) | 33.25 (0.25) | 29.76 (0.68) | 10.82 (2.01) | <0.0001∗ |
Cognitive Impairment defined as Overall cognitive composite T-score < 40, Row totals are reported for categorical variables; ∗indicates statistical significance at p < 0.05; ∗∗ISS Non-head has large amount of missing data; ∗∗∗ Percentages across groups do not sum to 100% due to participants having >1 lesion type recorded from CT head.
Table 1b.
Demographic and clinical variables by cognitive impairment status at 12 Months post-injury.
| 12 Month Cohort (n = 136) | ||||
|---|---|---|---|---|
| Not Cognitively Impaired (n = 68) | Cognitively Impaired (n = 57) | Cognitively Unable to Complete Testing (n = 11) | p-value | |
| Age at Injury, mean (SE) | 39.06 (2.31) | 38.65 (2.26) | 35.18 (5.09) | 0.7609 |
| Sex, Men (%) | 53 (77.94%) | 44 (32.35%) | 7 (63.34%) | 0.5754 |
| Race, n (%) | 0.4394 | |||
| Caucasian | 63 (92.65%) | 53 (92.98%) | 9 (81.82%) | |
| African American and Other | 5 (7.35%) | 4 (7.02%) | 2 (18.18%) | |
| Years of Education, Mean (SE) | 13.43 (0.24) | 12.59 (0.26) | 13.63 (0.53) | 0.0424∗ |
| Mechanism of injury, n (%) | 0.7405 | |||
| Automobile/MVA | 35 (51.47%) | 23 (40.35%) | 8 (72.73%) | |
| Fall | 17 (25.00%) | 16 (28.07%) | 1 (9.09%) | |
| Violent/Gunshot Wound | 1 (1.47%) | 1 (0.74%) | 0 (0.00%) | |
| Motorcycle | 10 (14.71%) | 12 (21.05%) | 2 (1.47%) | |
| Other | 5 (7.35%) | 5 (8.77%) | 0 (0.00%) | |
| Best in 24 GCS, Median (IQR) | 10 (7–11) | 7 (5.5–9) | 6 (4–9) | <0.0001∗ |
| Non-head ISS, Mean (SE)∗∗ | 10.26 (1.58) | 12.42 (1.57) | 15.14 (4.25) | 0.2728 |
| Acute Hospital Length of stay (days), Mean (SE) | 14.91 (1.06) | 24.33 (1.49) | 31.90 (4.32) | <0.0001∗ |
| CT Head Findings, % with finding∗∗∗ | ||||
| Subarachnoid Hemorrhage | 66% | 61% | 82% | 0.6801 |
| Subdural Hematoma | 59% | 70% | 91% | 0.0721 |
| Epidural Hematoma | 13% | 16% | 27% | 0.5559 |
| Intraventricular Hemorrhage | 25% | 35% | 27% | 0.3567 |
| Intraparenchymal Hemorrhage | 46% | 46% | 64% | 0.6763 |
| Intracerebral Hemorrhage | 2% | 2% | 0% | 0.8953 |
| Diffuse Axonal Injury | 7% | 16% | 27% | 0.1236 |
| Cerebral Contusion | 53% | 49% | 82% | 0.2490 |
| FIM Cognitive Subscale12 Months, Mean (SE) | 33.12 (0.26) | 30.51 (0.54) | 11.40 (2.86) | <0.0001∗ |
Cognitive Impairment defined as Overall cognitive composite T-score < 40; Row totals are reported for categorical variables; ∗indicates statistical significance at p < 0.05; ∗∗ISS Non-head has large amount of missing data; ∗∗∗ Percentages across groups do not sum to 100% due to participants having >1lesion type recorded from CT head.
Table 2.
Demographic and clinical bivariate associations with overall cognitive composite T score.
| 6 Months Cognitive Composite Score (n = 119) |
12 Months Cognitive Composite Score (n = 125) |
|||
|---|---|---|---|---|
| R | p-value | R | p-value | |
| Years of Education | 0.384 | <0.0001∗ | 0.295 | 0.0008∗ |
| Best in 24 GCS | 0.404 | <0.0001∗ | 0.405 | <0.0001∗ |
| Acute Hospital Length of stay | −0.511 | <0.0001∗ | −0.458 | <0.0001∗ |
| FIM Cognitive Subscale at 6 or 12 Months | 0.489 | <0.0001∗ | 0.537 | <0.0001∗ |
Excludes subjects cognitively unable to complete testing; ∗indicates statistical significance at p < 0.05.
3.2. Early chronic cytokine associations with cognitive outcome and ILS formation
Bivariate linear regression associations of median standardized inflammatory marker levels from 1-3 months and overall cognitive composite T-scores at 6 and 12 months are summarized in Table 3. Markers selected for ILS inclusion were based on our p-value criterion of p < 0.1 at both timepoints. Markers include IL-1β, TNF-α, sIL-4R, sIL-6R, RANTES, and MIP-1β. The median ± IQR of the uILS was 17 [15–20.25]. The median ± IQR of the wILS was -0.023 [-0.076–0.043] and -0.029 [-0.077–0.040] for the 6- and 12-month timepoints, respectively.
Table 3.
Biomarkers for ILS derived from bivariate regression models with overall composite scores.
| Biomarker 1–3 Month Median | 6 Month Overall Composite (n = 119) |
12 Month Overall Composite (n = 125) |
||
|---|---|---|---|---|
| β (Standard Error) | p-value | β (Standard Error) | p-value | |
| IL-1 β | −1.54 (0.83) | 0.066 | −1.35 (0.76) | 0.077 |
| IL-7 | −1.48 (0.86) | 0.090 | −1.63 (0.79) | 0.042 |
| TNF α | −1.38 (0.82) | 0.093 | −1.44 (0.74) | 0.055 |
| sIL-4R | 2.34 (0.92) | 0.012 | 1.54 (0.78) | 0.051 |
| sIL-6R | −1.92 (0.90) | 0.036 | −1.45 (0.81) | 0.077 |
| MIP-1b | −1.84 (0.82) | 0.026 | −1.79 (0.74) | 0.017 |
| RANTES | −1.62 (0.97) | 0.097 | −2.42 (0.82) | 0.004 |
We also evaluated bivariate biomarker associations with cognitive subdomains for 6 and 12 months post-TBI (Table 4a, Table 4ba and 4b). Markers are presented if p < 0.10. Memory was associated with the greatest number of biomarkers for both 6- and 12-month timepoints. Significant markers selected for the ILS above were associated with multiple subdomains of cognition. sIL-4R was positively associated with all subdomains, and RANTES was negatively associated with all but attention and processing speed subdomain at 6 months (Table 4a, Table 4ba). At 12 months, RANTES was negatively associated with all subdomains and MIP-1β was negatively associated with all but the executive function subdomain (Table 4b).
Table 4a.
Bivariate associations with standardized biomarkers 6-month cognitive composite domain scores.
| Biomarker 1–3 Month Median |
Subdomains |
|||||||
|---|---|---|---|---|---|---|---|---|
| Memory 6M (n = 124) |
Attention & Processing Speed 6M (n = 126) |
Verbal Fluency 6M (n = 123) |
Executive Function 6M (n = 125) |
|||||
| β (SE) | p-value | β (SE) | p-value | β (SE) | p-value | β (SE) | p-value | |
| IL-1 β | −2.11 (1.11) | 0.060 | ||||||
| IL-5 | 2.21 (1.23) | 0.087 | ||||||
| IL-7 | −2.37 (1.14) | 0.040 | ||||||
| IL-8 | −2.32 (1.04) | 0.028 | ||||||
| IL-10 | 2.78 (1.26) | 0.030 | ||||||
| IL-17 | 3.94 (1.96) | 0.046 | ||||||
| sIL-4R | 3.31 (1.19) | 0.007 | 2.54 (1.12) | 0.026 | 1.77 (1.02) | 0.085 | 2.30 (1.11) | 0.040 |
| sIL-6R | −1.88 (1.10) | 0.090 | −2.46 (0.97) | 0.013 | ||||
| MIP-1a | −2.86 (1.23) | 0.021 | ||||||
| MIP-1b | −3.10 (1.08) | 0.005 | ||||||
| sTNF-R1 | −2.28 (1.01) | 0.026 | ||||||
| sTNF-R2 | −2.01 (1.00) | 0.046 | ||||||
| sICAM1 | −2.46 (1.06) | 0.022 | ||||||
| RANTES | −2.35 (1.04) | 0.025 | ||||||
| sgp130:IL-6R | 2.21 (1.28) | 0.086 | ||||||
Table 4b.
Bivariate associations with standardized biomarkers and 12-month cognitive composite domain scores.
| Biomarker 1–3 Month Median | Subdomains |
|||||||
|---|---|---|---|---|---|---|---|---|
| Memory 12M (n = 131) |
Attention & Processing Speed 12M (n = 129) |
Verbal Fluency 12M (n = 130) |
Executive Function 12M (n = 129) |
|||||
| β (SE) | p-value | β (SE) | p-value | β (SE) | p-value | β (SE) | p-value | |
| IL-1 β | −1.87 (1.05) | 0.077 | −1.74 (1.04) | 0.097 | ||||
| IL-7 | −2.11 (1.08) | 0.052 | −1.80 (1.08) | 0.099 | ||||
| IL-8 | −2.22 (1.05) | 0.037 | ||||||
| TNF α | −1.87 (1.03) | 0.073 | ||||||
| sIL-2Ra | −1.74 (0.82) | 0.037 | ||||||
| sIL-4R | 2.78 (1.05) | 0.009 | 2.30 (1.04) | 0.029 | ||||
| MIP-1a | −2.28 (1.16) | 0.051 | ||||||
| MIP-1b | −2.76 (1.03) | 0.008 | −2.18 (1.02) | 0.035 | −1.45 (0.86) | 0.096 | ||
| MIP-3a | −1.98 (1.11) | 0.075 | ||||||
| sTNF-R1 | −1.62 (0.91) | 0.077 | −1.72 (0.80) | 0.034 | ||||
| GM CSF | −1.46 (0.87) | 0.096 | ||||||
| sICAM1 | −2.04 (0.98) | 0.040 | ||||||
| RANTES | −2.58 (1.14) | 0.025 | −2.35 (1.14) | 0.042 | −2.21 (0.95) | 0.023 | −1.81 (0.85) | 0.034 |
| sgp130:IL-6R | 2.24 (1.19) | 0.063 | 2.27 (0.88) | 0.011 | ||||
3.3. Inflammatory burden associations with overall cognitive performance
Table 5 shows the 6-month multivariable regression model assessing uILS associations with overall cognitive composite scores. A one unit increase in uILS was associated with 0.50 unit decrease in overall cognitive composite scores. Years of education (β = 1.54, p = 0.0004) and GCS (β = 0.95, p = 0.001) were also associated with cognitive performance. The adjusted R2 was 0.28—a 15.23% relative improvement over the base model without uILS including GCS, age, sex, and years of education (adjusted R2=0.243). Using wILS, the relative improvement in adjusted R2 was about 34% (adjusted R2 = 0.326) over the base model (adjusted R2=0.244), and a 0.1 unit increase in wILS was associated with a 2.25 unit decrease in overall cognitive composite (Table 6). Table 7 presents the multivariable regression evaluating uILS associations with 12-month overall cognitive composite scores. A one unit increase in uILS was associated with 0.62 unit decrease in overall cognitive composite scores. Years of education (β = 1.06, p = 0.006) and GCS (β = 0.92, p = 0.0002) were positively associated with 12-month composite scores. The adjusted R2 was 0.27, which was a 31.70% relative improvement over the base model (adjusted R2=0.205). Using wILS, the relative improvement in adjusted R2 was 36.59% over the base model, and a 0.1 unit increase in wILS was associated with a 2.16 unit decrease in overall cognitive composite (Table 8). Women tended to have worse composites than men in both of the wILS and uILS multivariable models at 12 months post-injury despite adjusting for sex specific normative values where possible in the component neuropsychological tests used to formulate composite scores. Together, the data suggest wILS scores improve model performance over uILS scores.
Table 5.
Unweighted ILS – multivariable linear regression with 6-month overall cognitive composite.
| Variable | β (Standard Error) | p-value | Adjusted R2 |
|---|---|---|---|
| Age | 0.004 (0.05) | 0.936 | 0.243 for base model |
| Sex | −2.46 (2.06) | 0.235 | 0.280 adding ILS |
| Years Education | 1.54 (0.42) | 0.0004 | Difference = 0.037 or 15.23% improvement |
| Best in 24 GCS | 0.95 (0.28) | 0.001 | |
| Unweighted ILS | −0.50 (0.20) | 0.013 |
n = 106; Results based on Quartiled Inflammatory Markers with 6M Overall Composite.
Table 6.
Weighted ILS – multivariable linear regression with 6-month overall cognitive composite.
| Variable | β (Standard Error) | p-value | Adjusted R2 |
|---|---|---|---|
| Age | 0.03 (0.05) | 0.614 | 0.244 for basemodel |
| Sex | −2.41 (1.99) | 0.229 | 0.326 adding ILS |
| Years Education | 1.71 (0.40) | <.0001 | Difference = 0.082 or 33.61% improvement |
| Best in 24 GCS | 0.89 (0.27) | 0.001 | |
| Weighted ILS | −22.52 (5.98) | 0.0003 |
n = 107; Results based on Ridge Regression with 6M Overall Composite with maximum regularization within 1 SE of minimum MSE.
Table 7.
Unweighted ILS – multivariable linear regression with 12-month overall cognitive composite.
| Variable | β (Standard Error) | p-value | Adjusted R2 |
|---|---|---|---|
| Age | −0.05 (0.43) | 0.266 | 0.205 for base model |
| Sex | −2.85 (1.83) | 0.122 | 0.270 adding ILS |
| Years Education | 1.06 (0.38) | 0.006 | Difference = 0.065 or 31.70% improvement |
| Best in 24 GCS | 0.92 (0.24) | 0.0002 | |
| Unweighted ILS | −0.62 (0.18) | 0.001 |
n = 112; Results based on Quartiled Inflammatory Markers with 12M Overall Composite.
Table 8.
Weighted ILS – multivariable linear regression with 12-month overall cognitive composite.
| Variable | β (Standard Error) | p-value | Adjusted R2 |
|---|---|---|---|
| Age | −0.03 (0.04) | 0.571 | 0.205 for base model |
| Sex | −2.93 (1.82) | 0.110 | 0.280 adding ILS |
| Years Education | 1.17 (0.37) | 0.002 | Difference = 0.075 or 36.59% improvement |
| Best in 24 GCS | 0.92 (0.23) | 0.0002 | |
| Weighted ILS | −21.57 (6.01) | 0.001 |
n = 112; Results based on Ridge Regression with 6M Overall Composite with maximum regularization within 1 SE of minimum MSE.
3.4. Early chronic cytokine associations with cognitive impairment status
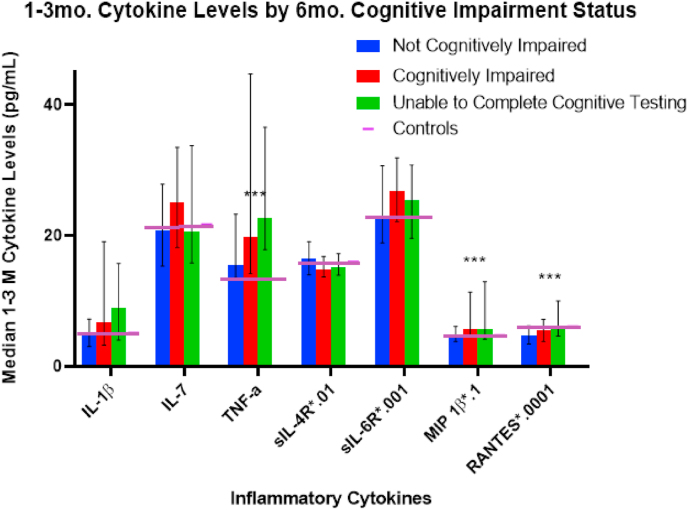
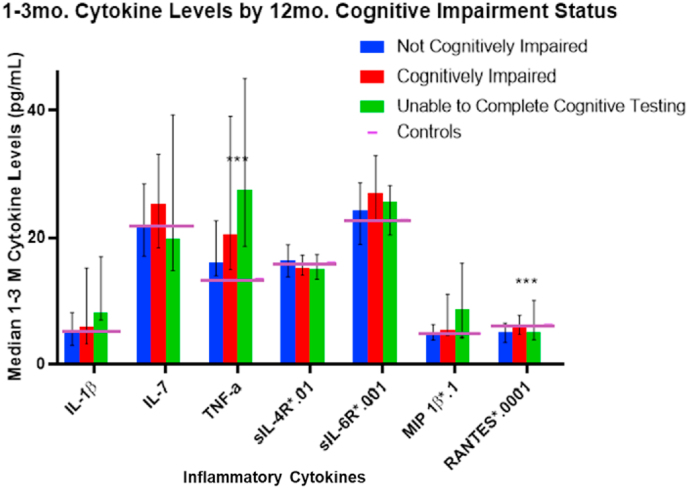
Fig. 1 depicts cytokine associations with 6-month cognitive impairment status, including those unable to complete cognitive testing. Median TNF-α levels for those with TBI was higher than among controls. Table 9 additionally shows the significant differences among groups for TNF-α (p = 0.007), RANTES (p = 0.0308), and MIP-1β (p = 0.045); trend level associations were noted for IL-1β and sIL-4R at 6 months post-injury. These bivariate results indicate that cognitively impaired and unable to complete groups had higher inflammatory burden. Similar patterns were noted for 12-month impairment status (Fig. 2) wherein TNF-α (p = 0.006) and RANTES (p = 0.019) differed by cognitive impairment status, and trend level associations were noted for IL-1β and sIL-6R at 12 months post-injury. Median TNF-α levels were higher than control levels in all TBI groups at both timepoints post-injury.
Fig. 1.
1- to 3-month Cytokine Median Levels by 6-Month Cognitive Impairment Status: Median marker levels are graphed by group membership, with levels scaled by a multiple of 10x to fit a 0 to 45 range. Error bars indicate 25th-75th percentile and control levels are included for reference. ∗∗∗ indicates statistical significance at p < 0.05.
Table 9.
1–3 M median cytokine levels by 6- and 12-month cognitive impairment status.
| 6-Month Cohort (n = 139) |
12-Month Cohort (n = 136) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Not Cognitively Impaired | Cognitively Impaired | Unable to Complete Testing | p-value | Not Cognitively Impaired | Cognitively Impaired | Unable to Complete Testing | p-value | |
| IL-1β | 4.98 | 6.82 | 8.92 | 0.099 | 5.23 | 5.80 | 8.16 | 0.074 |
| IL-7 | 20.80 | 25.05 | 20.68 | 0.169 | 21.40 | 25.19 | 19.89 | 0.368 |
| TNF-α | 15.51 | 19.80 | 22.61 | 0.007 | 15.93 | 20.47 | 27.53 | 0.006 |
| sIL-4R ∗.01 | 16.43 | 14.84 | 15.13 | 0.077 | 16.31 | 15.07 | 14.90 | 0.432 |
| sIL-6R ∗.001 | 22.95 | 26.81 | 25.47 | 0.143 | 24.23 | 27.08 | 25.58 | 0.067 |
| MIP 1β ∗.1 | 4.93 | 5.73 | 5.76 | 0.045 | 4.95 | 5.267 | 8.70 | 0.122 |
| RANTES∗.0001 | 4.78 | 5.60 | 5.67 | 0.031 | 4.91 | 5.99 | 5.01 | 0.019 |
Fig. 2.
1- to 3-month Cytokine Median Levels by 12-Month Cognitive Impairment Status: Median marker levels are graphed by group membership, with levels scaled by a multiple of 10x to fit a 0 to 45 range. Error bars indicate 25th-75th percentile and control levels are included for reference. ∗∗∗ indicates statistical significance at p < 0.05.
3.5. ILS associations with multidimensional outcomes
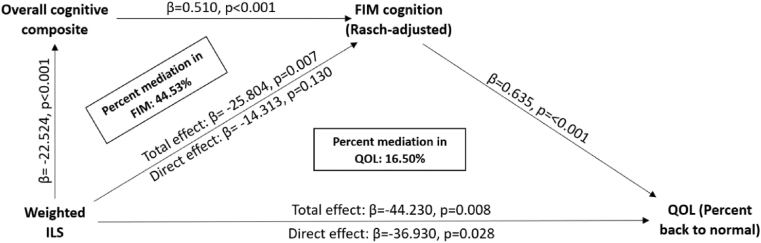
A covariate adjusted (age, sex, GCS, education, depression status) SEM was built with N = 108 subjects, and it was used to test the wILS association with 12-month QOL scores and the proportion of covariance in this relationship attributable to wILS associations with overall cognitive composite scores and FIM-Cog scores. The RMSEA and SRMR were <0.001 (p-value: 0.848) and 0.002 (recommended: p≤0.05), indicating small residuals. The CFI and TLI were 1.0 and 1.178, respectively, meeting the recommended >0.95 criteria, ensuring a satisfactory fit. Regression models showed significant wILS effects on QOL through FIM-Cog and cognitive composite scores (Fig. 3). The SEM indicated that at 12 months, 44.53% of the wILS and FIM-Cog (Rasch-adjusted) relationship was due to wILS relationship with overall cognitive composite, and 16.50% of the wILS and QOL relationship was due to wILS relationships with cognitive composite scores and FIM-Cog. Covariates in each of the paths are reported in Table 10. Notably, this 12-month model shows that in addition to education and injury severity, women performed significantly worse than men with respect to overall cognitive performance. Also, depression was a potent predictor of FIM-Cog scores and QOL, second in effect size only to ILS in both models.
Fig. 3.
SEM of QOL (12mo.) with ILS. FIM cognition (Rasch-adjusted) and overall cognitive composite (n = 108).
Table 10.
SEM of QOL (12mo.) with ILS, FIM cognition (Rasch-adjusted) and overall cognitive composite (n = 108).
| Regressions: | ||||
|---|---|---|---|---|
| Coefficients | Std. Err | z-value | P-value | |
| Percent back to normal ~ | ||||
| ILS | −36.93 | 16.822 | −2.195 | 0.028 |
| Age | −0.06 | 0.114 | −0.529 | 0.597 |
| Sex (Male) | −5.787 | 4.744 | −1.22 | 0.222 |
| Years of education | 0.55 | 0.966 | 0.569 | 0.569 |
| GCS | 0.3 | 0.621 | 0.483 | 0.629 |
| PTD | −12.392 | 5.474 | −2.264 | 0.024 |
| Overall cognitive composite ~ | ||||
| ILS | −22.524 | 6.264 | −3.596 | <0.001 |
| Age | −0.019 | 0.043 | −0.441 | 0.659 |
| Sex (Male) | −3.996 | 1.823 | −2.192 | 0.028 |
| Years of education | 1.284 | 0.368 | 3.485 | <0.001 |
| GCS | 0.86 | 0.235 | 3.658 | <0.001 |
| PTD | 0.289 | 2.004 | 0.144 | 0.885 |
| FIM Cog ~ | ||||
| Overall cognitive composite | 0.51 | 0.137 | 3.713 | <0.001 |
| ILS | −14.313 | 9.464 | −1.512 | 0.13 |
| Age | −0.084 | 0.062 | −1.356 | 0.175 |
| Sex (Male) | 3.742 | 2.66 | 1.407 | 0.159 |
| Years of education | 0.133 | 0.555 | 0.24 | 0.81 |
| GCS | 0.24 | 0.356 | 0.676 | 0.499 |
| PTD | −10.399 | 2.862 | −3.634 | <0.001 |
| Percent back to normal ~ | ||||
| FIM Cog | 0.635 | 0.165 | 3.853 | <0.001 |
| Defined Parameters: | ||||
| Indirect effect on FIM Cog | −11.491 | 4.448 | −2.583 | 0.01 |
| Total effect on FIM Cog | −25.804 | 9.498 | −2.717 | 0.007 |
| Indirect effect on QOL | −7.3 | 3.403 | −2.146 | 0.032 |
| Total effect on QOL | −44.23 | 16.686 | −2.651 | 0.008 |
4. Discussion
While acute neuroinflammation has been well accepted as a major feature of secondary injury after TBI, the role of systemic inflammation, particularly in the chronic phases of recovery, remains understudied. We have previously shown early, chronic, systemic inflammation maps to global recovery post-TBI (i.e. GOS scores at 6 and 12 months), yet no work published to date has focused on systemic inflammation effects on cognitive dysfunction and/or its downstream impacts on multiple dimensions of function after TBI. Few studies focus on inflammatory biomarker load score development or consider advanced statistical methodologies to deal with issues common to biomarker score formulation, such as collinearity among biologically interrelated markers with diverse functions.
Thus, our goals for this study were: 1) to identify relationships between early chronic inflammatory burden and long-term cognitive outcome after TBI; 2) to compare different methodologies of ILS formulation (weighted and unweighted score formulation) and their capacity to discriminate cognitive outcomes; and 3) assess cognitive performance derived ILS scores for their relationships to functional outcomes measures using a mediation model approach. Both ILS formulation methods captured additional variance in cognitive performance that was not accounted for by our base model of age, education, sex, and GCS, showing that systemic inflammatory burden in the early chronic phase of injury is independently related to cognitive outcome after TBI. The wILS accounted for a larger absolute and percent change in adjusted R2 than the uILS, and the ridge regression approach allowed for the derivation of stable β-weights when considering biologically diverse markers with a high degree of statistical collinearity. Further, the data show that the systemic inflammatory burden associated with objective cognitive performance deficits also impacts downstream measures of function, such as functional cognition and QOL metrics.
The work presented here is seminal to the TBI field, in that there are no previous reports of systemic inflammatory biomarkers and their capacity to predict cognitive performance in a clinical population with moderate-to-severe TBI. Cognitive functioning is a complex trait, and factors like age, sex, education, cognitive reserve, and comorbidities all play a role in affecting neuropsychological testing performance in the general as well as specific clinical populations (Green et al., 2008; Rabinowitz et al., 2018; Schneider et al., 2014; Brunner, 2005). Our models include many of these key contributors, even when adjusting for these factors using population normative data. Yet the additional biological information, in this case systemic inflammation, significantly improves overall model performance. Published statistical models capturing variation in neuropsychological testing performance in young healthy populations, such as those at risk for TBI are sparse, yet models exist predicting cognitive decline in late middle age using dementia, stroke, and cardiovascular disease risk scores (Kaffashian et al., 2013). Interestingly, a meta-analysis assessing risk for cognitive decline among subjects from large clinical studies involving elderly individuals shows inflammation and oxidative stress markers can considerably improve cognitive decline prediction models (Harrison et al., 2017). Other studies predict dementia risk and progression with variable effectiveness (Licher et al., 2018a, 2018b; Andrews et al., 2017, 2019; de Wolf et al., 2020). Similar to our study, however, significant model improvements in variance capture occur when adding biological information (Licher et al., 2018a; Lewczuk et al., 2018).
Notably, the weighted ILS approach uses a novel application of ridge regression to derive adjusted β-values, which allowed us to account for marker collinearity when creating our wILS (Hoerl and Kennard, 1970). The degree of collinearity observed within our cohort for the inflammatory panel presented would normally preclude using a large number of associated markers in the same regression model to derive adjusted β-weights because the regression coefficients can be unstable in presence of multicollinearity (Farrar and Glauber, 1967). We addressed the statistical issue of biologically interrelated, yet mechanistically diverse markers, by using ridge regression to derive coefficients for creating the wILS. The utility of ridge regression-derived β-coefficients is that the approach applies a “regularization” penalty term to each model term to limit the impact of collinearity on the β-coefficients generated for each marker (Hoerl and Kennard, 1970). In the context of statistical and machine learning models, the regularized model achieves more stable β-coefficients by substantially reducing the standard errors at the cost of a very small bias added to the parameter estimates. This novel application of ridge regression to generate stable β-coefficients for wILS formulation advances the biomarker field broadly. The work also builds upon our previous approach to uILS formation in the moderate-to-severe TBI population to demonstrate associations between a uILS and GOS after TBI (Kumar et al., 2014) by demonstrating the importance of systemic inflammatory burden to cognitive performance after TBI and the relevance of systemic inflammation to functional measures impacted by cognitive performance deficits.
The neuroimmune response is largely mediated by resident microglia that undergo rapid activation upon surveillance and detection of central nervous system (CNS) damage after TBI (Loane and Byrnes, 2010; Loane and Kumar, 2016). Activated microglia facilitate and perpetuate inflammatory cascades that promote systemic cellular immunity infiltration into the CNS to clear debris and dead neural tissues and cells (Jeong et al., 2013). Further, our work suggests that acute cortisol levels impact neuroinflammation, seemingly through divergent pathways, wherein increases in cerebrospinal fluid cortisol can facilitate a permissive immune response or promote a state of immunoparalysis, each of which can negatively impact outcome (Santarsieri et al., 2014a). This point is interesting because physiologically and in response to acute injury, the brain and the systemic immune system communicate via the sympathetic nervous system (SNS) (Elenkov et al., 2000; Kenney and Ganta, 2014) and via Hypothalamic-Pituitary-Adrenal (HPA) axis modulation (Elenkov et al., 2000). SNS activation has direct effects in lymphoid organs and liver that support cytokine production (Baumann and Gauldie, 1016; Dinarello, 1093; Heinrich et al., 1042; Kossmann et al., 1097). After the initial systemic inflammatory response, acute immunosuppression results from SNS activation and an innate immune response, which depletes lymphocytes and increases acute infection risk (Kourbeti et al., 2012; Schirmer-Mikalsen et al., 2013; Esnault et al., 2017). HPA activation drives excess CSF cortisol levels that impact neuroinflammation and also BDNF relationships with mortality and global outcome (Santarsieri et al., 2014a, 2014b; Munoz et al., 2017). Systemic infection often co-occurs with critical illness, propagates systemic inflammation, and perpetuates non-neurological organ dysfunction (Kemp et al., 2008; Zygun et al., 2005). For example, hospital acquired pneumonia (HAP) can occur in ~30%–33% of the population with moderate to severe TBI (Kesinger et al., 2015; Kumar et al., 2020). Together, these phenomena likely shape the early chronic systemic inflammatory profiles observed here after moderate-to-severe TBI. Inflammatory markers are quite variable in our TBI cohort, yet Fig. 1, Fig. 2 show many individuals have levels well above referenced controls, with higher values generally predicting lower cognitive composite scores and injury related deficits that preclude ability to complete cognitive testing. Some biomarker median values are not measurably different than controls, though, suggesting that biomarker levels do not necessarily have to be extremely “elevated” or “deficient” to be associated with chronic TBI pathology. Multiple inflammatory markers mapping to cognitive performance after TBI have also been implicated in cognition and in neurodegenerative and other diseases affecting cognition.
IL-1β and TNF-α have been mechanistically characterized in literature as mediators of cognitive impairment. For, example, CNS lipopolysaccharide (LPS) infusion studies show that TNF-α mediates chronic inflammation induced hippocampal dysfunction and cognitive impairment (Belarbi et al., 2012). Abnormal TNF-α activation is also associated with neurodegeneration, and increased systemic TNFR1 and IL-1β levels are implicated with Alzheimer’s disease risk in elderly adults (Tan et al., 2007; Diniz et al., 2010). Exogenous IL-1β infusion in mice also reduces spatial (i.e. hippocampal dependent) learning (Gibertini et al., 1995). Importantly, deficits in visuospatial learning in mouse TBI models may improve after IL-1β neutralization treatment (Clausen et al., 2011). We show high TNF-α levels as also associated with inability to complete cognitive testing. While higher TNF-α and IL-1β levels showed trends associated with overall cognitive composite scores at 6 and 12 months, both were highly associated with worse memory composite scores at 6 and 12 months post-TBI. Together with the literature, these data suggest a specific vulnerability of hippocampal function to inflammation, particularly with markers that propagate the innate immune response.
Soluble Interleukin-4 Receptor (sIL-4R) is uniquely and positively associated with both overall cognition and each cognitive sub-domain, which we report is a novel finding. Biologically, sIL-4R is generated via proteolytic cleavage of the transmembrane IL-4R and can modulate IL-4 and IL-13 signaling (Gessner and Röllinghoff, 2000; Andrews et al., 2006). sIL-4R competitively inhibits IL-4 signaling in a dose dependent manner by binding to IL-4 and preventing it from binding to its transmembrane receptor, but this soluble receptor also modulates IL-4 transmission by altering biodistribution of the cytokine (Gessner and Röllinghoff, 2000; Andrews et al., 2006; Sato et al., 1993). Growing evidence suggests IL-4 signaling, via Th2 type differentiated T-helper cellular release, is important for learning and memory (Karo-Atar et al., 2018). Derecki et al. reports that IL-4 producing Th2 cells accumulate in meninges after mice are exposed to the Morris Water Maze (MWM) as a visuospatial learning and memory task, and cognitive performance in IL-4 knockout mice improves after infusion of IL-4 producing Th2 cells (Derecki et al., 2010). Similar findings occur with IL-13 production after MWM, wherein both IL-4 and IL-13 stimulate astrocytes in the meninges and hippocampus (Brombacher et al., 2017) to improve cognitive function. After cerebral ischemia, IL-4R mRNA levels are increased, which may potentiate IL-4 signaling to stabilize cognitive performance after stroke (Liu et al., 2016). Further, other studies demonstrate IL-4 administration can improve cognitive performance after stroke in mice deficient in endogenous IL-4 production (Zhang et al., 2019). Despite data linking IL-4 signaling to cognition, to our knowledge, this is the first known study to identify consistent positive associations between sIL-4R levels and cognitive performance in any clinical population and represents a novel finding that warrants further study on its primary actions and as a potential treatment target affecting cognitive outcomes after TBI.
Another interesting finding is the significance of soluble Interleukin-6 Receptor (sIL-6R) with cognitive outcome, particularly overall cognition and verbal fluency. sIL-6R is generated by either proteolytic cleavage of membrane bound IL-6R or translation of alternatively spliced mRNA. It specifically binds with IL-6 and potentiates pro-inflammatory trans-signaling, with ubiquitous activity on any cell type (Rose-John et al., 2006; Morieri et al., 2017). IL-6 trans-signaling is a dominant mechanism driving many forms of CNS pathology (Campbell et al., 2014). CNS IL-6 signaling can also perpetuate blood brain barrier failure after TBI, meaning systemic IL-6 family cytokine levels are potentially relevant for TBI outcome prediction specifically (Shlosberg et al., 2010; Rochfort and Cummins, 2015). Increased sIL-6R trans-signaling is also associated with Alzheimer disease clinically, and inhibition of trans-signaling decreases amyloid plaque burden in mice (Hampel et al., 1999; Escrig et al., 2019). However, recent studies using a rodent model of experimental TBI suggest a potential role for sIL-6R trans-signaling in supporting adult neurogenesis and cognitive function through neuron-microglia interactions in rodent models (Willis et al., 2020). Yet sgp130 in a ratio with sIL-6R, has a positive association with memory scores in our study, which may be due to sgp130 effects in neutralizing sIL-6R trans-signaling (Morieri et al., 2017). Together, our data in combination with this literature suggests that IL-6 trans-signaling effects on CNS recovery, damage, and repair are likely both nuanced and complex.
MIP-1β and RANTES are important chemokines that facilitate immune cell chemotaxis; both act in the CNS and periphery through the CCR5 receptor to facilitate microglial and macrophage chemotaxis (Sorce et al., 2011). CNS MIP-1β levels are elevated in neurodegenerative and autoimmune disorders such as multiple sclerosis (MS), particularly in microglia near the white matter surrounding MS lesions (Simpson et al., 1998). RANTES specifically is elevated in the plasma and brain tissue of subjects with mild cognitive impairment or Alzheimer disease, but its effects can be both neurodegenerative through immune modulation via the CCR5 receptor or neuroprotective due to CCR5 effects on neuronal survival (Sorce et al., 2011; Marksteiner et al., 2011; Tripathy et al., 2010; Stuart and Baune, 2014). CSF MIP-1β levels in Alzheimer’s disease patients are associated with cognitive decline (Taipa et al., 2019). In our results, RANTES and MIP-1β are both associated with worse cognitive performance after TBI in overall composite scores and also across multiple sub-domains of cognition.
We show novel associations between IL-7 and worse overall cognitive and memory composite scores. IL-7 physiologically is essential for development of adaptive immune cells and modulates T-cell homeostasis, particularly augmenting autoimmunity (Lundström et al., 2012). Both low IL-7 and IL-7 receptor levels are associated with increased MS risk (Lundmark et al., 2007; Fernández-Paredes et al., 2017). In contrast, our recent clinical research has shown positive associations with chronic IgM class auto-antibody production after TBI that may be protective against later-occurring conditions like secondary hypogonadism, with auto-antibody production associated with systemic IL-7 levels (Vijapur et al., 2020). This is the first study, to our knowledge, to show an IL-7 association with memory and overall cognitive performance on neuropsychological testing in a clinical population. In addition, work from our group suggests that brief intermittent administration of rhIL-7 in mice can improve behavioral recovery after experimental TBI (in preparation). However, CSF IL-7 levels are reportedly elevated in Alzheimer disease and predict disease progression in frontotemporal dementia (Taipa et al., 2019). Yet with this current study, higher levels of endogenous IL-7 were associated with worse cognitive composite scores among those completing neuropsychological testing, while relatively lower IL-7 was associated with both good cognitive test performance as well as the inability to complete cognitive testing suggesting that IL-7 may have complex dose-dependent effects on other aspects of inflammation (e.g. innate immunity; auto-antibody production) that influence recovery (Vijapur et al., 2020; Sheikh and Abraham, 2019). Thus, further work will need to delineate the potential positive and negative effects of this molecule on cognitive recovery, specifically in the TBI population.
Memory is a large contributor to ILS relationships with overall performance in our analyses, and multiple additional markers not in ILS are associated with the memory sub-domain. Interestingly, levels IL-5, IL-10, and IL-17 are associated with higher memory subdomain scores, as well as sIL-4R and sgp130:sIL-6R ratios discussed above. IL-5 is important for B-cell and eosinophil maturation (Takatsu and Nakajima, 2008). However it is unclear exactly how IL-5 might influence TBI and/or cognition, though B-cell mediated autoantibody production may be relevant to tissue repair and recovery as noted above. IL-10 antagonizes the autoimmune effects of IL-6 and an increased ratio of IL-6:IL-10 has been previously shown by our group to have deleterious effects on global outcome (Kumar et al., 2014). IL-17 supports memory by increasing hippocampal long-term potentiation (LTP) and synaptic plasticity (Ribeiro et al., 2019). The hippocampus is particularly vulnerable to neuroinflammation due, in part, to its negative impacts on LTP, synaptic plasticity, and reduced neurotransmission, particularly with neuroinflammatory diseases like MS (Mancini et al., 2017). Systemic inflammation, including from aging, chronic intestinal inflammation, and other chronic disease states also influences hippocampal neurogenesis (Zonis et al., 2015; Chesnokova et al., 2016; Hill et al., 2019), a phenomenon also important to TBI recovery (Xiong et al., 2011; Blaya et al., 2019; Carlson and Saatman, 2018). The effects of chronic inflammation on hippocampal function after TBI may be one reason for strong relationships between memory and inflammatory markers in our analyses.
Literature from our group points to the relevance of biological heterogeneity in characterizing relationships between disease/injury and health/function, and we have conceptualized these relationships through the Rehabilomics research framework (Wagner, 2010, 2017; Wagner and Zitelli, 2013). Importantly, impairment in CNS body functions like depression also have ties to inflammation acutely after TBI (Juengst et al., 2015a), and both cognition and depression can have a profound impact on functional domains like activities of daily living, participation in social and societal roles (Failla et al., 2016; Juengst et al., 2015b). Our results suggest systemic inflammation, particularly as operationalized by the wILS, can impact CNS impairments like cognition and have resultant effects on functional measures, like FIM-Cog and QOL scores. While wILS was related to QOL (i.e. percent back to normal scores), overall cognitive composite and FIM-Cog scores were in the causal pathway, and depression was adjusted for in the various regression model components of the mediation. This finding emphasizes how systemic inflammation can indirectly influence function through its pathological effects on objective impairments, in this case neuropsychological test performance. These mediation models showing early chronic systemic inflammation effects on functional outcomes are seminal to the field as they provide direct evidence that biomarkers can capture complex relationships between disease, impairment, and function consistent with and as hypothesized with the Rehabilomics research model (Wagner, 2010, 2017; Wagner and Zitelli, 2013). Also, these findings show that mediation analyses are effective in dissecting complex relationships between biology and function.
Sex differences were noted at the trend level for 12-month multivariable models predicting cognitive composite test scores using the wILS and the uILS and was a significant predictor of cognitive deficits in the SEM associated regression (Table 10). Sex differences in computerized neurocognitive testing after concussion have been assessed in numerous reports, with many noting sex differences on visual spatial testing performance (Covassin et al., 2013; Majerske et al., 2008). However, variation with use and availability of normative data among child and adolescent athletes in score reporting, along with gender differences in post-concussive management, complicate the application of these findings (Majerske et al., 2008). Fewer studies have evaluated sex differences in test performance among those with moderate-to-severe TBI. Major studies in this population suggests women perform better in areas such as verbal memory, attention, and working memory (Ratcliff et al., 2007), however the lack of normative test scoreuse/reporting with neuropsychological data reporting complicates interpretation of TBI-specific sex differences. In fact, demographic differences in NIH toolbox neuropsychological test score performance among TBI/Stroke populations account for ~1/3 of the variance in scores, a finding that drops to ~5% when correcting for demographic differences in the general population (Nitsch et al., 2017). Our findings suggest that despite correcting for demographic influences on component score performance, women with TBI perform worse when adjusting for covariates like education and injury severity. Sex differences also exist with inflammatory response characteristics, including T-cell immunity, risk for autoimmune disease, and immune contributions to longevity, behavior, and dendritic cell function (Ahnstedt and McCullough, 2019; Gold et al., 2019; Lasselin et al., 2018; Laffont et al., 2017; Austad and Bartke, 2016). However, there were no significant interactions with sex and ILS scores in our study. Sex differences, with women as the risk group, have been reported in how dopamine genetics influence cognitive performance after TBI (Myrga et al., 2016), however future work is needed more broadly to better understand TBI-specific sex differences in cognitive performance.
Depression status, as measured by the PHQ-9, was associated with both QOL (percent back to normal) and functional cognition (FIM-Cog). These findings are consistent with other TBI research suggesting a link between mental health and life satisfaction (Juengst et al., 2015b; Rauen et al., 2020), as well as functional cognition metrics (Failla et al., 2016). We did not specifically assess inflammatory associations with PTD, even though reports exist suggesting inflammation as a contributor to PTD (Juengst et al., 2017) and that early inflammatory cascades influence later depression risk (Juengst et al., 2015a). Our future work will focus on how chronic inflammation affects PTD risk and its downstream impacts on functional outcomes.
Study limitations include the absence of pre-injury biomarker levels or neuropsychological testing results for our TBI cohort. However, normative reference data was used to develop cognitive composite T-scores, reducing the impact of this limitation and healthy control serum samples were measured to provide an inflammatory assay reference group. Although we identified differences in marker levels based on broad groupings of impairment status and those unable to complete cognitive testing, ILS analyses only included survivors and subjects cognitively and physically able to complete neuropsychological testing. Yet the inclusion of those cognitively unable to participate in formal neuropsychological testing suggests that some aspects of systemic inflammatory burden may be worse in these individuals. This work is also observational in nature and we cannot fully establish causality between changes in serum inflammatory biomarker levels and cognitive composite T-scores.
5. Conclusion
Despite these limitations, this is the first study examining the relationship between early chronic inflammation and cognitive outcome after TBI, and novel inflammatory marker associations with cognition have been identified through this work that may be useful in identifying modifiable treatment targets to reduce systemic inflammatory burden after TBI. We also demonstrate the potential importance of systemic inflammation in perpetuating neuroinflammatory burden, and future studies are necessary to characterize CNS-systemic inflammation relationships with neuroinflammation, as well as the mechanistic underpinnings for these observational findings. Additional work should validate ILS as a predictor of cognitive status after TBI and explore immunogenetic relationships to outcome. Further, inflammatory relationships with other secondary conditions after TBI should be explored as should their relationships with multiple domains of function. Importantly, the work presented here, linking inflammatory burden associated with cognitive deficits to functional outcome post-TBI, demonstrates the potential functional impact of immunotherapy interventions aimed at improving cognitive recovery post-TBI.
Funding support
This work was supported in part by NIDILRR (National Institute for Independent Living and Rehabilitation Research) USA 90DP004, CDC (Centers for Disease Control) USA R49 CCR 23155, DoD (Department of Defense) USA W81XWH-071-0701, DoD PT170100 W81XWH1810803, UPSOM (University of Pittsburgh School of Medicine) Dean’s Summer Research Program.
Declaration of competing interest
None.
Acknowledgements
The authors would like to thank Jessa Darwin for her editorial assistance in preparing this manuscript. This project used the UPCI Cancer Biomarkers Facility: Luminex Core Laboratory that is supported, in part, by the National Institute of Health [NIH P30CA047904].
References
- Ahnstedt H., McCullough L.D. The impact of sex and age on T cell immunity and ischemic stroke outcomes. Cell. Immunol. 2019;345:103960. doi: 10.1016/j.cellimm.2019.103960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andaluz-Ojeda D., Bobillo F., Iglesias V. A combined score of pro- and anti-inflammatory interleukins improves mortality prediction in severe sepsis. Cytokine. 2012;57(3):332–336. doi: 10.1016/j.cyto.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Andrews A.-L., Holloway J.W., Holgate S.T., Davies D.E. IL-4 receptor α is an important modulator of IL-4 and IL-13 receptor binding: implications for the development of therapeutic targets. J. Immunol. 2006;176(12):7456–7461. doi: 10.4049/jimmunol.176.12.7456. [DOI] [PubMed] [Google Scholar]
- Andrews S.J., Eramudugolla R., Velez J.I., Cherbuin N., Easteal S., Anstey K.J. Validating the role of the Australian National University Alzheimer’s Disease Risk Index (ANU-ADRI) and a genetic risk score in progression to cognitive impairment in a population-based cohort of older adults followed for 12 years. Alzheimer’s Res. Ther. 2017;9(1):16. doi: 10.1186/s13195-017-0240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews S.J., McFall G.P., Dixon R.A., Cherbuin N., Eramudugolla R., Anstey K.J. Alzheimer’s environmental and genetic risk scores are differentially associated with general cognitive ability and dementia severity. Alzheimer Dis. Assoc. Disord. 2019;33(2):95–103. doi: 10.1097/WAD.0000000000000292. [DOI] [PubMed] [Google Scholar]
- Austad S.N., Bartke A. Sex differences in longevity and in responses to anti-aging interventions: a mini-review. Gerontology. 2016;62(1):40–46. doi: 10.1159/000381472. [DOI] [PubMed] [Google Scholar]
- Baker S.P., O’Neill B., Haddon W., Jr., Long W.B. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J. Trauma. 1974;14(3):187–196. [PubMed] [Google Scholar]
- Baumann H, Gauldie J. The acute phase response. Immunol. today. doi:10.1016/0167-5699(94)90137-6. [DOI] [PubMed]
- Belarbi K., Jopson T., Tweedie D. TNF-α protein synthesis inhibitor restores neuronal function and reverses cognitive deficits induced by chronic neuroinflammation. J. Neuroinflammation. 2012;9:23. doi: 10.1186/1742-2094-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaya M.O., Wasserman J.M., Pieper A.A., Sick T.J., Bramlett H.M., Dietrich W.D. Neurotherapeutic capacity of P7C3 agents for the treatment of traumatic brain injury. Neuropharmacology. 2019;145:268–282. doi: 10.1016/j.neuropharm.2018.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkowski J.G., Benton A.L., Spreen O. Word fluency and brain damage. Neuropsychologia. 1967;5:135–140. [Google Scholar]
- Brombacher T.M., Nono J.K., Gouveia K.S.D. IL-13–Mediated regulation of learning and memory. J. Immunol. 2017;198(7):2681–2688. doi: 10.4049/jimmunol.1601546. [DOI] [PubMed] [Google Scholar]
- Brunner E.J. Social and biological determinants of cognitive aging. Neurobiol. Aging. 2005;26(1, Suppl. ment):17–20. doi: 10.1016/j.neurobiolaging.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Campbell Iain, Erta Maria, Lim Sue Ling, Frausto Ricardo, May Ulrich, Rose-John Stephan, Scheller Jurgen, Hidalgo Juan. Trans-signaling is a dominant mechanism for the pathogenic actions of interleukin-6 in the brain. J Neurosci. 2014 Feb 12;34(7):2503-13. doi: 10.1523/JNEUROSCI.2830-13.2014. Journal of Neuroscience. 2014;34(7):2503–2513. doi: 10.1523/JNEUROSCI.2830-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson S.W., Saatman K.E. Central infusion of insulin-like growth factor-1 increases hippocampal neurogenesis and improves neurobehavioral function after traumatic brain injury. J. Neurotrauma. 2018;35(13):1467–1480. doi: 10.1089/neu.2017.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnokova V., Pechnick R.N., Wawrowsky K. Chronic peripheral inflammation, hippocampal neurogenesis, and behavior. Brain Behav. Immun. 2016;58:1–8. doi: 10.1016/j.bbi.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen F., Hånell A., Israelsson C. Neutralization of interleukin-1β reduces cerebral edema and tissue loss and improves late cognitive outcome following traumatic brain injury in mice. Eur. J. Neurosci. 2011;34(1):110–123. doi: 10.1111/j.1460-9568.2011.07723.x. [DOI] [PubMed] [Google Scholar]
- Correia L.C.L., Andrade B.B., Borges V.M. Prognostic value of cytokines and chemokines in addition to the GRACE Score in non-ST-elevation acute coronary syndromes. Clin. Chim. Acta. 2010;411(7–8):540–545. doi: 10.1016/j.cca.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Covassin T., Elbin R.J., Crutcher B., Burkhart S. The management of sport-related concussion: considerations for male and female athletes. Transl Stroke Res. 2013;4(4):420–424. doi: 10.1007/s12975-012-0228-z. [DOI] [PubMed] [Google Scholar]
- de Wolf F., Ghanbari M., Licher S. Plasma tau, neurofilament light chain and amyloid-β levels and risk of dementia; a population-based cohort study. Brain. 2020;143(4):1220–1232. doi: 10.1093/brain/awaa054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis D.C. Psychological Corporation; 1987. CVLT-II, California Verbal Learning Test: Adult Version : Manual. [Google Scholar]
- Delis D., Kaplan E. The Psychological Corporation; 2001. Delis-Kaplan Executive Function System (DKEFS) [Google Scholar]
- Derecki N.C., Cardani A.N., Yang C.H. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J. Exp. Med. 2010;207(5):1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikmen S., Temkin N., McLean A., Wyler A., Machamer J. Memory and head injury severity. J. Neurol. Neurosurg. Psychiatry. 1987;50(12):1613–1618. doi: 10.1136/jnnp.50.12.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikmen S.S., Machamer J.E., Winn H.R., Temkin N.R. Neuropsychological outcome at 1-year post head injury. Neuropsychology. 1995;9(1):80–90. doi: 10.1037/0894-4105.9.1.80. [DOI] [Google Scholar]
- Dinarello CA. Interleukin-1. Rev. Infect. Dis.. doi:10.1093/clinids/6.1.51. [DOI] [PubMed]
- Diniz B.S., Teixeira A.L., Ojopi E.B. Higher serum sTNFR1 level predicts conversion from mild cognitive impairment to Alzheimer’s disease. J Alzheimers Dis JAD. 2010;22(4):1305–1311. doi: 10.3233/JAD-2010-100921. [DOI] [PubMed] [Google Scholar]
- Dodds T.A., Martin D.P., Stolov W.C., Deyo R.A. A validation of the functional independence measurement and its performance among rehabilitation inpatients. Arch. Phys. Med. Rehabil. 1993;74(5):531–536. doi: 10.1016/0003-9993(93)90119-u. [DOI] [PubMed] [Google Scholar]
- Elenkov I.J., Wilder R.L., Chrousos G.P., Vizi E.S. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol. Rev. 2000;52(4):595–638. [PubMed] [Google Scholar]
- Escrig A., Canal C., Sanchis P. IL-6 trans-signaling in the brain influences the behavioral and physio-pathological phenotype of the Tg2576 and 3xTgAD mouse models of Alzheimer’s disease. Brain Behav. Immun. 2019;82:145–159. doi: 10.1016/j.bbi.2019.08.005. [DOI] [PubMed] [Google Scholar]
- Esnault P., Nguyen C., Bordes J. Early-Onset ventilator-associated pneumonia in patients with severe traumatic brain injury: incidence, risk factors, and consequences in cerebral oxygenation and outcome. Neurocritical Care. 2017;27(2):187–198. doi: 10.1007/s12028-017-0397-4. [DOI] [PubMed] [Google Scholar]
- Failla M.D., Juengst S.B., Graham K.M., Arenth P.M., Wagner A.K. Effects of depression and antidepressant use on cognitive deficits and functional cognition following severe traumatic brain injury. J. Head Trauma Rehabil. 2016;31(6):E62–E73. doi: 10.1097/HTR.0000000000000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar D.E., Glauber R.R. Multicollinearity in regression analysis: the problem revisited. Rev. Econ. Stat. 1967;49(1):92–107. doi: 10.2307/1937887. [DOI] [Google Scholar]
- Fernández-Paredes L., Casrouge A., Decalf J. Multimarker risk stratification approach at multiple sclerosis onset. Clin Immunol Orlando Fla. 2017;181:43–50. doi: 10.1016/j.clim.2017.05.019. [DOI] [PubMed] [Google Scholar]
- Friedman J., Hastie T., Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Software. 2010;33(1):1–22. [PMC free article] [PubMed] [Google Scholar]
- Gessner A., Röllinghoff M. Biologic functions and signaling of the interleukin-4 receptor complexes. Immunobiology. 2000;201(3):285–307. doi: 10.1016/S0171-2985(00)80084-4. [DOI] [PubMed] [Google Scholar]
- Gibertini M., Newton C., Friedman H., Klein T.W. Spatial learning impairment in mice infected with Legionella pneumophila or administered exogenous interleukin-1-β. Brain Behav. Immun. 1995;9(2):113–128. doi: 10.1006/brbi.1995.1012. [DOI] [PubMed] [Google Scholar]
- Gold S.M., Willing A., Leypoldt F., Paul F., Friese M.A. Sex differences in autoimmune disorders of the central nervous system. Semin. Immunopathol. 2019;41(2):177–188. doi: 10.1007/s00281-018-0723-8. [DOI] [PubMed] [Google Scholar]
- Green R.E., Colella B., Christensen B. Examining moderators of cognitive recovery trajectories after moderate to severe traumatic brain injury. Arch. Phys. Med. Rehabil. 2008;89(12 Suppl. l):S16–24. doi: 10.1016/j.apmr.2008.09.551. [DOI] [PubMed] [Google Scholar]
- Hampel H., Teipel S.J., Padberg F. Discriminant power of combined cerebrospinal fluid tau protein and of the soluble interleukin-6 receptor complex in the diagnosis of Alzheimer’s disease. Brain Res. 1999;823(1–2):104–112. doi: 10.1016/s0006-8993(99)01146-4. [DOI] [PubMed] [Google Scholar]
- Harrison S.L., de Craen A.J.M., Kerse N. Predicting risk of cognitive decline in very old adults using three models: the Framingham stroke risk profile; the cardiovascular risk factors, aging, and dementia model; and oxi-inflammatory biomarkers. J. Am. Geriatr. Soc. 2017;65(2):381–389. doi: 10.1111/jgs.14532. [DOI] [PubMed] [Google Scholar]
- Heaton R.K. Psychol Assess Resour; 1981. Wisconsin Card Sorting Test Manual. Published online. [Google Scholar]
- Heinemann A.W., Michael Linacre J., Wright B.D., Hamilton B.B., Granger C. Measurement characteristics of the functional independence measure. Top. Stroke Rehabil. 1994;1(3):1–15. doi: 10.1080/10749357.1994.11754030. [DOI] [PubMed] [Google Scholar]
- Heinrich P, Castell J, Andus T. Interleukin-6 and the acute phase response. Biochem. J.. doi:10.1042/bj2650621. [DOI] [PMC free article] [PubMed]
- Hill J.D., Zuluaga-Ramirez V., Gajghate S., Winfield M., Sriram U., Persidsky Y. Activation of GPR55 induces neuroprotection of hippocampal neurogenesis and immune responses of neural stem cells following chronic, systemic inflammation. Brain Behav. Immun. 2019;76:165–181. doi: 10.1016/j.bbi.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerl A.E., Kennard R.W. ridge regression: biased estimation for nonorthogonal problems. Technometrics. 1970;12(1):55–67. doi: 10.1080/00401706.1970.10488634. [DOI] [Google Scholar]
- Jeong H.-K., Ji K., Min K., Joe E.-H. Brain inflammation and microglia: facts and misconceptions. Exp. Neurobiol. 2013;22(2):59–67. doi: 10.5607/en.2013.22.2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson V.E., Stewart J.E., Begbie F.D., Trojanowski J.Q., Smith D.H., Stewart W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain J. Neurol. 2013;136(Pt 1):28–42. doi: 10.1093/brain/aws322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juengst S.B., Kumar R.G., Failla M.D., Goyal A., Wagner A.K. Acute inflammatory biomarker profiles predict depression risk following moderate to severe traumatic brain injury. J. Head Trauma Rehabil. 2015;30(3):207–218. doi: 10.1097/HTR.0000000000000031. [DOI] [PubMed] [Google Scholar]
- Juengst S.B., Adams L.M., Bogner J.A. Trajectories of life satisfaction after traumatic brain injury: influence of life roles, age, cognitive disability, and depressive symptoms. Rehabil. Psychol. 2015;60(4):353–364. doi: 10.1037/rep0000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juengst S.B., Kumar R.G., Wagner A.K. A narrative literature review of depression following traumatic brain injury: prevalence, impact, and management challenges. Psychol. Res. Behav. Manag. 2017;10:175–186. doi: 10.2147/PRBM.S113264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaffashian S., Dugravot A., Elbaz A. Predicting cognitive decline. Neurology. 2013;80(14):1300–1306. doi: 10.1212/WNL.0b013e31828ab370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karo-Atar D., Bitton A., Benhar I., Munitz A. Therapeutic targeting of the interleukin-4/interleukin-13 signaling pathway: in allergy and beyond. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2018;32(3):201–220. doi: 10.1007/s40259-018-0280-7. [DOI] [PubMed] [Google Scholar]
- Kemp C.D., Johnson J.C., Riordan W.P., Cotton B.A. How we die: the impact of nonneurologic organ dysfunction after severe traumatic brain injury. Am. Surg. 2008;74(9):866–872. [PubMed] [Google Scholar]
- Kenney M.J., Ganta C.K. Autonomic nervous system and immune system interactions. Comp. Physiol. 2014;4(3):1177–1200. doi: 10.1002/cphy.c130051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesinger M.R., Kumar R.G., Wagner A.K. Hospital-acquired pneumonia is an independent predictor of poor global outcome in severe traumatic brain injury up to 5 years after discharge. J. Trauma Acute Care Surg. 2015;78(2):396–402. doi: 10.1097/TA.0000000000000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan F., Amatya B., Judson R. Factors associated with long-term functional and psychological outcomes in persons with moderate to severe traumatic brain injury. J. Rehabil. Med. 2016;48(5):442–448. doi: 10.2340/16501977-2084. [DOI] [PubMed] [Google Scholar]
- Kossmann T, Hans V, Imhof H, et al. Intrathecal and Serum Interleukin-6 and the Acute-phase Response in Patients with Severe Traumatic Brain Injuries. Shock (Augusta, Ga.). doi:10.1097/00024382-199511000-00001. [DOI] [PubMed]
- Kourbeti I.S., Vakis A.F., Papadakis J.A. Infections in traumatic brain injury patients. Clin. Microbiol. Infect. 2012;18(4):359–364. doi: 10.1111/j.1469-0691.2011.03625.x. [DOI] [PubMed] [Google Scholar]
- Kumar R.G., Boles J.A., Wagner A.K. Chronic inflammation after severe traumatic brain injury: characterization and associations with outcome at 6 and 12 Months postinjury. J. Head Trauma Rehabil. 2014 doi: 10.1097/HTR.0000000000000067. Published online June 4. [DOI] [PubMed] [Google Scholar]
- Kumar R.G., Diamond M.L., Boles J.A. Acute CSF interleukin-6 trajectories after TBI: associations with neuroinflammation, polytrauma, and outcome. Brain Behav. Immun. 2015;45:253–262. doi: 10.1016/j.bbi.2014.12.021. [DOI] [PubMed] [Google Scholar]
- Kumar R.G., Rubin J.E., Berger R.P., Kochanek P.M., Wagner A.K. Principal components derived from CSF inflammatory profiles predict outcome in survivors after severe traumatic brain injury. Brain Behav. Immun. 2016;53:183–193. doi: 10.1016/j.bbi.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R.G., Kesinger M.R., Juengst S.B. Effects of hospital-acquired pneumonia on long-term recovery and hospital resource utilization following moderate to severe traumatic brain injury. J. Trauma Acute Care Surg. 2020;88(4):491–500. doi: 10.1097/TA.0000000000002562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffont S., Seillet C., Guéry J.-C. Estrogen receptor-dependent regulation of dendritic cell development and function. Front. Immunol. 2017;8 doi: 10.3389/fimmu.2017.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannoo E., Colardyn F., Vandekerckhove T., De Deyne C., De Soete G., Jannes C. Subjective complaints versus neuropsychological test performance after moderate to severe head injury. Acta Neurochir. 1998;140(3):245–253. doi: 10.1007/s007010050091. [DOI] [PubMed] [Google Scholar]
- Lasselin J., Lekander M., Axelsson J., Karshikoff B. Sex differences in how inflammation affects behavior: what we can learn from experimental inflammatory models in humans. Front. Neuroendocrinol. 2018;50:91–106. doi: 10.1016/j.yfrne.2018.06.005. [DOI] [PubMed] [Google Scholar]
- Lee Y.-K., Hou S.-W., Lee C.-C., Hsu C.-Y., Huang Y.-S., Su Y.-C. Increased risk of dementia in patients with mild traumatic brain injury: a nationwide cohort study. PloS One. 2013;8(5) doi: 10.1371/journal.pone.0062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewczuk P., Ermann N., Andreasson U. Plasma neurofilament light as a potential biomarker of neurodegeneration in Alzheimer’s disease. Alzheimer’s Res. Ther. 2018;10 doi: 10.1186/s13195-018-0404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licher S., Leening M.J.G., Yilmaz P. Development and validation of a dementia risk prediction model in the general population: an analysis of three longitudinal studies. Am. J. Psychiatr. 2018;176(7):543–551. doi: 10.1176/appi.ajp.2018.18050566. [DOI] [PubMed] [Google Scholar]
- Licher S., Yilmaz P., Leening M.J.G. External validation of four dementia prediction models for use in the general community-dwelling population: a comparative analysis from the Rotterdam Study. Eur. J. Epidemiol. 2018;33(7):645–655. doi: 10.1007/s10654-018-0403-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Liu J., Zhao S. Interleukin-4 is essential for microglia/macrophage M2 polarization and long-term recovery after cerebral ischemia. Stroke J. Cereb. Circ. 2016;47(2):498–504. doi: 10.1161/STROKEAHA.115.012079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane D.J., Byrnes K.R. Role of microglia in neurotrauma. Neurother. J. Am. Soc. Exp. Neurother. 2010;7(4):366–377. doi: 10.1016/j.nurt.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane D.J., Kumar A. Microglia in the TBI brain: the good, the bad, and the dysregulated. Exp. Neurol. 2016;275(Pt 3):316–327. doi: 10.1016/j.expneurol.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundmark F., Duvefelt K., Iacobaeus E. Variation in interleukin 7 receptor alpha chain (IL7R) influences risk of multiple sclerosis. Nat. Genet. 2007;39(9):1108–1113. doi: 10.1038/ng2106. [DOI] [PubMed] [Google Scholar]
- Lundström W., Fewkes N.M., Mackall C.L. IL-7 in human health and disease. Semin. Immunol. 2012;24(3):218–224. doi: 10.1016/j.smim.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerske C.W., Mihalik J.P., Ren D. Concussion in sports: postconcussive activity levels, symptoms, and neurocognitive performance. J. Athl. Train. 2008;43(3):265–274. doi: 10.4085/1062-6050-43.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini A., Gaetani L., Di Gregorio M. Hippocampal neuroplasticity and inflammation: relevance for multiple sclerosis. Mult. Scler. Demyelinating Disord. 2017;2(1):2. doi: 10.1186/s40893-017-0019-1. [DOI] [Google Scholar]
- Mani K., Cater B., Hudlikar A. Cognition and return to work after mild/moderate traumatic brain injury: a systematic review. Work Read Mass. 2017;58(1):51–62. doi: 10.3233/WOR-172597. [DOI] [PubMed] [Google Scholar]
- Marksteiner J., Kemmler G., Weiss E.M. Five out of 16 plasma signaling proteins are enhanced in plasma of patients with mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging. 2011;32(3):539–540. doi: 10.1016/j.neurobiolaging.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh N.V. Cognitive functioning following traumatic brain injury: the first 5 years. NeuroRehabilitation. 2018;43(4):377–386. doi: 10.3233/NRE-182457. [DOI] [PubMed] [Google Scholar]
- McGarity S., Barnett S.D., Lamberty G. Community reintegration problems among veterans and active duty service members with traumatic brain injury. J. Head Trauma Rehabil. 2017;32(1):34–45. doi: 10.1097/HTR.0000000000000242. [DOI] [PubMed] [Google Scholar]
- Millis S.R., Rosenthal M., Novack T.A. Long-term neuropsychological outcome after traumatic brain injury. J. Head Trauma Rehabil. 2001;16(4):343–355. doi: 10.1097/00001199-200108000-00005. [DOI] [PubMed] [Google Scholar]
- Morieri M.L., Passaro A., Zuliani G. Interleukin-6 “trans-signaling” and ischemic vascular disease: the important role of soluble gp130. Mediat. Inflamm. 2017 doi: 10.1155/2017/1396398. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz M.J., Kumar R.G., Oh B.-M. Cerebrospinal fluid cortisol mediates brain-derived neurotrophic factor relationships to mortality after severe TBI: a prospective cohort study. Front. Mol. Neurosci. 2017;10:44. doi: 10.3389/fnmol.2017.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrga J.M., Failla M.D., Ricker J.H. A dopamine pathway gene risk score for cognitive recovery following traumatic brain injury: methodological considerations, preliminary findings, and interactions with sex. J. Head Trauma Rehabil. 2016;31(5):E15–29. doi: 10.1097/HTR.0000000000000199. [DOI] [PubMed] [Google Scholar]
- Nitsch K.P., Casaletto K.B., Carlozzi N.E., Tulsky D.S., Heinemann A.W., Heaton R.K. Uncorrected versus demographically-corrected scores on the NIH toolbox cognition battery in persons with traumatic brain injury and stroke. Rehabil. Psychol. 2017;62(4):485–495. doi: 10.1037/rep0000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS 9.4.
- R Core Team . 2019. R: A Language and Environment for Statistical Computing.http://www.R-project.org/ [Google Scholar]
- Osterrieth P.A. Le test de copie d’une figure complexe; contribution à l’étude de la perception et de la mémoire. [Test of copying a complex figure; contribution to the study of perception and memory.] Arch. Psychol. 1944;30:206–356. [Google Scholar]
- Ownsworth T., McKenna K. Investigation of factors related to employment outcome following traumatic brain injury: a critical review and conceptual model. Disabil. Rehabil. 2004;26(13):765–783. doi: 10.1080/09638280410001696700. [DOI] [PubMed] [Google Scholar]
- Powell J.M., Machamer J.E., Temkin N.R., Dikmen S.S. Self-report of extent of recovery and barriers to recovery after traumatic brain injury: a longitudinal study. Arch. Phys. Med. Rehabil. 2001;82(8):1025–1030. doi: 10.1053/apmr.2001.25082. [DOI] [PubMed] [Google Scholar]
- Rabinowitz A.R., Levin H.S. Cognitive sequelae of traumatic brain injury. Psychiatr. Clin. 2014;37(1):1–11. doi: 10.1016/j.psc.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz A.R., Hart T., Whyte J., Kim J. Neuropsychological recovery trajectories in moderate to severe traumatic brain injury: influence of patient characteristics and diffuse axonal injury. J Int Neuropsychol Soc JINS. 2018;24(3):237–246. doi: 10.1017/S1355617717000996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff J.J., Greenspan A.I., Goldstein F.C. Gender and traumatic brain injury: do the sexes fare differently? Brain Inj. 2007;21(10):1023–1030. doi: 10.1080/02699050701633072. [DOI] [PubMed] [Google Scholar]
- Rauen K., Reichelt L., Probst P. Quality of life up to 10 years after traumatic brain injury: a cross-sectional analysis. Health Qual. Life Outcome. 2020;18(1):166. doi: 10.1186/s12955-020-01391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R.M., Wolfson D. Neuropsychology Press; 1985. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. [Google Scholar]
- Ribeiro M., Brigas H.C., Temido-Ferreira M. Meningeal γδ T cell-derived IL-17 controls synaptic plasticity and short-term memory. Sci. Immunol. 2019;4(40) doi: 10.1126/sciimmunol.aay5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggio S., Wong M. Neurobehavioral sequelae of traumatic brain injury. Mt Sinai J. Med. N Y. 2009;76(2):163–172. doi: 10.1002/msj.20097. [DOI] [PubMed] [Google Scholar]
- Rochfort K.D., Cummins P.M. The blood-brain barrier endothelium: a target for pro-inflammatory cytokines. Biochem. Soc. Trans. 2015;43(4):702–706. doi: 10.1042/BST20140319. [DOI] [PubMed] [Google Scholar]
- Rose-John S., Scheller J., Elson G., Jones S.A. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J. Leukoc. Biol. 2006;80(2):227–236. doi: 10.1189/jlb.1105674. [DOI] [PubMed] [Google Scholar]
- Rosseel Y., Jorgensen T., Oberski D., Byrnes J., Vanbrabant L., Savalei V. 2017. Package ‘lavaan.’ Published Online. [Google Scholar]
- Ruff R.M., Marshall L.F., Crouch J. Predictors of outcome following severe head trauma: follow-up data from the Traumatic Coma Data Bank. Brain Inj. 1993;7(2):101–111. doi: 10.3109/02699059309008164. [DOI] [PubMed] [Google Scholar]
- Santarsieri M., Kumar R.G., Kochanek P.M., Berga S., Wagner A.K. Variable neuroendocrine-immune dysfunction in individuals with unfavorable outcome after severe traumatic brain injury. Brain Behav. Immun. 2014 doi: 10.1016/j.bbi.2014.09.003. Published online September 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarsieri M., Niyonkuru C., McCullough E.H. Cerebrospinal fluid cortisol and progesterone profiles and outcomes prognostication after severe traumatic brain injury. J. Neurotrauma. 2014;31(8):699–712. doi: 10.1089/neu.2013.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sashika H., Takada K., Kikuchi N. Rehabilitation needs and participation restriction in patients with cognitive disorder in the chronic phase of traumatic brain injury. Medicine (Baltim.) 2017;96(4) doi: 10.1097/MD.0000000000005968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T.A., Widmer M.B., Finkelman F.D. Recombinant soluble murine IL-4 receptor can inhibit or enhance IgE responses in vivo. J. Immunol. 1993;150(7):2717–2723. [PubMed] [Google Scholar]
- Schirmer-Mikalsen K., Moen K.G., Skandsen T., Vik A., Klepstad P. Intensive care and traumatic brain injury after the introduction of a treatment protocol: a prospective study. Acta Anaesthesiol. Scand. 2013;57(1):46–55. doi: 10.1111/j.1399-6576.2012.02785.x. [DOI] [PubMed] [Google Scholar]
- Schneider E.B., Sur S., Raymont V. Functional recovery after moderate/severe traumatic brain injury: a role for cognitive reserve? Neurology. 2014;82(18):1636–1642. doi: 10.1212/WNL.0000000000000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schretlen D.J., Shapiro A.M. A quantitative review of the effects of traumatic brain injury on cognitive functioning. Int. Rev. Psychiatr. Abingdon Engl. 2003;15(4):341–349. doi: 10.1080/09540260310001606728. [DOI] [PubMed] [Google Scholar]
- Sheikh A., Abraham N. Interleukin-7 receptor alpha in innate lymphoid cells: more than a marker. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.02897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlosberg D., Benifla M., Kaufer D., Friedman A. Blood–brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat. Rev. Neurol. 2010;6(7):393–403. doi: 10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J.E., Newcombe J., Cuzner M.L., Woodroofe M.N. Expression of monocyte chemoattractant protein-1 and other β-chemokines by resident glia and inflammatory cells in multiple sclerosis lesions. J. Neuroimmunol. 1998;84(2):238–249. doi: 10.1016/S0165-5728(97)00208-7. [DOI] [PubMed] [Google Scholar]
- Smith A. Western Psychological Corporation; 2002. Services (Firm) WP. Symbol Digit Modalities Test : Manual. Los Angeles, Calif. [Google Scholar]
- Sorce S., Myburgh R., Krause K.-H. The chemokine receptor CCR5 in the central nervous system. Prog. Neurobiol. 2011;93(2):297–311. doi: 10.1016/j.pneurobio.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Spitz G., Ponsford J.L., Rudzki D., Maller J.J. Association between cognitive performance and functional outcome following traumatic brain injury: a longitudinal multilevel examination. Neuropsychology. 2012;26(5):604–612. doi: 10.1037/a0029239. [DOI] [PubMed] [Google Scholar]
- Stuart M.J., Baune B.T. Chemokines and chemokine receptors in mood disorders, schizophrenia, and cognitive impairment: a systematic review of biomarker studies. Neurosci. Biobehav. Rev. 2014;42:93–115. doi: 10.1016/j.neubiorev.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Taipa R., das Neves S.P., Sousa A.L. Proinflammatory and anti-inflammatory cytokines in the CSF of patients with Alzheimer’s disease and their correlation with cognitive decline. Neurobiol. Aging. 2019;76:125–132. doi: 10.1016/j.neurobiolaging.2018.12.019. [DOI] [PubMed] [Google Scholar]
- Takatsu K., Nakajima H. IL-5 and eosinophilia. Curr. Opin. Immunol. 2008;20(3):288–294. doi: 10.1016/j.coi.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Tan Z.S., Beiser A.S., Vasan R.S. Inflammatory markers and the risk of alzheimer disease: the Framingham study. Neurology. 2007;68(22):1902–1908. doi: 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- Tate R.L., Fenelon B., Manning M.L., Hunter M. Patterns of neuropsychological impairment after severe blunt head injury. J. Nerv. Ment. Dis. 1991;179(3):117–126. doi: 10.1097/00005053-199103000-00001. [DOI] [PubMed] [Google Scholar]
- Teasdale G., Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Tripathy D., Thirumangalakudi L., Grammas P. RANTES upregulation in the Alzheimer’s disease brain: a possible neuroprotective role. Neurobiol. Aging. 2010;31(1):8–16. doi: 10.1016/j.neurobiolaging.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakil E., Greenstein Y., Weiss I., Shtein S. The effects of moderate-to-severe traumatic brain injury on episodic memory: a meta-analysis. Neuropsychol. Rev. 2019;29(3):270–287. doi: 10.1007/s11065-019-09413-8. [DOI] [PubMed] [Google Scholar]
- Vijapur S, Yang Z, Barton D, et al. Anti-pituitary and anti-hypothalamus autoantibody associations with inflammation and hypogonadotropic hypogonadism in men with traumatic brain injury. J. Neurotrauma. Published online February 28, 2020. doi:10.1089/neu.2019.6780. [DOI] [PMC free article] [PubMed]
- Wagner A.K. TBI translational rehabilitation research in the 21st Century: exploring a Rehabilomics research model. Eur. J. Phys. Rehabil. Med. 2010;46(4):549–556. [PubMed] [Google Scholar]
- Wagner A.K. TBI Rehabilomics research: an exemplar of a biomarker-based approach to precision care for populations with disability. Curr. Neurol. Neurosci. Rep. 2017;17(11):84. doi: 10.1007/s11910-017-0791-5. [DOI] [PubMed] [Google Scholar]
- Wagner A.K., Zitelli K.T. A Rehabilomics focused perspective on molecular mechanisms underlying neurological injury, complications, and recovery after severe TBI. Pathophysiol. Off. J. Int. Soc. Pathophysiol. ISP. 2013;20(1):39–48. doi: 10.1016/j.pathophys.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Wakelkamp I.M.M.J., Bakker O., Baldeschi L., Wiersinga W.M., Prummel M.F. TSH-R expression and cytokine profile in orbital tissue of active vs. inactive Graves’ ophthalmopathy patients. Clin. Endocrinol. 2003;58(3):280–287. doi: 10.1046/j.1365-2265.2003.01708.x. [DOI] [PubMed] [Google Scholar]
- Walker W.C., Pickett T.C. Motor impairment after severe traumatic brain injury: a longitudinal multicenter study. J. Rehabil. Res. Dev. 2007;44(7):975–982. doi: 10.1682/jrrd.2006.12.0158. [DOI] [PubMed] [Google Scholar]
- Wang H.-K., Lin S.-H., Sung P.-S. Population based study on patients with traumatic brain injury suggests increased risk of dementia. J. Neurol. Neurosurg. Psychiatry. 2012;83(11):1080–1085. doi: 10.1136/jnnp-2012-302633. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Psychological Corporation; 1997. WAIS-III, Wechsler Adult Intelligence Scale: Administration and Scoring Manual. [Google Scholar]
- Willis Emily, MacDonald Kelli, Nguyen Quan, Garrido Adahir, Gillespie Ellen, Harley Samuel, Bartlett Perry, Schroder Wayne, Yates Abi, Anthony Daniel, Rose-John Stephan, Ruitenberg Marc, Vukovic Jana. Repopulating Microglia Promote Brain Repair in an IL-6-Dependent Manner. Cell. 2020;180:833–846. doi: 10.1016/j.cell.2020.02.013. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Mahmood A., Zhang Y. Effects of posttraumatic carbamylated erythropoietin therapy on reducing lesion volume and hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome in rats following traumatic brain injury: laboratory investigation. J. Neurosurg. 2011;114(2):549–559. doi: 10.3171/2010.10.JNS10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Zhu W., Xu F. The interleukin-4/PPARγ signaling axis promotes oligodendrocyte differentiation and remyelination after brain injury. PLoS Biol. 2019;17(6) doi: 10.1371/journal.pbio.3000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonis S., Pechnick R.N., Ljubimov V.A. Chronic intestinal inflammation alters hippocampal neurogenesis. J. Neuroinflammation. 2015;12(1):65. doi: 10.1186/s12974-015-0281-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygun D.A., Kortbeek J.B., Fick G.H., Laupland K.B., Doig C.J. Non-neurologic organ dysfunction in severe traumatic brain injury∗. Crit. Care Med. 2005;33(3):654. doi: 10.1097/01.CCM.0000155911.01844.54. [DOI] [PubMed] [Google Scholar]