Abstract
The therapeutic approach of multifactorial complex diseases is always a challenge; Parkinson’s disease (PD) is a heterogeneous neurodegenerative disorder triggered by genetic and environmental factors, contributing to its etiology. Indeed, several pathogenic mechanisms lead to selective dopaminergic neuronal injury, including oxidative stress, mitochondrial dysfunction, alteration of endoplasmic reticulum-to-Golgi protein trafficking, excitotoxicity, and neuroinflammation. Current treatment approaches include mainly dopamine replacement therapy or optimizing dopaminergic transmission; however, these strategies that do not counteract the pathogenic mechanisms underlying PD symptoms and often are less effective over time. Recently, there has been growing interest in the therapeutic use of nutraceuticals, that could represent an integrative approach to the pharmacological standard therapy and specifically affect one or more pathogenic pathways. The intake of nutraceuticals or nutritional modifications are generally safe and can be combined with current common drug therapy in most cases to improve the patient’s quality of life and/or mitigate PD symptoms. The current review focuses on several key nutritional compounds and dietary modifications that are effective on several pathogenic pathways involved in PD onset and progression, and further highlights the rationale behind their potential use for the prevention and treatment of PD.
Keywords: Parkinson’s disease, Nutraceuticals, Mitochondrial dysfunction, Protein misfolding, Neuroinflammation, Gut-brain axis
Graphical abstract
Highlights
-
•
PD therapy has limited effectiveness acting only on mitigation of symptoms.
-
•
PD features include oxidative and ER stress, protein misfolding and inflammation.
-
•
Nutraceuticals own neuroprotective effect on PD with converging mechanisms.
-
•
Nutraceuticals may represent an adjuvant approach for PD management.
1. Introduction
The prevalence of Parkinson’s disease (PD) affects approximately 1–2% of the population, representing the second most recurring neurodegenerative disorder worldwide (Kalia and Lang, 2015). It is characterized by two main detrimental processes: a progressive reduction of dopaminergic neurons in the substantia nigra (SN) pars compacta (SNpc), and an intraneuronal accumulation of Lewy bodies, containing misfolded α-synuclein (α-Syn). Indeed, the marked decrease of dopamine (DA) metabolism (synthesis of striatal DA and its transporter) results in bradykinesia, rigidity, and resting tremor. A correlation between the progressive accumulation of α-Syn aggregates and PD onset was also shown and many symptoms of PD have been associated with the α-Syn-containing inclusions, Lewy bodies, within brain circuits (Braak and Del Tredici, 2008).
More recently, the potential role of the microbiome-gut-brain axis in the pathogenesis and severity of PD has been identified: disturbed gut microbiota could lead to gut barrier integrity disruption, local and systemic inflammation, impacting on blood-brain barrier (BBB) and causing neurodegeneration. Consistently, prodromal symptoms of altered gut function are often observed many years before motor ones.
Although many animal models of PD are currently available (Table 1), the preclinical research of new therapeutics is still a challenge. The lack of full translatability of experimental findings in PD patients has highlighted key drawbacks in recapitulating in animals the human pathology, due to disease complexity and degenerative nature. Even though the development of animal models has led to achieving new insight into the pathogenic mechanisms underlying PD, no significant progress has been obtained in drug discovery. Moreover, the profound neurodegeneration accompanying PD patients often requires symptomatic rather than curative therapy (Elkouzi et al., 2019). To date, the current gold-standard therapy remains the combination of levodopa and carbidopa. Otherwise, DA agonists, monoamine oxidase B inhibitors or anticholinergic drugs can be administered to manage motor dysfunction. However, this therapy aimed at replacing and/or balancing neurotransmitter homeostasis, does not target the underlying mechanisms of progressive PD-induced neurodegeneration and does not address non-dopaminergic functions or limit PD progression. Therefore, these treatments often become less effective over time and are not devoid of side effects. Notably, beyond cardinal motor symptoms, PD is also characterized by non-motor comorbidities, including mood and cognitive disorders, sleep disturbance, gastrointestinal ailments and hypertension (Chaudhuri and Schapira, 2009), that need to be counteracted as well to improve the patient’s quality of life. In this context, beside conventional therapy, many PD patients tend to use integrative support to control and/or limit the pathology and associated symptoms. Many natural products have been used based on “traditional” medicine to obtain beneficial effects in several neurodegenerative disorders, including PD, and only more recently, studies on the identification of the active principles and their mechanism of action have possibly led to therapeutic application based on sound scientific evidence.
Table 1.
The main animal models of Parkinson’s Disease (PD). The lack of full translatability renders preclinical research of PD still a challenge. To date, animal models are divided into three main groups: pharmacological, environmental and genetic. Although each model partially resembles PD features, the development of new experimental models would greatly contribute to our understanding in pathogenic mechanisms of PD.
| PD models | Type | Notes |
|---|---|---|
| Pharmacological | Reserpine | Induces transient parkinsonian symptoms without nigral dopaminergic neurodegeneration |
| Haloperidol |
Blocks striatal dopaminergic transmission |
|
| Environmental | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) | Widespread animal models. Rapid neurodegeneration does not mirror the chronic and progressive evolution of PD |
| 6-hydroxydopamine (6-OHDA) | ||
| Rotenone | Less frequently used models. Induce dopaminergic neuron loss and Lewy bodies. The main limitations are peripheral toxicity and extent inflammation | |
| Paraquat | ||
| Isoquinolines | ||
| Methamphetamine | ||
| Proteasome inhibitor I (PSI) | Induce nigrostriatal neuron loss and Lewy bodies with progressive motor disability. High variability and low reproducibility. | |
| Epoximicin | ||
| Lipopolysaccharide |
Induce inflammation and related nigrostriatal neuronal loss. Does not replicate PD features. |
|
| Genetic | Parkin | Knock-out mice for these genes show motor dysfunction, but no neurodegeneration |
| PTEN-induced kinase 1 (PINK1) | ||
| Protein deglycase (DJ-1) | ||
| Leucine-rich repeat kinase (LRRK) 2 | Knock-in mice show alteration of dopaminergic transmission, without clear behavioral deficits | |
| Vacuolar protein sorting-associated protein (VPS) 35 | Knock-out mice show a reduction in striatal dopamine level, accompanied by α-Synuclein accumulation and motor deficits | |
| Mitopark | The specific loss of the gene coding for mitochondrial transcription factor A in dopaminergic neurons leads to a progressive decline of neuronal function and slow development of PD-related behavior | |
| α-Synuclein models |
|
As verbatim reported by Andrew and Izzo (2017), “the term ‘nutraceutical’, a hybrid term introduced in 1989 to designate the link between ‘nutrition’ and ‘pharmaceutical agents’, has no universally accepted definition”. Generally, nutraceuticals comprise all naturally occurring products, including food or fortified or functional food, and food components or nutrients. To simplify the use of the “nutraceutical” term, herbal drug (as part of plants) or extract can be interchanged.
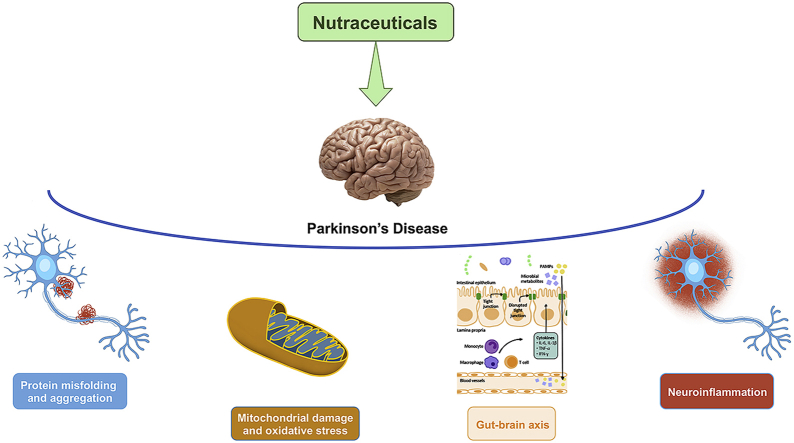
The unconventional approach based on nutraceuticals and/or supplements has becoming more and more frequent. Many nutraceuticals from natural sources have been substantiated to provide neuroprotection in experimental models through a specific mechanism of action or acting through multiple mechanistic pathways. Therefore, due to the multifactorial pathogenic nature underpinning PD symptoms, biological events that can be targeted by these therapeutic supplements are multiple, i.e. mitochondrial dysfunction, neuroinflammation, oxidative stress, endoplasmic reticulum (ER) stress, α-Syn aggregation and protein misfolding, and modulation of the gut-brain axis.
Although clinical support of nutraceutical potential in PD is often marginal or debatable, we summarize here the nutraceuticals showing an impact on essential molecular mechanisms involved in PD, that can be considered as part of adjunctive treatment to first-line therapy. (see Table 2, Table 3, Table 4)
Table 2.
Types of the nutraceuticals targeting oxidative and mitochondrial damage in PD. Food components and nutraceuticals limit PD progression by improving mitochondrial function and dynamics. Nutraceuticals acting on PD-related oxidative and mitochondrial stress are mainly divided into three types: nutrients, herbals and phytochemicals, and synthetic derivatives.
| Type | Nutraceutical | Notes |
|---|---|---|
| Nutrients | Coenzyme Q10 | Counteracts MPTP-induced neurotoxicity, blocking the electron transfer between complex 1 and other complexes |
| Resveratrol | Limits mitochondrial dysfunction and apoptosis in nigrostriatal cells, acting via protein kinase B (AKT)/glycogen synthase kinase-3β pathway | |
| Lycopene | Reduces oxidative stress, increasing NADH dehydrogenase and superoxide dismutase activity in the striatum, GSH, and reduces malondialdehyde levels | |
| Fish oil |
Enriched in ω-3 fatty acids, such as eicosapentaenoic and docosahexaenoic acids, that confer neuroprotective effects via multiple mechanisms |
|
| Herbals and phytochemicals | Epigallocatechin-3-gallate (EGCG) | Protects by toxic dopamine metabolites through its properties of radical scavenger and chelator of iron ions |
| Ginsenosides | Block dopaminergic neuronal death, reducing glutamate-induced excitotoxicity and promoting synaptic transmission in the nigrostriatal nucleus | |
| Vincamine | Own multiple mechanisms of action, including vasodilating effect, antioxidant and chelating activity | |
| Vinpocetine | ||
| Synthetic derivatives | MitoQ | Overcomes CoQ10’s restrictions, such as limited distribution to mitochondria linked to its hydrophobicity |
| Mito-apocynin | Limits not only the oxidative damage but also glial-mediated inflammation |
Table 3.
Classes of the nutraceuticals targeting ER stress in PD. Abnormal misfolded proteins are responsible for ER stress and the formation of aggregates. Many nutraceuticals carry out their beneficial effects on PD pattern through the anti-fibrillogenic activity or the enhancement of α-Syn disaggregation.
| Type | Nutraceutical | Notes |
|---|---|---|
| Nutrients | Palmitoylethanolamide | Inhibits BiP expression and the activation of PERK-eIF2α pathway |
| Vitamin A | Own anti-fibrillogenic effects | |
| β-Carotene | ||
| Coenzyme Q10 | ||
| Herbals and phytochemicals | Crocin | Decreases CHOP and BiP/Grp78 expressions, and inhibits the activation of pro-apoptotic factor caspase-12 |
| Epigallocatechin-3-gallate (EGCG) | Owns anti-fibrillogenic effects | |
| Baicalein | Prevents α-Syn fibrillation, induces autophagy, inhibits apoptosis, and reduces inflammation | |
| Rosmarinic acid | Owns anti-fibrillogenic effects | |
| Resveratrol | Enhances α-Syn autophagic degradation | |
| Gallic acid | Termed also as disaggregases, inhibit the aggregation of α-Syn fibrils | |
| Ginsenosides | ||
| Salidroside | Reduces α-Syn aggregation, enhancing the dephosphorylation of Ser129 |
Table 4.
Classification of the nutraceuticals targeting neuroinflammation in PD. Neuroinflammation represents a crucial event for PD progression, demonstrated by high levels of pro-inflammatory mediators and damaging molecules in the striatum of PD patients. Since the well-known anti-inflammatory properties, nutraceuticals may be useful in limiting inflammation-driven neurotoxicity.
| Type | Nutraceutical | Notes |
|---|---|---|
| Nutrients |
ω-3 polyunsaturated fatty acids |
Limit neuroinflammation without impacting neuronal apoptosis |
| Herbals and phytochemicals | Curcumin | Limits inflammation (NF-κB) and immune system activation (IFN regulatory factor 3, myeloid differentiation primary response 88, TLR4) |
| Ginsenosides | Limit astrogliosis and microgliosis and reduce the production of pro-inflammatory cytokines in SNpc | |
| Silymarin | Reduces apoptosis, through inhibition of TLR4 expression | |
| Asiatic acid | Reduces inflammatory cytokines, TLR2, TLR4, and NF-κB expression | |
| Glaucocalyxin B | Inhibits TLR/NF-κB activation and activated nuclear factor erythroid 2-related factor 2/heme oxygenase-1 pathway | |
| Extracts of Mucuna pruriens | Lessen neurotoxicity through the inhibition of NF-κB and pAkt pathway. Increase glial fibrillary acidic protein, iNOS, intercellular adhesion molecule, and TNF-α | |
| Quercetin |
2. Nutraceutical impact on PD pathogenic mechanisms
Since neurodegeneration in PD is the result of the combination of many processes occurring inside and/or outside the cells, its etiopathogenesis is not yet completely understood. While the mechanisms underlying neurotoxicity have been deeply analyzed, the factors driving or triggering these pathogenetic pathways and their interactions need to be addressed. Therefore, the understanding of basal ganglia physiopathology, the relevance of each pathological feature and their interplay would help in addressing the primary targets, and their pharmacological control. PD therapy firstly focused on the preservation of motor function and after on the control of non-motor symptoms. This approach contributed to improve PD symptoms but did not achieve the primary goal of blocking pathogenic mechanisms and preventing the progression of disease. Here, we examine the potential role of nutraceuticals targeting the underlying neurodegenerative processes of PD as adjunctive therapy to the first-line approach to delay the progression and reduce the burden of this disease. The natural compounds may exert their beneficial effects in PD, blunting different pathogenic events, such as neuroinflammation, oxidative stress, mitochondrial dysfunction, and apoptosis.
2.1. Targeting oxidative stress and mitochondrial dysfunction
Mitochondrial dysfunction and uncontrolled oxidative stress compromise cellular energy metabolism impacting brain functions in neurodegenerative disorders, including PD. However, whether mitochondrial dysfunction is a cause, or a consequence of neurodegeneration is still an open question. Although the exact mechanism causing mitochondrial dysfunction is disregarded, defective respiratory chain and mutation of mitochondrial DNA (mtDNA) in the dopaminergic neurons of PD patients have been hypothesized (Bose and Beal, 2016).
In neurons, mitochondria not only regulate ATP supply but also Ca2+ storage for neurotransmitter release and neuronal depolarization, protecting cells via fusion and fission. It was demonstrated a role of α-Syn not only in maintaining mitochondrial morphology but also in enhancing ATP synthase efficiency (Ludtmann et al., 2016); conversely, α-Syn aggregates could compromise mitochondrial bioenergetic function and increase reactive oxygen species (ROS) production, leading to an unbalanced oxidative status and neuronal death in rat primary neurons (Ludtmann et al., 2018).
In dopaminergic neurons, neuromelanin (NM) is a crucial pigment in protecting neurons against oxidative stress. Indeed, NM chelates several ions, including zinc and iron, maintaining the redox balance (Knorle, 2018). It is well known that excessive iron content represents a pathogenetic feature of PD (Mochizuki and Yasuda, 2012): when iron levels overcome the capability of NM binding, iron could aggravate neurotoxic events, triggering autoxidation of DA and causing neuroinflammation (Knorle, 2018). Consistently, several data showed a particular vulnerability of dopaminergic neurons containing NM to death or neurodegeneration in PD patients (Zucca et al., 2017).
Mitophagy machinery provides mitochondria turnover managing mitochondrial homeostasis and cell survival. Indeed, deletion or point mutation of genes encoding for PTEN induced kinase (PINK)1 and Parkin (PRKN) are common in familiar PD patients (Hamacher-Brady and Brady, 2016). A recent study confirmed the protective role of Parkin in a model of mtDNA mutation-induced mitochondrial dysfunction (Pickrell et al., 2015).
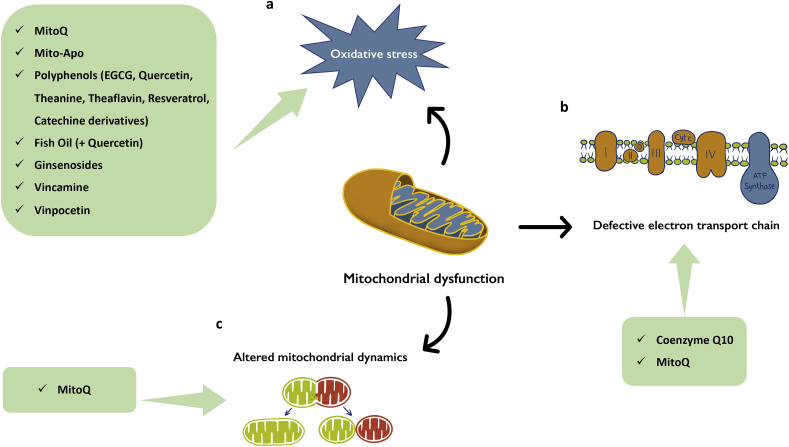
Several studies have shown that food components and nutraceuticals can prevent or delay PD progression by preserving mitochondrial function, further supporting the role of mitochondrial impairment as a major pathological mechanism of PD (Fato et al., 2008) (Fig. 1).
Fig. 1.
Nutraceuticals limit oxidative stress and impact on mitochondrial function and dynamics. Mitochondrial dysfunction characterizing PD pathogenesis can cause cellular energy dysmetabolism with several mechanisms impacting on brain functions. Nutraceuticals can re-establish mitochondrial homeostasis acting on oxidative stress (A), defective electron transport chain (B), and altered mitochondrial dynamics (C). The unbalanced oxidative status in PD depends on several mechanisms, including the formation of α-Syn aggregates (Ludtmann et al., 2018) and the reduction of neuromelanin levels (Knorle, 2018). Various nutraceutical compounds counteract mitochondrial dysfunction as one of the major pathological mechanism of PD, reducing oxidative stress and improving mitochondrial function and efficiency.
Considering preclinical evidence, targeting mitochondria has been identified as a promising therapeutic strategy (Thomas and Beal, 2010), even if to date no therapy is available as neuroprotective treatment (Athauda and Foltynie, 2015).
2.1.1. Nutrients
Among nutritional supplements, coenzyme Q10 (CoQ10) and fish oil, have been proposed as integrative strategies able to reduce PD progression (Shults, 2005). CoQ10, as a key component of the mitochondrial electron transport chain, actively involved in ATP production, counteracting 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced neurotoxicity, and blocking the electron transfer between complex 1 as well as other complexes (Cleren et al., 2008).
Polyphenols have demonstrated to counteract the multi-dimensional features of PD. These compounds, crossing the BBB, exert multiple favorable effects on PD pattern, including the improvement of motor and cognitive function, the protection of dopaminergic neurons, and the limitation of oxidative stress (Kujawska and Jodynis-Liebert, 2018).
Resveratrol showed antioxidant effects (Saiko et al., 2008). This nutraceutical induced an improvement in motor impairment in MPTP-treated mice (Guo et al., 2016). At a mechanistic level, an in vitro study showed resveratrol capability in limiting mitochondrial dysfunction and apoptosis in nigrostriatal cells, acting via protein kinase B (AKT)/glycogen synthase kinase-3β pathway (Zeng et al., 2017).
Lycopene, an acyclic lipid-soluble carotenoid occurring in red vegetables and fruits, mainly in tomatoes, exerted an antioxidant effect. Its neuroprotective effect was shown in MPTP-insulted mice fed with an enriched tomato powder diet, where the decrease of DA level in the striatum was prevented (Suganuma et al., 2002). Similar data were obtained by di Matteo et al. (2009) in 6-OHDA-challenged rats where a diet enriched in tomatoes preserved striatal dopaminergic neurons; as demonstrated in other models, the protective effect of lycopene was associated to a reduction of oxidative stress, accompanied by an improvement of neurobehavioral deficits and an increase in NADH dehydrogenase and superoxide dismutase activity at the striatal level, along with glutathione (GSH) level and a reduction in malondialdehyde levels (Prema et al., 2015).
As mentioned above, fish oil has demonstrated to limit the progression of PD. Because of richness in ω-3 fatty acids, such as eicosapentaenoic and docosahexaenoic acids, fish oil showed neuroprotective effects via multiple mechanisms. In MPTP-challenged mice, docosahexaenoic acid administration improved motor activity, reducing apoptosis of dopaminergic neurons and enhancing antioxidant defense (Parlak et al., 2018). The beneficial effects of ω-3 were confirmed in a double-blind randomizedclinical trial: 12-week supplementation with ω-3 and vitamin E in PD patients reduced inflammatory and oxidative biomarkers and limited metabolic impairment associated with PD (Taghizadeh et al., 2017). In another animal model of PD induced by chronic administration of rotenone, it has been shown that the combination of fish oil and quercetin increased the activity of antioxidant enzymes and GSH levels, also counteracting mitochondrial dysfunction and oxidative damage related to PD (Denny Joseph and Muralidhara, 2015).
2.1.2. Herbals and phytochemicals
Epigallocatechin-3-gallate (EGCG), the most prevalent polyphenol constituent of Camellia sinensis, has been shown neuroprotective activities in PD, since its capability in crossing the BBB. The protective effect of EGCG is due to its catechol-like structure, known to be a potent radical scavenger and chelator of iron ions (Gassen and Youdim, 1997), protecting by toxic DA metabolites. Moreover, EGCG improved motor coordination and limited neurotoxicity, increasing striatal DA amount in MPTP-insulted mice (Xu et al., 2017). The antioxidant and metal-chelating properties were also recognized in other polyphenolic compounds, such as quercetin, theanine (the amino acid analogs), and theaflavin, the catechin derivative able to protect from neuronal damage synergizing with anti-parkinsonian drugs (Dutta and Mohanakumar, 2015).
The Ginseng-derived ginsenosides have demonstrated a specific neuroprotective activity in several studies on PD. In MPTP-insulted mice, ginsenoside Rb1 exerted its neuroprotective and motor activity, blunting dopaminergic neuronal death, reducing excitotoxicity induced by glutamate, and promoting synaptic transmission in the nigrostriatal nucleus (Zhang et al., 2018). The anti-oxidative effect of ginsenoside seems to be related to its ability to manage glutathione levels and ROS-NF-kB pathway and to regulate the expression of iron transport proteins. This latter mechanism was shown to reduce the nigral iron content (Wang et al., 2009).
Vincamine, an alkaloid of Vinca minor, has been shown to improve PD through differentmechanisms of action. In particular, it has a vasodilating effect due to its capability to relax smooth muscle cells of the neuronal capillary (King, 1987). This event leads to an increase in nutrient flow and delivery to the brain: the increased free glucose is paralleled to an increase in ATP production via the Krebs cycle. Moreover, vincamine also reduces oxidative stress, being able to reduce Fe3+ brain concentration (Fayed, 2010). Its chelating activity of iron ions improves DA production and reduces neuronal damage. Therefore, vincamine as well as its semi-synthetic derivative vinpocetine, can be beneficial in the treatment of PD by increasing nutrient disposal, limiting ROS production, and chelating iron ions. Vinpocetine showed neuroprotective effects in a rotenone-induced PD model in rats by increasing DA levels and reducing oxidative stress (Zaitone et al., 2012).
2.1.3. Synthetic nutraceutical derivatives
Because of the hydrophobicity and limited distribution to mitochondria of CoQ10 (Kezic et al., 2016), MitoQ, a mitochondria-targeted antioxidant, can overcome CoQ10’s limitations. Indeed, the molecular structure of MitoQ contains a lipophilic triphenylphosphonium cation linked to the antioxidant CoQ10, which contributes to the maintenance of respiratory chain function (Orsucci et al., 2011). MitoQ effect on mitochondrial dynamics was shown in preclinical models of neurodegenerative diseases, including PD (Jin et al., 2014; Solesio et al., 2013). Nowadays, MitoQ is widely considered more effective than other untargeted antioxidants in terms of preventing mitochondrial oxidative damage (Feniouk and Skulachev, 2017). Recently, Xi et al. (2018) evidenced the protective effect of MitoQ on mitochondrial dysfunction in 6-hydroxydopamine (6-OHDA)-induced PD using in vitro and in vivo models. In particular, the Authors proposed a potential transcription mechanism underpinning MitoQ effect, i.e. increasing mitofusin 2 expression strictly via peroxisome proliferator-activated receptor gamma coactivator (PGC)-1α.
The effect of a newly synthesized derivative of apocynin, a natural antioxidant compound, named Mito-apocynin was also investigated. Targeting mitochondria, Mito-apocynin limits not only the oxidative damage but also glial-mediated inflammation and nigrostriatal neurodegeneration in MPTP-insulted mice (Langley et al., 2017).
2.2. Targeting endoplasmic reticulum (ER) stress pathway, protein misfolding and aggregation
Abnormal misfolded proteins lead to ER stress, evoking the unfolded protein response (UPR), the ER-associated degradation of aggregated proteins, and autophagy. The main goal of therapies targeting ER stress, aims at prevent aggregation and/or clear from misfolded proteins. The loss of neuronal capability in clearing aggregated proteins or damaged organelles leads to cell death and apoptosis, making neurons highly predisposed to neurodegeneration (Green and Levine, 2014).
In the central nervous system (CNS), UPR activation occurs through three ER stress pathways: 1) inositol-requiring protein- (IRE)1α, 2) activating transcription factor (ATF) 6 and 3) protein kinase RNA-like ER kinase (PERK) (Kaufman, 2002). Although UPR can induce positive effects, its over-activation triggers pro-apoptotic events managed by the anti-apoptotic factor B cell lymphoma (BCL)-2 family (Kim et al., 2008). Nigral dopaminergic neurons of PD patients have been found positive for ER stress markers and α-Syn accumulation, suggesting a positive correlation between ER stress and misfolded α-Syn (Hoozemans et al., 2007).
An in vitro study has underlined that α-Syn activates ATF6 pathway directly through protein-protein interaction (α-Syn-ATF6), and indirectly through ATF6 incorporation in vesicles, diminishing its transport from ER to Golgi (Credle et al., 2015). ATF6 is the neuroprotective branch of the UPR, so its reduction probably activates the pro-apoptotic IRE1α and PERK branches of the UPR, thus triggering apoptosis. ATF6 reduction is correlated to increased ER-associated degradation genes, responsible for consequent apoptosis and an impaired UPR signaling (Credle et al., 2015).
Interestingly, the treatment with two flavones found in fruits and vegetables, such as apigenin and luteolin, induces an up-regulation of ATF6 expression and improves locomotor and muscular activities in MPTP-insulted mice (Patil et al., 2014).
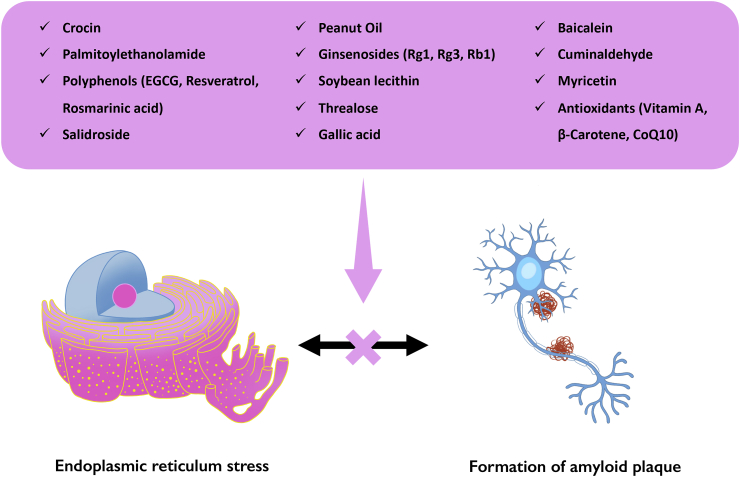
Regarding PERK pathway, high and prolonged phosphorylation of eukaryotic initiation factor (eIF)2α provokes long-lasting inhibition of protein synthesis, depleting essential short-lived proteins in ER (Harding et al., 1999). Moreover, it leads to the specific translation of ATF4, which upregulates genes involved in redox control, protein folding and apoptosis, such as the C/EBP-homologous protein (CHOP) and components of BCL-2 family (Tabas and Ron, 2011). Indeed, CHOP protects dopaminergic neurons against the 6-OHDA and MPTP in different experimental settings (Silva et al., 2005). On the other hand, low levels of phospho-eIF2α limit the temporary arrest in protein synthesis, leading to continuous protein storage that does not lessen ER stress. Exposure to 6-OHDA induces increased levels of phospho-eIF2α in neurons; moreover, sympathetic neurons from eIF2α null mice exhibit extended sensitivity to this neurotoxin (Ryu et al., 2002). In particular, 6-OHDA significantly increased ER stress response also by increasing in BiP expression and PERK-eIF2α pathway modulation at the striatal level. All followed-mentioned nutraceuticals with the aim at reducing ER stress and protein misfolding are shown in Fig. 2.
Fig. 2.
Nutraceuticals as therapeutics for PD, able to limit ER stress and protein misfolding and aggregation. The formation of misfolded proteins triggers the onset of ER stress, whose progression leads to the aggregation of proteins and eventually autophagy (Green and Levine, 2014). The main target of nutraceuticals as pharmacological treatment aims at clear misfolded proteins and reduce neurodegeneration.
2.2.1. Nutrients
Palmitoylethanolamide is an endogenous fatty acid ethanolamide, isolated from egg yolk as well as peanut oil and soybean lecithin, with the capability of modulating peripheral and central pathologic processes (Annunziata et al., 2020; Cristiano et al., 2018; Mattace Raso, Russo, Calignano and Meli, 2014; Russo et al., 2018). The treatment with palmitoylethanolamide, a PPAR-α ligand, reduces ER stress inhibiting BiP expression and the activation of PERK-eIF2α pathway in the striatum of 6-OHDA-challenged mice (Avagliano et al., 2016).
Moreover, it is well known that vitamins have been used in PD patients with some efficacy for their scavenger activity. However, hydrophobic antioxidants, such as vitamin A, β-carotene and CoQ10 may also have anti-fibrillogenic effects. In particular, vitamin A blocks intracellular α-Syn deposition in vivo (Ono and Yamada, 2007).
2.2.2. Herbals and phytochemicals
Crocin is a carotenoid compound responsible for the red color of the dried stigma of saffron (Crocus sativus L.) (Schmidt et al., 2007). Crocin exerts neuroprotective effects in several CNS disorders determined by many in vitro and in vivo studies; in particular, this carotenoid compound is able to decrease CHOP and binding-immunoglobulin protein (BiP)/Grp78 expressions and to inhibit the activation of pro-apoptotic factor caspase-12 in PC12 cells after MPP+ exposure (Zhang et al., 2015).
Beyond the beneficial effects on oxidative stress, EGCG also promotes the correct folding of α-Syn monomers into stable oligomers in a concentration-dependent manner (Sneideris et al., 2015). Moreover, it can remodel mature α-Syn fibrils and convert them into smaller, non-toxic ones, inducing a conformational change without disassembling α-Syn fibrils (Bieschke et al., 2010). More recently, it has been developed a combination of EGCG with specific α-Syn proteolytic peptide sequences for preventing α-Syn fibrillation and protecting dopaminergic neurons (Yoshida et al., 2013).
Baicalein is a flavone isolated from the roots of Scutellaria baicalensis Georgi (“Huang Qin” in Chinese), and from Scutellaria pinnatifida that grows in Iran. Baicalein prevents α-Syn fibrillation and protects SH-SY5Y and HeLa cells from neurotoxicity by preventing α-Syn oligomer formation (Lu et al., 2011). Furthermore, this flavone induces autophagy, inhibits apoptosis, reduces inflammation and restores DA in MPP+-induced PD model in mice (Hung et al., 2016).
The phenolic compound rosmarinic acid owns an activity on α-Syn oligomerization in mouse hippocampal slices in electrophysiological assays for long-term potentiation (Takahashi et al., 2015).
Resveratrol, a natural phytoestrogen, enhances α-Syn autophagic degradation in PC12 cells expressing α-Syn by induction of AMP-activated protein kinase mammalian silent information regulator 1 signaling mechanism (Wu et al., 2011). The induction of autophagy and apoptotic pathways represents an important approach in the therapeutic targeting of α-Syn, so resveratrol can represent a potential compound for a pharmaceutical approach, even if several factors, such as its stability and solubility at gastric pH, gut microflora, increased metabolic turnover, and a low permeability through the BBB, can compromise optimal bioavailability of polyphenol compounds in the brain (Scholz and Williamson, 2007).
Several chaperones called “disaggregases” play a crucial role in preserving the physiological status. Gallic acid, a type of phenolic acid chemically known as 3,4,5-trihydroxybenzoic acid, is found as a free form or as a part of the hydrolyzable tannins in sumac, witch hazel, tea leaves, and oak bark. In addition to inhibit aggregation, gallic acid disaggregates the preformed α-Syn fibrils in vitro (Ardah et al., 2014). A similar activity was shown by ginsenosides, named Rg1, Rg3, and Rb1, that represent the active constituents of ginseng (Panax ginseng, Araliaceae). In particular, Rb1 is a strong inhibitor of α-Syn fibrillation, disaggregating preformed fibrils and blocking the polymerization of α-Syn in vitro (Ardah et al., 2015).
Finally, salidroside, a glucoside present in Rhodiola rosea, was able to reduce α-Syn aggregation, enhancing the dephosphorylation at position Ser129, an enzymatic process strictly linked to Lewy bodies formation (Li et al., 2018).
2.3. Targeting neuroinflammation
The presence of high levels of proinflammatory cytokines, such as TNF-α, IL-1β, IL-6, and other damaging molecules was shown in the striatum of PD patients, and related to progressive degeneration of dopaminergic neurons and the consequent worsening of PD (Kaur et al., 2017). Banati et al. (1998) had already shown that post-mortem brains from PD patients examined revealed the activation of microglia, a crucial event of neuroinflammation. It has been reported that in Thy1-α-Syn mice overexpressing human α-synuclein, a strong activation of microglia in striatum (in 1-month old mice) and SN (later, at 5–6 months of age) was accompanied by high level of pro-inflammatory cytokines and toll-like receptor (TLR) expression (Watson et al., 2012), supporting the idea that neuroinflammation precedes the impairment of motor coordination.
During the neurodegenerative process of PD, the expression of TLRs stimulated by the presence of pathogen-associated molecular patterns or danger-associated molecular patterns, is induced. Among TLRs, TLR2 and TLR4 were found in high amount in post-mortem brains of PD patients (Drouin-Ouellet et al., 2014), precisely localized in Lewy bodies. In particular, TLR2 is responsible for the activation of microglia and the production of neurotoxic factors (Sanchez-Guajardo et al., 2015). Moreover, a high level of TLR4 in PD brain was associated with the production of pro-inflammatory factors (NF-κB, cyclooxygenase-2, iNOS) and cytokines (Trotta et al., 2014).
Several in vitro and in vivo studies have demonstrated the pivotal and detrimental role of inflammation in neurodegeneration, since non-steroidal anti-inflammatory drugs (NSAIDs), such as aspirin, counteracted dopaminergic neuronal loss, and improved PD symptoms (Esposito et al., 2007). However, precise doses and regimens have still to be determined. Consistently, the regular administration of NSAIDs was shown to reduce the risk of developing PD, while ibuprofen was shown to exert neuroprotective effects (Chen et al., 2003). Observational studies brought to the conclusion that NSAIDs or aspirin did not affect the risk of developing PD, while non-aspirin NSAIDs, particularly ibuprofen, reduced the risk of developing PD, although not significantly (Rees et al., 2011).
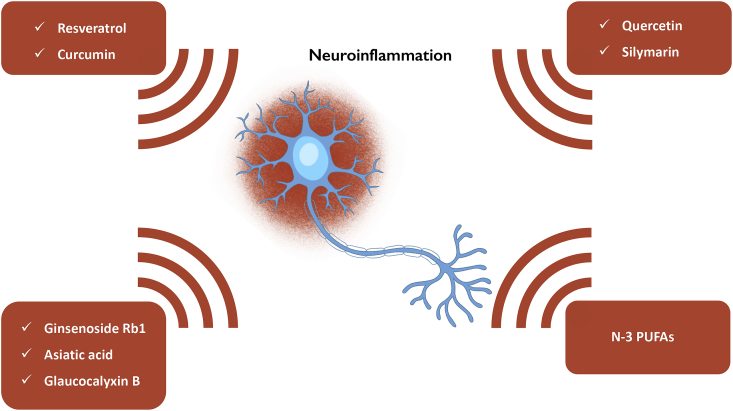
The nutraceuticals, as well as NSAIDs, are multi-target compounds with anti-inflammatory features useful for the treatment of neurodegenerative disorders (Fig. 3). Among natural products, resveratrol is characterized by an anti-inflammatory activity (Saiko et al., 2008), and several lines of evidence indicate its potential in neurodegenerative diseases; in particular, by limiting immune cell activation, reducing pro-inflammatory protein transcription (Das and Das, 2007), and exerting neuroprotective effect in dopaminergic neurons exposed to LPS (Zhang et al., 2010). Moreover, it also showed a protective effect by reducing microglia activation and the synthesis of pro-inflammatory mediators.
Fig. 3.
Nutraceuticals hold neuroprotective effects by reducing neuroinflammation. During the neurodegenerative process of PD, PAMPs and DAMPs induce the expression of TLRs, including TLR2 and TLR4. The activation of these immune mediators leads to the release of pro-inflammatory cytokines, responsible for the trigger of neuroinflammation (Kaur et al., 2017). The above-illustrated nutraceuticals limit the detrimental effects of inflammatory mediators, blunting the dopaminergic neuron loss.
2.3.1. Nutrients
ω-3 polyunsaturated fatty acids represent a potential approach for PD traits, because of their capability in limiting neuroinflammation and the loss of dopaminergic neurons. Indeed, 6-OHDA-animals fed with a diet supplemented with fish oil for 50 days or treated intragastrically with docosahexaenoic acid showed a reduced microgliosis and astrocytosis in SNpc (Hernando et al., 2019). Furthermore, the maternal supplement of ω-3 polyunsaturated fatty acids improved behavioral deficit in LPS-induced PD animals, limiting neuroinflammation without impacting on neuronal apoptosis (Delattre et al., 2017).
2.3.2. Herbals and phytochemicals
Curcumin is another phenolic compound, extracted from Curcuma longa roots, with excellent pharmacokinetic and activity for the treatment of neurodegenerative disorders. Firstly, Tegenge et colleagues (2014) demonstrated curcumin capability in protecting axons from neurodegeneration induced by nitric oxide. From this finding, other Authors investigated the potential role of curcumin in limiting neuroinflammatory features in PD models. In MPP+-stimulated astrocytes, curcumin limited inflammation (NF-κB) and immune system activation (IFN regulatory factor 3, myeloid differentiation primary response 88, TLR4) (Yu et al., 2016). The anti-inflammatory activity of curcumin was also confirmed in LPS-induced PD animals, where the polyphenol reduced levels of glial fibrillary acidic protein, NF-κB, iNOS, and pro-inflammatory cytokines (Sharma and Nehru, 2018). In 6-OHDA-treated animals, curcumin combined with the alkaloid piperine improved neurobehavioral features and reduced pro-inflammatory cytokine production (Singh and Kumar, 2017).
Beyond other above-mentioned mechanisms of action, ginsenosides, particularly Rb1, counteract PD features by limiting neuroinflammation. Indeed, Rb1 improved motor coordination altered by MPTP administration, limiting astrogliosis and microgliosis and reducing the production of pro-inflammatory cytokines in SNpc (TNF-α, IL-1β) (Heng et al., 2016). The beneficial effects of Rb1 in reducing neuroinflammation were also confirmed in LPS-induced PD animal model, reducing Iba-1 levels, the main factor of microgliosis, and the release of pro-inflammatory cytokines (Li et al., 2019). Sun et al. (2016) further elucidated the possible mechanism of action of Rb1. In particular, these Authors demonstrated that the antagonist of the glucocorticoid receptor RU486, partially limited the capability of Rb1 in inhibiting NF-κB pathway in SN.
Recently, Haddadi et al. (2018) have investigated the effect of a pre-treatment with silymarin, extracted from Sylibum marianum, on several pathogenic features in SNpc of 6-OHDA-insulted rats, showing a reduction in apoptosis, through inhibition of TLR4 expression.
It has been demonstrated that asiatic acid, a pentacyclic triterpene naturally occurring in medicinal plants, including Centella asiatica, was able to reduce striatal inflammation in MPTP-challenged mice, reducing inflammatory cytokines, TLR2, TLR4, and NF-κB expression (Chao et al., 2016). The Authors speculated that the reduction of α-Syn engagement by TLRs reduced NF-κB activation and thus inflammation. Moreover, they demonstrated an increase in neurotrophic factors, whose reduction sustained nigral dopaminergic neuron degeneration. Quite recently, a beneficial effect on LPS-induced PD symptoms has been demonstrated after glaucocalyxin B injection in rats. This entkaurane diterpenoid isolated from Rabdosia japonica inhibited TLR/NF-κB activation and activated nuclear factor erythroid 2-related factor 2/heme oxygenase-1 pathway in both in vivo and in vitro (Xu et al., 2017).
The seed extract of Mucuna pruriens owns anti-parkinsonian activity through the limitation of PD-induced neuroinflammation. Indeed, in MPTP mice model, the oral administration of an aqueous extract of Mucuna pruriens lessened neurotoxicity through the inhibition of NF-κB and pAkt pathway. Moreover, the MPTP-induced increase of other inflammatory parameters, including glial fibrillary acidic protein, iNOS, intercellular adhesion molecule, and TNF-α in mouse brains were significantly blunted by this natural product (Rai et al., 2017).
Among different phytochemicals, the compound with excellent anti-neuroinflammatory effects included in the seeds of Mucuna pruriens is quercetin. Bournival and colleagues (2012) were the first to identify the beneficial effects of quercetin in PD, limiting neuroinflammation. Later, other Authors demonstrated the capability of quercetin, also via new formulations such as nanocrystals, in limiting neuroinflammatory events triggered by PD progression (Ghaffari et al., 2018).
3. The role of gut-brain axis in PD: the gut-derived metabolites
Beyond the typical neurodegeneration, PD is also characterized by the impairment of gut homeostasis. In the last years, experimental data and clinical observations support the hypothesis that microbiota-gut-brain axis may play a role in the onset and progression of PD. Many central components assemble a complex network with the intestinal microbiota, where signals from the brain can influence the activity of the gut and vice versa (Grenham et al., 2011). Microbial-derived compounds impact CNS homeostasis, influencing neurogenesis and neuron survival (Ogbonnaya et al., 2015).
3.1. Microbial derivatives
Over the years, the description of gut microbiota composition in PD patients has become clearer. It is very complicated to associate each pathological feature (severity of disease, other disorders, ethnicity, dietary habits) to microbiota signature in PD and healthy humans, however, several studies underlying numerous differences in gut microbiota composition in PD have started to emerge (Keshavarzian et al., 2015; Unger et al., 2016). A very recent clinical study has analyzed the gut microbiota composition of 80 PD patients and 72 healthy individuals, demonstrating the deep diversity between the two groups (Pietrucci et al., 2019). Indeed, PD patients showed a gut microbiota characterized by overgrowth of Gram-negative bacteria, in particular Enterobacteriaceae, involved in the production of LPS. This endotoxin triggers the release of pro-inflammatory cytokines that reach the brain through the bloodstream and induce neuroinflammation, a starting step for the development of α-Syn-driven disease (Caputi and Giron, 2018).
The gut microbiota can also directly influence the production of α-Syn itself. Indeed, an in vivo study Chen et al. (2016) demonstrated that the exposure of bacterial amyloid protein curli induced an increased production of α-Syn in both periphery and CNS, subsequently enhancing both astrogliosis and microgliosis. Moreover, germ-free mice overexpressing α-Syn (GF Thy1-α-Syn mice) did not show typical features of PD, including α-synucleinopathy, impaired movement and microgliosis. Notably, their recolonization by fecal transplant of PD patients revealed a higher score in PD patterns (Sampson et al., 2016).
Further clinical trials are currently investigating additional links in the gut-brain axis in PD features (trial numbers: NCT03710668 and NCT03129451).
3.2. Short-chain fatty acids (SCFAs)
The PD-induced modifications not only influence gut microbiota composition, but also the production of microbial metabolites, such as SCFAs (Pietrucci et al., 2019; Unger et al., 2016). Several microbial genera are involved in the production of three main and neuroactive SCFAs, acetate, propionate, and butyrate through specific enzymatic pathways (Koh et al., 2016). Beyond the function of maintaining host homeostasis, SCFAs influence CNS function, acting as signaling molecules for the colonic biosynthesis of neuroactive compounds, including 5-hydroxytryptamine (Reigstad et al., 2015). Interestingly, microbial genera whose amount resulted modified in PD patients were directly involved in the metabolism of SCFAs. In the last years, many studies focused on the effects of SCFAs in PD mouse models. These gut-derived metabolites showed the capability to limit pathological features of PD, and, among all SCFAs, butyrate and its derivatives seem to own more interesting effects. In the rotenone-induced PD model, butyrate improved motor deficit by restoring DA levels in the brain, indicating the inhibition of histone deacetylase as an underlying mechanism (St Laurent, O’Brien and Ahmad, 2013). This finding was recently confirmed by Paiva et al. (2017), which demonstrated that the accumulation of α-Syn in dopaminergic neurons led to the reduction of acetylated histone 3 levels and that butyrate exerted its activity as histone deacetylase inhibitor, limiting DNA damage. In MPTP-treated mice, 3-week treatment with butyrate blunted dopaminergic degeneration, reduced BBB permeability and apoptosis markers (Liu et al., 2017). The beneficial effects of a butyrate derivative, β-hydroxybutyric acid, were also linked to its capability in reducing neuroinflammation through GPR109A (Fu et al., 2015). Although these findings would confirm the beneficial impact of SCFAs in PD, Sampson et al. (2016) have identified SCFAs as possible key drivers of microglial activation, triggering the impairment of motor coordination in mice. However, an ongoing clinical study (trial number: NCT03705520) aims at determining the amount of SCFAs in serum and stool samples of PD patients and healthy controls to clarify the role of these bacteria-derived metabolites in disease.
4. Conclusions
Despite the profound efforts in understanding PD pathological features and complexity, challenges to develop novel treatments to prevent dopaminergic loss and mitigate PD progression have to be still overcome due to several limits: the inability of animal models in recapitulating the pathological features of PD, the lack of reliable biomarkers to evaluate the efficacy of tested drugs, the inability in separating disease-modifying effects from long-lasting symptomatic relief in PD patients, or the selection of too advanced PD patients unresponsive to therapies. Among symptomatic drugs, apart levodopa, DA agonists are used as well. Nevertheless, DA reconstitution or DA agonist administration does not cure the disease and has no efficacy in limiting the progression of PD. Therefore, novel approaches have been identified trying to counteract the altered pathogenic pathways underlying PD onset and development, also in the view of the contribution of many interconnected events all converging in PD pathology. Notably, substantial evidence supports the potential of many nutraceuticals as adjunctive therapy for the prevention and treatment of PD, since acting on specific or more pathogenic mechanisms, nutraceuticals may sustain standard therapy contributing to neuroprotective effects.
The novel emerging therapeutic and integrative approach with nutraceuticals has opened a new scenario to deal with this multifactorial and complex disease. Indeed, it is becoming clear that multi-target, rather than single-drug approach must be considered, depending on disease etiology and progression.
Author contributions
AL, CP, CAv, CAn and GMR wrote the paper. MPM, AC, RM supervised the review editing. GMR decided the overall structure of the review, coordinating the working group.
Declaration of competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Contributor Information
Adriano Lama, Email: adriano.lama@unina.it.
Claudio Pirozzi, Email: claudio.pirozzi@unina.it.
Carmen Avagliano, Email: carmen.avagliano@unina.it.
Chiara Annunziata, Email: chiara.annunziata@unina.it.
Maria Pina Mollica, Email: mpmollic@unina.it.
Antonio Calignano, Email: calignan@unina.it.
Rosaria Meli, Email: meli@unina.it.
Giuseppina Mattace Raso, Email: mattace@unina.it.
References
- Andrew R., Izzo A.A. Principles of pharmacological research of nutraceuticals. Br. J. Pharmacol. 2017;174:1177–1194. doi: 10.1111/bph.13779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziata C., Lama A., Pirozzi C., Cavaliere G., Trinchese G., Di Guida F., Nitrato Izzo A., Cimmino F., Paciello O., De Biase D., Murru E., Banni S., Calignano A., Mollica M.P., Mattace Raso G., Meli R. Palmitoylethanolamide Counteracts Hepatic Metabolic Inflexibility Modulating Mitochondrial Function and Efficiency in Diet-Induced Obese Mice. The FASEB Journal. 2020;34:350–364. doi: 10.1096/fj.201901510RR. [DOI] [PubMed] [Google Scholar]
- Ardah M.T., Paleologou K.E., Lv G., Abul Khair S.B., Kazim A.S., Minhas S.T., Al-Tel T.H., Al-Hayani A.A., Haque M.E., Eliezer D., El-Agnaf O.M. Structure activity relationship of phenolic acid inhibitors of alpha-synuclein fibril formation and toxicity. Front. Aging Neurosci. 2014;6:197. doi: 10.3389/fnagi.2014.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardah M.T., Paleologou K.E., Lv G., Menon S.A., Abul Khair S.B., Lu J.H., Safieh-Garabedian B., Al-Hayani A.A., Eliezer D., Li M., El-Agnaf O.M. Ginsenoside Rb1 inhibits fibrillation and toxicity of alpha-synuclein and disaggregates preformed fibrils. Neurobiol. Dis. 2015;74:89–101. doi: 10.1016/j.nbd.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athauda D., Foltynie T. The ongoing pursuit of neuroprotective therapies in Parkinson disease. Nat. Rev. Neurol. 2015;11:25–40. doi: 10.1038/nrneurol.2014.226. [DOI] [PubMed] [Google Scholar]
- Avagliano C., Russo R., De Caro C., Cristiano C., La Rana G., Piegari G., Paciello O., Citraro R., Russo E., De Sarro G., Meli R., Mattace Raso G., Calignano A. Palmitoylethanolamide protects mice against 6-OHDA-induced neurotoxicity and endoplasmic reticulum stress: in vivo and in vitro evidence. Pharmacol. Res. 2016;113:276–289. doi: 10.1016/j.phrs.2016.09.004. [DOI] [PubMed] [Google Scholar]
- Banati R.B., Daniel S.E., Blunt S.B. Glial pathology but absence of apoptotic nigral neurons in long-standing Parkinson’s disease. Mov. Disord. 1998;13:221–227. doi: 10.1002/mds.870130205. [DOI] [PubMed] [Google Scholar]
- Bieschke J., Russ J., Friedrich R.P., Ehrnhoefer D.E., Wobst H., Neugebauer K., Wanker E.E. EGCG remodels mature alpha-synuclein and amyloid-beta fibrils and reduces cellular toxicity. Proc. Natl. Acad. Sci. U. S. A. 2010;107:7710–7715. doi: 10.1073/pnas.0910723107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose A., Beal M.F. Mitochondrial dysfunction in Parkinson’s disease. J. Neurochem. 2016;139(Suppl 1):216–231. doi: 10.1111/jnc.13731. [DOI] [PubMed] [Google Scholar]
- Bournival J., Plouffe M., Renaud J., Provencher C., Martinoli M.G. Quercetin and sesamin protect dopaminergic cells from MPP+-induced neuroinflammation in a microglial (N9)-neuronal (PC12) coculture system. Oxid. Med. Cell Longev. 2012;2012:921941. doi: 10.1155/2012/921941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., Del Tredici K. Invited Article: nervous system pathology in sporadic Parkinson disease. Neurology. 2008;70:1916–1925. doi: 10.1212/01.wnl.0000312279.49272.9f. [DOI] [PubMed] [Google Scholar]
- Caputi V., Giron M.C. Microbiome-gut-brain Axis and toll-like receptors in Parkinson’s disease. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19061689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao P.C., Lee H.L., Yin M.C. Asiatic acid attenuated apoptotic and inflammatory stress in the striatum of MPTP-treated mice. Food Funct. 2016;7:1999–2005. doi: 10.1039/c6fo00041j. [DOI] [PubMed] [Google Scholar]
- Chaudhuri K.R., Schapira A.H. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8:464–474. doi: 10.1016/S1474-4422(09)70068-7. [DOI] [PubMed] [Google Scholar]
- Chen H., Zhang S.M., Hernan M.A., Schwarzschild M.A., Willett W.C., Colditz G.A., Speizer F.E., Ascherio A. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch. Neurol. 2003;60:1059–1064. doi: 10.1001/archneur.60.8.1059. [DOI] [PubMed] [Google Scholar]
- Chen S.G., Stribinskis V., Rane M.J., Demuth D.R., Gozal E., Roberts A.M., Jagadapillai R., Liu R., Choe K., Shivakumar B., Son F., Jin S., Kerber R., Adame A., Masliah E., Friedland R.P. Exposure to the functional bacterial amyloid protein curli enhances alpha-synuclein aggregation in aged Fischer 344 rats and Caenorhabditis elegans. Sci. Rep. 2016;6:34477. doi: 10.1038/srep34477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleren C., Yang L., Lorenzo B., Calingasan N.Y., Schomer A., Sireci A., Wille E.J., Beal M.F. Therapeutic effects of coenzyme Q10 (CoQ10) and reduced CoQ10 in the MPTP model of Parkinsonism. J. Neurochem. 2008;104:1613–1621. doi: 10.1111/j.1471-4159.2007.05097.x. [DOI] [PubMed] [Google Scholar]
- Credle J.J., Forcelli P.A., Delannoy M., Oaks A.W., Permaul E., Berry D.L., Duka V., Wills J., Sidhu A. alpha-Synuclein-mediated inhibition of ATF6 processing into COPII vesicles disrupts UPR signaling in Parkinson’s disease. Neurobiol. Dis. 2015;76:112–125. doi: 10.1016/j.nbd.2015.02.005. [DOI] [PubMed] [Google Scholar]
- Cristiano C., Pirozzi C., Coretti L., Cavaliere G., Lama A., Russo R., Lembo F., Mollica M.P., Meli R., Calignano A., Mattace Raso G. Palmitoylethanolamide counteracts autistic-like behaviours in BTBR T+tf/J mice: contribution of central and peripheral mechanisms. Brain Behav. Immun. 2018;74:166–175. doi: 10.1016/j.bbi.2018.09.003. [DOI] [PubMed] [Google Scholar]
- Das S., Das D.K. Anti-inflammatory responses of resveratrol. Inflamm. Allergy - Drug Targets. 2007;6:168–173. doi: 10.2174/187152807781696464. [DOI] [PubMed] [Google Scholar]
- Delattre A.M., Carabelli B., Mori M.A., Kempe P.G., Rizzo de Souza L.E., Zanata S.M., Machado R.B., Suchecki D., Andrade da Costa B.L.S., Lima M.M.S., Ferraz A.C. Maternal omega-3 supplement improves dopaminergic system in pre- and postnatal inflammation-induced neurotoxicity in Parkinson’s disease model. Mol. Neurobiol. 2017;54:2090–2106. doi: 10.1007/s12035-016-9803-8. [DOI] [PubMed] [Google Scholar]
- Denny Joseph K.M., Muralidhara Combined oral supplementation of fish oil and quercetin enhances neuroprotection in a chronic rotenone rat model: relevance to Parkinson’s disease. Neurochem. Res. 2015;40:894–905. doi: 10.1007/s11064-015-1542-0. [DOI] [PubMed] [Google Scholar]
- di Matteo V., Pierucci M., Di Giovanni G., Dragani L.K., Murzilli S., Poggi A., Esposito E. Intake of tomato-enriched diet protects from 6-hydroxydopamine-induced degeneration of rat nigral dopaminergic neurons. J. Neural Transm. 2009;Suppl:333–341. doi: 10.1007/978-3-211-92660-4_28. [DOI] [PubMed] [Google Scholar]
- Drouin-Ouellet J., St-Amour I., Saint-Pierre M., Lamontagne-Proulx J., Kriz J., Barker R.A., Cicchetti F. Toll-like receptor expression in the blood and brain of patients and a mouse model of Parkinson’s disease. Int. J. Neuropsychopharmacol. 2014;18 doi: 10.1093/ijnp/pyu103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta D., Mohanakumar K.P. Tea and Parkinson’s disease: constituents of tea synergize with antiparkinsonian drugs to provide better therapeutic benefits. Neurochem. Int. 2015;89:181–190. doi: 10.1016/j.neuint.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Elkouzi A., Vedam-Mai V., Eisinger R.S., Okun M.S. Emerging therapies in Parkinson disease - repurposed drugs and new approaches. Nat. Rev. Neurol. 2019;15:204–223. doi: 10.1038/s41582-019-0155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito E., Di Matteo V., Benigno A., Pierucci M., Crescimanno G., Di Giovanni G. Non-steroidal anti-inflammatory drugs in Parkinson’s disease. Exp. Neurol. 2007;205:295–312. doi: 10.1016/j.expneurol.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Fato R., Bergamini C., Leoni S., Strocchi P., Lenaz G. Generation of reactive oxygen species by mitochondrial complex I: implications in neurodegeneration. Neurochem. Res. 2008;33:2487–2501. doi: 10.1007/s11064-008-9747-0. [DOI] [PubMed] [Google Scholar]
- Fayed A.H. Brain trace element concentration of rats treated with the plant alkaloid, vincamine. Biol. Trace Elem. Res. 2010;136:314–319. doi: 10.1007/s12011-009-8550-3. [DOI] [PubMed] [Google Scholar]
- Feniouk B.A., Skulachev V.P. Cellular and molecular mechanisms of action of mitochondria-targeted antioxidants. Curr. Aging Sci. 2017;10:41–48. doi: 10.2174/1874609809666160921113706. [DOI] [PubMed] [Google Scholar]
- Fu S.P., Wang J.F., Xue W.J., Liu H.M., Liu B.R., Zeng Y.L., Li S.N., Huang B.X., Lv Q.K., Wang W., Liu J.X. Anti-inflammatory effects of BHBA in both in vivo and in vitro Parkinson’s disease models are mediated by GPR109A-dependent mechanisms. J. Neuroinflammation. 2015;12:9. doi: 10.1186/s12974-014-0230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassen M., Youdim M.B. The potential role of iron chelators in the treatment of Parkinson’s disease and related neurological disorders. Pharmacol. Toxicol. 1997;80:159–166. doi: 10.1111/j.1600-0773.1997.tb00390.x. [DOI] [PubMed] [Google Scholar]
- Ghaffari F., Hajizadeh Moghaddam A., Zare M. Neuroprotective effect of quercetin nanocrystal in a 6-hydroxydopamine model of Parkinson disease: biochemical and behavioral evidence. Basic Clin. Neurosci. 2018;9:317–324. doi: 10.32598/bcn.9.5.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D.R., Levine B. To be or not to be? How selective autophagy and cell death govern cell fate. Cell. 2014;157:65–75. doi: 10.1016/j.cell.2014.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenham S., Clarke G., Cryan J.F., Dinan T.G. Brain-gut-microbe communication in health and disease. Front. Physiol. 2011;2:94. doi: 10.3389/fphys.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y.J., Dong S.Y., Cui X.X., Feng Y., Liu T., Yin M., Kuo S.H., Tan E.K., Zhao W.J., Wu Y.C. Resveratrol alleviates MPTP-induced motor impairments and pathological changes by autophagic degradation of alpha-synuclein via SIRT1-deacetylated LC3. Mol. Nutr. Food Res. 2016;60:2161–2175. doi: 10.1002/mnfr.201600111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddadi R., Nayebi A.M., Eyvari Brooshghalan S. Silymarin prevents apoptosis through inhibiting the Bax/caspase-3 expression and suppresses toll like receptor-4 pathway in the SNc of 6-OHDA intoxicated rats. Biomed. Pharmacother. 2018;104:127–136. doi: 10.1016/j.biopha.2018.05.020. [DOI] [PubMed] [Google Scholar]
- Hamacher-Brady A., Brady N.R. Mitophagy programs: mechanisms and physiological implications of mitochondrial targeting by autophagy. Cell. Mol. Life Sci. 2016;73:775–795. doi: 10.1007/s00018-015-2087-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding H.P., Zhang Y., Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- Heng Y., Zhang Q.S., Mu Z., Hu J.F., Yuan Y.H., Chen N.H. Ginsenoside Rg1 attenuates motor impairment and neuroinflammation in the MPTP-probenecid-induced parkinsonism mouse model by targeting alpha-synuclein abnormalities in the substantia nigra. Toxicol. Lett. 2016;243:7–21. doi: 10.1016/j.toxlet.2015.12.005. [DOI] [PubMed] [Google Scholar]
- Hernando S., Requejo C., Herran E., Ruiz-Ortega J.A., Morera-Herreras T., Lafuente J.V., Ugedo L., Gainza E., Pedraz J.L., Igartua M., Hernandez R.M. Beneficial effects of n-3 polyunsaturated fatty acids administration in a partial lesion model of Parkinson’s disease: the role of glia and NRf2 regulation. Neurobiol. Dis. 2019;121:252–262. doi: 10.1016/j.nbd.2018.10.001. [DOI] [PubMed] [Google Scholar]
- Hoozemans J.J., van Haastert E.S., Eikelenboom P., de Vos R.A., Rozemuller J.M., Scheper W. Activation of the unfolded protein response in Parkinson’s disease. Biochem. Biophys. Res. Commun. 2007;354:707–711. doi: 10.1016/j.bbrc.2007.01.043. [DOI] [PubMed] [Google Scholar]
- Hung K.C., Huang H.J., Wang Y.T., Lin A.M. Baicalein attenuates alpha-synuclein aggregation, inflammasome activation and autophagy in the MPP(+)-treated nigrostriatal dopaminergic system in vivo. J. Ethnopharmacol. 2016;194:522–529. doi: 10.1016/j.jep.2016.10.040. [DOI] [PubMed] [Google Scholar]
- Jin H., Kanthasamy A., Ghosh A., Anantharam V., Kalyanaraman B., Kanthasamy A.G. Mitochondria-targeted antioxidants for treatment of Parkinson’s disease: preclinical and clinical outcomes. Biochim. Biophys. Acta. 2014;1842:1282–1294. doi: 10.1016/j.bbadis.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia L.V., Lang A.E. Parkinson’s disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- Kaufman R.J. Orchestrating the unfolded protein response in health and disease. J. Clin. Investig. 2002;110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur K., Gill J.S., Bansal P.K., Deshmukh R. Neuroinflammation - a major cause for striatal dopaminergic degeneration in Parkinson’s disease. J. Neurol. Sci. 2017;381:308–314. doi: 10.1016/j.jns.2017.08.3251. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A., Green S.J., Engen P.A., Voigt R.M., Naqib A., Forsyth C.B., Mutlu E., Shannon K.M. Colonic bacterial composition in Parkinson’s disease. Mov. Disord. 2015;30:1351–1360. doi: 10.1002/mds.26307. [DOI] [PubMed] [Google Scholar]
- Kezic A., Spasojevic I., Lezaic V., Bajcetic M. Mitochondria-targeted antioxidants: future perspectives in kidney ischemia reperfusion injury. Oxid. Med. Cell Longev. 2016;2016:2950503. doi: 10.1155/2016/2950503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I., Xu W., Reed J.C. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- King G.A. Protective effects of vinpocetine and structurally related drugs on the lethal consequences of hypoxia in mice. Arch. Int. Pharmacodyn. Ther. 1987;286:299–307. [PubMed] [Google Scholar]
- Knorle R. Neuromelanin in Parkinson’s disease: from Fenton reaction to calcium signaling. Neurotox. Res. 2018;33:515–522. doi: 10.1007/s12640-017-9804-z. [DOI] [PubMed] [Google Scholar]
- Koh A., De Vadder F., Kovatcheva-Datchary P., Backhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- Kujawska M., Jodynis-Liebert J. Polyphenols in Parkinson’s disease: a systematic review of in vivo studies. Nutrients. 2018;10 doi: 10.3390/nu10050642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley M., Ghosh A., Charli A., Sarkar S., Ay M., Luo J., Zielonka J., Brenza T., Bennett B., Jin H., Ghaisas S., Schlichtmann B., Kim D., Anantharam V., Kanthasamy A., Narasimhan B., Kalyanaraman B., Kanthasamy A.G. Mito-apocynin prevents mitochondrial dysfunction, microglial activation, oxidative damage, and progressive neurodegeneration in MitoPark transgenic mice. Antioxidants Redox Signal. 2017;27:1048–1066. doi: 10.1089/ars.2016.6905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D.W., Zhou F.Z., Sun X.C., Li S.C., Yang J.B., Sun H.H., Wang A.H. Ginsenoside Rb1 protects dopaminergic neurons from inflammatory injury induced by intranigral lipopolysaccharide injection. Neural Regen. Res. 2019;14:1814–1822. doi: 10.4103/1673-5374.257536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Feng Y., Yang R., Wu L., Li R., Huang L., Yang Q., Chen J. Salidroside promotes the pathological alpha-synuclein clearance through ubiquitin-proteasome system in SH-SY5Y cells. Front. Pharmacol. 2018;9:377. doi: 10.3389/fphar.2018.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Wang F., Liu S., Du J., Hu X., Xiong J., Fang R., Chen W., Sun J. Sodium butyrate exerts protective effect against Parkinson’s disease in mice via stimulation of glucagon like peptide-1. J. Neurol. Sci. 2017;381:176–181. doi: 10.1016/j.jns.2017.08.3235. [DOI] [PubMed] [Google Scholar]
- Lu J.H., Ardah M.T., Durairajan S.S., Liu L.F., Xie L.X., Fong W.F., Hasan M.Y., Huang J.D., El-Agnaf O.M., Li M. Baicalein inhibits formation of alpha-synuclein oligomers within living cells and prevents Abeta peptide fibrillation and oligomerisation. Chembiochem. 2011;12:615–624. doi: 10.1002/cbic.201000604. [DOI] [PubMed] [Google Scholar]
- Ludtmann M.H., Angelova P.R., Ninkina N.N., Gandhi S., Buchman V.L., Abramov A.Y. Monomeric alpha-synuclein exerts a physiological role on brain ATP synthase. J. Neurosci. 2016;36:10510–10521. doi: 10.1523/JNEUROSCI.1659-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludtmann M.H.R., Angelova P.R., Horrocks M.H., Choi M.L., Rodrigues M., Baev A.Y., Berezhnov A.V., Yao Z., Little D., Banushi B., Al-Menhali A.S., Ranasinghe R.T., Whiten D.R., Yapom R., Dolt K.S., Devine M.J., Gissen P., Kunath T., Jaganjac M., Pavlov E.V., Klenerman D., Abramov A.Y., Gandhi S. alpha-synuclein oligomers interact with ATP synthase and open the permeability transition pore in Parkinson’s disease. Nat. Commun. 2018;9:2293. doi: 10.1038/s41467-018-04422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattace Raso G., Russo R., Calignano A., Meli R. Palmitoylethanolamide in CNS health and disease. Pharmacol. Res. 2014;86:32–41. doi: 10.1016/j.phrs.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Mochizuki H., Yasuda T. Iron accumulation in Parkinson’s disease. J Neural Transm (Vienna) 2012;119:1511–1514. doi: 10.1007/s00702-012-0905-9. [DOI] [PubMed] [Google Scholar]
- Ogbonnaya E.S., Clarke G., Shanahan F., Dinan T.G., Cryan J.F., O’Leary O.F. Adult hippocampal neurogenesis is regulated by the microbiome. Biol. Psychiatry. 2015;78:e7–9. doi: 10.1016/j.biopsych.2014.12.023. [DOI] [PubMed] [Google Scholar]
- Ono K., Yamada M. Vitamin A potently destabilizes preformed alpha-synuclein fibrils in vitro: implications for Lewy body diseases. Neurobiol. Dis. 2007;25:446–454. doi: 10.1016/j.nbd.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Orsucci D., Mancuso M., Ienco E.C., LoGerfo A., Siciliano G. Targeting mitochondrial dysfunction and neurodegeneration by means of coenzyme Q10 and its analogues. Curr. Med. Chem. 2011;18:4053–4064. doi: 10.2174/092986711796957257. [DOI] [PubMed] [Google Scholar]
- Paiva I., Pinho R., Pavlou M.A., Hennion M., Wales P., Schutz A.L., Rajput A., Szego E.M., Kerimoglu C., Gerhardt E., Rego A.C., Fischer A., Bonn S., Outeiro T.F. Sodium butyrate rescues dopaminergic cells from alpha-synuclein-induced transcriptional deregulation and DNA damage. Hum. Mol. Genet. 2017;26:2231–2246. doi: 10.1093/hmg/ddx114. [DOI] [PubMed] [Google Scholar]
- Parlak H., Ozkan A., Dilmac S., Tanriover G., Ozsoy O., Agar A. Neuronal nitric oxide synthase phosphorylation induced by docosahexaenoic acid protects dopaminergic neurons in an experimental model of Parkinson’s disease. Folia Histochem. Cytobiol. 2018;56:27–37. doi: 10.5603/FHC.a2018.0005. [DOI] [PubMed] [Google Scholar]
- Patil S.P., Jain P.D., Sancheti J.S., Ghumatkar P.J., Tambe R., Sathaye S. Neuroprotective and neurotrophic effects of Apigenin and Luteolin in MPTP induced parkinsonism in mice. Neuropharmacology. 2014;86:192–202. doi: 10.1016/j.neuropharm.2021.108876. [DOI] [PubMed] [Google Scholar]
- Pickrell A.M., Huang C.H., Kennedy S.R., Ordureau A., Sideris D.P., Hoekstra J.G., Harper J.W., Youle R.J. Endogenous Parkin preserves dopaminergic substantia nigral neurons following mitochondrial DNA mutagenic stress. Neuron. 2015;87:371–381. doi: 10.1016/j.neuron.2015.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrucci D., Cerroni R., Unida V., Farcomeni A., Pierantozzi M., Mercuri N.B., Biocca S., Stefani A., Desideri A. Dysbiosis of gut microbiota in a selected population of Parkinson’s patients. Park. Relat. Disord. 2019;65:124–130. doi: 10.1016/j.parkreldis.2019.06.003. [DOI] [PubMed] [Google Scholar]
- Prema A., Janakiraman U., Manivasagam T., Thenmozhi A.J. Neuroprotective effect of lycopene against MPTP induced experimental Parkinson’s disease in mice. Neurosci. Lett. 2015;599:12–19. doi: 10.1016/j.neulet.2015.05.024. [DOI] [PubMed] [Google Scholar]
- Rai S.N., Birla H., Singh S.S., Zahra W., Patil R.R., Jadhav J.P., Gedda M.R., Singh S.P. Mucuna pruriens protects against MPTP intoxicated neuroinflammation in Parkinson’s disease through NF-kappaB/pAKT signaling pathways. Front. Aging Neurosci. 2017;9:421. doi: 10.3389/fnagi.2017.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees K., Stowe R., Patel S., Ives N., Breen K., Clarke C.E., Ben-Shlomo Y. Non-steroidal anti-inflammatory drugs as disease-modifying agents for Parkinson’s disease: evidence from observational studies. Cochrane Database Syst. Rev. 2011 doi: 10.1002/14651858.CD008454.pub2. [DOI] [PubMed] [Google Scholar]
- Reigstad C.S., Salmonson C.E., Rainey J.F., 3rd, Szurszewski J.H., Linden D.R., Sonnenburg J.L., Farrugia G., Kashyap P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015;29:1395–1403. doi: 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo R., Cristiano C., Avagliano C., De Caro C., La Rana G., Raso G.M., Canani R.B., Meli R., Calignano A. Gut-brain Axis: role of lipids in the regulation of inflammation, pain and CNS diseases. Curr. Med. Chem. 2018;25:3930–3952. doi: 10.2174/0929867324666170216113756. [DOI] [PubMed] [Google Scholar]
- Ryu E.J., Harding H.P., Angelastro J.M., Vitolo O.V., Ron D., Greene L.A. Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson’s disease. J. Neurosci. 2002;22:10690–10698. doi: 10.1523/JNEUROSCI.22-24-10690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiko P., Szakmary A., Jaeger W., Szekeres T. Resveratrol and its analogs: defense against cancer, coronary disease and neurodegenerative maladies or just a fad? Mutat. Res. 2008;658:68–94. doi: 10.1016/j.mrrev.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Sampson T.R., Debelius J.W., Thron T., Janssen S., Shastri G.G., Ilhan Z.E., Challis C., Schretter C.E., Rocha S., Gradinaru V., Chesselet M.F., Keshavarzian A., Shannon K.M., Krajmalnik-Brown R., Wittung-Stafshede P., Knight R., Mazmanian S.K. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167:1469–1480. doi: 10.1016/j.cell.2016.11.018. e1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Guajardo V., Tentillier N., Romero-Ramos M. The relation between alpha-synuclein and microglia in Parkinson’s disease: recent developments. Neuroscience. 2015;302:47–58. doi: 10.1016/j.neuroscience.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Schmidt M., Betti G., Hensel A. Saffron in phytotherapy: pharmacology and clinical uses. Wien. Med. Wochenschr. 2007;157:315–319. doi: 10.1007/s10354-007-0428-4. [DOI] [PubMed] [Google Scholar]
- Scholz S., Williamson G. Interactions affecting the bioavailability of dietary polyphenols in vivo. Int. J. Vitam. Nutr. Res. 2007;77:224–235. doi: 10.1024/0300-9831.77.3.224. [DOI] [PubMed] [Google Scholar]
- Sharma N., Nehru B. Curcumin affords neuroprotection and inhibits alpha-synuclein aggregation in lipopolysaccharide-induced Parkinson’s disease model. Inflammopharmacology. 2018;26:349–360. doi: 10.1007/s10787-017-0402-8. [DOI] [PubMed] [Google Scholar]
- Shults C.W. Therapeutic role of coenzyme Q(10) in Parkinson’s disease. Pharmacol. Ther. 2005;107:120–130. doi: 10.1016/j.pharmthera.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Silva R.M., Ries V., Oo T.F., Yarygina O., Jackson-Lewis V., Ryu E.J., Lu P.D., Marciniak S.J., Ron D., Przedborski S., Kholodilov N., Greene L.A., Burke R.E. CHOP/GADD153 is a mediator of apoptotic death in substantia nigra dopamine neurons in an in vivo neurotoxin model of parkinsonism. J. Neurochem. 2005;95:974–986. doi: 10.1111/j.1471-4159.2005.03428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Kumar P. Neuroprotective potential of curcumin in combination with piperine against 6-hydroxy dopamine induced motor deficit and neurochemical alterations in rats. Inflammopharmacology. 2017;25:69–79. doi: 10.1007/s10787-016-0297-9. [DOI] [PubMed] [Google Scholar]
- Sneideris T., Baranauskiene L., Cannon J.G., Rutkiene R., Meskys R., Smirnovas V. Looking for a generic inhibitor of amyloid-like fibril formation among flavone derivatives. PeerJ. 2015;3 doi: 10.7717/peerj.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solesio M.E., Prime T.A., Logan A., Murphy M.P., Del Mar Arroyo-Jimenez M., Jordan J., Galindo M.F. The mitochondria-targeted anti-oxidant MitoQ reduces aspects of mitochondrial fission in the 6-OHDA cell model of Parkinson’s disease. Biochim. Biophys. Acta. 2013;1832:174–182. doi: 10.1016/j.bbadis.2012.07.009. [DOI] [PubMed] [Google Scholar]
- St Laurent R., O’Brien L.M., Ahmad S.T. Sodium butyrate improves locomotor impairment and early mortality in a rotenone-induced Drosophila model of Parkinson’s disease. Neuroscience. 2013;246:382–390. doi: 10.1016/j.neuroscience.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganuma H., Hirano T., Arimoto Y., Inakuma T. Effect of tomato intake on striatal monoamine level in a mouse model of experimental Parkinson’s disease. J. Nutr. Sci. Vitaminol. 2002;48:251–254. doi: 10.3177/jnsv.48.251. [DOI] [PubMed] [Google Scholar]
- Sun X.C., Ren X.F., Chen L., Gao X.Q., Xie J.X., Chen W.F. Glucocorticoid receptor is involved in the neuroprotective effect of ginsenoside Rg1 against inflammation-induced dopaminergic neuronal degeneration in substantia nigra. J. Steroid Biochem. Mol. Biol. 2016;155:94–103. doi: 10.1016/j.jsbmb.2015.09.040. [DOI] [PubMed] [Google Scholar]
- Tabas I., Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghizadeh M., Tamtaji O.R., Dadgostar E., Daneshvar Kakhaki R., Bahmani F., Abolhassani J., Aarabi M.H., Kouchaki E., Memarzadeh M.R., Asemi Z. The effects of omega-3 fatty acids and vitamin E co-supplementation on clinical and metabolic status in patients with Parkinson’s disease: a randomized, double-blind, placebo-controlled trial. Neurochem. Int. 2017;108:183–189. doi: 10.1016/j.neuint.2017.03.014. [DOI] [PubMed] [Google Scholar]
- Takahashi R., Ono K., Takamura Y., Mizuguchi M., Ikeda T., Nishijo H., Yamada M. Phenolic compounds prevent the oligomerization of alpha-synuclein and reduce synaptic toxicity. J. Neurochem. 2015;134:943–955. doi: 10.1111/jnc.13180. [DOI] [PubMed] [Google Scholar]
- Tegenge M.A., Rajbhandari L., Shrestha S., Mithal A., Hosmane S., Venkatesan A. Curcumin protects axons from degeneration in the setting of local neuroinflammation. Exp. Neurol. 2014;253:102–110. doi: 10.1016/j.expneurol.2013.12.016. [DOI] [PubMed] [Google Scholar]
- Thomas B., Beal M.F. Mitochondrial therapies for Parkinson’s disease. Mov. Disord. 2010;25(Suppl 1):S155–S160. doi: 10.1002/mds.22781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta T., Porro C., Calvello R., Panaro M.A. Biological role of Toll-like receptor-4 in the brain. J. Neuroimmunol. 2014;268:1–12. doi: 10.1016/j.jneuroim.2014.01.014. [DOI] [PubMed] [Google Scholar]
- Unger M.M., Spiegel J., Dillmann K.U., Grundmann D., Philippeit H., Burmann J., Fassbender K., Schwiertz A., Schafer K.H. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Park. Relat. Disord. 2016;32:66–72. doi: 10.1016/j.parkreldis.2016.08.019. [DOI] [PubMed] [Google Scholar]
- Wang J., Xu H.M., Yang H.D., Du X.X., Jiang H., Xie J.X. Rg1 reduces nigral iron levels of MPTP-treated C57BL6 mice by regulating certain iron transport proteins. Neurochem. Int. 2009;54:43–48. doi: 10.1016/j.neuint.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Watson M.B., Richter F., Lee S.K., Gabby L., Wu J., Masliah E., Effros R.B., Chesselet M.F. Regionally-specific microglial activation in young mice over-expressing human wildtype alpha-synuclein. Exp. Neurol. 2012;237:318–334. doi: 10.1016/j.expneurol.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Li X., Zhu J.X., Xie W., Le W., Fan Z., Jankovic J., Pan T. Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson’s disease. Neurosignals. 2011;19:163–174. doi: 10.1159/000328516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Y., Feng D., Tao K., Wang R., Shi Y., Qin H., Murphy M.P., Yang Q., Zhao G. MitoQ protects dopaminergic neurons in a 6-OHDA induced PD model by enhancing Mfn2-dependent mitochondrial fusion via activation of PGC-1alpha. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2018;1864:2859–2870. doi: 10.1016/j.bbadis.2018.05.018. [DOI] [PubMed] [Google Scholar]
- Xu Q., Langley M., Kanthasamy A.G., Reddy M.B. Epigallocatechin gallate has a neurorescue effect in a mouse model of Parkinson disease. J. Nutr. 2017;147:1926–1931. doi: 10.3945/jn.117.255034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Zheng D., Liu Y., Li J., Yang L., Shang X. Glaucocalyxin B alleviates lipopolysaccharide-induced Parkinson’s disease by inhibiting TLR/NF-kappaB and activating Nrf2/HO-1 pathway. Cell. Physiol. Biochem. 2017;44:2091–2104. doi: 10.1159/000485947. [DOI] [PubMed] [Google Scholar]
- Yoshida W., Kobayashi N., Sasaki Y., Ikebukuro K., Sode K. Partial peptide of alpha-synuclein modified with small-molecule inhibitors specifically inhibits amyloid fibrillation of alpha-synuclein. Int. J. Mol. Sci. 2013;14:2590–2600. doi: 10.3390/ijms14022590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S., Wang X., He X., Wang Y., Gao S., Ren L., Shi Y. Curcumin exerts anti-inflammatory and antioxidative properties in 1-methyl-4-phenylpyridinium ion (MPP(+))-stimulated mesencephalic astrocytes by interference with TLR4 and downstream signaling pathway. Cell Stress Chaperones. 2016;21:697–705. doi: 10.1007/s12192-016-0695-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitone S.A., Abo-Elmatty D.M., Elshazly S.M. Piracetam and vinpocetine ameliorate rotenone-induced Parkinsonism in rats. Indian J. Pharmacol. 2012;44:774–779. doi: 10.4103/0253-7613.103300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W., Zhang W., Lu F., Gao L., Gao G. Resveratrol attenuates MPP(+)-induced mitochondrial dysfunction and cell apoptosis via AKT/GSK-3beta pathway in SN4741 cells. Neurosci. Lett. 2017;637:50–56. doi: 10.1016/j.neulet.2016.11.054. [DOI] [PubMed] [Google Scholar]
- Zhang F., Shi J.S., Zhou H., Wilson B., Hong J.S., Gao H.M. Resveratrol protects dopamine neurons against lipopolysaccharide-induced neurotoxicity through its anti-inflammatory actions. Mol. Pharmacol. 2010;78:466–477. doi: 10.1124/mol.110.064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.F., Zhang Y., Zhao G. Crocin protects PC12 cells against MPP(+)-induced injury through inhibition of mitochondrial dysfunction and ER stress. Neurochem. Int. 2015;89:101–110. doi: 10.1016/j.neuint.2015.07.011. [DOI] [PubMed] [Google Scholar]
- Zhang Y.L., Liu Y., Kang X.P., Dou C.Y., Zhuo R.G., Huang S.Q., Peng L., Wen L. Ginsenoside Rb1 confers neuroprotection via promotion of glutamate transporters in a mouse model of Parkinson’s disease. Neuropharmacology. 2018;131:223–237. doi: 10.1016/j.neuropharm.2017.12.012. [DOI] [PubMed] [Google Scholar]
- Zucca F.A., Segura-Aguilar J., Ferrari E., Munoz P., Paris I., Sulzer D., Sarna T., Casella L., Zecca L. Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Prog. Neurobiol. 2017;155:96–119. doi: 10.1016/j.pneurobio.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]