Abstract
Although valuable and effective in decreasing disease burden, influenza vaccination has low rates of efficacy, especially in those at most risk. Studies have shown that acute exercise can improve vaccine responses, most consistently with weaker antigens. Here we examined the effect of resistance exercise on the acute and longer-term responses to influenza vaccination among healthy older adults. Forty-six participants (47.8% male, mean 73.4 ± 6.6 years) were randomised to perform one 45-min moderate-intensity resistance exercise session or sit quietly prior to the receipt of influenza vaccination.
Acute exercise reduced vaccine reactions but had no effect on either antibody responses or development of influenza-like symptoms during six months of follow-up. Psychosocial and behavioural characteristics were examined for potential associations with the responses to vaccination. Participants (n = 36) vaccinated in the previous year had higher baseline antibody titres but not follow-up titres nor more frequent experience of influenza-like symptoms over 6 months compared to those unvaccinated in the previous year. These findings provide further support for the ability of acute exercise to reduce vaccine reactions and suggest risk factors for vaccine responses for future exploration.
Keywords: Acute exercise, Vaccination, Immune function, Older adults
Highlights
-
•
Acute exercise reduced influenza vaccine reactions in older adults.
-
•
Acute exercise did not change antibody titres.
-
•
Psychosocial and behavioural factors had limited association with responses.
1. Introduction
Globally, seasonal influenza is estimated to affect 3–5 million persons annually and cause approximately 650,000 deaths (World Health Organization). Vaccination is the most effective method of preventing influenza virus infections, however the vaccine effectiveness remains low at 30%–60% across different cohorts (European Centre for Disease Prevention and Control, 2019). Low effectiveness and perceived risk of vaccine-related side effects are among the barriers contributing to low coverage rates, particularly among at-risk populations (Australian Institute of Health and Welfare, 2009; World Health Organization; World Health Organization, 2016; Centers for Disease Control and Prevention, 2019). Thus, improvements in both the vaccination experience and in vaccine effectiveness are important to increase vaccine uptake and improve population health.
There are some indications that particular health conditions may affect vaccination responses. For example, obesity has been associated with weaker antibody responses to influenza vaccines in children (Lin et al., 2017) and adults (Tam et al., 2007; Neidich et al., 2017; Moehling et al., 2018), while vaccinated obese persons are more likely than vaccinated normal weight peers to contract influenza or an influenza-like illness (Neidich et al., 2017). Insomnia is associated with poor antibody responses to influenza vaccination (Taylor et al., 2017), but in older persons sleep quality may, curiously, be inversely related to antibody response (Segerstrom et al., 2012). Numerous studies have shown reductions in influenza antibody responses associated with chronic stress and there are also links between immune function and depression (Whittaker, 2018). Similarly, poor health-related behaviours (e.g., smoking and low physical activity) are related to poor vaccination rates and weaker responses. For example, although alcohol use does not appear to alter vaccination effectiveness at preventing illness (Woolpert et al., 2014), whether this is also true with smoking is uncertain as studies on the effectiveness of vaccination to prevent influenza hospitalizations among smokers have had inconsistent (Cruijff et al., 1999; Woolpert et al., 2014; Godoy et al., 2018). Physical activity is generally associated with improved antibody responses to influenza vaccination (Schuler et al., 2003; Stewart et al., 2018), but little is known about modalities and doses of physical activity which may be most beneficial. Thus, there is little consensus on effective approaches to optimization of uptake and effectiveness of influenza vaccination strategies via lifestyle recommendations.
In addition to the heterogenous evidence regarding lifestyle behaviours and vaccination efficacy, the role of previous vaccination history and its relationship to potential lifestyle factors is controversial. For example, although influenza vaccination is recommended annually, there is conflicting evidence as to whether receiving the vaccination yearly reduces effectiveness (Bartoszko et al., 2018; Ramsay et al., 2019). Given the relatively poor effectiveness of vaccination overall and the significant impact of influenza in terms of morbidity, mortality, and costs (Gordon and Reingold, 2018), particularly among older and at-risk populations, further investigation of risk factors for poor vaccination responses and of methods to improve responsiveness is warranted.
Recent studies have employed acute exercise as a potential behavioural adjuvant for vaccinations. Positive effects on responses to influenza vaccination have been reported following a bout of exercise relative to controls, though the effects have varied with strain and sex. For example, A/Panama H3N2 titre levels showed greater increase in young adult women, but not men, following a 45-min bout of aerobic exercise (Edwards et al., 2006); H1N1 titres showed greater increase in older women, but not men, who completed 40 min aerobic exercise (Ranadive et al., 2014); and A/Wyoming H3N2 and B/Jiangsu titre levels increased in young adult women following 25 min of upper-body resistance exercise, while interferon-ϒ levels were higher in men (Edwards et al., 2007a). Other studies have not reported any exercise effect on influenza antibody levels in young or older adults following 45 min of brisk walking, or in young adults following 25 min of eccentric exercise (Campbell et al., 2010; Long et al., 2012). Importantly, benefits from acute exercise are most apparent in those with low immunogenic responses (Edwards et al., 2012) and therefore older adults, who generally have weaker responses, may stand to benefit most. In addition to its potential to improve antibody response, acute exercise may reduce local and systemic reactions to vaccination. For example, a randomised trial employing 15 min of moderate intensity resistance exercise prior to or following influenza vaccination was shown to significantly decrease local swelling and fever incidence and improve appetite suppression among young adults compared to a resting control group (Lee et al., 2018). As one barrier to vaccination uptake is a fear of such adverse effects (Ho et al., 2017), investigating the potential for acute exercise to mitigate such responses is warranted.
1.1. Aim
Much of the literature on exercise effects on influenza vaccination responses is concentrated in young adults with the two studies in older (50–75 years) populations (Long et al., 2012; Ranadive et al., 2014) reporting discordant results; therefore, the primary purpose of this study was to test the effect of acute exercise vaccination responses among older adults. We hypothesized that one bout of resistive exercise prior to vaccination would improve antibody response and decrease rates of vaccine reactions and influenza-like symptoms. Given the growing literature on factors associated with immunity, and the vulnerability of our study population, we also decided, a priori, to examine the potential for other factors to influence specific and non-specific responses to influenza vaccination.
2. Methods
2.1. Participants
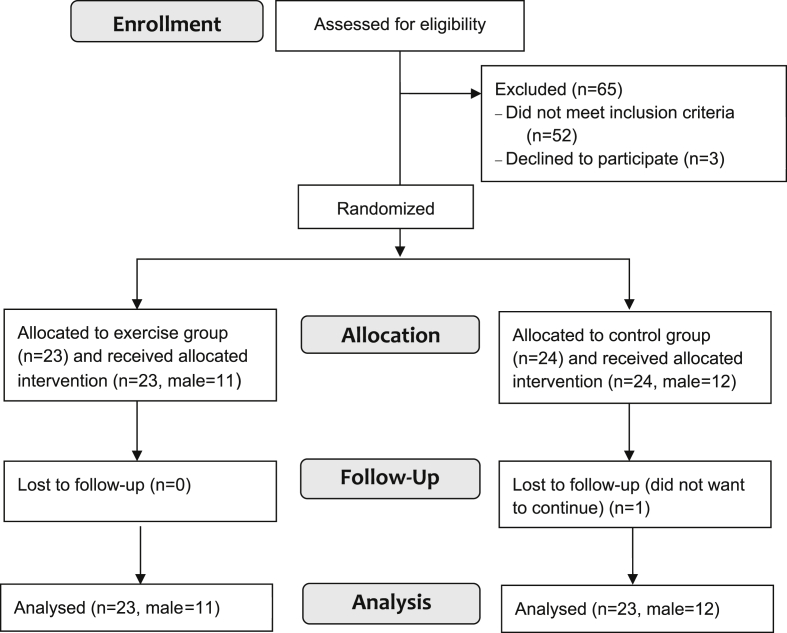
Forty-seven healthy older adults were recruited for a 6-month randomized controlled trial on the acute effects of exercise on influenza vaccination responses. Participants were recruited through local media and community-based groups and facilities (Fig. 1).
Fig. 1.
Participant flow chart.
Potential participants were screened by phone and their eligibility reviewed by the study team. Inclusion criteria included community-dwelling men or women, age 65 years or older, and apparently healthy or with no unstable diseases. Exclusion criteria included serious vaccine allergy or allergy to egg or chicken; receipt of the current season’s influenza vaccine; current performance of resistance training at least once per week; physical impairment (e.g., injury or amputation) to upper or lower body exercise; current immune disorder or unstable medical condition or a new-onset event within the past six months (e.g., rheumatoid arthritis, asthma or aneurysm); terminal or rapidly progressive illness; or steroid or anticoagulant medication usage.
Participants provided written informed consent and their general practitioner was notified of involvement in the study. Participants were offered a travel reimbursement (AUD60) for study visits.
The project was approved by the Ethics Review Committee (RPAH Zone) of the Sydney Local Health District (X11-0370) and registered with the Australian and New Zealand clinical trials registry (ACTRN12611001119987). Recruitment took place March–June 2012.
2.2. Procedure
At study visit one, participants attended the clinic between 8 and 10 a.m. in a fasted state (no food or fluids except water for 8 h prior) for baseline measurements. Fasting-state blood samples were collected as described below.
Barefoot participants were measured using a wall-mounted stadiometer and electronic scale for height and weight, respectively, and the average of three measurements recorded. Body mass was calculated using the equation from Lukaski et al. (1986), based on the average of 3 measurements from a BIA analyser (RJL Systems, Inc., Clinton, MI). (Further measurements were obtained as detailed in the supplement.) Following a meal, muscular strength (one repetition maximum (1RM) tests for seated row, leg press, standing biceps curl, knee extension and standing triceps pushdown, as detailed in De Vos et al. (2005), were conducted. Participants completed questionnaires (described below) and were then randomly allocated to exercise or control groups. Randomisation was at the level of participant and stratified by gender using computer-generated randomized assignment; sealed envelopes containing the assignment were prepared by an independent researcher and opened by the participant at the end of study visit one.
At study visit two, on average seven days (range 3–17days) after visit one, participants performed the exercise or control task as described below, and immediately thereafter received a full dose influenza vaccination (2012 Southern Hemisphere influenza vaccine Vaxigrip, Sanofi Pasteur 0.5 mL, batch no. H8333, expiry 06/2012) via intra-muscular injection into the deltoid muscle of the non-dominant arm. Vaccinations and all blood samples were performed by trained phlebotomists [researchers EM and AP]. Blood samples were obtained 30 min post vaccination and again one month (range 27–42 days) later at study visit 3.
2.3. Intervention and control conditions
Exercise participants (n = 23) performed one bout of exercise which consisted of the five separate resistance exercises at moderate-intensity (60% 1RM) interspersing repetitions (8) with 2–3 s recovery and sets (3) with 60–90 s recovery. Two minutes of rest were provided between each of the five exercises, for a total exercise time of approximately 45 min. Participants randomized to the control resting task (n = 24) were provided reading material about health, exercise and nutrition during 30 min of seated rest.
2.4. Blood sampling and assays
Serum was collected at baseline and follow-up sessions in 9 ml vacutainers which were stored at room temperature until centrifugation (4000 rpm for 15 min at 4 °C), followed by storage at −80 °C until for later assessment. Anti-influenza antibody titres were determined using hemagglutination inhibition (HI) as described previously (Kok et al., 2011). Paired pre- and post-immunisation samples for each individual were tested in parallel against the antigens influenza A/California/7/2009 (H1N1), A/Perth/16/2009 (H3N2) and B/Brisbane/60/2008. The HI titre is the reciprocal of the highest dilution of serum that completely inhibited agglutination of red blood cells.
Plasma interleukin-6 (IL-6) measurement used high-sensitivity ELISA (Quantikine HS Human IL-6 ELISA, R&D Systems) according to manufacturer’s instructions. The reported sensitivity of the assay was 0.039 pg/mL, with recorded intra-assay and inter-assay variation both <10%.
2.5. Questionnaires
Participants completed a series of research staff-administered questionnaires at baseline: demographics, alcohol intake and smoking status, the Physical Activity Scale for the Elderly (PASE) (Washburn et al., 1993), a life event inventory (LEI) (Phillips et al., 2008) excluding the health events section as these were assessed at screening, Geriatric Depression Scale (GDS) (Yesavage et al., 1982), and the Pittsburgh Sleep Quality Index (PSQI) (Buysse et al., 2006). The demographics, GDS and LEI questionnaires were completed at study visit 1; the PASE and PSQI were completed following vaccination (study visit 2).
2.6. Vaccine reactions and influenza-like symptoms
Participants were phoned about 48 h (range 1–3 days) after their vaccination to record any vaccine reaction (defined as pain, redness, or swelling at injection site or mention of a symptom/illness).
At clinic visit 2, participants were given a symptom log in which to report any influenza-like symptoms (e.g., fever, myalgias, gastrointestinal symptoms, cough and/or rhinorrhoea) and these logs were returned at the subsequent study visits. Participants were called fortnightly to remind them to use the log and for reporting of symptoms during the previous fortnight (up to 6 months post vaccination).
2.7. Statistical analysis
One participant (male, control) discontinued participation following baseline measures. Analysis was therefore conducted on the remaining 46 participants.
Symptom frequency was calculated as the number of fortnights in which any symptom was reported divided by the number fortnights of reporting; response rate for the fortnightly reports averaged 85.5% (range 50–100%, n = 45) over the 6 months.
Antibody levels were analysed as titres which were log-transformed due to skewed distribution. Levels were also reported as seroprotective (with seroprotection defined as an antibody titre ≥40) and seroconverted (with seroconversion defined as a four-fold increase from baseline level).
Alcohol intake was converted to a binomial variable of drinking less than once per week or at least once per week, a dividing point close to the average Australian alcohol intake (The National Centre for Education and Training on Addiction, 2019).
There were only two current smokers (one exercise, one control); both were male. Smoking was therefore classified as a binomial variable of never having smoked versus current or past smoking.
Statistical analysis was performed using IBM SPSS 25.0 with alpha set at 0.05. Group differences at each timepoint in antibody titres and symptom frequency were first tested by t-test. A repeated ANOVA was then used to evaluate change in antibody titres over time as well as potential group interaction. A repeated ANOVA was used to test for changes over time and group differences in symptoms over time. Correlations between symptom frequency and antibody titres with the factors of interest were performed; where factors were dichotomous variables, a t-test was used instead. Stepwise multiple regression was then performed with variables with correlates of p ≤ 0.200 and significant dichotomous variables; significance values were corrected using the Bonferroni method.
3. Results
3.1. Baseline measures
The study comprised 46 participants (47.8% male) with a mean age of 73.4 ± 6.6 years. As expected, the two groups (exercise and control) were not different for sex, age, body fat, sleep quality, physical activity, vaccination history, alcohol intake, smoking, stress, depressive symptom measures, as shown in Table 1.
Table 1.
Baseline measures. Baseline measures of exercise and control groups: mean ± standard deviation or % (n).
| Measure | Exercise (n = 23) | Control (n = 23) | P value |
|---|---|---|---|
| Male % (#) | 47.8% (11) | 47.8% (11) | 1.0 |
| Age (years) | 74.4 ± 6.5 | 72.3 ± 6.7 | .289 |
| Body fat % | 32.3 ± 9.3 | 35.9 ± 7.9 | .169 |
| Depressive symptom score (GDS units) | 2.9 ± 2.9 | 3.1 ± 3.8 | .861 |
| Physical activity (PASE scores) | 113.1 ± 52.2 | 89.5 ± 42.7 | .105 |
| Stress (# stressful events in past year) | 1.9 ± 1.7 | 2.9 ± 2.9 | .160 |
| Sleep quality (PSQI score) | 4.2 ± 3.0 | 5.7 ± 4.6 | .192 |
| Had prior year’s vaccination | 82.6% (19) | 73.9% (17) | .475 |
| Drink alcohol weekly or more frequently | 78.3% (18) | 78.3% (18) | 1.0 |
| Smoke (ever) | 47.8% (11) | 45.5% (10) | .873 |
3.2. Vaccine reactions and influenza-like symptoms
No participant experienced a severe vaccine reaction; however, control participants were more likely to experience a minor side effect (e.g., fever) than the exercise group (p = 0.036), as shown in Table 2.
Table 2.
Outcome measures by intervention group. Comparison of log-transformed HI titres at baseline and one month, 4-fold conversion and seroprotection rates for antibodies, IL-6 levels, and vaccine reaction and influenza-like symptom reporting for exercise and control groups. Values are expressed as means ± standard deviation or as percentages (n); p values are for chi-square or t tests.
| Outcomes | Exercise (n = 23) | Control (n = 23) | p value | |
|---|---|---|---|---|
| A/California/7/2009 | Baseline | 0.74 ± 0.642 | 0.80 ± 0.730 | .774 |
| 1 month | 1.58 ± 0.647 | 1.81 ± 0.0653 | .244 | |
| 4-fold at 1 month | 34.8% (8) | 28.6% (6) | .659 | |
| seroprotection at 1 month | 60.9% (14) | 71.4% (15) | .460 | |
| A/Perth/16/2009 | Baseline | 0.94 ± 0.815 | 1.21 ± 0.717 | .258 |
| 1 month | 1.91 ± 0.556 | 2.10 ± 0.460 | .222 | |
| 4-fold at 1 month | 52.2% (12) | 59.1% (13) | .641 | |
| seroprotection at 1 month | 95.5% (21) | 91.3% (21) | .577 | |
| B/Brisbane/60/2008 | Baseline | 1.23 ± 0.501 | 1.17 ± 0.619 | .752 |
| 1 month | 1.70 ± 0.484 | 1.83 ± 0.500 | .372 | |
| 4-fold at 1 month | 4.3% (1) | 18.2% (4) | .140 | |
| seroprotection at 1 month | 87.0% (20) | 72.7% (19) | .233 | |
| IL-6 (pg/mL) | Baseline | 1.40 ± 0.652 | 1.53 ± 0.659 | .513 |
| 30 min post vaccination | 1.92 ± 1.238 | 1.35 ± 0.711 | .020 | |
| Vaccine reaction | 0% (0) | 17.4% (4) | .036 | |
| Symptom frequency | 28.1 ± 21.23% | 18.8 ± 20.16% | .142 | |
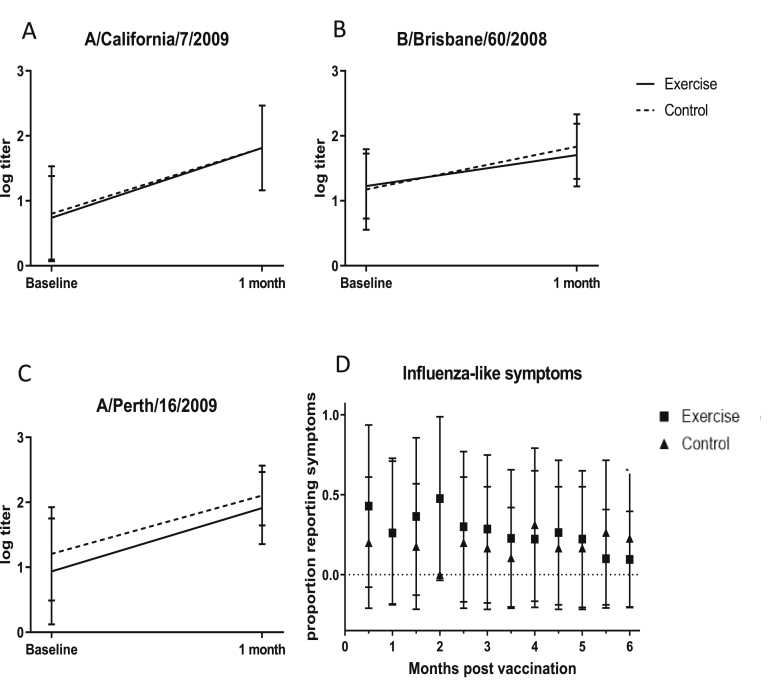
There were no group differences in symptom frequency: the exercise group reported influenza-like symptoms 28.1% (±21.2 standard deviation) of the time over the 6-month reporting period compared to 18.8% (±20.2) among controls (p = 0.142). The mean symptoms reported at each fortnightly interval is shown in Fig. 2. ANOVA revealed there was no difference in symptom frequency over time (p = .472), nor was there a time×group interaction (p = .472). Depressive symptom scores were the only factor of interest correlated with symptom frequency (Pearson r = .334, p = .025). However, no factor significantly predicted symptoms in the final regression model.
Fig. 2.
Effects of acute exercise. A-C. Influenza strain antibody titre levels (log transformed HI titre) at baseline and 1 month. D. Proportion reporting influenza-like symptoms per fortnight.
3.3. Antibody and interleukin-6 responses
Antibody titres did not differ between groups at either time point for any strain (p ≥ 0.222) as shown in Table 2. Also as expected, IL-6 levels did not differ prior to vaccination (p = 0.513) and were higher in the exercise group, compared to controls, 30 min after vaccination (p = 0.020).
As expected, antibody titres for all three strains increased from baseline to one-month post vaccination (p < 0.001; Table 3) but there was no group × time effect (Fig. 2). Observed IL-6 measurements also met expectations, with a significant group × time effect (p = .034).
Table 3.
Effects of acute exercise. ANOVA analysis results (p values) on effects of time and time×group on influenza strain antibody titres (baseline to 1-month), IL-6 levels (baseline to 30 min post vaccination) and influenza-like symptoms (fortnightly experience of symptoms from baseline to 6 months).
| Time | Group × time | |
|---|---|---|
| A/California/7/2009 | <.001 | .601 |
| A/Perth/16/2009 | <.001 | .642 |
| B/Brisbane/60/2008 | <.001 | .253 |
| IL-6 | .347 | .034 |
| Symptoms | .472 | .704 |
There were few relationships between vaccination responses and factors of interest. Participants having received the influenza vaccination in the prior year had higher baseline antibody titres for all strains (A/California/7/2009, p < .001; A/Perth/8/2009, p = 0.020; B/Brisbane/60/2008, p < .007). As shown in Table 4, there was no difference in titres between those previously vaccinated, and those not vaccinated, at one-month follow-up (p ≥ .280) for all strains. Baseline titre was related to alcohol intake for the A/Perth/8/2009 strain (p = .047) only, while depressive symptom score was correlated with the A/California/7/2009 strain baseline titre (r = 0.338, p = .029). Stress and sleep quality were correlated with one-month A/Perth/8/2009 strain titres (r = 0.322 and 0.323, respectively, p = .033 for each), but there were no other factors correlated with any strain.
Table 4.
Outcomes by vaccination history. Comparison of log-transformed HI titre levels at baseline and one month, 4-fold conversion and seroprotection rates for antibodies, and vaccine reaction and influenza-like symptom reporting for prior year’s vaccination status. Values are expressed as means ± standard deviation or as percentages (n); p values are for chi-square or t tests.
| Prior year | ||||
|---|---|---|---|---|
| Outcomes | Vaccinated (n = 36) | Not vaccinated (n = 11) | p value | |
| A/California/7/2009 | Baseline | 0.98 ± 0.612 | 0.00 ± 0.000 | <.001 |
| 1 month | 1.65 ± 0.665 | 1.84 ± 0.617 | .461 | |
| 4-fold at 1 month | 25.7% (9) | 55.6% (5) | .086 | |
| seroprotection at 1 month | 65.7% (23) | 66.7% (6) | .957 | |
| A/Perth/16/2009 | Baseline | 1.20 ± 0.699 | 0.53 ± 0.856 | .020 |
| 1 month | 2.05 ± 0.529 | 1.84 ± 0.446 | .280 | |
| 4-fold at 1 month | 52.8% (19) | 66.7% (6) | .453 | |
| seroprotection at 1 month | 94.4% (34) | 88.9% (8) | .550 | |
| B/Brisbane/60/2008 | Baseline | 1.35 ± 0.445 | 0.62 ± 0.602 | .007 |
| 1 month | 1.79 ± 0.466 | 1.67 ± 0.598 | .508 | |
| 4-fold at 1 month | 5.6% (2) | 33.3% (3) | .018 | |
| seroprotection at 1 month | 86.1% (31) | 55.6% (5) | .040 | |
| Illness within 48 h | 8.3% (3) | 10.0% (1) | .869 | |
| Symptom frequency | 23.7 ± 21.67% | 22.9 ± 19.26% | .925 | |
Multiple regression analysis revealed only that baseline HI titre was a significant predictor for one-month levels, and only for A/California/7/2009 (adjusted r2 = 0.073, p = .047) and B/Brisbane/60/2008 (adjusted r2 = 0.210, p = .001) strains. Although sleep quality and depressive symptoms were positively correlated with A/Perth/8/2009 antibody response at one-month (p = .033 for each), neither these nor baseline titres were significant predictors in the final multiple regression model.
4. Discussion
In this study involving older adults, a bout of acute exercise prior to influenza vaccination reduced vaccine reactions in the 48 h following immunization. Antibody titres were higher for each strain one month after vaccination compared to baseline levels, as expected, but there were no effects of exercise on the antibody responses. This contrasts to several studies which reported exercise-induced beneficial effects on antibody titres following influenza vaccination in younger cohorts (Edwards et al., 2007a, 2010; Campbell et al., 2010), which could suggest an age-related difference in effect. It is also possible the selected exercise was insufficient at producing an exercise-induced response, as has been theorized in another study in which brisk walking at >55% heart rate maximum did not elicit an enhancement in antibody response among older adults (Long et al., 2012); however the intensity here was 60% 1RM, a level shown to elicit similar antibody responses to exercising at 85% or 110% (Edwards et al., 2010).
A proposed mechanism by which exercise acts as an adjuvant involves exercise-induced increases in IL-6 from muscle cells (Edwards et al., 2007b). Here, although IL-6 levels increased in the exercise group in agreement with the hypothesized mechanism, there was no increase in antibody levels relative to that in controls, indicating circulating IL-6 was not associated with subsequent development of antibody response. Another potential mechanism involves exercise-induced increases in lymph flow leading to the adjuvant effect (Edwards et al., 2007b; Edwards and Campbell, 2011). Studies of exercise effects on lymph flow show that the initial period of exercise causes a large increase in lymph flow rate (5 fold with moderate intensity exercise), which then reduces to a 2–3 fold increase over rest during longer duration of exercise (Lane et al., 2005). Previous resistance exercise tasks that have shown an effect on antibody response were shorter (15 min, with only 30–60 s rest periods) than the exercise task here which was 45 min including rest periods of 60–90 s between sets and 2 min between exercises. Therefore, it is possible that the spread of the exercise over a longer period and longer rest periods reduced the rate of lymph flow at the time of vaccine injection, reducing the efficiency of antigen clearance to lymph nodes.
As a better understanding of vaccination responses is needed to improve vaccine effectiveness and ultimately reduce the incidence of influenza, this study also examined potential risk factors for specific and non-specific responses to influenza vaccination in older adults. Aside from vaccination history, no other investigated factor correlated with antibody titres at one-month post vaccination. The data did indicate initial correlations between increase in antibody titre and both good sleep quality and experiencing a higher number of stressful events, but these did not remain significant in the final model. Our sample’s characteristics and measurement differences could explain contradictions between these findings and the literature. For example, whereas measurement of sleep quality via a single item question did lead to a positive correlation with antibody response in other samples (Segerstrom et al., 2012), we used a potentially more discriminating instrument, the PSQI, and did not find any association. We did find an inverse correlation between antibody response and stress, but we measured only the quantity of stressful events and not the perceived stress associated with them. In contrast, studies frequently observe reductions in antibody response but use indicators of perceived stress (Whittaker, 2018). Studies with a larger sample size or designed specifically to assess the impact of these factors in those with stress or sleep disorders could better investigate these potential relationships.
The one factor which correlated to all antibody titres was vaccination history. Those participants who had received the prior year’s identical influenza vaccination (hereafter referred to as ‘consecutive vaccination’) had significantly higher titres at baseline compared to those who had not (‘naïve’). We found there was no reduction in vaccine effectiveness for the influenza A strains with consecutive vaccination when measured by titres, four-fold increases or seroprotection at one month. For the influenza B strain, there were no observed differences between consecutive and naïve groups in titre level at follow-up, but a higher percentage of those receiving consecutive vaccinations attained seroprotection (p = .040). However, the observed difference should be interpreted with caution due to low seroconversion overall (n = 5). In contrast, a previous study observed fewer responders for the influenza B strain among those with repeated vaccinations compared to those with no previous vaccination (Strindhall et al., 2016). Similar to the present study, vaccination history did not, however, affect seroconversion rates in the A strain (Strindhall et al., 2016).
We found few associations between investigated factors and experiencing influenza symptoms. In a previous study depression predicted occurrence of influenza-like symptoms (Gidron et al., 2005), supporting our initial observed correlation between depressive symptoms scores and influenza symptoms. We observed no association between repeated vaccination and experiencing influenza symptoms, suggesting repeated vaccination results in no serological or clinical difference. A recent meta-analysis of studies with laboratory-confirmed influenza virus infection as the outcome measure also found no reduction with repeated vaccination (Bartoszko et al., 2018), however, a second meta-analysis concluded having the previous and current year vaccinations was less effective than having only the current vaccine for A/H3N2 and B strains but had no effect on A/H1N1 (Ramsay et al., 2019). We note vaccine effectiveness was not tested through laboratory confirmed diagnoses in our study.
It should be acknowledged that the present study was limited by its small sample size and the healthy nature of the older adult population, precluding detailed exploration of lifestyle and health status in relationship to vaccination responses.
4.1. Conclusion
Acute exercise had no detrimental effect on vaccination response in healthy older adults and it reduced vaccine reactions experienced in the first days following influenza vaccination. These findings support the ability of pre-exposure to one bout of resistive exercise to reduce post-vaccination vaccine reactions in older adults, and future studies may determine whether chronic exposure to such exercise or other modalities, including shorter, intense exercise bouts, may enhance immune responsiveness and efficacy of vaccinations in older adults. The mechanism for exercise-effects on vaccine reactions is likely different to those for antibody generation and immunocompetence and symptom responses. Given that little is known about factors effecting vaccine reactions, further work to elucidate the mechanical factors of vaccine reactions is warranted. Only vaccination history influenced antibody response. Repeated vaccination was no less effective in boosting antibody titres and resulted in higher seroprotection rates for the B strain antigen. Thus, the data support the recommendation for annual influenza vaccinations.
Declaration of competing interest
The authors declare that they have no known conflicting or competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This study was supported by an establishment grant from the Clive and Vera Ramaciotti Foundation (3344/2011 K. Edwards).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2019.100009.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Australian Institute of Health and Welfare Adult vaccination survey: summary results. 2009. https://www.aihw.gov.au/reports/health-care-quality-performance/2009-adult-vaccination-survey-summary-results/contents/table-of-contents from.
- Bartoszko J.J., McNamara I.F., Aras O.A.Z., Hylton D.A., Zhang Y.B., Malhotra D. Does consecutive influenza vaccination reduce protection against influenza: a systematic review and meta-analysis. Vaccine. 2018;36(24):3434–3444. doi: 10.1016/j.vaccine.2018.04.049. [DOI] [PubMed] [Google Scholar]
- Buysse D.J., Ancoli-Israel S., Edinger J.D., Lichstein K.L., Morin C.M. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29(9):1155–1173. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- Campbell J.P., Edwards K.M., Ring C., Drayson M.T., Bosch J.A., Inskip A. The effects of vaccine timing on the efficacy of an acute eccentric exercise intervention on the immune response to an influenza vaccine in young adults. Brain Behav. Immun. 2010;24(2):236–242. doi: 10.1016/j.bbi.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Estimates of influenza vaccination coverage among adults—United States, 2017–18 flu season. 4 May, 2019. https://wwwcdcgov/flu/fluvaxview/coverage-1718estimateshtm#figure1 from.
- Cruijff M., Thijs C., Govaert T., Aretz K., Dinant G.J., Knottnerus A. The effect of smoking on influenza, influenza vaccination efficacy and on the antibody response to influenza vaccination. Vaccine. 1999;17(5):426–432. doi: 10.1016/S0264-410X(98)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos N.J., Singh N.A., Ross D.A., Stavrinos T.M., Orr R., Singh M.A.F. Optimal load for increasing muscle power during explosive resistance training in older adults. J. Gerontol. Ser. A Biol. Med. Sci. 2005;60(5):638–647. doi: 10.1093/gerona/60.5.638. [DOI] [PubMed] [Google Scholar]
- Edwards K.M., Campbell J.P. Acute exercise as an adjuvant to influenza vaccination. Am. J. Lifestyle Med. 2011;5(6):512–517. [Google Scholar]
- Edwards K.M., Burns V.E., Reynolds T., Carroll D., Drayson M., Ring C. Acute stress exposure prior to influenza vaccination enhances antibody response in women. Brain Behav. Immun. 2006;20(2):159–168. doi: 10.1016/j.bbi.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Edwards K.M., Burns V.E., Allen L.M., McPhee J.S., Bosch J.A., Carroll D. Eccentric exercise as an adjuvant to influenza vaccination in humans. Brain Behav. Immun. 2007;21(2):209–217. doi: 10.1016/j.bbi.2006.04.158. [DOI] [PubMed] [Google Scholar]
- Edwards K.M., Burns V.E., Carroll D., Drayson M., Ring C. The acute stress-induced immunoenhancement hypothesis. Exerc. Sport Sci. Rev. 2007;35(3):150–155. doi: 10.1097/JES.0b013e3180a031bd. [DOI] [PubMed] [Google Scholar]
- Edwards K.M., Campbell J.P., Ring C., Drayson M.T., Bosch J.A., Downes C. Exercise intensity does not influence the efficacy of eccentric exercise as a behavioural adjuvant to vaccination. Brain Behav. Immun. 2010;24(4):623–630. doi: 10.1016/j.bbi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Edwards K.M., Pung M.A., Tomfohr L.M., Ziegler M.G., Campbell J.P., Drayson M.T. Acute exercise enhancement of pneumococcal vaccination response: a randomised controlled trial of weaker and stronger immune response. Vaccine. 2012;30(45):6389–6395. doi: 10.1016/j.vaccine.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control . 17April, 2019. Influenza Vaccine Effectiveness. European Centre for Disease Prevention and Control.https://ecdc.europa.eu/en/seasonal-influenza/prevention-and-control/vaccine-effectiveness from. [Google Scholar]
- Gidron Y., Hassid A., Yisrael H., Biderman A. Do psychological factors predict occurrence of influenza-like symptoms in vaccinated elderly residents of a sheltered home? Br. J. Health Psychol. 2005;10(3):411–420. doi: 10.1348/135910704X20026. [DOI] [PubMed] [Google Scholar]
- Godoy P., Castilla J., Soldevila N., Mayoral J.M., Toledo D., Martín V. Smoking may increase the risk of influenza hospitalization and reduce influenza vaccine effectiveness in the elderly. Eur. J. Public Health. 2018;28(1):150–155. doi: 10.1093/eurpub/ckx130. [DOI] [PubMed] [Google Scholar]
- Gordon A., Reingold A. The burden of influenza: a complex problem. Curr. Epidemiol. Rep. 2018;5(1):1–9. doi: 10.1007/s40471-018-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho H.J., Chan Y.Y., Ibrahim M.A.B., Wagle A.A., Wong C.M., Chow A. A formative research-guided educational intervention to improve the knowledge and attitudes of seniors towards influenza and pneumococcal vaccinations. Vaccine. 2017;35(47):6367–6374. doi: 10.1016/j.vaccine.2017.10.005. [DOI] [PubMed] [Google Scholar]
- Kok J., Tudo K., Blyth C., Foo H., Hueston L., DE D. Pandemic (H1N1) 2009 influenza virus seroconversion rates in HIV-infected individuals. J. Acquir. Immune Defic. Syndr. 2011;56(2):91–94. doi: 10.1097/QAI.0b013e318204a1c3. [DOI] [PubMed] [Google Scholar]
- Lane K., Worsley D., McKenzie D. Exercise and the lymphatic system. Sport. Med. 2005;35(6):461–471. doi: 10.2165/00007256-200535060-00001. [DOI] [PubMed] [Google Scholar]
- Lee V.Y., Booy R., Skinner S.R., Fong J., Edwards K.M. The effect of exercise on local and systemic adverse reactions after vaccinations – outcomes of two randomized controlled trials. Vaccine. 2018;36(46):6995–7002. doi: 10.1016/j.vaccine.2018.09.067. [DOI] [PubMed] [Google Scholar]
- Lin C.J., Martin J.M., Cole K.S., Zimmerman R.K., Susick M., Moehling K.K. Are children’s vitamin D levels and BMI associated with antibody titers produced in response to 2014–2015 influenza vaccine? Hum. Vaccines Immunother. 2017;13(7):1661–1665. doi: 10.1080/21645515.2017.1299837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J.E., Ring C., Drayson M., Bosch J., Campbell J.P., Bhabra J. Vaccination response following aerobic exercise: can a brisk walk enhance antibody response to pneumococcal and influenza vaccinations? Brain Behav. Immun. 2012;26(4):680–687. doi: 10.1016/j.bbi.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Lukaski H.C., Bolonchuk W.W., Hall C.B., Siders W. Validation of tetrapolar bioelectrical impedance method to assess human body composition. J. Appl. Physiol. 1986;60:1327–1332. doi: 10.1152/jappl.1986.60.4.1327. [DOI] [PubMed] [Google Scholar]
- Moehling K.K., Nowalk M.P., Lin C.J., Bertolet M., Ross T.M., Carter C.E. The effect of frailty on HAI response to influenza vaccine among community-dwelling adults ≥ 50 years of age. Hum. Vaccines Immunother. 2018;14(2):361–367. doi: 10.1080/21645515.2017.1405883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidich S.D., Green W.D., Rebeles J., Karlsson E.A., Schultz-Cherry S., Noah T.L. Increased risk of influenza among vaccinated adults who are obese. Int. J. Obes. 2017;41(9):1324–1330. doi: 10.1038/ijo.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips A.C., Der G., Carroll D. Stressful life-events exposure is associated with 17-year mortality, but it is health-related events that prove predictive. Br. J. Health Psychol. 2008;13(4):647–657. doi: 10.1348/135910707X258886. [DOI] [PubMed] [Google Scholar]
- Ramsay L.C., Buchan S.A., Stirling R.G., Cowling B.J., Feng S., Kwong J.C. The impact of repeated vaccination on influenza vaccine effectiveness: a systematic review and meta-analysis. BMC Med. 2019;17(1) doi: 10.1186/s12916-018-1239-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranadive S.M., Cook M., Kappus R.M., Yan H., Lane A.D., Woods J.A. Effect of acute aerobic exercise on vaccine efficacy in older adults. Med. Sci. Sport. Exerc. 2014;46(3):455–461. doi: 10.1249/MSS.0b013e3182a75ff2. [DOI] [PubMed] [Google Scholar]
- Schuler P.B., Leblanc P.A., Marzilli T.S. Effect of physical activity on the production of specific antibody in response to the 1998-99 influenza virus vaccine in older adults. J. Sport. Med. Phys. Fit. 2003;43(3):404–408. [PubMed] [Google Scholar]
- Segerstrom S.C., Hardy J.K., Evans D.R., Greenberg R.N. Vulnerability, distress, and immune response to vaccination in older adults. Brain Behav. Immun. 2012;26(5):747–753. doi: 10.1016/j.bbi.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A., Vanderkooi O.G., Reimer R.A., Doyle-Baker P.K. Immune response in highly active young men to the 2014/2015 seasonal influenza vaccine. Appl. Physiol. Nutr. Metabol. 2018;43(8):769–774. doi: 10.1139/apnm-2017-0683. [DOI] [PubMed] [Google Scholar]
- Strindhall J., Ernerudh J., Mörner A., Waalen K., Löfgren S., Matussek A. Humoral response to influenza vaccination in relation to pre-vaccination antibody titres, vaccination history, cytomegalovirus serostatus and CD4/CD8 ratio. Infect. Dis. 2016;48(6):436–442. doi: 10.3109/23744235.2015.1135252. [DOI] [PubMed] [Google Scholar]
- Tam J.S., Capeding M.R.Z., Lum L.C.S., Chotpitayasunondh T., Jiang Z., Huang L.M. Efficacy and safety of a live attenuated, cold-adapted influenza vaccine, trivalent against culture-confirmed influenza in young children in Asia. Pediatr. Infect. Dis. J. 2007;26(7):619–628. doi: 10.1097/INF.0b013e31806166f8. [DOI] [PubMed] [Google Scholar]
- Taylor D.J., Kelly K., Kohut M.L., Song K.S. Is insomnia a risk factor for decreased influenza vaccine response? Behav. Sleep Med. 2017;15(4):270–287. doi: 10.1080/15402002.2015.1126596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The National Centre for Education and Training on Addiction . 13 June, 2019. National Alcohol & Drug Knowledgebase.http://nadk.flinders.edu.au/kb/alcohol/consumption-patterns/frequency-consumption/how-often-do-australians-drink-alcohol/ from. [Google Scholar]
- Washburn R.A., Smith K.W., Jette A.M., CA J. The physical activity scale for the elderly (PASE): development and evaluation. J. Clin. Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- Whittaker A.C. The vaccination model in psychoneuroimmunology research: a review. Methods Mol. Biol. 2018;1781:309–326. doi: 10.1007/978-1-4939-7828-1_16. [DOI] [PubMed] [Google Scholar]
- Woolpert T., Phillips C.J., Sevick C., Crum-Cianflone N.F., Blair P.J., Faix D. Health-related behaviors and effectiveness of trivalent inactivated versus live attenuated influenza vaccine in preventing influenza-like illness among young adults. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0102154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; 2016. Barriers of Influenza Vaccination Intention and Behavior: a Systematic Review of Influenza Vaccine Hesitancy 2005 – 2016.https://apps.who.int/iris/handle/10665/251671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Influenza (seasonal) https://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal) from. (Accessed 17 April 2019)
- World Health Organization Influenza vaccination coverage and effectiveness. http://www.euro.who.int/en/health-topics/communicable-diseases/influenza/vaccination/influenza-vaccination-coverage-and-effectiveness from.
- Yesavage J.A., Brink T.L., Rose T.L., Lum O., Huang V., Adey M., Leirer V.O. Development and validation of a geriatric depression screening scale: a preliminary report. J. Psychiatr. Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.