Abstract
Traditional aerobic exercise reduces the risk of developing chronic diseases by inducing immune, metabolic, and myokine responses. Following traditional exercise, both the magnitude and time-course of these beneficial responses are different between obese compared to normal weight individuals. Although obesity may affect the ability to engage in traditional exercise, whole body vibration (WBV) has emerged as a more tolerable form of exercise . The impact of WBV on immune, metabolic, and myokine responses as well as differences between normal weight and obese individuals, however, is unknown.
Purpose
To determine if WBV elicits differential magnitudes and time-courses of immune, metabolic, and myokine responses between obese and normal weight individuals.
Methods
21 participants [Obese (OB): n = 11, Age: 33 ± 4 y, percent body fat (%BF): 39.1 ± 2.4% & Normal weight (NW) n = 10, Age: 28 ± 8 y, %BF: 17.4 ± 2.1%] engaged in 10 cycles of WBV exercise [1 cycle = 1 min of vibration followed by 30 s of rest]. Blood samples were collected pre-WBV (PRE), immediately (POST), 3 h (3H), and 24 h (24H) post-WBV and analyzed for leukocytes, insulin, glucose, and myokines (IL-6, decorin, myostatin).
Results
The peak (3H) percent change in neutrophil counts (OB: 13.9 ± 17.4 vs. NW: 47.2 ± 6.2%Δ; p = 0.007) was different between groups. The percent change in neutrophil percentages was increased in NW (POST: -1.6 ± 2.0 vs. 3H: 13.0 ± 7.2%Δ, p = 0.019) but not OB (p > 0.05). HOMA β-cell function was increased at 24H (PRE: 83.4 ± 5.4 vs. 24H: 131.0 ± 14.1%; p = 0.013) in NW and was not altered in OB (p > 0.05). PRE IL-6 was greater in OB compared to NW (OB: 2.7 ± 0.6 vs. NW: 0.6 ± 0.1 pg/mL; p = 0.011); however, the percent change from PRE to peak (3H) was greater in NW (OB: 148.1 ± 47.9 vs. NW: 1277.9 ± 597.6 %Δ; p = 0.035). Creatine kinase, decorin, and myostatin were not significantly altered in either group (p > 0.05).
Conclusion
Taken together, these data suggest that acute whole body vibration elicits favorable immune, metabolic, and myokine responses and that these responses differ between obese and normal weight individuals.
Keywords: Leukocytes, Whole body vibration, Myokines, Glucose
Highlights
-
•
Whole body vibration (WBV) normalizes lymphocytes in obese.
-
•
10 min of WBV facilitates increases in neutrophils in normal weight but not obese.
-
•
WBV produces myokine IL-6 in both obese and normal weight.
-
•
WBV improves glucose metabolism in obese.
-
•
Improvements in glucose metabolism correspond to peak IL-6 concentrations.
1. Introduction
Cardiovascular disease is the leading cause of death worldwide (Benjamin et al., 2018). The most common form of cardiovascular disease, atherosclerosis, often leads to cardiovascular events such as heart attack and/or stroke (Benjamin et al., 2018). Atherosclerosis, hallmarked by the accumulation of lipid rich immune “foam” cells within the arterial intima, is characterized as a pro-inflammatory, immune-mediated disease (Moore and Tabas, 2011).
Obesity is an independent risk factor for the development of atherosclerosis (Poirier et al., 2006). One of the most effective methods to prevent both obesity and atherosclerosis is regular exercise (Schuler et al., 2013). A single (acute) exercise session elicits a rapid, acute immune response that includes monocytosis, lymphocytosis, and an increased presence of circulating neutrophils (Foster et al., 1986; Steel et al., 1987). Repeated bouts of acute exercise eventually lead to enhanced production of anti-inflammatory cytokines (i.e. IL-10), muscle repair, and increased leukocyte turnover, all of which contribute to reduced inflammation and the overall prevention of atherosclerosis (Schuler et al., 2013; Beavers et al., 2010). In addition, acute bouts of exercise increase skeletal muscle production of small proteins, known as myokines (Leal et al., 2018). Myokines, act in paracrine, endocrine, and autocrine manners, and mediate a variety of health benefits including improved glucose metabolism (Glund et al., 2007), reduced pro-inflammatory cytokine production (Mizuhara et al., 1994), and increased anti-inflammatory cytokine production (Steensberg et al., 2003). Interleukin-6 (IL-6) is arguably the most studied myokine. Although basal concentrations of IL-6 are positively related to adiposity and thought to contribute to insulin resistance (Kim et al., 2009), IL-6 produced by skeletal muscle in response to exercise improves insulin sensitivity and appears to have similar benefits for both obese and normal weight individuals (Christiansen et al., 2013). However, differences in the myokine response to aerobic and resistance exercise have been observed between obese and normal weight individuals (Hjorth et al., 2016; Marqueti et al., 2018).
While the cardiometabolic benefits of exercise are plentiful, most obese individuals do not or cannot engage in regular exercise (Pietilainen et al., 2008). Often, obese individuals struggle with starting and/or maintaining an exercise regime due to physical limitations (i.e. joint pain) that may occur with traditional forms of exercise, such as treadmill walking or cycle ergometery (Okifuji and Hare, 2015; Bish et al., 2007). Recently, whole body vibration (WBV) has emerged as a potential alternative to traditional exercise modalities (Totosy de et al., 2009; Zago et al., 2018), and due to the low-impact may be more tolerable (Cardinale and Wakeling, 2005). Although there have been promising investigations into the beneficial outcomes of WBV therapy for obese individuals, no studies to date have examined the potential mechanisms responsible for these benefits (Zago et al., 2018). Therefore, the purpose of this investigation was to examine the immune, metabolic, and myokine responses to acute WBV and to explore potential differences between obese and normal weight individuals.
2. Methods
2.1. Participants
Twenty-one apparently healthy men and women ages 18–45y were recruited to take part in this study. Participants were excluded if they were active smokers or quit within the previous 6 months, had a clinical diagnosis of cardiovascular disease, hypertension, metabolic disease, or were taking any vasoactive medications (i.e. nitrates, beta-blockers, ACE inhibitors, etc.). All study protocols were approved by the Institutional Review Board at Augusta University.
2.2. Experimental design
All participants reported to the Laboratory of Integrated Vascular and Exercise Physiology (LIVEP) at the Georgia Prevention Institute for a preliminary visit that consisted of the informed consent process, anthropometric measures, and body composition assessment. For the experimental visit, participants reported to the LIVEP in the morning following an overnight fast, and having abstained from moderate-to-vigorous physical activity for 24 hours prior to investigation.
2.3. Participant characteristics and clinical laboratory values
Height and weight, determined using a stadiometer and standard platform scale (CN20, DETECTO©, Webb City, MO), were used for calculation of body mass index (BMI). Total body fat, fat-mass, and fat-free mass were determined using dual energy X-ray absorptiometry (QDR-4500W; Hologic, Waltham, MA). Resting systolic and diastolic blood pressures were evaluated using established protocols (Kapuku et al., 1999). Resting oxygen saturation was obtained using an Onyx II fingertip sensor (Nonin Medical, Plymouth, MN). Heart rate, stroke volume, and cardiac output were assessed using thoracic impedance cardiography (PhysioFlow Enduro, Manatec Biomedical, Bristol, PA). An intravenous catheter was inserted into an antecubital vein and a 10 mL blood sample (PRE) was obtained. Fasting concentrations of total cholesterol (TC), high-density lipoproteins (HDL), low-density lipoproteins (LDL), triglycerides (TG), and glucose were obtained using a Cholestech LDX point of care analyzer (Alere Inc., Scarborough, ME). Hemoglobin and hematocrit were determined using a HemoPoint H2 analyzer (Stanbio Laboratories). Leukocyte counts and concentrations as well as insulin, platelet counts, concentrations of creatine kinase (CK), and high-sensitivity C-reactive protein (hsCRP) were obtained using standard core laboratory techniques (Laboratory Corporation of America Holdings, Burlington, NC). The homeostasis model assessments for insulin resistance (HOMA-IR) and beta-cell function (HOMA-β-cell function) were calculated using glucose and insulin measurements as follows: HOMA-IR = glucose (mmol/mL) x insulin (μIU/mL])/22.5 & HOMA-β-cell function = 20 x insulin (μIU/mL)/glucose (mmol/mL) – 3.5 (Matthews et al., 1985). Insulin sensitivity was calculated as the inverse of HOMA-IR (Levy et al., 1998). Additional blood samples were obtained immediately following (POST) and 3 hours (3H) post-WBV. Participants returned to the LIVEP in a fasted state 24 hours (24H) following WBV and a venous blood sample was obtained. Percentage of body fat (%BF) was used to assign participants into obese (OB: males > 25%; females > 35%) or normal weight (NW: men ≤ 20%; women ≤ 30%) groups (Pasco et al., 2014).
2.4. Whole body vibration protocol
An oscillating side alternating whole body vibration platform (RS3000, Rock Solid Wholesale, Atlantic Beach, FL) was used for the study. Participants were instructed to remove any footwear and stand mid-center on the platform with a loose grip on the front rails. Vibration frequency was set to 14 Hz as this frequency has been demonstrated to elicit muscle activation, yet is well below the frequency in which potentially harmful side effects may occur (Cardinale and Wakeling, 2005). Vibration amplitude was set to 2.5 mm. These settings yielded a root mean squared acceleration of 20.19 m/s (≈2.1 g). Based on extensive pilot data collection, the applied protocol consisted of 10 cycles of 1 min of vibration exercise followed by 30 s of standing rest. During the vibration portion of the protocol, participants were instructed to stand in a static squat position, consisting of knee flexion (~60°) with a stable non-flexed trunk.
2.5. Myokine analyses
Blood was separated via centrifugation and plasma samples were aliquoted, flash frozen in liquid nitrogen, and stored at −80 °C until further analysis. Plasma concentrations of IL-6 were determined using Simple Plex cartridges run on the Ella platform (ProteinSimple, San Jose, CA) according to manufacturer’s instructions. High sensitivity ELISA kits were used to determine the plasma concentrations of myostatin (R&D Systems, Minneapolis, MN) and decorin (Abcam, Cambridge, United Kingdom) according to manufacturer’s instructions.
2.6. Statistical analyses
All analyses were performed using SPSS version 25 (IBM Corporation, Somers, NY). Independent t-tests were performed to identify group differences in demographics and clinical laboratory markers. Due to the prognostic value of handgrip strength to risk stratify for all-cause mortality in the general population (Leong et al., 2015) as well as the relationship between handgrip strength and muscle strength (Trosclair et al., 2011), handgrip strength was analyzed as a covariate. A two-way (group by time) analysis of covariance (ANCOVA) was used to test for pre- to post-vibration differences in immune, metabolic, and myokine parameters. If a significant pre-WBV between group difference was observed, the percent change from pre-WBV was calculated in order to determine the relative magnitude of the response. Effect sizes (partial eta squared [η_Pˆ2]) are reported for the interaction terms of the ANCOVA, where values of 0.01, 0.06, and 0.14 correspond to small, medium, and large effects, respectively (Cohen, 1988). Values are presented as mean ± SEM unless otherwise noted. An alpha <0.05 was considered statistically significant for all analyses.
3. Results
Participant demographics and laboratory values are presented in Table 1. The obese group had significantly greater body fat percentage, BMI, weight, C-reactive protein, and lower handgrip strength compared to the normal weight group. All other characteristics and clinical laboratory values were similar between groups.
Table 1.
Participant demographics and laboratory values.
| Normal Weight (NW) n = 10 | Obese (OB) n = 11 | P-value | |
|---|---|---|---|
| Sex (men/women) | 7/3 | 3/8 | n/a |
| Ethnicity (Black/White/Other) | 2/6/2 | 6/4/1 | n/a |
| Age (yrs) | 28.1 ± 2.4 | 32.5 ± 4.3 | 0.112 |
| Height (cm) | 173.3 ± 2.6 | 169.1 ± 2.4 | 0.238 |
| Weight (kg) | 69.7 ± 3.9 | 91.9 ± 9.2 | 0.045* |
| Body mass index (kg/m2) | 23.1 ± 1.1 | 32.0 ± 3.6 | 0.045* |
| Body Fat (%) | 24.7 ± 2.0 | 39.2 ± 2.3 | <0.001* |
| Handgrip (kg) | 43.4 ± 3.9 | 34.7 ± 3.9 | 0.036* |
| Total Cholesterol (mg/dL) | 159.8 ± 9.7 | 163.6 ± 6.5 | 0.743 |
| HDL (mg/dL) | 49.5 ± 4.4 | 56.5 ± 4.8 | 0.297 |
| LDL (mg/dL) | 93.2 ± 9.8 | 84.3 ± 6.8 | 0.458 |
| Triglycerides (mg/dL) | 74.6 ± 8.3 | 91.8 ± 26.1 | 0.532 |
| Total Cholesterol/HDL | 3.4 ± 0.3 | 3.1 ± 1.1 | 0.574 |
| Fasting glucose (mg/dL) | 86.5 ± 2.1 | 92.9 ± 5.0 | 0.268 |
| HbA1c (%) | 5.3 ± 0.1 | 5.6 ± 0.1 | 0.140 |
| Hemoglobin (g/dL) | 14.0 ± 0.5 | 13.6 ± 0.6 | 0.665 |
| Hematocrit (%) | 41.0 ± 1.6 | 40.8 ± 1.7 | 0.946 |
| C-reactive protein (mg/L) | 0.4 ± 0.1 | 3.7 ± 1.0 | 0.007* |
*p < 0.05; Independent samples t-tests; High density lipoprotein (HDL), low density lipoprotein (LDL), hemoglobin A1c (HbA1c).
3.1. Immune response to WBV
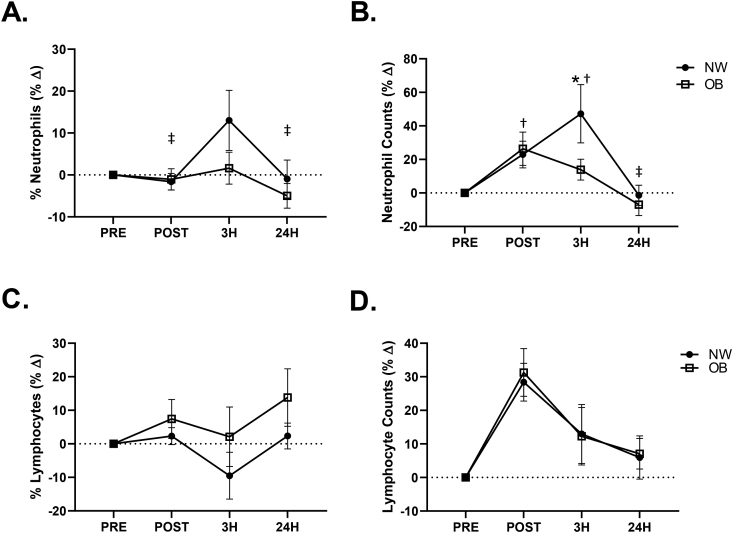
There was a significant between group difference in neutrophil percentages (p = 0.022, η = 0.26) as well as neutrophil counts (p = 0.048, η = 0.20). Baseline (PRE) (OB: 62.8 ± 2.0 vs. NW: 49.8 ± 2.9%; p = 0.006) and POST (OB: 62.0 ± 2.1 vs. NW: 48.9 ± 2.8%; p = 0.008) neutrophil percentages as well as PRE (OB: 4.3 ± 0.5 vs. NW: 2.7 ± 0.3 × 103 cells/μL; p = 0.011) and POST (OB: 5.2 ± 0.6 vs. NW: 3.3 ± 0.5 × 103 cells/μL; p = 0.028) neutrophil counts were elevated in OB compared to NW. A significant between group difference was also observed in lymphocyte percentages (p = 0.049, η = 0.18) despite there being no difference in lymphocyte counts (p > 0.05). Lymphocyte percentages were reduced in OB compared to NW at PRE (OB: 28.2 ± 1.9 vs. NW: 39.0 ± 2.8%; p = 0.015). The time-course of the percent change from pre-WBV for both neutrophils and lymphocytes expressed as percentages and absolute counts are presented in Fig. 1. A significant group by time effect was observed for the percent change from PRE in neutrophil percentages (p = 0.027, η = 0.15) (Fig. 1A) and neutrophil counts (p = 0.008, η = 0.20) (Fig. 1B). In the NW group the percent change in neutrophil percentages was significantly elevated at 3H when compared to POST (POST: -1.6 ± 2.0 vs. 3H: 13.0 ± 7.2%Δ; p = 0.019) and 24H (3H: 13.0 ± 7.2 vs. 24H: -1.0 ± 5.0%Δ; p = 0.031). Additionally, when compared to PRE, the percent change in neutrophil counts was significantly elevated at POST (22.9 ± 8.0%Δ; p = 0.045) and 3H (47.2 ± 17.4%Δ; p = 0.001) and significantly lower at 24H when compared to 3H (3H: 47.2 ± 17.4 vs. 24H: -1.4 ± 6.0%Δ; p = 0.001) in the NW group. In the OB group, no significant changes were observed in the percent change in neutrophil percentages or counts (p > 0.05). There was a between group difference in the percent change from PRE in neutrophil counts (p = 0.017, η = 0.28) (Fig. 1B). The OB had a significantly lower percent change in neutrophil counts when compared to the NW group at 3H (OB: 13.9 ± 6.2 vs. NW: 47.2 ± 17.4%Δ; p = 0.007). No additional leukocyte changes were observed in either group following WBV.
Fig. 1.
Percent change from baseline (PRE) in neutrophil percentages (A) and neutrophil counts (B) as well as lymphocyte percentages (C) and lymphocyte counts (D) in normal weight (NW) and obese (OB). *p < 0.05 between groups, †p < 0.05 vs. PRE within NW group, ‡p < 0.05 vs. 3H within NW group; 2 × 4 factorial ANCOVA.
3.2. Metabolic response to WBV
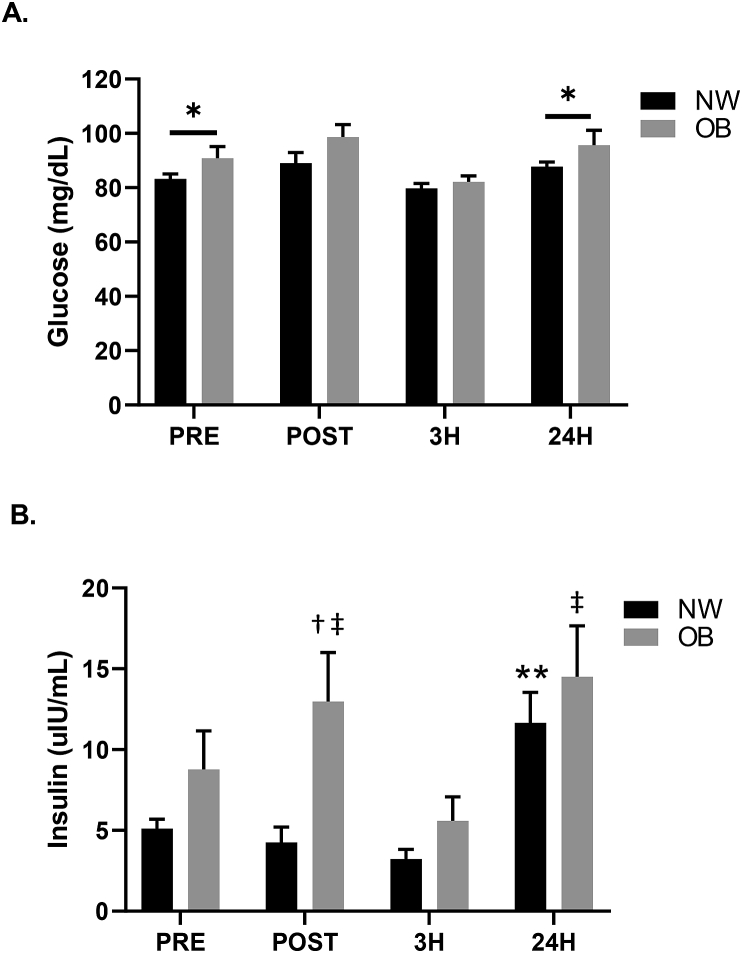
Fig. 2 presents the time-course response of glucose and insulin to WBV. A significant difference between groups was observed for glucose (p = 0.037, η = 0.22) (Fig. 2A). Glucose was significantly greater in the OB group when compared to the NW group at PRE (OB: 90.8 ± 4.4 vs. NW: 83.2 ± 1.9 mg/dL; p = 0.029) and 24H (OB: 95.9 ± 5.6 vs. NW: 87.7 ± 1.8 mg/dL; p = 0.048). Glucose was not different between groups at POST and 3H (p > 0.05). No significant between or within group differences were observed in the percent change in glucose. However, a group by time interaction was observed for insulin (p = 0.025, η = 0.17) (Fig. 2B). Insulin was significantly elevated 24 hours post-WBV in NW compared to all other time points (p < 0.05). In the OB group, insulin was elevated immediately post-WBV compared to PRE (PRE: 7.7 ± 2.4 vs. POST: 11.3 ± 3.0 uIU/mL; p = 0.029). However, at 3H, insulin returned to PRE concentrations (p > 0.05) and when compared to POST was significantly lower in OB (POST: 11.3 ± 3.0 vs. 3H: 5.6 ± 1.5 uIU/mL; p = 0.033). In response to WBV, a group by time interaction was found for HOMA-IR (p = 0.025, η = 0.17) (Table 2) as well as for HOMA-β-cell function (p = 0.040, η = 0.15) (Table 2). HOMA-IR in the NW group was increased at 24H in comparison to all other time points (p < 0.05). In the OB group, HOMA-IR was increased at POST (PRE: 1.2 ± 0.3 vs. POST: 1.7 ± 0.4; p = 0.036) and had returned to PRE values by 3H (Table 2). Additionally, in the OB group, HOMA-IR was lower at 3H when compared to POST (POST: 1.7 ± 0.4 vs. 3H: 0.8 ± 0.2; p = 0.025) and was elevated at 24H in comparison to 3H (3H: 0.8 ± 0.2 vs. 24H: 1.9 ± 0.4; p = 0.012). HOMA-IR at 24H was not significantly different from PRE (p > 0.05) in OB. Following WBV, HOMA-β-cell function was increased at 24H compared to all other time points (p < 0.05) in the NW group; however, WBV did not alter HOMA-β-cell function in the OB group (Table 2). Insulin sensitivity was not different between groups nor was insulin sensitivity altered in response to WBV exercise in either group.
Fig. 2.
Time-course of glucose (A) and insulin (B) responses for normal weight (NW) and obese (OB) participants. *p < 0.05 between groups, **p < 0.05 vs. all time points within NW group; †p < 0.05 PRE vs. POST within OB group, ‡p < 0.05 vs. 3H within OB group; 2 × 4 factorial ANCOVA.
Table 2.
Time-course of homeostatic model assessment (HOMA) of insulin resistance, beta-cell function, and insulin sensitivity responses to whole body vibration in normal weight (NW) and obese groups (OB).
| Insulin Resistance |
Beta-Cell Function (%) |
Insulin Sensitivity (%) |
||||
|---|---|---|---|---|---|---|
| NW | OB | NW | OB | NW | OB | |
| PRE | 0.7 ± 0.1 | 1.2 ± 0.3 | 83.4 ± 5.4 | 90.3 ± 20.7 | 173.0 ± 20.0 | 281.2 ± 143.0 |
| POST | 0.6 ± 0.1 | 1.7 ± 0.4†,‡ | 60.6 ± 12.5 | 105.9 ± 18.8 | 347.1 ± 150.1 | 99.4 ± 22.9 |
| 3H | 0.5 ± 0.1 | 0.8 ± 0.2 | 62.8 ± 8.2 | 84.4 ± 18.9 | 372.8 ± 128.7 | 205.5 ± 44.0 |
| 24H | 1.5 ± 0.2** | 1.9 ± 0.4‡ | 131.0 ± 14.1** | 123.3 ± 20.6 | 83.7 ± 14.5 | 97.5 ± 28.5 |
**p < 0.05 vs. all time points within NW group; †p < 0.05 PRE vs. POST within OB group, ‡p < 0.05 vs. 3H within OB group; 2 × 4 factorial ANCOVA.
3.3. Myokine response to WBV
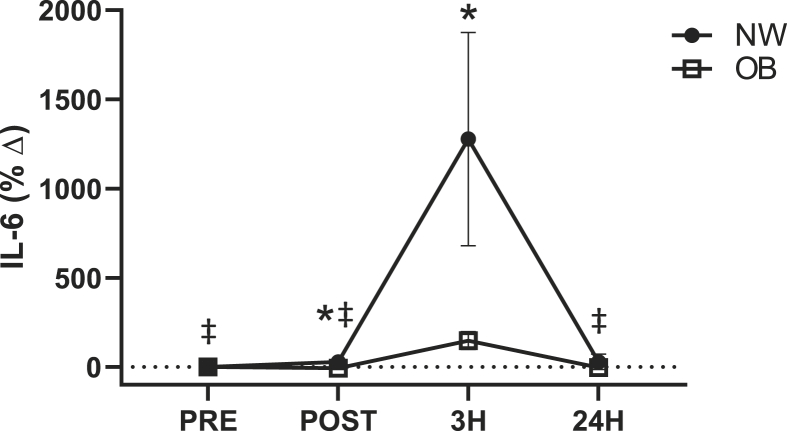
Overall, when both groups were analyzed together, IL-6 was increased in response to WBV exercise (p = 0.042, η = 0.15). IL-6 concentrations were significantly greater at 3H when compared to PRE (PRE: 1.8 ± 0.4 vs. 3H: 4.9 ± 0.7 pg/mL; p < 0.001). IL-6 concentrations were significantly different between groups (p = 0.046, η = 0.22). IL-6 was significantly greater in the OB group at PRE, POST, and 24H when compared to the NW group (p < 0.05). Additionally, a significant group by time interaction (p = 0.002, η = 0.25) (Fig. 3) was observed for the percent change in IL-6 from pre-WBV. In the NW group, the percent change in IL-6 was significantly greater at 3H compared to all time points (p < 0.05). No significant changes were observed in the percent change of IL-6 in the OB group. A significant difference between groups was observed for the percent change of IL-6 (p = 0.032, η = 0.24). The percent change of IL-6 was significantly greater in the NW group at POST (OB: -5.8 ± 8.1 vs. NW: 28.7 ± 21.8%Δ; p = 0.037) and 3H (OB: 148.1 ± 47.9 vs. NW: 1277.9 ± 597.6%Δ; p = 0.030) when compared to the OB group. No differences were observed in creatine kinase or the myokine concentrations of decorin, myostatin following WBV.
Fig. 3.
Percent change of IL-6 from pre-WBV for normal weight (NW) and obese (OB) individuals in response to whole body vibration. *p < 0.05 between groups, ‡p < 0.05 vs. 3H within NW group; 2 × 4 factorial ANCOVA.
4. Discussion
Traditional aerobic exercise reduces the risk of developing chronic diseases by inducing immune, metabolic, and myokine responses. Whether or not WBV produces similar physiological effects is unknown. The purpose of the present investigation was to determine if WBV elicits differential magnitudes and time-courses of immune, metabolic, and myokine responses between obese and normal weight individuals. Findings of the present study demonstrate that an acute bout of WBV exercise elicits immune, metabolic, and myokine responses in both obese and normal weight individuals. However, the magnitude and time-courses of the immune, metabolic, and myokine responses are different between groups. For the first time, we demonstrate that the responses to a single session of WBV are similar to those that occur in response to an acute bout of moderate intensity traditional aerobic exercise, such as treadmill running or cycle ergometry.
4.1. Immune response to WBV
Basal elevations in lymphocytes and neutrophils were observed in obese compared to normal weight individuals prior to WBV. These findings, combined with elevated basal CRP (Table 1), are consistent with previous investigations that have demonstrated the relationship between excessive adiposity and heightened inflammation, mainly due to inflammatory cytokine production by adiopocytes (Furuncuoglu et al., 2016; Visser et al., 1999). Despite greater basal inflammation in obese individuals, the neutrophil response was lower 3 hours following WBV in obese compared to normal weight participants. This blunted neutrophil response may be due to 1) a reduction in bone marrow production of neutrophils (Wang, 2018), or 2) increased neutrophil infiltration into tissue, such as muscle, in order to facilitate tissue repair and remodeling (Nunes-Silva et al., 2014; Murakami et al., 2010). Neutrophils have been shown to be key contributors to immune-mediated inflammation due to pro-inflammatory cytokine production as well as increased inflammatory activation in response to inflammatory cytokines and adipokines (Furuncuoglu et al., 2016; Kwon and Pessin, 2013). In addition, a greater neutrophil response to acute exercise has been associated with a greater pro-inflammatory response (Fielding et al., 1993). Therefore, although the current investigation did not examine neutrophil subsets or inflammatory cytokines in response to WBV, the blunted neutrophil response observed in obese individuals likely helps to mitigate the exercise-induced inflammatory response. Taken together, the lower WBV induced neutrophil response in obese individuals may improve cardiovascular health by blunting and resolving immune-mediated inflammation (Welsh et al., 2017).
A key benefit of traditional moderate intensity exercise is a heightened adaptive immune defense (Walsh et al., 2011). In the present study, a nearly significant (p = 0.083) time dependent increase in the percentage of lymphocytes in response to WBV exercise was observed. Previous investigations have repeatedly demonstrated increases in all lymphocyte subpopulations, as well as reductions in the CD4+/CD8+ ratio, that are dependent on exercise intensity and not the specific mode of exercise (Keast et al., 1988; Nieman, 1994; Pedersen and Hoffman-Goetz, 2000). In order to achieve greater tolerability of acute WBV exercise, the intensity of WBV in the current investigation was relativity low in comparison to prior investigations (Tossige-Gomes et al., 2012; Milanese et al., 2018). Therefore, it is plausible that WBV of greater intensity may have induced changes in lymphocytes similar to those observed in prior investigations using traditional forms of exercise. Furthermore, increased lymphocyte mobilization in response to exercise is thought to be dependent upon catecholamine production (Benschop et al., 1996). Although the current investigation did not examine catecholamines, previous investigations have shown a blunted catecholamine response to exercise in obese individuals (Zouhal et al., 2013). It is plausible that for obese individuals, the WBV intensity used in the current investigation did not stimulate a catecholamine response great enough to elicit an increase in lymphocytes. Although basal lymphocyte percentage was higher in normal weight individuals, following WBV, no group differences were observed in lymphocyte counts, percentages, or percent change from pre-WBV. The nadir in lymphocyte percentages occurred 3 hours post-WBV, which corresponded to a significant increase in the percentage of neutrophils in normal weight participants; however, this response was not observed in obese participants. In obese participants, greater basal inflammation likely blunted the increase in neutrophil percentage, thus preventing a reduction in lymphocyte percentage. Although the percentage of lymphocytes appeared to be lower at 3 hours post-WBV in normal weight participants, lymphocyte counts were not significantly reduced below pre-WBV levels. Therefore, WBV induced immune suppression was unlikely. Taken together, these data suggest that the adaptive immune profile of obese and normal weight individuals in response to acute WBV exercise is not negatively impacted and WBV may have a favorable impact on the immune profile of obese individuals by normalizing and preserving the adaptive immune response.
4.2. Metabolic response to WBV
Obese individuals often display impaired fasting blood glucose in comparison to their normal weight counterparts, even in the absence of apparent clinical metabolic dysfunction (Martyn et al., 2008). Elevated fasting glucose in obese individuals is thought to be caused by reduced glycemic sensing and insulin signaling in response to hormones and cytokines produced by excessive adipocytes (Martyn et al., 2008). Basal blood glucose was found to be elevated in obese participants prior to WBV exercise, however, no significant differences between groups were observed immediately and 3 hours post-WBV. These data suggest that blood glucose was normalized in obese participants following WBV. Although glucose transport was not measured in the current investigation, traditional exercise has been shown to elicit an increase in insulin sensitivity that results in enhanced GLUT4 glucose uptake into skeletal muscle (Marliss and Vranic, 2002; Ebeling et al., 1998). Therefore, it is likely that this mechanism contributed to normalizing blood glucose following WBV in obese individuals. GLUT4 glucose transport has been demonstrated to be impaired in obese individuals (Cusi et al., 2000). As such, despite potential improvements in response to WBV, the observed post-WBV increase in insulin in obese individuals may have been a compensatory attempt at glucose regulation due to impaired GLUT4 transport. Additionally, this mechanism is likely the reason no significant change in insulin sensitivity and an increase in HOMA-IR were observed immediately following WBV exercise in obese individuals. However, HOMA-IR was returned to pre-WBV at 3H and both HOMA-IR and insulin sensitivity remained stable at 24H post-WBV. In normal weight individuals, it is plausible that subtle post-WBV improvements in insulin sensitivity, and subsequently GLUT4 transport, led to a reduced need for insulin in order to facilitate glucose uptake. This, combined with improved beta-cell function, may explain the increase in HOMA-IR that was observed 24 hours following WBV in NW. Despite increased insulin, HOMA-beta-cell function was not significantly altered following WBV in obese participants. These data suggest that acute WBV exercise does not place harmful metabolic strain on the pancreas of obese individuals and may help to maintain normal pancreatic beta-cell function. Acute WBV may normalize blood glucose in obese individuals; however, future studies that evaluate both acute and chronic WBV in a larger cohort of obese individuals are certainly warranted. Nonetheless, these data suggest that acute WBV elicits changes in glucose metabolism that may contribute to long-term improvements in glucose regulation in both obese and normal weight individuals.
4.3. Myokine response
An acute bout of WBV elicited significant muscle activation in both obese and normal weight participants as indicated by the IL-6 response. Although basal IL-6 has been associated with increased low-grade inflammation (Kim et al., 2009), IL-6 in response to muscle activation (i.e. exercise) has been demonstrated to have anti-inflammatory benefits, including increased anti-inflammatory cytokine production (IL-10), and reduced pro-inflammatory cytokine production (TNF-α, IL-1β) by leukocytes (Mizuhara et al., 1994; Steensberg et al., 2003). In addition, repeated acute increases in IL-6, such as those that occur with exercise training, reduce circulating concentrations of IL-6 and contribute to an attenuation of basal inflammation (Beavers et al., 2010; Warnberg et al., 2010). Moreover, IL-6 is involved in glucose metabolism by increasing glucose uptake into sarcomeres and hepatocytes as well as increasing skeletal muscle glucose oxidation (Glund et al., 2007). Additionally, muscle production of IL-6 has been thought to act as an “energy sensor” due to its increased production when muscle glycogen is low, reduced production when blood glucose is high, and its ability to increase skeletal muscle lipolysis (Pedersen, 2012). Therefore, the reduced percent change in IL-6 that was observed in obese compared to normal weight participants may have been due to elevated blood glucose in the obese group. In addition, chronically elevated IL-6 has been shown to be inversely related to muscle (Rieusset et al., 2004) and hepatic IL-6 sensitivity (Gavito et al., 2016). It is plausible that WBV exercise enhanced tissue IL-6 sensitivity, thereby reducing the need for exercise induced increases in IL-6. Although no significant changes in myostatin and decorin were observed following WBV, our study was designed to capture peak IL-6, leukocyte, and metabolic changes in a feasible time frame. It is possible; however, that alterations in these other myokines may have occurred outside of the observed time course, as peak reductions in myostatin have been reported 12 hours post-exercise (Louis et al., 2007) and peak increases in decorin have been observed 4 hours post-exercise (Sullivan et al., 2009). Beneficial changes in myostatin and decorin may occur over time in response to repeated bouts of exercise; however, future studies are warranted to test this hypothesis following WBV training. Additionally, myostatin has been shown to act as an energy regulator by increasing glycolysis and inhibiting glycogen synthesis (Chen et al., 2010). Although participants in the current investigation were asked to fast overnight, they were also asked to refrain from strenuous physical activity for 24 hours prior to participating in the investigation. Accordingly, glycogen stores were likely normal and as such, may have affected the myostatin response to WBV in both obese and normal weight participants. Moreover, in-vitro studies have shown increased myokine production of decorin in response to low frequency (1 Hz) stretch (Kanzleiter et al., 2014). Therefore, the greater contractile frequency experienced during WBV (14 Hz) may have inhibited or altered the time-course of decorin production by skeletal muscle. No significant changes were observed in creatine kinase for either group. As post-exercise creatine kinase is considered to be a marker of muscle damage, it is likely that the intensity of WBV in the current investigation did not induce muscle damage, thus supporting the safety of WBV exercise (Cardinale and Wakeling, 2005). Despite the lack of observable changes in myostatin and decorin, the known effects of muscle derived IL-6 likely contributed to the altered glucose metabolism that was observed following WBV exercise. As acute increases in IL-6 have been demonstrated to promote an anti-inflammatory immune response, combined with its beneficial effects on glucose metabolism, IL-6 produced in response to WBV may act to reduce atherosclerotic risk in both obese and normal weight individuals (Steensberg et al., 2003).
5. Conclusion
This is the first investigation to demonstrate that an acute bout of WBV exercise stimulates favorable immune, metabolic, and myokine responses in obese and normal weight individuals. Perhaps most importantly, the responses to WBV appear to be different between obese and normal weight individuals. Excessive adiposity in addition to energy status may have affected hormone, cytokine, and metabolic signaling in obese individuals, thereby contributing to the observed differences in the immune, metabolic, and myokine responses between groups. Despite these differences, the overall response in both groups appears to be favorable. Although the current investigation examined the responses following a single bout of WBV, based on this data it is likely that an accumulation of repeated bouts of WBV exercise would lead to long-term responses and adaptations and an improvement in inflammatory status. Future studies are warranted to investigate the potential impact of repeated bouts of WBV upon leukocyte subset phenotype and function, metabolic responses, and inflammatory status. Nonetheless, these data suggest that whole body vibration may be an effective exercise mimetic to elicit physiological alterations that are similar to those observed with traditional modes of exercise.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The project was supported in part by an Augusta University Collaborative Proposal Award (RAH and DWS) and NIH 1R01DK117365 (RAH).
References
- Beavers K.M., Brinkley T.E., Nicklas B.J. Effect of exercise training on chronic inflammation. Clin. Chim. Acta. 2010;411(11–12):785–793. doi: 10.1016/j.cca.2010.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin E.J., Virani S.S., Callaway C.W. Heart disease and stroke statistics-2018 update: a report from the American heart association. Circulation. 2018;137(12):e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- Benschop R.J., Rodriguez-Feuerhahn M., Schedlowski M. Catecholamine-induced leukocytosis: early observations, current research, and future directions. Brain Behav. Immun. 1996;10(2):77–91. doi: 10.1006/brbi.1996.0009. [DOI] [PubMed] [Google Scholar]
- Bish C.L., Blanck H.M., Maynard L.M., Serdula M.K., Thompson N.J., Khan L.K. Activity/participation limitation and weight loss among overweight and obese US adults: 1999 to 2002 NHANES. MedGenMed. 2007;9(3):63. [PMC free article] [PubMed] [Google Scholar]
- Cardinale M., Wakeling J. Whole body vibration exercise: are vibrations good for you? Br. J. Sports Med. 2005;39(9):585–589. doi: 10.1136/bjsm.2005.016857. discussion 589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Ye J., Cao L., Zhang Y., Xia W., Zhu D. Myostatin regulates glucose metabolism via the AMP-activated protein kinase pathway in skeletal muscle cells. Int. J. Biochem. Cell Biol. 2010;42(12):2072–2081. doi: 10.1016/j.biocel.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Christiansen T., Bruun J.M., Paulsen S.K. Acute exercise increases circulating inflammatory markers in overweight and obese compared with lean subjects. Eur. J. Appl. Physiol. 2013;113(6):1635–1642. doi: 10.1007/s00421-013-2592-0. [DOI] [PubMed] [Google Scholar]
- Cohen J. second ed. 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- Cusi K., Maezono K., Osman A. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J. Clin. Investig. 2000;105(3):311–320. doi: 10.1172/JCI7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebeling P., Koistinen H.A., Koivisto V.A. Insulin-independent glucose transport regulates insulin sensitivity. FEBS Lett. 1998;436(3):301–303. doi: 10.1016/s0014-5793(98)01149-1. [DOI] [PubMed] [Google Scholar]
- Fielding R.A., Manfredi T.J., Ding W., Fiatarone M.A., Evans W.J., Cannon J.G. Acute phase response in exercise. III. Neutrophil and IL-1 beta accumulation in skeletal muscle. Am. J. Physiol. 1993;265(1 Pt 2):R166–R172. doi: 10.1152/ajpregu.1993.265.1.R166. [DOI] [PubMed] [Google Scholar]
- Foster N.K., Martyn J.B., Rangno R.E., Hogg J.C., Pardy R.L. Leukocytosis of exercise: role of cardiac output and catecholamines. J. Appl. Physiol. 1986;61(6):2218–2223. doi: 10.1152/jappl.1986.61.6.2218. 1985. [DOI] [PubMed] [Google Scholar]
- Furuncuoglu Y., Tulgar S., Dogan A.N., Cakar S., Tulgar Y.K., Cakiroglu B. How obesity affects the neutrophil/lymphocyte and platelet/lymphocyte ratio, systemic immune-inflammatory index and platelet indices: a retrospective study. Eur. Rev. Med. Pharmacol. Sci. 2016;20(7):1300–1306. [PubMed] [Google Scholar]
- Gavito A.L., Bautista D., Suarez J. Chronic IL-6 administration desensitizes IL-6 response in liver, causes hyperleptinemia and aggravates steatosis in diet-induced-obese mice. PLoS One. 2016;11(6) doi: 10.1371/journal.pone.0157956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glund S., Deshmukh A., Long Y.C. Interleukin-6 directly increases glucose metabolism in resting human skeletal muscle. Diabetes. 2007;56(6):1630–1637. doi: 10.2337/db06-1733. [DOI] [PubMed] [Google Scholar]
- Hjorth M., Pourteymour S., Gorgens S.W. Myostatin in relation to physical activity and dysglycaemia and its effect on energy metabolism in human skeletal muscle cells. Acta Physiol. 2016;217(1):45–60. doi: 10.1111/apha.12631. [DOI] [PubMed] [Google Scholar]
- Kanzleiter T., Rath M., Gorgens S.W. The myokine decorin is regulated by contraction and involved in muscle hypertrophy. Biochem. Biophys. Res. Commun. 2014;450(2):1089–1094. doi: 10.1016/j.bbrc.2014.06.123. [DOI] [PubMed] [Google Scholar]
- Kapuku G.K., Treiber F.A., Davis H.C., Harshfield G.A., Cook B.B., Mensah G.A. Hemodynamic function at rest, during acute stress, and in the field: predictors of cardiac structure and function 2 years later in youth. Hypertension. 1999;34(5):1026–1031. doi: 10.1161/01.hyp.34.5.1026. [DOI] [PubMed] [Google Scholar]
- Keast D., Cameron K., Morton A.R. Exercise and the immune response. Sport. Med. 1988;5(4):248–267. doi: 10.2165/00007256-198805040-00004. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Bachmann R.A., Chen J. Interleukin-6 and insulin resistance. Vitam. Horm. 2009;80:613–633. doi: 10.1016/S0083-6729(08)00621-3. [DOI] [PubMed] [Google Scholar]
- Kwon H., Pessin J.E. Adipokines mediate inflammation and insulin resistance. Front. Endocrinol. 2013;4:71. doi: 10.3389/fendo.2013.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal L.G., Lopes M.A., Batista M.L., Jr. Physical exercise-induced myokines and muscle-adipose tissue crosstalk: a review of current knowledge and the implications for health and metabolic diseases. Front. Physiol. 2018;9:1307. doi: 10.3389/fphys.2018.01307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong D.P., Teo K.K., Rangarajan S. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015;386(9990):266–273. doi: 10.1016/S0140-6736(14)62000-6. [DOI] [PubMed] [Google Scholar]
- Levy J.C., Matthews D.R., Hermans M.P. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21(12):2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- Louis E., Raue U., Yang Y., Jemiolo B., Trappe S. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J. Appl. Physiol. 2007;103(5):1744–1751. doi: 10.1152/japplphysiol.00679.2007. 1985. [DOI] [PubMed] [Google Scholar]
- Marliss E.B., Vranic M. 2002. Intense Exercise Has Unique Effects on Both Insulin Release and its Roles in Glucoregulation: Implications for Diabetes. (0012-1797 (Print)) [DOI] [PubMed] [Google Scholar]
- Marqueti R.C., Durigan J.L.Q., Oliveira A.J.S. Effects of aging and resistance training in rat tendon remodeling. FASEB J. 2018;32(1):353–368. doi: 10.1096/fj.201700543R. [DOI] [PubMed] [Google Scholar]
- Martyn J.A., Kaneki M., Yasuhara S. Obesity-induced insulin resistance and hyperglycemia: etiologic factors and molecular mechanisms. Anesthesiology. 2008;109(1):137–148. doi: 10.1097/ALN.0b013e3181799d45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Milanese C., Cavedon V., Sandri M. Metabolic effect of bodyweight whole-body vibration in a 20-min exercise session: a crossover study using verified vibration stimulus. PLoS One. 2018;13(1) doi: 10.1371/journal.pone.0192046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuhara H., O’Neill E., Seki N. T cell activation-associated hepatic injury: mediation by tumor necrosis factors and protection by interleukin 6. J. Exp. Med. 1994;179(5):1529–1537. doi: 10.1084/jem.179.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K.J., Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145(3):341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S., Kurihara S., Titchenal C.A., Ohtani M. Suppression of exercise-induced neutrophilia and lymphopenia in athletes by cystine/theanine intake: a randomized, double-blind, placebo-controlled trial. J. Int. Soc. Sport. Nutr. 2010;7(1):23. doi: 10.1186/1550-2783-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieman D.C. Exercise, upper respiratory tract infection, and the immune system. Med. Sci. Sport. Exerc. 1994;26(2):128–139. doi: 10.1249/00005768-199402000-00002. [DOI] [PubMed] [Google Scholar]
- Nunes-Silva A., Bernardes P.T., Rezende B.M. Treadmill exercise induces neutrophil recruitment into muscle tissue in a reactive oxygen species-dependent manner. An intravital microscopy study. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0096464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okifuji A., Hare B.D. The association between chronic pain and obesity. J. Pain Res. 2015;8:399–408. doi: 10.2147/JPR.S55598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasco J.A., Holloway K.L., Dobbins A.G., Kotowicz M.A., Williams L.J., Brennan S.L. Body mass index and measures of body fat for defining obesity and underweight: a cross-sectional, population-based study. BMC Obes. 2014;1:9. doi: 10.1186/2052-9538-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen B.K. Muscular interleukin-6 and its role as an energy sensor. Med. Sci. Sport. Exerc. 2012;44(3):392–396. doi: 10.1249/MSS.0b013e31822f94ac. [DOI] [PubMed] [Google Scholar]
- Pedersen B.K., Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiol. Rev. 2000;80(3):1055–1081. doi: 10.1152/physrev.2000.80.3.1055. [DOI] [PubMed] [Google Scholar]
- Pietilainen K.H., Kaprio J., Borg P. Physical inactivity and obesity: a vicious circle. Obesity. 2008;16(2):409–414. doi: 10.1038/oby.2007.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier P., Giles T.D., Bray G.A. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American heart association scientific statement on obesity and heart disease from the obesity committee of the council on nutrition, physical activity, and metabolism. Circulation. 2006;113(6):898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- Rieusset J., Bouzakri K., Chevillotte E. Suppressor of cytokine signaling 3 expression and insulin resistance in skeletal muscle of obese and type 2 diabetic patients. Diabetes. 2004;53(9):2232–2241. doi: 10.2337/diabetes.53.9.2232. [DOI] [PubMed] [Google Scholar]
- Schuler G., Adams V., Goto Y. Role of exercise in the prevention of cardiovascular disease: results, mechanisms, and new perspectives. Eur. Heart J. 2013;34(24):1790–1799. doi: 10.1093/eurheartj/eht111. [DOI] [PubMed] [Google Scholar]
- Steel J.M., Steel C.M., Johnstone F.D. Leukocytosis induced by exercise. Br. Med. J. 1987;295(6606):1135–1136. doi: 10.1136/bmj.295.6606.1135-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensberg A., Fischer C.P., Keller C., Moller K., Pedersen B.K. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am. J. Physiol. Endocrinol. Metab. 2003;285(2):E433–E437. doi: 10.1152/ajpendo.00074.2003. [DOI] [PubMed] [Google Scholar]
- Sullivan B.E., Carroll C.C., Jemiolo B. Effect of acute resistance exercise and sex on human patellar tendon structural and regulatory mRNA expression. J. Appl. Physiol. 2009;106(2):468–475. doi: 10.1152/japplphysiol.91341.2008. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tossige-Gomes R., Avelar N.C., Simao A.P. Whole-body vibration decreases the proliferativeb response of TCD4(+) cells in elderly individuals with knee osteoarthritis. Braz. J. Med. Biol. Res. 2012;45(12):1262–1268. doi: 10.1590/S0100-879X2012007500139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totosy de Zepetnek JO., Giangregorio L.M., Craven B.C. Whole-body vibration as potential intervention for people with low bone mineral density and osteoporosis: a review. J. Rehabil. Res. Dev. 2009;46(4):529–542. doi: 10.1682/jrrd.2008.09.0136. [DOI] [PubMed] [Google Scholar]
- Trosclair D., Bellar D., Judge L.W., Smith J., Mazerat N., Brignac A. Hand-grip strength as a predictor of muscular strength and endurance. J. Strength Cond. Res. 2011;25:S99. [Google Scholar]
- Visser M., Bouter L.M., McQuillan G.M., Wener M.H., Harris T.B. Elevated C-reactive protein levels in overweight and obese adults. J. Am. Med. Assoc. 1999;282(22):2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- Walsh N.P., Gleeson M., Shephard R.J. Position statement. Part one: immune function and exercise. Exerc. Immunol. Rev. 2011;17:6–63. [PubMed] [Google Scholar]
- Wang J. Neutrophils in tissue injury and repair. Cell Tissue Res. 2018;371(3):531–539. doi: 10.1007/s00441-017-2785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnberg J., Cunningham K., Romeo J., Marcos A. Physical activity, exercise and low-grade systemic inflammation. Proc. Nutr. Soc. 2010;69(3):400–406. doi: 10.1017/S0029665110001928. [DOI] [PubMed] [Google Scholar]
- Welsh P., Grassia G., Botha S., Sattar N., Maffia P. Targeting inflammation to reduce cardiovascular disease risk: a realistic clinical prospect? Br. J. Pharmacol. 2017;174(22):3898–3913. doi: 10.1111/bph.13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zago M., Capodaglio P., Ferrario C., Tarabini M., Galli M. Whole-body vibration training in obese subjects: a systematic review. PLoS One. 2018;13(9) doi: 10.1371/journal.pone.0202866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouhal H., Lemoine-Morel S., Mathieu M.E., Casazza G.A., Jabbour G. Catecholamines and obesity: effects of exercise and training. Sport. Med. 2013;43(7):591–600. doi: 10.1007/s40279-013-0039-8. [DOI] [PubMed] [Google Scholar]