Abstract
Parkinson’s disease (PD) is one of the most common neurodegenerative movement disorders which is characterised neuropathologically by progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and the presence of Lewy bodies (made predominately of α-synuclein) in the surviving neurons. Animal models of PD have improved our understanding of the disease and have played a critical role in the development of neuroprotective agents. Neuroinflammation has been strongly implicated in the pathogenesis of PD, and recent studies have used lipopolysaccharide (LPS), a component of gram-negative bacteria and a potent activator of microglia cells, to mimic the inflammatory events in clinical PD. Modulating the inflammatory response could ameliorate PD associated complications and thus, it is essential to understand the extent to which LPS models reflect human PD. This review will outline the routes of administration of LPS such as stereotaxic, systemic and intranasal, their ability to recapitulate neuropathological markers of PD, and mechanisms of LPS induced toxicity. We will also discuss the ability of the models to replicate motor symptoms and non-motor symptoms of PD such as gastrointestinal dysfunction, olfactory dysfunction, anxiety, depression and cognitive dysfunction.
Keywords: Animal models, Motor symptoms, Non-motor symptoms, Parkinson’s disease, Lipopolysaccharide
Highlights
-
•
This review describes stereotaxic, intraperitoneal and intranasal LPS animal models of PD.
-
•
The ability of the models to replicate motor symptoms and non-motor symptoms of PD are analysed.
-
•
The possible mechanisms of LPS induced toxicity and their relevance to clinical PD are discussed.
-
•
Currently most evidence exists for the stereotaxic LPS model of PD.
-
•
Further characterisation of LPS models of PD is warranted.
1. Introduction
Parkinson’s disease (PD) is the most common neurodegenerative movement disorder, highly prevalent in the elderly population 65 years old and over (de Lau and Breteler, 2006; Obeso et al., 2010; Stoker and Greenland, 2018). The diagnosis of PD in patients is based on the core motor symptoms such as resting tremor, rigidity, slow movement and postural instability. In addition, PD is associated with non-motor symptoms such as olfactory deficits, gastrointestinal dysfunction, impaired regulation of sleep-wake cycle, anxiety, depression and cognitive decline, and it is evident that non-motor symptoms precede the onset of motor symptoms (Obeso et al., 2010; Poewe, 2008). Pathologically, PD is characterised by progressive degeneration of dopaminergic neurons in substantia nigra pars compacta (SNpc) and loss of these neurons is predominately associated with the core motor symptoms (Dawson and Dawson, 2003; Sulzer and Surmeier, 2013). The other neuronal systems that are affected in PD include dopaminergic neurons in the ventral tegmental area (VTA), dopaminergic neurons in the enteric nervous system (ENS), noradrenergic neurons in the locus coeruleus (LC), serotonergic neurons in the raphe nuclei, the dorsal motor nucleus of the vagus nerve (DMV), olfactory system, pedunculopontine nuclei, the nucleus basalis of Meynert and various cortical areas (Obeso et al., 2010; Sulzer and Surmeier, 2013). Dysfunction of these neuronal systems could contribute to non-motor symptoms of PD. Another pathological hallmark of PD is the formation of Lewy bodies and Lewy neurites (Lewy pathology) in the surviving neurons, which are inclusions made predominately of aggregated α-synuclein protein (Dawson and Dawson, 2003). The function of normal α-synuclein protein is not entirely understood but it is mostly a presynaptic protein implicated in neuronal differentiation, dopamine biosynthesis and vesicle trafficking (Dawson and Dawson, 2003; Emamzadeh, 2016).
Genetic factors account for 5–10% of PD cases and the rest of the cases have no known cause. It is evident both clinically and in animal models of PD that neuroinflammation, oxidative stress, mitochondrial dysfunction and defects in protein clearance are fundamental in the aetiology of the disease (Halliday and McCann, 2010; Hauser and Hastings, 2013; Obeso et al., 2010; Stoker and Greenland, 2018). Animal models of PD broadly encompass genetic based models, neurotoxin based models, inflammatory models and pharmacological models which are beyond the scope of this review; please see (Duty and Jenner, 2011) for more details. Briefly, the genetic models of PD are based on the genes associated with monogenic PD such as α-synuclein (α-syn), leucine rich repeat kinase 2 (LRRK2), parkin, PTEN-induced putative kinase 1 (PINK1), ubiquitin carboxyl terminal hydroxylase isozyme L1 (UCH-L1) and protein deglycase DJ-1, and most of these genetic models do not display clear degeneration of dopaminergic neurons in the SN or motor deficits (Duty and Jenner, 2011; Stoker and Greenland, 2018). Neurotoxin-based models include exposing rodents and non-human primates to toxins including 6-hydroxydopamine (6-OHDA) which is a dopamine and norepinephrine analogue; 1-methyl – 4 phenyl −1, 2, 3, 6 tetrahydropyridine (MPTP) a prodrug to MPP+ which is toxic to dopaminergic neurons; a pesticide known as rotenone or a herbicide known as paraquat. 6-OHDA, MPTP and rotenone inhibit mitochondrial complex I, resulting in reduced energy metabolism, and leakage of electrons that can form reactive oxygen species (ROS) and consequently, resulting in oxidative stress. Moreover, paraquat induced neurotoxicity is mediated by ROS generated from redox cycling of this herbicide (He et al., 2013). The ability of these neurotoxins to recapitulate the features of PD in animal models is dependent on the route of administration, dosage and species. It should also be emphasized that none of the neurotoxin models can replicate all the core features of PD namely: (1) progressive degeneration of dopaminergic neurons in the SN and striatum, (2) α-synuclein aggregation and (3) motor deficits.
Apart from genetic and neurotoxin models, there is strong evidence to suggest that neuroinflammation is critical in clinical PD (Tansey and Goldberg, 2010). Positron emission tomography indicates increased activation of microglia cells in the basal ganglia, brainstem and frontal and temporal cortices in patients with PD compared to healthy patients (Stoker and Greenland, 2018). Consistent with these findings, pro-inflammatory cytokines are elevated in the serum and cerebrospinal fluid of PD patients (Hirsch and Hunot, 2009; Stoker and Greenland, 2018), with post-mortem analysis finding microglia and complement activation, T-lymphocyte infiltration and elevated pro-inflammatory cytokines in the SN and striatum (Hirsch and Hunot, 2009; McGeer et al., 1988; Stoker and Greenland, 2018). Polymorphisms in neuroinflammation associated genes increase the risk of PD and these reports are consistent with epidemiological studies indicating reduced risk of PD in regular users of non-steroidal anti-inflammatory drugs compared to non-users (Hirsch and Hunot, 2009). The two most common inflammatory models of PD are those using polyinosinic polycytidylic acid (poly (I:C)) and lipopolysaccharide (LPS), which activate toll-like receptors 3 and 4 respectively. Toll-like receptors (TLR) recognise pathogen-associated molecular patterns, initiating an immune response and promoting production of pro-inflammatory cytokines, chemokines and oxidative factors. TLR3 recognizes RNA and viruses, whereas TLR4 mediates the response to bacterial endotoxins. Injection of poly (I:C) into the SN leads to a sustained inflammatory reaction in the SN and dorsal striatum (Bobyn et al., 2012, Deleidi et al., 2010, Olsen et al., 2019), with intrastriatal injection producing profound contralateral motor deficits at 28 days post-injection accompanied by striatal neuroinflammation (Concannon et al., 2016). LPS as a model of PD has been more thoroughly characterised and thus is the focus of this review, given its ability to recapitulate some pathological features of human PD such as degeneration of dopaminergic neurons in the SN and motor deficits (Badshah et al., 2016; Beier et al., 2017; Bodea et al., 2014; Khan et al., 2018; Wang et al., 2015a). Indeed, some PD patients have elevated serum LPS levels, which may reflect increased intestinal permeability evident in the early stage of the diseases. Consistent with these findings, it has been shown that the gut in PD patients is colonized by LPS producing bacteria such as Helicobacter Pylori, and eradication of these bacteria improves PD symptoms (Brown, 2019). Thus, not only do LPS models allow further understanding of PD related pathologies but also provide the platform to understand LPS as a risk factor for PD. Previous reviews on LPS models of PD have outlined the most common routes of administration of LPS including stereotaxic, systemic and intranasal depending on the review, inflammation induced consequences (e.g. microglia activation, production of inflammatory cytokines and free radicals) and the ability to produce neuropathological markers of PD (e.g. degeneration of dopaminergic neurons and α-synuclein pathology) (Batista et al., 2019; Liu and Bing, 2011; Stojkovska et al., 2015). Our review provides a comprehensive update and critical analysis of these routes of administration and their ability to reproduce PD related pathologies, motor symptoms and non-motor symptoms such as gastrointestinal dysfunction, olfactory dysfunction, anxiety, depression and cognitive dysfunction. We also provide a latest update on the possible mechanisms of toxicity of LPS, and their relevance to clinical PD.
2. LPS models of PD: an overview
LPS as an initiator for PD was first reported when a laboratory worker, accidently exposed to 10 μg of LPS from Salmonella minnesota through an open wound, developed a PD-like phenotype characterised by tremor, rigidity and bradykinesia three weeks after the incident, with positron emission tomography demonstrating damage to the SN and cerebral cortex a few years later (Niehaus and Lange, 2003). In pre-clinical research, Castano and colleagues first used stereotaxic surgery, a form of surgical intervention which utilises a three-dimensional coordinate system to locate a small target in the brain, to administer LPS into the SN in rats (Castano et al., 1998). Post-mortem evidence from this study showed that intranigral LPS induced microglia activation and degeneration of dopaminergic neurons in the SN, and these findings are consistent with later studies (Arimoto et al., 2007; Bing et al., 1998; Hernández-Romero et al., 2008) using the same route of administration. These early studies set the foundation for stereotaxic LPS models of PD, with extension to intrastriatal, intrapallidal and intracerebroventricular (ICV) models. ICV and intrapallidal administration of LPS can be used to model PD, with the ability to induce microglial activation in the SN and subsequently, degeneration of dopaminergic neurons. However, utilisation of these routes of administration is limited as the effects are not specific to PD, with ICV administration of LPS also used to model Alzheimer’s disease (AD) (Bardou et al., 2014). Following the establishment of intracranial LPS injections to model PD, Qin et al. (2007) demonstrated for the first time that systemic administration of a single dose of LPS via an intraperitoneal (i.p) injection induced self-sustained neuroinflammation and specific degeneration of dopaminergic neurons in the SN, and these findings were confirmed by later studies (Chen et al., 2017; Jiang et al., 2017; Liu et al., 2008; Qin et al., 2013). Subsequently, He et al. (2013) established that administration of LPS via the nasal cavity can induce PD-like pathology. The ability of LPS models to reproduce specific features of PD based on the routes of administration (e.g. stereotaxic, systemic and intranasal) and dosage regimens will be discussed in this review.
2.1. Stereotaxic LPS models
2.1.1. Degeneration of nigrostriatal dopaminergic pathway, motor dysfunction and α-synuclein pathology in stereotaxic LPS models
Unilateral administration of a single dose of LPS into the SN or striatum which are the most common regions for stereotaxic administration of LPS, induces degeneration of dopaminergic neurons in the SN and their neuronal projections to the striatum. Intranigral administration of 10 μg of LPS in rats induces a 50–80% reduction in the number of tyrosine hydroxylase (TH) positive cells in the SN compared to shams at 4 weeks post-treatment (Bao et al., 2018; Chen et al., 2017; Fu et al., 2015; He et al., 2016a; Wang et al., 2015a). This lesion to the nigrostriatal pathway was illustrated by increased rotational behaviour in the apomorphine and amphetamine induced rotational test which was not evident in the sham group. Moreover, striatal dopamine was reduced by 34–50%, along with motor function which was assessed with the rotarod test, commonly used to evaluate motor skills based on the ability of the animals to maintain themselves on a rotating rod (Bao et al., 2018; Chen et al., 2017; Fu et al., 2015; He et al., 2016a; Wang et al., 2015a). Furthermore, according to Choi et al. (2009), intrastriatal administration of 30 μg of LPS in rats induces a significant reduction in the number of TH positive cells in SN by 21%, 38% and by 41% at 1, 2 and 4 weeks respectively post-treatment compared to the non-injected side. Studies performed by Hunter and colleagues using the same dose in rats further illustrated that LPS induced 21% reduction in TH positive cells in the SN at 1 week post-treatment, indicating reproducibility of the model in the same species (Hunter et al., 2007, 2017). Similar findings are reported in mouse models of stereotaxic PD, with intrastriatal administration of 20 μg of LPS in C57BL/6 mice inducing degeneration in SN with a 26%, 72% and 81% reduction in TH positive cells at weeks 1, 4 and 12 respectively (Hunter et al., 2009). Moreover, Hunter et al. (2009) performed the rotarod test weekly for four weeks after intrastriatal LPS administration in mice and showed that LPS administration progressively reduced the time spent on the rotarod starting at 2 weeks post-treatment and that latency time correlated with depletion of striatal dopamine, which was alleviated through the administration of levodopa. Stereotaxic injections of LPS in mice to model PD are not as common as stereotaxic LPS in rat models and to our best knowledge, there are only 3 studies as illustrated in Table 1. Therefore, further characterisation of stereotaxic mouse models of LPS is required. Stereotaxic LPS in rats also increases accumulation of cytoplasmic α-synuclein protein in nigral TH positive neurons, which has a pathological significance (Choi et al., 2009). Choi and co-authors showed using double immunostaining that there was increased accumulation of cytoplasmic α-synuclein protein in nigral TH-positive neurons following intrastriatal administration of LPS (30 μg) in rats (Choi et al., 2009). This finding is resemblant of α-synuclein pathology in PD; however, α-synuclein protein is not widely explored in stereotaxic models of LPS and further investigation is needed to solidify this finding. The mechanisms responsible for α-synuclein hyperphosphorylation and aggregation are not clearly understood in human PD, but free radicals such as nitric oxide (NO), superoxide and peroxynitrite could modify α-synuclein through nitrosylation/oxidization and decrease its solubility, forming aggregates (Gao et al., 2011; Gao et al., 2008,Gao et al., 2002). Neuronal α-synuclein can be excreted into the surrounding medium and it can activate microglia through nicotinamide adenosine dinucleotide phosphate (NADPH) oxidase and TLR4, leading to further production of ROS and pro-inflammatory cytokines (Zhang et al., 2017). Microglia cells are essential in the clearance of α-synuclein; however, exacerbated production of α-synuclein could overwhelm the clearance pathway and consequently, can increase extracellular α-synuclein (Zhang et al., 2017).
Table 1.
Degeneration of nigrostriatal dopaminergic pathway, α-synuclein pathology, motor deficits, extranigral pathology and non-motor deficits in stereotaxic, systemic and intranasal LPS models.
| Route of administration | Species | Dose of LPS | Duration of experiment Day (d), week (w) month (m) |
TH positive neurons in SN or TH protein |
Striatal dopamine | Αlpha-synuclein protein/aggregates in SN | Motor deficits | Extranigral pathology | Non-motor deficits |
|---|---|---|---|---|---|---|---|---|---|
| Stereotaxic (single dose) | Rats | 0.6–16 μg (intranigral) | 1w6,7, 3w4,8, 4w1,2,5, and 6w3 | TH neurons 50–80% ↓1,2,3,4,5 | 34–50% ↓3,4 | Not assessed | ↑ rotational behaviour1,2,3,4,6,7 and ↓ time on the rotarod3,5 | Not assessed | Impaired memory6, Anxiety and depressive-like behaviour7, and Gastric dysmotility8 |
| Rats | 30–32 μg (intra-striatal) | 1w9,10,11, 2w and 4w9 | TH neurons 21% ↓ in 1w9,10,11, 38 and 41% ↓ in 2w and 4w9 |
40–42% ↓9,10 | ↑ α-syn protein9 | ↑ rotational behaviour9 | Not assessed | Not assessed | |
| C57BL/6 Mice | 20 μg (intrastriatal) |
1w, 4w and 12w12 | TH neurons 26% ↓ in 1w, 72% ↓ in 4w and 81% ↓ in 12w12 | 42% ↓ in 4w12 | Not assessed | ↓ time on the rotarod12 | Not assessed | Not assessed | |
| B6.129S6-Cybbtm 1Din mice, non-transgenic mice (nTg) (C57BL and 129/Sv) and transgenic mice expressing human WT (M7KO) and A53T mutant α-syn (M83KO) | 5 μg (intranigral) | 2w34, 4w35 | TH neurons 44% ↓34, TH neurons 35% ↓ nTg, 48% ↓ M7KO and M83KO 65% ↓35 | Not assessed | α-syn aggregates in TH neurons35 | Not assessed | Not assessed | Not assessed | |
|
(No studies for repeated LPS injections in rats) Systemic (i.p) |
C57BL/6 mice | 250 μg/kg daily for 7 days | 7d15, 13d13, 14d14 | TH protein 87% ↓13 | Not assessed | 3.8-fold ↑ α-syn protein13 | Not assessed | 4.2-fold ↑ in α-syn protein13 and 8-fold ↓ in TH protein in hippocampus13. | Impaired memory13,14 |
| C57BL/6 mice | 1 mg/kg daily for 4 days | 19d16,17 | TH neurons 36%16 and 40% ↓17 | 73% ↓ at day 1916 | Not assessed | Not assessed | Not assessed | Not assessed | |
| C57BL/6 mice | 2.5–5 mg/kg (once) | 2m25, (1–5m)23, 718,19,20,21,22,24,26,27, and 10m26 | TH neurons 18–22% ↓18,19,20,21,22,34, 50% ↓26 and 46% ↓27 | Not assessed | 2.2 and 1.7-fold ↑ α-syn protein in young and aged mice compared to age matched controls at 7 m20, 4-fold ↑ α-syn protein27 | ↓ voluntary movement in the open field27 and ↓ time in the rotarod test21,24,27 | ↑ α-syn expression in myenteric plexus23. 42% and 54% ↓ NE neurons in LC (7m, 10m), 28% ↓ Neun+ in motor cortex (7m) and 30% ↓ in hippocampus (10m)26 | ↑ gut permeability23 | |
| Rats | 5 mg/kg (once) | 15d28 | TH protein 50% ↓28 | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed | |
| Intranasal (no studies for rats) | C57BL/6 mice | 1 mg/ml (every other day) | 21d32, 1m29,30,31, 5m33 | TH neurons 38–55%29,30,31, 79% ↓33 | ≈40% ↓33 | 2.229 and 2.4-fold30 in α-syn protein | ↓ voluntary movement in the open field29,30,33 and bradykinesia (pole test)30,31 | 44% ↓ in TH protein and 2.75-fold ↑ in α-syn protein in OB30, 31.9% ↓ in TH neurons in OB32, 1.75-fold ↑ in α-syn protein in OB33 | Not assessed |
Abbreviations: TH, tyrosine hydroxylase; SN, substantia nigra; OB, olfactory bulb; LC, locus coeruleus; NE, noradrenaline; α-syn, α-synuclein.
References: 1 (Chen et al., 2017); 2 (Wang et al., 2015c, Wang et al., 2015b, Wang et al., 2015a); 3 (He et al., 2016b, He et al., 2016a); 4 (Fu et al., 2015); 5 (Bao et al., 2018); 6 (Hritcu and Ciobica, 2013); 7 (Hritcu and Gorgan, 2014); 8 (Zheng et al., 2013b, Zheng et al., 2013a); 9 (Choi et al., 2009); 10 (Hunter et al., 2007); 11 (Hunter et al., 2017); 12 (Zheng et al., 2013b, Zheng et al., 2013a); 13(Zhang and Xu, 2018); 14 (Khan et al., 2018); 15 (Badshah et al., 2016); 16 (Beier et al., 2017), 17 (Bodea et al., 2014), 18 (Qin et al., 2007); 19(Qin et al., 2013); 20 (Zheng et al., 2013b, Zheng et al., 2013a); 21 (Liu et al., 2008); 22 (Reinert et al., 2014); 23Kelly et al., 2014; 24 (Jiang et al., 2017); 25 (Zheng et al., 2013b, Zheng et al., 2013a), 26 (Song et al., 2018); 27 (Chen et al., 2018; 28 (Gasparotto et al., 2018):29 (Li et al., 2015); 30 (He et al., 2016b, He et al., 2016a); 31 (Zheng et al., 2013b, Zheng et al., 2013a); 32 (Hasegawa-Ishii et al., 2017); 33 (He et al., 2013),34 (Qin et al., 2004); 35 (Gao et al., 2008).
2.1.2. Possible mechanisms of neurotoxicity and their relevance to clinical PD
Microglia constantly monitor the microenvironment of the brain and they respond rapidly to immunological stimuli or neuronal injury by becoming activated whereby they transform from a ramified state to an amoeboid morphology (circular in shape, lacking processes and containing phagocytic vacuoles) (Arimoto et al., 2007; Boche et al., 2013). Chronic infusion or single stereotaxic administration of LPS (e.g. into the SN or striatum) activates resident microglia resulting in the release of molecules such as tumour necrosis factor α (TNF-α), interleukin-6 (IL-6), interleukin-1β (IL-1β), NO and superoxide (O2-) in the brain, and such molecules are toxic to neurons (Arimoto et al., 2007; Block et al., 2007; Castano et al., 1998; Gao et al., 2002; Wang et al., 2015a). It has been identified in post-mortem brain samples of PD patients that there are increased levels of TNF-α, IL-1β, IL-6, inducible nitric oxidase synthase (iNOS) and cyclooxygenase 2 (COX2) which is consistent with the findings in stereotaxic LPS models (Hirsch and Hunot, 2009; Stoker and Greenland, 2018; Wang et al., 2015b). Moreover, it has been reported that SN is one of the brain regions highly populated by microglia and this is consistent with pre-clinical animal experiments in rodents (Kim et al., 2000; Saijo et al., 2009). Thus, the abundance of microglia as well as other innate properties, could underlie the vulnerability of dopaminergic neurons in PD (Kim et al., 2000; Saijo et al., 2009). Following their activation, microglia facilitate activation of astrocytes, and activated astrocytes augment the inflammatory response and degeneration of dopaminergic neurons through further production of inflammatory cytokines (Hoban et al., 2013; Kuter et al., 2018; Liu and Bing, 2011).
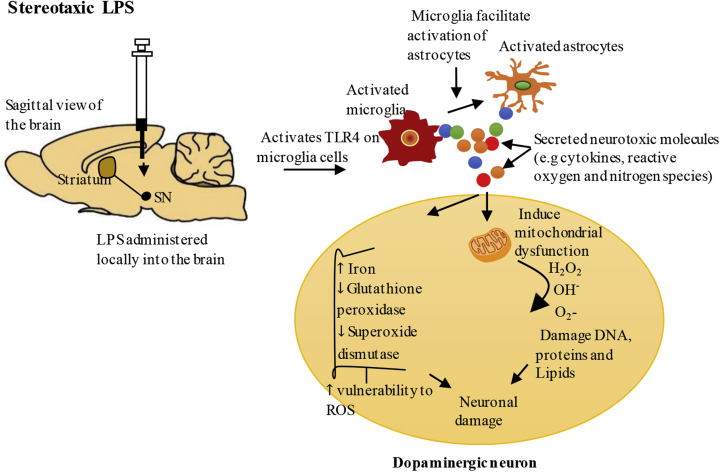
Neuroinflammation may also drive oxidative stress through microglial activation and release of NO, with iNOS the enzyme essential for its production increased. NO can cause neuronal mitochondrial dysfunction (Chitnis and Weiner, 2017; Qin et al., 2013) and mitochondrial defects lead to impaired energy metabolism and excessive production of ROS, which is problematic especially for dopaminergic neurons due to their high energy demands and reduced antioxidant capacity (Park et al., 2018; Villacé et al., 2017). Stereotaxic administration of LPS reduces the activity of mitochondrial complex I of the electron transport chain (ETC) in both the SN and striatum, consistent with reports on reduced activity of mitochondrial complex I in the SN of PD patients (Choi et al., 2009; Hauser and Hastings, 2013; Hunter et al., 2017; Reeve et al., 2018). Abnormal function of mitochondrial complex I leads to excessive production of superoxide, which is toxic for neurons (Puspita et al., 2017). Indeed, stereotaxic administration of LPS upregulates the expression of microglial NADPH oxidase in the SN and striatum in mice and rats, the enzyme essential to produce superoxide (Choi et al., 2009; Chung et al., 2010, 2012; Wang et al., 2015a). Superoxide (O2-) and hydrogen peroxide (H2O2) can participate in the Fenton reaction catalysed by iron to yield hydroxyl radicals (OH−) which are highly reactive. Such events could have detrimental effects on neurons considering the evidence that iron is increased, and glutathione peroxidase, a major antioxidant which limits the harmful effects of hydrogen peroxide, and superoxide dismutase, an antioxidant essential for cellular defence against superoxide, are reduced in the SN of stereotaxic LPS models (Ferreira Mello et al., 2013; Hauser and Hastings, 2013; Oakley et al., 2007; Zhang et al., 2014). These studies are consistent with the findings that iron is increased and glutathione peroxide is reduced in PD patients (Wang et al., 2015a; Zhang et al., 2014). Reducing ROS by blockage of NADPH oxidase and iNOS in stereotaxic LPS models attenuates degeneration of dopaminergic neurons, thereby validating the importance of these enzymes in neurodegeneration (Chung et al., 2010, 2012; Hunter et al., 2009). The possible mechanisms of stereotaxic LPS induced toxicity are summarised in Fig. 1.
Fig. 1.
Stereotaxicadministration ofLPS – the proposed mechanisms leading todopaminergicneuron degeneration.
Stereotaxic administration of LPS (delivered directly into the local brain environment) can activate Toll-like receptor 4 (TLR4) on microglia cells resulting in the production of inflammatory cytokines (e.g.TNF-α, IL-1β, IL-6, IFN-γ) and free radicals (e.g. NO and O2-) in the brain (Liu and Bing, 2011). Activated microglia facilitate the activation of astrocytes which augment the inflammatory response through additional production of inflammatory cytokines, and reactive oxygen and nitrogen species. Excessive production of these neurotoxic molecules could impair mitochondrial function, resulting in impaired energy metabolism and additional production of reactive oxygen species (ROS), and can reduce antioxidants such as glutathione peroxidase and superoxide dismutase in the brain (Hauser and Hastings, 2013; Park et al., 2018; Villacé et al., 2017). Stereotaxic LPS also increases iron levels in the substantia nigra (SN), which could catalyse the Fenton reaction of superoxide (O2-) and hydrogen peroxide (H2O2) to yield hydroxyl radicals (OH−) which are toxic to the neurons (Hauser and Hastings, 2013).
2.2. Systemic LPS models
2.2.1. Degeneration of nigrostriatal dopaminergic pathway, motor dysfunction and α-synuclein pathology in systemic LPS models
Systemic administration of LPS via the intraperitoneal cavity in C57BL/6 mice induces degeneration of dopaminergic neurons, α-synuclein pathology and motor deficits; however, the onset of the disease in these animal models depends on the nature of the dose. It has been identified by multiple studies in mice that systemic administration of a single high dosage (5 mg/kg) of LPS induces a delayed progressive degeneration of TH positive neurons in the SN in male mice with the onset around 7 months post-treatment (Liu et al., 2008; Qin et al., 2007, 2013; Zheng et al., 2013a). According to Liu et al. (2008), this dose does not cause neurodegeneration in female C57BL/6 mice, potentially due to anti-inflammatory actions of oestrogen, and this phenomenon is consistent with clinical PD considering that male patients outnumber the female patients (Jankovic, 2008). Nonetheless in male mice, Liu and colleagues found progressive loss of dopaminergic neurons (18%, 37% and 55% at 6, 9 and 20 months consecutively) which correlated with worsening performance in the rotarod test and could be alleviated by systemic administration of levodopa (Liu et al., 2008). Increased α-synuclein protein levels in the midbrain [91, 92] were also noted (Liu et al., 2008; Zheng et al., 2013a), mirroring the clinical situation. This study was not replicated by Gasparotto et al. (2018), where a single injection of 5 mg/kg of LPS in Wistar rats only significantly reduced TH protein levels in the SN at 15 days with recovery to sham level at 30 and 60 days post-injury, with further investigation of species specific effects needed. In contrast to acute single injection models, repeated daily systemic injections of 250 μg/kg LPS for 7 days in mice significantly reduced TH protein level (87%) and increased α-synuclein protein levels (3.8-fold) in the SN compared to the sham group at one week after the last LPS injection (Zhang and Xu, 2018). Moreover, it was shown by Beier et al. (2017) and Bodea et al. (2014) that repeated systemic administration of LPS (1 mg/kg, daily for 4 days), significantly reduced TH positive neurons in SN by 36% and 40% respectively at 19 days post-treatment, with no further significant reduction at 36 days. According to these studies, there was increased production of pro-inflammatory cytokines and insignificant changes in anti-inflammatory cytokines at day 5 and day 19 following systemic administration of LPS. However, at day 36 post-treatment, there was a shift in cytokine production with an increased production of anti-inflammatory cytokines and reduced pro-inflammatory cytokines indicating that at later time the animals would recover. These findings were consistent with reports that repeated systemic administration of LPS induces tolerance by causing a switch from a neurotoxic pro-inflammatory profile to a beneficial anti-inflammatory profile (Beier et al., 2017). Thus, the value of such model may be limited due to the inability to cause progressive degeneration of dopaminergic neurons but it can be used to investigate the effects of systemic inflammation on driving inflammation within the nigrostriatal pathway.
2.2.2. Possible mechanisms of toxicity and their relevance to clinical PD
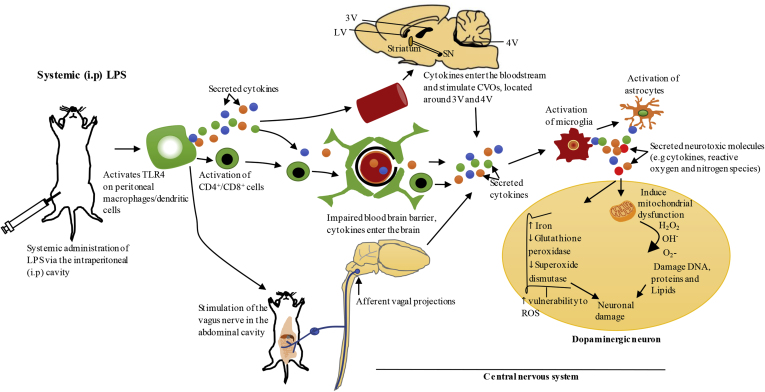
It has been proposed that PD could begin in the gastrointestinal (GI) tract, with GI complications such as delayed gastric emptying and constipation being early symptoms of PD (Fasano et al., 2015; Hawkes et al., 2007). The GI tract is directly connected to the CNS through the vagus nerve, which originates in medulla oblongata (Forsyth et al., 2011). Based on Braak hypothesis, the dorsal motor nucleus of the vagus nerve (DMV) is one of the first regions that exhibits Lewy bodies in clinical PD (Halliday and McCann, 2010). In addition, it has been shown that α-synuclein lysates from PD patients can travel in a time dependent manner from the vagal nerve to other regions of the DMV connected to the brainstem when injected into the intestinal wall of rats; thus, supporting the involvement of this nerve in propagation of peripheral pathology to the brain (Holmqvist et al., 2014). Systemic LPS models are suitable to understand the propagation of systemic inflammation and how it can induce neuroinflammation and neurodegeneration in PD. It is known that the immune system communicates with the CNS; however, a thorough investigation is needed to understand the underlying mechanisms (Elmquist et al., 1997). In systemic LPS models (chronic and acute), the passage of LPS into the brain is limited by the blood brain barrier (BBB), and it is likely that systemic LPS causes neuroinflammation indirectly (Qin et al., 2007). Systemic administration of LPS can lead to activation of TLR4 on the surface of peritoneal macrophages/dendritic cells at the site of injection resulting in production of pro-inflammatory cytokines such as TNF-α, IL-6 and IL-1 (Block et al., 2007). These pro-inflammatory cytokines are significantly elevated in the serum of PD patients as well as in the brain (Arimoto et al., 2007; Dobbs et al., 1999; Reale et al., 2009). TNF-α, IL-6 and IL-1 are essential in mediation of systemic inflammation and for the immune system to communicate with the brain (Perry et al., 2007; Perry, 2010). Firstly, it has been demonstrated that IL-1 produced by peritoneal macrophages/dendritic cells in response to systemic LPS can activate the IL-1 receptor on the vagal nerve that innervates the abdominal cavity, which stimulates the CNS (Block et al., 2007; Konsman et al., 1999; Lane, 2009). The vagal nerve terminates in the nucleus of solitary tract (NTS) located in the brainstem, which projects to other brain regions that mediate the acute sickness response (Konsman et al., 1999; Vitkovic et al., 2000). Secondly, in neurological diseases including PD, the BBB could lose its integrity, and peripheral immune cells such as CD4+/CD8+ T-lymphocytes and pro-inflammatory cytokines in the bloodstream could enter the brain and promote inflammation (Dantzer et al., 2008; Flores-Martinez et al., 2018; Hoban et al., 2013). Consistent with this concept, it has been shown using positron emission tomography that PD patients have an impaired BBB, and the regions shown to have impaired BBB overlapped with the regions evident to have neuronal damage in post-mortem brains of PD patients such as SN, VTA, locus coeruleus and raphe nucleus (Gonçalves et al., 2018; Kortekaas et al., 2005). Moreover, Gonçalves et al. (2018) demonstrated that the concentration of a tracing dye known as sodium fluorescein was increased in the striatum following systemic administration of LPS, indicating impaired BBB permeability. Lastly, inflammatory cytokines in the bloodstream can communicate with circumventricular organs (CVOs) in the CNS, which are a group of structures located around the third and fourth ventricles that lack the BBB, leading to activation of microglia and astrocytes in these regions (Perry, 2010). CVOs such as the median eminence and area postrema lack a tight barrier between the blood and cerebrospinal fluid (CSF); therefore, inflammatory molecules produced in these regions could access ventricular CSF and propagate to other regions of the brain via volume transmission (Vitkovic et al., 2000). Systemic administration of LPS leads to activation of microglia and astrocytes in the SN and striatum, and exacerbated inflammation in these brain regions can cause degeneration of dopaminergic neurons as proposed in Fig. 1 and Fig. 2 (Beier et al., 2017; Bodea et al., 2014; Liu et al., 2008; Qin et al., 2013; Reinert et al., 2014; Song et al., 2018).
Fig. 2.
Systemic administration of LPS via intraperitoneal cavity-the proposed pathways of propagation of systemic inflammation to the brain.
Systemic administration of LPS via intraperitoneal (i.p) cavity activates Toll-like receptor 4 (TLR4) on peritoneal macrophages/dendritic cells which results in local secretion of inflammatory cytokines such as IL-1, TNF-α and IL-6 (Block et al., 2007; Konsman et al., 1999). Systemic inflammation could be communicated to the brain through several mechanisms. Firstly, IL-1 produced by peritoneal macrophages/dendritic cells at the site of injection in response to systemic LPS, can activate IL-1 receptor on the vagus nerve that innervates the abdominal cavity. This nerve terminates in the nucleus of solitary tract located in the brainstem, which projects to other brain regions that mediate acute sickness response (Konsman et al., 2002; Vitkovic et al., 2000). Secondly, the blood brain barrier (BBB) could lose its integrity under pathological conditions, resulting in the entry of peripheral immune cells such as CD4+/CD8+ T-lymphocytes and inflammatory cytokines into the brain, and these molecules can mediate neuroinflammation through activation of microglia and astrocytes (Hoban et al., 2013). Furthermore, systemic cytokines can communicate with circumventricular organs (CVOs) in the CNS, which are a group of structures located around the third and fourth ventricles that lack BBB, which can also bring about the activation of microglia and astrocytes in these regions. Inflammatory molecules produced in the CVOs could access ventricular cerebrospinal fluid and propagate to other regions of the brain via volume transmission (Perry, 2010; Vitkovic et al., 2000). Exacerbated neuroinflammation in the substantia nigra (SN) could cause degeneration of dopaminergic neurons as outlined in Fig. 1. LV, lateral ventricle; 3V, third ventricle; 4V, fourth ventricle; ROS, reactive oxygen species.
2.3. Intranasal LPS models
2.3.1. Degeneration of nigrostriatal dopaminergic pathway, motor dysfunction and α-synuclein pathology in intranasal LPS models
According to He et al., 2016a, He et al., 2016b, unilateral intranasal administration of LPS (1 mg/ml, 10l) in C57BL/6 mice for a period of one month resulted in a 55% and 50% reduction in TH positive neurons in the SN and striatum respectively on the affected side. These authors also reported that α-synuclein protein expression in the SN was increased by 2.4-fold in the LPS group compared to saline controls. Similarly, a study by Li et al. (2015) with a similar regimen indicated that LPS instillation for one month reduced TH positive neurons by 38% and increased α-synuclein protein expression by 2.2-fold in the SN compared to saline controls. Both studies showed by using the open field test that LPS instillation impairs voluntary movement. In addition, Li et al. (2015) demonstrated by using the pole and adhesive removal tests, that LPS induces bradykinesia and sensorimotor impairment, respectively. Moreover, Zhao et al. (2018), used a bilateral instillation of LPS (1 mg/ml) which resulted in a 41.4% reduction in TH positive neurons in the SN of young mice, and was further exacerbated in aged mice (60% reduction). Like the other studies, Zhao and colleagues used the pole and adhesive removal tests to investigate impaired motor function in the LPS group, which was worse in aged mice (Zhao et al., 2018). These results demonstrated that aging is a risk factor for the development of PD and these results are consistent with the fact that PD is most prevalent in the aged population.
2.3.2. Possible mechanisms of toxicity and their relevance to clinical PD
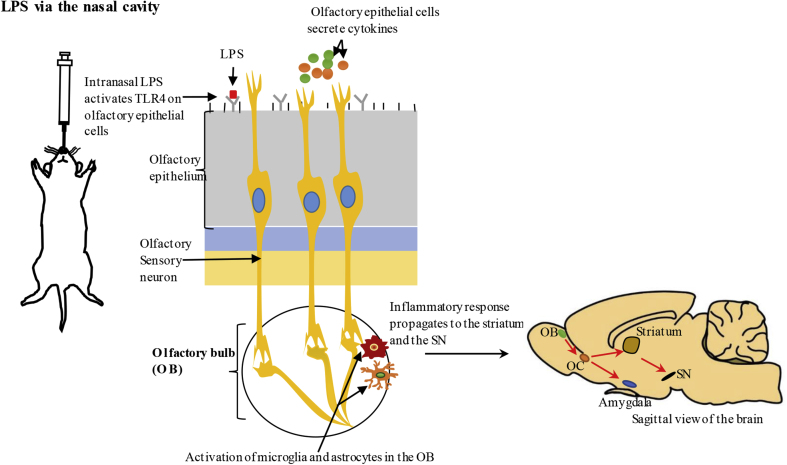
The olfactory vector hypothesis postulates that PD could be caused or catalysed by agents that use the olfactory system to gain access to the brain (He et al., 2013). Consistent with this hypothesis, the earliest signs of α-synuclein pathology in PD patients are seen in the olfactory bulb (OB), anterior olfactory nucleus and secondary olfactory structures (Doty, 2012; Hawkes et al., 2007). Also, olfactory dysfunction is one of the earliest non-motor symptoms of PD (Kranick and Duda, 2008; Masala et al., 2018). Therefore, understanding the relationship between the olfactory system and PD could be helpful in predicting the future development of PD which can aid in early diagnosis and treatment. Intranasal LPS models are suitable to understand the pathways to which environmental pathogens via the nasal cavity, could cause neurodegeneration. Intranasal models developed by chronic administration of LPS were only recently adopted, and our understanding of the pathological events is still limited. The olfactory epithelial cells express pattern recognition receptors, like TLR4, and these cells secrete inflammatory mediators in response to immune stimuli in the airway lumen. In addition, olfactory epithelial cells mediate recruitment of resident immune cells such as macrophages, dendritic cells and mucosal lymphocytes to the inflammatory site (Lane, 2009). It has been shown that intranasal LPS activates neutrophils, macrophages and T-lymphocytes in the olfactory mucosa (olfactory epithelium and lamina propria) resulting in the production of inflammatory cytokines (e.g. IL-1β) (Hasegawa-Ishii et al., 2017). The inflammatory response propagates to the OB which is part of the CNS, whereby microglia and astrocytes are activated (Hasegawa-Ishii et al., 2017; He et al., 2016b). Activated microglia and astrocytes in the OB could induce neurodegeneration as outlined in Fig. 1 and Fig. 3. The olfactory sensory neurons are located in the olfactory epithelium and they project directly to the neurons in the OB. The olfactory mucosa and OB pathway could act as a brain-immune interface possibly through cell to cell interaction between olfactory sensory neurons and neuronal dendrites in the OB or other inflammatory mediators. The neurons in the OB project to various regions of the brain (e.g. the olfactory cortex) and thus could propagate the inflammatory response (Doty, 2012). Intranasal administration of LPS (every second day for 1 month or more) induces inflammation and neurodegeneration in the SN, indicating propagation of intranasal inflammation to this region (He et al., 2013, 2016b; Li et al., 2015).
Fig. 3.
Intranasaladministration ofLPS – the proposed pathways of propagation of intranasal inflammation to the brain.
LPS administered via the nasal cavity can activate Toll-like receptor 4 (TLR4) expressed on olfactory epithelial cells, stimulating these cells to produce inflammatory cytokines. Olfactory epithelial cells can also recruit resident immune cells such as macrophages, dendritic cells and mucosal lymphocytes to the inflammatory site (Lane, 2009). Intranasal LPS can activate microglia and astrocytes in the olfactory bulb (OB) located in the CNS and the inflammatory response initiated by olfactory epithelial cells could propagate to the OB through cell to cell interaction between olfactory sensory neurons embedded in olfactory epithelium and neuronal dendrites in the OB (Hasegawa-Ishii et al., 2017; He et al., 2016b). The neurons in the OB project to various regions in the brain such as olfactory cortex (OC) which can propagate inflammation to the substantia nigra (SN) (Doty, 2012). Exacerbated neuroinflammation in the SN could cause degeneration of dopaminergic neurons as outlined in Fig. 1.
3. Extranigral neuropathology and non-motor deficits in LPS models of PD
Beside motor symptoms that define its diagnosis, PD is also associated with a plethora of non-motor symptoms such as olfactory dysfunction, gastrointestinal dysfunction, cardiovascular dysfunction, sleep disturbances and neuropsychiatric disturbances (Jankovic, 2008; Stoker and Greenland, 2018). Currently, it is difficult to treat non-motor symptoms of PD because the majority of the symptoms are resistant to dopamine replacement therapy and deep brain stimulation, which are the only treatments available for the management of PD (Obeso et al., 2010). Throughout the course of PD, α-synuclein pathology propagates to different regions of the CNS, peripheral nervous system and the ENS, and these changes could underlie some non-motor symptoms of PD (Halliday and McCann, 2010; Sulzer and Surmeier, 2013). Administration of LPS in animal models can induce some extranigral neuropathology and non-motor symptoms of PD and these will be discussed in further detail.
3.1. Gastrointestinal (GI) dysfunction
The entire GI tract is affected in patients with PD resulting in a plethora of complications such as drooling, dysphagia, delayed gastric emptying and constipation, which is the most common GI complication (Fasano et al., 2015). The mechanisms responsible for GI complications are not well understood; however, abnormal accumulation of α-synuclein is evident throughout the GI tract in patients with PD and could be involved in the pathogenesis (Fasano et al., 2015). Currently, there is a limited understanding of GI complications in LPS models of PD. A study by Zheng et al. (2013) indicated that a bilateral intranigral administration of LPS (16 μg) induced gastric dysmotility, measured with a strain gauge force transducer and this finding was consistent with human PD. Gastric dysmotility was related to an imbalance of neurotransmitters within the DMV with an increase in dopamine, which has inhibitory effects on muscle relaxation, and a decrease in acetylcholine, an excitatory neurotransmitter essential for smooth muscle contraction and peristalsis in the GI tract (Zheng et al., 2013b). Kelly and colleagues administered systemically a single dose of 2.5 mg/kg of LPS, which was below the threshold for neurodegeneration in the brain, to investigate the effects of LPS on the GI tract and they indicated that this dose induced hyperpermeability, especially in the large intestine at 4 months post-treatment, consistent with clinical findings that patients with PD have increased gut permeability (Forsyth et al., 2011; Kelly et al., 2014). In addition, Kelly et al. (2014) found increased phosphorylated serine 129 α-synuclein in colonic myentric neurons, which is the form that makes up the bulk of phosphorylated α-synuclein in Lewy bodies. It is yet to be investigated in LPS models and clinical PD whether the presence of abnormal α-synuclein is associated with neuronal loss in the ENS (Kelly et al., 2014). Future studies using LPS models of PD should examine α-synuclein pathology, inflammatory markers, oxidative stress markers and neurodegeneration in the ENS compared to nigrostriatal pathway, and correlate these pathological events with GI complications.
3.2. Olfactory dysfunction
Olfactory dysfunction is evident in approximately 90% of PD cases and it occurs prior to motor dysfunction (Doty, 2012). It has been shown that PD patients have impairments in odour threshold, odour identification and odour detection (Kranick and Duda, 2008; Masala et al., 2018). The cause of olfactory dysfunction is not clearly understood; however, Lewy bodies are detected in the OB and olfactory tract in almost all PD subjects and could have a role in the pathogenesis (Rey et al., 2018). According to Hasegawa-Ishii et al. (2017) unilateral administration of LPS (1 mg/ml, 3 times per a week) into the nasal cavity in C57BL/6 mice for 21 days induced inflammation in olfactory mucosa. A follow- up study by this group noted that although a subpopulation of neurons in the OB can regenerate in adulthood (Hasegawa-Ishii et al., 2018), as seen by recovery of the OB atrophy following cessation of LPS treatment, olfactory sensory neurons did not regenerate completely and could underlie progression of pathology. Studies by He and colleagues demonstrated that intranasal LPS can also increase the expression of α-synuclein protein (1.75 and 2.75-fold) and decrease expression of TH protein (44%) in the OB (He et al., 2013, 2016b). Collectively, intranasal LPS models of PD showed neuropathology in the OB; however, these studies did not perform any olfactory tests like the buried food test or olfactory habituation/cross habituation test, and it is yet to be determined whether these changes are associated with defects in olfaction that precede motor deficits. Future studies could use intranasal LPS models to thoroughly understand the relationship between direct damage to the olfactory system and PD. In addition, stereotaxic and systemic models of PD that do not directly damage the components of the olfactory systems could be helpful to elucidate the indirect mechanisms that could contribute to olfactory dysfunction.
3.3. Anxiety and depression
Anxiety and depression are highly prevalent in PD patients compared to the general population, with a rate of 20–49% and 40–50% respectively (Gallagher and Schrag, 2012; Jankovic, 2008; Stoker and Greenland, 2018). It is difficult to distinguish anxiety and depression but unlike depression, the major feature of anxiety is excessive fear, usually associated with particular situations or threats (Djamshidian and Friedman, 2014). In contrast, depression in PD is characterised by irritability, pessimism, sadness, dysphoria and suicidal ideation (Fontoura et al., 2017). The pathophysiology of anxiety and depression in PD is not thoroughly understood; however, cumulative evidence indicates that these disorders could be associated with lesions in dopaminergic, serotonergic and noradrenergic systems (Gallagher and Schrag, 2012; Thobois et al., 2017). Indeed, according to Braak hypothesis, deposition of Lewy bodies occurs in key brainstem structures like the serotonergic raphe nuclei and noradrenergic locus coeruleus, which have been implicated in mood and emotional behaviour, before the SN of PD patients (Gratwicke et al., 2015; Halliday and McCann, 2010). Indeed, Song et al. (2018) found that a single systemic administration of LPS (5 mg/kg) in mice induced progressive degeneration in noradrenergic neurons in the locus coeruleus as early as at 4 months (27% reduction) prior to loss of dopaminergic neurons in SN at 7 months (34% reduction). Some LPS PD studies have directly examined anxiety and depressive-like behaviour with intranigral administration of LPS (3 μg/kg and 10 μg/kg) and were associated with increased anxiety-like behaviour as signified by a significant reduction in the time spent in the open arm in the elevated plus maze test and increased depressive-like behaviour with increased immobility time in the forced swim test (Hritcu and Ciobica, 2013). Anxiety and depressive-like behaviour were linked to a reduction in the brain derived neurotrophic factor (BDNF) which is crucial for neuronal growth, survival and maintenance as well as synaptic plasticity and memory processes (Hritcu and Ciobica, 2013; Obeso et al., 2010).
3.4. Cognitive dysfunction
Subtle disturbances in planning, working memory and attention are observed in the early stages of PD; however, in the later stages of the disease, cognitive impairments become more severe and can progress to dementia, which affects up to 90% of PD patients in the long-term (Gratwicke et al., 2015; Pagonabarraga and Kulisevsky, 2012). The cause of dementia in PD is not well understood; however, it could be associated with dysfunction in the dopaminergic, noradrenergic and cholinergic systems (Gratwicke et al., 2015; Pagonabarraga and Kulisevsky, 2012). Degeneration of nigrostriatal pathway induced by unilateral intranigral administration of LPS (3μg/kg and 10 μg/kg) impaired working memory as seen by decreased percentage alternation in the Y-Maze test and increased errors in the radial arm maze test, as well as long-term memory indicated by an increased number of reference memory errors in the radial arm maze test. This may occur becuase the dentate gyrus of the hippocampus, a region essential for memory formation, storage and retrieval, receives neuronal projects from the SN. Indeed, intranigral LPS decreased the levels of superoxide dismutase and glutathione peroxidase and increased the levels of malondialdehyde, a marker of lipid peroxidation in the hippocampus. These findings indicated that reduced antioxidant capacity and increased ROS in the hippocampus could lead to cognitive dysfunction (Hritcu and Ciobica, 2013). Similar findings have been reported with systemic administration of LPS (250 μg/kg daily for 7 days) which reduced TH positive cells in the SN (Badshah et al., 2016; Khan et al., 2018; Zhang and Xu, 2018) and caused cognitive impairment indicated by increased latency time and reduced time in the target quadrant in the Morris water maze test. This was related to a decrease in synaptic proteins such as synaptophysin, postsynaptic density protein 95 (PSD-95) and synaptosomal associated protein of 25 kDA (SNAP-25). A single, systemic administration of LPS (5 mg/kg) could also induce neuronal loss in the hippocampus (30% reduction) at 10 months post-treatment and this finding coincided with cognitive impairment (Song et al., 2018). Future studies could focus on understanding the neuropathology in the hippocampus and specific cortical regions that are essential for cognition.
4. Discussion
LPS models of PD allow modelling of specific aspects of clinical PD pathogenesis; however, each route of administration of LPS has specific advantages and disadvantages.
Firstly, stereotaxic LPS which is commonly delivered into the SN and striatum, is useful to study the specific effects of inflammation on dopaminergic neurons in the SN and their neuronal projections to the striatum, with these models showing selective degeneration of dopaminergic neurons in the SN and their neuronal projections to the striatum. This is accompanied by an increase in cytoplasmic α-synuclein in the SN, and motor abnormalities which improve with levodopa administration which are evident in clinical PD. It should be noted however, that due to the small size of the SN and dense distribution of dopaminergic neurons, intranigral injection of LPS itself can cause mechanical injury to the neurons; thus, this site of administration may not be optimal. On the other hand, the striatum is larger when compared to the SN which limits the effects of mechanical injury and in clinical PD, degeneration of dopaminergic neurons starts with these neuronal terminals (Hunter et al., 2007). Therefore, intrastriatal injection of LPS could be a more suitable model compared to intranigral injection. The advantage of stereotaxic models is that neurodegeneration occurs in a progressive manner causing 21% loss of TH positive neurons at one week, 38% loss at two weeks, and 41% at four weeks in rats (Choi et al., 2009) and from 26% at one week, 72% at four weeks, and 81% at twelve weeks in mice (Hunter et al., 2009). However, the progression of changes in these models appears to be relatively rapid, which can be considered both advantageous (for screening novel therapeutics) and disadvantageous (for investigating PD pathogenesis); thus, researchers should consider carefully if this model is suitable to answer their research question. The major setback of stereotaxic injections is that the complexity of the required surgery could be a deterrent for many laboratories. Also, this route is not representative of environmental exposure because it bypasses the physical defence system of the animal.
Secondly, the systemic LPS model of PD could be a better representation of clinical disease because systemic LPS does not bypass the physical defence of the brain and could mimic environmental exposure to toxic agents. Unlike stereotaxic injection, systemic administration of LPS via i.p injection is not complex and thus easy to implement for researchers, with no specialised equipment required. It is important to note that many effects of the LPS when injected systemically may not be PD specific acutely, with the acute phase response including sickness behaviour such as depression, disturbed olfaction, and reduced locomotion which resemble PD symptoms. However, the most frequently used systemic LPS models investigate PD pathology and symptoms long after the acute phase response subsided and can reproduce PD specific pathology and behavioural abnormalities. For example, a single high dose of LPS (5 mg/kg) in mice causes progressive degeneration of dopaminergic neurons in the SN 7 months post-treatment, and it does not induce a significant reduction in GABAergic neurons in the SN and dopaminergic neurons in VTA, indicating selectivity for neurons in the SN (Beier et al., 2017; Liu et al., 2008; Qin et al., 2007). The major disadvantage with single systemic injection in mice is that it takes a long time to induce degeneration of dopaminergic neurons in the SN (approximately 7 months) (Liu et al., 2008; Qin et al., 2007, 2013). Furthermore this pathology could not be replicated in Wistar rats (Gasparotto et al., 2018). Daily systemic injections (for 4 days) in mice were able to reduce the time taken to induce degeneration of dopaminergic neurons in the SN, but this model did not cause progressive neurodegeneration and the animals tended to recover (Beier et al., 2017; Bodea et al., 2014). Further investigation is needed to determine an optimal LPS regimen, and this may also be species specific. Other limitations of the systemic LPS models are that systemic LPS can cause severe toxicity and death (high dose LPS from E. coli 0111: B4, 1 mg/kg for 4 consecutive days) based on own observations. In contrast, repeated administration of a lower dose (LPS from E. coli 0111: B4, 0.3 mg/kg for 4 consecutive days) caused less toxicity but did not cause significant degeneration of dopaminergic neurons in the midbrain or any motor deficits (unpublished observations). Moreover, different batches and manufacturers of LPS can produce significantly different results, which makes it difficult to compare published results. Therefore, for any new study we recommend that researchers should conduct a small pilot study to establish the sub-lethal dose of the LPS stock.
Thirdly, environmental toxins capable of inducing degeneration in the SN can enter the brain through the nose, bypassing the BBB. Thus, intranasal LPS models are useful to understand the pathways by which these substances can initiate neurodegeneration. It is important to note that inflammation induced via intranasal LPS is not localised only to the SN, but this model resembles certain aspects specific to PD such as degeneration of dopaminergic neurons and increased expression of α-synuclein protein in the SN and motor dysfunction which need to be elucidated further. There has been limited attention towards characterisation of intranasal models of LPS and it is yet to be clarified whether this route of administration can induce progressive degeneration of dopaminergic neurons in the SN.
It is also fundamental that the neuropathology observed in LPS models of PD is congruent with the behaviour. It has been shown in stereotaxic and systemic LPS models that the lesion to the nigrostriatal pathway is reflected in the performance in the drug induced rotational test and rotarod test which are common motor tests used for such purpose. In these models, the severity of the nigrostriatal lesion correlated with poor performance in rotarod test, which improved upon administration of levodopa (Choi et al., 2009; Hunter et al., 2009; Liu et al., 2008). These results are consistent with clinical PD; thus, solidifying the relevance of pre-clinical behavioural data to clinical PD. In addition, it has been demonstrated using the elevated plus maze, forced swim test and Morris water maze test that specific LPS regimens in both the stereotaxic and systemic LPS models can induce anxiety, depression and cognitive decline (Badshah et al., 2016; Hritcu and Gorgan, 2014; Khan et al., 2018; Zhang and Xu, 2018). Moreover, these models can replicate the gastric dysmotility and gut hyperpermeability which are seen in clinical PD (Forsyth et al., 2011; Kelly et al., 2014; Zheng et al., 2013b). There is less attention towards non-motor symptoms in LPS and other animal models of PD and considering that the onset of non-motor symptoms precedes the onset of motor symptoms, it is important to understand non-motor aspects of PD which could aid in early diagnosis.
One of the useful applications of the LPS PD models is to assess the potential beneficial effects of anti-inflammatory agents, and this has been carried out particularly in stereotaxic LPS models. For example, treatment with minocycline inhibits the activation of microglia reducing the expression of cytokines including IL-1 and TNF-α within the SN leading to a reduction in dopaminergic neuronal loss (Tomás-Camardiel et al., 2004). Similarly, chronic simvastatin treatment reduces astroglial and microglial activation, decreasing pro-inflammatory cytokine release and hence dopaminergic degeneration (Hernández-Romero et al., 2008; Wang et al., 2015c). Evaluation of the TRPV1 agonist, capsaicin found that injection simultaneously with LPS led to a shift in the pro-inflammatory M1 microglia/macrophage population to an anti-inflammatory M2 state as demonstrated by decreased expression of M1 markers (i.e., iNOS and IL-6) and elevated expression of M2 markers (i.e., arginase 1 and CD206) in the SN (Bok et al., 2018). Given that TRPV1 activation promotes the neurogenic inflammatory response via release of substance P and calcitonin gene related peptide (Bok et al., 2018), it suggests that activation of this pathway can modify the classical inflammation initiated by LPS and potentially provide a novel treatment. The efficacy of a number of nutraceuticals with anti-inflammatory properties has also been studied in the intranigral LPS PD model. These include the flavonoids farrerol (obtained from rhododendron) (Li et al., 2019), curcumin (from turmeric) (Sharma and Nehru, 2018), licochalcone A (from licorice) (Huang et al., 2017) and polydatin (from Polygonum cuspidatum) (Huang et al., 2018), which were all able to reduce LPS-induced inflammation within the SN and prevent dopaminergic cell loss. This corresponded to improved motor performance in tasks including locomotion in the open field (Huang et al., 2018) and apomorphine induced turns (Huang et al., 2017). It should be noted that the aforementioned studies all included a pre-treatment phase prior to LPS injection and hence the efficacy once neuroinflammation has already been initiated is not yet known.
In conclusion, stereotaxic, systemic and intranasal LPS models can replicate degeneration of dopaminergic neurons and increased expression of α-synuclein protein in the SN with accompanying motor dysfunction which are the classical features of human PD. Although LPS models can recapitulate some of the classical features of PD, the models are still new and require further examination of the neuropathology. Also, future studies using LPS models should focus on assessing motor symptoms and non-motor symptoms of PD such as olfactory dysfunction, gastrointestinal dysfunction, anxiety, depression and cognitive dysfunction. These symptoms should be correlated with degeneration of dopaminergic neurons, abnormal α-synuclein expression and inflammatory mediators in the SN as well as in other regions implicated in PD. Understanding the pathological changes in the central and peripheral nervous system associated with specific symptoms of PD, could aid in developing specific treatments. We hope that this review will encourage further characterisation of the LPS models and positively contribute to PD research.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the China-Australia Centre for Health Research (University of South Australia, Australia and Shandong University, China). Isaac Deng is a recipient of University of South Australia Postgraduate Award (USAPA), Australia.
References
- Hernández-Romero M.d.C., Argüelles S., Villarán R.F., De Pablos R.M., Delgado-Cortés M.J., Santiago M., Herrera A.J., Cano J., Machado A. Simvastatin prevents the inflammatory process and the dopaminergic degeneration induced by the intranigral injection of lipopolysaccharide. J. Neurochem. 2008;105:445–459. doi: 10.1111/j.1471-4159.2007.05148.x. [DOI] [PubMed] [Google Scholar]
- Arimoto T., Choi D.-Y., Lu X., Liu M., Nguyen X.V., Zheng N., Stewart C.A., Kim H.-C., Bing G. Interleukin-10 protects against inflammation-mediated degeneration of dopaminergic neurons in substantia nigra. Neurobiol. Aging. 2007;28:894–906. doi: 10.1016/j.neurobiolaging.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Badshah H., Ali T., Rehman S.-u., Amin F.-u., Ullah F., Kim T.H., Kim M.O. Protective effect of lupeol against lipopolysaccharide-induced neuroinflammation via the p38/c-Jun N-terminal kinase pathway in the adult mouse brain. J. Neuroimmune Pharmacol. 2016;11:48–60. doi: 10.1007/s11481-015-9623-z. [DOI] [PubMed] [Google Scholar]
- Bao L.-H., Zhang Y.-N., Zhang J.-N., Gu L., Yang H.-M., Huang Y.-Y., Xia N., Zhang H. Urate inhibits microglia activation to protect neurons in an LPS-induced model of Parkinson’s disease. J. Neuroinflammation. 2018;15:131. doi: 10.1186/s12974-018-1175-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardou I., Kaercher R.M., Brothers H.M., Hopp S.C., Royer S., Wenk G.L. Age and duration of inflammatory environment differentially affect the neuroimmune response and catecholaminergic neurons in the midbrain and brainstem. Neurobiol. Aging. 2014;35:1065–1073. doi: 10.1016/j.neurobiolaging.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista C.R.A., Gomes G.F., Candelario-Jalil E., Fiebich B.L., de Oliveira A.C.P. Lipopolysaccharide-induced neuroinflammation as a bridge to understand neurodegeneration. Int. J. Mol. Sci. 2019;20:2293. doi: 10.3390/ijms20092293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier E.E., Neal M., Alam G., Edler M., Wu L.-J., Richardson J.R. Alternative microglial activation is associated with cessation of progressive dopamine neuron loss in mice systemically administered lipopolysaccharide. Neurobiol. Dis. 2017;108:115–127. doi: 10.1016/j.nbd.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing G., Lu X., Zheng N., Jin L., Floyd R., Kim H. Pergamon-Elsevier Science Ltd The Boulevard; Langford Lane, Kidlington: 1998. Microglia Mediated Dopaminergic Cell Death in the Substantia Nigra: A New Animal Model for Parkinson’s Disease. Free Radical Biology and Medicine. S44-S44. [Google Scholar]
- Block M.L., Zecca L., Hong J.-S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007;8:57. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Boche D., Perry V., Nicoll J. Activation patterns of microglia and their identification in the human brain. Neuropathol. Appl. Neurobiol. 2013;39:3–18. doi: 10.1111/nan.12011. [DOI] [PubMed] [Google Scholar]
- Bodea L.-G., Wang Y., Linnartz-Gerlach B., Kopatz J., Sinkkonen L., Musgrove R., Kaoma T., Muller A., Vallar L., Di Monte D.A., Balling R., Neumann H. Neurodegeneration by activation of the microglial complement–phagosome pathway. J. Neurosci. 2014;34:8546–8556. doi: 10.1523/jneurosci.5002-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok E., Chung Y.C., Kim K.-S., Baik H.H., Shin W.-H., Jin B.K. Modulation of M1/M2 polarization by capsaicin contributes to the survival of dopaminergic neurons in the lipopolysaccharide-lesioned substantia nigra in vivo. Exp. Mol. Med. 2018;50:1–14. doi: 10.1038/s12276-018-0111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G.C. The endotoxin hypothesis of neurodegeneration. J. Neuroinflammation. 2019;16:180. doi: 10.1186/s12974-019-1564-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castano A., Herrera A., Cano J., Machado A. Lipopolysaccharide intranigral injection induces inflammatory reaction and damage in nigrostriatal dopaminergic system. J. Neurochem. 1998;70:1584–1592. doi: 10.1046/j.1471-4159.1998.70041584.x. [DOI] [PubMed] [Google Scholar]
- Chen Y., Jiang M., Li L., Ye M., Yu M., Zhang L., Wei D. DL‑3‑n‑butylphthalide reduces microglial activation in lipopolysaccharide‑induced Parkinson’s disease model mice. Mol. Med. Rep. 2018;17:3884–3890. doi: 10.3892/mmr.2017.8332. [DOI] [PubMed] [Google Scholar]
- Chen G., Liu J., Jiang L., Ran X., He D., Li Y., Huang B., Wang W., Fu S. Galangin reduces the loss of dopaminergic neurons in an LPS-evoked model of Parkinson’s disease in rats. Int. J. Mol. Sci. 2017;19:12. doi: 10.3390/ijms19010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis T., Weiner H.L. CNS inflammation and neurodegeneration. J. Clin. Invest. 2017;127:3577–3587. doi: 10.1172/JCI90609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D.-Y., Liu M., Hunter R.L., Cass W.A., Pandya J.D., Sullivan P.G., Shin E.-J., Kim H.-C., Gash D.M., Bing G. Striatal neuroinflammation promotes parkinsonism in rats. PloS One. 2009;4 doi: 10.1371/journal.pone.0005482. e5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E.S., Chung Y.C., Bok E., Baik H.H., Park E.S., Park J.-Y., Yoon S.-H., Jin B.K. Fluoxetine prevents LPS-induced degeneration of nigral dopaminergic neurons by inhibiting microglia-mediated oxidative stress. Brain Res. 2010;1363:143–150. doi: 10.1016/j.brainres.2010.09.049. [DOI] [PubMed] [Google Scholar]
- Chung E.S., Bok E., Chung Y.C., Baik H.H., Jin B.K. Cannabinoids prevent lipopolysaccharide-induced neurodegeneration in the rat substantia nigra in vivo through inhibition of microglial activation and NADPH oxidase. Brain Res. 2012;1451:110–116. doi: 10.1016/j.brainres.2012.02.058. [DOI] [PubMed] [Google Scholar]
- Concannon R.M., Okine B.N., Finn D.P., Dowd E. Upregulation of the cannabinoid CB2 receptor in environmental and viral inflammation-driven rat models of Parkinson’s disease. Exp. Neurol. 2016;283:204–212. doi: 10.1016/j.expneurol.2016.06.014. [DOI] [PubMed] [Google Scholar]
- Dantzer R., O’Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9:46. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson T.M., Dawson V.L. Molecular pathways of neurodegeneration in Parkinson’s disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- de Lau L.M.L., Breteler M.M.B. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5:525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- Deleidi M., Hallett P.J., Koprich J.B., Chung C.-Y., Isacson O. The Toll-like receptor-3 agonist polyinosinic: polycytidylic acid triggers nigrostriatal dopaminergic degeneration. J. Neurosci. 2010;30:16091–16101. doi: 10.1523/JNEUROSCI.2400-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djamshidian A., Friedman J.H. Anxiety and depression in Parkinson’s disease. Curr. Treat. Options Neurol. 2014;16:285. doi: 10.1007/s11940-014-0285-6. [DOI] [PubMed] [Google Scholar]
- Dobbs R., Charlett A., Purkiss A., Dobbs S., Weller C., Peterson D. Association of circulating TNF-α and IL-6 with ageing and parkinsonism. Acta Neurol. Scand. 1999;100:34–41. doi: 10.1111/j.1600-0404.1999.tb00721.x. [DOI] [PubMed] [Google Scholar]
- Doty R.L. Olfactory dysfunction in Parkinson disease. Nat. Rev. Neurol. 2012;8:329. doi: 10.1038/nrneurol.2012.80. [DOI] [PubMed] [Google Scholar]
- Duty S., Jenner P. Animal models of Parkinson’s disease: a source of novel treatments and clues to the cause of the disease. Br. J. Pharmacol. 2011;164:1357–1391. doi: 10.1111/j.1476-5381.2011.01426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmquist J.K., Scammell h.E., Saper C.B. Mechanisms of CNS response to systemic immune challenge: the febrile response. Trends Neurosci. 1997;20:565–570. doi: 10.1016/S0166-2236(97)01138-7. [DOI] [PubMed] [Google Scholar]
- Emamzadeh F.N. Alpha-synuclein structure, functions, and interactions. J. Res. Med. Sci. : Off. J. Isfahan Univ. Med. Sci. 2016;21:29. doi: 10.4103/1735-1995.181989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A., Visanji N.P., Liu L.W., Lang A.E., Pfeiffer R.F. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2015;14:625–639. doi: 10.1016/S1474-4422(15)00007-1. [DOI] [PubMed] [Google Scholar]
- Ferreira Mello B.S., Monte A.S., McIntyre R.S., Soczynska J.K., Custódio C.S., Cordeiro R.C., Chaves J.H., Mendes Vasconcelos S.M., Nobre Júnior H.V., Florenço de Sousa F.C., Hyphantis T.N., Carvalho A.F., Macêdo D.S. Effects of doxycycline on depressive-like behavior in mice after lipopolysaccharide (LPS) administration. J. Psychiatr. Res. 2013;47:1521–1529. doi: 10.1016/j.jpsychires.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Flores-Martinez Y.M., Fernandez-Parrilla M.A., Ayala-Davila J., Reyes-Corona D., Blanco-Alvarez V.M., Soto-Rojas L.O., Luna-Herrera C., Gonzalez-Barrios J.A., Leon-Chavez B.A., Gutierrez-Castillo M.E., Martínez-Dávila I.A., Martinez-Fong D. Acute neuroinflammatory response in the substantia nigra pars compacta of rats after a local injection of lipopolysaccharide. J. Immunol. Res. 2018;2018:1838921. doi: 10.1155/2018/1838921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontoura J.L., Baptista C., Pedroso F.d.B., Pochapski J.A., Miyoshi E., Ferro M.M. Depression in Parkinson’s disease: the contribution from animal studies. Parkinson’s Dis. 2017;2017 doi: 10.1155/2017/9124160. 9124160-9124160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth C.B., Shannon K.M., Kordower J.H., Voigt R.M., Shaikh M., Jaglin J.A., Estes J.D., Dodiya H.B., Keshavarzian A. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PloS One. 2011;6 doi: 10.1371/journal.pone.0028032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S.-P., Wang J.-F., Xue W.-J., Liu H.-M., Liu B.-r., Zeng Y.-L., Li S.-N., Huang B.-X., Lv Q.-K., Wang W., Liu J.-X. Anti-inflammatory effects of BHBA in both in vivo and in vitro Parkinson’s disease models are mediated by GPR109A-dependent mechanisms. J. Neuroinflammation. 2015;12:9. doi: 10.1186/s12974-014-0230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher D.A., Schrag A. Psychosis, apathy, depression and anxiety in Parkinson’s disease. Neurobiol. Dis. 2012;46:581–589. doi: 10.1016/j.nbd.2011.12.041. [DOI] [PubMed] [Google Scholar]
- Gao H.M., Jiang J., Wilson B., Zhang W., Hong J.S., Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson’s disease. J. Neurochem. 2002;81:1285–1297. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- Gao H.M., Kotzbauer P.T., Uryu K., Leight S., Trojanowski J.Q., Lee V. M.-Y. Neuroinflammation and oxidation/nitration of α-synuclein linked to dopaminergic neurodegeneration. J. Neurosci. 2008;28:7687–7698. doi: 10.1523/Jneurosci.0143-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H.-M., Zhang F., Zhou H., Kam W., Wilson B., Hong J.-S. Neuroinflammation and α-synuclein dysfunction potentiate each other, driving chronic progression of neurodegeneration in a mouse model of Parkinson’s disease. Environ. Health Perspect. 2011;119:807–814. doi: 10.1289/ehp.1003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparotto J., Ribeiro C.T., da Rosa-Silva H.T., Bortolin R.C., Rabelo T.K., Peixoto D.O., Moreira J.C.F., Gelain D.P. Systemic inflammation changes the site of RAGE expression from endothelial cells to neurons in different brain areas. Mol. Neurobiol. 2018 doi: 10.1007/s12035-018-1291-6. [DOI] [PubMed] [Google Scholar]
- Gonçalves C., dos Santos D.B., Portilho S.S., Lopes M.W., Ghizoni H., de Souza V., Mack J.M., Naime A.A., Dafre A.L., de Souza Brocardo P., Prediger R.D., Farina M.J.N.R. vol. 43. 2018. pp. 745–759. (Lipopolysaccharide-Induced Striatal Nitrosative Stress and Impaired Social Recognition Memory Are Not Magnified by Paraquat Coexposure). [DOI] [PubMed] [Google Scholar]
- Gratwicke J., Jahanshahi M., Foltynie T. Parkinson’s disease dementia: a neural networks perspective. Brain. 2015;138:1454–1476. doi: 10.1093/brain/awv104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday G.M., McCann H. The progression of pathology in Parkinson’s disease. Ann. N. Y. Acad. Sci. 2010;1184:188–195. doi: 10.1111/j.1749-6632.2009.05118.x. [DOI] [PubMed] [Google Scholar]
- Hasegawa-Ishii S., Shimada A., Imamura F. Lipopolysaccharide-initiated persistent rhinitis causes gliosis and synaptic loss in the olfactory bulb. Sci. Rep. 2017;7:11605. doi: 10.1038/s41598-017-10229-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa-Ishii S., Shimada A., Imamura F. Neuroplastic changes in the olfactory bulb associated with nasal inflammation in mice. J. Allergy Clin. Immunol. 2018 doi: 10.1016/j.jaci.2018.09.028. [DOI] [PubMed] [Google Scholar]
- Hauser D.N., Hastings T.G. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease and monogenic parkinsonism. Neurobiol. Dis. 2013;51:35–42. doi: 10.1016/j.nbd.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes C.H., Del Tredici K., Braak H. Parkinson’s disease: a dual-hit hypothesis. Neuropathol. Appl. Neurobiol. 2007;33:599–614. doi: 10.1111/j.1365-2990.2007.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q., Yu W., Wu J., Chen C., Lou Z., Zhang Q., Zhao J., Wang J., Xiao B. Intranasal LPS-mediated Parkinson’s model challenges the pathogenesis of nasal cavity and environmental toxins. PloS One. 2013;8 doi: 10.1371/journal.pone.0078418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H., Guo W.-W., Xu R.-R., Chen X.-Q., Zhang N., Wu X., Wang X.-M. Alkaloids from piper longum protect dopaminergic neurons against inflammation-mediated damage induced by intranigral injection of lipopolysaccharide. BMC Compl. Alternative Med. 2016;16:412. doi: 10.1186/s12906-016-1392-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q., Li Y.h., Guo S.s., Wang Y., Lin W., Zhang Q., Wang J., Ma C.g., Xiao B.G. Inhibition of Rho-kinase by Fasudil protects dopamine neurons and attenuates inflammatory response in an intranasal lipopolysaccharide-mediated Parkinson’s model. Eur. J. Neurosci. 2016;43:41–52. doi: 10.1111/ejn.13132. [DOI] [PubMed] [Google Scholar]
- Hirsch E.C., Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- Hoban D.B., Connaughton E., Connaughton C., Hogan G., Thornton C., Mulcahy P., Moloney T.C., Dowd E. Further characterisation of the LPS model of Parkinson’s disease: a comparison of intra-nigral and intra-striatal lipopolysaccharide administration on motor function, microgliosis and nigrostriatal neurodegeneration in the rat. Brain Behav. Immun. 2013;27:91–100. doi: 10.1016/j.bbi.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Holmqvist S., Chutna O., Bousset L., Aldrin-Kirk P., Li W., Björklund T., Wang Z.-Y., Roybon L., Melki R., Li J.-Y. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128:805–820. doi: 10.1007/s00401-014-1343-6. [DOI] [PubMed] [Google Scholar]
- Hritcu L., Ciobica A. Intranigral lipopolysaccharide administration induced behavioral deficits and oxidative stress damage in laboratory rats: relevance for Parkinson’s disease. Behav. Brain Res. 2013;253:25–31. doi: 10.1016/j.bbr.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Hritcu L., Gorgan L.D. Intranigral lipopolysaccharide induced anxiety and depression by altered BDNF mRNA expression in rat hippocampus. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2014;51:126–132. doi: 10.1016/j.pnpbp.2014.01.016. [DOI] [PubMed] [Google Scholar]
- Huang B., Liu J., Ju C., Yang D., Chen G., Xu S., Zeng Y., Yan X., Wang W., Liu D., Fu S. Licochalcone A prevents the loss of dopaminergic neurons by inhibiting microglial activation in lipopolysaccharide (LPS)-Induced Parkinson’s disease models. Int. J. Mol. Sci. 2017;18:2043. doi: 10.3390/ijms18102043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B., Liu J., Meng T., Li Y., He D., Ran X., Chen G., Guo W., Kan X., Fu S., Wang W., Liu D. Polydatin prevents lipopolysaccharide (LPS)-Induced Parkinson’s disease via regulation of the AKT/GSK3β-Nrf2/NF-κB signaling axis. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.02527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter R.L., Dragicevic N., Seifert K., Choi D.Y., Liu M., Kim H.C., Cass W.A., Sullivan P.G., Bing G. Inflammation induces mitochondrial dysfunction and dopaminergic neurodegeneration in the nigrostriatal system. J. Neurochem. 2007;100:1375–1386. doi: 10.1111/j.1471-4159.2006.04327.x. [DOI] [PubMed] [Google Scholar]
- Hunter R.L., Cheng B., Choi D.-Y., Liu M., Liu S., Cass W.A., Bing G. Intrastriatal lipopolysaccharide injection induces parkinsonism in C57/B6 mice. J. Neurosci. Res. 2009;87:1913–1921. doi: 10.1002/jnr.22012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter R., Ojha U., Bhurtel S., Bing G., Choi D.-Y. Lipopolysaccharide-induced functional and structural injury of the mitochondria in the nigrostriatal pathway. Neurosci. Res. 2017;114:62–69. doi: 10.1016/j.neures.2016.09.007. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Parkinson’s disease: clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatr. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- Jiang M.J., Chen Y.H., Li L., Xu L., Liu H., Qu X.L., Xu J.J., Ge B.B., Qu H.D. Protective effects of DL-3-n-butylphthalide in the lipopolysaccharide-induced mouse model of Parkinson’s disease. Mol. Med. Rep. 2017;16:6184–6189. doi: 10.3892/mmr.2017.7352. [DOI] [PubMed] [Google Scholar]
- Kelly L.P., Carvey P.M., Keshavarzian A., Shannon K.M., Shaikh M., Bakay R.A.E., Kordower J.H. Progression of intestinal permeability changes and alpha-synuclein expression in a mouse model of Parkinson’s disease. Mov. Disord. : Off. J. Mov. Disord. Soc. 2014;29:999–1009. doi: 10.1002/mds.25736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.S., Ali T., Kim M.W., Jo M.H., Chung J.I., Kim M.O. Anthocyanins improve hippocampus-dependent memory function and prevent neurodegeneration via JNK/Akt/GSK3β signaling in LPS-treated adult mice. Mol. Neurobiol. 2018 doi: 10.1007/s12035-018-1101-1. [DOI] [PubMed] [Google Scholar]
- Kim W.-G., Mohney R.P., Wilson B., Jeohn G.-H., Liu B., Hong J.-S. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. J. Neurosci. 2000;20:6309–6316. doi: 10.1523/jneurosci.20-16-06309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konsman J.P., Kelley K., Dantzer R. Temporal and spatial relationships between lipopolysaccharide-induced expression of fos, interleukin-1 β and inducible nitric oxide synthase in rat brain. Neuroscience. 1999;89:535–548. doi: 10.1016/S0306-4522(98)00368-6. [DOI] [PubMed] [Google Scholar]
- Konsman J.P., Parnet P., Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25:154–159. doi: 10.1016/S0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- Kortekaas R., Leenders K.L., van Oostrom J.C., Vaalburg W., Bart J., Willemsen A.T., Hendrikse N.H. Blood–brain barrier dysfunction in parkinsonian midbrain in vivo. Ann. Neurol. 2005;57:176–179. doi: 10.1002/ana.20369. [DOI] [PubMed] [Google Scholar]
- Kranick S.M., Duda J.E. Olfactory dysfunction in Parkinson’s disease. Neurosignals. 2008;16:35–40. doi: 10.1159/000109757. [DOI] [PubMed] [Google Scholar]
- Kuter K., Olech Ł., Głowacka U. Prolonged dysfunction of astrocytes and activation of microglia accelerate degeneration of dopaminergic neurons in the rat substantia nigra and block compensation of early motor dysfunction induced by 6-OHDA. Mol. Neurobiol. 2018;55:3049–3066. doi: 10.1007/s12035-017-0529-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane A.P. The role of innate immunity in the pathogenesis of chronic rhinosinusitis. Curr. Allergy Asthma Rep. 2009;9:205–212. doi: 10.1007/s11882-009-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.-H., He Q., Yu J.-z., Liu C.-y., Feng L., Chai Z., Wang Q., Zhang H.-z., Zhang G.-X., Xiao B.-g., Ma C.-g. Lipoic acid protects dopaminergic neurons in LPS-induced Parkinson’s disease model. Metab. Brain Dis. 2015;30:1217–1226. doi: 10.1007/s11011-015-9698-5. [DOI] [PubMed] [Google Scholar]
- Li Y., Zeng Y., Meng T., Gao X., Huang B., He D., Ran X., Du J., Zhang Y., Fu S., Hu G. Farrerol protects dopaminergic neurons in a rat model of lipopolysaccharide-induced Parkinson’s disease by suppressing the activation of the AKT and NF-κB signaling pathways. Int. Immunopharm. 2019;75:105739. doi: 10.1016/j.intimp.2019.105739. [DOI] [PubMed] [Google Scholar]
- Liu M., Bing G. Lipopolysaccharide animal models for Parkinson’s disease. Parkinson’s Dis. 2011;2011 doi: 10.4061/2011/327089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Qin L., Wilson B., Wu X., Qian L., Granholm A.-C., Crews F.T., Hong J.-S. Endotoxin induces a delayed loss of TH-IR neurons in substantia nigra and motor behavioral deficits. Neurotoxicology. 2008;29:864–870. doi: 10.1016/j.neuro.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masala C., Solla P., Liscia A., Defazio G., Saba L., Cannas A., Cavazzana A., Hummel T., Haehner A. Correlation among olfactory function, motors’ symptoms, cognitive impairment, apathy, and fatigue in patients with Parkinson’s disease. J. Neurol. 2018;265:1764–1771. doi: 10.1007/s00415-018-8913-9. [DOI] [PubMed] [Google Scholar]
- McGeer P.L., Itagaki S., Boyes B.E., McGeer E. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38 doi: 10.1212/wnl.38.8.1285. 1285-1285. [DOI] [PubMed] [Google Scholar]
- Niehaus I., Lange J. Endotoxin: is it an environmental factor in the cause of Parkinson’s disease? Occup. Environ. Med. 2003;60 doi: 10.1136/oem.60.5.378. 378-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley A., Collingwood J., Dobson J., Love G., Perrott H., Edwardson J., Elstner M., Morris C. Individual dopaminergic neurons show raised iron levels in Parkinson disease. Neurology. 2007;68:1820–1825. doi: 10.1212/01.wnl.0000262033.01945.9a. [DOI] [PubMed] [Google Scholar]
- Obeso J.A., Rodriguez-Oroz M.C., Goetz C.G., Marin C., Kordower J.H., Rodriguez M., Hirsch E.C., Farrer M., Schapira A.H., Halliday G. Missing pieces in the Parkinson’s disease puzzle. Nat. Med. 2010;16:653. doi: 10.1038/nm.2165. [DOI] [PubMed] [Google Scholar]
- Olsen L.K., Cairns A.G., Ådén J., Moriarty N., Cabre S., Alamilla V.R., Almqvist F., Dowd E., McKernan D.P. Viral mimetic priming enhances α-synuclein-induced degeneration: Implications for Parkinson’s disease. Brain Behav. Immun. 2019;80:525–535. doi: 10.1016/j.bbi.2019.04.036. [DOI] [PubMed] [Google Scholar]
- Pagonabarraga J., Kulisevsky J. Cognitive impairment and dementia in Parkinson’s disease. Neurobiol. Dis. 2012;46:590–596. doi: 10.1016/j.nbd.2012.03.029. [DOI] [PubMed] [Google Scholar]
- Park J.-S., Davis R.L., Sue C.M. Mitochondrial dysfunction in Parkinson’s disease: new mechanistic insights and therapeutic perspectives. Curr. Neurol. Neurosci. Rep. 2018;18:21. doi: 10.1007/s11910-018-0829-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry V.H.J.A.N. vol. 120. 2010. pp. 277–286. (Contribution of Systemic Inflammation to Chronic Neurodegeneration). [DOI] [PubMed] [Google Scholar]
- Perry V.H., Cunningham C., Holmes C.J.N.R.I. vol. 7. 2007. p. 161. (Systemic Infections and Inflammation Affect Chronic Neurodegeneration). [DOI] [PubMed] [Google Scholar]
- Poewe W. Non-motor symptoms in Parkinson’s disease. Eur. J. Neurol. 2008;15:14–20. doi: 10.1111/j.1468-1331.2008.02056.x. [DOI] [PubMed] [Google Scholar]
- Puspita L., Chung S.Y., Shim J.-w. Oxidative stress and cellular pathologies in Parkinson’s disease. Mol. Brain. 2017;10:53. doi: 10.1186/s13041-017-0340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L., Liu Y., Wang T., Wei S.-J., Block M.L., Wilson B., Liu B., Hong J.-S. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J. Biol. Chem. 2004;279:1415–1421. doi: 10.1074/jbc.M307657200. [DOI] [PubMed] [Google Scholar]
- Qin L., Wu X., Block M.L., Liu Y., Breese G.R., Hong J.S., Knapp D.J., Crews F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L., Liu Y., Hong J.S., Crews F.T. NADPH oxidase and aging drive microglial activation, oxidative stress, and dopaminergic neurodegeneration following systemic LPS administration. Glia. 2013;61:855–868. doi: 10.1002/glia.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reale M., Iarlori C., Thomas A., Gambi D., Perfetti B., Di Nicola M., Onofrj M. Peripheral cytokines profile in Parkinson’s disease. Brain Behav. Immun. 2009;23:55–63. doi: 10.1016/j.bbi.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Reeve A.K., Grady J.P., Cosgrave E.M., Bennison E., Chen C., Hepplewhite P.D., Morris C.M. Mitochondrial dysfunction within the synapses of substantia nigra neurons in Parkinson’s disease. npj Parkinson’s Dis. 2018;4:9. doi: 10.1038/s41531-018-0044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert K.R.S., Umphlet C.D., Quattlebaum A., Boger H.A. Short-term effects of an endotoxin on substantia nigra dopamine neurons. Brain Res. 2014;1557:164–170. doi: 10.1016/j.brainres.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey N.L., Wesson D.W., Brundin P. The olfactory bulb as the entry site for prion-like propagation in neurodegenerative diseases. Neurobiol. Dis. 2018;109:226–248. doi: 10.1016/j.nbd.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo K., Winner B., Carson C.T., Collier J.G., Boyer L., Rosenfeld M.G., Gage F.H., Glass C.K. A nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009;137:47–59. doi: 10.1016/j.cell.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N., Nehru B. Curcumin affords neuroprotection and inhibits α-synuclein aggregation in lipopolysaccharide-induced Parkinson’s disease model. Inflammopharmacology. 2018;26:349–360. doi: 10.1007/s10787-017-0402-8. [DOI] [PubMed] [Google Scholar]
- Song S., Jiang L., Oyarzabal E.A., Wilson B., Li Z., Shih Y.-Y.I., Wang Q., Hong J.-S. Loss of brain norepinephrine elicits neuroinflammation-mediated oxidative injury and selective caudo-rostral neurodegeneration. Mol. Neurobiol. 2018:1–17. doi: 10.1007/s12035-018-1235-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojkovska I., Wagner B.M., Morrison B.E. Parkinson’s disease and enhanced inflammatory response. Exp. Biol. Med. 2015;240:1387–1395. doi: 10.1177/1535370215576313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker T.B., Greenland J.C. Codon Publications; 2018. Parkinson’s Disease: Pathogenesis and Clinical Aspects. [PubMed] [Google Scholar]
- Sulzer D., Surmeier D.J. Neuronal vulnerability, pathogenesis, and Parkinson’s disease. Mov. Disord. 2013;28:715–724. doi: 10.1002/mds.25187. [DOI] [PubMed] [Google Scholar]
- Tansey M.G., Goldberg M.S. Neuroinflammation in Parkinson’s disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol. Dis. 2010;37:510–518. doi: 10.1016/j.nbd.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thobois S., Prange S., Sgambato-Faure V., Tremblay L., Broussolle E. Imaging the etiology of apathy, anxiety, and depression in Parkinson’s disease: implication for treatment. Curr. Neurol. Neurosci. Rep. 2017;17:76. doi: 10.1007/s11910-017-0788-0. [DOI] [PubMed] [Google Scholar]
- Tomás-Camardiel M., Rite I., Herrera A.J., de Pablos R.M., Cano J., Machado A., Venero J.L. Minocycline reduces the lipopolysaccharide-induced inflammatory reaction, peroxynitrite-mediated nitration of proteins, disruption of the blood–brain barrier, and damage in the nigral dopaminergic system. Neurobiol. Dis. 2004;16:190–201. doi: 10.1016/j.nbd.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Villacé P., Mella R.M., Kortazar D. Mitochondria in the context of Parkinson’s disease. Neural Regen. Res. 2017;12:214–215. doi: 10.4103/1673-5374.200802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitkovic L., Konsman J., Bockaert J., Dantzer R., Homburger V., Jacque C. Cytokine signals propagate through the brain. Mol. Psychiatr. 2000;5:604. doi: 10.1038/sj.mp.4000813. [DOI] [PubMed] [Google Scholar]
- Wang J., He C., Wu W.-Y., Chen F., Wu Y.-Y., Li W.-Z., Chen H.-Q., Yin Y.-Y. Biochanin A protects dopaminergic neurons against lipopolysaccharide-induced damage and oxidative stress in a rat model of Parkinson’s disease. Pharmacol. Biochem. Behav. 2015;138:96–103. doi: 10.1016/j.pbb.2015.09.013. [DOI] [PubMed] [Google Scholar]
- Wang Q., Liu Y., Zhou J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl. Neurodegener. 2015;4:19. doi: 10.1186/s40035-015-0042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Cao X.-B., Chen X.-W., Huang P.-P., Zhang T., Chen Z.-B., Tang B.-S. Influence of simvastatin on dopaminergic neurons of lipopolysaccharide–induced rat model of Parkinson’s disease. Asian Pac. J. Trop. Med. 2015;8:64–67. doi: 10.1016/S1995-7645(14)60189-9. [DOI] [PubMed] [Google Scholar]
- Zhang F.-X., Xu R.-S. Juglanin ameliorates LPS-induced neuroinflammation in animal models of Parkinson’s disease and cell culture via inactivating TLR4/NF-κB pathway. Biomed. Pharmacother. 2018;97:1011–1019. doi: 10.1016/j.biopha.2017.08.132. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Hou L., Song J.L., Song N., Sun Y.J., Lin X., Wang X.L., Zhang F.Z., Ge Y.L. Pro-inflammatory cytokine-mediated ferroportin down-regulation contributes to the nigral iron accumulation in lipopolysaccharide-induced Parkinsonian models. Neuroscience. 2014;257:20–30. doi: 10.1016/j.neuroscience.2013.09.037. [DOI] [PubMed] [Google Scholar]
- Zhang Q.-S., Heng Y., Yuan Y.-H., Chen N.-H. Pathological α-synuclein exacerbates the progression of Parkinson’s disease through microglial activation. Toxicol. Lett. 2017;265:30–37. doi: 10.1016/j.toxlet.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Zhao Y.-F., Qiong-Zhang J.-F.Z., Lou Z.-Y., Zu H.-B., Wang Z.-G., Zeng W.-C., Kai-Yao B.-G.X. The synergy of aging and LPS exposure in a mouse model of Parkinson’s disease. Aging Dis. 2018;9:785. doi: 10.14336/AD.2017.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H.-F., Yang Y.-P., Hu L.-F., Wang M.-X., Wang F., Cao L.-D., Li D., Mao C.-J., Xiong K.-P., Wang J.-D., Liu C.-F. Autophagic impairment contributes to systemic inflammation-induced dopaminergic neuron loss in the midbrain. PloS One. 2013;8 doi: 10.1371/journal.pone.0070472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L.-F., Zhang Y., Chen C.-L., Song J., Fan R.-F., Cai Q.-Q., Wang Z.-Y., Zhu J.-X. Alterations in TH- and ChAT-immunoreactive neurons in the DMV and gastric dysmotility in an LPS-induced PD rat model. Auton. Neurosci. 2013;177:194–198. doi: 10.1016/j.autneu.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Bobyn J., Mangano E.N., Gandhi A., Nelson E., Moloney K., Clarke M., Hayley S. Viral-toxin interactions and Parkinson’s disease: poly (I: C) priming enhanced the neurodegenerative effects of paraquat. J. Neuroinflammation. 2012;9:86. doi: 10.1186/1742-2094-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]