Abstract
Background
Pregnant veterans are a subpopulation known to be at elevated risk of developing mental health symptoms, such as depression and suicidal ideation. Inflammation has been associated with depression, specifically during the perinatal period. Critical changes in estradiol, cortisol, and inflammatory cytokines are necessary for the progression of a healthy pregnancy, which are then rapidly altered in the postpartum period. We explored changes in estradiol, cortisol, and pro-inflammatory cytokines relative to depressive symptoms and suicidal thoughts across pregnancy and postpartum in this pilot and feasibility study.
Methods
We measured estradiol, cortisol, and the inflammatory cytokines IL-1β, IL-6, IL-8, IFN-γ, and TNF-α in 18 pregnant veterans and analyzed the data using descriptive statistics, dependent t-tests, and correlation analyses. We assessed depression severity with the Edinburgh Postnatal Depression Scale and suicidality with the Columbia-Suicide Severity Rating Scale. Thirteen of the women repeated assessments in the early postpartum period at an average of 6.7 weeks after birth.
Results
As anticipated, estradiol (t(12) = 12.47, p < .001) and cortisol (t(12) = 9.43, p < .001) significantly decreased from pregnancy to postpartum. There were no differences in the means of gestational and postpartum IL-1β, IL-6, TNF-α, or IFN-γ, but IL-8 was significantly increased from pregnancy to postpartum (t(12) = −4.60, p = .001). Estradiol during pregnancy was positively correlated with IL-6 levels both during pregnancy (rp = .656, p = .008) and postpartum (r = 0.648, p = .023). Elevated IL-1β was associated with suicidal thoughts during pregnancy (r = 0.529, p = .029). Although not statistically significant, depressive symptom severity trended towards a positive association with larger increases in IL-1β (r = 0.535, p = .09) and TNF-α (r = 0.501, p = .08) from pregnancy to postpartum.
Conclusion
This preliminary study suggests the feasibility of our approach for exploring a complex interplay between hormonal and pro-inflammatory changes from pregnancy to postpartum, and their relationship with depressive symptoms. Given our small sample and the relatively exploratory nature of our analyses, additional investigation focusing on hormonal and inflammatory changes and their potential associations with perinatal mental health is necessary to confirm and extend our preliminary findings and examine additional potential covariates.
Keywords: Pregnancy, Postpartum, Depression, Inflammatory cytokines
Fig. 1.

Mercedes Szpunar, MD, PhD
Dr. Szpunar completed the Medical Scientist Training Program at the University of Rochester School of Medicine & Dentistry in 2014. Her doctoral thesis research investigated the impact of stress, namely sympathetic nervous system activation, on breast cancer progression and metastasis at the level of the tumor microenvironment. This project evaluated inflammatory cytokines and structural changes of breast tumors after adrenergic receptor stimulation and blockade. Dr. Szpunar received a trainee travel award to present this work at the PNIRS annual meeting. After completion of medical school, she subsequently trained in the University of California San Diego (UCSD) Psychiatry Residency Research Track and the UCSD Women’s Reproductive Mental Health clinical training program. She went on to a post-doctoral fellowship in the Advanced Fellowship in Women’s Health at the Department of Veterans Affairs (VA) San Diego.
Trained as a clinician-scientist, Dr. Szpunar is interested in delineating biological mechanisms of reproductive mood disorders. With her mentors, she designed the perinatal women veteran pilot study described in this Brain Behavior Immunity - Health special issue. In 2021, she joined the faculty at the Massachusetts General Hospital Center for Women’s Mental Health. She plans continue her investigation of neuroendocrine and inflammatory changes relative to stress and mood alterations during the female reproductive life cycle.
1. Introduction
Adaptational changes in hormones (Bloch et al., 2003) and immune cytokines (Erlebacher, 2013; Munoz-Suano et al., 2011) are critical to the progression of a healthy pregnancy. Circulating levels of estradiol, progesterone, and cortisol progressively increase as pregnancy advances until parturition. Gestational changes of these hormones contribute to maternal cellular immunity tolerance by downregulating T cell-dependent inflammatory responses to prevent fetal rejection and promote anti-inflammatory responses (Robinson and Klein, 2012). Circulating levels of estradiol and progesterone plummet and cortisol levels decrease and remain at low levels after delivery (Bloch et al., 2003), ceasing their inhibitory effect on the release of pro-inflammatory cytokines. In the early postpartum period, interleukin-6 (IL-6) and interleukin-8 (IL-8) have been shown to increase in women on postpartum days 1 and 3 compared to nonpregnant women (Maes et al., 2000, 2002). Another study found significant increases in IL-6 and tumor necrosis factor-α (TNF-α) at 4–6 weeks postpartum compared to the 3rd trimester of pregnancy (Christian and Porter, 2014). During pregnancy, high pro-inflammatory cytokines, such as IL-8, may have detrimental effects in the development of preeclampsia (Ahn et al., 2011; Arikan et al., 2012; Cudihy and Lee, 2009) and preterm labor and birth (Coussons-Read et al., 2012; Wei et al., 2010).
Inflammatory cytokines have been increasingly implicated in the pathogenesis of mood disorders in women and specifically in perinatal depression (Christian et al., 2009; Haeri et al., 2013; Leff-Gelman et al., 2016; Leff Gelman et al., 2019; Osborne et al., 2019). Perinatal depression affects up to 15 % of women (Gaynes et al., 2005), and pregnant veterans are twice as likely as their non-pregnant counterparts to develop depression, anxiety, and other mental health issues (Mattocks et al., 2010). Similarly, the prevalence of mental health disorders in postpartum women veterans is about 30 % (Miller and Ghadiali, 2018; Nillni et al., 2020). In addition to depression, women veterans are at risk for trauma and other stress-related disorders (Lehavot et al., 2018; Zinzow et al., 2007) and suicide mortality (Kimerling et al., 2016). Given their propensity for mental health pathology including perinatal depression, women veterans are an important subpopulation who warrant further examination of inflammatory cytokines during the perinatal period.
Research addressing the link between inflammatory cytokines and perinatal depression in civilian samples has had varying results. Although Blackmore et al. (2011) did not find any evidence that a pro-inflammatory state was associated with depression during the 2nd or 3rd trimesters of pregnancy, multiple investigations have reported a significant relationship between depression and 1) IL-6 and TNF-α during the 1st and 2nd trimesters of pregnancy (Christian et al., 2009; Haeri et al., 2013) and 2) interleukin-1β (IL-1β) during the 2nd trimester of pregnancy (Cassidy-Bushrow et al., 2012). During the 3rd trimester, Leff Gelman et al. (2019) reported that pregnancy IL-6, TNF-α and interferon-γ (IFN-γ) levels were significantly increased in women with depression and anxiety compared with healthy pregnant women without mood symptoms. In the postpartum period, women with depressive symptoms had significantly increased IL-6, IL-8, and TNF-α but no change in IL-1β compared with non-depressed controls (Achtyes et al., 2020).
Similarly, there have been conflicting results regarding pro-inflammatory cytokines during pregnancy and the development of postpartum depressive symptoms. Although Simpson et al. (2016) did not observe a relationship between inflammatory cytokines and depression during late pregnancy, in the early postpartum period, or across time, they did find an association between late pregnancy IL-6 levels and postpartum depressive symptoms. Despite the observation of a pro-inflammatory burst in women with depression and anxiety during the 3rd trimester, Osborne et al. (2019) did not determine any discernible patterns regarding the relationship of inflammatory cytokines and depression throughout the perinatal period. Importantly, the authors encouraged longitudinal investigation of hormonal and inflammatory changes in relation to perinatal mood changes. To our knowledge, inflammatory cytokines in pregnant women veterans with depression has yet to be formally evaluated.
As the primary stress hormone, cortisol was previously hypothesized to be elevated in postpartum depression; however, a morning reduction in cortisol levels has been the most consistent finding - with no differences at other times of the day - in postpartum women with major depressive disorder compared with healthy women (Szpunar and Parry, 2018). It is well-established that hypothalamic-pituitary-adrenal (HPA) hyperactivation in response to a stressor results in high cortisol levels and inhibits the hypothalamic-pituitary-gonadal (HPG) axis. It is conceivable that dysregulation of both HPA and HPG axes may play a role in the development of mood disorders during reproductive phases, including the perinatal period (Schweizer-Schubert et al., 2021).
Thus, we sought to explore changes in inflammatory cytokines during the perinatal period relative to depressive symptoms, cortisol, and estradiol among women veterans. Given the alterations noted by previous investigators, we analyzed the pro-inflammatory cytokines IL-6, IL-8, IL-1β, TNF-α, and IFN-γ. We hypothesized that IL-6, TNF-α, and IFN-γ would be associated with increased depressive symptoms during pregnancy, and we explored whether depression was associated with increased pro-inflammatory cytokine levels from the 3rd trimester of pregnancy to the postpartum period. Secondary analyses included exploratory analyses of individual cytokines and their associations with suicidal ideation as well as cytokine correlations with cortisol and estradiol.
2. Materials and methods
2.1. Study participants
The Department of Veterans Affairs (VA) San Diego Healthcare System Institutional Review Board approved all study procedures. Participants were assessed with a psychological assessment battery followed by biospecimen collection. From November 2017 until October 2018, 18 pregnant veterans were recruited from a census maintained by the VA maternity care coordinator. We have previously described recruitment details as part of a larger study (Szpunar et al., 2020). Briefly, written informed consent as well as sociodemographic, medical, obstetrical, and military history were obtained from the participants at the initial encounter during the 3rd trimester of pregnancy, mean 36.4 weeks gestation (SD = 1.8 weeks), and in the postpartum period at 6.7 weeks post-birth (SD = 0.9 weeks). Participants received modest compensation (gift card) after completion of each visit.
Inclusion criteria for participation for this study included: 1) female gender; 2) age 18 or older; 3) veteran status; 4) pregnant at the time of recruitment; and 5) willingness to provide biospecimens. Exclusion criteria included gestational age greater than 38 weeks and significant medical illness, such as inflammatory or autoimmune conditions. Thirteen women completed the postpartum visit.
2.2. Assessments
The Edinburgh Postnatal Depression Scale (EPDS) is 10-item scale that was developed to assess for depressive symptoms specifically during the perinatal period. Women report symptoms over the past week, with scores ranging from 0 to 30 (Cox et al., 1987). It has good psychometric properties both during pregnancy (Bergink et al., 2011) and postpartum (Boyd et al., 2005), and these investigators have established that a score of 10 or above identifies clinically relevant symptoms.
Suicidal ideation was assessed using the Columbia-Suicide Severity Rating Scale (C-SSRS), an instrument with good validity and reliability (Posner et al., 2011). During the 3rd trimester visit, the women veterans were assessed for suicidal ideation and behavior since determination of their pregnancy, and at the postpartum visit, they were assessed since having the baby. The C-SSRS queries wishes to be dead (i.e., to fall asleep and not wake up) and non-specific suicidal thoughts (i.e., “I've thought about killing myself”). If the response to either is affirmative, additional questions assess suicidal thoughts regarding methods, intent, or plan. The C-SSRS also assesses for any past suicide attempts, including actual, interrupted, or aborted attempts (Posner et al., 2011).
Participants with active symptoms at time of the assessments were allowed to continue in this study, and the women were offered treatment resources and referral within the VA for formal assessment. One participant accepted the referral, and the remainder with active symptoms were already engaging in treatment.
2.3. Blood collection
Following completion of psychological assessments, blood samples were collected into vacutainer tubes between 10:30 a.m. and 1:00 p.m. under aseptic conditions and immediately placed on wet ice and then centrifuged under refrigeration for 20 min. Plasma was aliquoted into Eppendorf tubes and stored at −80 °C for future use.
2.4. Cytokine analyses
Serum cytokines IL-6, IL-8, IL-1β, TNF-α and IFN-γ were determined by multi-cytokine array using the Meso Scale Discovery platform (MSD, Gaithersburg, MD). Plates were read by Sector 600 measuring electrochemiluminescence in accordance with the manufacturer's instructions. Sample concentrations were extrapolated from a standard curve calculated using MSD software, and samples were run in duplicate. Inter-assay coefficients of variation (CV) were: IL-6 = 12.1 %; IL-8 = 2.1 %; IL-1β = 26.9 %; TNF-α = 6.3%; and IFN-γ = 6.9 %. Intra-assay CV were: IL-6 = 11.1 %; IL-8 = 10.9 %; IL-1β = 1.1 %; TNF-α = 3.5 %; and IFN-γ = 4.1 %. Lower limit of quantifications for the individual cytokines were as follows: IL-6 0.136 pg/mL; IL-8 0.0890 pg/mL; IL-1β 0.0130 pg/mL; TNF-α 0.0394 pg/mL; and IFN-γ 1.50 pg/mL. No values were above the upper limit of detection. Samples below level of detection were not included in analyses (2 samples for IL-1β and 5 samples for IL-6).
2.5. Hormone analyses
Plasma concentrations of cortisol and estradiol were determined by enzyme-linked immunosorbent assay (ELISA) following manufacturers' protocols, IBL America (Minneapolis, MN) and R&D Systems (Minneapolis, MN), respectively. Samples were tested in duplicate and derived using a standard curve generated with each assay provided by the manufacturer using optical density at 450 nm generated by an automatic microplate reader (BioTek). For cortisol, the samples were undiluted, and intra- and interassay CV was <10 % with sensitivity of 3.8 ng/mL. For estradiol, the samples were diluted per manufacturer's instructions by a factor of 1.6. Several samples were above the upper limit of detection and hence repeated with an additional 1:10 dilution. Intra- and interassay CV was <10 % with sensitivity of 12.1 pg/mL.
2.6. Analytic plan
Statistical analyses were performed using SPSS software (Version 27). Missing data were dropped case-wise, by analysis. Descriptive statistics were calculated for demographic and clinical variables. Data were also examined for outliers, and one case was removed due to being an extreme outlier from our analyses of IFN-γ postpartum. As noted above, samples below detection level were dropped from analyses case-wise, and n's are reported by analysis below. Next, we calculated descriptive statistics and first-order correlations (two-tailed) for the hormone and cytokine levels during pregnancy and postpartum followed by dependent samples t-tests to examine changes in hormones and cytokines of interest from pregnancy to postpartum. Finally, we calculated Pearson's correlations (r) and partial correlations (rp) to examine associations between hormones and cytokines and psychosocial/clinical variables of interest, including depressive symptom severity, suicidal thoughts, and lifetime suicide attempt history while controlling for potential confounding variables, such as gestational age and parity.
3. Results
3.1. Participant characteristics
Participants were on average 31.1 years of age (SD = 4.0 years, range 26–41 years) and relatively diverse with many women identifying as non-white or biracial/multiracial (see Table 1). The majority of women were married or partnered. In terms of parity, 44.4 % were nulliparous, 44.4 % had living children, and 11.1 % had only previous miscarriage(s). We examined correlations between several demographic characteristics and our variables of interest (changes in inflammatory cytokines in the perinatal period, depressive symptoms, suicidal thoughts, cortisol, and estradiol) to consider potential covariates (e.g., maternal age, ancestry, parity, gestational age). Age of the women veterans and ancestry were not significantly associated with any measures of interest, and hence, they were not included as covariates of interest. Parity was significantly, negatively correlated with estradiol levels during pregnancy, r = −0.540, p = .021, large effect size (ES) (N = 18). Parity was included as a covariate where applicable.
Table 1.
Participant demographics.
| M (SD), %, or weeks | |
|---|---|
| Age | 31.1 (4.0) |
| Race/Ethnicity | |
| Caucasian/White | 50 % |
| Hispanic | 11 % |
| African-American/Black | 5.6 % |
| Asian American | 5.6 % |
| More than one race | 28 % |
| Gestational age at 1st assessment | 36.4 weeks |
| Weeks postpartum at 2nd assessment First pregnancy |
6.7 weeks 44.4 % |
| Marital Status | |
| Married/Partnered | 83.3 % |
| Divorced | 11.1 % |
| Single | 5.6 % |
| Education | |
| High school or equivalent | 100 % |
| Bachelor's Degree | 38.9 % |
| Graduate Degree | 11.1 % |
| Average EPDS during pregnancy Pregnancy EPDS 10 or above Average EPDS postpartum |
8.7 (4.9) 50 % 8.2 (4.6) |
| Postpartum EPDS 10 or above Thoughts of suicide during pregnancy Thoughts of suicide postpartum |
38.4 % 11.1 % 7.7 % |
EPDS = Edinburgh Postnatal Depression Scale.
Mean EPDS score during the 3rd trimester was 8.7 (range 1–17) compared with postpartum mean score of 8.2 (range 1–19). Fifty percent of the women veterans had an EPDS score of 10 or higher during pregnancy compared with 38.4 % postpartum. Gathered by the C-SSRS, 11.1 % had suicidal thoughts during pregnancy; one participant had passive thoughts (i.e., a wish to be dead with no intent or plan), and one had active thoughts (intent, but no plan). At the postpartum assessment, 7.7 % had passive suicidal thoughts. None of the women exhibited suicidal behaviors or plans.
3.2. Pregnancy to postpartum changes in hormones and cytokines
Table 2 provides descriptive statistics for the hormones and cytokines during the 3rd trimester of pregnancy and postpartum. Among women who attended both assessment visits, dependent samples t-tests (n = 13) indicated significant decreases in estradiol (t(12) = 12.47, p < .001), Cohen's d = 2.61, large ES) and cortisol (t(12) = 9.43, p < .001, Cohen's d = 3.58, large ES) from pregnancy to postpartum.
Table 2.
Mean levels of estradiol, cortisol, IL-1β, IL-6, IL-8, IFN-γ, and TNF-α
| 3rd Trimester of Pregnancy M(SD) |
Postpartum M(SD) |
|
|---|---|---|
| Estradiol | 6643.7 (2199.4) pg/mL | 114.9 (32.4) pg/mLa |
| Cortisol | 236.62 (51.38) ng/mL | 86.34 (24.13) ng/mLa |
| IL-1β | 0.0344 (0.0181) pg/mL | 0.0424 (0.0238) pg/mL |
| IL-6 | 0.722 (0.526) pg/mL | 0.739 (0.459) pg/mL |
| IL-8 | 2.302 (0.713) pg/mL | 3.287 (0.991) pg/mLa |
| IFN-γ | 5.92 (3.57) pg/mL | 7.57 (3.73) pg/mL |
| TNF-α | 1.616 (0.328) pg/mL | 1.783 (0.285) pg/mL |
Change from pregnancy to postpartum was significant, p < .05.
There were no significant differences from pregnancy to postpartum in levels of the inflammatory cytokines IL-1β, IL-6, IFN-γ or TNF-α (see Table 2). However, among 13 women veterans who attended both assessment visits (n = 5 dropped), IL-8 was significantly elevated postpartum (t(12) = −4.60, p = .001, Cohen's d = −1.26, large ES).
3.3. Associations among hormones and inflammatory cytokines
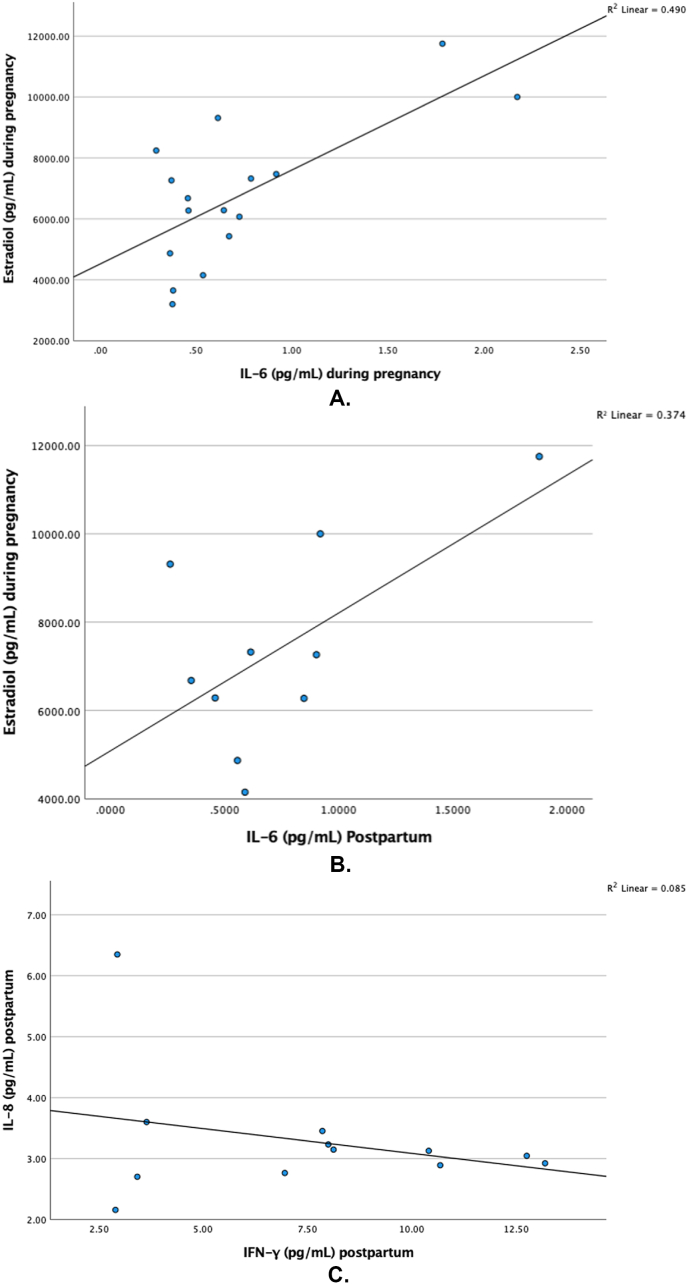
Among 16 pregnant women (n = 2 excluded due to below detectable range), IL-6 and estradiol levels were significantly positively correlated, rp = .667, p = .009, large ES, after controlling for number of weeks gestation at time of assessment and parity (Fig. 2A). During pregnancy (N = 18), cortisol and estradiol were not significantly correlated, r = 0.045, p = .859; however, at postpartum (n = 13), there was a significant negative correlation, r = −0.549, p = .05, large ES.
Fig. 2.
A. Levels of estradiol and IL-6 during pregnancy.
Fig. 2B. Levels of estradiol during pregnancy and IL-6 postpartum.
Fig. 2C. Levels of postpartum IL-8 and IFN-γ.
Estradiol during pregnancy was significantly positively correlated with IL-6 postpartum, r = 0.648, p = .023, large ES (Fig. 2B), where n = 10 women who attended both assessment visits (n = 5 excluded) and had IL-6 values within detectable range (an additional n = 3 excluded); however, this was attenuated when controlling for parity, rp = .570, p = .11, large ES. Lastly, among postpartum women (n = 13), there was a significant negative correlation between IL-8 and IFN-γ, r = −0.585, p = .046, large ES (Fig. 2C).
3.4. Correlations between depressive symptoms and inflammatory cytokines
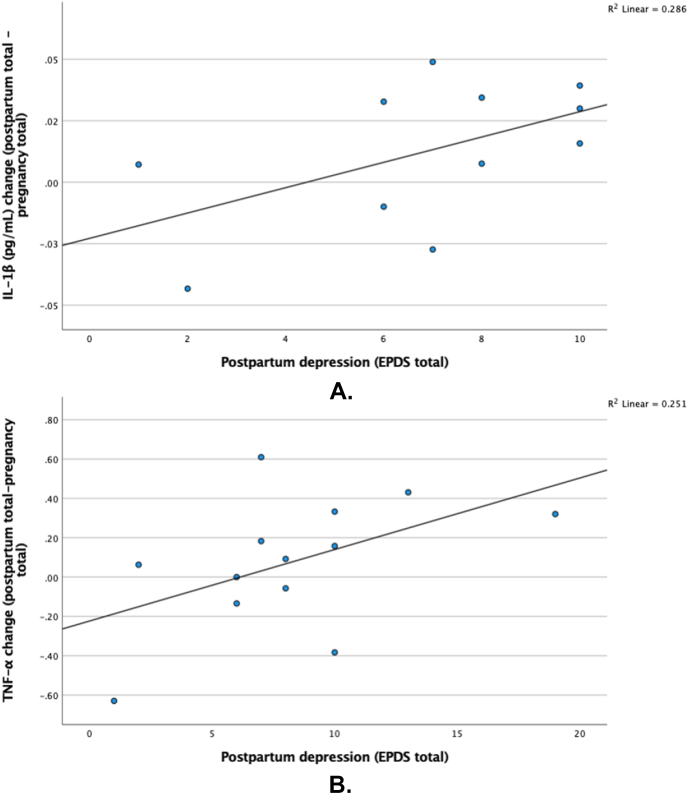
Depressive symptom severity trended towards a positive association with larger increases in IL-1β from pregnancy to postpartum (n = 11; 2 excluded for below level of detection), r = 0.535, p = .09, large ES, although this finding was not statistically significant (Fig. 3A). Similarly, depressive symptom severity trended towards a positive association with larger increases in TNF-α from pregnancy to postpartum (n = 13), r = 0.501, p = .08, large ES, although not statistically significant (Fig. 3B).
Fig. 3.
A. Change in IL-1β levels from pregnancy to postpartum and postpartum depressive symptoms (per EPDS).
Fig. 3B. Change in TNF-α levels from pregnancy to postpartum and postpartum depressive symptoms (per EPDS).
3.5. Correlations between suicidality and inflammatory cytokines
During pregnancy (n = 17; 1 excluded for below level of detection), IL-1β levels were significantly elevated among women veterans with suicidal thoughts compared to those without, r = 0.529, p = .029, large ES. There were some trends towards elevated cytokines among women with at least one lifetime suicide attempt compared with those did not attempt suicide in the past. Specifically, there were trends toward elevated IL-8 during pregnancy (t(16) = −1.94, p = .07, Cohen's d = 0.66, medium ES) and postpartum IL-6 (t(16) = −1.96, p = .09, Cohen's d = 0.40, small ES) among women with a history of suicide attempts(s) compared to those without such history.
4. Discussion and conclusions
The goal of this preliminary study was to investigate interactions of estradiol and cortisol with pro-inflammatory cytokines in this unique and highly vulnerable population, perinatal women veterans. To our knowledge, this analysis is the first to compare inflammatory cytokines and depression in women veterans across the perinatal period. Our study had several important design strengths, including the longitudinal evaluation of hormones and inflammatory cytokines from the 3rd trimester of pregnancy to postpartum and examination of important clinical variables of depression and suicidality. Contrary to our hypothesis, we did not determine any relationship between pro-inflammatory cytokine levels and depression during pregnancy. However, we found that levels of the pro-inflammatory cytokine IL-8 increase after estradiol and cortisol plummet to low levels in the early postpartum period. Glucocorticoids have been reported to have a biphasic immunoregulatory action whereby high levels of cortisol have an anti-inflammatory effect whereas lower levels may promote a pro-inflammatory state (Yeager et al., 2011). Similar to our findings, Christian and Porter (2014) observed an increase in IL-8 from pregnancy to postpartum, but they also found increases in IL-6 and TNF-α at 6–8 weeks postpartum among obese women.
As predicted, we observed a dramatic 60-fold reduction in estradiol levels in parallel with a nearly 3-fold reduction in cortisol levels from pregnancy to postpartum. Interestingly, we found significant correlations between pregnancy estradiol and both pregnancy and postpartum IL-6. This correlation is consistent with emerging evidence of crosstalk between HPG and HPA axes, which has been proposed as a mechanism of reproductive mood disorders (Schweizer-Schubert et al., 2021). It is plausible that inflammation may also contribute to this pathogenic process, but more research is needed to probe the complex interplay between inflammation and neuroendocrine mediation of mood disorders.
We found some preliminary evidence of elevated IL-1β among pregnant women with thoughts of suicide compared to those without suicidal thoughts. Previous research regarding the relationship between IL-1β and suicide has been inconclusive, with elevated IL-1β levels being associated with increased risk of suicide in some studies (Bastos et al., 2017; Monfrim et al., 2014) but not others (Achtyes et al., 2020; Ganança et al., 2020). Cassidy-Bushrow et al. (2012) found elevated IL-1β - but not other inflammatory cytokines - during the 2nd trimester of pregnancy in African-American women with depressive symptoms. The potential association of IL-1β with depressive symptoms and thoughts of suicide during pregnancy warrants further evaluation in perinatal populations with attention to ancestry.
Our findings provide some insight into the feasibility of recruitment and retention methods for research on pregnant women veterans and to guide the design of future, larger-scale research. Despite considerable efforts at retention, 5 out of 18 (27 %) of the women veterans did not complete the postpartum assessment. We acknowledge that these missing data are a limitation of the present study and recommend additional efforts at retention for future research (e.g., scheduling the assessment visit at the same time as another clinic visit). We recommend inclusion of at least one additional timepoint for data collection in order to allow for more nuanced longitudinal methodology and a more effective handling of missing data. Our initial study design included a 2nd trimester assessment, but our participants were unwilling to present at that time due to occupational and/or childcare obligations. Additionally regarding feasibility, although we attempted to strictly constrain the timing of cortisol collection to minimize differences from diurnal variation, the practical realities of the assessments and phlebotomy scheduling were not conducive to strict adherence to these procedures. Based on a detailed analysis of circadian cortisol profiles, cortisol levels decrease ~18 % from 10:30 a.m. to 1:00 p.m. (Debono et al., 2009), and it is unlikely that the circadian-related changes during our 2.5-h blood sampling time altered our data given the magnitude of difference observed from the 3rd trimester of pregnancy to the postpartum period. Nonetheless, we plan to adopt additional strategies to attempt for better control of collection time in future studies.
These exploratory analyses have limitations, most notably our small sample size and the inability to differentiate between true non-associations and low statistical power; we encourage cautious interpretation of inferential findings and hope that observed effect sizes and lessons related to feasibility will inform future research. These results may not be generalizable to populations outside of perinatal women veterans. Our recruitment method (voluntary but incentivized) may have introduced a self-selection bias. We allowed the participants to engage in treatment, if warranted, which may have impacted our postpartum results. As previously noted, assessment at additional time points during pregnancy or postpartum may have provided more detailed information pertinent to the dynamic nature of the various hormones and cytokines during the perinatal period. We only included a self-report measure of depressive symptom severity (EPDS); our findings could be further bolstered and expanded by use of clinician-administered interviews. Finally, given the small sample size and exploratory nature of this pilot study, we were unable to comprehensively assess and control for all potentially confounding variables, such as current enrollment in psychiatric treatment or weight status; future larger-scale studies should be designed and powered to more carefully control for these and other potentially confounding variables.
In conclusion, these findings suggest that the rapid loss of high circulating levels of estradiol and cortisol after parturition may permit an upregulation of some pro-inflammatory cytokines, which may confer heightened vulnerability to depression in women during the early postpartum period. These preliminary findings require replication to elucidate their significance in this specific population and more generally for reproductive mood disorders.
Disclaimer
The authors are currently or were formerly Federal employees and performed this work as part of their Federal job duties.
Funding
This material is the result of work supported with resources and the use of facilities at the VA San Diego Healthcare System, VA Center of Excellence for Stress and Mental Health (CESAMH), the Majda Foundation (San Diego, CA), and the VA Advanced Fellowship in Women's Health. Dr. Malaktaris is supported by the VA Clinical Science Research and Development Service under Career Development Award 1IK2CX002041.
Declaration of competing interest
None.
Acknowledgements
Biospecimen collection and hormone analyses were performed by CESAMH BioBank Core staff Sandra Braun and Tanya Shekhtman and financially supported by CESAMH. Cytokine analyses were performed by Dr. Suzi Hong's UCSD Integrative Health and Mind-Body Biomarker Laboratory by Christopher Pruitt. We would also like to thank Drs. Caroline Nievergelt and Victoria Risbrough for their support.
References
- Achtyes E., Keaton S.A., Smart L. Inflammation and kynurenine pathway dysregulation in post-partum women with severe and suicidal depression. Brain Behav. Immun. 2020;83:239–247. doi: 10.1016/j.bbi.2019.10.017. PMID: 31698012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn H., Gilman-Sachs A., Kwak-Kim J. Immunologic characteristics of preeclampsia: a comprehensive review. Am. J. Reprod. Immunol. 2011;65:377–394. doi: 10.1111/j.1600-0897.2010.00913.x. PMID: 20825381. [DOI] [PubMed] [Google Scholar]
- Arikan D.C., Aral M., Coskun A., Ozer A. Plasma IL-4, IL-8, IL-12, interferon-γ and CRP levels in pregnant women with preeclampsia, and their relation in severity of disease and fetal birth weight. J. Matern. Fetal Neonatal Med. 2012;25:1569–1573. doi: 10.3109/14767058.2011.648233. PMID: 22185464. [DOI] [PubMed] [Google Scholar]
- Bastos C.R., Gazal M., Quevedo L.A. Polymorphism in CRHR1 gene affects the IL-1beta levels in suicidal attempters. J. Psychiatr. Res. 2017;86:34–38. doi: 10.1016/j.jpsychires.2016.11.009. PMID: 27894002. [DOI] [PubMed] [Google Scholar]
- Bergink V., Kooistra L., Lambregtse-van den Berg M.P. Validation of the Edinburgh depression scale during pregnancy. J. Psychosom. Res. 2011;70:385–389. doi: 10.1016/j.jpsychores.2010.07.008. PMID: 21414460. [DOI] [PubMed] [Google Scholar]
- Blackmore E.R., Moynihan J.A., Rubinow D.R. Psychiatric symptoms and proinflammatory cytokines in pregnancy. Psychosom. Med. 2011;73:656–663. doi: 10.1097/PSY.0b013e31822fc277. PMID: 21949424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch M., Daly R.C., Rubinow D.R. Endocrine factors in the etiology of postpartum depression. Compr. Psychiatr. 2003;44:234–246. doi: 10.1016/S0010-440X(03)00034-8. PMID: 12764712. [DOI] [PubMed] [Google Scholar]
- Boyd R.C., Le H.N., Somberg R. Review of screening instruments for postpartum depression. Arch Womens Ment Health. 2005;8:141–153. doi: 10.1007/s00737-005-0096-6. PMID: 16133785. [DOI] [PubMed] [Google Scholar]
- Cassidy-Bushrow A.E., Peters R.M., Johnson D.A., Templin T.N. Association of depressive symptoms with inflammatory biomarkers among pregnant African-American women. J. Reprod. Immunol. 2012;94(2):202–209. doi: 10.1016/j.jri.2012.01.007. PMID: 22386525. [DOI] [PubMed] [Google Scholar]
- Christian L.M., Porter K. Longitudinal changes in serum proinflammatory markers across pregnancy and postpartum: effects of maternal body mass index. Cytokine. 2014;70:134–140. doi: 10.1016/j.cyto.2014.06.018. PMID: 25082648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian L.M., Franco A., Glaser R., Iams J.D. Depressive symptoms are associated with elevated serum proinflammatory cytokines among pregnant women. Brain Behav. Immun. 2009;23:750–754. doi: 10.1016/j.bbi.2009.02.012. PMID: 19258033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussons-Read M.E., Lobel M., Carey J.C. The occurrence of preterm delivery is linked to pregnancy specific distress and elevated inflammatory markers across gestation. Brain Behav. Immun. 2012;26:650–659. doi: 10.1016/j.bbi.2012.02.009. PMID: 22426431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J., Holden J., Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh postnatal depression scale. Br. J. Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. PMID: 3651732. [DOI] [PubMed] [Google Scholar]
- Cudihy D., Lee R.V. The pathophysiology of pre-eclampsia: current clinical concepts. J Obstet Gynecol. 2009;29:576–582. doi: 10.1080/01443610903061751. PMID: 19757258. [DOI] [PubMed] [Google Scholar]
- Debono M., Ghobadi C., Rostami-Hodjegan A. Modified-release hydrocortisone to provide circadian cortisol profiles. J. Clin. Endocrinol. Metab. 2009;94(5):1548–1554. doi: 10.1210/jc.2008-2380. PMID: 19223520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlebacher A. Immunology of the maternal-fetal interface. Annu. Rev. Immunol. 2013;31:387–411. doi: 10.1146/annurev-immunol-032712-100003. PMID: 23298207. [DOI] [PubMed] [Google Scholar]
- Ganança L., Galfalvy H.C., Cisneros-Trujullo S. Relationships between inflammatory markers and suicide risk status in major depression. J. Psychiatr. Res. 2020;134:192–199. doi: 10.1016/j.jpsychires.2020.12.029. PMID: 33388702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynes B.N., Gavin N., Meltzer-Brody S. Perinatal depression: prevalence, screen accuracy, and screen outcomes. Evid. Rep. Technol. Assess. 2005;119:1–8. doi: 10.1037/e439372005-001. PMID: 15760246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeri S., Baker A.M., Ruano R. Do pregnant women with depression have a pro-inflammatory profile? J. Obstet. Gynaecol. Res. 2013;39:948–952. doi: 10.1111/jog.12017. PMID: 23509887. [DOI] [PubMed] [Google Scholar]
- Kimerling R., Makin-Byrd K., Louzon S. Military sexual trauma and suicide mortality. Am. J. Prev. Med. 2016;50(6):684–691. doi: 10.1016/j.amepre.2015.10.019. PMID: 26699249. [DOI] [PubMed] [Google Scholar]
- Leff Gelman P., Mancillia-Herrera I., Flores-Ramos M. The cytokine profile of women with severe anxiety and depression during pregnancy. BMC Psychiatr. 2019;19:104. doi: 10.1186/s12888-019-2087-6. PMID: 30943938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff-Gelman P., Mancilla-Herrera I., Flores-Ramos M. The immune system and the role of inflammation in perinatal depression. Neurosci Bull. 2016;32:398–420. doi: 10.1007/s12264-016-0048-3. PMID: 27432060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehavot K., Katon J.G., Chen J.A. Post-traumatic stress disorder by gender and veteran status. Am. J. Prev. Med. 2018;54:e1–9. doi: 10.1016/j.amepre.2017.09.008. PMID: 29254558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M., Lin A., Ombelet W. Immune activation in the early puerperium is related to postpartum anxiety and depressive symptoms. Psychoneuroendocrinology. 2000;25:121–137. doi: 10.1016/s0306-4530(99)00043-8. PMID: 10674277. [DOI] [PubMed] [Google Scholar]
- Maes M., Verkerk R., Bonaccorso S. Depressive and anxiety symptoms in the early puerperium are related to increased degradation of tryptophan into kynurenine, a phenomenon which is related to immune activation. Life Sci. 2002;71:1837–1848. doi: 10.1016/s0024-3205(02)01853-2. PMID: 12175700. [DOI] [PubMed] [Google Scholar]
- Mattocks K.M., Skanderson M., Goulet J.L. Pregnancy and mental health among women veterans returning from Iraq and Afghanistan. J Womens Health (Larchmt) 2011;19:2159–2166. doi: 10.1089/jwh.2009.1892. PMID: 21039234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L.J., Ghadiali N.Y. Mental health across the reproductive cycle in women veterans. Mil. Med. 2018;183:e140–e146. doi: 10.1093/milmed/usx094. PMID: 29415146. [DOI] [PubMed] [Google Scholar]
- Monfrim X., Gazal M., De Leon P.B. Immune dysfunction in bipolar disorder and suicide risk: is there an association between peripheral corticotropin-releasing hormone and interleukin-1β? Bipolar Disord. 2014;16:741–747. doi: 10.1111/bdi.12214. PMID: 24862833. [DOI] [PubMed] [Google Scholar]
- Munoz-Suano A., Hamilton A.B., Betz A.G. Gimme shelter: the immune system during pregnancy. Immunol. Rev. 2011;241:20–38. doi: 10.1111/j.1600-065X.2011.01002.x. PMID: 21488887. [DOI] [PubMed] [Google Scholar]
- Osborne L.M., Yenokyan G., Fei K. Innate immune activation and depressive and anxious symptoms across the peripartum: an exploratory study. Psychoneuroendocrinology. 2019;99:80–86. doi: 10.1016/j.psyneuen.2018.08.038. PMID: 30195110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner K., Brown G.K., Stanley B. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am. J. Psychiatr. 2011;168:1266–1277. doi: 10.1176/appi.ajp.2011.10111704. PMID: 22193671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D.P., Klein S.L. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm. Behav. 2012;62(3):263–271. doi: 10.1016/j.yhbeh.2012.02.023. PMID: 22406114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer-Schubert S., Gordon J.L., Eisenlohr-Moul T.A. Steroid hormone sensitivity in reproductive mood disorders: on the role of the GABAA receptor complex and stress during hormonal transitions. Front. Med. 2021;7:479646. doi: 10.3389/fmed.2020.479646. PMID: 33585496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson W., Steiner M., Coote M., Frey B.N. Relationship between inflammatory biomarkers and depressive symptoms during late pregnancy and the early postpartum period: a longitudinal study. Br. J. Psychiatr. 2016;38:190–196. doi: 10.1590/1516-4446-2015-1899. PMID: 27579595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar M.J., Parry B.L. A systematic review of cortisol, thyroid-stimulating hormone, and prolactin in peripartum women with major depression. Arch Womens Ment Health. 2018;21:149–161. doi: 10.1007/s00737-017-0787-9. PMID: 29022126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar M.J., Crawford J.N., Baca S.A., Lang A.J. Suicidal ideation in pregnant and postpartum women veterans: an initial clinical needs assessment. Mil. Med. 2020;185:e105–e111. doi: 10.1093/milmed/usz171. PMID: 31287881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S.Q., Fraser W., Luo Z.C. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review. Obstet. Gynecol. 2010;116:393–401. doi: 10.1097/AOG.0b013e3181e6dbc0. PMID: 20664401. [DOI] [PubMed] [Google Scholar]
- Yeager M.P., Pioli P.A., Guyre P.M. Cortisol exerts bi-phasic regulation of inflammation in humans. Dose Response. 2011;9:322–347. doi: 10.2203/dose-response.10-013.Yeager. PMID: 22013396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nillni Y.I., Shayani D.R., Finley E. The impact of posttraumatic stress disorder and moral injury on women veterans' perinatal outcomes following separation from military service. J. Trauma Stress. 2020;33:248–256. doi: 10.1002/jts.22509. PMID: 32291816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinzow H.M., Grubaugh A.L., Monnier J. Trauma among female veterans: a critical review. Trauma Violence Abuse. 2007;8:384–400. doi: 10.1177/1524838007307295. PMID: 17846179. [DOI] [PubMed] [Google Scholar]