Abstract
Women suffer from major depressive disorder (MDD) more often than men and report greater MDD symptom severity. Mounting evidence suggests that sex differences in MDD may be driven, in part, by sex-specific neurobiological mechanisms. Chronic stress is a significant risk factor in MDD, and preclinical rodent models show differential patterns of stress-induced neural remodeling and cognitive-behavioral dysfunction in males and females. For instance, chronic stress leads to synapse loss in the medial prefrontal cortex in male rodents yet has either no effect on- or increases-synapse number in females. Recent reports have implicated microglia, the immune cells of the brain, in MDD, and findings demonstrate sex-specific microglial signatures in both preclinical stress models and MDD patients. Given that microglia can remodel neural architecture, modulate synaptic transmission, and affect subsequent changes in behavior, it is plausible that microglial pathways contribute to differential stress effects on neuroplasticity and function in males and females. As such, this review examines the evidence for sex-specific microglia-neuron interactions in preclinical stress models and in patients with MDD. Discoveries highlighted herein demonstrate divergent microglial contributions in males and females and suggest that future studies investigating stress-linked disorders should be guided by sex-dependent neurobiological and behavioral findings. Examining these pathways represents a clear avenue toward both a richer understanding of brain, behavior, and immunity, and innovative psychoneuroimmunology-based applications in personalized medicine.
Keywords: Sex difference, Microglia, Neuroimmune, Depression, Female
1. Introduction
Women are twice as likely to suffer from major depressive disorder (MDD), are nearly four times as likely to experience multiple depressive episodes, and are two- to three-times as likely to attempt suicide as compared to men (Weissman et al., 1996; Maciejewski et al., 2001; Kessler et al., 2005; Seedat et al., 2009). Moreover, women report greater depression severity, alongside higher rates of mood disorder-associated hypersomnia, psychomotor disturbances, decreased energy, interpersonal sensitivity, and rumination, amongst other symptoms (Kornstein et al., 2000; Fava et al., 2003; Marcus et al., 2005; Shors et al., 2017). This disparity is driven in part by gender based differences in psychological factors, interpersonal violence, and structural gender inequities (Kessler, 2003; Goodwin and Gotlib, 2004; Bulloch et al., 2017; Kuehner, 2017). Beyond psychosocial discrepancies however, mounting evidence indicates that biological sex is a crucial factor in the etiology of MDD, and suggests the potential for sex-specific interventions in the treatment of mood disorders (Kessler, 2003; Kuehner, 2017).
Chronic stress is a significant risk factor in numerous mental health disorders, including MDD (Maciejewski et al., 2001; Gotlib et al., 2021). Preclinical rodent models show patterns of stress-induced synapse loss, structural atrophy, and cognitive-behavioral dysfunction that mirror findings in patients with severe stressor exposure and MDD (Vythilingam et al., 2002; Hercher et al., 2009; Christoffel et al., 2011; Kaul et al., 2020). Considering this, rodent models represent a powerful tool in understanding the sex-specific neurobiological mechanisms associated with stress-linked disorders. Indeed, preclinical findings indicate sex-dependent stress effects on a number of brain regions implicated in depression. For instance, chronic stress reduces dendritic length and synapse number in the medial prefrontal cortex (mPFC) in males but leads to either no change- or dendritic growth-in females (Garrett and Wellman, 2009; Shansky et al., 2010; Moench and Wellman, 2017; Wohleb et al., 2018). This coincides with reductions in prefrontal activity and cognitive-behavioral dysfunction in male- but not female-rodents (Wei et al., 2014).
Alongside sex differences in neuroplasticity and behavioral function, stress has been shown to differentially affect microglia in males and females. Microglia are the resident immune cells of the central nervous system. These cells exhibit a unique morphological structure consisting of a largely stable soma with numerous thin, highly motile processes (Eyo et al., 2018). Microglia extend and retract these processes, constantly surveying the brain microenvironment. In fact, microglial processes make contact with any given synapse once every hour, effectively ‘checking up’ on neuronal health (Wake et al., 2009). As such, microglia are uniquely attuned to neuronal activity, and are responsive to various neurotransmitters, cytokines, purines, and hormones (reviewed in Eyo and Wu, 2013; Marinelli et al., 2019). Using these signals, microglia can orient their processes toward signs of distress, aiding neurons via the release of soluble factors, modulation of neurotransmission, phagocytosis of dendritic elements, and induction of synaptic growth (Weinhard et al., 2018a; Marinelli et al., 2019). Given their proximity to neurons and ability to regulate neuronal shape and function, microglia are primed to serve as critical mediators of sex-specific stress effects on neuroplasticity, cognition, and behavior – interactions which may be critical in MDD pathogenesis. Therefore, this brief review will examine stress effects on microglia in both males and females, the sex specific role of microglia in stress-induced synaptic remodeling, and sex-dependent microglial signatures in depression (see Fig. 1).
Fig. 1.

Dr. Justin L Bollinger. Dr. Bollinger completed his PhD under the mentorship of Dr. Cara Wellman at Indiana University. His graduate research explored sex-specific stress effects on microglia in the mPFC, amongst other brain regions. These studies uncovered divergent patterns of microglial remodeling in males and females exposed to stress and established a role for estradiol in modulating stress effects on microglia in females. Following this, Dr. Bollinger pursued postdoctoral training with Dr. Eric Wohleb at the University of Cincinnati, where he is currently examining pathways which guide microglia-neuron interaction in stress, including that of neuronal activity and purinergic signaling. In addition to these microglia-oriented projects, he is investigating the role of astrocytes in stress-induced vascular remodeling and blood-brain-barrier compromise. This work is supported by a Ruth L. Kirschstein National Research Service Award from the National Institute of Mental Health. The majority of research examining stress effects on the brain adheres to a neuron-centric viewpoint, leaving a critical void in our knowledge of non-neuronal contributions to brain health and dysfunction. Likewise, studies exploring stress effects on brain and behavior have largely overlooked females. Dr. Bollinger fully believes that basic research addressing these gaps has the potential to uncover innovative treatment targets in stress-linked psychological disorders. In line with this, he plans to integrate previous research aims into a broad program studying glial mechanisms underlying vascular alterations in stress, their contributions to energy maintenance, and cross-talk between the cardiovascular system and brain in both males and females.
2. Sex-specific stress effects on microglia and neuroplasticity: Preclinical models
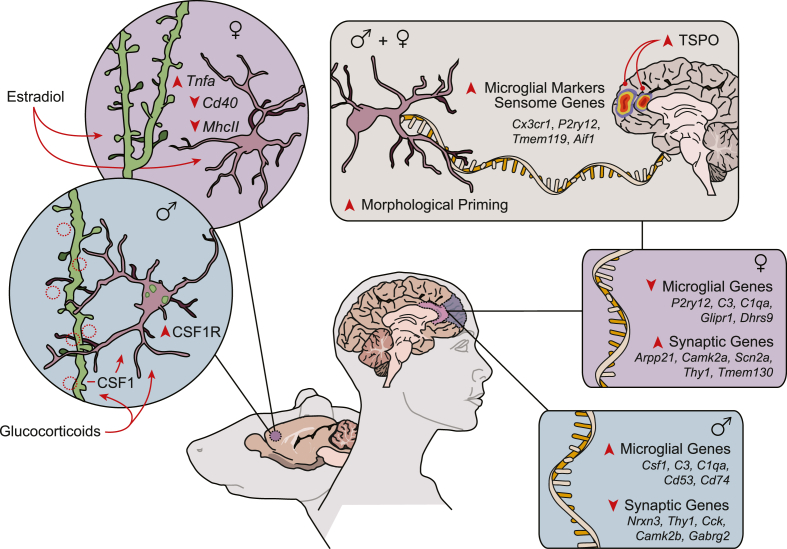
Sex differences in stress effects on microglia have been reported in various brain regions with perhaps the most pronounced divergence characterized in the mPFC. Interestingly, this region shows basal sex differences in microglial state, neuroimmune factor expression, and neuronal architecture. Findings indicate an increased ratio of microglia with broadened morphology (large cell body with thick processes)-to-surveillant microglia (small cell body with thin, highly ramified processes) in the mPFC in female as compared to male rats, suggesting microglial process engagement (Bollinger et al., 2016). Females also show increased levels of prefrontal Cx3cl1 and Cx3cr1 transcript alongside greater Csf1r, Cd11b, and Tgfbr1 expression in frontal cortex microglia (Bollinger et al., 2016; Woodburn et al., 2021). These gene pathways are particularly relevant to microglia-neuron interaction and are important regulators of microglial survival, migration, and synaptic pruning (Paolicelli et al., 2011; Schafer et al., 2012; Elmore et al., 2014). Such sex differences in microglial shape and transcriptional profile mirror sex differences in neuronal morphology. Female rats have larger dendritic arbors and greater dendritic spine density in the mPFC as compared to males (Garrett and Wellman, 2009). Considering that microglia are intimately attuned to the neuronal environment and are capable of regulating neuronal structure, larger dendritic arbors in females would suggest an increased need for microglial surveillance and maintenance in the mPFC. In line with this, females show heightened microglial complexity and neuron-microglia gene expression in this region.
Whereas chronic stress increases microglial ramification and induces neuroimmune signaling in males (Tynan et al., 2010; Hinwood et al., 2012), the opposite has been shown in females: chronic stress reduces the ratio of broadened-to-surveillant microglia in the mPFC and inhibits expression of prefrontal MhcII and Cd40, both factors associated with canonical immune activation (Bollinger et al., 2016, 2019). Similar alterations in microglial morphology are seen in the mPFC of female prairie voles following partner loss (Pohl et al., 2021). Collectively, these findings suggest divergent transcriptional and morphological effects of stress on microglia in the mPFC of male and female rodents. While not detailed here, other brain structures also show sex-dependent stress effects on microglial morphology and immunoregulatory factor expression, including the orbitofrontal cortex (OFC), basolateral amygdala, hippocampus, and nucleus accumbens (Bollinger et al., 2017; Fonken et al., 2018; Liu et al., 2019; Gaspar et al., 2021; Picard et al., 2021).
Congruent with prior morphological findings, groundbreaking work by Wohleb et al. (2018) confirmed a sex-dependent role for microglia in regulating stress effects on neuroplasticity. In this study, chronic stress increased microglial phagocytosis of dendritic elements in the mPFC, leading to decreased dendritic spine density and cognitive-behavioral dysfunction in male- but not female-mice. Moreover, chronic stress led to increased neuronal CSF1 and microglial CSF1R in the mPFC of males, suggesting a sex-specific pathway toward microglia-neuron interaction. Blocking this pathway inhibited microglia-mediated dendritic remodeling and ameliorated stress effects on neuroplasticity and behavior. These findings implicate microglia in stress-induced neuronal remodeling in male- but not female-mice, and further suggest that CSF1 signaling may guide sex-dependent stress effects on microglia-neuron interaction in the mPFC. Additional studies have demonstrated a role for glucocorticoid signaling and neural activity in mediating stress effects on microglia function in males (Horchar and Wohleb, 2019; Bollinger et al., 2020). Collectively, this work indicates that microglia play an important role in regulating sex-specific patterns of stress-induced synaptic remodeling and subsequent cognitive-behavioral change.
Recently, a study by Woodburn et al. (2021) revealed a sex-specific and temporally-dynamic role for microglia in guiding stress effects on neuroplasticity in the mPFC. As previously reported, chronic stress (14-days) induces microglia-mediated dendritic remodeling in the mPFC and behavioral dysfunction in male- but not female-mice. However, males exposed to prolonged chronic stress (28-days) exhibit the same pattern of spine loss, absent microglial engagement at this time point. This suggests that microglia remodel dendritic structures earlier in stress and that these microglial contributions to dendritic morphology are – with continued stressor exposure – enduring in the mPFC. Earlier time points in stress are marked by heightened neuronal activity and increased glutamatergic transmission (Yuen et al., 2009). Microglia are exceedingly responsive to these signals, thus, initial bursts in neuronal activity may drive stress effects on the microglial landscape and spur microglia-neuron interaction in males. Interestingly, prolonged chronic stress induces behavioral dysfunction in female mice, with relatively little change in neuroimmune factor expression or microglial engagement in the mPFC. These findings, again, suggest that microglia direct stress effects on dendritic structure and function in males, yet play a largely divergent or, at present, unknown role in regulating neuroplasticity in females.
Though few mechanisms underlying these divergent stress effects are known, gonadal hormones appear to be important. Microglia express estrogen receptors, can express androgen receptors, and are responsive to other hormonal pathways that differ between the sexes (Sierra et al., 2008; Carrillo-de Sauvage et al., 2013; Horchar and Wohleb, 2019). Findings show that manipulation of gonadal hormones in male rats shifts the morphological footprint of microglia in the mPFC yet has little effect on microglial stress responsivity (Bollinger et al., 2019). In contrast, removal of gonadal hormones via ovariectomy blocks chronic stress-induced reductions in microglial morphological area in females, whereas supplemental estradiol facilitates stress effects on microglial structure. Gonadal hormones similarly guide stress effects on neuroplasticity in the mPFC in females: ovariectomy prevents stress-induced dendritic growth, whereas estradiol facilitates stress induced increases in dendritic morphology and spine density (Garrett and Wellman, 2009; Shansky et al., 2010). This indicates a significant role for gonadal hormones in mediating stress effects on microglia and neural architecture in the mPFC, particularly in female rats. Additional studies will be needed to delineate direct androgenic- and estrogenic-actions in microglia-neuron engagement.
3. Microglia across development: Leveraging periods of plasticity to understand sex differences in stress-linked disorders
Psychological disorders are complicated, with MDD symptomology and severity likely rooted in a confluence of factors, including that of ongoing stressors, early life experiences, psychosocial factors, and biological predispositions, amongst others. While not all of these can be addressed in this short review, studies suggest that adverse early life experiences can induce lasting changes in neuroplasticity and behavior which contribute to MDD later in life (reviewed in Chen and Baram, 2015; Gumusoglu and Stevens, 2019; LeMoult et al., 2020). The brain undergoes significant growth and remodeling well into young adulthood. This timeframe is marked by a number of ‘sensitive periods’, wherein the central nervous system is more susceptible to insult. Preclinical models show that microglia play a substantial role in sculpting neuronal pathways during these ‘sensitive’ windows (Schafer et al., 2012; Sipe et al., 2016). Microglia have also been shown to undergo sex-dependent developmental trajectories, with differences in density, morphology, transcriptional profile, and phagocytic capacity observed across various brain regions (Schwarz et al., 2012; Hanamsagar et al., 2017; Weinhard et al., 2018b; Villa et al., 2019). Given these divergent trajectories and the role of microglia in synaptic sculpting, it is plausible that microglia guide sex-specific patterns of early life stress effects on brain structure, stress responsivity, and behavior. Indeed, findings indicate that adverse early life experiences and adolescent stress differentially affect microglia in male and female rodents (Diz-Chaves et al., 2012; Gildawie et al., 2020; Bekhbat et al., 2021; Gaspar et al., 2021). For instance, prenatal stress heightens the responsivity of hippocampal microglia to a peripheral immune challenge in adult female, but not male, mice (Diz-Chaves et al., 2012; Saavedra et al., 2021). A similar sex-specific pattern is seen in rodents exposed to adolescent stress, suggesting heightened susceptibility in females during these unique developmental windows (Bekhbat et al., 2019, 2021). Interestingly, both sexes appear to be vulnerable to early postnatal adversity, with studies showing altered microglia-synapse engagement and later hyperactive fear responsivity in adulthood (Bolton et al., 2021; Zetter et al., 2021). Studies have yet to examine the effects of early life adversity on microglia in humans. Together, these findings suggest that prenatal- and postnatal-stressors may program unique, sex-specific patterns of microglia-neuron interaction.
The mechanisms underlying this, alongside the potential implications of these altered microglial functions, remain largely unknown. However, it is easy to speculate that these developmental shifts in microglia may very well rewire the brain and subsequent responses to stress. For instance, loss of microglia or inhibition of microglia-neuron signaling during early postnatal development impairs typical patterns of synaptic remodeling, differentially alters behavior in males and females, and reduces levels of acute stress-induced corticosterone release in female – but not male – rats (Parkhurst et al., 2013; VanRyzin et al., 2016; Nelson and Lenz, 2017; Lowery et al., 2021). Moving forward, significant efforts will be needed to investigate sex differences in microglia across development, alongside the enduring effects of early life adversity on microglial function, neuroplasticity, and behavior in both preclinical models and in human subjects.
It should also be noted that certain life events, including pregnancy, the post-partum period, and menopause are unique to the female sex. These female-specific life events have been associated with higher rates of MDD in women (Freeman et al., 2004; Le Strat et al., 2011), with rodent models finding similar increases in MDD-associated behavior, neuroimmune signaling, and brain reorganization (Leuner and Sabihi, 2016; Haim et al., 2017; Sherer et al., 2017). This includes reductions in microglial density and area alongside alterations in synaptic plasticity in numerous brain structures implicated in MDD, including the mPFC, amygdala, and hippocampus, in pregnant and postpartum animals (Kinsley et al., 2006; Haim et al., 2017; Flores-Vivaldo et al., 2019). All of these life events are associated with dramatic shifts in gonadal hormones known to affect microglial function and neuronal structure, including estrogens and progestogens (Shansky et al., 2010; Bollinger et al., 2019; Rahimian et al., 2019). Given this, investigating microglia and stress responsivity during these dynamic life events may yield insight into the complex interplay between endocrine factors, immune signals, brain, and behavior.
4. Microglia, sex, and depression: Post-mortem reports and clinical findings
Clinical researchers have long suspected a role for peripheral immune signals in depressed mood and psychopathology (reviewed in Dantzer, 2017). Only recently have reports implicated microglia and neuroimmune pathways in depression. For instance, post-mortem findings show a greater proportion of broadened-to-surveillant microglia alongside heighted expression of the microglia-associated gene Aif1 (Iba1) in the dorsal anterior cingulate cortex (dACC) of patients with MDD who died by suicide (Torres-Platas et al., 2014). No alterations in inflammatory cytokine expression were detected in this study, suggesting increased microglial engagement in depression absent neuroinflammation. In line with this, recent transcriptomic analyses indicate a non-inflammatory, sensome-associated microglial signature in MDD (Böttcher et al., 2020; Snijders et al., 2020). The ‘sensome’ represents a cluster of genes which aid microglia in ‘sensing’ changes in the brain microenvironment (Hickman et al., 2013). In these studies, microglia were isolated from various brain regions, including the frontal cortex and thalamus, and microglial gene expression was characterized. There were similar levels of neuroinflammation-associated transcript (e.g. Il-1β, Il-6, TNFα, Cd11b, Trem2) in microglia isolated from patients with MDD and healthy controls. However, analyses revealed increased levels of microglial Cx3cr1, P2ry12, and Tmem119 expression, suggesting an upregulation of microglial sensome-functions directed toward neuronal interaction and regulation of the extracellular milieu in depressive disorders.
In addition to these data, a recent series of in vivo positron emission tomography (PET) studies found brain region-specific differences in translocator protein (TSPO)-binding in patients with ongoing MDD. TSPO is largely expressed by neurons, microglia, astrocytes, and endothelial cells in the brain and is often considered a biomarker of microglial engagement. That said, the primary function of TSPO is cholesterol trafficking in steroidogenesis, a pathway which does not necessarily reflect altered neuroimmune function (Rupprecht et al., 2010). In fact, recent data show that induced neuronal activity increases TSPO in neurons, but not glia (Notter et al., 2020). Findings indicate increased levels of TSPO-binding in the PFC and ACC in patients suffering a major depressive episode (Setiawan et al., 2015). Moreover, heightened TSPO-binding in the ACC correlates with depression severity, with increased TSPO in the PFC, ACC, and insula associated with MDD duration (Setiawan et al., 2018). Interestingly, prolonged treatment with antidepressants reduces TSPO-binding in these regions, pointing toward possible glial contributions in both depression severity and antidepressant responsivity. Similar to post-mortem findings, TSPO-binding in the ACC does not correlate with measures of mild-peripheral inflammation in MDD, again, lending support to a non-neuroinflammatory phenotype in depressed mood (Schubert et al., 2021). Though compelling, these studies lacked the sample size necessary to characterize microglial profile and TSPO-binding in both men and women, a critical step in understanding the sex-specific role of microglia in depression.
Nonetheless, a few post-mortem studies have compared molecular signatures of MDD in both sexes, with significant sex differences in pathways relevant to neuronal structure and microglial function detected across dorsolateral PFC (dlPFC), ventromedial PFC (vmPFC), ACC, and OFC (Labonté et al., 2017; Seney et al., 2018). Comparing connectivity between genes in brain samples derived from patients with MDD and healthy controls, Labonté et al. (2017) identified a gain of connectivity (GOC) in various neuronal, microglial, astrocytic, and endothelial gene modules in men, yet a loss of connectivity (LOC) in other neuronal modules. However, in women, numerous neuronal modules exhibited a GOC, whereas glial modules were largely conserved or comparable to healthy controls. Though similar gene modules were often engaged in men and women with MDD, these modules appear to be organized and expressed differentially between the sexes, pointing toward sex-dependent molecular pathways in MDD. Likewise, additional studies have found that genes associated with neuroplasticity and synaptic function are downregulated in the dlPFC and ACC in men- yet upregulated in women-with MDD, whereas genes associated with microglia and neuroimmune signaling are upregulated in men but downregulated in women (Seney et al., 2018). Differential gene expression analyses even show an increase in complement-associated expression pathways in men with MDD, yet reduced expression of microglial P2ry12 and complement genes in women. Together, these findings indicate that microglial and neuronal contributions in MDD differ between the sexes and suggest that therapeutics targeting microglia in psychiatric medicine may need to be tailored to – at the very least – patient sex (see Fig. 2).
Fig. 2.
Sex differences in microglial morphology, function, and transcriptional profile in stress and depression. This figure highlights chronic stress effects on microglia and neuroplasticity in prefrontal cortex (anatomical location noted in purple) in preclinical rodent models alongside microglial and neuronal alterations in dorsolateral prefrontal cortex (blue) and anterior cingulate cortex (pink) in men and women with MDD. Blue panel (circle): chronic stress increases microglial area and ramification and induces microglial phagocytosis of dendritic elements and dendritic spine loss (red dashed-circle) in males. This is mediated, in part, by glucocorticoids and CSF1 signaling. Purple panel (circle): in females, chronic stress reduces microglial profile, increases microglial TNFα, and induces dendritic growth. These effects on microglial function and neuroplasticity are estradiol dependent. Tan panel (rectangle): mixed-sex cohorts show an increased number of microglia with broadened morphology, heightened expression of microglial markers and sensome genes, and increased TSPO binding in MDD. Blue panel (rectangle): when men and women are compared separately, neuron-associated transcript levels are reduced in men with MDD, whereas microglia-associated transcript levels are increased. Purple panel (rectangle): the opposite occurs in women – neuron-associated transcript levels are increased in MDD, whereas microglia-associated transcript levels are decreased. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
5. Bridging translational gaps: a broader discussion of stress and adaptation in model systems
As examined here, sex differences in stress effects on microglia in rodent model systems largely align with those seen in men and women with MDD. Reports indicate an upregulation of microglial genes in the dlPFC and ACC in men with MDD, yet the opposite in women. Similarly, chronic stress increases transcripts associated with microglia-neuron interaction in the mPFC in male rodents but downregulates- or has limited effects on-microglial gene expression in females (see Fig. 2). Despite these findings, a troubling paradox exists within the literature. Whereas women suffer from greater rates of MDD and heightened symptom severity (see section 2 Introduction), rarely do studies report greater stress-induced deficits in cognition or behavior in female rats and mice. In fact, a number of studies indicate improvements in anhedonia-associated behaviors and enhanced cognitive function in female rodents exposed to chronic stress (Bowman et al., 2001, 2002; Conrad et al., 2003; Kitraki et al., 2004; Huynh et al., 2011; Wei et al., 2014). At present, these contradictory patterns make it difficult to extrapolate neurobiological findings in female rats and mice to those in women with MDD, raising a number of questions. Are current model systems accurately capturing stress effects across both sexes? And how might we extend these models to better examine the role of microglia and neuroimmune function in stress-linked psychopathology?
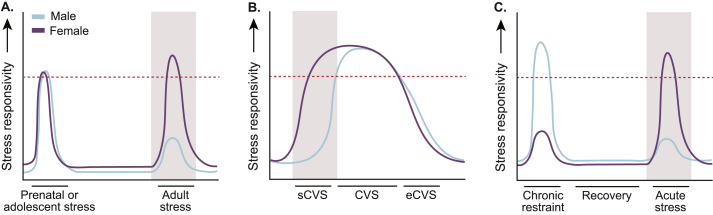
Though many potential answers exist, findings suggest that rodents experience temporal patterns of stress-induced neurobiological and behavioral dysfunction which are sex-specific, and that certain stress paradigms may be more appropriate in female rats and mice. The majority of stress models were originally designed using male rats and mice (Shansky, 2019). These stress regimens typically last for 14–21 days, with effects on brain and behavior examined immediately after stressor cessation. However, findings suggest that female rodents may exhibit deficits in behavior at earlier time points in stress, in response to specific stressors, and during stress recovery (see Fig. 3). For instance, subchronic variable stress (1 severe stressor/day for 6 days) leads to reductions in grooming, greater rates of novelty suppressed feeding, and disrupted stress coping in female mice, with males showing behavioral changes only after longer stress exposures (Hodes et al., 2015; Zhang et al., 2018; Williams et al., 2020; Johnson et al., 2021). As such, this model may be particularly useful in assessing the shared- and divergent-neurobiological mechanisms underlying stress effects on cognition and behavior in both sexes.
Fig. 3.
Differing stressor types, durations, and timepoints unmask sex-specific periods of vulnerability in rodent preclinical models. Studies examining (A) developmental stress, (B) specific stress regimens, and (C) stress recovery have revealed multiple timepoints wherein female rodents appear to be more vulnerable to adversity. Heightened stress responsivity, be it neurobiological or behavioral, is represented by either a blue (male)- or purple (female)- line crossing the red-dotted threshold. Shaded regions denote greater vulnerability or stress responsivity in females as compared to males. A. Females exposed to prenatal- or adolescent-stress mount a heightened neuroimmune response during bouts of peripheral inflammation or stress in adulthood. B. Females exhibit behavioral changes in response to sub-chronic variable stress (sCVS, 6-days), whereas behavioral shifts only emerge in males with longer durations of CVS (28-days). Acclimation occurs in both sexes following extended-CVS (eCVS, 56-days). C. Female rats previously exposed to chronic restraint stress show alterations in behavior when challenged with a subsequent acute stressor, whereas male rats do not. These scenarios highlight just three of the many nuances underlying sex differences in stress biology. A confluence of factors contributes to MDD in humans. Thus, it is likely that rodent models examining a confluence of temporal factors (including those presented in panels A–C) will lend greater translational insight into sex differences in stress neurobiology and psychopathology. Future studies aimed at understanding stress effects on brain, behavior, and immunity should take advantage of these paradigms, amongst others. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Outside of variable stress, recent reports utilizing chronic restraint indicate sex-specific neurobiological and behavioral alterations in stress recovery. Male rats exposed to chronic restraint show stress-induced behavioral changes which quickly recover following stressor cessation (Moench et al., 2019, 2020). When exposed to a subsequent mild stress, these same males display blunted neuronal activity and limited behavioral change, suggesting adaptation. In contrast, females recovering from chronic stress show increased neuronal activity and cognitive impairment in response to a subsequent stressor. This hyperactive response may reflect a lack of stress adaptation in females and, consequently, heightened vulnerability to later bouts of adversity. Considering this, what we perceive as dysfunction during- and immediately following-chronic stress may, in the long term, represent an adaptive process in males, yet a failure to adapt in females. The role of microglia and neuroimmune signals in subchronic variable stress and stress recovery remain to be studied. Likewise, studies have yet to examine stress effects on microglia and microglia-neuron interaction in other model species, including non-human primates (Deak et al., 2017). Future efforts will be needed to rectify these gaps and to more broadly explore, design, and integrate female-relevant stress models into neuroscience research (for further discussion, see Lopez and Bagot, 2021).
6. A call to action: Investigating sex-dependent pathways in psychoneuroimmunology research
This review demonstrates differing – and often opposite – stress effects on microglia and neuroplasticity in males and females, with similar neuroimmune patterns seen in MDD (see Fig. 2). While not covered here, stress has also been shown to alter peripheral immune cell composition and signaling in a sex-specific manner, with data suggesting a role for these factors in various mood disorders (reviewed in Bekhbat and Neigh, 2017; Rainville and Hodes, 2019; Martinez-Muniz and Wood, 2020). Together, such findings point toward sex-dependent, stress-associated immunoregulatory pathways in depression symptomology, and underscore the fact that heterogenous neuroimmune mechanisms – irrespective of microglia – may produce similar divergences in neurobiology, cognition, and behavior. Understanding these pathways represents an important goal in developing appropriate, sex-specific interventions in psychiatric care.
Though only one example is illustrated in this review, sex is a variable intrinsic to every mammalian cell, tissue, organ, and organ system, and is an important factor in health and disease. Studying sex differences informs our understanding of brain, behavior, and immunity, it unmasks new hypotheses and fosters a richer body of scientific knowledge. Examining males and females elucidates both important convergent pathways and equally important divergent – or sex-specific – pathways regulating brain structure and function. Preclinical research has been plagued by a fundamental bias toward the male brain and male-typical behavior, often ignoring females, failing to develop or utilize female-appropriate assays, or erroneously assuming that circulating gonadal hormones heighten variability in female data (Prendergast et al., 2014; Becker et al., 2016; Shansky, 2019). These preclinical studies both inform- and are informed by-clinical findings. Given this reciprocal relationship, an imbalance in the preclinical literature only stands to contribute to an imbalance at the clinical level, likely hindering novel advances in diagnosis and treatment (Seydel, 2021). Surely, we continue to harm both sexes by ignoring the differences between them. As researchers in the field of psychoneuroimmunology, we strive to understand the connections between immune function, neurobiology, and behavior, and to define the mechanisms whereby these connections lead to disorder. All of these connected nodes can – and often do – vary by sex. Using this understanding, it is our goal to innovate and to ultimately develop therapeutics aimed at helping people in the most effective manner possible. The new frontier of medicine is precision – to treat disorders based on patient-specific criteria. Indeed, examining divergent sex-specific pathways in biomedical research represents a clear first-step toward this goal, giving the field of psychoneuroimmunology and broader scientific community the ability to better understand and treat pathology.
Funding sources
This work was supported by the National Institute of Mental Health (F32MH123051).
Declaration of competing interest
Author has no conflict of interest to disclose.
Acknowledgments
I would like to thank Dr. Eric Wohleb, Dr. Teresa Reyes, and Samuel Woodburn for providing kind and constructive feedback on this manuscript.
References
- Becker J.B., Prendergast B.J., Liang J.W. Female rats are not more variable than male rats: a meta-analysis of neuroscience studies. Biol. Sex Differ. 2016;7:34. doi: 10.1186/s13293-016-0087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhbat M., Howell P.A., Rowson S.A., Kelly S.D., Tansey M.G., Neigh G.N. Chronic adolescent stress sex-specifically alters central and peripheral neuro-immune reactivity in rats. Brain Behav. Immun. 2019;76:248–257. doi: 10.1016/j.bbi.2018.12.005. https://pubmed.ncbi.nlm.nih.gov/30550932/ Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhbat M., Mukhara D., Dozmorov M.G., Stansfield J.C., Benusa S.D., Hyer M.M., Rowson S.A., Kelly S.D., Qin Z., Dupree J.L., Tharp G.K., Tansey M.G., Neigh G.N. Adolescent stress sensitizes the adult neuroimmune transcriptome and leads to sex-specific microglial and behavioral phenotypes. Neuropsychopharmacology. 2021:1–10. doi: 10.1038/s41386-021-00970-2. http://www.nature.com/articles/s41386-021-00970-2 Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhbat M., Neigh G.N. Sex differences in the neuro-immune consequences of stress: focus on depression and anxiety. Brain Behav. Immun. 2017 doi: 10.1016/j.bbi.2017.02.006. http://www.sciencedirect.com/science/article/pii/S0889159117300478 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger J.L., Burns C.M.B., Wellman C.L. Differential effects of stress on microglial cell activation in male and female medial prefrontal cortex. Brain Behav. Immun. 2016;52:88–97. doi: 10.1016/j.bbi.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger J.L., Collins K.E., Patel R., Wellman C.L. Behavioral stress alters corticolimbic microglia in a sex- and brain region-specific manner. PloS One. 2017;12 doi: 10.1371/journal.pone.0187631. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger J.L., Horchar M.J., Wohleb E.S. Diazepam limits microglia-mediated neuronal remodeling in the prefrontal cortex and associated behavioral consequences following chronic unpredictable stress. Neuropsychopharmacology. 2020;45:1766–1776. doi: 10.1038/s41386-020-0720-1. Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger J.L., Salinas I., Fender E., Sengelaub D.R., Wellman C.L. Gonadal hormones differentially regulate sex-specific stress effects on glia in the medial prefrontal cortex. J. Neuroendocrinol. 2019 doi: 10.1111/jne.12762. https://onlinelibrary.wiley.com/doi/abs/10.1111/jne.12762 Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton J.L., Short A.K., Othy S., Kooiker C.L., Shao M., Gunn B.G., Beck J., Bai X., Law S.M., Savage J.C., Lambert J.J., Belelli D., Tremblay M.-E., Cahalan M.D., Baram T.Z. ) Impaired developmental microglial pruning of excitatory synapses on CRH-expressing hypothalamic neurons exacerbates stress responses throughout life. 2021. https://www.biorxiv.org/content/10.1101/2021.07.21.453252v1 bioRxiv:2021.07.21. [DOI] [PMC free article] [PubMed]
- Böttcher C., Fernández-Zapata C., Snijders G.J.L., Schlickeiser S., Sneeboer M.A.M., Kunkel D., De Witte L.D., Priller J. Single-cell mass cytometry of microglia in major depressive disorder reveals a non-inflammatory phenotype with increased homeostatic marker expression. Transl. Psychiatry. 2020;10:1–11. doi: 10.1038/s41398-020-00992-2. Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman R.E., Ferguson D., Luine V.N. Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience. 2002;113:401–410. doi: 10.1016/s0306-4522(02)00156-2. [DOI] [PubMed] [Google Scholar]
- Bowman R.E., Zrull M.C., Luine V.N. Chronic restraint stress enhances radial arm maze performance in female rats. Brain Res. 2001;904:279–289. doi: 10.1016/s0006-8993(01)02474-x. [DOI] [PubMed] [Google Scholar]
- Bulloch A.G.M., Williams J.V.A., Lavorato D.H., Patten S.B. The depression and marital status relationship is modified by both age and gender. J. Affect. Disord. 2017;223:65–68. doi: 10.1016/j.jad.2017.06.007. [DOI] [PubMed] [Google Scholar]
- Carrillo-de Sauvage M.A., Maatouk L., Arnoux I., Pasco M., Sanz Diez A., Delahaye M., Herrero M.T., Newman T.A., Calvo C.F., Audinat E., Tronche F., Vyas S. Potent and multiple regulatory actions of microglial glucocorticoid receptors during CNS inflammation. Cell Death Differ. 2013;20:1546–1557. doi: 10.1038/cdd.2013.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Baram T.Z. Toward understanding how early-life stress reprograms cognitive and emotional brain networks. Neuropsychopharmacology. 2015;411 41:197–206. doi: 10.1038/npp.2015.181. https://www.nature.com/articles/npp2015181 2016. Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel D.J., Golden S.A., Russo S.J. Structural and synaptic plasticity in stress-related disorders. Rev. Neurosci. 2011;22:535–549. doi: 10.1515/RNS.2011.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad C.D., Grote K.A., Hobbs R.J., Ferayorni A. Sex differences in spatial and non-spatial Y-maze performance after chronic stress. Neurobiol. Learn. Mem. 2003;79:32–40. doi: 10.1016/s1074-7427(02)00018-7. [DOI] [PubMed] [Google Scholar]
- Dantzer R. 2017. Neuroimmune interactions: from the brain to the immune system and vice versa.https://journals.physiology.org/doi/abs/10.1152/physrev.00039.2016 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak T., Kudinova A., Lovelock D.F., Gibb B.E., Hennessy M.B. A multispecies approach for understanding neuroimmune mechanisms of stress. Dialogues Clin. Neurosci. 2017;19:37. doi: 10.31887/DCNS.2017.19.1/tdeak. Available at: /pmc/articles/PMC5442363/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diz-Chaves Y., Pernía O., Carrero P., Garcia-Segura L.M. Prenatal stress causes alterations in the morphology of microglia and the inflammatory response of the hippocampus of adult female mice. J. Neuroinflammation. 2012;9:1–10. doi: 10.1186/1742-2094-9-71. https://link.springer.com/articles/10.1186/1742-2094-9-71 Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore M.R., Najafi A.R., Koike M.A., Dagher N.N., Spangenberg E.E., Rice R.A., Kitazawa M., Matusow B., Nguyen H., West B.L., Green K.N. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 2014;82:380–397. doi: 10.1016/j.neuron.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo U.B., Mo M., Yi M.-H., Murugan M., Liu J., Yarlagadda R., Margolis D.J., Xu P., Wu L.-J. P2Y12R-Dependent translocation mechanisms gate the changing microglial landscape. Cell Rep. 2018;23:959–966. doi: 10.1016/j.celrep.2018.04.001. http://www.sciencedirect.com/science/article/pii/S2211124718305308 Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo U.B., Wu L.J. Bidirectional microglia-neuron communication in the healthy brain. Neural Plast. 2013 doi: 10.1155/2013/456857. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M., Rush A.J., Trivedi M.H., Nierenberg A.A., Thase M.E., Sackeim H.A., Quitkin F.M., Wisniewski S., Lavori P.W., Rosenbaum J.F., Kupfer D.J. Background and rationale for the sequenced treatment alternatives to relieve depression (STAR∗D) study. Psychiatr Clin North Am. 2003;26:457–494. doi: 10.1016/s0193-953x(02)00107-7. [DOI] [PubMed] [Google Scholar]
- Flores-Vivaldo Y.M., Camacho-Abrego I., Picazo O., Flores G. Pregnancies alters spine number in cortical and subcortical limbic brain regions of old rats. Synapse. 2019:73. doi: 10.1002/syn.22100. https://pubmed.ncbi.nlm.nih.gov/30958589/ Available at. [DOI] [PubMed] [Google Scholar]
- Fonken L.K., Frank M.G., Gaudet A.D., D'Angelo H.M., Daut R.A., Hampson E.C., Ayala M.T., Watkins L.R., Maier S.F. Neuroinflammatory priming to stress is differentially regulated in male and female rats. Brain Behav. Immun. 2018;70:257–267. doi: 10.1016/j.bbi.2018.03.005. http://www.sciencedirect.com/science/article/pii/S0889159118300497 Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman E.W., Sammel M.D., Liu L., Gracia C.R., Nelson D.B., Hollander L. Hormones and menopausal status as predictors of depression in womenin transition to menopause. Arch. Gen. Psychiatr. 2004;61:62–70. doi: 10.1001/archpsyc.61.1.62. https://jamanetwork.com/journals/jamapsychiatry/fullarticle/481940 Available at. [DOI] [PubMed] [Google Scholar]
- Garrett J.E., Wellman C.L. Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. Neuroscience. 2009;162:195–207. doi: 10.1016/j.neuroscience.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar R., Soares-Cunha C., Domingues A.V., Coimbra B., Baptista F.I., Pinto L., Ambrósio A.F., Rodrigues A.J., Gomes C.A. Resilience to stress and sex-specific remodeling of microglia and neuronal morphology in a rat model of anxiety and anhedonia. Neurobiol Stress. 2021 doi: 10.1016/j.ynstr.2021.100302. https://linkinghub.elsevier.com/retrieve/pii/S2352289521000102 Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gildawie K.R., Orso R., Peterzell S., Thompson V., Brenhouse H.C. Sex differences in prefrontal cortex microglia morphology: Impact of a two-hit model of adversity throughout development. Neurosci. Lett. 2020;738 doi: 10.1016/j.neulet.2020.135381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin R.D., Gotlib I.H. Gender differences in depression: the role of personality factors. Psychiatr. Res. 2004;126:135–142. doi: 10.1016/j.psychres.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Gotlib I.H., Borchers L.R., Chahal R., Gifuni A.J., Teresi G.I., Ho T.C. Early life stress predicts depressive symptoms in adolescents during the COVID-19 pandemic: the mediating role of perceived stress. Front. Psychol. 2021;11:3864. doi: 10.3389/fpsyg.2020.603748. https://www.frontiersin.org/articles/10.3389/fpsyg.2020.603748/full Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumusoglu S.B., Stevens H.E. Maternal inflammation and neurodevelopmental programming: a review of preclinical outcomes and implications for translational psychiatry. Biol. Psychiatr. 2019;85:107–121. doi: 10.1016/j.biopsych.2018.08.008. http://www.biologicalpsychiatryjournal.com/article/S0006322318317803/fulltext Available at. [DOI] [PubMed] [Google Scholar]
- Haim A., Julian D., Albin-Brooks C., Brothers H.M., Lenz K.M., Leuner B. A survey of neuroimmune changes in pregnant and postpartum female rats. Brain Behav. Immun. 2017;59:67–78. doi: 10.1016/j.bbi.2016.09.026. [DOI] [PubMed] [Google Scholar]
- Hanamsagar R., Alter M.D., Block C.S., Sullivan H., Bolton J.L., Bilbo S.D. Generation of a microglial developmental index in mice and in humans reveals a sex difference in maturation and immune reactivity. Glia. 2017;65:1504–1520. doi: 10.1002/glia.23176. https://onlinelibrary.wiley.com/doi/full/10.1002/glia.23176 Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercher C., Turecki G., Mechawar N. Through the looking glass: examining neuroanatomical evidence for cellular alterations in major depression. J. Psychiatr. Res. 2009;43:947–961. doi: 10.1016/j.jpsychires.2009.01.006. http://www.sciencedirect.com/science/article/pii/S0022395609000168 Available at. [DOI] [PubMed] [Google Scholar]
- Hickman S.E., Kingery N.D., Ohsumi T.K., Borowsky M.L., Wang L.C., Means T.K., El Khoury J. The microglial sensome revealed by direct RNA sequencing. Nat. Neurosci. 2013;16:1896–1905. doi: 10.1038/nn.3554. https://pubmed.ncbi.nlm.nih.gov/24162652/ Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinwood M., Morandini J., Day T.A., Walker F.R. Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex. Cerebr. Cortex. 2012;22:1442–1454. doi: 10.1093/cercor/bhr229. [DOI] [PubMed] [Google Scholar]
- Hodes G.E. Sex differences in nucleus accumbens transcriptome profiles associated with susceptibility versus resilience to subchronic variable stress. J. Neurosci. 2015;35:16362–16376. doi: 10.1523/JNEUROSCI.1392-15.2015. https://www.jneurosci.org/content/35/50/16362 Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horchar M.J., Wohleb E.S. Glucocorticoid receptor antagonism prevents microglia-mediated neuronal remodeling and behavioral despair following chronic unpredictable stress. Brain Behav. Immun. 2019 doi: 10.1016/j.bbi.2019.06.030. [DOI] [PubMed] [Google Scholar]
- Huynh T.N., Krigbaum A.M., Hanna J.J., Conrad C.D. Sex differences and phase of light cycle modify chronic stress effects on anxiety and depressive-like behavior. Behav. Brain Res. 2011;222:212–222. doi: 10.1016/j.bbr.2011.03.038. [DOI] [PubMed] [Google Scholar]
- Johnson A., Rainville J.R., Rivero-Ballon G.N., Dhimitri K., Hodes G.E. Testing the limits of sex differences using variable stress. Neuroscience. 2021;454:72–84. doi: 10.1016/j.neuroscience.2019.12.034. [DOI] [PubMed] [Google Scholar]
- Kaul D., Smith C.C., Stevens J., Fröhlich A.S., Binder E.B., Mechawar N., Schwab S.G., Matosin N. Severe childhood and adulthood stress associates with neocortical layer-specific reductions of mature spines in psychiatric disorders. Neurobiol Stress. 2020;13 doi: 10.1016/j.ynstr.2020.100270. https://linkinghub.elsevier.com/retrieve/pii/S2352289520300606 Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C. Epidemiology of women and depression. J. Affect. Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Walters E.E. Lifetime prevalence and age-of-onset distributions' of DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatr. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kinsley C.H., Trainer R., Stafisso-Sandoz G., Quadros P., Marcus L.K., Hearon C., Meyer E.A.A., Hester N., Morgan M., Kozub F.J., Lambert K.G. Motherhood and the hormones of pregnancy modify concentrations of hippocampal neuronal dendritic spines. Horm. Behav. 2006;49:131–142. doi: 10.1016/j.yhbeh.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Kitraki E., Kremmyda O., Youlatos D., Alexis M.N., Kittas C. Gender-dependent alterations in corticosteroid receptor status and spatial performance following 21 days of restraint stress. Neuroscience. 2004;125:47–55. doi: 10.1016/j.neuroscience.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Kornstein S.G., Schatzberg A.F., Thase M.E., Yonkers K.A., McCullough J.P., Keitner G.I., Gelenberg A.J., Ryan C.E., Hess A.L., Harrison W., Davis S.M., Keller M.B. Gender differences in chronic major and double depression. J. Affect. Disord. 2000;60:1–11. doi: 10.1016/s0165-0327(99)00158-5. [DOI] [PubMed] [Google Scholar]
- Kuehner C. Why is depression more common among women than among men? The Lancet Psychiatry. 2017;4:146–158. doi: 10.1016/S2215-0366(16)30263-2. [DOI] [PubMed] [Google Scholar]
- Labonté B. Sex-specific transcriptional signatures in human depression. Nat. Med. 2017;23:1102. doi: 10.1038/nm.4386. Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Strat Y., Dubertret C., Le Foll B. Prevalence and correlates of major depressive episode in pregnant and postpartum women in the United States. J. Affect. Disord. 2011;135:128–138. doi: 10.1016/j.jad.2011.07.004. [DOI] [PubMed] [Google Scholar]
- LeMoult J., Humphreys K.L., Tracy A., Hoffmeister J.A., Ip E., Gotlib I.H. Meta-analysis: exposure to early life stress and risk for depression in childhood and adolescence. J. Am. Acad. Child Adolesc. Psychiatry. 2020;59:842–855. doi: 10.1016/j.jaac.2019.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B., Sabihi S. The birth of new neurons in the maternal brain: hormonal regulation and functional implications. Front. Neuroendocrinol. 2016;41:99–113. doi: 10.1016/j.yfrne.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L.L., Li J.M., Su W.J., Wang B., Jiang C.L. Sex differences in depressive-like behaviour may relate to imbalance of microglia activation in the hippocampus. Brain Behav. Immun. 2019;81:188–197. doi: 10.1016/j.bbi.2019.06.012. Available at. [DOI] [PubMed] [Google Scholar]
- Lopez J., Bagot R.C. Defining valid chronic stress models for depression with female rodents. Biol. Psychiatr. 2021 doi: 10.1016/j.biopsych.2021.03.010. http://www.biologicalpsychiatryjournal.com/article/S000632232101115X/fulltext Available at. [DOI] [PubMed] [Google Scholar]
- Lowery R.L., Mendes M.S., Sanders B.T., Murphy A.J., Whitelaw B.S., Lamantia C.E., Majewska A.K. Loss of P2Y12 has behavioral effects in the adult mouse. Int. J. Mol. Sci. 2021;22:1868. doi: 10.3390/ijms22041868. https://www.mdpi.com/1422-0067/22/4/1868 Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejewski P.K., Prigerson H.G., Mazure C.M. Sex differences in event-related risk for major depression. Psychol. Med. 2001;31:593–604. doi: 10.1017/s0033291701003877. [DOI] [PubMed] [Google Scholar]
- Marcus S.M., Young E.A., Kerber K.B., Kornstein S., Farabaugh A.H., Mitchell J., Wisniewski S.R., Balasubramani G.K., Trivedi M.H., Rush A.J. Gender differences in depression: findings from the STAR∗D study. J. Affect. Disord. 2005;87:141–150. doi: 10.1016/j.jad.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Marinelli S., Basilico B., Marrone M.C., Ragozzino D. Microglia-neuron crosstalk: signaling mechanism and control of synaptic transmission. Semin. Cell Dev. Biol. 2019;94:138–151. doi: 10.1016/j.semcdb.2019.05.017. [DOI] [PubMed] [Google Scholar]
- Martinez-Muniz G.A., Wood S.K. Sex differences in the inflammatory consequences of stress: implications for pharmacotherapy. J. Pharmacol. Exp. Therapeut. 2020;375:161–174. doi: 10.1124/jpet.120.266205. https://jpet.aspetjournals.org/content/early/2020/08/05/jpet.120.266205 Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moench K.M., Breach M.R., Wellman C.L. Chronic stress produces enduring sex- and region-specific alterations in novel stress-induced c-Fos expression. Neurobiol Stress. 2019:10. doi: 10.1016/j.ynstr.2019.100147. Available at: /pmc/articles/PMC6430515/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moench K.M., Breach M.R., Wellman C.L. Prior stress followed by a novel stress challenge results in sex-specific deficits in behavioral flexibility and changes in gene expression in rat medial prefrontal cortex. Horm. Behav. 2020;117 doi: 10.1016/j.yhbeh.2019.104615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moench K.M., Wellman C.L. Differential dendritic remodeling in prelimbic cortex of male and female rats during recovery from chronic stress. Neuroscience. 2017;357:145–159. doi: 10.1016/j.neuroscience.2017.05.049. http://www.sciencedirect.com/science/article/pii/S0306452217303834 Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson L.H., Lenz K.M. Microglia depletion in early life programs persistent changes in social, mood-related, and locomotor behavior in male and female rats. Behav. Brain Res. 2017;316:279. doi: 10.1016/j.bbr.2016.09.006. Available at: /pmc/articles/PMC6103217/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notter T., Schalbetter S.M., Clifton N.E., Mattei D., Richetto J., Thomas K., Meyer U., Hall J. Neuronal activity increases translocator protein (TSPO) levels. Mol. Psychiatr. 2020:1–13. doi: 10.1038/s41380-020-0745-1. Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli R.C., Bolasco G., Pagani F., Maggi L., Scianni M., Panzanelli P., Giustetto M., Ferreira T.A., Guiducci E., Dumas L., Ragozzino D., Gross C.T. Synaptic pruning by microglia is necessary for normal brain development. Science (80- ) 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Parkhurst C.N., Yang G., Ninan I., Savas J.N., Yates J.R., Lafaille J.J., Hempstead B.L., Littman D.R., Gan W.B. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard K., St-Pierre M.-K., Vecchiarelli H.A., Bordeleau M., Tremblay M.-È. Neuroendocrine, neuroinflammatory and pathological outcomes of chronic stress: a story of microglial remodeling. Neurochem. Int. 2021 doi: 10.1016/j.neuint.2021.104987. https://linkinghub.elsevier.com/retrieve/pii/S0197018621000334 Available at. [DOI] [PubMed] [Google Scholar]
- Pohl T.T., Jung O., Di Benedetto B., Young L.J., Bosch O.J. Microglia react to partner loss in a sex- and brain site-specific manner in prairie voles. Brain Behav. Immun. 2021;96:168–186. doi: 10.1016/j.bbi.2021.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast B.J., Onishi K.G., Zucker I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 2014;40:1–5. doi: 10.1016/j.neubiorev.2014.01.001. https://pubmed.ncbi.nlm.nih.gov/24456941/ Available at. [DOI] [PubMed] [Google Scholar]
- Rahimian R., Cordeau P., Kriz J. Brain response to Injuries: when microglia go sexist. Neuroscience. 2019;405:14–23. doi: 10.1016/j.neuroscience.2018.02.048. [DOI] [PubMed] [Google Scholar]
- Rainville J.R., Hodes G.E. Inflaming sex differences in mood disorders. Neuropsychopharmacology. 2019;44:184–199. doi: 10.1038/s41386-018-0124-7. https://pubmed.ncbi.nlm.nih.gov/29955150/ Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht R., Papadopoulos V., Rammes G., Baghai T.C., Fan J., Akula N., Groyer G., Adams D., Schumacher M. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat. Rev. Drug Discov. 2010;9:971–988. doi: 10.1038/nrd3295. www.nature.com/reviews/drugdisc Available at. [DOI] [PubMed] [Google Scholar]
- Saavedra L.M., Hernández-Velázquez M.G., Madrigal S., Ochoa-Zarzosa A., Torner L. Long-term activation of hippocampal glial cells and altered emotional behavior in male and female adult rats after different neonatal stressors. Psychoneuroendocrinology. 2021;126 doi: 10.1016/j.psyneuen.2021.105164. [DOI] [PubMed] [Google Scholar]
- Schafer D.P., Lehrman E.K., Kautzman A.G., Koyama R., Mardinly A.R., Yamasaki R., Ransohoff R.M., Greenberg M.E., Barres B.A., Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert J.J., Veronese M., Fryer T.D., Manavaki R., Kitzbichler M.G., Nettis M.A., Mondelli V., Pariante C.M., Bullmore E.T., Turkheimer F.E. A modest increase in 11C-PK11195-PET TSPO binding in depression is not associated with serum C-reactive protein or body mass index. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021 doi: 10.1016/j.bpsc.2020.12.017. https://linkinghub.elsevier.com/retrieve/pii/S2451902221000252 Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J.M., Sholar P.W., Bilbo S.D. Sex differences in microglial colonization of the developing rat brain. J. Neurochem. 2012;120:948–963. doi: 10.1111/j.1471-4159.2011.07630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seedat S. Cross-National associations between gender and mental disorders in the world health organization world mental health surveys. Arch. Gen. Psychiatr. 2009;66:785–795. doi: 10.1001/archgenpsychiatry.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seney M.L., Huo Z., Cahill K., French L., Puralewski R., Zhang J., Logan R.W., Tseng G., Lewis D.A., Sibille E. Opposite molecular signatures of depression in men and women. Biol. Psychiatr. 2018;84:18–27. doi: 10.1016/j.biopsych.2018.01.017. http://www.sciencedirect.com/science/article/pii/S0006322318300659 Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan E., Attwells S., Wilson A.A., Mizrahi R., Rusjan P.M., Miler L., Xu C., Sharma S., Kish S., Houle S., Meyer J.H. Association of translocator protein total distribution volume with duration of untreated major depressive disorder: a cross-sectional study. The Lancet Psychiatry. 2018;5:339–347. doi: 10.1016/S2215-0366(18)30048-8. https://pubmed.ncbi.nlm.nih.gov/29496589/ Available at. [DOI] [PubMed] [Google Scholar]
- Setiawan E., Wilson A.A., Mizrahi R., Rusjan P.M., Miler L., Rajkowska G., Suridjan I., Kennedy J.L., Rekkas V., Houle S., Meyer J.H. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. Jama Psychiatry. 2015;72:268–275. doi: 10.1001/jamapsychiatry.2014.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydel C. The missing sex. Nat. Biotechnol. 2021 doi: 10.1038/s41587-021-00844-4. http://www.nature.com/articles/s41587-021-00844-4 Available at. [DOI] [PubMed] [Google Scholar]
- Shansky R.M. Are hormones a “female problem” for animal research? Science (80- ) 2019;364:825. doi: 10.1126/science.aaw7570. http://science.sciencemag.org/content/364/6443/825.abstract Available at. [DOI] [PubMed] [Google Scholar]
- Shansky R.M., Hamo C., Hof P.R., Lou W., McEwen B.S., Morrison J.H. Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cerebr. Cortex. 2010;20:2560–2567. doi: 10.1093/cercor/bhq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer M.L., Posillico C.K., Schwarz J.M. An examination of changes in maternal neuroimmune function during pregnancy and the postpartum period. Brain Behav. Immun. 2017;66:201–209. doi: 10.1016/j.bbi.2017.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors T.J., Millon E.M., Chang H.Y.M., Olson R.L., Alderman B.L. Do sex differences in rumination explain sex differences in depression? J. Neurosci. Res. 2017;95:711–718. doi: 10.1002/jnr.23976. [DOI] [PubMed] [Google Scholar]
- Sierra A., Gottfried-Blackmore A., Milner T.A., McEwen B.S., Bulloch K. Steroid hormone receptor expression and function in microglia. Glia. 2008;56:659–674. doi: 10.1002/glia.20644. [DOI] [PubMed] [Google Scholar]
- Sipe G.O., Lowery R.L., Tremblay M., Kelly E.A., Lamantia C.E., Majewska A.K. Microglial P2Y12 is necessary for synaptic plasticity in mouse visual cortex. Nat. Commun. 2016;7:1–15. doi: 10.1038/ncomms10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders G.J.L.J., Sneeboer M.A.M., Fernández-Andreu A., Udine E., Boks M.P., Ormel P.R., van Berlekom A.B., van Mierlo H.C., Bӧttcher C., Priller J., Raj T., Hol E.M., Kahn R.S., de Witte L.D. Distinct non-inflammatory signature of microglia in post-mortem brain tissue of patients with major depressive disorder. Mol. Psychiatr. 2020:1–14. doi: 10.1038/s41380-020-00896-z. Available at. [DOI] [PubMed] [Google Scholar]
- Torres-Platas S.G., Cruceanu C., Chen G.G., Turecki G., Mechawar N. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav. Immun. 2014;42:50–59. doi: 10.1016/j.bbi.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Tynan R.J., Naicker S., Hinwood M., Nalivaiko E., Buller K.M., Pow D.V., Day T.A., Walker F.R. Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain Behav. Immun. 2010;24:1058–1068. doi: 10.1016/j.bbi.2010.02.001. [DOI] [PubMed] [Google Scholar]
- VanRyzin J.W., Yu S.J., Perez-Pouchoulen M., McCarthy M.M. Temporary depletion of microglia during the early postnatal period induces lasting sex-dependent and sex-Independent effects on behavior in rats. eNeuro. 2016:3. doi: 10.1523/ENEURO.0297-16.2016. https://www.eneuro.org/content/3/6/eneuro.0297-16.2016 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa A., Della Torre S., Maggi A. Sexual differentiation of microglia. Front. Neuroendocrinol. 2019;52:156–164. doi: 10.1016/j.yfrne.2018.11.003. [DOI] [PubMed] [Google Scholar]
- Vythilingam M., Heim C., Newport J., Miller A.H., Anderson E., Bronen R., Brummer M., Staib L., Vermetten E., Charney D.S., Nemeroff C.B., Bremner J.D. 2002. Childhood trauma associated with smaller hippocampal volume in women with major depression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H., Moorhouse A.J., Jinno S., Kohsaka S., Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of Ischemic terminals. J. Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J., Yuen E.Y., Liu W., Li X., Zhong P., Karatsoreos I.N., McEwen B.S., Yan Z. Estrogen protects against the detrimental effects of repeated stress on glutamatergic transmission and cognition. Mol. Psychiatr. 2014;19:588–598. doi: 10.1038/mp.2013.83. [DOI] [PubMed] [Google Scholar]
- Weinhard L., di Bartolomei G., Bolasco G., Machado P., Schieber N.L., Neniskyte U., Exiga M., Vadisiute A., Raggioli A., Schertel A., Schwab Y., Gross C.T. Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat. Commun. 2018;9:1228. doi: 10.1038/s41467-018-03566-5. Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhard L., Neniskyte U., Vadisiute A., di Bartolomei G., Aygün N., Riviere L., Zonfrillo F., Dymecki S., Gross C. Sexual dimorphism of microglia and synapses during mouse postnatal development. Dev Neurobiol. 2018;78:618–626. doi: 10.1002/dneu.22568. https://pubmed.ncbi.nlm.nih.gov/29239126/ Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman M.M., Bland R.C., Canino G.J., Faravelli C., Greenwald S., Hwu H.G., Joyce P.R., Karam E.G., Lee C.K., Lellouch J., Lepine J.P., Newman S.C., RubioStipec M., Wells J.E., Wickramaratne P.J., Wittchen H.U., Yeh E.K. Cross-national epidemiology of major depression and bipolar disorder. Jama-Journal Am Med Assoc. 1996;276:293–299. [PubMed] [Google Scholar]
- Williams E.S., Manning C.E., Eagle A.L., Swift-Gallant A., Duque-Wilckens N., Chinnusamy S., Moeser A., Jordan C., Leinninger G., Robison A.J. Androgen-dependent excitability of mouse ventral hippocampal afferents to nucleus accumbens underlies sex-specific susceptibility to stress. Biol. Psychiatr. 2020;87:492–501. doi: 10.1016/j.biopsych.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb E.S., Terwilliger R., Duman C.H., Duman R.S. Stress-induced neuronal colony stimulating factor 1 provokes microglia-mediated neuronal remodeling and depressive-like behavior. Biol. Psychiatr. 2018;83:38–49. doi: 10.1016/j.biopsych.2017.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodburn S.C., Bollinger J.L., Wohleb E.S. Synaptic and behavioral effects of chronic stress are linked to dynamic and sex-specific changes in microglia function and astrocyte dystrophy. Neurobiol Stress. 2021 doi: 10.1016/j.ynstr.2021.100312. https://linkinghub.elsevier.com/retrieve/pii/S2352289521000205 Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen E.Y., Liu W., Karatsoreos I.N., Feng J., McEwen B.S., Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc. Natl. Acad. Sci. U. S. A. 2009;106:14075–14079. doi: 10.1073/pnas.0906791106. www.pnas.org/cgi/content/full/ Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetter M.A., Hernández V.S., Roque A., Hernández-Pérez O.R., Gómora M.J., Ruiz-Velasco S., Eiden L.E., Zhang L. Microglial synaptic pruning on axon initial segment spines of dentate granule cells: sexually dimorphic effects of early-life stress and consequences for adult fear response. J. Neuroendocrinol. 2021:e12969. doi: 10.1111/jne.12969. https://onlinelibrary.wiley.com/doi/full/10.1111/jne.12969 Available at. [DOI] [PubMed] [Google Scholar]
- Zhang S., Zhang H., Ku S.M., Juarez B., Morel C., Tzavaras N., Montgomery S., Hodes G.E., Brancato A., Russo S.J., Cao J.L., Han M.H. Sex differences in the neuroadaptations of reward-related circuits in response to subchronic variable stress. Neuroscience. 2018;376:108–116. doi: 10.1016/j.neuroscience.2018.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]